Physico-Chemical and Sensory Characteristics of Extruded Cereal Composite Flour Porridge Enriched with House Crickets (Acheta domesticus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Procurement and Preparation
2.2. Extrusion Processing
2.3. Color
2.4. Aroma Profile
2.4.1. Sample Preparation
2.4.2. Analysis of Volatile Compounds
2.5. Pasting Properties of Flours
2.6. Rheological Properties of Porridges
2.7. Sensory Characteristics of Porridge
2.8. Data Analysis
3. Results and Discussion
3.1. Color of the Composite Flours
3.2. Aroma Profile of the Composite Flours
3.3. Pasting Properties of Flour
3.4. Rheological Properties of Porridge
3.4.1. Aging Characteristics
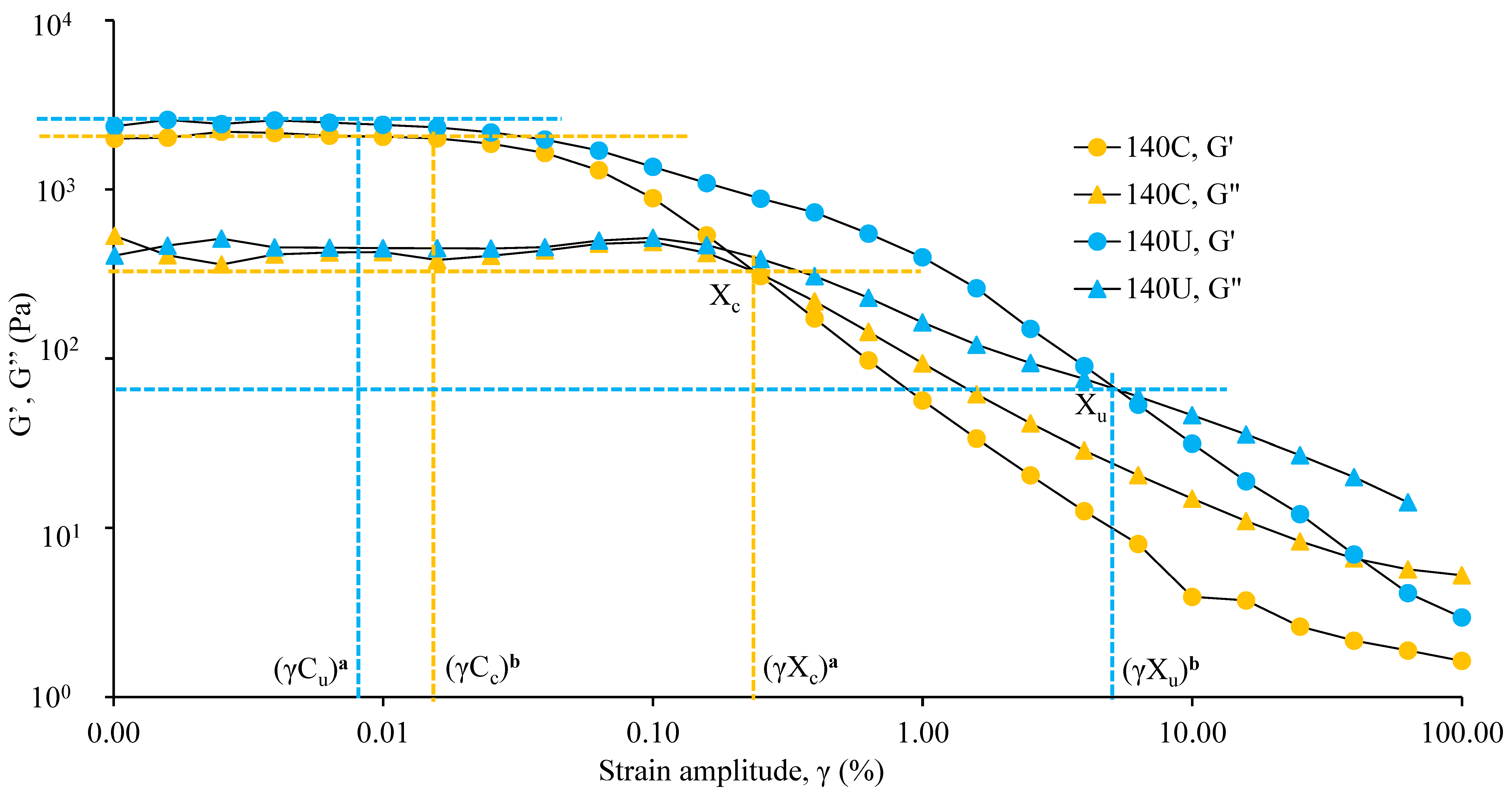
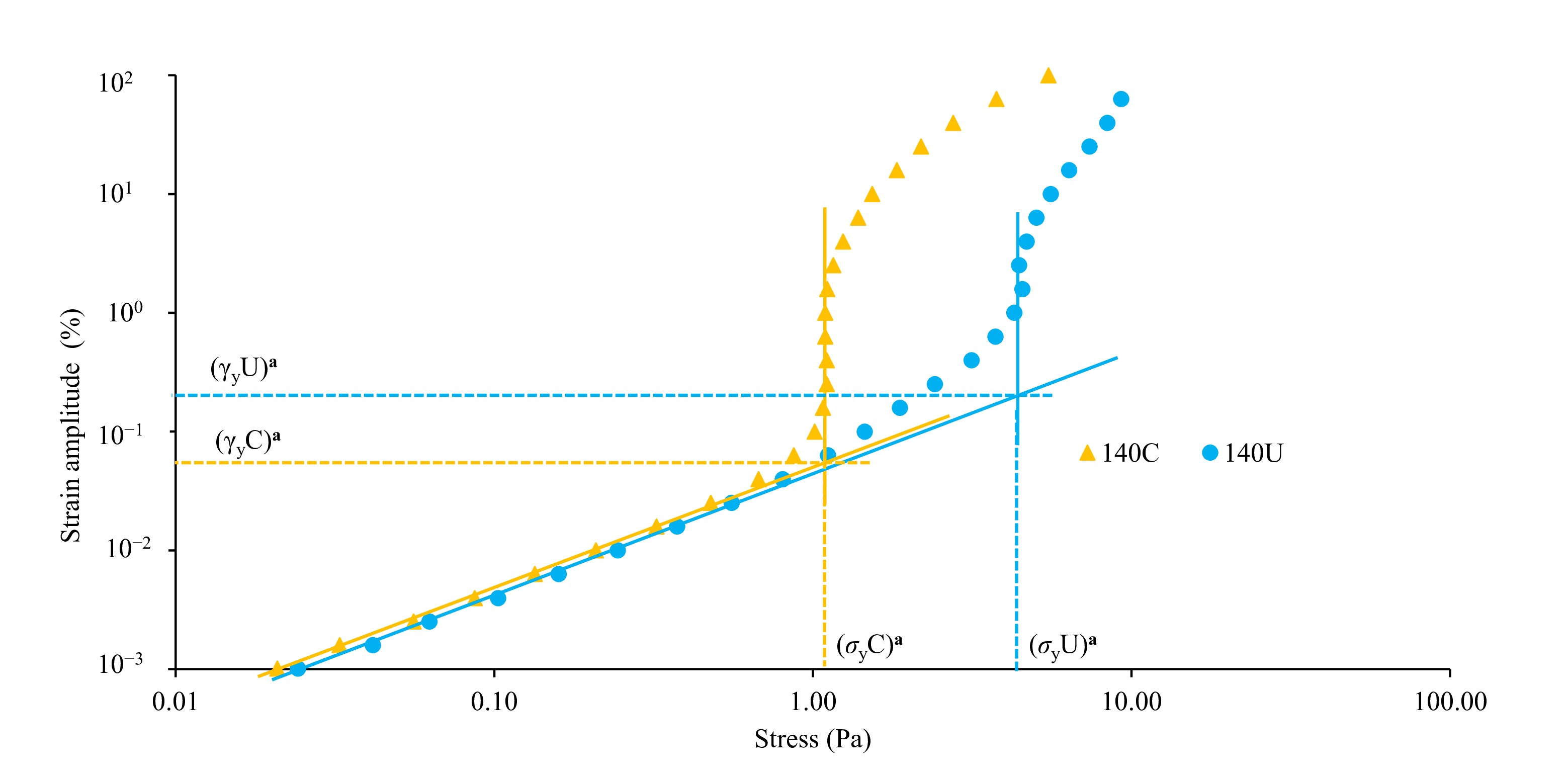
3.4.2. Variation of Visco-Elastic Moduli with Amplitude Strain and Yielding Behavior
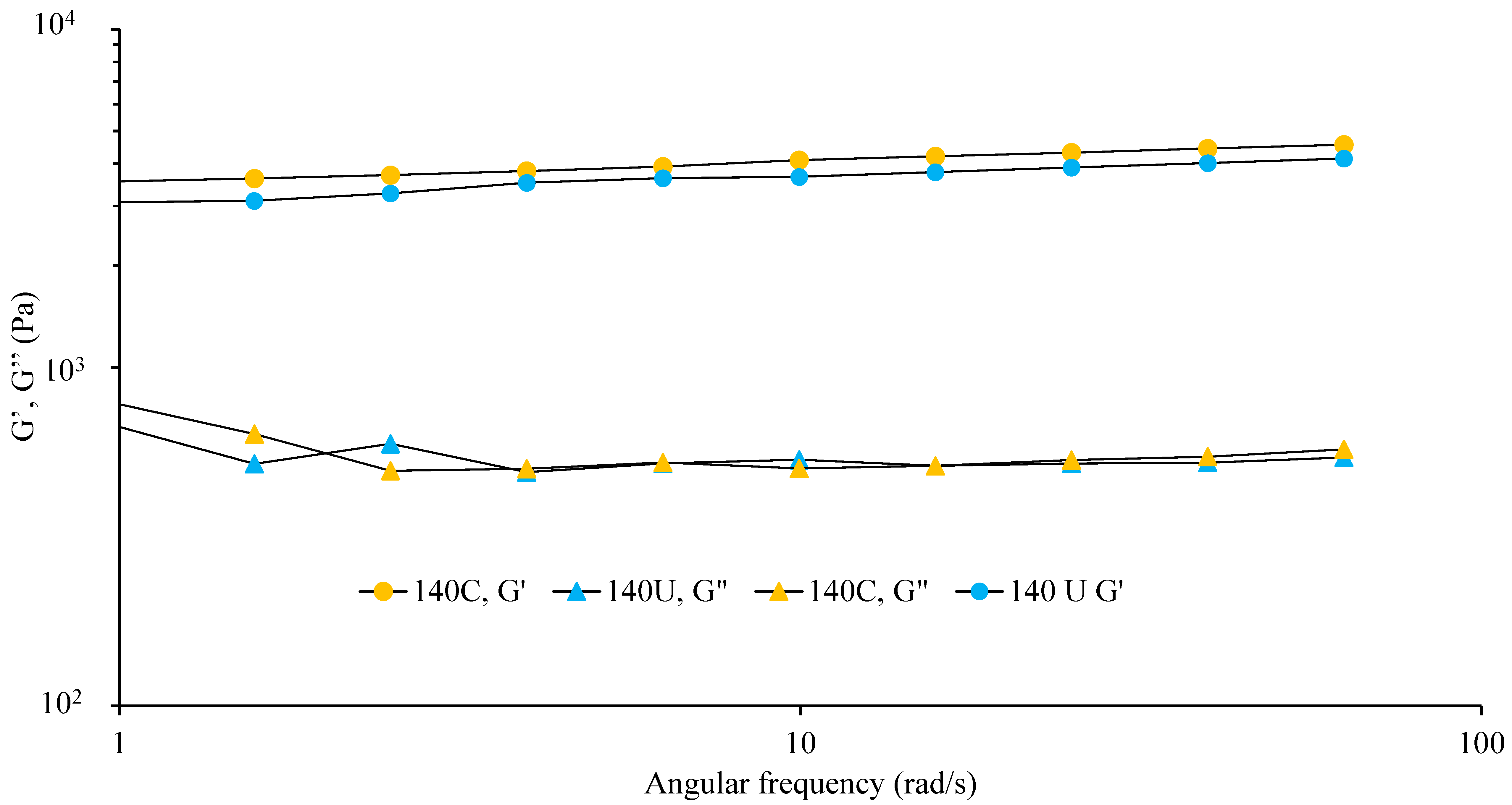
3.4.3. Angular Frequency Dependence of Visco-Elastic Moduli
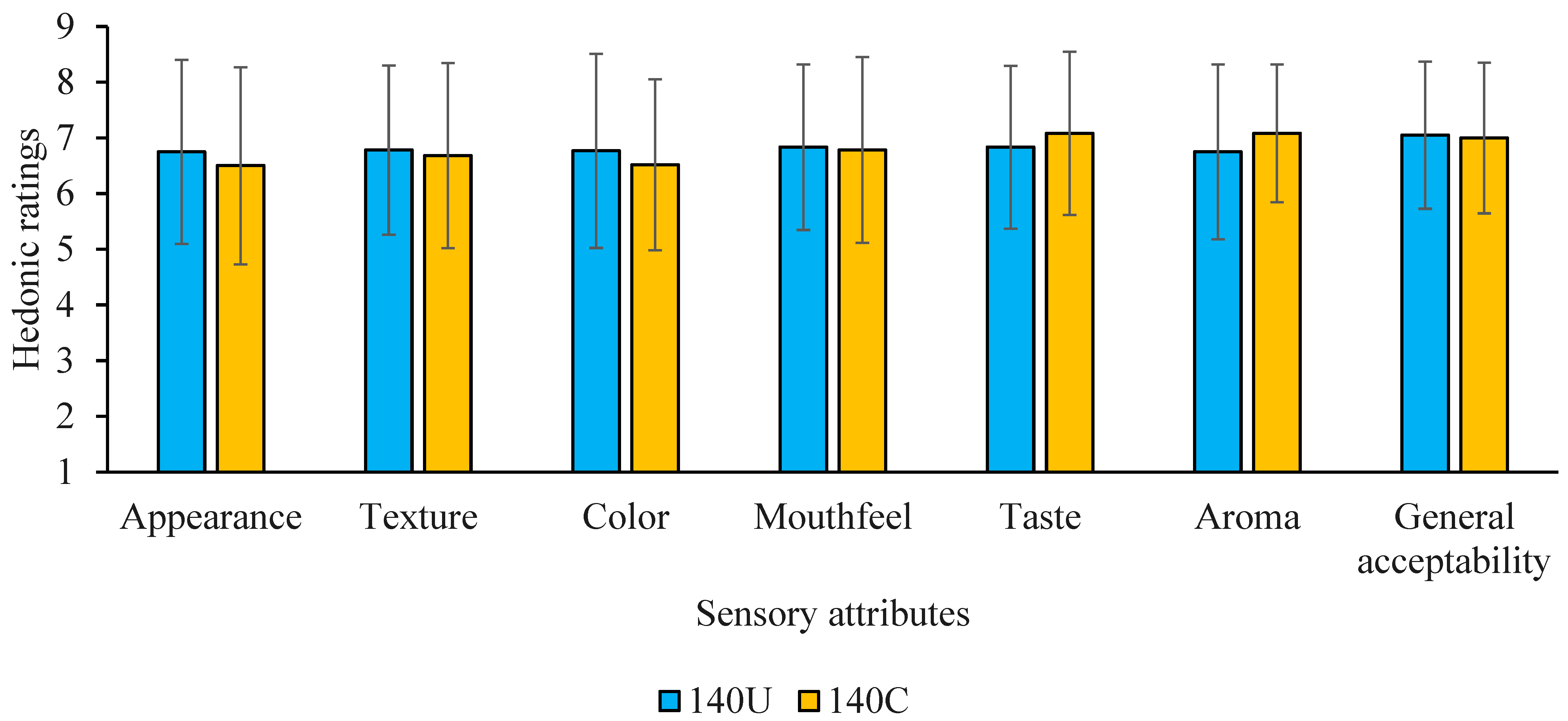
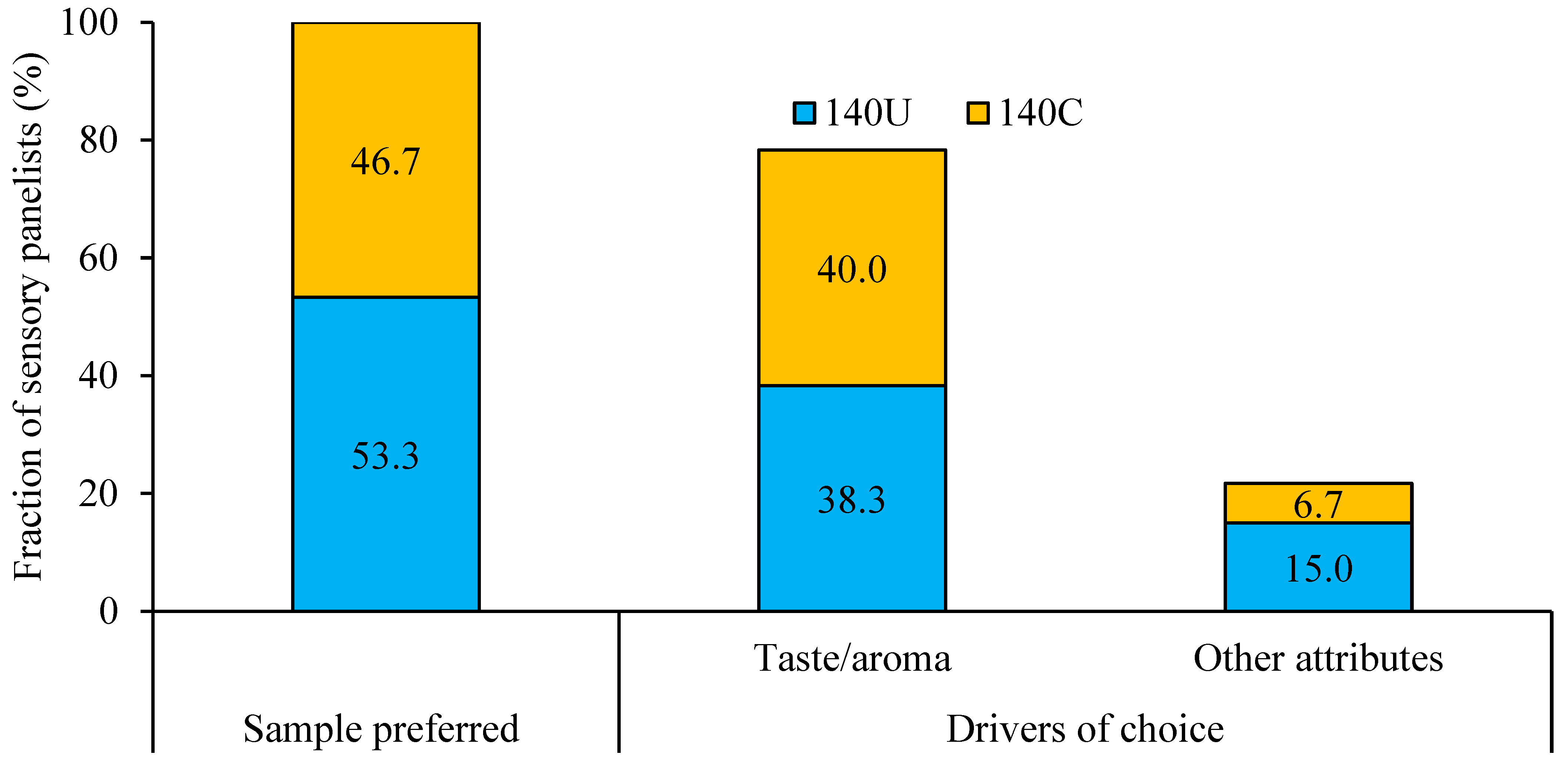
3.5. Sensory Attributes and Preference of Porridges
