3.1. Orange Residue Flour and Initial Emulsion Characteristics
The chemical composition of ORF has been previously described in the methodology section. Additionally, its solubility at pH 3.8 was measured, yielding a value of 38 ± 2%. The water-soluble fraction of ORF at pH 3.8 (38 ± 2%) is probably composed of pectins, simple sugars, and other soluble dietary fibre components such as arabinans and galactans, together with a small fraction of soluble proteins and phenolic compounds. The remaining insoluble fraction is primarily constituted by cellulose, hemicelluloses with low solubility, and pectins strongly bound to the cell wall matrix. Although pectins are generally considered water-soluble, their dissolution is often slow and incomplete [
40]. In composite materials like ORF, this process is further hindered by the structural association of pectins with other cell wall components. The solubility observed in this study is within the upper range reported by Garau et al. [
41] for orange peel and pulp dried at moderate temperatures (25–38%) when measured in non-acidified water. Although the values are not directly comparable due to methodological differences, including the use of acidified water in our case, their results support the idea that a large proportion of orange residues remains insoluble under typical aqueous extraction.
From a functional perspective, this partial solubility means that a fraction of ORF remains as suspended particles in the aqueous phase, which can contribute to emulsion stability via Pickering-type stabilisation, as we previously reported for ORF [
12] and other partially insoluble plant materials, such as artichoke bracts [
27] and mushroom flour [
28,
42]. In these cases, the presence of insoluble polysaccharides enhanced resistance to droplet coalescence and improved oxidative stability after spray drying.
Regarding the emulsions, the soy protein pre-emulsion at pH 3.8 exhibited a ζ-potential of 6.5 ± 3.0 mV, confirming that at this pH the protein carries a net positive charge (above its isoelectric point). These conditions were, therefore, suitable for the preparation of a double-layer emulsion. After emulsification with ORF, the system showed a ζ-potential of −23.5 ± 0.6 mV, indicating that the negatively charged pectins of the ORF successfully formed the secondary layer, contributing predominantly to the overall surface charge. These results are consistent with Locali et al. [
17], who reported that the isoelectric point of soy proteins is close to pH 4.0 and that working below this value promotes electrostatic attraction with negatively charged pectins, facilitating bilayer formation. In line with our findings, Moser et al. [
16,
18] also observed a shift in ζ-potential from positive values in protein-only systems to negative values after pectin adsorption, confirming the successful deposition of the secondary layer.
3.2. Powder Yield
A three-way ANOVA was performed to evaluate the effects of inlet temperature (Tin), feed rate (FR), and powder collection position (chamber or collector) on the yield and the properties of the powder and the reconstituted emulsions. The results are summarised in
Table 2. For yield, all main factors were significant (
p < 0.05), as well as the interactions Tin × Position and FR × Position.
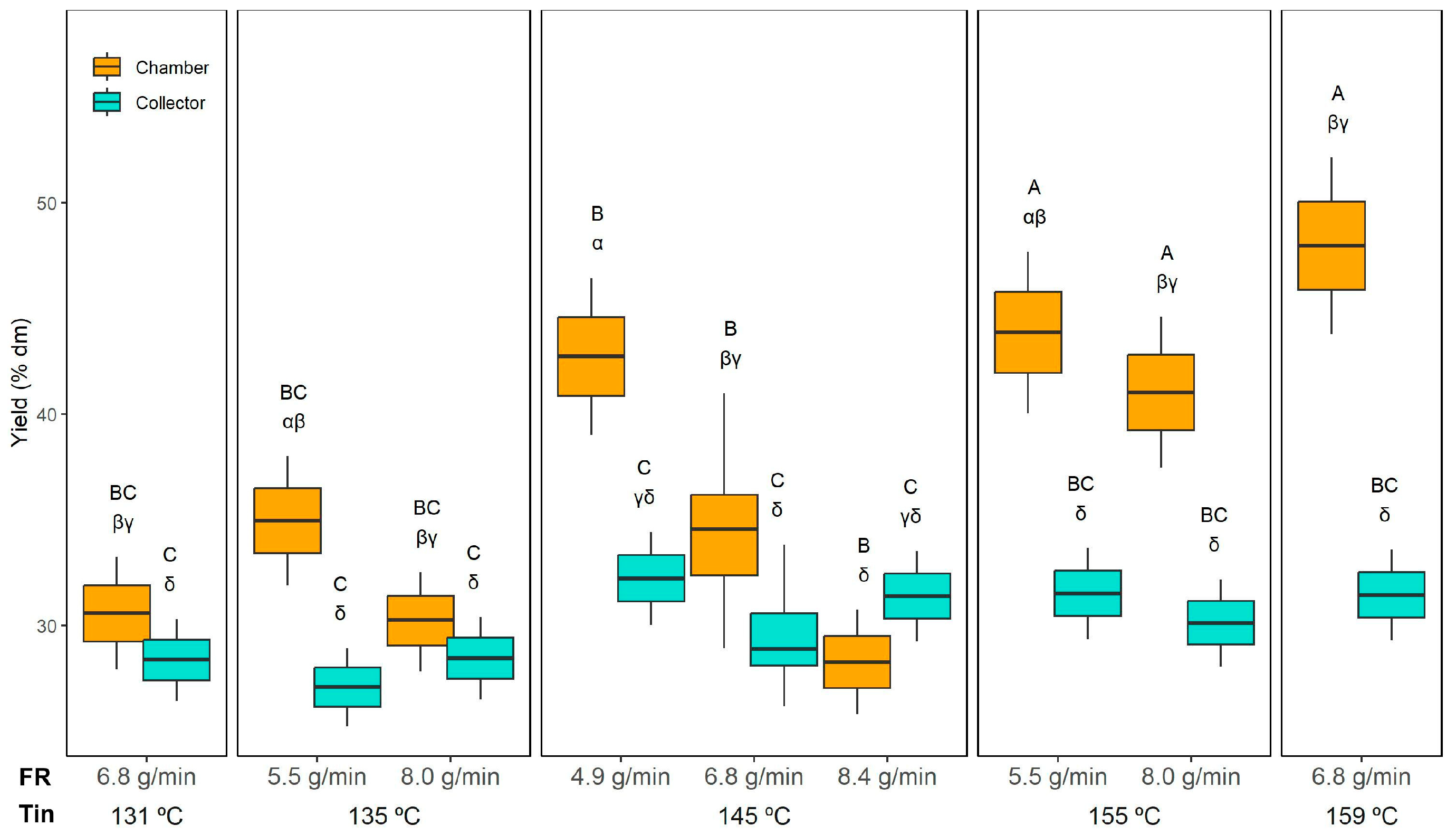
Figure 1 shows the yield results. Interestingly, the collector yield was practically unaffected by Tin or FR, averaging 30 ± 2% dm across all experiments. The collector yield remained stable because it primarily captures fine, dried particles carried by the exhaust airflow, which are less sensitive to Tin and FR variations. In contrast, the chamber yield varied widely, from 28 ± 2% dm (145 °C and 8.4 g/min) to 48 ± 4% dm (159 °C and 6.8 g/min). When comparing the powder collected from the collector and the drying chamber, significantly higher yields were obtained in the chamber than in the collector, except for the run at 145 °C with 8.4 g/min, where the yield decreased.
Regarding the effect of FR, when comparing samples dried at the same temperature (e.g., 145 °C), a significant decrease in chamber yield was only observed when FR reached the highest value (8.4 g/min). Therefore, 8.4 g/min likely exceeded the dryer’s optimal capacity, introducing too much liquid for the available thermal energy. As a result, the larger droplets formed at this extreme FR required longer drying times, favouring particle stickiness and wall deposition.
Increasing the Tin to 155 °C or 159 °C resulted in significantly higher chamber yields compared to drying at 131 °C or 135 °C. This effect aligns with the outlet temperatures (Tout) recorded under these conditions (
Table 1), where higher Tin corresponded to higher Tout, indicating a more efficient drying environment. At elevated Tin, the faster evaporation rate promotes quicker crust formation on droplet surfaces, reducing particle stickiness and wall deposition, thereby enhancing chamber yield. Conversely, at lower Tin (131–135 °C), the lower Tout suggests slower drying kinetics, leaving particles partially wet and prone to adhesion on the chamber walls, which reduces yield.
Overall, these results highlight the importance of optimising Tin and FR to minimise wall deposition in the chamber while maintaining a stable cyclone collection.
Moser et al. [
16], who dried a bilayer emulsion containing buriti in a Büchi B-290 at inlet temperatures between 154 °C and 196 °C and feed rates up to 6.8 mL/min, reported total yields ranging from 33% to 54%. In our study, the total yield (calculated as the sum of the powder recovered from the collector and the drying chamber) ranged from 59 ± 2% (135 °C and 8.0 g/min) to 79 ± 6% (159 °C and 6.8 g/min). In both studies, higher inlet temperatures resulted in significantly higher yields. The higher total yields obtained in this study compared to previous works with similar bilayer emulsions may reflect the favourable drying behaviour of the ORF. Similarly, Quintero-Gamero et al. [
43], who spray-dried buriti oil emulsions at Tin ranging from 140 °C to 180 °C, achieved total yields between 72% and 96%. As in the present study, higher inlet temperatures favoured higher yields.
3.3. Characteristics of the Powders
3.3.1. Encapsulation Efficiency
Encapsulation efficiency (EE) is a key parameter in microencapsulation processes, as it reflects the proportion of oil effectively protected by the wall material. As shown in
Table 2, EE was significantly affected by the Tin and the powder collection position, while the interaction FR × Position also had a significant influence (
p < 0.05). The EE values are shown in
Figure 2. As can be seen, among the evaluated factors, collection position had the strongest effect, with the powder collected in the drying chamber exhibiting significantly higher EE. This parameter varied in the chamber from 80.3 ± 3.3% (149 °C and 4.9 g/min) to 86 ± 1.2% (145 °C and 6.8 g/min). For the collector, the lowest encapsulation efficiency was 69.8 ± 2.4% (155 °C and 8.0 g/min), while the highest was 76.8 ± 0.1% (145 °C and 4.9 g/min). Although Tin had a statistically significant effect, it was less pronounced than the influence of the collection position. Nevertheless, a slight decrease in EE was observed at 155 °C compared to 135 °C in both the chamber and collector, regardless of the feed rate. This reduction may be attributed to increased thermal stress at higher temperatures, which can promote partial coalescence or surface oil formation, thereby lowering encapsulation efficiency.
