Thermal Modulation of Musalais Wine Characteristics: Volatile Profiles and Chemical Composition at Different Brix Levels
Abstract
1. Introduction
2. Materials and Methods
2.1. Fermentation Assays
2.2. Determination of the Basic Physicochemical Indicators of Musalais Wine
2.2.1. Determination of Total Acidity (TA)
2.2.2. Alcohol Concentration
2.2.3. pH
2.2.4. Residual Sugar Content
2.2.5. Vitamin C (VC)
2.3. Analysis of Polyphenol Profile
2.3.1. Total Polyphenol Content (TPC)
2.3.2. Total Flavonoid Content (TFC)
2.3.3. Total Anthocyanin Content
2.4. Analysis of Volatile Compounds by GC-IMS
2.5. Statistical Analysis
3. Results and Discussion
3.1. Basic Physicochemical Properties of Musalais Wine
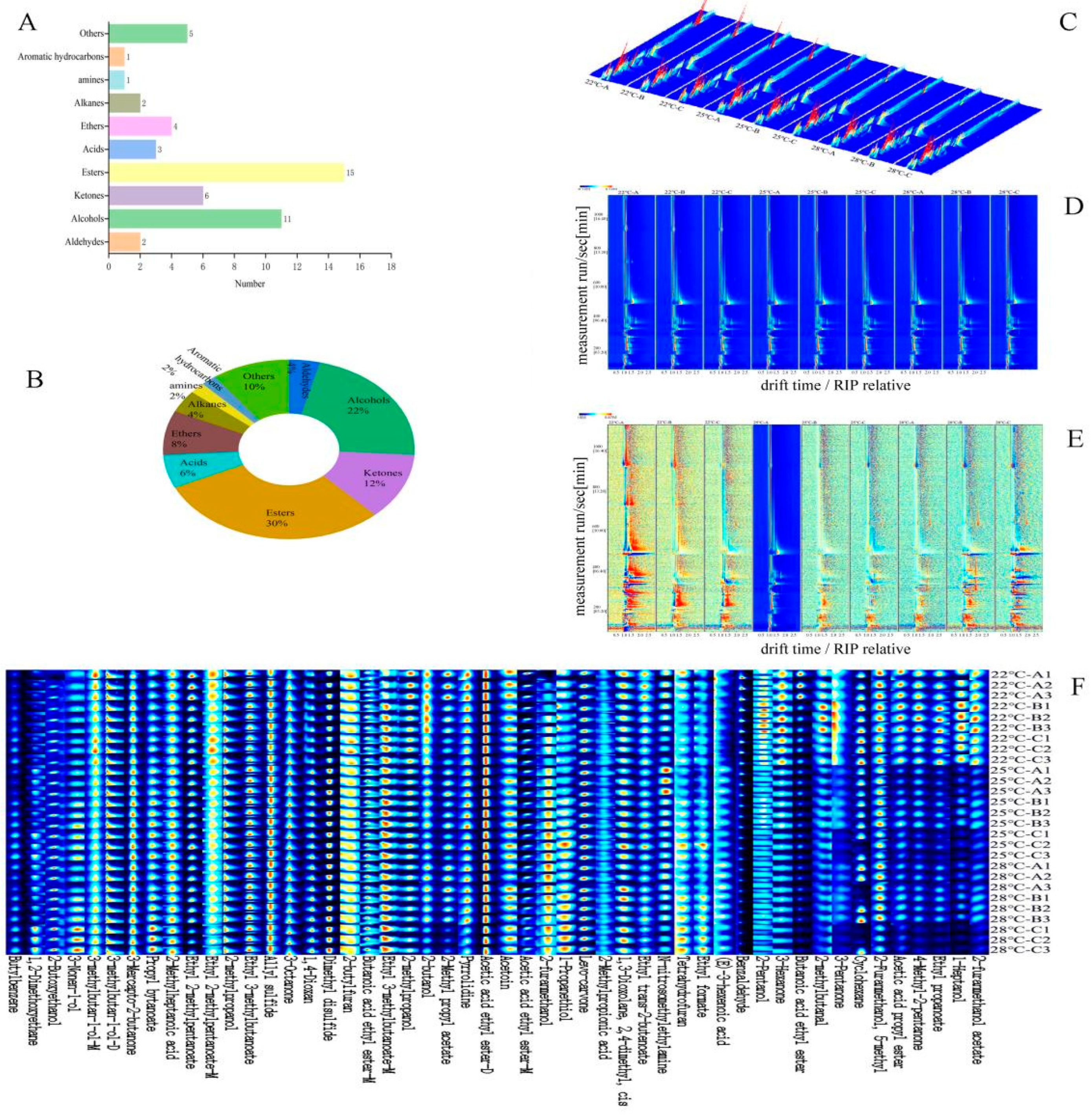
3.2. Volatile Compounds Identified by GC-IMS
3.3. Graph Analysis
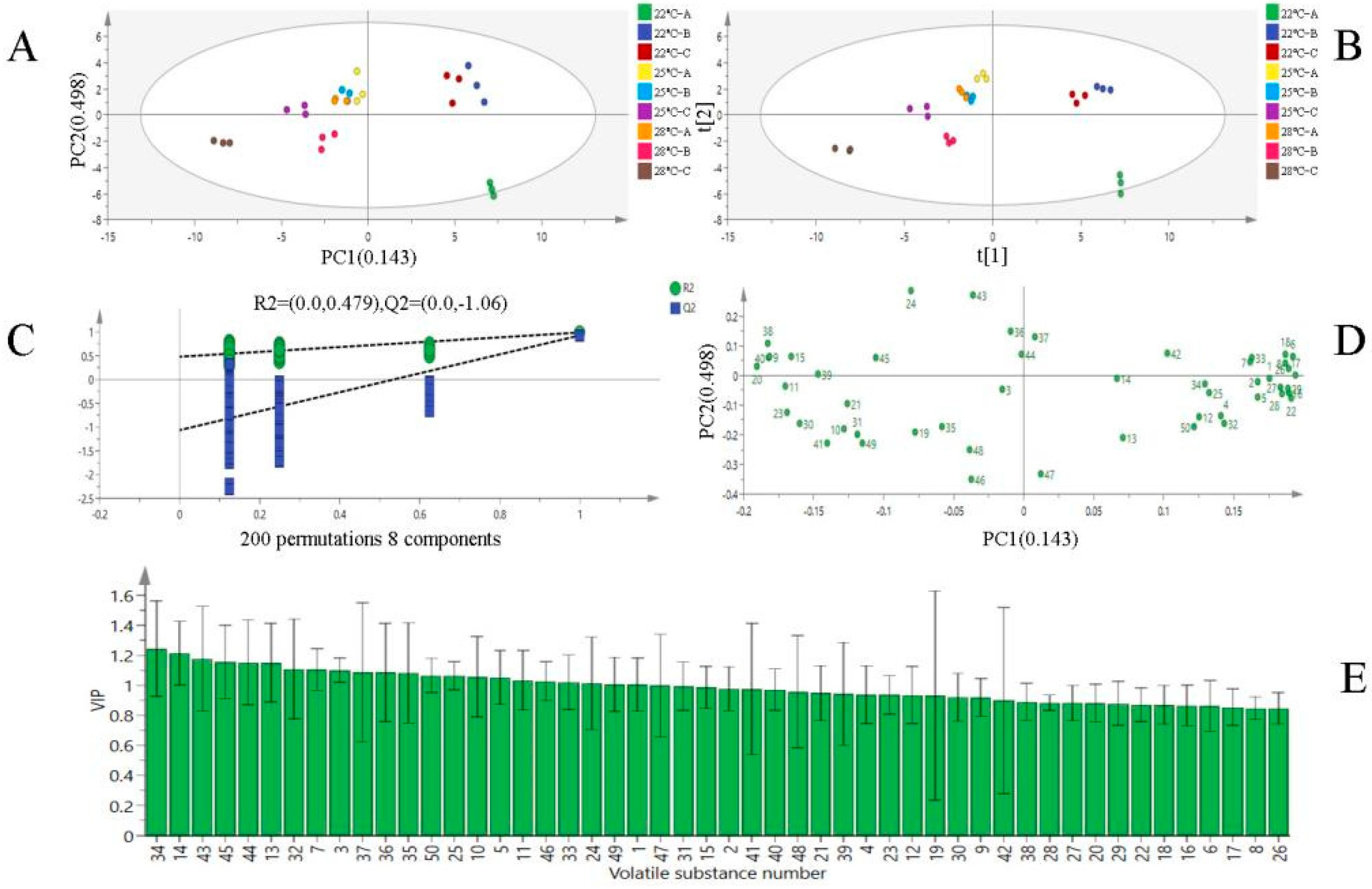
3.4. Multivariate Statistical Analysis of Volatile Substances
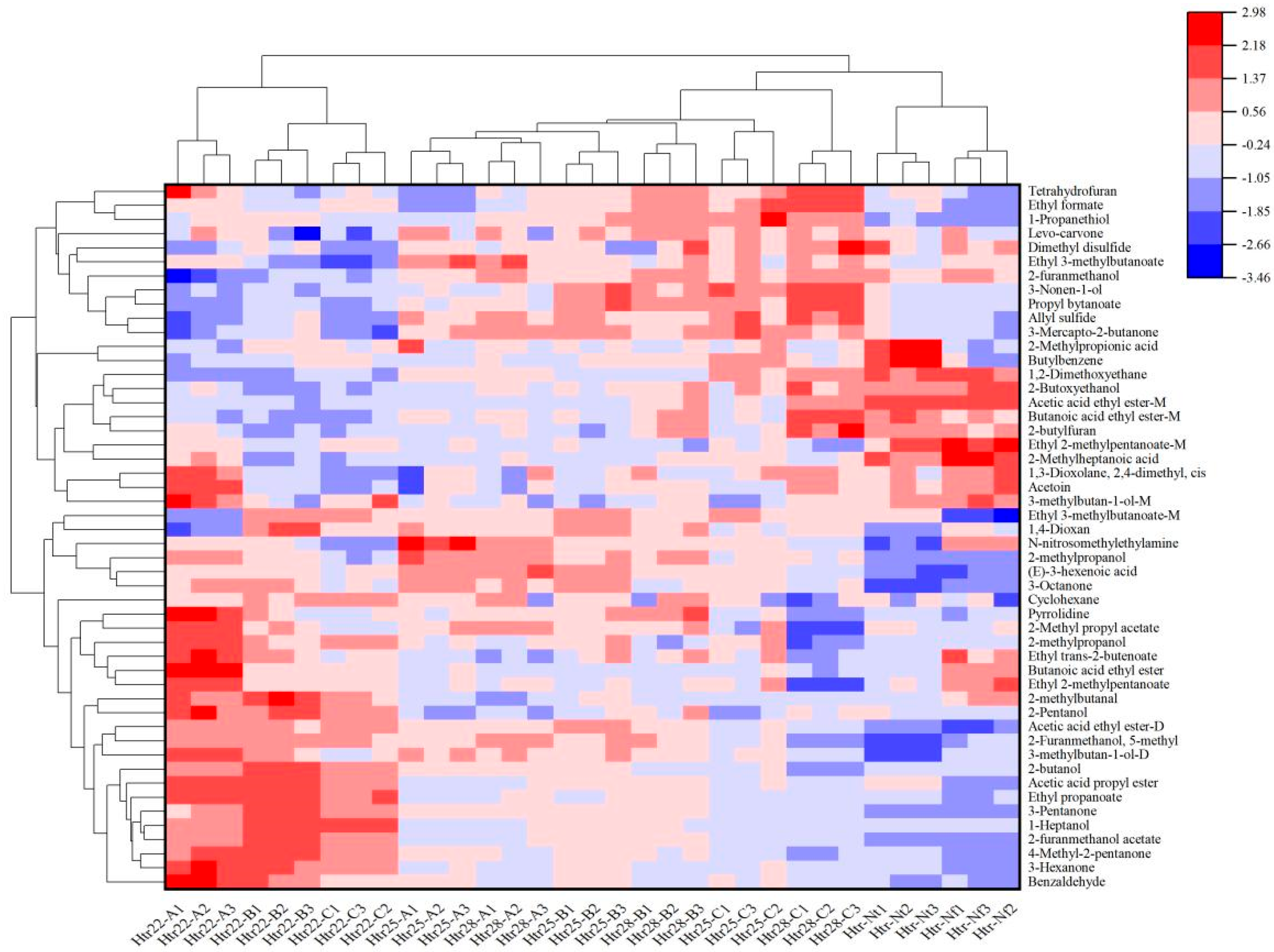
3.5. Cluster Analysis of VOCs from Musalais Wine with Different Brewing and Fermentation Conditions
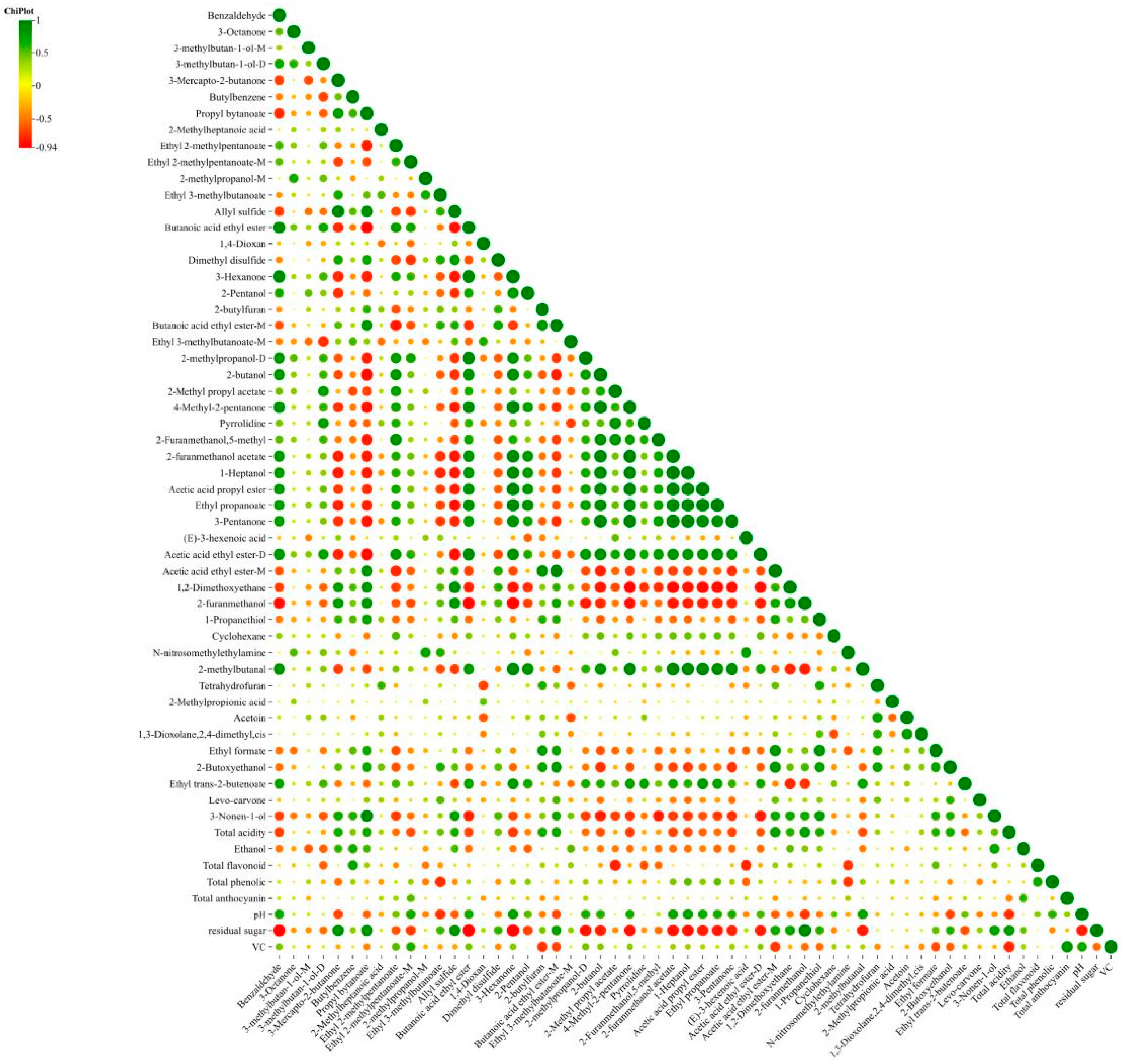
3.6. Correlation Analysis Between Physicochemical Indices and Volatile Substances
