Ultrasonic-Assisted Ginkgo biloba Leaves Extract as Natural Antioxidant on Oxidative Stability of Oils During Deep-Frying
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Ginkgo biloba Leaves Extract
2.3. Optimization of the Extraction Process by Response Surface Methodology (RSM)
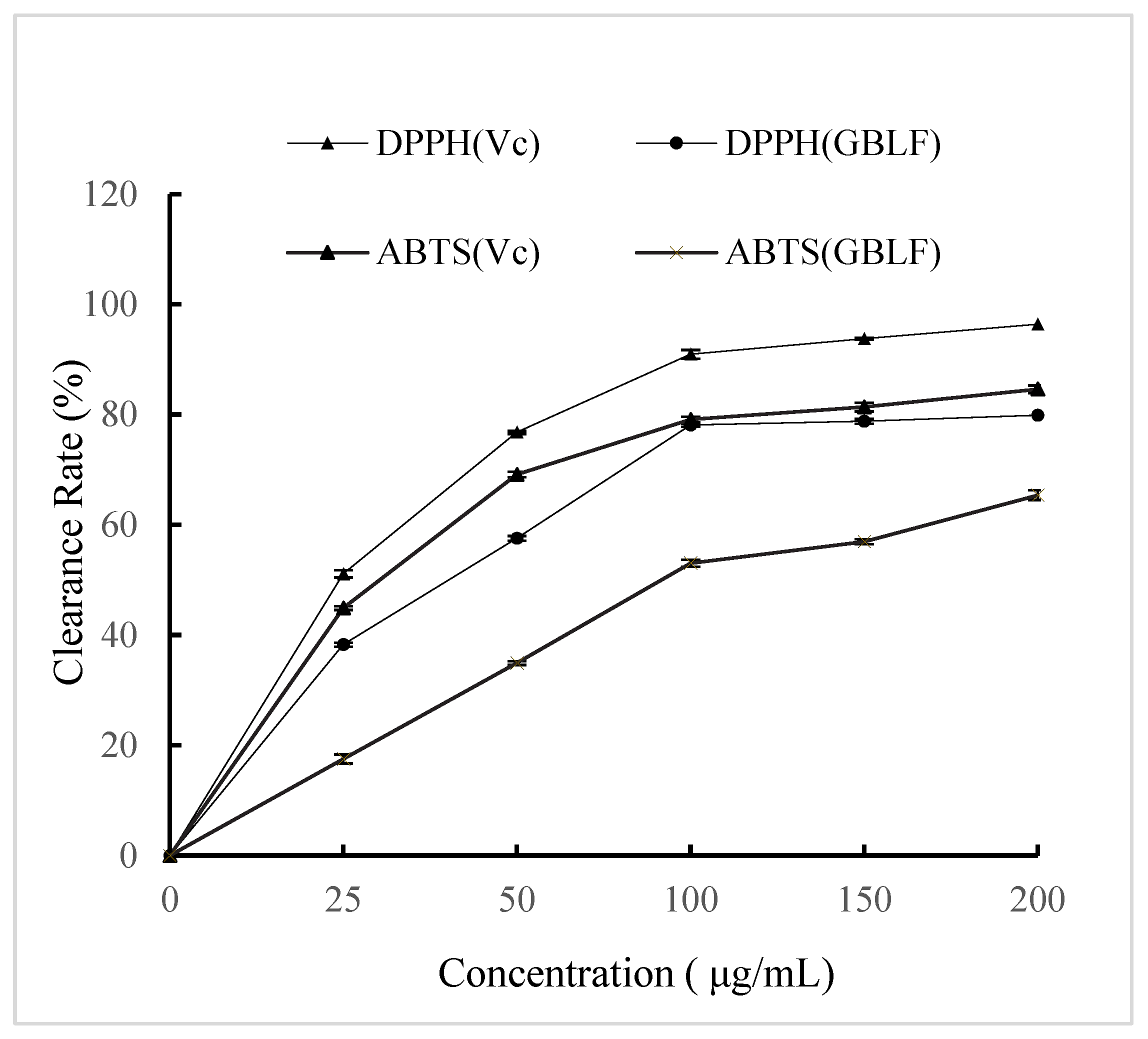
2.4. Determination of GBL Antioxidant Activity
2.5. Preparation of Frying Oil Protocol
2.6. Determination of Chemical Property of Frying Oil
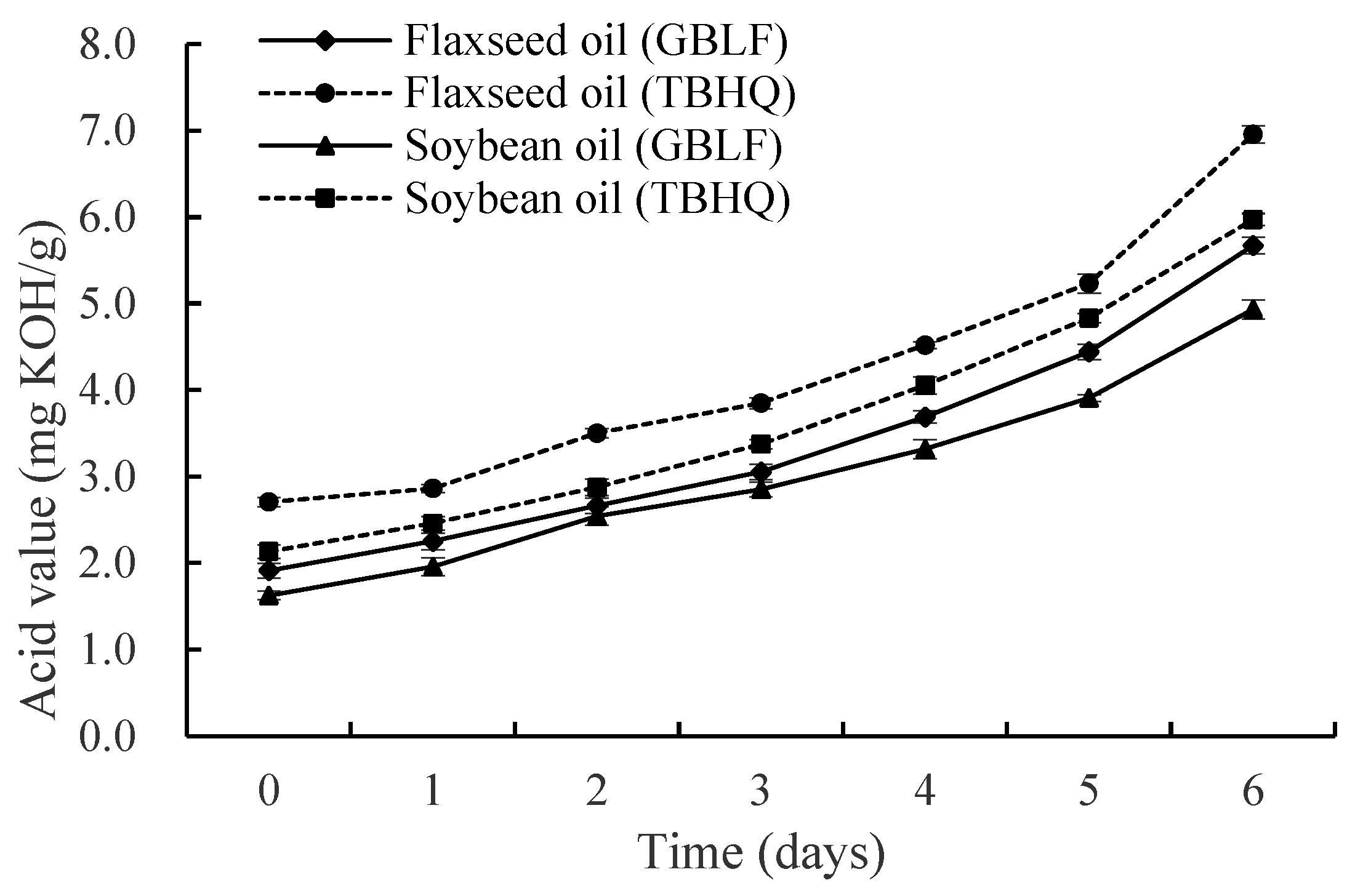
2.6.1. Determination of the Acid Value (AV)
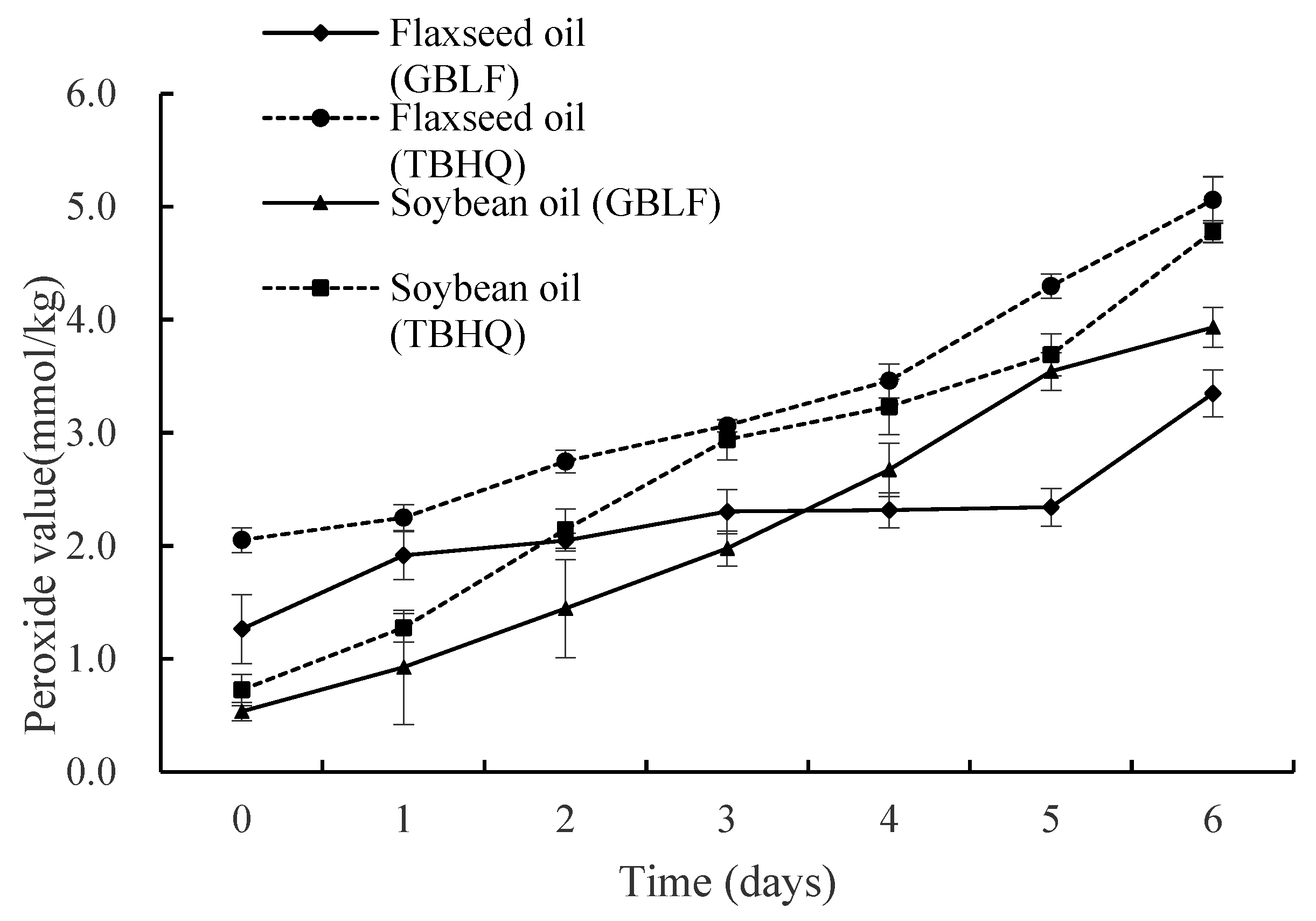
2.6.2. Determination of the Peroxide Value (PV)
2.6.3. Determination of Anisidine Value (p-AV)
2.6.4. Polar Compounds (PC)
2.7. Determination of Tocopherol Contents of Frying Oil
2.8. Determination of Fatty Acid Contents of Frying Oil
2.9. Statistical Analyses
3. Results and Discussion
3.1. Ultrasonic Assisted Extraction of Flavonoid from GBL
3.2. Antioxidant Activity of Extract
3.3. Changes of Frying Oils Oxidative Stability During Repeated Frying Cycles
3.3.1. Acid Value
3.3.2. Peroxide Value
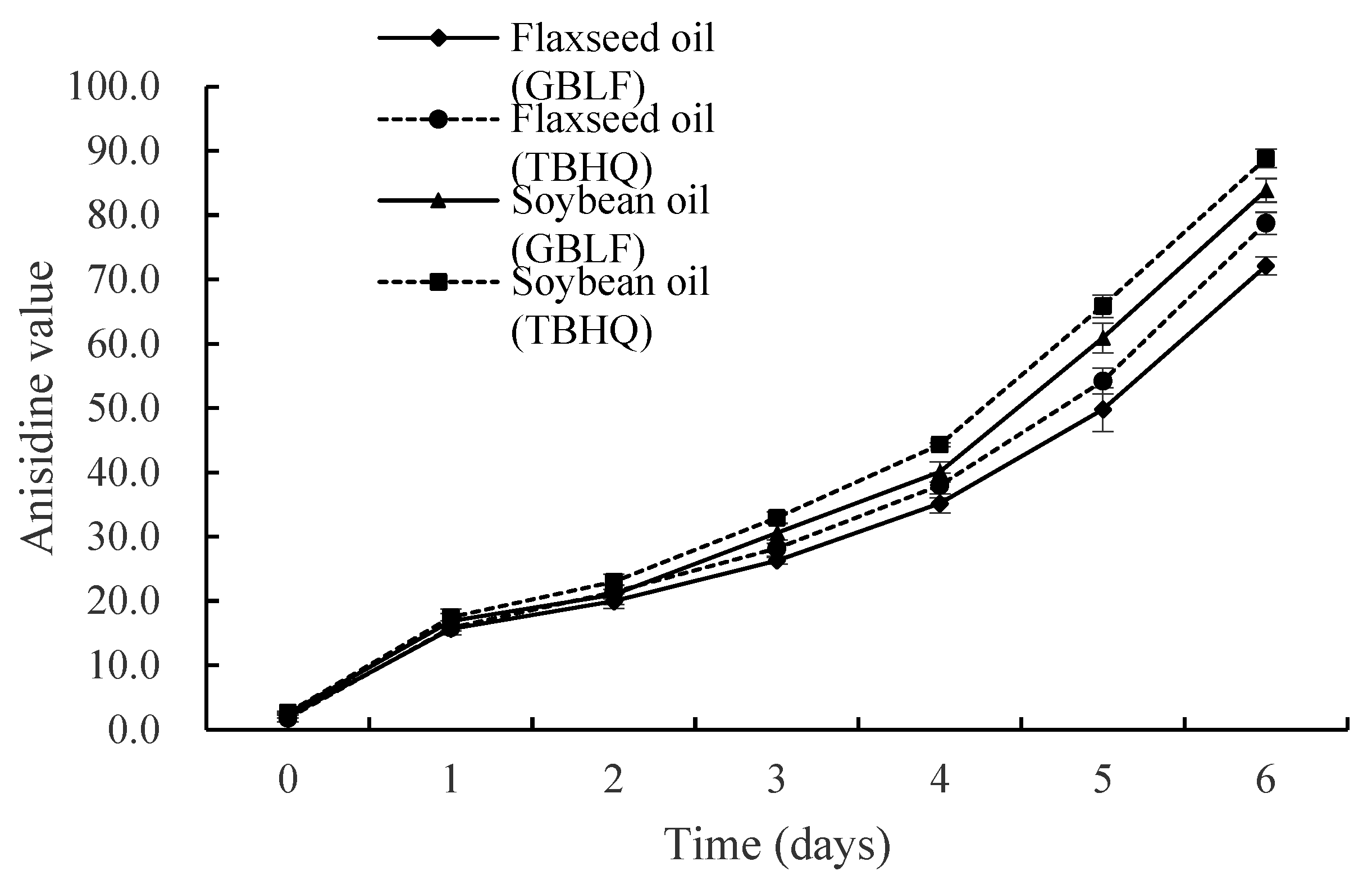
3.3.3. Anisidine Value
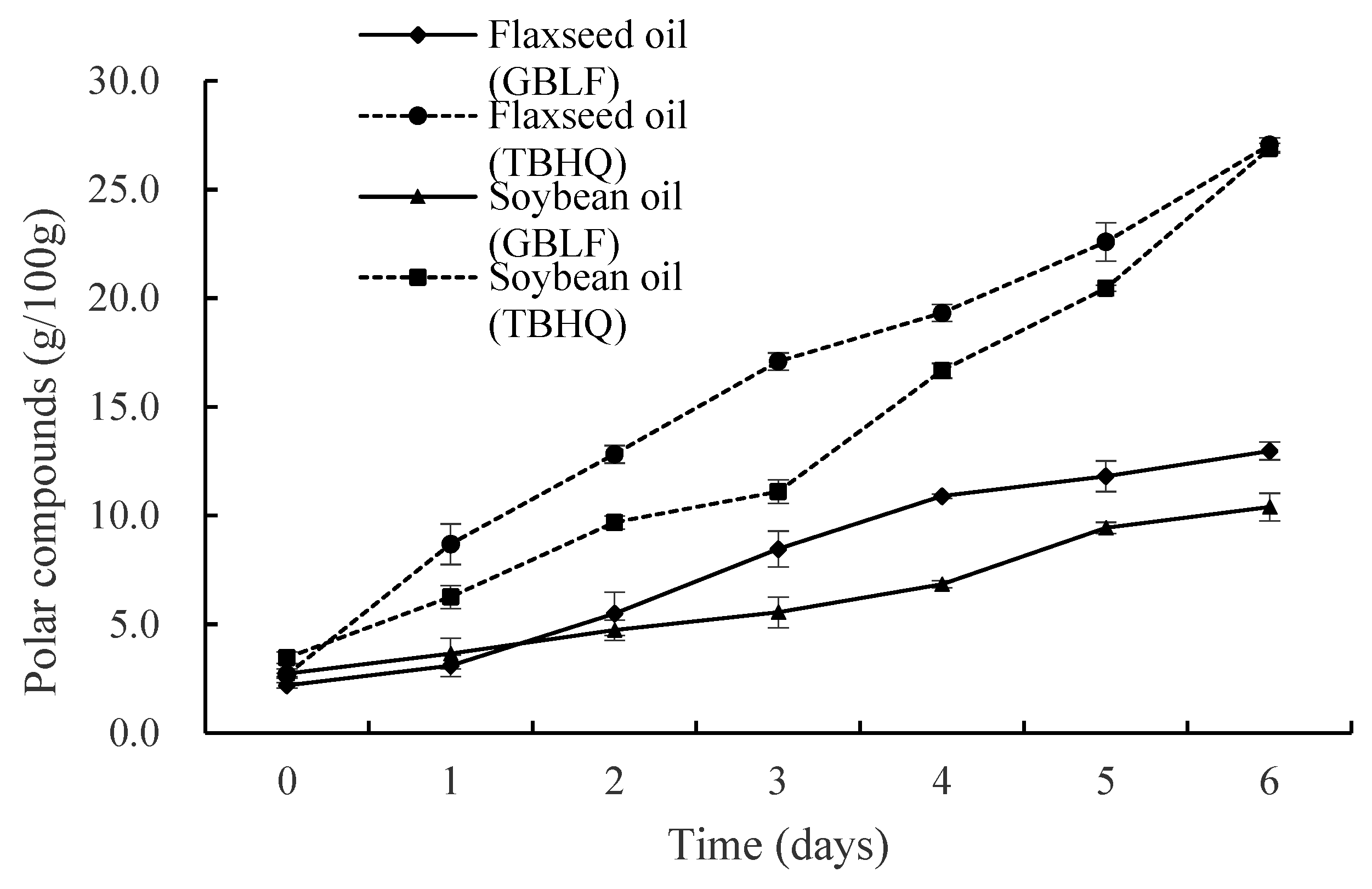
3.3.4. Polar Compounds
3.3.5. Tocopherol Contents
3.3.6. Fatty Acid Contents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, F.; Boateng, I.D.; Chen, S.; Yang, X.M.; Soetanto, D.A.; Liu, W. Pulsed Light Irradiation Improves Degradation of Ginkgolic Acids and Retainment of Ginkgo Flavonoids and Terpene Trilactones in Ginkgo biloba Leaves. Ind. Crops Prod. 2023, 204, 117297. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Dahija, S.; Hassan, S.T.S. Biflavonoids: Important Contributions to the Health Benefits of Ginkgo (Ginkgo biloba L.). Plants 2022, 11, 1381. [Google Scholar] [CrossRef]
- Wang, L.T.; Fan, X.H.; Jian, Y.; Dong, M.Z.; Yang, Q.; Meng, D.; Fu, Y.J. A sensitive and selective multiple reaction monitoring mass spectrometry method for simultaneous quantification of flavonol glycoside, terpene lactones, and biflavonoids in Ginkgo biloba leaves. J. Pharm. Biomed. Anal. 2019, 170, 335–340. [Google Scholar] [CrossRef]
- Liu, L.M.; Wang, Y.T.; Zhang, J.C.; Wang, S.F. Advances in the chemical constituents and chemical analysis of Ginkgo biloba leaf, extract, and phytopharmaceuticals. J. Pharm. Biomed. Anal. 2021, 30, 113704. [Google Scholar] [CrossRef] [PubMed]
- Boateng, I.D.; Li, F.; Yang, X.M. Development, Validation, and Application of High-Performance Liquid Chromatography with Diode-Array Detection Method for Simultaneous Determination of Ginkgolic Acids and Ginkgols in Ginkgo biloba. Foods 2024, 13, 1250. [Google Scholar] [CrossRef]
- Boateng, I.D. Ginkgols and Bilobols in Ginkgo biloba L. A Review of Their Extraction and Bioactivities. Phyther. Res. 2023, 37, 3211–3223. [Google Scholar] [CrossRef]
- Yang, X.M.; Wang, Y.F.; Li, Y.Y.; Ma, H.L. Thermal Stability of Ginkgolic Acids from Ginkgo biloba and the Effects of Ginkgol C17:1 on the Apoptosis and Migration of SMMC7721 Cells. Fitoterapia. 2014, 98, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Cheng, K.C.; Hsu, R.J.; Hsieh, C.W.; Wang, H.T.; Ting, Y. Enzymatic Degradation of Ginkgolic Acid by Laccase Immobilized on Novel Electrospun Nanofiber Mat. J. Sci. Food Agric. 2020, 100, 2705–2712. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Committee. Ginkgo Leaves Extract; China Medical Science and Technology Press: Beijing, China, 2020; Volume 1. [Google Scholar]
- Boateng, I.D. A critical review of ginkgolic acids in Ginkgo biloba leaf extract (EGb): Toxicity and technologies to remove ginkgolic acids and their promising bioactivities. Food Funct. 2022, 13, 9226–9242. [Google Scholar] [CrossRef]
- Hua, Z.; Wu, C.; Fan, G.; Tang, Z.; Cao, F. The antibacterial activity and mechanism of ginkgolic acid C15:1. BMC Biotechnol. 2017, 17, 5. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhou, W.; Wu, A.; Qian, X.; Xie, L.; Zhou, X.; Zhang, L. Effect of Ginkgo Biloba Powder on the Physicochemical Properties and Quality Characteristics of Wheat Dough and Fresh Wet Noodles. Foods 2022, 11, 698. [Google Scholar] [CrossRef]