The reported values are similar but slightly lower than those achieved in the investigation of Moser et al. [
16], who also dried double-layer emulsions combining proteins and polysaccharides (79–90%). They expressed that probably the bilayer formed at the oil droplet surface contributed to the high encapsulation efficiency. Our results are also comparable to those reported for other emulsions containing vegetable oils spray dried at the laboratory scale. For instance, sea buckthorn berry oil emulsions processed in a Labplant SD-06 at 120–180 °C achieved EE values ranging from 73 to 93%, with higher Tin and the use of β-cyclodextrin improving retention [
21]. In contrast, retinoic acid emulsions spray dried in a Büchi B-290 at 115–150 °C showed much lower EE (27–65%) due to the extreme thermal sensitivity of the compound and the less stable emulsions [
44]. They observed that intermediate Tin (~130 °C) maximised encapsulation efficiency, while higher Tin (150 °C) led to a decline due to thermal stress and weaker wall integrity.
The higher EE observed in the chamber powder compared to the collector is in general agreement with the observations of Donz et al. [
23]. In this study whey–palm oil emulsions with a very high fat content (50 g/100 g) were spray dried at an industrial scale, and powders were collected at different stages of the process: directly from the drying chamber, from the first and second cyclones, and as the final mixed product. They observed that the free surface fat (meaning a low encapsulation efficiency) content increased significantly in the powders processed through the cyclones, with the second cyclone showing the highest free fat levels (around 37%) compared to only ~8% in the final powder and ~3% in the chamber powder without fines. This accumulation of surface fat was attributed to mechanical stress and particle selection in the cyclones, which either released fat droplets from the powder matrix or preferentially carried lighter fat-rich particles.
3.3.2. Moisture Content and Water Activity
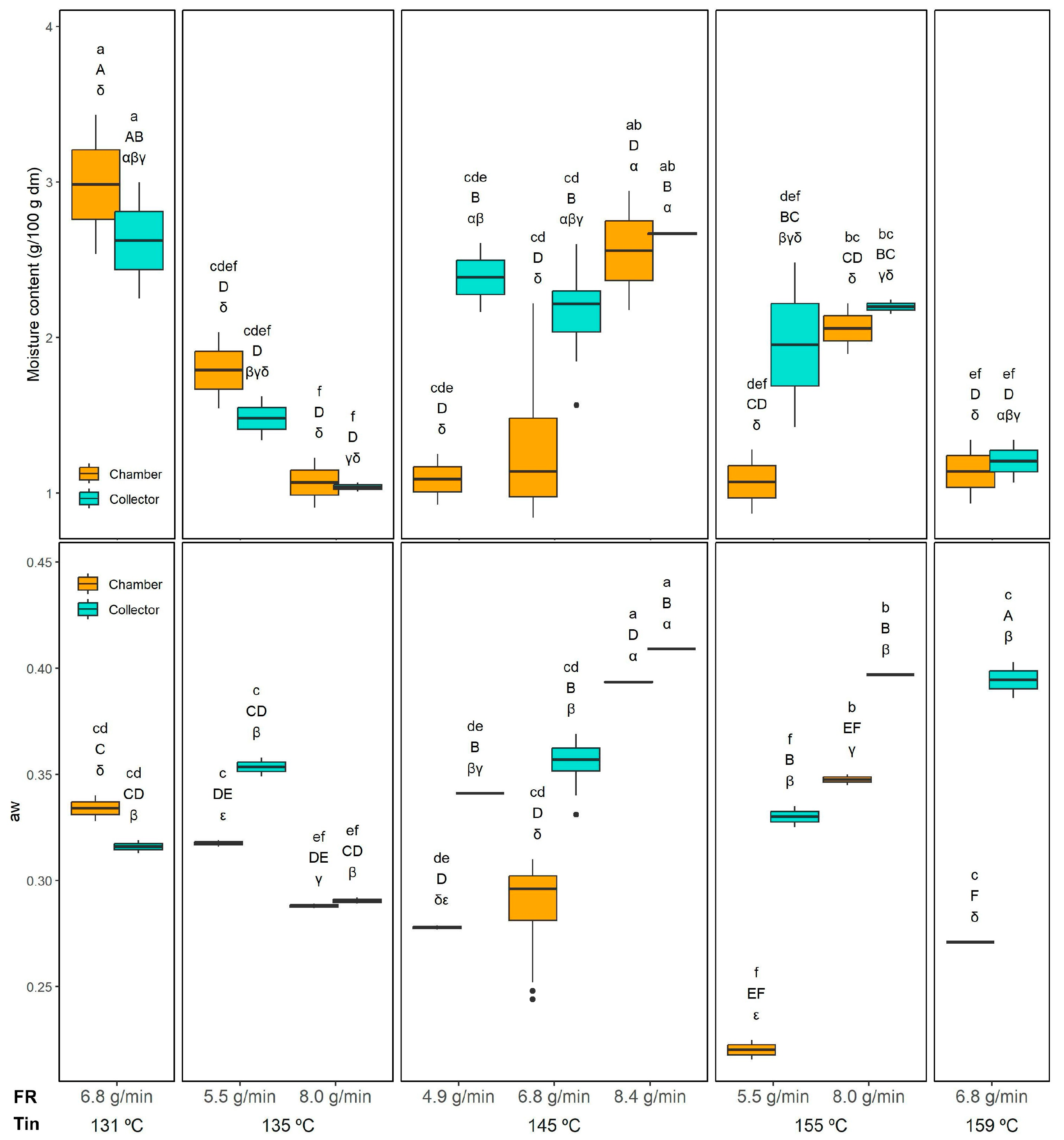
Regarding the moisture content (MC), all the evaluated factors (Tin, FR, and powder collection position) showed a significant effect (
p < 0.05), as well as all two-way interactions. The MC and the aw results are shown in
Figure 3. In contrast, the three-way interaction Tin × FR × Position was not significant. The MC ranged from 1.04 ± 0.03 (155 °C 8.0 g/min) to 2.67 ± 0.01 g/100 g dm (145 °C and 8.4 g/min) in the collector and from 0.99 ± 0.15 (145 °C and 6.8 g/min) to 2.98 ± 0.21 g/100 g dm (131 °C and 6.8 g/min) in the chamber. Significant differences between chamber and collector powders were observed only when drying at 145 °C with feed rates of 4.9 and 6.8 g/min, where the chamber powder presented a lower MC, likely due to its longer exposure to higher temperatures inside the drying chamber. At a constant Tin of 145 °C, the MC of the chamber increased when the FR was raised from 4.9 and 6.8 g/min to 8.4 g/min, probably because this higher feed rate exceeded the drying capacity, resulting in insufficient moisture removal. Interestingly, this FR effect was not observed in the collector.
Regarding the Tin effect, when comparing chamber samples dried at the same FR (6.8 g/min), a significant decrease in the MC of the chamber was observed when increasing Tin from 131 °C to 145 °C and 159 °C, which is probably relates to the higher Tout (
Table 1) observed in this conditions (145 and 159 compared to 131 °C). A similar trend was also evident in the collector powders, where the MC at 131 °C was significantly higher than at 159 °C, confirming that higher Tin enhances moisture removal efficiency. These results align with the drying dynamics in spray drying. Higher Tin accelerates water evaporation and promotes the rapid formation of a dry outer layer, which reduces overall moisture content. Conversely, excessively high feed rates increase the droplet size and shorten the effective residence time, limiting moisture removal and resulting in higher residual MC.
Comparable MC values have been reported for other emulsions spray dried at the laboratory scale. For instance, sea buckthorn berry oil powders processed at 120–180 °C showed MC values ranging from 0.23 to 3.7%, also decreasing with higher Tin [
21]. In contrast, Moser et al. [
16] obtained much lower MC values (0.4–1.0%) for bilayer buriti oil emulsions dried at 154–196 °C, likely due to the higher Tin and the use of partial vacuum. Nevertheless, they observed a similar trend with FR, where increasing the feed rate resulted in higher residual MC, although in their case, Tin did not show a clear effect.
Regarding the aw (
Figure 3), all the evaluated factors (Tin, FR, and collection position) as well as their two-way interactions were significant (
p < 0.05), while the three-way interaction Tin × FR × Position was not. The lowest aw in the collector was observed at 135 °C and 8.0 g/min (0.29 ± 0.01), while the highest aw was obtained at 145 °C and 8.4 g/min (0.41 ± 0.01). In the chamber, the lowest aw was recorded at 155 °C and 5.5 g/min (0.22 ± 0.01), and the highest at 145 °C and 8.4 g/min (0.39 ± 0.01). The effect of collection position was not very consistent. Overall, higher aw values were generally observed in the collector compared to the chamber, consistent with the shorter residence time of particles collected in the cyclone. However, this trend was not consistent under all conditions. For instance, at the lowest drying temperatures (131 °C and 135 °C), the chamber and collector exhibited similar aw values, likely because the lower thermal input in the chamber was insufficient to evaporate free water as effectively as at higher temperatures.
The effect of FR was clearer. At 145 °C, the highest FR (8.4 g/min) produced a significantly higher aw than the lower FR conditions (4.9 and 6.8 g/min), both in the chamber and in the collector, which is in agreement with the MC results.