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 140U | Composite cereal flour containing undried house crickets, extruderd at 140 °C |
| 140C | Composite cereal flour without crickets, extruded at 140 °C |
| WHO | World Health Organization |
| FAO | Food and Agricultural Organization |
| Pa | Pascals |
| mPa.s | Millipascal seconds |
| Hz | Hertz |
| AU | Peak area units |
| BI | Browning index |
| CIE L*a*b* | Color space defined by the International Commission on Illumination |
| L* | Lightness |
| b* | Yellowness/blueness |
| a* | Redness/greenness |
| Cab | Chroma values calculated using the a* and b* values |
| ΔC | Chroma change/difference |
| ΔE | Total color difference |
| GC | Gas chromatography |
| KIexp | Experimental Kovat’s indices |
| KIlit | Kovat’s indices reported in the literature |
| LVE | Linear visco-elastic region |
| G′ | Elastic/storage modulus |
| G″ | Viscous/loss modulus |
| γc | Critical strain |
| γX | Strain at cross-over point, X |
| σy | Yield stress |
| γy | Yield strain |
References
- Acire, P.V.; Bagonza, A.; Opiri, N. The Misbeliefs and Food Taboos during Pregnancy and Early Infancy: A Pitfall to Attaining Adequate Maternal and Child Nutrition Outcomes among the Rural Acholi Communities in Northern Uganda. BMC Nutr. 2023, 9, 126. [Google Scholar] [CrossRef] [PubMed]
- Bbosa, T.; Nakimbugwe, D.; Matthys, C.; Vandeweyer, D.; Grauwet, T.; Van Der Borght, M. Influence of Processing on Nutritional and Microbiological Quality of Maize and Millet Composite Flours Enriched with House Crickets (Acheta domesticus). Appl. Food Res. 2025, 5, 100848. [Google Scholar] [CrossRef]
- Mujinda, G.; Manhokwe, S.; Chawafambira, A.; Mugadza, D.T.; Chagwena, D.T.; Jombo, T.Z. Optimisation of Nutritional Composition of Traditional Porridges Produced from Blended Pearl Millet, Cowpeas, and Wild Loquat and Velvet Wild Medlar Fruits. Food Chem. Adv. 2023, 3, 100478. [Google Scholar] [CrossRef]
- Kinyuru, J.; Kipkoech, C.; Imathiu, S.; Konyole, S.; Roos, N. Acceptability of Cereal-Cricket Porridge Compared to Cereal and Cereal-Milk- Porridges among Caregivers and Nursery School Children in Uasin Gishu, Kenya. Int. J. Trop. Insect Sci. 2021, 41, 2007–2013. [Google Scholar] [CrossRef]