- Soetanto, D.A.; Li, F.N.; Boateng, I.D.; Yang, X.M. Thermal fixation technologies affect phenolic profile, ginkgolides, bilobalide, product quality, and ginkgolic acids in Ginkgo biloba leaf tea. J. Food Sci. 2024, 89, 4093–4108. [Google Scholar] [CrossRef]
- Oschmann, R.; Waimer, F.; Hauer, H. Preparation of Ginkgo biloba Extract Used for Treating e.g., Dementia or Cerebral Disorder, Involves Extracting Aqueous Alcoholic Solution of Ginkgo biloba Leaves with Heptane to Remove Alkyl Phenol Compounds. DE102006019863A1, 16 November 2006. [Google Scholar]
- Boateng, I.D.; Yang, X.M. Effect of different drying methods on product quality, bioactive and toxic components of Ginkgo biloba L. seed. J. Sci. Food Agric. 2021, 101, 3290–3297. [Google Scholar] [CrossRef]
- Wang, J.H.; Cao, F.L.; Su, E.Z.; Zhao, L.G.; Qin, W.S. Improvement of Animal Feed Additives of Ginkgo Leaves through Solid-state Fermentation using Aspergillus niger. Int. J. Biol. Sci. 2018, 14, 736–747. [Google Scholar] [CrossRef]
- Biernacka, P.; Adamska, I.; Felisiak, K. The Potential of Ginkgo biloba as a Source of Biologically Active Compounds-A Review of the Recent Literature and Patents. Molecules 2023, 28, 3993. [Google Scholar] [CrossRef] [PubMed]
- Amoussa, A.M.O.; Zhang, L.; Lagnika, C.; Riaz, A.; Zhang, L.; Liu, X.; Beta, T. Effects of preheating and drying methods on pyridoxine, phenolic compounds, ginkgolic acids, and antioxidant capacity of Ginkgo biloba nuts. J. Wood Sci. 2021, 86, 4197–4208. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.S.; Yoon, N.; Oh, B.S.; Jeong, H.S.; Yeoun, P.S.; Kim, D.J. Variation of Toxin Content in Ginkgo Fruits According to Thermal Treatment. Natl. Acad. Sci. Lett. 2020, 43, 673–676. [Google Scholar] [CrossRef]
- Fan, G.J.; Wang, X.; Wu, C.E.; Pan, H.M.; Yang, J.T.; Li, T.T.; Tang, Z.X.; Cao, F.L. Effect of heating on the content and composition of ginkgolic acids in ginkgo seeds. Qual. Assur. Saf. Crops Foods. 2017, 9, 195–199. [Google Scholar] [CrossRef]
- Wong, K.H.; Tan, W.L.; Serra, A.; Xiao, T.S.; Sze, S.K.; Yang, D.W.; Tam, J.P. Ginkgotides: Proline-Rich Hevein-Like Peptides from Gymnosperm Ginkgo biloba. Front. Plant Sci. 2016, 7, 1693. [Google Scholar] [CrossRef]
- Song, J.P.; Li, X.Y.; Zhang, S.J.; Li, Y.; Chen, L.; Wang, Z.W.; Cheng, G.Q.; Yu, Q.; Han, Y.X. Ginkgo biloba leaf extract: A natural and eco-friendly stabilizer for enhancing the thermal and oxidative stability of polyethylene. Polym. Res. 2024, 31, 341. [Google Scholar] [CrossRef]
- Wong, K.H.; Tan, W.L.; Xiao, T.S.; Tam, J.P. β-Ginkgotides: Hyperdisulfide-constrained peptides from Ginkgo biloba. Sci. Rep. 2017, 7, 6140. [Google Scholar] [CrossRef]
- Boateng, I.D.; Yang, X.M. Do non-thermal pretreatments followed by intermediate-wave infrared drying affect toxicity, allergenicity, bioactives, functional groups, and flavor components of Ginkgo biloba seed? A case study. Ind. Crops Prod. 2021, 165, 113421. [Google Scholar] [CrossRef]
- Rohn, S.; Buchner, N.; Driemel, G.; Rauser, M.; Kroh, L.W. Thermal degradation of onion quercetin glucosides under roasting conditions. J. Agric. Food Chem. 2007, 55, 1568–1573. [Google Scholar] [CrossRef]
- Laib, I.; Barkat, M. Impact of cooking and conservation for twelve days on total polyphenols content, antioxidant and anticholinesterase activities of red onion. Afr. J. Pharm. Pharmcol. 2016, 10, 270–277. [Google Scholar] [CrossRef]
- Sharma, K. Temperature-dependent studies on the total phenolics, flavonoids, antioxidant activities, and sugar content in six onion varieties. J. Food Drug Anal. 2015, 23, 243–252. [Google Scholar] [CrossRef]
- Jin, W.B.; Zhou, T.; Li, G.K. Recent advances of modern sample preparation techniques for traditional Chinese medicines. J. Chromatogr. 2019, 1606, 460377. [Google Scholar] [CrossRef]