When maintaining the FR constant at 6.8 g/min, increasing Tin from 131 °C to 159 °C significantly reduced the aw in the chamber. In contrast, this trend was not observed in the collector, where aw increased at 159 °C. There is a lack of direct correlation between MC and aw particularly evident in that experiment (159 °C and 6.8 g/min), where the collector powder showed the highest aw despite not having a high MC. The higher aw in this powder, despite a moderate MC, can be explained by the faster surface drying of maltodextrin-based particles at high Tin (and high Tout,
Table 1), which reduces total moisture but leaves a larger fraction of the residual water accessible on the particle surface, especially in the finer particles collected in the collector.
Comparable aw values have been reported for other spray-dried emulsions. Čulina et al. [
21] observed aw values ranging from 0.22 to 0.35, with slightly lower values at higher Tin. Moser et al. [
16], drying bilayer emulsions of buriti oil, obtained even lower aw values (0.18–0.27) due to the higher Tin and the use of partial vacuum. Similarly, Gonçalves et al. [
44] reported aw values in the range of 0.20–0.30 for bilayer buriti oil emulsions, where FR had a more pronounced effect than Tin. Despite the differences in absolute values, likely due to the specific wall material composition, all studies confirm that higher FR tends to increase aw. In contrast, the effect of Tin is weaker or only evident at lower FR.
3.3.3. Oxidation of the Encapsulated Oil
The oxidation level of the initial and the encapsulated oil was evaluated by measuring the specific absorbance of conjugated dienes (SA at 234 nm), which are primary oxidation products of unsaturated fatty acids [
45]. This parameter is critical, as it reflects both the oxidative stability of the final product and the protective capacity of the wall materials during the spray drying process.
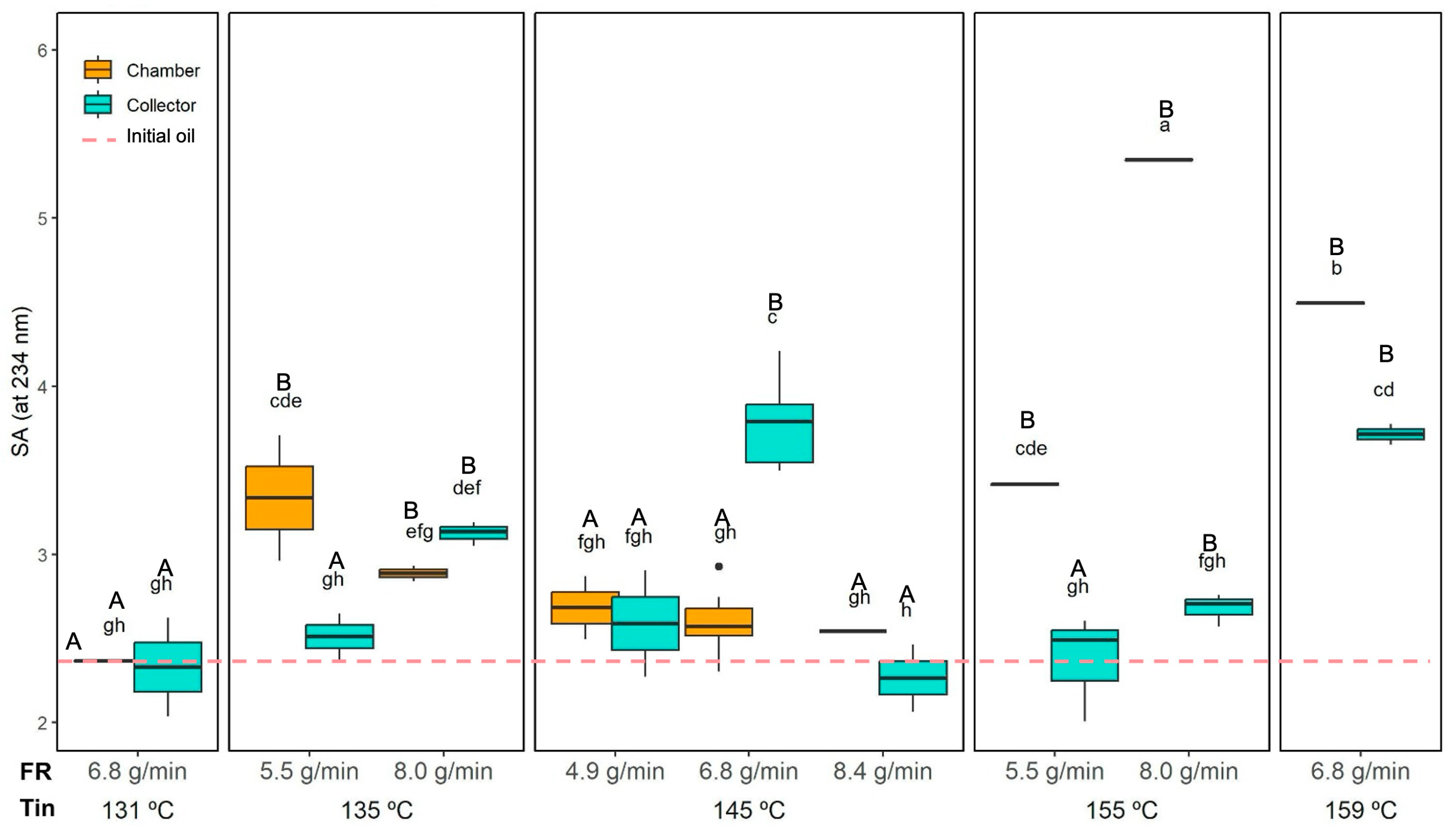
As shown in
Table 2, Tin and FR significantly affected oil oxidation, while the collection position alone did not exhibit a strong effect. However, all two- and three-way interactions were also significant (
p < 0.05), indicating a complex relationship between drying conditions and oxidative stability.
The SA results are presented in
Figure 4. The initial oil, before emulsion preparation and spray drying, had a baseline SA of 2.3 ± 0.1. Most of the dried powders exhibited values not significantly different from those of the initial oil, indicating that the process preserved the oil’s integrity. However, some conditions led to significantly higher oxidation levels. In the collector, samples dried at 135 °C and 8.0 g-min, 145 °C and 6.8 g/min, 155 °C and 8.0 g/min, and 159 °C and 6.8 g/min exhibited significantly higher SA than the baseline oil. In the chamber, all powders dried at 135 °C and 155 °C, as well as those at 159 °C (regardless of FR), also showed significantly higher oxidation.
Across all conditions, SA ranged from 2.3 ± 0.2 (145 °C and 8.4 g-min) to 3.8 ± 0.3 (145 °C and 6.8 g/min) in the collector, and from 2.4 ± 0.1 (131 °C and 6.8 g/min) to 5.4 ± 0.1 (155 °C and 8.0 g/min) in the chamber. Significantly higher SA values (p < 0.05) were observed in the chamber compared to the collector when drying at 135 °C and 5.5 g/min, at 155 °C (regardless of FR), and 159 °C. This suggests that the longer residence time and greater thermal exposure in the drying chamber promoted oil oxidation compared to the collector. Nevertheless, the higher encapsulation efficiency (EE) of chamber powders may have provided a more effective oxygen barrier, partially offsetting thermal degradation.
In some cases, increasing FR led to higher oxidation (e.g., at 155 °C, chamber powders showed higher SA at 8.0 g/min compared to 5.5 g/min), while in other cases, FR had no significant impact or even reduced oxidation. Conversely, the Tin effect was more consistent: the highest Tin (159 °C) produced significantly higher oxidation in both the chamber and collector compared to the lowest Tin (131 °C) at the same FR. The Tin effect was more pronounced in the chamber where the highest SA values were observed at the highest temperatures (155 and 159 °C; in these conditions, Tout was close to or even higher than 100 °C; see
Table 1).
Oxidation patterns varied with collection position. In the chamber, SA values increased steadily with Tin, as longer residence time and higher temperatures promoted cumulative thermal degradation. In contrast, collector powders, with lower encapsulation efficiency and more exposed surface oil, were more sensitive to oxygen. Oxidation peaked at 145 °C and 6.8 g/min because this condition represented the least favourable balance between surface oil protection and oxidative exposure. At this intermediate feed rate, droplet size and moisture content during drying were such that crust formation was slower than at 4.9 g/min, leaving more unprotected oil exposed to oxygen, while the residence time was still sufficient for oxidation to occur. In contrast, at the same temperature but with a lower feed rate (4.9 g/min), smaller droplets and faster crust formation reduced oxygen access, lowering oxidation. Similarly, at a higher feed rate (8.4 g/min), although crust formation was also slower, the shorter residence time in the chamber limited oxygen contact, resulting in lower oxidation. At 155 °C, faster crust formation further restricted oxygen diffusion, slightly reducing oxidation, whereas at 159 °C, extreme thermal stress once again promoted oil degradation despite crust presence.
Similar observations were reported by Javed et al. [
46], who spray dried designer egg powder and found that higher inlet temperatures (≥180 °C) significantly increased peroxide values and accelerated PUFA degradation, highlighting the thermal sensitivity of lipids. Likewise, Huang et al. [
47] showed that higher Tin (110–123 °C) and lower encapsulation efficiency in tilapia oil powders led to increased hydroperoxide formation. Although different matrices and markers were used (peroxide values or hydroperoxides vs. SA of conjugated dienes), both studies confirm that elevated inlet temperatures promote lipid oxidation.