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Izquierdo-Cañas, P.M.; González Viñas, M.A.; Mena-Morales, A.; Pérez Navarro, J.; García-Romero, E.; Marchante-Cuevas, L.; Gómez-Alonso, S.; Sánchez-Palomo, E. Effect of fermentation temperature on volatile compounds of Petit Verdot red wines from the Spanish region of La Mancha (central-southeastern Spain). Eur. Food Res. Technol. 2020, 246, 1153–1165. [Google Scholar] [CrossRef]
- Spranger, M.I.; Climaco, M.C.; Sun, B.; Eiriz, N.; Fortunato, C.; Nunes, A.; Leandro, C.; Avelar, M.L.; Belchior, A.P. Differentiation of red winemaking technologies by phenolic and volatile composition. Anal. Chim. Acta 2004, 513, 151–161. [Google Scholar] [CrossRef]
- Tang, R.; Abudureheman, B.; Zhang, J.L.; Chen, L.; Li, H.Y.; Zhu, S.; Guo, M.Q.; Huang, J.L.; Zhu, X.; Ye, X.Q. Fermentation dynamics: Microbial and metabolite shifts in Musalais wine. J. Agric. Food Res. 2025, 23, 102204. [Google Scholar] [CrossRef]
- Yang, F.; Chen, C.; Ni, D.R.; Yang, Y.B.; Tian, J.H.; Li, Y.Y.; Chen, S.G.; Ye, X.Q.; Wang, L. Effects of fermentation on bioactivity and the composition of polyphenols contained in polyphenol-rich foods: A Review. Foods 2023, 12, 3315. [Google Scholar] [CrossRef]