- Cong, J.X.; Wang, S.Y.; Zhang, W.; Tang, X.D. Ultrasonic-Assisted Extraction of Total Flavonoids and Lactones from Ginkgo Biloba Powder. Adv. Mat. Res. 2013, 641–642, 867–870. [Google Scholar] [CrossRef]
- Zhou, G.; Ma, J.; Tang, Y.; Wang, X.; Zhang, J.; Duan, J.A. Multi-Response Optimization of Ultrasonic Assisted Enzymatic Extraction Followed by Macroporous Resin Purification for Maximal Recovery of Flavonoids and Ginkgolides from Waste Ginkgo biloba Fallen Leaves. Molecules 2018, 23, 1029. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Badawi, M.H. Comparison of Antioxidant and Antimicrobial Properties for Ginkgo biloba and Rosemary (Rosmarinus officinalis L.) from Egypt. Not. Bot. Horti. Agrobo. 2013, 41, 126–135. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Gao, Y.J.; Ding, H.; Liu, S.J.; Han, X.; Gui, J.Z.; Liu, D. Subcritical ethanol extraction of flavonoids from Moringa oleifera leaf and evaluation of antioxidant activity. Food Chem. 2017, 218, 152–158. [Google Scholar] [CrossRef]
- Zhang, W.L.; Rizkiyah, D.N.; Putra, N.R. Innovative Techniques in Sandalwood Oil Extraction: Optimizing Phenolic and Flavonoid Yields with Subcritical Ethanol. Separations 2024, 11, 201. [Google Scholar] [CrossRef]
- Gong, Q.Y.; Guo, Z.D.; Sun, Z.H.; Gong, J.G.; Wei, F.Y. Graphene oxide-assisted ethanol reflux extraction of total flavonoids from Ginkgo biloba leaves: Study of kinetics and mechanism. Chem. Pap. 2019, 74, 971–984. [Google Scholar] [CrossRef]
- Shan, B.; Xie, J.H.; Zhu, J.H.; Peng, Y. Ethanol modified supercritical carbon dioxide extraction of flavonoids from Momordica charantia L. and its antioxidant activity. Food Bioprod. Process. 2012, 90, 579–587. [Google Scholar] [CrossRef]
- Chen, M.Y.; Zhang, T.T.; Sun, D.Y.; Wu, S.F.; Wang, K.Z.; He, L.; Wang, X.; Chen, Y.R. Study on extraction technology of flavonoids from natural green cotton fiber. J. Text. Inst. 2019, 111, 869–873. [Google Scholar] [CrossRef]
- Ge, C.Y.; Zhang, J.L.; Wang, M.S. Extraction and electrochemical fingerprinting of total flavonoids from Hovenia spp. Int. J. Electrochem. Sci. 2021, 16, 211236. [Google Scholar] [CrossRef]
- Lei, J.; Jiang, Y.M.; Luo, X.R.; Zheng, Y.; Zhu, L.; Sun, C.X.; Lang, L.H.; Qin, C.; Gang, W. Ultrasonic-Assisted Ionic Liquid Extraction of Four Biflavonoids from Ginkgo biloba L. Chemistryselect 2021, 6, 3297–3307. [Google Scholar] [CrossRef]
- Ko, Y.C.; Lee, R.J.; Feng, H.T.; Lee, M.R. Development of a Liquid Chromatography Tandem Mass Spectrometry Method for Identification of Flavonoids in Ginkgo biloba. J. Chin. Chem. Soc. 2013, 60, 1333–1338. [Google Scholar] [CrossRef]
- Kovtun, E.; Pogrebnyak, L.; Stepanova, E.; Pogrebnyak, A.; Morozov, Y.; Bokov, D. Preparation of Components of Ultrasonic Extract of Ginkgo Biloba, Physico-chemical and Pharmacological Analysis and Molecular Design. Arch. Euromedica 2022, 12, 26–28. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Zanqui, A.B.; de Morais, D.R.; da Silva, C.M.; Santos, J.M.; Gomes, S.T.M.; Visentainer, J.V.; Eberlin, M.N.; Cardozo-Filho, L.; Matsushita, M. Subcritical extraction of flaxseed oil with n-propane: Composition and purity. Food Chem. 2015, 188, 452–458. [Google Scholar] [CrossRef]
- Hou, J.C.; Zhou, X.; Yu, T.; Sop, R.T.; Ma, J.G.; Wang, M.L.; Wu, Q.R.; Zheng, X.Y.; Jiang, Z.M. Zixiphi spinosae semen oil enhance the oxidative stability of soybean oil under frying conditions. Eur. J. Lipid Sci. Technol. 2021, 123, 2100060. [Google Scholar] [CrossRef]
- Iwegbue, C.M.A.; Osijaye, K.O.; Igbuku, U.A.; Egobueze, F.E.; Tesi, G.O.; Bassey, F.I.; Martincigh, B.S. Effect of the number of frying cycles on the composition, concentrations and risk of polycyclic aromatic hydrocarbons (PAHs) in vegetable oils and fried fish. J. Food Compos. Anal. 2020, 94, 103633. [Google Scholar] [CrossRef]
- Xie, H.Y.; Wang, J.R.; Yau, L.F.; Liu, Y.; Liu, L.; Han, Q.B.; Zhao, Z.Z.; Jiang, Z.H. Quantitative analysis of the flavonoid glycosides and terpene trilactones in the extract of Ginkgo biloba and evaluation of their inhibitory activity towards fibril formation of β-amyloid peptide. Molecules 2014, 19, 4466–4478. [Google Scholar] [CrossRef]