3.3.4. Size and Microstructure of the Reconstituted Emulsions
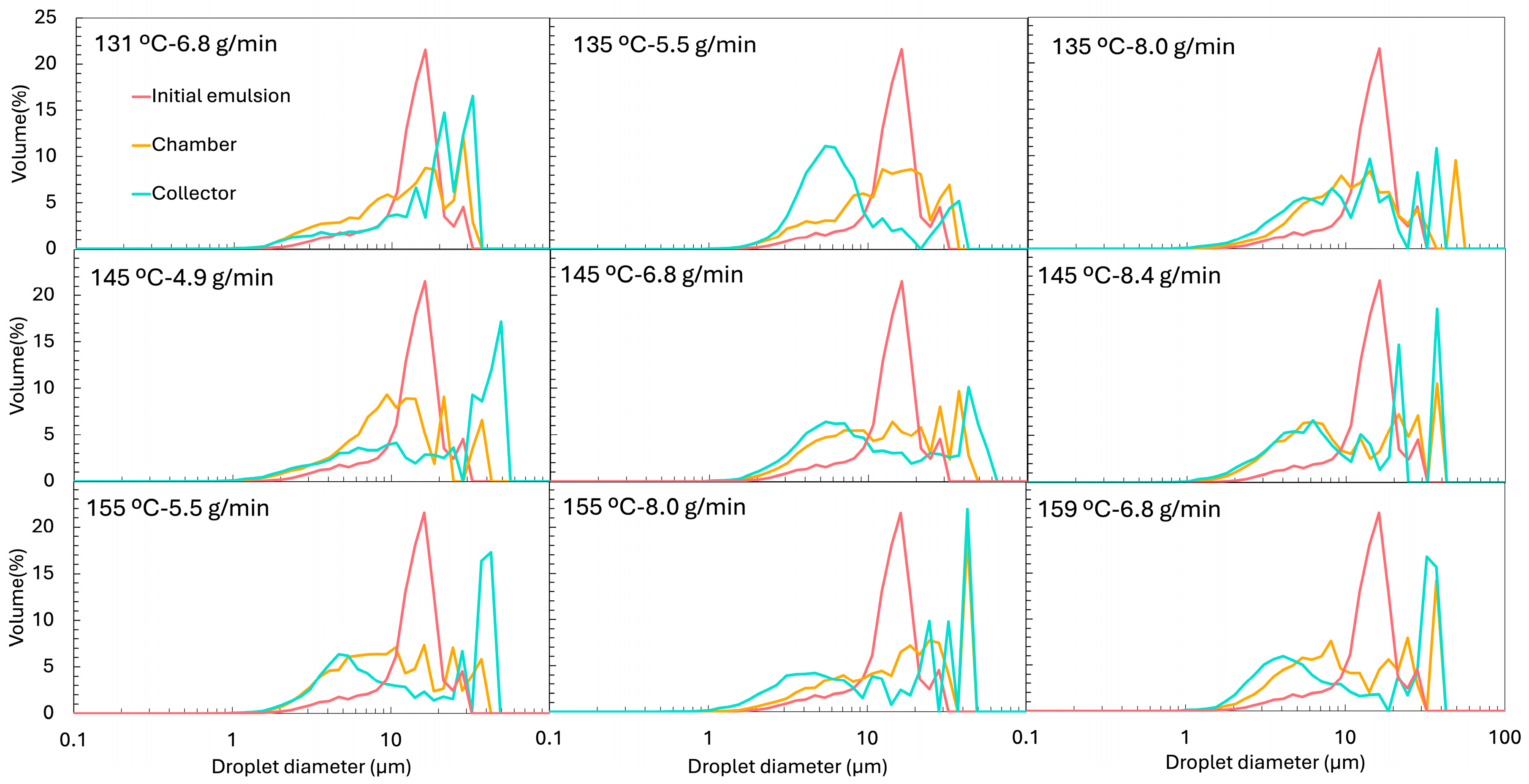
The particle size distribution (PSD) of the initial emulsion (before spray drying) is presented in
Figure 5 and exhibits a clear bimodal profile. The first peak, around 10 µm, corresponds to the oil droplets. In contrast, the second, broader peak (~79 µm) originates from the insoluble particles of ORF, which is only partially soluble (measured solubility was 38 ± 2%). The PSD of the oil-free ORF suspension, also included in
Figure 5, confirms its contribution to the large-size population of the initial emulsion.
This compositional complexity complicates the interpretation of the reconstituted emulsions, since the insoluble ORF particles remain present after drying and reconstitution. To distinguish between droplets and insoluble particles, two complementary techniques were used: (i) laser diffraction and dynamic image analysis (LD), which detects all dispersed elements (droplets + fibres) (
Figure 5), and (ii) optical microscopy coupled with image analysis (IA), where insoluble ORF particles were visually excluded, so only oil droplets were analysed (
Figure 6 for the micrograph and
Figure 7 for the PSD). From the LD PSDs in
Figure 5, marked differences were observed between emulsions reconstituted from chamber and collector powders. Collector-derived emulsions showed an additional population of very fine droplets/particles (<1 µm), absent in chamber emulsions. This likely originates from the atomisation step, where intense shear and turbulence can generate submicron droplets that, once dried, are more prone to being carried to the collector. As described by Höhne and Gaukel [
48], the atomisation step itself can lead to oil droplet breakup and consequently alter the oil droplet size distribution before the drying stage. A similar effect was reported by Peng et al. [
49], who observed a ~65% reduction in droplet size after spray drying, which they attributed to atomiser speed and nozzle size.
Thus, the formation of finer droplets during atomisation may result in a broader PSD in the reconstituted emulsion compared to the original feed. Conversely, the large-size peak associated with insoluble ORF particles was much more prominent in chamber samples but was nearly absent in the collector, suggesting that the heavier insoluble particles were retained in the drying chamber rather than transported to the cyclone. It is possible that, in the chamber powders, the very large ORF particles dominate the volume-weighted PSD, making the few smaller droplets (<1 µm) undetectable.
These compositional differences are corroborated by the micrographs in
Figure 6, where chamber-derived emulsions exhibit a higher prevalence of irregular particles (“I”), which are attributed to the insoluble fraction of the ORF. Quantification of droplet sphericity (
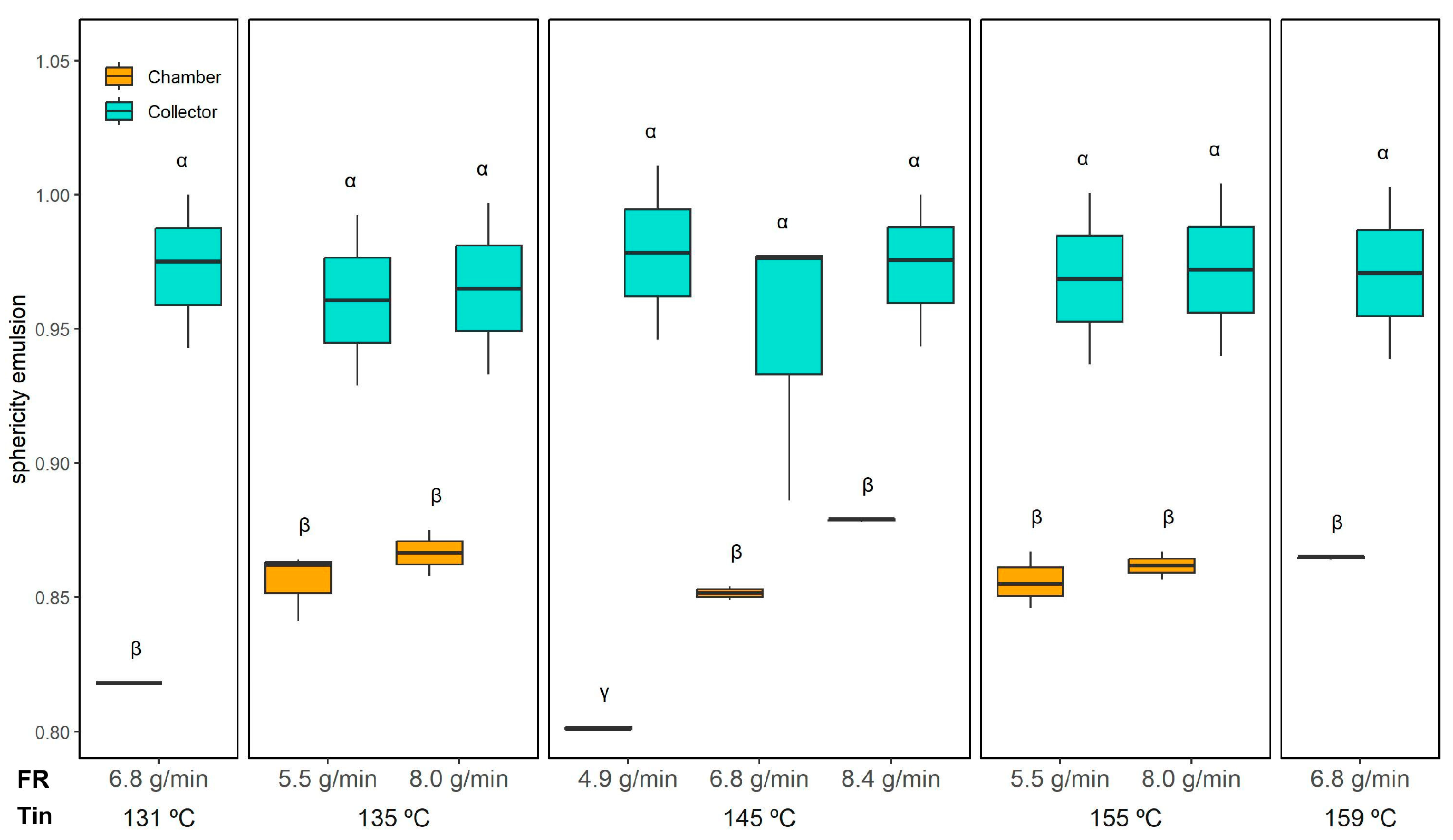
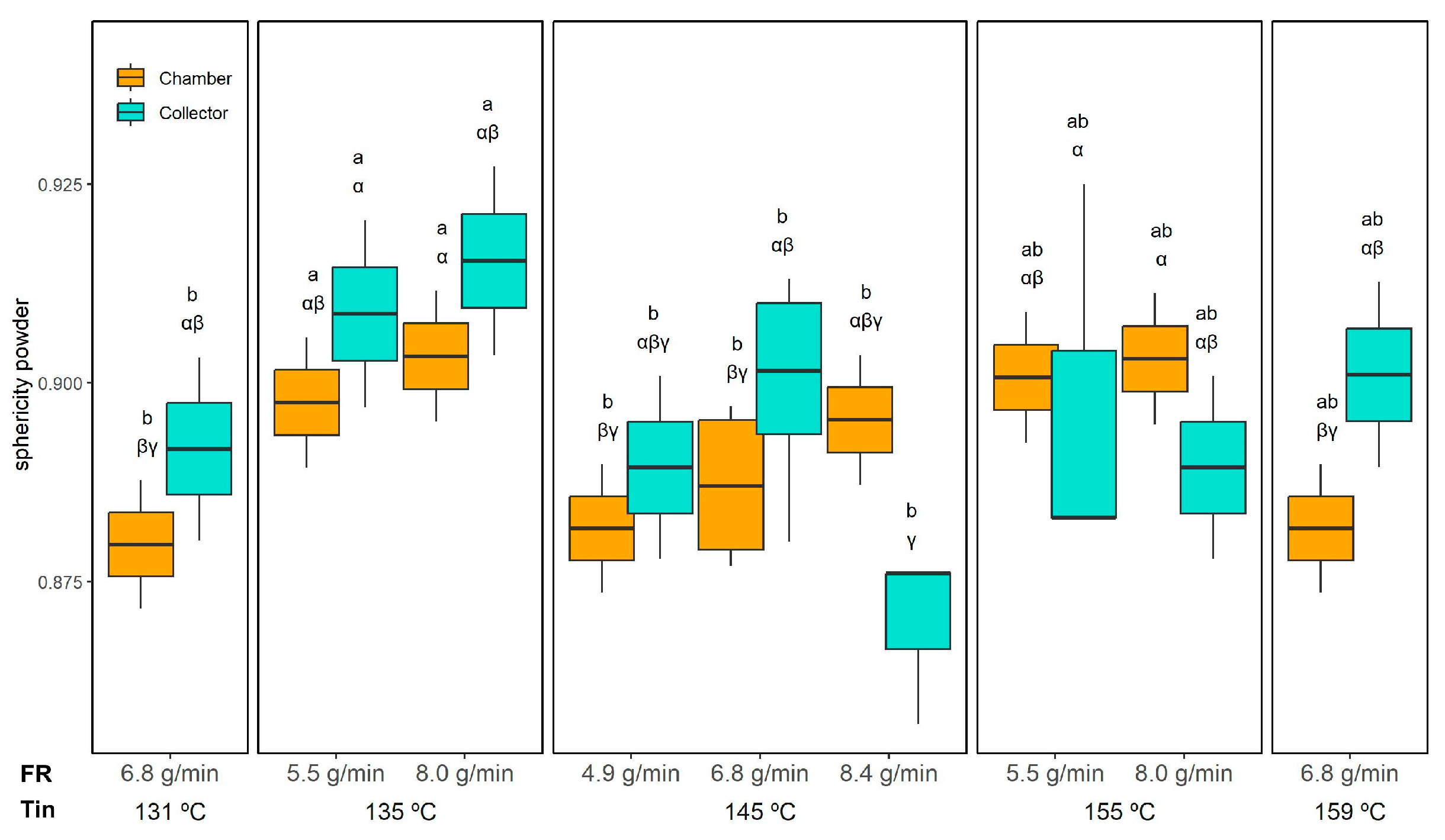
Figure 8) supports this observation: droplets in reconstituted emulsions from collector powder maintained high sphericity (0.97 ± 0.02, unaffected by drying conditions), whereas those from the chamber powder had lower sphericity varying from 0.80 ± 0.01 (145 °C and 4.9 g/min) to 0.88 ± 0.01 (145 °C and 8.4 g/min), similar to the initial emulsion (0.85 ± 0.01), indicating a greater presence of irregular ORF particles.
When examining only the droplets via IA (
Figure 7), the PSD profiles of reconstituted emulsions from chamber and collector powders became similar. This was also evident when comparing the d50 values of the PSD. These values are shown in
Figure 9, presenting the d50 obtained by LD (a) and by IA (b). The d50 of the reconstituted emulsions obtained by IA (
Figure 9b) was very similar between the chamber and the collector powders. The collector showed values ranging from 7.5 ± 1.2 µm to 18.5 ± 3.0 µm, while the chamber ranged from 9.3 ± 2.3 µm to 18.6 ± 4.4 µm, without any clear systematic difference. However, when using LD, which includes all dispersed particles, a clear difference between positions emerged. The chamber samples exhibited much higher d50 values (47.5–69.3 µm) compared to those of the collector (11.6–30.7 µm). This disparity reflects the contribution of larger and irregular ORF particles retained in the chamber, which were excluded from the image-based measurement.
It is noteworthy that the chamber powders also exhibited higher encapsulation efficiency as previously described (80–86% vs. 70–77% in the collector), which is consistent with their higher retention of ORF insoluble particles. This higher retention of ORF insoluble particles in the chamber powders can be explained by their relatively high density and larger size, which reduces their likelihood of being entrained in the cyclone airflow and collected in the cyclone vessel. As a result, these particles remain predominantly in the chamber fraction. Their presence contributes to a more continuous and complete wall matrix around the oil droplets, improving encapsulation efficiency. Furthermore, ORF particles are rich in bioactive compounds with antioxidant activity (as described in the methodology regarding ORF composition), which may enhance oxidative stability by limiting oxygen access to the encapsulated oil. This could explain why, despite being exposed to harsher thermal conditions, the oil in the chamber powders showed similar or even lower oxidation levels compared to the collector powders in some conditions (
