Exploring the Integration of Anthocyanins with Functional Materials in Smart Food Packaging: From Stabilization to Application
Abstract
1. Introduction
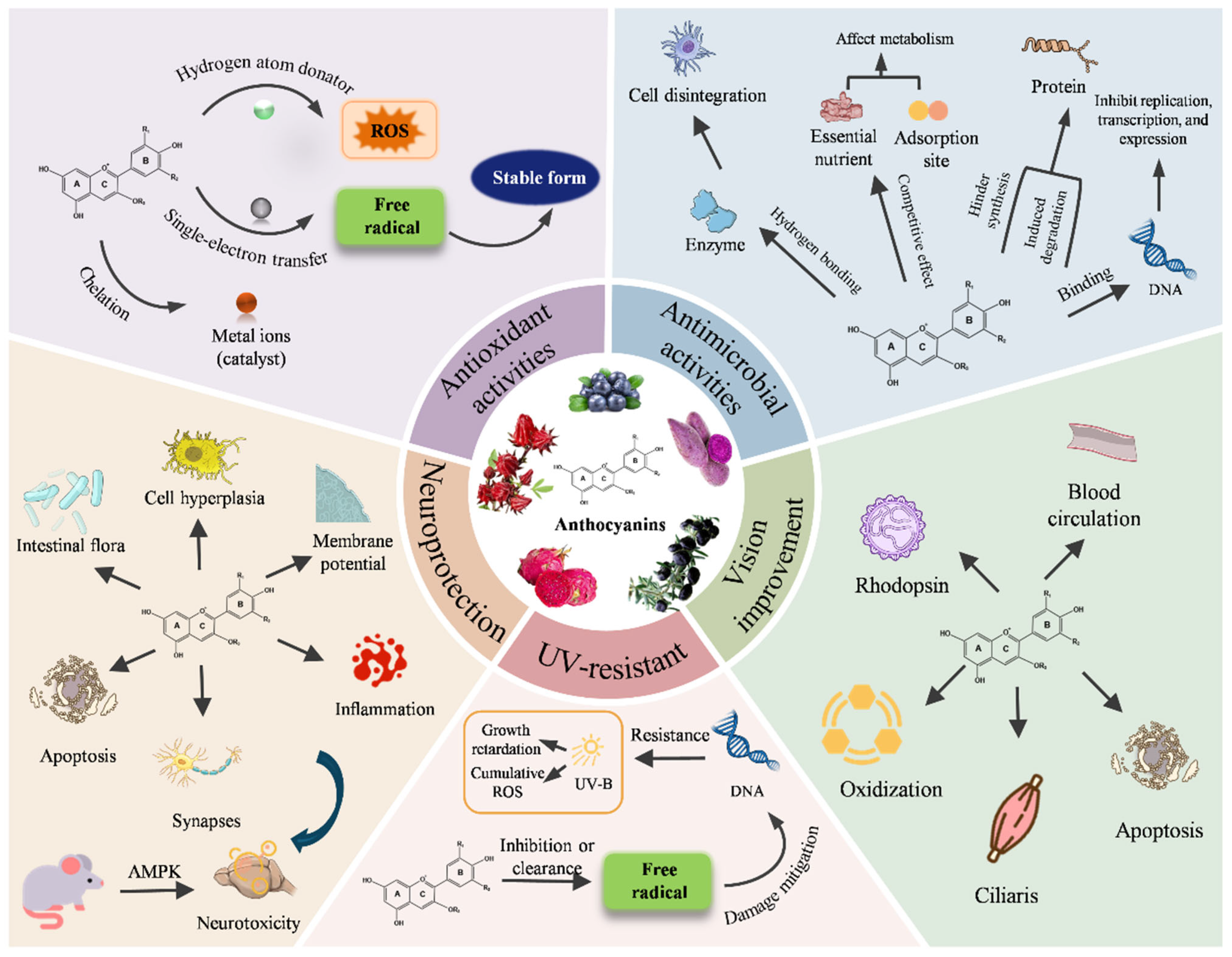
2. The Properties of Anthocyanins in Food Packaging
2.1. Antioxidant Activities
2.2. Antimicrobial Activities
2.3. UV-Resistant
2.4. Neuroprotection
2.5. Vision Improvement
3. Functional Materials in Anthocyanin-Based Food Packaging
3.1. Natural Polymer Materials
3.1.1. Polysaccharides
3.1.2. Proteins
3.1.3. Liposomes
3.1.4. Natural Polymer Composites
3.2. Engineering Nanomaterials
3.2.1. Nanoemulsions
3.2.2. Nanoparticles
Metallic Nanoparticles
Porous Organic Frameworks (POFs)
Quantum Dots
Biopolymeric Nanoparticles
3.2.3. Nanoclays
3.2.4. Polymer Nanomicelles (PNMs)
4. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, J.; Zhang, J.; Huang, X.; Shi, J.; Muhammad, A.; Zhai, X.; Xiao, J.; Li, Z.; Povey, M.; Zou, X. Study on cinnamon essential oil release performance based on pH-triggered dynamic mechanism of active packaging for meat preservation. Food Chem. 2023, 400, 134030. [Google Scholar] [CrossRef]
- Surendhiran, D.; Li, C.; Cui, H.; Lin, L. Fabrication of high stability active nanofibers encapsulated with pomegranate peel extract using chitosan/PEO for meat preservation. Food Packag. Shelf Life 2020, 23, 100439. [Google Scholar] [CrossRef]
- Huang, X.; Du, L.; Li, Z.; Xue, J.; Shi, J.; Tahir, H.E.; Zhai, X.; Zhang, J.; Zhang, N.; Sun, W. A visual bi-layer indicator based on mulberry anthocyanins with high stability for monitoring Chinese mitten crab freshness. Food Chem. 2023, 411, 135497. [Google Scholar] [CrossRef]
- Jiao, X.; Huang, X.; Yu, S.; Wang, L.; Wang, Y.; Zhang, X.; Ren, Y. A novel composite colorimetric sensor array for quality characterization of shrimp paste based on indicator displacement assay and etching of silver nanoprisms. J. Food Process Eng. 2023, 46, e14195. [Google Scholar] [CrossRef]
- Li, M.; Hong, X.; Qiu, X.; Yang, C.; Mao, Y.; Li, Y.; Liu, Z.; Du, D. Ultrasensitive monitoring strategy of PCR-like levels for zearalenone contamination based DNA barcode. J. Sci. Food Agric. 2021, 101, 4490–4497. [Google Scholar] [CrossRef]
- Huang, S.; Chen, Y.; Liu, D.; Xue, S.; Zhang, T.; Qiu, F.; Yang, D. A reconstructed cellulose packaging with heat dissipating and water-resistant properties for functional applications. Int. J. Biol. Macromol. 2025, 320, 1. [Google Scholar] [CrossRef]
- Lin, H.; Kang, W.; Han, E.; Chen, Q. Quantitative analysis of colony number in mouldy wheat based on near infrared spectroscopy combined with colorimetric sensor. Food Chem. 2021, 354, 129545. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, B.; Wu, S.; Wang, S.; He, M.; Bai, H.; Wang, H.; Shen, Y. A coupled theoretical and experimental study: Construction, properties, and freshness preservation applications of Coumarinic acid/cyclodextrin metal-organic frameworks. Innov. Food Sci. Emerg. Technol. 2025, 104, 104151. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Shi, J.; Li, Z.; Wang, X.; Hu, X.; Gong, Y.; Zou, X. A Novel Gas Sensor for Detecting Pork Freshness Based on PANI/AgNWs/Silk. Foods 2022, 11, 2372. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Jayan, H.; Cai, J.; El-Seedi, H.R.R.; Guo, Z.; Zou, X. Spoilage Monitoring and Early Warning for Apples in Storage Using Gas Sensors and Chemometrics. Foods 2023, 12, 2968. [Google Scholar] [CrossRef]
- Huang, X.; Sun, W.; Li, Z.; Shi, J.; Zhang, N.; Zhang, Y.; Zhai, X.; Hu, X.; Zou, X. Hydrogen sulfide gas sensing toward on-site monitoring of chilled meat spoilage based on ratio-type fluorescent probe. Food Chem. 2022, 396, 133654. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Zhang, Z.H.; Qiao, J.Q.; Qu, W.J.; Wang, M.S.; Gao, X.L.; Zhang, C.S.; Brennan, C.S.; Qi, X.H. Improvement of betalains stability extracted from red dragon fruit peel by ultrasound-assisted microencapsulation with maltodextrin. Ultrason. Sonochem. 2022, 82, 105897. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Hu, H.; Xiong, Z.; Wang, L.; Yuan, L.; Gao, R. Application and Mechanism of High-Sensitivity Indicator Film for Monitoring Fish Freshness. Food Sci. 2023, 44, 150–158. [Google Scholar]
- Herrera-Balandrano, D.D.; Chai, Z.; Beta, T.; Feng, J.; Huang, W. Blueberry anthocyanins: An updated review on approaches to enhancing their bioavailability. Trends Food Sci. Technol. 2021, 118, 808–821. [Google Scholar] [CrossRef]
- Hashim, S.B.H.; Tahir, H.E.; Li, L.; Zhang, J.; Zhai, X.; Mahdi, A.A.; Awad, F.N.; Hassan, M.M.; Zou, X.; Shi, J. Intelligent colorimetric pH sensoring packaging films based on sugarcane wax/agar integrated with butterfly pea flower extract for optical tracking of shrimp freshness. Food Chem. 2022, 373, 131514. [Google Scholar] [CrossRef]
- Gao, R.C.; Hu, H.L.; Shi, T.; Bao, Y.L.; Sun, Q.C.; Wang, L.; Ren, Y.H.; Jin, W.G.; Yuan, L. Incorporation of gelatin and Fe2+ increases the pH-sensitivity of zein-anthocyanin complex films used for milk spoilage detection. Curr. Res. Food Sci. 2022, 5, 677–686. [Google Scholar] [CrossRef]
- Song, S.; Yu, Y.; Song, S.; Zhang, X.; Zhang, W. Effect of co-pigments on anthocyanins of wild cranberry and investigation of interaction mechanisms. Food Chem. 2025, 466, 142212. [Google Scholar] [CrossRef]
- Huang, J.; Hu, Z.; Chin, Y.; Pei, Z.; Yao, Q.; Chen, J.; Li, D.; Hu, Y. Improved thermal stability of roselle anthocyanin by co-pigmented with oxalic acid: Preparation, characterization and enhancement mechanism. Food Chem. 2023, 410, 135407. [Google Scholar] [CrossRef]
- Wang, Y.; Julian McClements, D.; Chen, L.; Peng, X.; Xu, Z.; Meng, M.; Ji, H.; Zhi, C.; Ye, L.; Zhao, J.; et al. Progress on molecular modification and functional applications of anthocyanins. Crit. Rev. Food Sci. Nutr. 2024, 64, 11409–11427. [Google Scholar] [CrossRef]