- Snoussi, A.; Essaidi, I.; Koubaier, H.B.; Zrelli, H.; Alsafari, I.; Zivoslav, T.; Mihailovic, J.; Khan, M.; El Omri, A.; Velickovic, T.C.; et al. Drying methodology effect on the phenolic content, antioxidant activity of Myrtus communis L. leaves ethanol extracts and soybean oil oxidative stability. BMC Chem. 2021, 15, 31. [Google Scholar] [CrossRef]
- Hwang, H.S.; Winkler-Moser, J.K.; Tisserat, B.; Harry-O’kuru, R.E.; Berhow, M.A.; Liu, S.X. Antioxidant Activity of Osage Orange Extract in Soybean Oil and Fish Oil during Storage. J. Am. Oil Chem. Soc. 2021, 98, 73–87. [Google Scholar] [CrossRef]
- Jahanfar, S.; Gahavami, M.; Khosravi-Darani, K.; Jahadi, M.; Mohsin, H.; Todorov, S.D.; Tripathid, A.D. The effect of antioxidant properties of free and encapsulated rosemary extract in liposome on the oxidation process of canola oil. Int. J. Food Sci. Technol. 2023, 58, 5521–5529. [Google Scholar] [CrossRef]
- Molaee, M.; Sahari, M.A.; Kenari, R.E.; Amirkaveei, S.; Arbidar, E. Antioxidant Properties of Avicennia marina Leaf Extract in Soybean Oil System. Curr. Nutr. Food Sci. 2020, 16, 1245–1251. [Google Scholar] [CrossRef]
- Okhli, S.; Mirzaei, H.; Hosseini, S.E. Antioxidant activity of citron peel (Citrus medica L.) essential oil and extract on stabilization of sunflower oil. OCL 2020, 27, 32. [Google Scholar] [CrossRef]
- Demydova, A.; Yevlash, V.; Aksonova, O.; Tkachenko, O.; Kameneva, N. Antioxidant activity of plants extracts of ukrainian origin and their effect on the oxidative stability of sunflower oil. Food Sci. Technol. 2022, 16, 55–64. [Google Scholar] [CrossRef]
- Chen, X.J.; Ren, S.M.; Dong, J.Z.; Qiu, C.G.; Chen, Y.W.; Tao, H.L. Ginkgo biloba extract-761 protects myocardium by regulating Akt/Nrf2 signal pathway. Drug Des. Dev. Ther. 2019, 13, 647–655. [Google Scholar] [CrossRef]
- de Souza, G.A.; de Marqui, S.V.; Matias, J.N.; Guiguer, E.L.; Barbalho, S.M. Effects of Ginkgo biloba on Diseases Related to Oxidative Stress. Planta Med. 2020, 86, 376–386. [Google Scholar] [CrossRef]
- Guimaraes, N.S.S.; Ramos, V.S.; Prado-Souza, L.F.L.; Lopes, R.M.; Arini, G.S.; Feitosa, L.G.P.; Silva, R.R.; Nantes, I.L.; Damasceno, D.C.; Lopes, N.P. Rosemary (Rosmarinus officinalis L.) Glycolic Extract Protects Liver Mitochondria from Oxidative Damage and Prevents Acetaminophen-Induced Hepatotoxicity. Antioxidants 2023, 12, 628. [Google Scholar] [CrossRef]
- Xiang, Q.S.; Liu, Z.G.; Wang, Y.T.; Xiao, H.F.; Wu, W.Q.; Xiao, C.X.; Liu, X.B. Carnosic acid attenuates lipopolysaccharide-induced liver injury in rats via fortifying cellular antioxidant defense system. Food Chem. Toxicol. 2013, 53, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.J.; Yang, Z.B.; Ding, X.; Yang, C.W. Effects of Ginkgo biloba leaves (Ginkgo biloba) and Ginkgo biloba extract on nutrient and energy utilization of broilers. Poult. Sci. 2018, 97, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Li, W.Y.; Yang, S.B.; Duo, J.; Li, Q.; Xue, Y.Z. Detection and Development on Total Flavonoids by the Way of Al(NO3)3 Ultraviolet Colorimetry. Res. Sports Med. 2020, 7, RISM.000658. [Google Scholar] [CrossRef]
- Liu, C.; Liu, S.; Zhang, L.; Wang, X.; Ma, L. Partition Behavior in Aqueous Two-Phase System and Antioxidant Activity of Flavonoids from Ginkgo biloba. Appl. Sci. 2018, 8, 2058. [Google Scholar] [CrossRef]
- Sadawarte, P.D.; Annapure, U.S. Study of the behavior and properties of frying oil on repetitive deep frying. J. Food Sci. Tech. Mys. 2023, 60, 2549–2556. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, X.; Huang, J.; Zhao, C.W.; Qi, J.F.; Jin, Q.Z.; Wang, X.G. Correlations between polycyclic aromatic hydrocarbons and polar components in edible oils during deep frying of peanuts. Food Control. 2018, 87, 109–116. [Google Scholar] [CrossRef]
- Matthäus, B.; Babiker, E.E.; Özcan, M.M.; Al-Juhaimi, F.Y.; Ahmed, I.A.M.; Ghafoor, K. Changes in Fatty Acid, Tocopherol and Sterol Contents of Oils Extracted from Several Vegetable Seeds. J. Oleo Sci. 2021, 70, 1607–1614. [Google Scholar] [CrossRef]