Figure 4, e.g., at 131 °C and 145 °C). Moreover, the chamber powders generally have larger particle sizes compared to the collector fraction (as discussed in
Section 3.3.5); it is the combined effect of particle composition and structure that appears to play a dominant role in the improved encapsulation efficiency and oxidative stability observed in the chamber samples.
The initial emulsion, analysed by IA, showed a d50 of 12.7 ± 2.7 µm (
Figure 9b), which did not differ significantly from the d50 values of the reconstituted emulsions, whether from the chamber or the collector. However, relying solely on d50 is somewhat limiting, as it only reflects the median and does not capture the full distribution of droplet sizes. For this reason, we also presented the full PSD curves (
Figure 7). These curves reveal that the initial emulsion exhibited a much narrower, more monomodal distribution, while the reconstituted emulsions developed broader, bimodal-like distributions, with additional populations shifted both to the left (smaller droplets) and right (larger droplets). This suggests that, during drying and reconstitution, droplet rearrangements and partial destabilisation occur, even if the median size remained unchanged.
Moreover,
Figure 6 reveals that the reconstituted emulsions, particularly those from the collector, exhibited some large droplets (marked as “B”), with sizes exceeding 20 µm. Interestingly, the initial emulsion, when analysed by LD, showed an intermediate d50 value (43.9 ± 5.5 µm), positioned between the reconstituted emulsions from the chamber and the collector (
Figure 9a).
When comparing the different experiments, no clear or consistent trend was observed regarding the effect of Tin or FR on the droplet size (d50) obtained by IA. Laser diffraction provides complementary information to image analysis, as optical microscopy is limited in detecting very small particles (<0.2 µm) [
50], whereas laser diffraction can cover a much broader size range, from approximately 0.01 to 4000 µm [
51]. In the collector samples, where microscopy and sphericity results confirmed that most dispersed particles were indeed droplets, the LD results are reliable (
Figure 9a). In this case, the highest feed rates (e.g., 145 °C and 8.4 g/min and 155 °C and 8.0 g/min showed d50 of 30.7 ± 8.3 µm and 28.3 ± 7.6 µm, respectively) tended to produce larger d50 values. This trend was also evident in the PSD shown in
Figure 5, where the peaks corresponding to larger droplets become more pronounced in these samples, while the peaks associated with smaller droplets diminish. This can be explained by two combined effects: a higher FR reduced atomisation efficiency, producing larger initial spray droplets, and simultaneously lowered the outlet temperature, slowing the drying kinetics, and the longer drying time allowed more droplet collision and coalescence before the particle structure was locked. These observations are in agreement with the results reported by Höhne and Gaukel [
48], who spray dried a model oil-in-water emulsion stabilised with modified starch under controlled conditions, systematically varying inlet (180–220 °C) and outlet temperatures (65–85 °C). They observed that higher outlet and inlet temperatures, which increased the drying rate, led to smaller oil droplets in the reconstituted powders. The explanation was that faster drying caused earlier skin formation on the droplet surface, reducing the time for collisions and coalescence during the drying step.
3.3.5. Size, Morphology, and Colour of the Powders
The PSD of the dried powders is shown in
Figure 10, while the median particle size (d50) derived from these distributions is presented in
Figure 11. Representative SEM micrographs of the microcapsules are provided in
Figure 12, allowing a detailed visualisation of their morphology. In this study, a number-based particle size distribution was used instead of a volume-weighted one. Maltodextrin-based powders tend to form mild agglomerates, which strongly bias volume-weighted metrics, as even a small fraction of large particles disproportionately increases the mean diameter. A number distribution is less affected by these agglomerates and better represents the dominant population of individual microcapsules, providing a more realistic comparison between drying conditions.
Across all figures, a consistent trend is evident: powders collected in the chamber displayed substantially larger particle sizes compared to those recovered from the collector.