- Xue, H.; Zhao, J.; Wang, Y.; Shi, Z.; Xie, K.; Liao, X.; Tan, J. Factors affecting the stability of anthocyanins and strategies for improving their stability: A review. Food Chem.-X 2024, 24, 101883. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Fan, L.J.; Chen, X.Y.; Guo, Z.T.; Zhang, B.X. Microencapsulation of Lonicera caerulea pomace extract by spray drying: Characterization and stability studies. LWT-Food Sci. Technol. 2025, 223, 117778. [Google Scholar] [CrossRef]
- Cai, D.; Li, X.; Chen, J.; Jiang, X.; Ma, X.; Sun, J.; Tian, L.; Vidyarthi, S.K.; Xu, J.; Pan, Z.; et al. A comprehensive review on innovative and advanced stabilization approaches of anthocyanin by modifying structure and controlling environmental factors. Food Chem. 2022, 366, 130611. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Xu, J.; Zhang, L.; Liu, L.; Zhang, L. Nanoencapsulation of anthocyanin by an amphiphilic peptide for stability enhancement. Food Hydrocoll. 2021, 118, 106741. [Google Scholar] [CrossRef]
- Liu, D.; Zhong, Y.; Li, X.; Pu, Y.; Chen, S.; Zhang, C. Indicator films based on anthocyanins loaded on Metal-Organic Framework carriers and BP neural network for monitoring meat freshness. Food Hydrocoll. 2023, 145, 109106. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Gao, X.K.; Gao, X.N.; Jiang, Z.M.; Alhomrani, M.; Alamri, A.S.; Alsanie, W.F.; Cui, H.Y. Development of polysaccharide based intelligent packaging system for visually monitoring of food freshness. Int. J. Biol. Macromol. 2024, 277, 134588. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Y.; Zhang, J.; Shi, J.; Liu, L.; Huang, X.; Song, W.; Li, Z.; Zou, X.; Povey, M. High-Stability Bi-Layer Films Incorporated with Liposomes @Anthocyanin/Carrageenan/Agar for Shrimp Freshness Monitoring. Foods 2023, 12, 732. [Google Scholar] [CrossRef]
- Yue, X.J.; Xiong, Q.; Dai, Y.T.; Yang, D.Y.; Xue, S.L.; Zhang, T.; Qiu, F.X. Double-oil-capture design of upcycling waste PP into pH sensitivity and sustainable aerogel for oil-water separation. J. Environ. Chem. Eng. 2025, 13, 115204. [Google Scholar] [CrossRef]
- Wang, H.X.; Li, B.; Ding, F.Y.; Ma, T.L. Improvement of properties of smart ink via chitin nanofiber and application as freshness indicator. Prog. Org. Coat. 2020, 149, 115921. [Google Scholar] [CrossRef]
- Mazzoni, L.; Giampieri, F.; Alvarez Suarez, J.M.; Gasparrini, M.; Mezzetti, B.; Forbes Hernandez, T.Y.; Battino, M.A. Isolation of strawberry anthocyanin-rich fractions and their mechanisms of action against murine breast cancer cell lines. Food Funct. 2019, 10, 7103–7120. [Google Scholar] [CrossRef]
- Gao, Q.; Li, Y.; Li, Y.; Liang, Y.; Zhang, Z. Profile of anthocyanins in purple vegetables commonly consumed in China and their relationship with antioxidant abilities. J. Food Meas. Charact. 2022, 16, 1659–1673. [Google Scholar] [CrossRef]
- Tena, N.; Martín, J.; Asuero, A.G. State of the Art of Anthocyanins: Antioxidant Activity, Sources, Bioavailability, and Therapeutic Effect in Human Health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef]
- Chen, Z.L.; Ma, J.; Li, P.; Wen, B.; Wang, Y.; Ma, Y.H.; Huang, W.Y. Preparation of hypoglycemic anthocyanins from mulberry (Fructus mori) fruits by ultrahigh pressure extraction. Innov. Food Sci. Emerg. Technol. 2023, 84, 130255. [Google Scholar] [CrossRef]
- Wang, L.; Peng, F.J.; Yang, S.F.; Yang, Y.Y.; Jiang, H.Z.; Huang, W.Y.; Bian, Y.Y.; Li, B. Antioxidant capacity and in vitro lipid-lowering effect of purple corn (Zea mays L.) processed by different methods. Ind. Crops Prod. 2024, 222, 120084. [Google Scholar] [CrossRef]
- Aalim, H.; Hashim, S.B.H.; Zhou, C.G.; Zou, X.B.; Luo, Z.S. Matrix characteristics modulate black rice phenolic compounds bioaccessibility and antioxidant activity during simulated gastrointestinal digestion. Food Biosci. 2024, 58, 103628. [Google Scholar] [CrossRef]
- Petruskevicius, A.; Viskelis, J.; Urbonaviciene, D.; Viskelis, P. Anthocyanin Accumulation in Berry Fruits and Their Antimicrobial and Antiviral Properties: An Overview. Horticulturae 2023, 9, 88. [Google Scholar] [CrossRef]
- Pattananandecha, T.; Apichai, S.; Sirilun, S.; Julsrigival, J.; Sawangrat, K.; Ogata, F.; Kawasaki, N.; Sirithunyalug, B.; Saenjum, C. Anthocyanin Profile, Antioxidant, Anti-Inflammatory, and Antimicrobial against Foodborne Pathogens Activities of Purple Rice Cultivars in Northern Thailand. Molecules 2021, 26, 5234. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-h.; Zhou, T.-t.; Wei, C.-h.; Lan, W.-q.; Zhao, Y.; Pan, Y.-j.; Wu, V.C.H. Antibacterial effect and mechanism of anthocyanin rich Chinese wild blueberry extract on various foodborne pathogens. Food Control 2018, 94, 155–161. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, C.; Zhong, W.; Shu, Y.; Zhang, Y.; Yang, D. Antibacterial effect and mechanism of anthocyanin from Lycium ruthenicum Murr. Front. Microbiol. 2022, 13, 974602. [Google Scholar] [CrossRef] [PubMed]
- Regolo, L.; Giampieri, F.; Battino, M.; Diaz, Y.A.; Mezzetti, B.; Elexpuru-Zabaleta, M.; Mazas, C.; Tutusaus, K.; Mazzoni, L. From by-products to new application opportunities: The enhancement of the leaves deriving from the fruit plants for new potential healthy products. Front. Nutr. 2024, 11, 1083759. [Google Scholar] [CrossRef]
- Xu, L.; Tang, Z.; Herrera-Balandrano, D.D.; Qiu, Z.; Li, B.; Yang, Y.; Huang, W. In vitro fermentation characteristics of blueberry anthocyanins and their impacts on gut microbiota from obese human. Food Res. Int. 2024, 176, 113761. [Google Scholar] [CrossRef]
- Deng, H.; Zhu, J.; Tong, Y.; Kong, Y.; Tan, C.; Wang, M.; Wan, M.; Meng, X. Antibacterial characteristics and mechanisms of action of Aronia melanocarpa anthocyanins against Escherichia coli. LWT-Food Sci. Technol. 2021, 150, 112018. [Google Scholar] [CrossRef]