- Du, X.; Myracle, A.D. Fermentation alters the bioaccessible phenolic compounds and increases the alpha-glucosidase inhibitory effects of aronia juice in a dairy matrix following in vitro digestion. Food Funct. 2018, 9, 2998–3007. [Google Scholar] [CrossRef]
- Zhu, S.L.; Chen, Q.L.; Zhang, W.X.E.; He, J.; Guo, S.T.; Kan, L.P.; Yang, X.Y. Effect of different fermentation methods on the aroma and sensory characteristics of Musalais wine. Zhongguo Niangzao 2025, 446, 163–170. [Google Scholar]
- Wu, R.M.; Chen, Q.L.; Aypari, A.; Guliziba, A.; Zhang, X.; Yang, X.Y. Fermentation process optimization and quality analysis of red grape Musalais in Xinjiang. Zhongguo Niangzao 2024, 43, 182–188. [Google Scholar]
- Li, Z.J.; Zhang, X.D.; Long, B.X.; Cao, Y.; Hou, X.J. Research on product development and response surface optimization of Msalais pear. Agric. Prod. Process. 2025, 3, 1–10. [Google Scholar]
- Zhu, L.X.; Wang, L.L.; Song, H.Z.; Guo, D.Q.; Fan, Y.G.; Hou, C.H.; Xue, J.L. Qualitative analysis of the main aroma compounds associated with traditional Musalais processing in Xinjiang, China. J. Inst. Brew. 2012, 118, 236–242. [Google Scholar] [CrossRef]