- Han, S.; Chio, C.; Ma, T.; Kognou, A.L.M.; Shrestha, S.; Chen, F.; Qin, W. Extracting flavonoid from Ginkgo biloba using lignocellulolytic bacteria Paenarthrobacter sp. and optimized via response surface methodology. Biotechnol. Appl. Microbiol. 2021, 15, 867–878. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.Y.; Zhang, Z.L.; Ragman, K.; Wang, S.J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Wong, K.H.; Li, G.Q.; Li, K.M.; Razmovski-Naumovski, V.; Chan, K. Optimisation of Pueraria isoflavonoids by response surface methodology using ultrasonic-assisted extraction. Food Chem. 2017, 231, 231–237. [Google Scholar] [CrossRef]
- Liu, J.; Mu, T.H.; Sun, H.N.; Fauconnier, M.L. Optimization of ultrasonic-microwave synergistic extraction of flavonoids from sweet potato leaves by response surface methodology. J. Food Process. Pres. 2019, 43, e13928. [Google Scholar] [CrossRef]
- Kang, M.; Wang, X.J. Macroporous resin purification and quantification by LC-MS of bioactive flavonoids in Ginkgo biloba leaves. CYTA-J. Food 2025, 23, 2517329. [Google Scholar] [CrossRef]
- Wanasundara, U.N.; Shahidi, F. Stabilization of canola oil with flavonoids. Food Chem. 1994, 50, 393–396. [Google Scholar] [CrossRef]
- Liu, X.G.; Lu, X.; Gao, W.; Li, P.; Yang, H. Structure, Synthesis, Biosynthesis, and Activity of the Characteristic Compounds from Ginkgo biloba L. Nat. Prod. Rep. 2022, 3, 474–511. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, C.; Liu, X.; Su, E.; Cau, F.; Zhao, L. Study on Synergistic Antioxidant Effect of Typical Functional Components of Hydroethanolic Leaf Extract from Ginkgo Biloba In Vitro. Molecules 2022, 27, 439. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Zhang, T.; Jiang, B.; Mu, W.M.; Miao, M. Characterization and antioxidant activity of Ginkgo biloba exocarp polysaccharides. Carbohydr. Polym. 2012, 87, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Kuncoro, H.; Farabi, K.; Rijai, L.; Julaeha, E.; Supratman, U.; Shiono, Y. Flavonoid Compounds from Krokot Herb (Lygodium microphyllum) and their Antioxidant Activity against DPPH. J. Math. Fundam. Sci. 2018, 50, 192–202. [Google Scholar] [CrossRef]
- Li, D.N.; Li, B.; Ma, Y.; Sun, X.Y.; Lin, Y.; Meng, X.J. Polyphenols, anthocyanins, and flavonoids contents and the antioxidant capacity of various cultivars of highbush and half-high blueberries. J. Food Compost. Anal. 2017, 62, 84–93. [Google Scholar] [CrossRef]
- Kim, D.O.; Lee, K.W.; Lee, H.J.; Lee, C.Y. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J. Agric. Food Chem. 2002, 50, 3713–3717. [Google Scholar] [CrossRef]
- Mutailifu, P.; Nuerxiati, R.; Lu, C.F.; Huojiaaihemaiti, H.; Abuduwaili, A.; Yili, A. Extraction, purifcation, and characterization of polysaccharides from Alhagi pseudoalhagi with antioxidant and hypoglycemic activities. Process Biochem. 2022, 121, 339–348. [Google Scholar] [CrossRef]
- Zheng, J.; Long, Y.Y.; Chen, W.; Zhi, W.L.; Xu, T.T.; Wang, L.; Hu, A.J. Quality changes of repeatedly fried palm oil and extracted oil from fried loach. Int. J. Food Eng. 2022, 18, 371–381. [Google Scholar] [CrossRef]
- Silva, J.A.; Dorocz, E.L.; Sanchez, J.L.; Santos, L.D.D.; Beneti, S.C.; Tanamati, A.; Bona, E.; Tanamati, A.A.C. Multi-block analysis of the oxidative stability of the palm olein and hydrogenated soybean oil during the industrial deep-frying process. J. Food Compos. Anal. 2024, 126, 105897. [Google Scholar] [CrossRef]
- Romano, R.; Filosa, G.; Pizzolongo, F.; Durazzo, A.; Lucarini, M.; Severino, P.; Santini, A. Oxidative stability of high oleic sunflower oil during deep-frying process of purple potato Purple Majesty. Heliyon 2021, 7, e06294. [Google Scholar] [CrossRef]
- Endo, Y. Analytical Methods to Evaluate the Quality of Edible Fats and Oils: The JOCS Standard Methods for Analysis of Fats, Oils and Related Materials (2013) and Advanced Methods. J. Oleo Sci. 2018, 67, 1–10. [Google Scholar] [CrossRef]