- Hu, J.; Fang, H.; Wang, J.; Yue, X.; Su, M.; Mao, Z.; Zou, Q.; Jiang, H.; Guo, Z.; Yu, L.; et al. Ultraviolet B-induced MdWRKY72 expression promotes anthocyanin synthesis in apple. Plant Sci. 2020, 292, 110377. [Google Scholar] [CrossRef]
- Xue, S.; Zang, Y.; Chen, J.; Shang, S.; Gao, L.; Tang, X. Ultraviolet-B radiation stress triggers reactive oxygen species and regulates the antioxidant defense and photosynthesis systems of intertidal red algae Neoporphyra haitanensis. Front. Mar. Sci. 2022, 9, 1043462. [Google Scholar] [CrossRef]
- Li, X.L.; Ren, Q.L.; Zhao, W.X.; Liao, C.C.; Wang, Q.; Ding, T.H.; Hu, H.; Wang, M. Interaction between UV-B and plant anthocyanins. Funct. Plant Biol. 2023, 50, 599–611. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, N.M.; Batista, P.B.; Batista, A.G.; Marostica Junior, M.R. Current evidence on cognitive improvement and neuroprotection promoted by anthocyanins. Curr. Opin. Food Sci. 2019, 26, 71–78. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Cappellini, F.; Reiner, Z.; Zorzan, D.; Imran, M.; Sener, B.; Kilic, M.; El-Shazly, M.; Fahmy, N.M.; et al. The Therapeutic Potential of Anthocyanins: Current Approaches Based on Their Molecular Mechanism of Action. Front. Pharmacol. 2020, 11, 1300. [Google Scholar] [CrossRef]
- Ali, T.; Kim, T.; Rehman, S.U.; Khan, M.S.; Amin, F.U.; Khan, M.; Ikram, M.; Kim, M.O. Natural Dietary Supplementation of Anthocyanins via PI3K/Akt/Nrf2/HO-1 Pathways Mitigate Oxidative Stress, Neurodegeneration, and Memory Impairment in a Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2018, 55, 6076–6093. [Google Scholar] [CrossRef]
- Wang, D.; Ho, L.; Faith, J.; Ono, K.; Janle, E.M.; Lachcik, P.J.; Cooper, B.R.; Jannasch, A.H.; D’Arcy, B.R.; Williams, B.A.; et al. Role of intestinal microbiota in the generation of polyphenol-derived phenolic acid mediated attenuation of Alzheimer’s disease -amyloid oligomerization. Mol. Nutr. Food Res. 2015, 59, 1025–1040. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Xu, J.; Yang, M.; Hussain, M.; Liu, X.; Feng, F.; Guan, R. Protective Effect of Anthocyanins against Neurodegenerative Diseases through the Microbial-Intestinal-Brain Axis: A Critical Review. Nutrients 2023, 15, 496. [Google Scholar] [CrossRef]
- Suresh, S.; Begum, R.F.; Singh, A.S.; Chitra, V. Anthocyanin as a therapeutic in Alzheimer’s disease: A systematic review of preclinical evidences. Ageing Res. Rev. 2022, 76, 101595. [Google Scholar] [CrossRef]
- Shah, S.A.; Ul Amin, F.; Khan, M.; Abid, M.N.; Rehman, S.U.; Kim, T.H.; Kim, M.W.; Kim, M.O. Anthocyanins abrogate glutamate-induced AMPK activation, oxidative stress, neuroinflammation, and neurodegeneration in postnatal rat brain. J. Neuroinflamm. 2016, 13, 16. [Google Scholar] [CrossRef]
- Casedas, G.; Les, F.; Lopez, V. Anthocyanins: Plant Pigments, Food Ingredients or Therapeutic Agents for the CNS? A Mini-Review Focused on Clinical Trials. Curr. Pharm. Des. 2020, 26, 1790–1798. [Google Scholar] [CrossRef]
- Duan, H.; Wang, D.; Zheng, Y.; Zhou, Y.; Yan, W. The powerful antioxidant effects of plant fruits, flowers, and leaves help to improve retinal damage and support the relief of visual fatigue. Heliyon 2024, 10, 299. [Google Scholar] [CrossRef]
- Hsieh, F.C.; Hung, C.T.; Cheng, K.C.; Wu, C.Y.; Chen, Y.C.; Wu, Y.J.; Liu, W.; Chiu, C.C. Protective Effects of Lycium barbarum Extracts on UVB-Induced Damage in Human Retinal Pigment Epithelial Cells Accompanied by Attenuating ROS and DNA Damage. Oxidative Med. Cell. Longev. 2018, 2018, 4814928. [Google Scholar] [CrossRef]
- Szumny, D.; Kucharska, A.Z.; Czajor, K.; Bernacka, K.; Ziolkowska, S.; Krzyzanowska-Berkowska, P.; Magdalan, J.; Misiuk-Hojlo, M.; Sozanski, T.; Szelag, A. Extract from Aronia melanocarpa, Lonicera caerulea, and Vaccinium myrtillus Improves near Visual Acuity in People with Presbyopia. Nutrients 2024, 16, 926. [Google Scholar] [CrossRef] [PubMed]
- Ohguro, I.; Ohguro, H.; Nakazawa, M. Effects of Anthocyanins in Black Currant on Retinal Blood Flow Circulation of Patients with Normal Tension Glaucoma. A Pilot Study. Hirosaki Med. J. 2008, 59, 23–32. [Google Scholar] [CrossRef]
- Nomi, Y.; Iwasaki-Kurashige, K.; Matsumoto, H. Therapeutic Effects of Anthocyanins for Vision and Eye Health. Molecules 2019, 24, 3311. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Cheng, Z.R.; Duan, Y.Q.; Hu, K.; Cai, M.H.; Zhang, H.H. Effect of subcritical water temperature on the chain conformation and immune activity of ginger polysaccharides. Int. J. Biol. Macromol. 2024, 261, 129833. [Google Scholar] [CrossRef]
- Rostamabadi, H.; Demirkesen, I.; Colussi, R.; Roy, S.; Tabassum, N.; de Oliveira Filho, J.G.; Bist, Y.; Kumar, Y.; Nowacka, M.; Galus, S.; et al. Recent trends in the application of films and coatings based on starch, cellulose, chitin, chitosan, xanthan, gellan, pullulan, Arabic gum, alginate, pectin, and carrageenan in food packaging. Food Front. 2024, 5, 350–391. [Google Scholar] [CrossRef]
- Zhong, R.; Wan, X.; Wang, D.; Zhao, C.; Liu, D.; Gao, L.; Wang, M.; Wu, C.; Nabavid, S.M.; Daglia, M. Polysaccharides from marine Enteromorpha: Structure and function. Trends Food Sci. Technol. 2020, 99, 11–20. [Google Scholar] [CrossRef]
- Wang, C.; Yu, Y.-B.; Chen, T.-T.; Wang, Z.-W.; Yan, J.-K. Innovative preparation, physicochemical characteristics and functional properties of bioactive polysaccharides from fresh okra (Abelmoschus esculentus (L.) Moench). Food Chem. 2020, 320, 126647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, Z.; Shi, J.; Zou, X.; Zhai, X.; Huang, X.; Li, Z.; Holmes, M.; Daglia, M.; Xiao, J. Physical properties and bioactivities of chitosan/gelatin-based films loaded with tannic acid and its application on the preservation of fresh-cut apples. LWT-Food Sci. Technol. 2021, 144, 111223. [Google Scholar] [CrossRef]