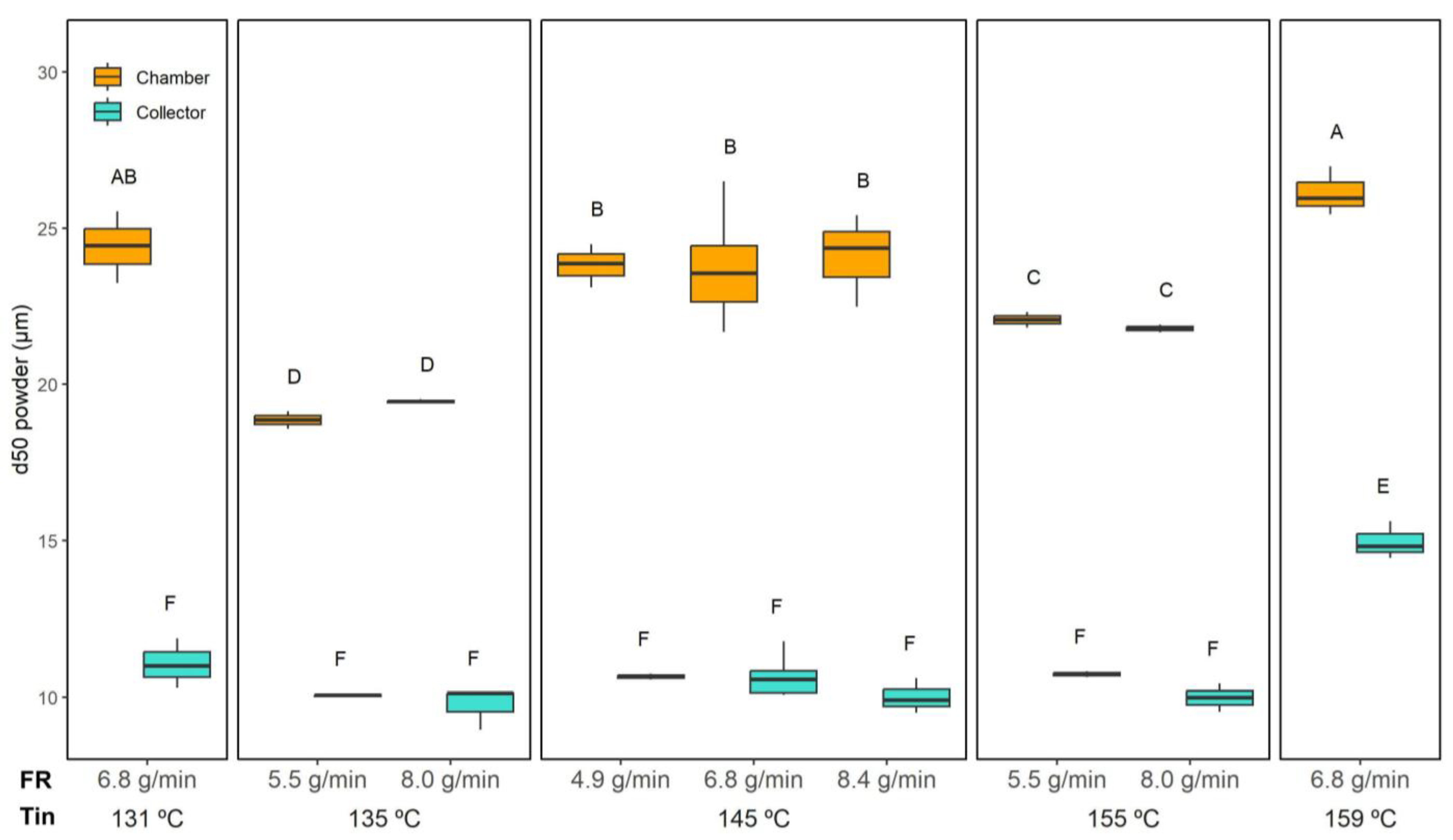
According to the three-way ANOVA (
Table 2), both the collection position and the Tin significantly affected d50 (
p < 0.05), as did their interaction, whereas FR alone showed no significant effect. In the collector, the PSD was highly uniform, with d50 values remaining remarkably consistent for most conditions (10.4 ± 0.5 µm). The only exception was observed at the highest drying temperature tested (159 °C and 6.8 g/min), where d50 increased to 15.0 ± 0.6 µm.
Both Moser et al. [
16] and Gonçalves et al. [
44] reported trends that align with this effect of Tin observed in the collector. In Moser et al.’s [
16] study, spray-drying buriti oil bilayer emulsions at inlet temperatures between 154 and 196 °C and feed rates from 1.2 to 6.8 mL/min resulted in particle sizes ranging from 17.9 to 32.8 µm (volume-weighted mean diameter). They observed that increasing Tin and FR consistently produced larger particles due to faster crust formation and particle expansion. Similarly, Gonçalves et al. [
44], working with retinoic-acid-loaded emulsions encapsulated with different biopolymers, showed that powders dried at higher temperatures (130–150 °C) presented larger d50 values (volume-weighted mean diameter up to ~13 µm with modified chitosan), while milder conditions (115 °C) produced smaller, more uniform particles (~1.7 µm).
In contrast, powders collected from the chamber exhibited greater variability. The largest d50 values were found at the extreme drying conditions, namely, the lowest (131 °C) and highest (159 °C) Tin, both yielding around 25.2 ± 1.2 µm. Intermediate Tin values (135 and 155 °C) resulted in comparatively smaller particles collected in the chamber (20.5 ± 1.6 µm). This pattern suggests that at very low Tin, incomplete atomisation and early deposition promoted larger agglomerates, while at very high Tin, rapid crust formation around partially dried droplets may have led to hollow, bulkier particles. Conversely, mid-range temperatures facilitated more controlled droplet shrinkage, yielding finer particles.
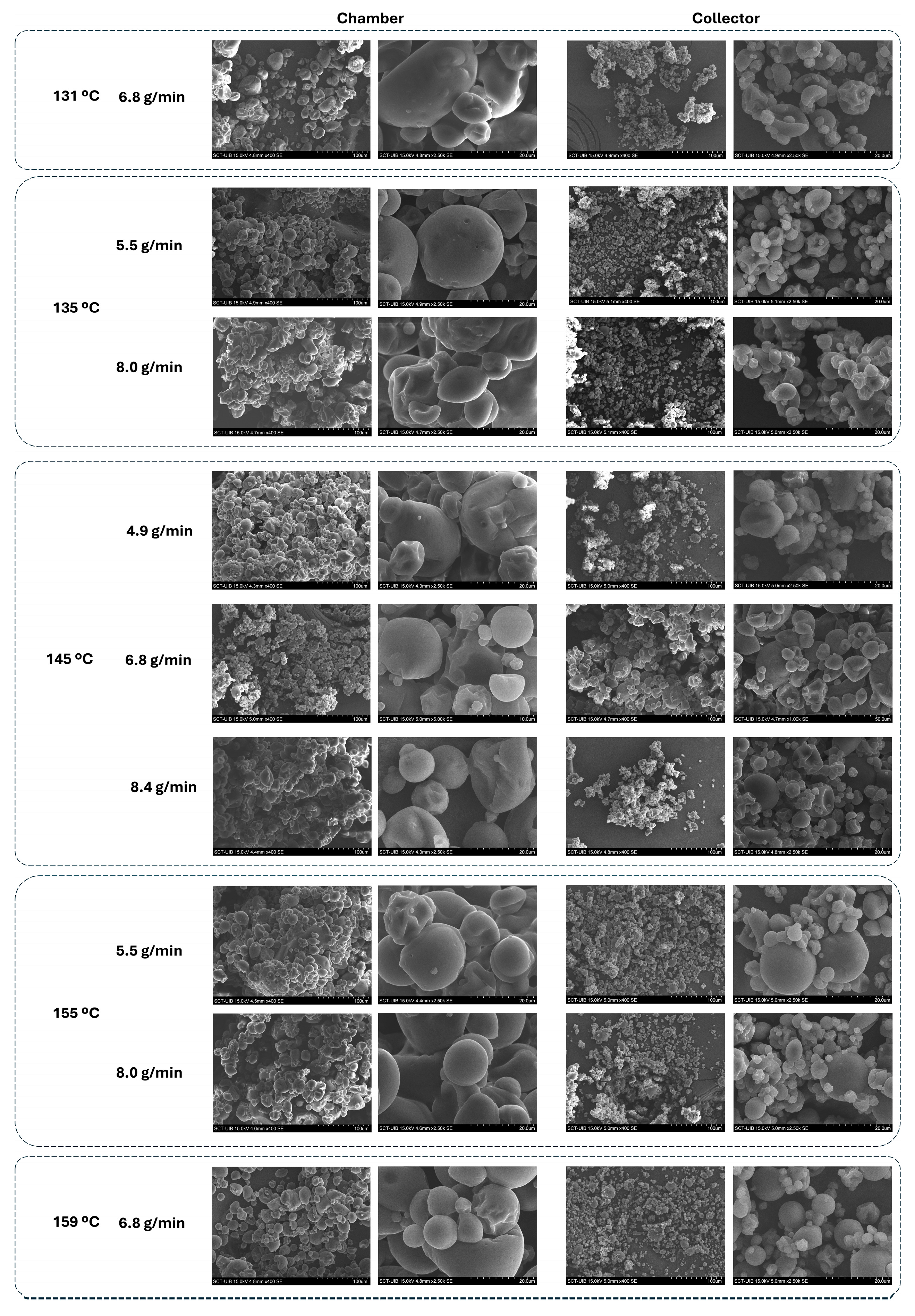
The SEM micrographs (
Figure 12) revealed that the powders were generally spherical with smooth surfaces, and no visible fibres from the ORF were detected externally. This suggests that the ORF components were successfully embedded within the microcapsule matrix, likely contributing to crust formation rather than remaining as free particles. The larger sizes in the chamber powders could be linked to their higher encapsulation efficiency, as the additional ORF material retained in the chamber may have thickened the wall structure and promoted particle growth.
Sphericity measurements (
Figure 13) confirmed that all powders maintained a relatively high degree of roundness, with only subtle differences across conditions. The three-way ANOVA indicated significant effects of Tin, collection position, and the FR × Position interaction; however, these effects were minor. Overall, sphericity ranged from 0.88 ± 0.01 (collector, 145 °C and 8.4 g/min) to 0.91 ± 0.01 (collector, 135 °C/8.0 g/min), indicating well-formed spherical microcapsules across all conditions.