- Ding, X.P.; Qi, J.; Chang, Y.X.; Mu, L.L.; Zhu, D.N.; Yu, B.Y. Quality control of flavonoids in Ginkgo biloba leaves by high-performance liquid chromatography with diode array detection and on-line radical scavenging activity detection. J. Chromatogr. A 2009, 1216, 2204–2210. [Google Scholar] [CrossRef] [PubMed]
- Metzner Ungureanu, C.R.; Poiana, M.A.; Cocan, I.; Lupitu, A.I.; Alexa, E.; Moigradean, D. Strategies to improve the thermo-oxidative stability of sunfower oil by exploiting the antioxidant potential of blueberries processing by products. Molecules 2020, 25, 5688. [Google Scholar] [CrossRef]
- Ahmad, S.N.A.; Tarmizi, A.H.A.; Razak, R.A.A.; Jinap, S.; Norliza, S.; Sulaiman, R.; Sanny, M. Selection of Vegetable Oils and Frying Cycles Influencing Acrylamide Formation in the Intermittently Fried Beef Nuggets. Foods 2021, 10, 257. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Singh, B.; Kaur, A.; Yadav, M.P.; Singh, N. Impact of intermittent frying on chemical properties, fatty acid composition, and oxidative stability of 10 different vegetable oil blends. J. Food Process. Pres. 2021, 45, e16015. [Google Scholar] [CrossRef]
- Hosseini, H.; Ghorbani, M.; Meshginfar, N.; Mahoonak, S.A. A review on frying: Procedure, fat, deterioration progress and health hazards. J. Am. Oil Chem. Soc. 2016, 93, 445–466. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.Y.; Li, Q.; Wang, M.Z.; Dong, Y.Y.; Yu, X.Z. Comparative study on the evolution of polar compound composition of four common vegetable oils during different oxidation processes. LWT-Food Sci.Technol. 2020, 129, 109538. [Google Scholar] [CrossRef]
- Elaine, E.; Fong, E.L.; Pui, L.P.; Goh, K.M.; Nyam, K.L. The frying stability comparison of refined palm oil, canola oil, corn oil, groundnut oil, and sunflower oil during intermittent frying of french fries. J. Food Meas. Charact. 2023, 17, 518–526. [Google Scholar] [CrossRef]
- Xu, L.R.; Yang, F.; Li, X.; Zhao, C.W.; Jin, Q.Z.; Huang, J.H.; Wang, X.G. Evaluation of polar compound distribution in edible oils under restaurant deep frying. J. Food Compos. Anal. 2022, 106, 104297. [Google Scholar] [CrossRef]
- Mba, O.I.; Dumont, M.J.; Ngadi, M. Characterization of tocopherols, tocotrienols and total carotenoids in deep-fat fried French fries. J. Food Compos. Anal. 2018, 69, 78–86. [Google Scholar] [CrossRef]
- Zeb, A.; Nisar, P. Effects of High Temperature Frying of Spinach Leaves in Sunflower Oil on Carotenoids, Chlorophylls, and Tocopherol Composition. Front. Chem. 2017, 5, 19. [Google Scholar] [CrossRef]
- Aladedunye, F.A. Natural antioxidants as stabilizers of frying oils. Eur. J. Lipid Sci. Technol. 2014, 116, 688–706. [Google Scholar] [CrossRef]
- Blekas, G.; Psomiadou, E.; Tsimidou, M.; Boskou, D. On the importance of total polar phenols to monitor the stability of Greek virgin olive oil. Eur. J. Lipid Sci. Technol. 2002, 104, 340–346. [Google Scholar] [CrossRef]
- Baldioli, M.; Servili, M.; Perretti, G.; Montedoro, G.F. Antioxidant activity of tocopherols and phenolic compounds of virgin olive oil. J. Am. Oil Chem. Soc. 1996, 73, 1589–1593. [Google Scholar] [CrossRef]
- Li, P.; Yang, X.; Lee, W.J.; Huang, F.R.; Wang, Y.; Li, Y. Comparison between synthetic and rosemary-based antioxidants for the deep frying of French fries in refined soybean oils evaluated by chemical and non-destructive rapid methods. Food Chem. 2021, 335, 127638. [Google Scholar] [CrossRef]
- Manzoor, S.; Masoodi, F.A.; Rashid, R.; Dar, M.M. Improving oxidative stability of soybean oil by apple pomace extract during deep frying of French fries. Food Biosci. 2022, 49, 101874. [Google Scholar] [CrossRef]
- Xin, L.; Hu, M.M.; Ma, X.; Wu, S.M.; Yoong, J.H.; Chen, S.Q.; Tarmizi, A.H.A.; Zhang, G.W. Selection of 12 vegetable oils influences the prevalence of polycyclic aromatic hydrocarbons, fatty acids, tocol homologs and total polar components during deep frying. J. Food Compos. Anal. 2022, 114, 104840. [Google Scholar] [CrossRef]