- Wang, H.; Ding, F.; Ma, L.; Zhang, Y. Recent advances in gelatine and chitosan complex material for practical food preservation application. Int. J. Food Sci. Technol. 2021, 56, 6279–6300. [Google Scholar] [CrossRef]
- Godana, E.A.; Yang, Q.; Wang, K.; Zhang, H.; Zhang, X.; Zhao, L.; Abdelhai, M.H.; Legrand, N.N.G. Bio-control activity of Pichia anomala supplemented with chitosan against Penicillium expansum in postharvest grapes and its possible inhibition mechanism. LWT-Food Sci. Technol. 2020, 124, 109188. [Google Scholar] [CrossRef]
- Mesgari, M.; Aalami, A.H.; Sahebkar, A. Antimicrobial activities of chitosan/titanium dioxide composites as a biological nanolayer for food preservation: A review. Int. J. Biol. Macromol. 2021, 176, 530–539. [Google Scholar] [CrossRef]
- Liu, W.; Kang, S.; Zhang, Q.; Chen, S.; Yang, Q.; Yan, B. Self-assembly fabrication of chitosan-tannic acid/MXene composite film with excellent antibacterial and antioxidant properties for fruit preservation. Food Chem. 2023, 410, 135405. [Google Scholar] [CrossRef]
- Halasz, K.; Csoka, L. Black chokeberry (Aronia melanocarpa) pomace extract immobilized in chitosan for colorimetric pH indicator film application. Food Packag. Shelf Life 2018, 16, 185–193. [Google Scholar] [CrossRef]
- Zhang, Q.; Lin, G.; Wang, H.; Jin, M.C.; Dang, H.; Zhang, J.; Guo, R.; Yan, H.; Niu, B.; Wang, H. Development of smart packaging film incorporated with sodium alginate-chitosan quaternary ammonium salt nanocomplexes encapsulating anthocyanins for monitoring milk freshness. Int. J. Biol. Macromol. 2024, 263, 130336. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, S.; Feng, H.; Zhuang, D.; Zhu, J. An intelligent chitosan/gelatin film via improving the anthocyanin-induced color recognition accuracy for beef sub-freshness differentiation monitoring. Food Hydrocoll. 2024, 146, 109219. [Google Scholar] [CrossRef]
- Yang, Z.; Zou, X.; Li, Z.; Huang, X.; Zhai, X.; Zhang, W.; Shi, J.; Tahir, H.E. Improved Postharvest Quality of Cold Stored Blueberry by Edible Coating Based on Composite Gum Arabic/Roselle Extract. Food Bioprocess Technol. 2019, 12, 1537–1547. [Google Scholar] [CrossRef]
- Piryaei, M.; Azimi, S. Preparation and evaluation of smart food packaging films with anthocyanin Sardasht black grape based on Astragalus gummifer and chitosan nanoparticles. Int. J. Biol. Macromol. 2024, 254, 127974. [Google Scholar] [CrossRef] [PubMed]
- Moshfegh, N.; Niakousary, M.; Hosseini, S.M.H.; Mazloomi, S.M.; Abbasi, A. Effect of maltodextrin and Persian gum as wall materials and tannic acid as copigment on some properties of encapsulated sour cherry anthocyanin microcapsules. Food Chem. 2025, 463, 141165. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.; Qayum, A.; Bacha, S.A.S.; Liang, Q.; Liu, Y.; Kang, L.; Chi, Z.; Chi, R.; Han, X.; Ekumah, J.N.; et al. Preparation and functional characterization of pullulan-sodium alginate composite film enhanced with ultrasound-assisted clove essential oil Nanoemulsions for effective preservation of cherries and mushrooms. Food Chem. 2024, 457, 140048. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zheng, Y.; Ai, C.; Cao, H.; Xiao, J.; El-Seedi, H.; Chen, L.; Teng, H. Effect of carboxymethyl cellulose (CMC) on some physico-chemical and mechanical properties of unrinsed surimi gels. LWT-Food Sci. Technol. 2023, 180, 114653. [Google Scholar] [CrossRef]
- Jang, Y.; Koh, E. Characterisation and storage stability of aronia anthocyanins encapsulated with combinations of maltodextrin with carboxymethyl cellulose, gum Arabic, and xanthan gum. Food Chem. 2023, 405, 135002. [Google Scholar] [CrossRef]
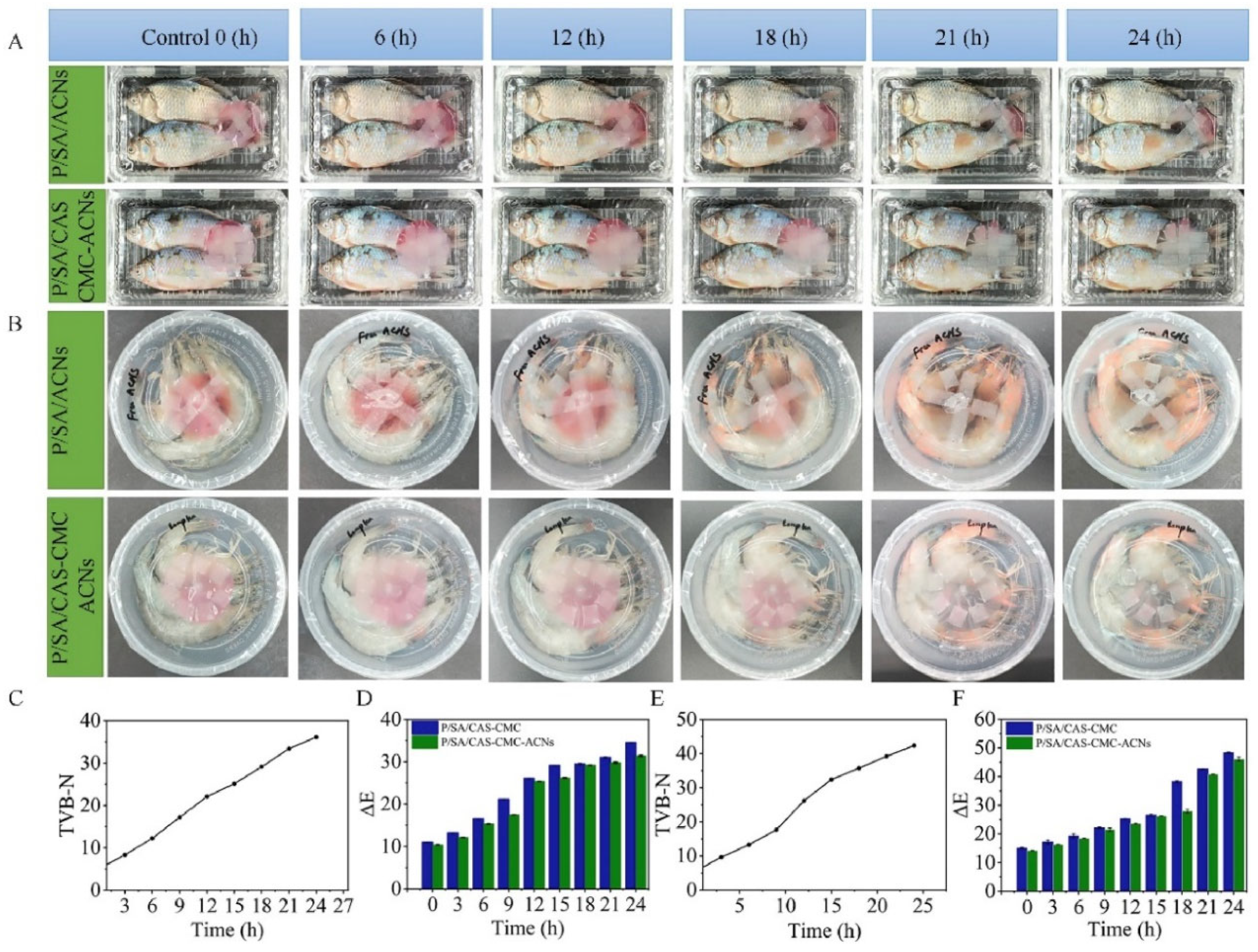
- Zhang, K.; Li, Z.; Khan, S.; Shishir, M.R.I.; Zheng, H.; Gao, L.; Shi, J.; Huang, X.; Zou, X. Natural silk improves the physico-mechanical properties of the colorimetric aerogel composed of anthocyanin, carboxymethyl cellulose and sodium alginate for fish freshness indication. Int. J. Biol. Macromol. 2024, 297, 138198. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, H.H.; Chen, L.; Zhou, W.B.; Zhou, S.W. Construction of homologous branched oligomer megamolecules based on linker-directed protein assembly. Soft Matter 2024, 20, 6889–6893. [Google Scholar] [CrossRef]
- Wu, H.; Oliveira, G.; Lila, M.A. Protein-binding approaches for improving bioaccessibility and bioavailability of anthocyanins. Compr. Rev. Food Sci. Food Saf. 2023, 22, 333–354. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, L.; Zhao, F.; Yu, A.; Zhou, Y.; Wen, Q.; Wang, J.; Zheng, T.; Chen, P. Protein and Peptide-Based Nanotechnology for Enhancing Stability, Bioactivity, and Delivery of Anthocyanins. Adv. Healthc. Mater. 2023, 12, 2300473. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, Y.; Li, L.; Qi, B.; Ju, M.; Xu, Y.; Zhang, Y.; Sui, X. Covalent conjugates of anthocyanins to soy protein: Unravelling their structure features and in vitro gastrointestinal digestion fate. Food Res. Int. 2019, 120, 603–609. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, L.; Li, J.; Oliveira, H.; Yang, N.; Jin, W.; Zhu, Z.; Li, S.; He, J. Microencapsulation of anthocyanins extracted from grape skin by emulsification/internal gelation followed by spray/freeze-drying techniques: Characterization, stability and bioaccessibility. LWT 2020, 123, 109097. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Zhang, L. Study on the mechanism of non-covalent interaction between rose anthocyanin extracts and whey protein isolate under different pH conditions. Food Chem. 2022, 384, 132492. [Google Scholar] [CrossRef]