The colour of the powders was evaluated both visually and instrumentally. Since no major visual differences were observed across drying conditions,
Figure 14 presents a representative example of the powders from the collector and chamber at the central drying condition (145 °C and 6.8 g/min). The chamber powder appears darker and more intensely yellow, with a slightly coarser texture and more irregularly shaped agglomerates. In contrast, the collector powder looks lighter, almost pale yellow to off-white, and seems finer with a more uniform, less compact structure. To quantify these observations, the CIELab* coordinates were determined, and from them, the Browning index (BI) was calculated (Equations (2) and (3)) (
Figure 15).
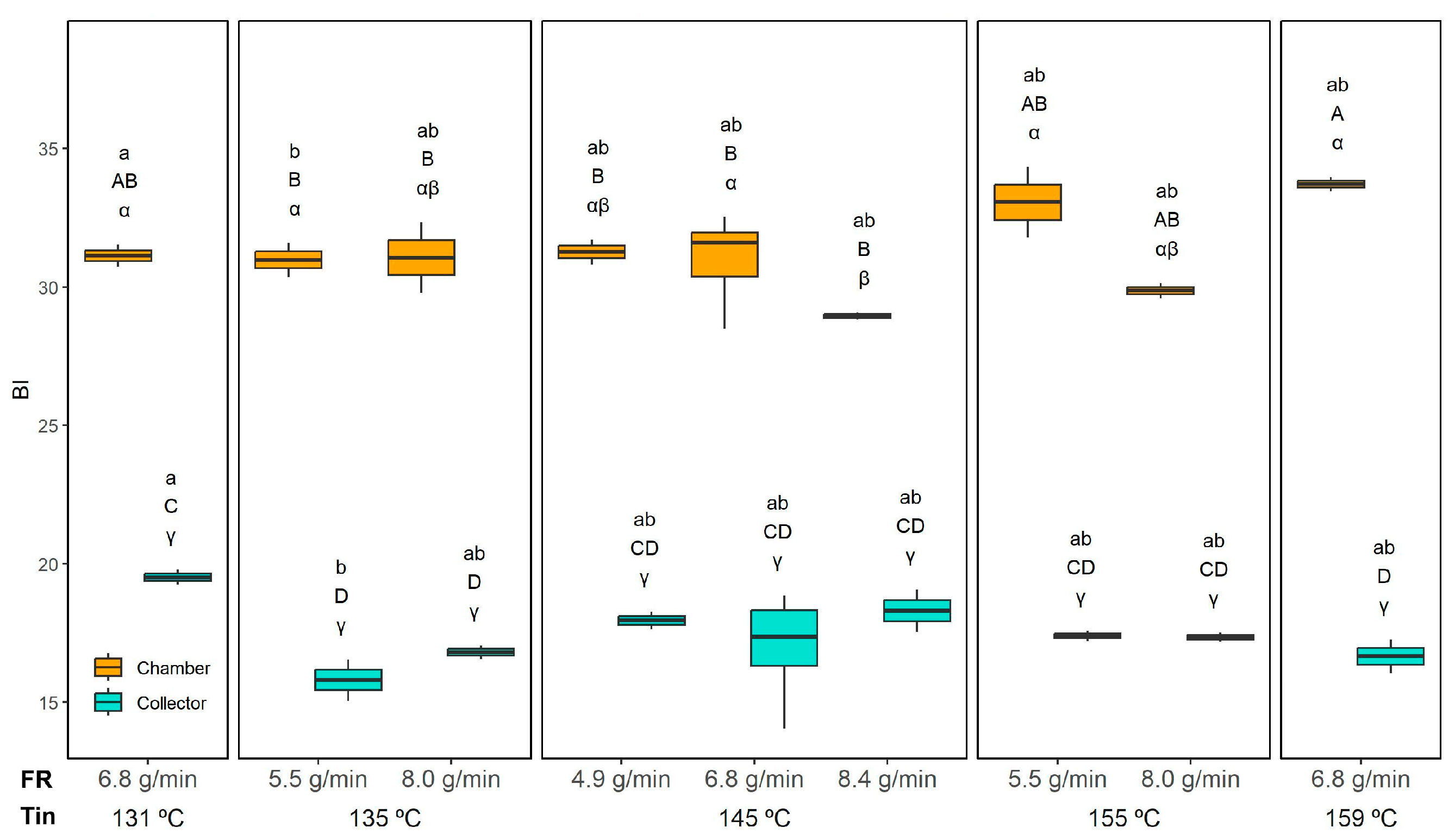
The BI values (
Figure 15) were significantly influenced by the interaction of all studied variables (Tin, FR, and position). As expected, the collection position had the strongest effect, with chamber powders consistently exhibiting higher BI values than those collected in the collector. In the chamber, the BI ranged from 29.0 ± 0.1 (145 °C and 8.4 g/min) to 33.7 ± 0.3 (159 °C and 6.8 g/min), whereas in the collector, it ranged from 15.8 ± 0.7 (135 °C and 5.5 g/min) to 19.5 ± 0.3 (131 °C and 6.8 g/min).
This difference can be explained by three main factors. First, the powders collected in the chamber contain a higher proportion of orange residue flour (ORF), which inherently imparts a stronger colour due to its natural pigments. Second, ORF is rich in sugars that can enhance browning reactions, further increasing the BI. Third, the longer residence time of particles in the chamber exposes them to prolonged heat, favouring Maillard-type reactions.
Within the chamber powders, the FR showed only a minor effect: at the highest FR (8.4 g/min), the BI slightly decreased, likely because faster drying reduced the residence time and thermal load and also because a higher FR can reduce the Tout. This is in agreement with Moser et al.’s [
16] observations; they reported that buriti oil bilayer emulsions spray dried with varying Tin (154–196 °C) and FR (1.2–6.8 mL/min) showed pigment degradation and colour changes with higher Tin, whereas a higher FR mitigated these effects by reducing exposure time. A similar trend was reported for spray-dried spinach juice, with the BI increasing significantly at higher inlet temperatures (160–200 °C) but decreasing when the outlet temperature was reduced from 100 to 80 °C by increasing the feed rate [
52,
53,
54]. In any case, this effect was negligible compared to the strong influence of collection position in our study. In the collector, no clear trend was observed for either the FR or Tin, with BI values remaining relatively uniform.
3.4. Modelling
Response surface methodology (RSM) was applied to evaluate the effects of spray-drying process variables (Tin and FR) on the characteristics of the powders and the reconstituted emulsions. Experimental data were fitted to a second-order polynomial model, and the regression coefficients for each response are shown in
Table 3. Only responses for which the proposed model was statistically significant (
p < 0.05) are reported. Model adequacy was evaluated through R
2 and adjusted R
2 values, and only models with acceptable explanatory power (R
2 > 0.5) were considered.
For the chamber fraction, the model was statistically significant and exhibited acceptable explanatory power for yield, aw, specific absorbance of conjugated dienes (SA), and browning index (BI). In contrast, for the collector fraction, these conditions were met for aw, SA, and the d50 of the reconstituted emulsion (LD).
For the chamber fraction, the yield was well described by the model, with an R2 close to 0.9, indicating very good predictive accuracy. Tin was the most influential variable, appearing in both linear (negative) and quadratic terms, while FR had only an inverse linear effect. The experimental results indicate that increasing FR led to lower chamber yields, whereas the highest yields were obtained at the highest Tin value, which is described in the equation with the positive quadratic term.
The aw was mainly affected by the FR, showing a negative linear and a positive quadratic effect, meaning moderate increases in FR reduced aw, but extreme values raised it again. Tin had only a minor negative linear influence, slightly lowering aw, while its positive interaction with the FR suggests combined conditions can increase aw. This model also showed a relatively high explanatory power (R2 > 0.8). Similarly, the SA was well described by the model (R2 > 0.8). In this case, Tin was the most relevant factor, appearing as a linear, quadratic, and interactive term. This aligns with the experimental results, where powders collected in the chamber showed higher oxidation levels at higher temperatures, likely due to the longer residence time under heat. Finally, the model also adequately described the BI of the chamber powders, with R2 values above 0.8. The BI was mainly affected by Tin, as higher temperatures promoted more intense browning, most likely due to Maillard reactions. An inverse effect of the FR was also detected, which is consistent with the previously discussed results: a higher FR shortened the residence time of the powders in the chamber, reducing browning intensity.
Regarding the collector, the aw of powder showed an inverse linear effect of both Tin and FR, as well as a significant positive Tin × FR interaction. This model had an R2 above 0.7, indicating a relatively good predictive ability. The SA model also showed an R2 above 0.7 and highlighted the strong influence of both Tin and the FR, although the FR appeared to have a more pronounced effect. The positive coefficients for the linear terms suggest that increasing either Tin or the FR leads to higher SA values, indicating greater oil oxidation. However, the negative quadratic coefficient implies that this effect is not strictly linear; beyond a certain point, further increases in Tin or the FR could attenuate or even reverse the trend. The droplet size (d50) of the reconstituted emulsion from the collector powder was well described by the model (R2 > 0.8), indicating good predictive ability. Among the variables, the FR had the strongest influence, appearing as a significant linear and quadratic term, as well as in the Tin × FR interaction. This suggests that both very low and very high feed rates tend to produce larger droplets, while an intermediate FR minimises droplet size. Tin also played an important role, showing a negative linear effect, indicating that increasing inlet temperature reduces droplet size. Still, this trend was moderated by a small positive quadratic effect, suggesting a slight curvature at extreme temperatures. Together, these results indicate a complex, non-linear response where the combined effects of Tin and the FR determine the final droplet size of the reconstituted emulsion.
The total yield was also successfully modelled, showing good agreement with the experimental data (R2 > 0.8). The response exhibited a direct linear effect of Tin. Conversely, the FR showed a linear inverse impact, indicating that higher feed rates slightly reduced the total yield.