- Martínez-Yusta, A.; Guillén, M.D. Deep-frying. A study of the influence of the frying medium and the food nature, on the lipidic composition of the fried food, using 1H nuclear magnetic resonance. Food Res. Int. 2014, 62, 998–1007. [Google Scholar] [CrossRef]
- Jiang, X.F.; Huang, R.F.; Wu, S.M.; Wang, Q.; Zhang, Z.H. Correlations between 1H NMR and conventional methods for evaluating soybean oil deterioration during deep frying. J. Food Meas. Charact. 2018, 12, 1420–1426. [Google Scholar] [CrossRef]
- Li, L.; Zhang, M.X.; Wang, X.Y.; Yang, Y.L.; Gong, X.; Wang, C.C.; Xu, J.F.; Li, M.H. Assessment of components of Gingko biloba leaves collected from different regions of China that contribute to its antioxidant effects for improved quality monitoring. Food Sci. Technol. 2021, 41, 676–683. [Google Scholar] [CrossRef]
- Adan, M.; Rasul, A.; Hussain, G.; Shah, M.A.; Zahoor, M.K.; Anwar, H.; Sarfraz, I.; Riaz, A.; Manzoor, M.; Adem, S.; et al. Ginkgetin: A natural biflavone with versatile pharmacological activities. Food Chem. Toxicol. 2020, 145, 111642. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Guo, C.; Liu, L. Compound Gingko Health-Care Tea and Preparation Method Thereof. CN114766573A, 22 July 2022. [Google Scholar]
- Liu, E. Ginkgo and Tartary Buckwheat Vinegar. CN103981077A, 13 August 2014. [Google Scholar]
- Wang, F.; Cui, X.; Liu, G.; Lan, X.; He, Y. Ginkgo Beverage and Preparation Method Thereof. CN104473273B, 5 December 2014. [Google Scholar]
- Qian, L. Preparation Method of Ginkgo Drink. CN104770812A, 15 July 2015. [Google Scholar]
- Yunrong, K. Method for Extracting Gingko Powder from Gingkoes and Preparing Gingko Healthy Wine. CN101597553A, 9 December 2009. [Google Scholar]
- Lyu, Q. Manufacturing Method of Ginkgo Health-Care Wine. CN103773660A, 7 May 2014. [Google Scholar]
- Cao, W. Brewing Method of Gingko Wine. CN113831990A, 24 December 2021. [Google Scholar]
- Zhang, Z.; Zhang, J. Gingko Wine and Production Method Thereof. CN113105986A, 13 July 2021. [Google Scholar]
- Yang, X. Process for Preparing Gingko Wine. CN114292718A, 8 April 2022. [Google Scholar]
- Zhou, W. Ginkgo Healthful Drink. CN103054116A, 24 April 2013. [Google Scholar]
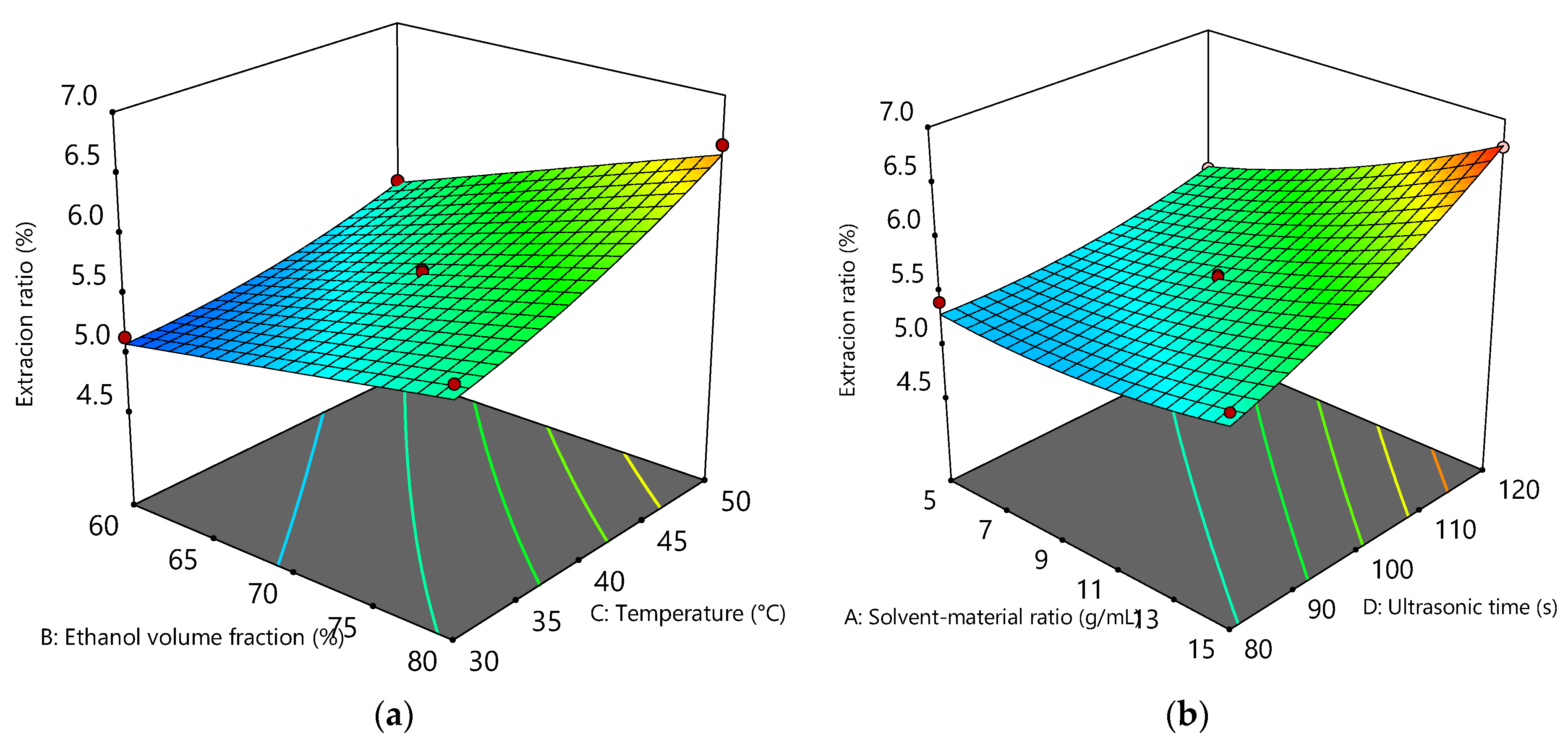

| Factor | Coded Symbol | Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Solvent: material ratio | X1 | 1:5 | 1:10 | 1:15 |
| Ethanol volume fraction | X2 | 60% | 70% | 80% |
| Extraction temperature | X3 | 30 °C | 40 °C | 50 °C |
| Ultrasonic time | X4 | 80 s | 100 s | 120 s |
| Run | Solvent: Material Ratio (mL/g) X1 | Ethanol Volume Fraction (%) X2 | Extraction Temperature (°C) X3 | Ultrasonic Time (s) X4 | Extraction Rate of Flavonoids (%) Y |
|---|---|---|---|---|---|
| 1 | −1 | −1 | 0 | 0 | 5.11 |
| 2 | 1 | −1 | 0 | 0 | 5.60 |
| 3 | −1 | 1 | 0 | 0 | 5.71 |
| 4 | 1 | 1 | 0 | 0 | 6.51 |
| 5 | 0 | 0 | −1 | −1 | 5.05 |
| 6 | 0 | 0 | 1 | −1 | 5.66 |
| 7 | 0 | 0 | −1 | 1 | 5.75 |
| 8 | 0 | 0 | 1 | 1 | 6.76 |
| 9 | −1 | 0 | 0 | −1 | 5.42 |
| 10 | 1 | 0 | 0 | −1 | 5.61 |
| 11 | −1 | 0 | 0 | 1 | 5.63 |
| 12 | 1 | 0 | 0 | 1 | 6.75 |
| 13 | 0 | −1 | −1 | 0 | 5.14 |
| 14 | 0 | 1 | −1 | 0 | 5.70 |
| 15 | 0 | −1 | 1 | 0 | 5.56 |
| 16 | 0 | 1 | 1 | 0 | 6.59 |
| 17 | −1 | 0 | −1 | 0 | 5.11 |
| 18 | 1 | 0 | −1 | 0 | 5.68 |
| 19 | −1 | 0 | 1 | 0 | 5.64 |
| 20 | 1 | 0 | 1 | 0 | 6.39 |
| 21 | 0 | −1 | 0 | −1 | 4.94 |
| 22 | 0 | 1 | 0 | −1 | 5.48 |
| 23 | 0 | −1 | 0 | 1 | 5.68 |
| 24 | 0 | 1 | 0 | 1 | 6.44 |
| 25 | 0 | 0 | 0 | 0 | 5.63 |
| 26 | 0 | 0 | 0 | 0 | 5.61 |
| 27 | 0 | 0 | 0 | 0 | 5.55 |
| 28 | 0 | 0 | 0 | 0 | 5.50 |
| 29 | 0 | 0 | 0 | 0 | 5.61 |
| Source | Sum of Squares | df | df | Mean Square | F-Value | Prob > F |
|---|---|---|---|---|---|---|
| Model | 6.81 | 14 | 14 | 0.49 | 46.69 | <0.0001 |
| X1 | 1.29 | 1 | 1 | 1.29 | 123.55 | <0.0001 |
| X2 | 1.61 | 1 | 1 | 1.61 | 155.01 | <0.0001 |
| X3 | 1.44 | 1 | 1 | 1.44 | 137.77 | <0.0001 |
| X4 | 1.96 | 1 | 1 | 1.96 | 188.48 | <0.0001 |
| X1X4 | 0.21 | 1 | 1 | 0.21 | 20.49 | 0.0005 |
| X2X3 | 0.056 | 1 | 1 | 0.056 | 5.41 | 0.0355 |
| X12 | 0.076 | 1 | 1 | 0.076 | 7.34 | 0.0170 |