- Guo, Z.; Huang, Y.; Huang, J.; Li, S.; Zhu, Z.; Deng, Q.; Cheng, S. Formation of protein-anthocyanin complex induced by grape skin extracts interacting with wheat gliadins: Multi-spectroscopy and molecular docking analysis. Food Chem. 2022, 385, 132702. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jia, L.; Xie, Y.; He, T.; Wang, S.; Jin, X.; Xie, F. Deciphering the interaction mechanism between soy protein isolate and fat-soluble anthocyanin on experiments and molecular simulations. Int. J. Biol. Macromol. 2024, 266, 131308. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, I.; Nilsuwan, K.; Prodpran, T.; Benjakul, S. Covalently phenolated-β-lactoglobulin-pullulan as a green halochromic biosensor efficiency monitored Barramundi fish’s spoilage. Int. J. Biol. Macromol. 2023, 243, 125189. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Inhibition of Escherichia coli O157:H7 biofilm on vegetable surface by solid liposomes of clove oil. LWT-Food Sci. Technol. 2020, 117, 108656. [Google Scholar] [CrossRef]
- Chen, B.-H.; Inbaraj, B.S. Nanoemulsion and Nanoliposome Based Strategies for Improving Anthocyanin Stability and Bioavailability. Nutrients 2019, 11, 1052. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, Z.; Yang, Y.; Adu-Frimpong, M.; Chen, L.; Ji, H.; Toreniyazov, E.; Wang, Q.; Yu, J.; Xu, X. Preparation, characterization, pharmacokinetics, and antirenal injury activity studies of Licochalcone A-loaded liposomes. J. Food Biochem. 2022, 46, e14007. [Google Scholar] [CrossRef]
- Chen, J.; Fang, W.; Liu, W.; Liu, J.; Gong, P. Microcapsules and Nanoliposomes Based Strategies to Improve the Stability of Blueberry Anthocyanins. Molecules 2023, 28, 7344. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Q.; Oliveira, H.; Mateus, N.; Ye, S.; Jiang, S.; He, J.; Wu, M. Preparation of nanoliposomes loaded with anthocyanins from grape skin extracts: Stability, gastric absorption and antiproliferative properties. Food Funct. 2022, 13, 10912–10922. [Google Scholar] [CrossRef]
- Song, J.; Yu, Y.; Chen, M.; Ren, Z.; Chen, L.; Fu, C.; Ma, Z.f.; Li, Z. Advancement of Protein- and Polysaccharide-Based Biopolymers for Anthocyanin Encapsulation. Front. Nutr. 2022, 9, 938829. [Google Scholar] [CrossRef]
- Rashid, A.; Qayum, A.; Shah Bacha, S.A.; Liang, Q.; Liu, Y.; Kang, L.; Chi, Z.; Chi, R.; Han, X.; Ekumah, J.-N.; et al. Novel pullulan-sodium alginate film incorporated with anthocyanin-loaded casein-carboxy methyl cellulose nanocomplex for real-time fish and shrimp freshness monitoring. Food Hydrocoll. 2024, 156, 110356. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhao, S.; Yang, M.; Song, M.; Li, J.; Zheng, J. Structurally stable sustained-release microcapsules stabilized by self-assembly of pectin-chitosan-collagen in aqueous two-phase system. Food Hydrocoll. 2022, 125, 107413. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhao, S.; Fan, Z.; Zhao, C.; Zhang, L.; Liu, D.; Bao, Y.; Zheng, J. A novel all-natural (collagen+pectin)/chitosan aqueous two-phase microcapsule with improved anthocyanin loading capacity. Food Hydrocoll. 2022, 134, 107984. [Google Scholar] [CrossRef]
- Sarabandi, K.; Jafari, S.M.; Mohammadi, M.; Akbarbaglu, Z.; Pezeshki, A.; Heshmati, M.K. Production of reconstitutable nanoliposomes loaded with flaxseed protein hydrolysates: Stability and characterization. Food Hydrocoll. 2019, 96, 442–450. [Google Scholar] [CrossRef]
- Javadi, B.; Farahmand, A.; Soltani-Gorde-Faramarzi, S.; Hesarinejad, M.A. Chitosan-coated nanoliposome: An approach for simultaneous encapsulation of caffeine and roselle-anthocyanin in beverages. Int. J. Biol. Macromol. 2024, 275, 133469. [Google Scholar] [CrossRef]
- Homayoonfal, M.; Mousavi, M.; Kiani, H.; Askari, G.; Desobry, S.; Arab-Tehrany, E. Modifying the stability and surface characteristic of anthocyanin compounds Incorporated in the nanoliposome by chitosan biopolymer. Pharmaceutics 2022, 14, 1622. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Li, X.J.; Wang, L.; Li, B.; Tian, J.L. Design of a liposome casein hydrogel as an efficient front-end homeostatic anthocyanin loading system. Int. J. Biol. Macromol. 2024, 278, 134928. [Google Scholar] [CrossRef]
- Shen, C.; Chen, W.; Li, C.; Aziz, T.; Cui, H.; Lin, L. Topical advances of edible coating based on the nanoemulsions encapsulated with plant essential oils for foodborne pathogen control. Food Control 2022, 145, 109419. [Google Scholar] [CrossRef]
- Zhang, J.; Hamadou, A.H.; Chen, C.; Xu, B. Encapsulation of phenolic compounds within food-grade carriers and delivery systems by pH-driven method: A systematic review. Crit. Rev. Food Sci. Nutr. 2021, 63, 4153–4174. [Google Scholar] [CrossRef]
- Taheri-Yeganeh, A.; Ahari, H.; Mashak, Z.; Jafari, S.M. Monitor the freshness of shrimp by smart halochromic films based on gelatin/pectin loaded with pistachio peel anthocyanin nanoemulsion. Food Chem.-X 2024, 21, 101217. [Google Scholar] [CrossRef]
- Nazareth, M.; Shreelakshmi, S.; Rao, P.; Shetty, N. Micro and nanoemulsions of Carissa spinarum fruit polyphenols, enhances anthocyanin stability and anti-quorum sensing activity: Comparison of degradation kinetics. Food Chem. 2021, 359, 129876. [Google Scholar] [CrossRef]
- Liang, T.; Jing, P.; He, J. Nano techniques: An updated review focused on anthocyanin stability. Crit. Rev. Food Sci. Nutr. 2024, 64, 11985–12008. [Google Scholar] [CrossRef] [PubMed]
- Kanha, N.; Jaimun, R.; Rattanamato, B.; Laokuldilok, T. Novel indicator film incorporating Dendrobium orchid extract and TiO2 nanoparticles for seafood freshness monitoring. Future Foods 2024, 10, 100512. [Google Scholar] [CrossRef]
- Cai, M.; Zhang, G.; Wang, J.; Li, C.; Cui, H.; Lin, L. Application of glycyrrhiza polysaccharide nanofibers loaded with tea tree essential oil/gliadin nanoparticles in meat preservation. Food Biosci. 2021, 43, 101270. [Google Scholar] [CrossRef]