It is worth noting that not all parameters could be modelled with the same accuracy for the collector and chamber fractions because their behaviours were inherently different. For example, the yield remained nearly constant for the collector, while the drying conditions strongly influenced it in the chamber. Likewise, the BI was more affected in the chamber fraction because the powders were exposed to high temperatures for longer periods, enhancing browning reactions.
These clear differences highlight the importance of characterising the powders from the collector and the chamber separately. Each fraction undergoes distinct drying dynamics and thermal exposure, leading to different functional and physicochemical properties.
Figure S1 shows the plots of standardised residuals versus the calculated values for each response variable. In all cases, the residuals were randomly scattered around zero, indicating no evident systematic patterns or model misspecification. Most points were within the ±2 range, with only a few approaching ±3, suggesting the absence of strong outliers or influential observations. The dispersion of residuals appeared relatively constant across the predicted range.
3.5. Multifactorial Optimisation
Optimisation was carried out only including the parameters significantly affected by the drying variables and well described by the regression model (R2 > 0.5). Three separate optimisations were carried out.
First, the drying conditions were identified to optimise the chamber powder characteristics, with the objective of maximising the yield, which was strongly affected by the drying conditions, while simultaneously minimising aw, SA, and the browning index (BI).
Similarly, the Tin and FR conditions were identified to optimise the collector powder. Since its yield remained very stable regardless of the conditions, the objective function focused on minimising aw, the SA, and the droplet size of the reconstituted emulsion. This approach aimed to obtain a dry powder with low oxidation levels and good emulsion stability.
Finally, a global optimisation was proposed, considering the most relevant characteristics of both powders. In this case, the total yield was maximised while minimising the aw of the collector powder. This criterion was chosen because the chamber powder was inherently drier due to the higher temperatures in the drying chamber; indeed, from 145 °C onward, the chamber powder consistently showed lower aw values than the collector, which was more sensitive to this parameter. The SA and BI of the chamber powders were minimised to reduce oxidation and browning. The SA of the collector powder was not included to avoid duplicating this parameter. Moreover, the highest levels of oil oxidation were observed in the chamber powder, particularly at the highest temperatures (155 °C and 159 °C), indicating that this fraction was more sensitive to oxidative damage.
The desirability functions were defined for each parameter, assigning the same relative importance to all in the overall desirability function.
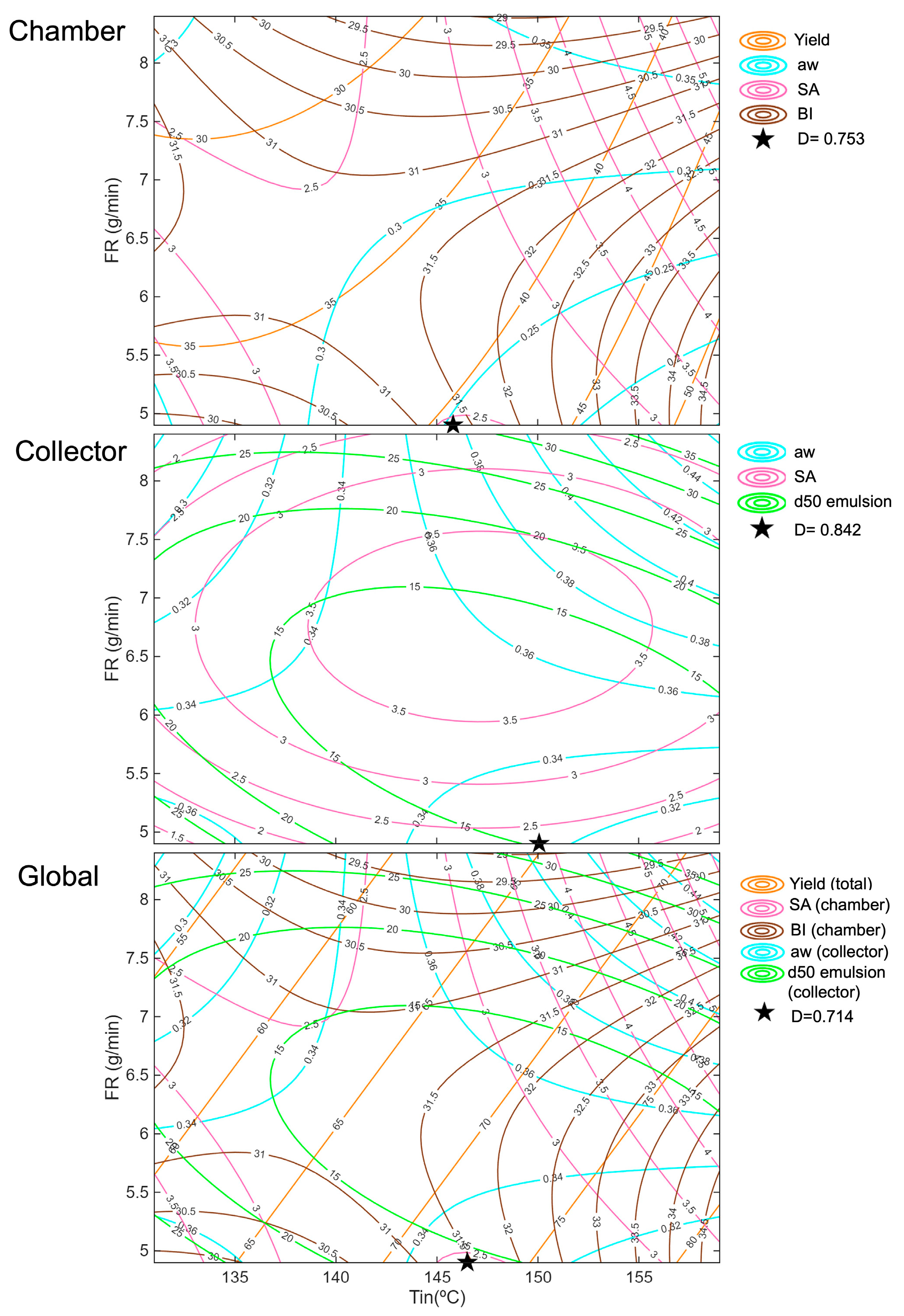
The results of the optimisation, including the overall desirability values for each case, are presented in
Table 4. Moreover,
Figure 16 illustrates the position of the overall desirability in the studied region for each optimisation, along with all the parameters considered in the optimisation process. The overall desirability values obtained for the optimised conditions were 0.753 for the chamber, 0.842 for the collector, and 0.714 for the global optimisation. These values indicate that the collector conditions achieved the best overall compromise among the targeted responses, while the chamber and global optimisations were still satisfactory.
The optimisation for the chamber represents a balanced compromise between yield, BI, SA, and aw because it selects conditions where none of the responses reach their extremes (intermediate Tin of 146 °C and the lowest FR of 4.9 g/min). Very high temperatures increased yield but also intensified oxidation (SA), while low temperatures reduced oxidation but resulted in poor yield. Similarly, the effect of FR was non-linear: in some cases, increasing FR reduced browning due to shorter residence times, but in others, it increased aw and even worsened oxidation. The chosen optimum at moderate Tin and low FR achieves a middle ground; yield is enhanced compared to a very high FR, while oxidation and browning are kept lower than at the highest temperatures, and aw remains stable. This reflects the trade-off captured by the model, prioritising overall product quality without excessively sacrificing any single parameter.
For the collector powder, optimisation focused on minimising aw, SA, and the droplet size of the reconstituted emulsion. The optimum corresponded to a low FR (4.9 g/min) combined with an intermediate Tin (150 °C, similar conditions to the chamber with slightly higher Tin), which represented the best compromise among the three parameters. According to the model and experimental results, an excessively high FR increased aw and, in some cases, promoted oil oxidation (SA) due to inefficient drying and higher residual moisture. Similarly, a high FR produced larger droplets, while an intermediate-to-low FR minimised d50, improving emulsion stability. Regarding temperature, higher Tin generally reduced droplet size due to faster crust formation but also increased oxidation beyond a certain point. Conversely, too low Tin risked incomplete drying and higher aw. Thus, the optimisation favoured a moderate Tin, which balanced proper drying without excessive thermal damage, together with a lower FR.
The global optimisation closely matched the individual optimisations, aligning perfectly with the chamber fraction. This is because the chamber responses, yield, oxidation (SA), and browning (BI), were more sensitive to drying conditions, especially temperature, making them dominant in the global solution. While the collector focused mainly on minimising aw, SA, and droplet size, its influence was negligible. Thus, the global optimum reflects a compromise but primarily favours the chamber’s critical parameters while keeping acceptable conditions for the collector.
Similar trends have been reported in the literature, supporting the low feed rates identified in our optimisation. For example, Himmetagaoglu and Erbay [
55], who spray-dried high-fat dairy emulsions to produce cream powders, observed that higher feed rates produced powders with lower quality, higher surface fat, and wettability. Likewise, Yadav et al. [
56], working on probiotic microencapsulation via spray drying, showed that increasing the feed rate reduced yield and worsened aw due to insufficient drying time inside the chamber. These findings align with our results, where an excessively high FR increased aw, occasionally promoted oxidation, and produced larger droplets. At the same time, a low FR ensured better drying, improved emulsion stability, and minimised quality losses.
To validate the model predictions, experiments were carried out under the optimal spray-drying conditions determined for each powder fraction. As shown in
Table 4, the experimental values closely matched the predicted responses across most of the evaluated parameters. For the chamber fraction, yield, SA, and BI exhibited good agreement with the model, confirming the reliability of the predicted optimum conditions. The experimental value for aw was slightly larger but still acceptably similar to the predicted one. Similarly, for the collector powder, the predicted and experimental values of aw, SA, and d50 of the reconstituted emulsion fell within acceptable deviation margins. In the case of the global optimisation, the experimentally measured responses of d50 were slightly higher than predicted but still within reasonable agreement. These findings support the validity of the models and confirm their applicability for guiding process optimisation under different recovery positions, reinforcing the robustness of the methodological approach used.