- Gao, X.Y.; Bai, Y.J.; Zheng, W.C.; Chen, L.H.; Fan, S.H.; Feng, Z.S. Study on the quality and aroma components of Musalais by different treatments. Food Mach. 2019, 35, 32–39. [Google Scholar]
- Zhang, L.; Zhang, Y.; Huang, Y.; Xuan, Y.; Hou, X.J. Changes of volatile components in Musalais by E-nose and GC-MS. Sci. Technol. Food Ind. 2018, 39, 242–249. [Google Scholar] [CrossRef]
- Yang, B.; He, S.; Liu, Y.; Liu, B.; Ju, Y.; Kang, D.; Sun, X.; Fang, Y. Transcriptomics integrated with metabolomics reveals the effect of regulated deficit irrigation on anthocyanin biosynthesis in Cabernet Sauvignon grape berries. Food Chem. 2020, 314, 126170. [Google Scholar] [CrossRef]
- Cai, Y.N.; Li, X.; Ding, J.; Huang, J.Y.; Bao, X.Y. Uncertainty evaluation for the determination of alcohol content in re-test compound wine by pycnometric method. J. Food Saf. Qual. 2021, 12, 1766–1772. [Google Scholar]
- Association of Official Analytical Chemists Inc. Changes in official methods of analysis of the Association of Official Analytical Chemists, Second supplement, 1991 to the fifteenth edition 1991, pp. 61–117. Available online: https://www.scirp.org/reference/referencespapers?referenceid=440931 (accessed on 8 July 2025).