- Yang, Z.; Zhai, X.; Zhang, C.; Shi, J.; Huang, X.; Li, Z.; Zou, X.; Gong, Y.; Holmes, M.; Povey, M. Agar/TiO2/radish anthocyanin/neem essential oil bionanocomposite bilayer films with improved bioactive capability and electrochemical writing property for banana preservation. Food Hydrocoll. 2022, 123, 107187. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Huang, X.; Shi, J.; Liu, L.; Song, W.; Zhai, X.; Xiao, J.; Hashim, S.B.H.; Li, Z. A visual bi-layer sensor based on Agar/TiO2/butterfly bean flower anthocyanin/κ-carrageenan with photostability for monitoring Penaeus chinensis freshness. Int. J. Biol. Macromol. 2023, 235, 123706. [Google Scholar] [CrossRef] [PubMed]
- Yi, F.; Hou, F.; Zhan, S.; Song, L.; Chen, X.; Zhang, R.; Gao, M.; Han, X.; Wang, X.; Liu, Z. Effect of nano-TiO2 particle size on the performance of chitosan/zein/red radish anthocyanin composite film for visual monitoring of mushroom freshness. Postharvest Biol. Technol. 2024, 211, 112809. [Google Scholar] [CrossRef]
- Ren, X.; Wang, J.; Rashid, A.; Hou, T.; Ma, H.; Liang, Q. Characterization of Nano-SiO2/Zein Film Prepared Using Ultrasonic Treatment and the Ability of the Prepared Film to Resist Different Storage Environments. Foods 2023, 12, 3056. [Google Scholar] [CrossRef]
- Pang, H.-Q.; Fan, T.-H.; Xia, T.; Qiao, W.-W.; Gao, Y.-F. Modeling and measurement of effective thermal conductivity of core-shell-structured SiO2 MHSPs-silica aerogel composite. J. Non-Cryst. Solids 2022, 593, 121791. [Google Scholar] [CrossRef]
- Shi, S.; Wu, X.; Wang, Y.; Li, W.; Zhang, H.; Lou, X.; Xia, X.; Liang, W. Sodium-alginate-based indicator film containing a hydrophobic nanosilica layer for monitoring fish freshness. Int. J. Biol. Macromol. 2024, 265, 130714. [Google Scholar] [CrossRef]
- Yang, Z.; Li, M.; Li, Y.; Li, Z.; Huang, X.; Wang, X.; Shi, J.; Zou, X.; Zhai, X.; Povey, M. Improving properties of Litsea cubeba oil Pickering emulsion-loaded gelatin-based bio-nanocomposite film via optimizing blending ratio: Application for mango preservation. Food Hydrocoll. 2023, 145, 109052. [Google Scholar] [CrossRef]
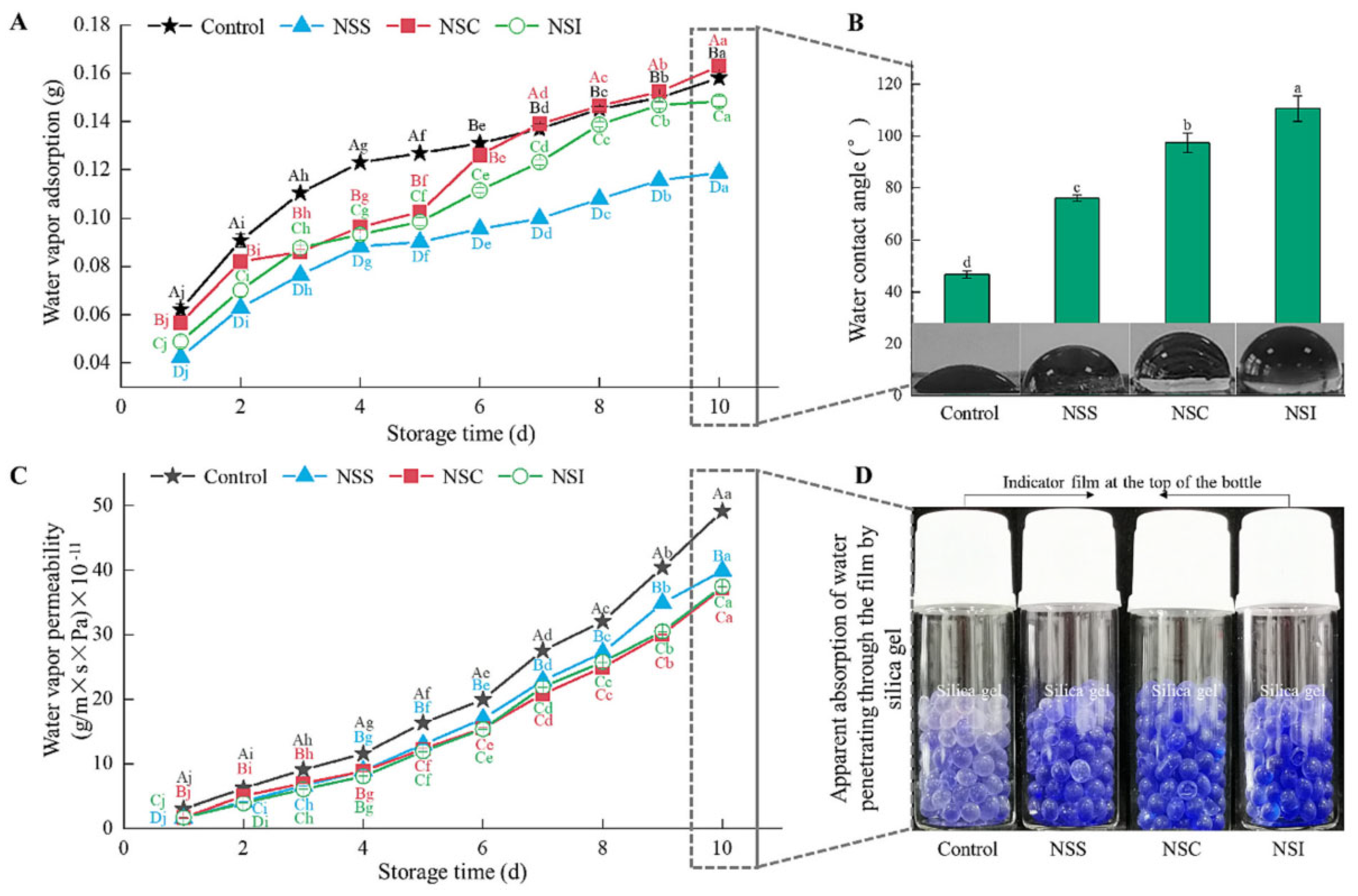
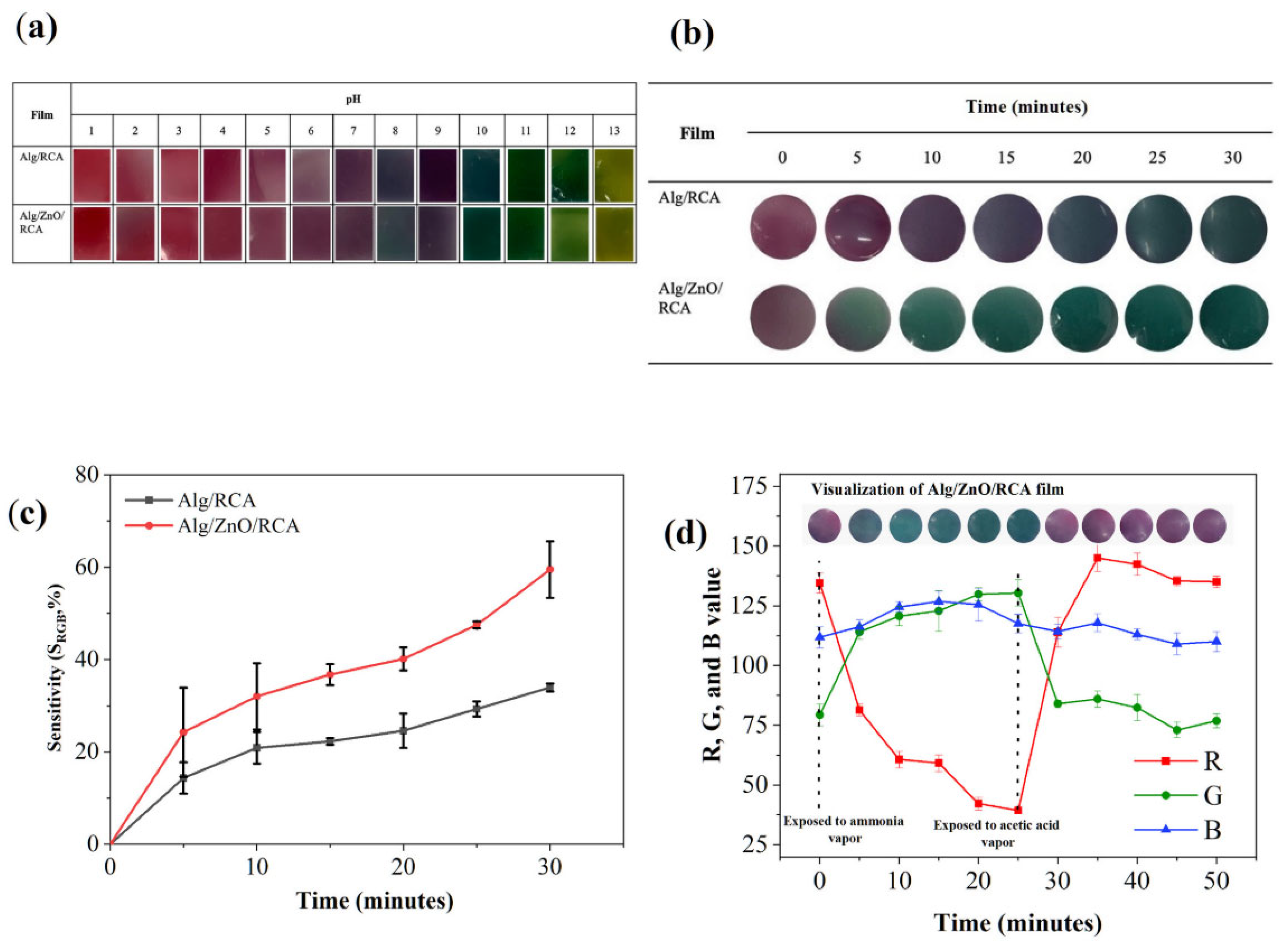
- Anugrah, D.S.B.; Darmalim, L.V.; Sinanu, J.D.; Pramitasari, R.; Subali, D.; Prasetyanto, E.A.; Cao, X.T. Development of alginate-based film incorporated with anthocyanins of red cabbage and zinc oxide nanoparticles as freshness indicator for prawns. Int. J. Biol. Macromol. 2023, 251, 126203. [Google Scholar] [CrossRef]
- Waqas, M.; Chen, Z.; Abbas, Y.; Farooq, A.; Han, X.; Zhong, H.; Ke, X.; Li, H.; Liu, X. Highly sensitive zinc oxide nanoparticle composite film with deep learning-assisted mobile technology for enhanced food freshness monitoring. Food Biosci. 2024, 62, 105541. [Google Scholar] [CrossRef]
- He, M.; Ou, X.; Wang, Y.; Chen, Z.; Li, D.; Chen, B.; Hu, B. Porous organic frameworks-based (micro) extraction. J. Chromatogr. A 2020, 1609, 460477. [Google Scholar] [CrossRef]
- Chen, Q.; Han, Y.; Wang, Y.; Wang, S.; Wei, J.; Jiao, T.; Chen, X.; Yuan, S.; Li, D.; Chen, Q. A natural pigment-based nanosized colorimetric sensor for freshness evaluation of aquatic products. Food Chem. 2025, 465, 141945. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, J.; Li, Y.; Zhang, L.; Bi, N.; Gou, J.; Zhu, T.; Jia, L. A novel intelligently integrated MOF-based ratio fluorescence sensor for ultra-sensitive monitoring of TC in water and food samples. Food Chem. 2023, 405, 134899. [Google Scholar] [CrossRef]
- Kang, L.; Liang, Q.; Abdul, Q.; Rashid, A.; Ren, X.; Ma, H. Preparation technology and preservation mechanism of γ-CD-MOFs biaological packaging film loaded with curcumin. Food Chem. 2023, 420, 136142. [Google Scholar] [CrossRef]
- Su, Q.; Su, W.; Xing, S.; Tan, M. Enhanced stability of anthocyanins by cyclodextrin–metal organic frameworks: Encapsulation mechanism and application as protecting agent for grape preservation. Carbohydr. Polym. 2024, 326, 121645. [Google Scholar] [CrossRef]
- Li, P.; Deng, Y.; Zou, W.; Ma, Z.; Yang, X.; Zhao, Q. Biosafe Cu-MOF loaded chitosan/gelatin-based multifunctional packaging film for monitoring shrimp freshness. Food Hydrocoll. 2025, 160, 110721. [Google Scholar] [CrossRef]
- Kang, Y.; Zhao, D.; Cai, D.; Jia, B.; Fu, J.; Li, X.; Hu, J.; Li, L. Novel copper-based metal–organic skeleton smart tags that respond to ammonia for real-time visual freshness monitoring of shrimp. Chem. Eng. J. 2024, 495, 153388. [Google Scholar] [CrossRef]
- Fang, S.-Y.; Zhang, P.; Gong, J.-L.; Tang, L.; Zeng, G.-M.; Song, B.; Cao, W.-C.; Li, J.; Ye, J. Construction of highly water-stable metal-organic framework UiO-66 thin-film composite membrane for dyes and antibiotics separation. Chem. Eng. J. 2020, 385, 123400. [Google Scholar] [CrossRef]
- Wu, W.; Liu, L.; Zhou, Y.; Shao, P. Highly ammonia-responsive starch/PVA film with gas absorption system as the ‘bridge’for visually spoilage monitoring of animal-derived food. Food Chem. 2024, 430, 137032. [Google Scholar] [CrossRef]
- Fang, H.; Cao, L.; Sui, J.; Lin, H.; Wang, L.; Wang, X.; Wang, K. Multifunctional metal-organic framework-enhanced sodium alginate-based intelligent indicator: Mechanism and application for freshness monitoring. Int. J. Biol. Macromol. 2024, 276, 133914. [Google Scholar] [CrossRef]
- Younas, R.; Jubeen, F.; Bano, N.; Andreescu, S.; Zhang, H.; Hayat, A. Covalent organic frameworks (COFs) as carrier for improved drug delivery and biosensing applications. Biotechnol. Bioeng. 2024, 121, 2017–2049. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, L.; He, B.; Lin, Y.; Wang, J.; Li, M. 8-Hydroxyquinoline functionalized covalent organic framework as a pH sensitive carrier for drug delivery. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 117, 111243. [Google Scholar] [CrossRef] [PubMed]