| X32 | 0.056 | 1 | 1 | 0.056 | 5.36 | 0.0362 |
| X42 | 0.079 | 1 | 1 | 0.079 | 7.54 | 0.0158 |
| Lack of Fit | 0.13 | 10 | 10 | 0.013 | 4.84 | 0.0710 |
| Fatty Acids | Flaxseed Oil | C16:0 | C18:0 | C18:1 | C18:2 | C18:3 |
|---|---|---|---|---|---|---|
| 0 days | TBHQ | 5.48 ± 0.03 d | 3.41 ± 0.01 f | 18.18 ± 0.12 a | 16.48 ± 0.24 a | 48.49 ± 0.13 a |
| GBLF | 5.87 ± 0.06 d | 4.05 ± 0.10 f | 20.64 ± 0.56 a | 16.57 ± 0.35 a | 51.29 ± 0.51 a | |
| 1 days | TBHQ | 5.53 ± 0.05 d | 4.11 ± 0.11 e | 16.62 ± 0.23 b | 14.41 ± 0.32 b | 46.57 ± 0.33 b |
| GBLF | 5.88 ± 0.05 d | 4.54 ± 0.07 e | 18.37 ± 0.34 b | 14.33 ± 0.29 b | 49.76 ± 0.60 b | |
| 2 days | TBHQ | 5.86 ± 0.09 c | 4.30 ± 0.13 d | 14.75 ± 0.17 c | 14.16 ± 0.16 b | 42.64 ± 0.26 c |
| GBLF | 5.87 ± 0.06 d | 4.74 ± 0.08 cd | 17.26 ± 0.18 c | 14.18 ± 0.33 b | 43.42 ± 0.47 c | |
| 3 days | TBHQ | 6.14 ± 0.11 bc | 4.41 ± 0.06 cd | 14.58 ± 0.38 c | 13.45 ± 0.38 c | 42.36 ± 0.55 c |
| GBLF | 6.00 ± 0.05 d | 4.66 ± 0.08 de | 16.45 ± 0.41 d | 13.41 ± 0.33 c | 41.19 ± 0.42 d | |
| 4 days | TBHQ | 6.25 ± 0.26 b | 4.54 ± 0.07 c | 14.58 ± 0.39 c | 13.34 ± 0.49 cd | 40.77 ± 0.59 d |
| GBLF | 6.24 ± 0.06 c | 4.87 ± 0.06 bc | 14.52 ± 0.40 e | 13.37 ± 0.46 c | 41.16 ± 0.22 d | |
| 5 days | TBHQ | 6.26 ± 0.26 b | 4.94 ± 0.14 b | 14.43 ± 0.36 c | 12.85 ± 0.26 d | 39.86 ± 1.17 d |
| GBLF | 6.45 ± 0.12 b | 4.94 ± 0.09 b | 14.15 ± 0.74 e | 12.54 ± 0.42 d | 40.22 ± 0.30 e | |
| 6 days | TBHQ | 6.73 ± 0.23 a | 5.18 ± 0.05 a | 11.84 ± 0.28 d | 10.33 ± 0.30 e | 29.71 ± 0.61 e |
| GBLF | 6.88 ± 0.11 a | 5.37 ± 0.07 a | 12.56 ± 0.42 f | 10.38 ± 0.34 e | 32.82 ± 0.75 f |
| Fatty Acids | Soybean Oil | C16:0 | C18:0 | C18:1 | C18:2 | C18:3 |
|---|---|---|---|---|---|---|
| 0 days | TBHQ | 10.45 ± 0.51 g | 4.07 ± 0.07 c | 29.62 ± 0.56 a | 51.45 ± 0.90 a | 6.28 ± 0.25 a |
| GBLF | 11.06 ± 0.45 g | 4.85 ± 0.09 cd | 30.23 ± 0.55 a | 50.88 ± 0.38 a | 6.85 ± 0.09 a | |
| 1 days | TBHQ | 12.70 ± 0.40 f | 4.07 ± 0.08 c | 28.67 ± 0.33 b | 50.76 ± 0.46 ab | 5.84 ± 0.11 b |
| GBLF | 13.38 ± 0.31 f | 4.82 ± 0.15 cd | 29.76 ± 0.34 a | 50.78 ± 0.81 a | 6.04 ± 0.10 b | |
| 2 days | TBHQ | 16.55 ± 0.32 e | 4.13 ± 0.07 bc | 27.10 ± 0.35 c | 49.65 ± 0.68 b | 5.43 ± 0.12 c |
| GBLF | 18.28 ± 0.32 e | 4.83 ± 0.10 cd | 28.55 ± 0.34 b | 50.75 ± 0.56 a | 5.84 ± 0.07 b | |
| 3 days | TBHQ | 20.49 ± 0.44 d | 4.22 ± 0.10 abc | 25.77 ± 0.20 d | 47.46 ± 0.51 c | 4.85 ± 0.12 d |
| GBLF | 22.32 ± 0.40 d | 4.71 ± 0.14 d | 25.95 ± 0.16 c | 48.59 ± 0.96 b | 5.03 ± 0.14 c | |
| 4 days | TBHQ | 22.24 ± 0.60 c | 4.30 ± 0.17 ab | 26.64 ± 0.31 e | 45.47 ± 0.64 d | 4.39 ± 0.21 e |
| GBLF | 25.58 ± 0.44 c | 5.02 ± 0.13 bc | 25.22 ± 0.19 d | 46.60 ± 0.42 c | 4.94 ± 0.10 c | |
| 5 days | TBHQ | 24.31 ± 0.64 b | 4.41 ± 0.12 a | 23.79 ± 0.33 f | 40.28 ± 0.70 e | 3.80 ± 0.13 f |
| GBLF | 26.25 ± 0.28 b | 5.29 ± 0.15 ab | 23.81 ± 0.22 e | 41.11 ± 0.38 d | 4.36 ± 0.42 d | |
| 6 days | TBHQ | 27.48 ± 0.36 a | 4.31 ± 0.18 ab | 23.35 ± 0.37 f | 32.36 ± 0.53 f | 2.76 ± 0.12 g |
| GBLF | 28.45 ± 0.39 a | 5.42 ± 0.26 a | 23.89 ± 0.23 e | 34.24 ± 0.54 e | 2.90 ± 0.18 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, M.; Zulkurnain, M. Ultrasonic-Assisted Ginkgo biloba Leaves Extract as Natural Antioxidant on Oxidative Stability of Oils During Deep-Frying. Foods 2025, 14, 2958. https://doi.org/10.3390/foods14172958
Kang M, Zulkurnain M. Ultrasonic-Assisted Ginkgo biloba Leaves Extract as Natural Antioxidant on Oxidative Stability of Oils During Deep-Frying. Foods. 2025; 14(17):2958. https://doi.org/10.3390/foods14172958
Chicago/Turabian StyleKang, Min, and Musfirah Zulkurnain. 2025. "Ultrasonic-Assisted Ginkgo biloba Leaves Extract as Natural Antioxidant on Oxidative Stability of Oils During Deep-Frying" Foods 14, no. 17: 2958. https://doi.org/10.3390/foods14172958
APA StyleKang, M., & Zulkurnain, M. (2025). Ultrasonic-Assisted Ginkgo biloba Leaves Extract as Natural Antioxidant on Oxidative Stability of Oils During Deep-Frying. Foods, 14(17), 2958. https://doi.org/10.3390/foods14172958