- Yadav, D.K.; Chand, K.; Kumari, P. Effect of fermentation parameters on physicochemical and sensory properties of Burans wine. Syst. Microbiol. Biomanufacturing 2022, 2, 380–392. [Google Scholar] [CrossRef]
- Varo, M.A.; Martin-Gomez, J.; Serratosa, M.P.; Merida, J. Effect of potassium metabisulphite and potassium bicarbonate on color, phenolic compounds, vitamin C and antioxidant activity of blueberry wine. LWT 2022, 163, 113585. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, Y.; Yuan, T.; Xie, J.; Yu, Q.; Chen, Y. Polyphenol compounds contributing to the improved bioactivities of fermented Rubus chingii Hu. Food Res. Int. 2024, 197, 115218. [Google Scholar] [CrossRef]
- Sharma, A.; Yadav, M.; Sharma, N.; Kumari, A.; Kaur, S.; Meenu, M.; Garg, M. Comparison of wheatgrass juices from colored wheat (white, black, blue, and purple) for health promoting phytochemicals. Food Res. Int. 2022, 161, 111833. [Google Scholar] [CrossRef]
- Jiang, P.; Miao, X.; Li, J.; Qi, H.; Shang, S.; Dong, X. Volatile flavor characteristics of scallops (Chlamys farreri) with different drying methods were analyzed based on GC-IMS and GC-O-QTOF. Food Chem. X 2024, 24, 101960. [Google Scholar] [CrossRef] [PubMed]
- Liu, W. A Study on Ohmic Heating Properties and Its Physical-Chemical Characters of Fruit Juice with Fruit Particles. Ph.D. Thesis, Jilin University, Changchun, China, 2005. [Google Scholar]
- Du, Q.; Zhi, R.J.; Zang, X.M.; Qu, R.; Ye, D.Q.; Nan, H.; Liu, Y.L. Reshaping yeast metabolism and enhancing the quality of fresh-style red wine through low-temperature fermentation. LWT-Food Sci. Technol. 2024, 191, 115705. [Google Scholar] [CrossRef]
- Don, S.M.; Rambli, M.; Nore, B.F. Antioxidant content following fermentation of lemongrass for herbal beverage development. J. Food Sci. Technol. 2024, 61, 1–14. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, D.; Chen, X.; Kilmartin, P.; Quek, S.Y. The influence of vinification methods and cultivars on the volatile and phenolic profiles of fermented alcoholic beverages from cranberry. Antioxidants 2019, 8, 144. [Google Scholar] [CrossRef]
- Ampofo, J.; Ngadi, M.; Ramaswamy, H.S. The impact of temperature treatments on elicitation of the phenylpropanoid pathway, phenolic accumulations and antioxidative capacities of common bean (Phaseolus vulgaris) sprouts. Food Bioprocess Technol. 2020, 13, 1544–1555. [Google Scholar] [CrossRef]
- Zhou, Q.E.; Wang, X.Y.; Tian, C.R.; Guo, Y.R. Comparison of enological characteristics between fresh apple juice and juice concentrate. Sci. Technol. Food Ind. 2011, 32, 225–227. [Google Scholar]
- Zhang, H.; Jiang, Y.; Lv, Y.; Pan, J.; Duan, Y.; Huang, Y.; Geng, K. Effect of water quality on the main components in Fuding white tea infusions. J. Food Sci. Technol. 2017, 54, 1206–1211. [Google Scholar] [CrossRef]
- Qiao, T.T.; Zhu, L.X. Aroma threshold and binary synergy of furanone, 5-methylfurfural and 3-methylthiopropanol in Musalais wine. Food Sci. 2023, 44, 176–183. [Google Scholar]
- Miao, X.; Li, S.; Shang, S.; Sun, N.; Dong, X.; Jiang, P. Characterization of volatile flavor compounds from fish maw soaked in five different seasonings. Food Chem. X 2023, 19, 100805. [Google Scholar] [CrossRef]
- Lu, X.K.; Yang, B.; Sun, Y.; He, J.N.; Fan, B.M.; Sun, H.; Sun, X.T. Feature analysis and identification of Baijiu based on gas chromatography-ion migration spectrometry. J. Chin. Inst. Food Sci. Technol. 2023, 23, 278–295. [Google Scholar]
- Hou, R.; Jelley, R.E.; van Leeuwen, K.A.; Pinu, F.R.; Fedrizzi, B.; Deed, R.C. Hydrogen sulfide production during early yeast fermentation correlates with volatile sulfur compound biogenesis but not thiol release. FEMS Yeast Res. 2023, 23, foad031. [Google Scholar] [CrossRef] [PubMed]