- Akyuz, L. An imine based COF as a smart carrier for targeted drug delivery: From synthesis to computational studies. Microporous Mesoporous Mater. 2020, 294, 109850. [Google Scholar] [CrossRef]
- Li, D.-M.; Zhang, S.-Y.; Wan, J.-Y.; Zhang, W.-Q.; Yan, Y.-L.; Tang, X.-H.; Zheng, S.-R.; Cai, S.-L.; Zhang, W.-G. A new hydrazone-linked covalent organic framework for Fe(iii) detection by fluorescence and QCM technologies. Crystengcomm 2021, 23, 3594–3601. [Google Scholar] [CrossRef]
- Yao, R.; Deng, B.; Li, Z.; Xie, L.; Li, J.; Tuo, K.; Fan, C.; Pu, S. A covalent organic framework rich in lanthanide Eu3+ binding sites for sensitive and selective determination of tetracycline. Dye. Pigment. 2023, 213, 111159. [Google Scholar] [CrossRef]
- Xu, H.; Guo, Y.; Zhou, S.; Wang, J.; Lu, F.; Wang, S.; Deng, Q. Colorimetric Covalent Organic Framework Gel as Thermal History Indicators for Food Freshness Monitoring. Food Bioprocess Technol. 2024, 17, 4927–4938. [Google Scholar] [CrossRef]
- Zou, Y.; Shi, Y.; Wang, T.; Ji, S.; Zhang, X.; Shen, T.; Huang, X.; Xiao, J.; Farag, M.A.; Shi, J. Quantum dots as advanced nanomaterials for food quality and safety applications: A comprehensive review and future perspectives. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13339. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Li, L.; Luo, L.; Liu, X.; Li, J.; You, T. A ratiometric fluorescence aptasensor based on photoinduced electron transfer from CdTe QDs to WS2 NTs for the sensitive detection of zearalenone in cereal crops. Food Chem. 2022, 385, 132657. [Google Scholar] [CrossRef]
- Zhang, Y.; Kim, G.; Zhu, Y.; Wang, C.; Zhu, R.; Lu, X.; Chang, H.-C.; Wang, Y. Chiral graphene quantum dots enhanced drug loading into small extracellular vesicles. ACS Nano 2023, 17, 10191–10205. [Google Scholar] [CrossRef]
- Badıllı, U.; Mollarasouli, F.; Bakirhan, N.K.; Ozkan, Y.; Ozkan, S.A. Role of quantum dots in pharmaceutical and biomedical analysis, and its application in drug delivery. TrAC Trends Anal. Chem. 2020, 131, 116013. [Google Scholar] [CrossRef]
- Hu, X.; Li, Y.; Xu, Y.; Gan, Z.; Zou, X.; Shi, J.; Huang, X.; Li, Z.; Li, Y. Green one-step synthesis of carbon quantum dots from orange peel for fluorescent detection of Escherichia coli in milk. Food Chem. 2021, 339, 127775. [Google Scholar] [CrossRef]
- Liang, N.; Hu, X.; Li, W.; Mwakosya, A.W.; Guo, Z.; Xu, Y.; Huang, X.; Li, Z.; Zhang, X.; Zou, X. Fluorescence and colorimetric dual-mode sensor for visual detection of malathion in cabbage based on carbon quantum dots and gold nanoparticles. Food Chem. 2021, 343, 128494. [Google Scholar] [CrossRef]
- Shojaeiarani, J.; Shirzadifar, A.; Shine, C.; Reisi, A.M. Hybrid nanocomposite packaging films from cellulose nanocrystals, zinc sulfide quantum dots reinforced polylactic acid with fluorescent and antibacterial properties. Polym. Eng. Sci. 2022, 62, 1562–1570. [Google Scholar] [CrossRef]
- Grzebieniarz, W.; Nowak, N.; Khachatryan, G.; Krzan, M.; Krystyjan, M.; Kosiński, J.; Khachatryan, K. The preparation and characterization of quantum dots in polysaccharide carriers (starch/chitosan) as elements of smart packaging and their impact on the growth of microorganisms in food. Materials 2021, 14, 7732. [Google Scholar] [CrossRef]
- Ghadari, R.; Ghanbari, S.; Mohammadzadeh, Y. A computational study on the interactions between a layered imine-based COF structure and selected anticancer drugs. J. Mol. Model. 2021, 27, 44. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, J.; Hu, X.; Huang, X.; Zhang, X.; Zou, X.; Shi, J. Preparation of edible antibacterial films based on corn starch /carbon nanodots for bioactive food packaging. Food Chem. 2024, 444, 138467. [Google Scholar] [CrossRef]
- Hadavifar, S.; Abedi-Firoozjah, R.; Bahramian, B.; Jafari, N.; Sadeghi, S.M.; Majnouni, S.; Ebrahimi, B.; Ehsani, A.; Tavassoli, M. Multifunctional performance of chitosan/soy protein isolation-based films impregnated carbon dots/anthocyanin derived from purple hull pistachio for tracking and extending the shelf life of fish. Food Hydrocoll. 2025, 159, 110678. [Google Scholar] [CrossRef]
- Qin, W.; Zou, L.; Hou, Y.; Wu, Z.; Loy, D.; Lin, D. Characterization of novel anthocyanins film @ carbon quantum dot nanofiber intelligent active double-layer film, physicochemical properties and fresh-keeping monitoring in Ictalurus punctatus fish. Chem. Eng. J. 2024, 496, 154041. [Google Scholar] [CrossRef]
- Liu, T.; Jiang, L.; Wang, Y.; Li, M.; Li, Z.; Liu, Y. Bilayer pH-sensitive colorimetric indicator films based on chitosan/purple carrot extract and gellan gum/Mg-carbon dots for visual monitoring of pork freshness. Food Packag. Shelf Life 2024, 45, 101336. [Google Scholar] [CrossRef]
- Khan, A.; Riahi, Z.; Kim, J.T.; Rhim, J.-W. Carrageenan-based multifunctional packaging films containing Zn-carbon dots/anthocyanin derived from Kohlrabi peel for monitoring quality and extending the shelf life of shrimps. Food Chem. 2024, 432, 137215. [Google Scholar] [CrossRef]
- Qiu, C.; Qin, Y.; Zhang, S.; Xiong, L.; Sun, Q. A comparative study of size-controlled worm-like amylopectin nanoparticles and spherical amylose nanoparticles: Their characteristics and the adsorption properties of polyphenols. Food Chem. 2016, 213, 579–587. [Google Scholar] [CrossRef]
- Ahari, H.; Golestan, L.; Anvar, S.A.A.; Cacciotti, I.; Garavand, F.; Rezaei, A.; Sani, M.A.; Jafari, S.M. Bio-nanocomposites as food packaging materials; the main production techniques and analytical parameters. Adv. Colloid Interface Sci. 2022, 310, 102806. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Liu, L.; Yu, J.; Farag, M.A.; Shao, P. Intelligent starch/chitosan-based film incorporated by anthocyanin-encapsulated amylopectin nanoparticles with high stability for food freshness monitoring. Food Control 2023, 151, 109798. [Google Scholar] [CrossRef]