- Kopriva, S.; Malagoli, M.; Takahashi, H. Sulfur nutrition: Impacts on plant development, metabolism, and stress responses. J. Exp. Bot. 2019, 70, 4069–4073. [Google Scholar] [CrossRef]
- Sirisena, S.; Chan, S.; Roberts, N.; Dal Maso, S.; Gras, S.L.; Martin, G. Influence of yeast growth conditions and proteolytic enzymes on the amino acid profiles of yeast hydrolysates: Implications for taste and nutrition. Food Chem. 2024, 437, 137906. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, D.; Li, G.; Hao, J.; Jiang, W.; Liu, Z.; Qin, Q. Flavor contribution of esters in lager beers and an analysis of their flavor thresholds. J. Am. Soc. Brew. Chem. 2017, 75, 201–206. [Google Scholar] [CrossRef]
- Alves, V.; Gonçalves, J.; Figueira, J.A.; Ornelas, L.P.; Branco, R.N.; Câmara, J.S.; Pereira, J.A. Beer volatile fingerprinting at different brewing steps. Food Chem. 2020, 326, 126856. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhao, P.; Du, G.; Zhai, J.; Guo, Y.; Wang, X. Advancements and challenges for brewing aroma-enhancement fruit wines: Microbial metabolizing and brewing techniques. Food Chem. 2024, 456, 139981. [Google Scholar] [CrossRef] [PubMed]
- Alises, M.O.; Sanchez-Palomo, E.; Gonz’alez-Vinas, M.A. Effects of winemaking techniques on the volatile compounds of Chelva wines. Food Biosci. 2023, 59, 104121. [Google Scholar] [CrossRef]
- Cao, W.Y.; Shu, N.; Yang, Y.M.; Wen, J.L.; Lu, W.P. Comprehensive evaluation of nine grape varieties based on fundamental physical and chemical indicators, color and volatile compounds. Res. Rep. 2023, 13, 121–144. [Google Scholar] [CrossRef]
- Sanchez-Palomo, E.; Perez-Coello, M.S.; Díaz-Maroto, M.C.; Gonzalez-Vinas, M.A.; Cabezudo, M.D. Contribution of free and glycosidically-bound volatile compounds to the aroma of muscat “a petit grains” wines and effect of skin contact. Food Chem. 2006, 95, 279–289. [Google Scholar] [CrossRef]
- Xu, N.; Lu, H.; Feng, L.G.; Huang, X.-H. Volatile components analysed by HS-SPME-GC-MS in different parts of fruiting bodies and nutritional composition of Oudemansiella raphanipes. Mycosystema 2020, 39, 1933–1947. [Google Scholar]
- Jin, W.G.; Liu, J.X.; Sun, H.Y.; He, L.L.; Pei, J.J.; Cheng, H.; Jiang, P.F. Characterization of volatile organic compounds of Giant Salamander (Andrias davidianus) oil adulterated with different amounts of peanut oil by gas chromatography-ion mobility spectrometry combined with chemometrics. Food Sci. 2023, 44, 368–376. [Google Scholar] [CrossRef]
- He, P.; Hassan, M.M.; Tang, F.; Jiang, H.; Chen, M.; Liu, R.; Lin, H.; Chen, Q. Total fungi counts and metabolic dynamics of volatile organic compounds in paddy contaminated by Aspergillus niger during storage employing gas chromatography-ion mobility spectrometry. Food Anal. Methods 2022, 15, 1638–1651. [Google Scholar] [CrossRef]
- Shao, S.X.; Xu, M.M.; Lin, Y.P.; Chen, X.M.; Fang, D.Y.; Cai, J.Y.; Wang, J.H.; Jin, S.; Ye, N.X. Differential analysis of aroma components of Huangguanyin Oolong tea from different geographical origins using electronic nose and headspace solid-phase microextraction-gas chromatography-mass spectrometry. Food Sci. 2023, 44, 232–239. [Google Scholar] [CrossRef]
- Ni, R.J.; Zhang, P.; Tian, H.L. Effects of frying time on volatile flavor compounds in fried pepper (Zanthoxylum bungeanum) oil as analyzed by gas chromatography-ion mobility spectrometry and multivariate statistical analysis. Food Sci. 2022, 43, 279–286. [Google Scholar]
- Zhang, Y.; Tong, X.Y.; Chen, B.J.; Wu, S.H.; Wang, X.; Zheng, Q.; Jiang, F.; Qiao, Y.J. Novel application of HS-GC-IMS for characteristic fingerprints and flavor compound variations in citrus reticulatae pericarpium during storage with different Aspergillus niger fermentation. Food Chem. X 2023, 18, 100653. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Liu, Y.; Jin, W.; Zhu, K.; Miao, X.; Dong, X.; Jiang, P. Effects of curing concentration and drying time on flavor and microorganisms in dry salted Spanish mackerel. Food Chem. X 2024, 21, 101126. [Google Scholar] [CrossRef]

| Serial Number | Volatile Compounds | Aroma Description | CAS Number | Molecular Formula | RI | RT/s | Dt/ms |
|---|---|---|---|---|---|---|---|
| Aldehydes | |||||||
| 1 | Benzaldehyde | Almond-like, sweet, caramel-like | C100527 | C7H6O | 1478.2 | 931.37 | 1.49817 |
| 2 | 2-methylbutanal | Pungent, malty–nutty | C96173 | C5H10O | 844.1 | 161.886 | 1.15319 |
| Alcohols | |||||||
| 3 | 2-methylpropanol-M | Jasmine aroma, apple aroma, rose aroma | C78831 | C4H10O | 1106.3 | 330.378 | 1.37197 |
| 4 | 2-Pentanol | A pungent, fruity/alcoholic | C6032297 | C5H12O | 1092.2 | 315.058 | 1.205 |
| 5 | 2-methylpropanol-D | subtle apple aroma | C78831 | C4H10O | 1052.2 | 277.953 | 1.38121 |
| 6 | 2-butanol | Almond-like, sweet, caramel-like | C78922 | C4H10O | 1034.8 | 263.244 | 1.34048 |
| 7 | 2-Furanmethanol, 5-methyl | Caramel, bread-like aroma | C3857258 | C6H8O2 | 982.7 | 225.841 | 1.2658 |
| 8 | 1-Heptanol | Heavy, oily, green | C111706 | C7H16O | 976.3 | 222.389 | 1.39717 |
| 9 | 2-furanmethanol | Caramel, bread-like aroma | C98000 | C5H6O2 | 882.1 | 177.387 | 1.12604 |
| 10 | 1-Propanethiol | Intensely pungent, sulfurous aroma | C107039 | C3H8S | 844.1 | 161.907 | 1.17181 |
| 11 | 3-Nonen-1-ol | Cucumber–melon freshness | C10340235 | C9H18O | 1158.7 | 396.965 | 1.37738 |
| 12 | 3-methylbutan-1-ol-D | pungent, fruity/alcoholic | C123513 | C5H12O | 1221.7 | 492.456 | 1.49817 |
| 13 | 3-methylbutan-1-ol-M | A pungent, fruity–fusel aroma with complex alcoholic and fermented nuances | C123513 | C5H12O | 1231.1 | 508.095 | 1.24796 |
| Ketones | |||||||
| 14 | 3-Octanone | Creaminess, rose, jasmine | C106683 | C8H16O | 1241.1 | 525.188 | 1.31526 |
| 15 | 3-Mercapto-2-butanone | Pungent, savory, sulfurous | C40789988 | C4H8OS | 1267.4 | 573.086 | 1.12763 |
| 16 | 3-Hexanone | Green, fruity, slightly pungent | C589388 | C6H12O | 1052.4 | 278.16 | 1.45111 |
| 17 | 4-Methyl-2-pentanone | Sharp, sweet, solvent-like | C108101 | C6H12O | 1033.2 | 261.984 | 1.47626 |
| 18 | 3-Pentanone | Mild, sweet, ethereal | C96220 | C5H10O | 998.9 | 235.309 | 1.35641 |
| 19 | Levo-carvone | Cool, herbaceous, subtly sweet | C6485401 | C10H14O | 1213.1 | 478.463 | 1.31052 |
| Esters | |||||||
| 20 | Propyl butanoate | Banana scent, pineapple scent | C105668 | C7H14O2 | 1154.6 | 391.363 | 1.25786 |
| 21 | Ethyl 3-methylbutanoate-D | Refined, ultra-fruity | C108645 | C7H14O2 | 1116.9 | 342.873 | 1.24933 |
| 22 | Butanoic acid ethyl ester-D | Apple aroma, buttery aroma | C105544 | C6H12O2 | 1056.4 | 281.632 | 1.55951 |
| 23 | Butanoic acid ethyl ester-M | Pineapple aroma, banana aroma | C105544 | C6H12O2 | 1063.6 | 288.04 | 1.20243 |
| 24 | Ethyl 3- methylbutanoate-M | Vibrantly fruity, tropical–sweet | C108645 | C7H14O2 | 1054.1 | 279.634 | 1.27258 |
| 25 | 2-Methyl propyl acetate | Bright, fruity, slightly floral | C110190 | C6H12O2 | 1029.6 | 259.042 | 1.61204 |
| 26 | 2-furanmethanol acetate | Warm, balsamic–sweet | C623176 | C7H8O3 | 999.8 | 235.928 | 1.41516 |
| 27 | Acetic acid propyl ester | Fresh, fruity, slightly herbal | C109604 | C5H10O2 | 993.8 | 231.938 | 1.48052 |
| 28 | Ethyl propanoate | Bright, fruity, and rum-like | C105373 | C5H10O2 | 974.7 | 221.546 | 1.45274 |
| 29 | Acetic acid ethyl ester-D | Fruity-sweet, wine-like, brandy undertone | C141786 | C4H8O2 | 902.9 | 186.439 | 1.34715 |