- Li, Y.; Liang, W.; Huang, M.; Huang, W.; Feng, J. Green preparation of holocellulose nanocrystals from burdock and their inhibitory effects against α-amylase and α-glucosidase. Food Funct. 2022, 13, 170–185. [Google Scholar] [CrossRef]
- Zheng, D.; Cao, S.; Li, D.; Wu, Y.; Duan, P.; Liu, S.; Li, X.; Zhang, X.; Chen, Y. Fabrication and characterization of chitosan/anthocyanin intelligent packaging film fortified by cellulose nanocrystal for shrimp preservation and visual freshness monitoring. Int. J. Biol. Macromol. 2024, 264, 130692. [Google Scholar] [CrossRef] [PubMed]
- Padil, V.V.; Kumar, K.A.; Murugesan, S.; Torres-Mendieta, R.; Wacławek, S.; Cheong, J.Y.; Černík, M.; Varma, R.S. Sustainable and safer nanoclay composites for multifaceted applications. Green Chem. 2022, 24, 3081–3114. [Google Scholar] [CrossRef]
- Leandro, G.C.; Capello, C.; Koop, B.L.; Garcez, J.; Monteiro, A.R.; Valencia, G.A. Adsorption-desorption of anthocyanins from jambolan (Syzygium cumini) fruit in laponite® platelets: Kinetic models, physicochemical characterization, and functional properties of biohybrids. Food Res. Int. 2021, 140, 109903. [Google Scholar] [CrossRef]
- Elias Machado, J.P.; de Freitas, R.A.; Wypych, F. Layered clay minerals, synthetic layered double hydroxides and hydroxide salts applied as pickering emulsifiers. Appl. Clay Sci. 2019, 169, 10–20. [Google Scholar] [CrossRef]
- Koop, B.L.; Soares, L.S.; Cesca, K.; Souza, V.G.L.; Valencia, G.A.; Monteiro, A.R. Enhancing the stability of anthocyanins extracts through adsorption into nanoclays—Development of a smart biohybrid sensor for intelligent food packaging or as natural food additive/preservative. Food Bioprod. Process. 2024, 147, 315–326. [Google Scholar] [CrossRef]
- Gutierrez, T.J.; Leon, I.E.; Ponce, A.G.; Alvarez, V.A. Active and pH-Sensitive Nanopackaging Based on Polymeric Anthocyanin/Natural or Organo-Modified Montmorillonite Blends: Characterization and Assessment of Cytotoxicity. Polymers 2022, 14, 4881. [Google Scholar] [CrossRef]
- Li, S.E.; Mu, B.; Ding, J.J.; Zhang, H.; Wang, X.W.; Wang, A.Q. Fabrication of Anthocyanin/Montmorillonite Hybrid Pigments to Enhance Their Environmental Stability and Application in Allochroic Composite Films. Clays Clay Miner. 2021, 69, 142–151. [Google Scholar] [CrossRef]
- Abdullah, Z.W.; Dong, Y. Biodegradable and Water Resistant Poly(vinyl) Alcohol (PVA)/Starch (ST)/Glycerol (GL)/Halloysite Nanotube (HNT) Nanocomposite Films for Sustainable Food Packaging. Front. Mater. 2019, 6, 58. [Google Scholar] [CrossRef]
- Fizir, M.; Dramou, P.; Dahiru, N.S.; Ruya, W.; Huang, T.; He, H. Halloysite nanotubes in analytical sciences and in drug delivery: A review. Microchim. Acta 2018, 185, 389. [Google Scholar] [CrossRef]
- Ghavami, A.; Zamiri, B.; Mohebali, A. Anthocyanin/halloysite hybrid pigments with enhanced thermal and acid stability. Dye. Pigment. 2024, 231, 112356. [Google Scholar] [CrossRef]
- Ruiz, A.I.; Ruiz-García, C.; Ruiz-Hitzky, E. From old to new inorganic materials for advanced applications: The paradigmatic example of the sepiolite clay mineral. Appl. Clay Sci. 2023, 235, 106874. [Google Scholar] [CrossRef]
- Marquez-Rodriguez, A.S.; Carrion, A.; Trejo, F.; Esparza-Ponce, H.E.; Napoles-Duarte, J.M.; Ballinas-Casarrubias, M.L.; Fuentes-Cobas, L.E.; Salas, E.; Palomares-Baez, J.P.; Fuentes-Montero, M.E. Anthocyanins stabilization of Hibiscus sabdariffa extract with sepiolite: Analytical and reactive force fields approaches. Sustain. Chem. Pharm. 2024, 42, 101831. [Google Scholar] [CrossRef]
- Raji, M.; El Foujji, L.; Mekhzoum, M.E.M.; El Achaby, M.; Essabir, H.; Bouhfid, R.; Qaiss, A.e.K. pH-indicative Films Based on Chitosan-PVA/Sepiolite and Anthocyanin from Red Cabbage: Application in Milk Packaging. J. Bionic Eng. 2022, 19, 837–851. [Google Scholar] [CrossRef]
- Qi, X.D.; Jia, X.L.; Song, Y.M. Preparation and Characterization of Florfenicol/Chitosan-stearic Acid Polymer Nanomicelle and Its Antibiotic Properties. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2018, 33, 1007–1013. [Google Scholar] [CrossRef]
- Tawfik, S.M.; Azizov, S.; Elmasry, M.R.; Sharipov, M.; Lee, Y.-I. Recent advances in nanomicelles delivery systems. Nanomaterials 2020, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- Farhoudi, L.; Kesharwani, P.; Majeed, M.; Johnston, T.P.; Sahebkar, A. Polymeric nanomicelles of curcumin: Potential applications in cancer. Int. J. Pharm. 2022, 617, 121622. [Google Scholar] [CrossRef]
- Hatamipour, M.; Sahebkar, A.; Alavizadeh, S.H.; Dorri, M.; Jaafari, M.R. Novel nanomicelle formulation to enhance bioavailability and stability of curcuminoids. Iran. J. Basic Med. Sci. 2019, 22, 282–289. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Zhang, K.; Li, Z.; Zhang, J.; Zhai, X.; Zhang, N.; Du, L.; Qin, Z. Exploring the Integration of Anthocyanins with Functional Materials in Smart Food Packaging: From Stabilization to Application. Foods 2025, 14, 2896. https://doi.org/10.3390/foods14162896
Huang X, Zhang K, Li Z, Zhang J, Zhai X, Zhang N, Du L, Qin Z. Exploring the Integration of Anthocyanins with Functional Materials in Smart Food Packaging: From Stabilization to Application. Foods. 2025; 14(16):2896. https://doi.org/10.3390/foods14162896
Chicago/Turabian StyleHuang, Xiaowei, Ke Zhang, Zhihua Li, Junjun Zhang, Xiaodong Zhai, Ning Zhang, Liuzi Du, and Zhou Qin. 2025. "Exploring the Integration of Anthocyanins with Functional Materials in Smart Food Packaging: From Stabilization to Application" Foods 14, no. 16: 2896. https://doi.org/10.3390/foods14162896
APA StyleHuang, X., Zhang, K., Li, Z., Zhang, J., Zhai, X., Zhang, N., Du, L., & Qin, Z. (2025). Exploring the Integration of Anthocyanins with Functional Materials in Smart Food Packaging: From Stabilization to Application. Foods, 14(16), 2896. https://doi.org/10.3390/foods14162896