| 30 | Acetic acid ethyl ester-M | Fruity, pineapple-like, sweet, green, waxy | C141786 | C4H8O2 | 892.1 | 181.664 | 1.09893 |
| 31 | Ethyl formate | Sharp, fruity–ethereal | C109944 | C3H6O2 | 849.8 | 164.123 | 1.22488 |
| 32 | ethyl (E)-2-butenoate | Sharp, fruity–pungent | C623701 | C6H10O2 | 1141.7 | 373.98 | 1.54672 |
| 33 | Ethyl 2-methylpentanoate-D | Bright, fruity, slightly herbal | C39255328 | C8H16O2 | 1139.7 | 371.392 | 1.74581 |
| 34 | Ethyl 2-methylpentanoate-M | Fruity, tropical, slightly green | C39255328 | C8H16O2 | 1141.1 | 373.325 | 1.30848 |
| Acids | |||||||
| 35 | 2-Methylheptanoic acid | Pungent, earthy–musky, faintly fruity | C1188029 | C8H16O2 | 1145.1 | 378.479 | 1.40971 |
| 36 | (E)-3-hexenoic acid | Green, fatty, slightly sweaty | C1577180 | C6H10O2 | 1000.4 | 236.432 | 1.2286 |
| 37 | 2-Methylpropionic acid | Vinegary with a buttery undertone, sharp, sweaty, dairy-like | C79312 | C4H8O2 | 784.8 | 140.397 | 1.15387 |
| Ethers | |||||||
| 38 | Allyl sulfide | Pungent, intensely garlic-like | C592881 | C6H10S | 1121.1 | 348.048 | 1.13028 |
| 39 | Dimethyl disulfide | Pungent, sulfurous aroma | C624920 | C2H6S2 | 1092.1 | 315 | 1.13104 |
| 40 | 1,2-Dimethoxyethane | Sweetly ethereal, faintly fruity | C110714 | C4H10O2 | 930.8 | 199.358 | 1.09708 |
| 41 | 2-Butoxyethanol | Mild, sweet, slightly floral–ethereal | C111762 | C6H14O2 | 893.1 | 182.097 | 1.20459 |
| Alkanes | |||||||
| 42 | Cyclohexane | Mild, sweet | C110827 | C6H12 | 709.5 | 117.184 | 1.11184 |
| 43 | 1,4-Dioxan | Etheric/clean, slightly sweet and fruity | C123911 | C4H8O2 | 1097.3 | 320.214 | 1.13104 |
| Amines | |||||||
| 44 | N-nitrosomethylethylamine | Slightly sweet but offensive | C10595956 | C3H8N2O | 841.6 | 160.951 | 1.11657 |
| Aromatic hydrocarbons | |||||||
| 45 | Butylbenzene | Neroli, jasmine, pineapple | C104518 | C10H14 | 1301.3 | 636.777 | 1.20606 |
| others | |||||||
| 46 | Tetrahydrofuran | Ethereal, sweet, and slightly pungent | C109999 | C4H8O | 851.2 | 164.698 | 1.06385 |
| 47 | Acetoin | Buttery or creamy, caramel, vanilla, sweet | C513860 | C4H8O2 | 731.1 | 123.429 | 1.34026 |
| 48 | 1,3-Dioxolane, 2,4-dimethyl, cis | Sweet and fruity, scent of fresh flowers | C3390123 | C5H10O2 | 734.5 | 124.436 | 1.3821 |
| 49 | 2-butylfuran | Overripe pear or dried fruit | C4466244 | C8H12O | 1110.4 | 335.237 | 1.17524 |
| 50 | Pyrrolidine | Strong, sharp odor | C123751 | C4H9N | 998.6 | 235.087 | 1.27258 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abudureheman, B.; Guo, M.; Zhang, J.; Chen, L.; Li, Q.; Long, T.; Lv, Z.; Huang, J.; Fang, D.; Jiang, L.; et al. Thermal Modulation of Musalais Wine Characteristics: Volatile Profiles and Chemical Composition at Different Brix Levels. Foods 2025, 14, 2956. https://doi.org/10.3390/foods14172956
Abudureheman B, Guo M, Zhang J, Chen L, Li Q, Long T, Lv Z, Huang J, Fang D, Jiang L, et al. Thermal Modulation of Musalais Wine Characteristics: Volatile Profiles and Chemical Composition at Different Brix Levels. Foods. 2025; 14(17):2956. https://doi.org/10.3390/foods14172956
Chicago/Turabian StyleAbudureheman, Buhailiqiemu, Minqiang Guo, Jianlin Zhang, Lin Chen, Qian Li, Tiantian Long, Zhuanzhuan Lv, Junli Huang, Dandan Fang, Luxi Jiang, and et al. 2025. "Thermal Modulation of Musalais Wine Characteristics: Volatile Profiles and Chemical Composition at Different Brix Levels" Foods 14, no. 17: 2956. https://doi.org/10.3390/foods14172956
APA StyleAbudureheman, B., Guo, M., Zhang, J., Chen, L., Li, Q., Long, T., Lv, Z., Huang, J., Fang, D., Jiang, L., Ye, X., & Pan, H. (2025). Thermal Modulation of Musalais Wine Characteristics: Volatile Profiles and Chemical Composition at Different Brix Levels. Foods, 14(17), 2956. https://doi.org/10.3390/foods14172956