- House, J. Consumer Acceptance of Insect-Based Foods in the Netherlands: Academic and Commercial Implications. Appetite 2016, 107, 47–58. [Google Scholar] [CrossRef]
- Henchion, M.; Hayes, M.; Mullen, A.; Fenelon, M.; Tiwari, B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef]
- Batat, W.; Peter, P. The Healthy and Sustainable Bugs Appetite: Factors Affecting Entomophagy Acceptance and Adoption in Western Food Cultures. J. Consum. Mark. 2020, 37, 291–303. [Google Scholar] [CrossRef]
- Ayustaningwarno, F.; Ayu, A.M.; Syiffah, L.; Muthia, H.; Amalina, F.A.; Afifah, D.N.; Nindita, Y.; Maharani, N.; Widyastuti, N.; Anjani, G.; et al. Physicochemical and Sensory Properties of Cookies with Cricket Powder as an Alternative Snack to Prevent Iron Deficiency Anemia and Chronic Energy Deficiency. Appl. Food Res. 2024, 4, 100485. [Google Scholar] [CrossRef]
- Grossmann, K.K.; Merz, M.; Appel, D.; De Araujo, M.M.; Fischer, L. New Insights into the Flavoring Potential of Cricket (Acheta domesticus) and Mealworm (Tenebrio molitor) Protein Hydrolysates and their Maillard Products. Food Chem. 2021, 364, 130336. [Google Scholar] [CrossRef]
- Balet, S.; Guelpa, A.; Fox, G.; Manley, M. Rapid Visco Analyser (RVA) as a Tool for Measuring Starch-Related Physiochemical Properties in Cereals: A Review. Food Anal. Methods 2019, 12, 2344–2360. [Google Scholar] [CrossRef]
- Aboge, D.O.; Orinda, M.A.; Konyole, S.O. Acceptability of Complementary Porridge Enriched with Crickets (Acheta domesticus) among Women of Reproductive Age in Alego-Usonga Sub-County, Kenya. Int. J. Health Allied Sci. 2021, 7, 23–30. [Google Scholar] [CrossRef]
- Ssepuuya, G.; Nakimbugwe, D.; De Winne, A.; Smets, R.; Claes, J.; Van Der Borght, M. Effect of Heat Processing on the Nutrient Composition, Colour, and Volatile Odour Compounds of the Long-Horned Grasshopper Ruspolia differens Serville. Food Res. Int. 2020, 129, 108831. [Google Scholar] [CrossRef]
- Fernández-Artigas, P.; Guerra-Hernández, E.; García-Villanova, B. Browning Indicators in Model Systems and Baby Cereals. J. Agric. Food Chem. 1999, 47, 2872–2878. [Google Scholar] [CrossRef]
- Perez-Santaescolastica, C.; De Winne, A.; Devaere, J.; Fraeye, I. Comparing Aroma Profile of Seven Unheated Edible Insects. Food Res. Int. 2023, 164, 112389. [Google Scholar] [CrossRef] [PubMed]
- Viste, A. NIST Chemistry WebBook, NIST Standard Reference Database Number 69. Available online: https://webbook.nist.gov/chemistry/ (accessed on 4 August 2025).
- Khatun, H.; Van Der Borght, M.; Akhtaruzzaman, M.; Claes, J. Rheological Characterization of Chapatti (Roti) Enriched with Flour or Paste of House Crickets (Acheta domesticus). Foods 2021, 10, 2750. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Gunes, D.Z.; Farrés, I.F. Rheological Control of Pea Fibre Dispersions in Oil: The Role of Particle and Water Volume Fractions. Food Hydrocoll. 2021, 121, 106988. [Google Scholar] [CrossRef]
- Onyango, C.; Wanjala, G.W. Quality of Porridge from sub-Saharan Africa Evaluated Using Instrumental Techniques and Descriptive Sensory Lexicon. Part 2: Thin Porridge. Afr. J. Food Sci. 2018, 12, 104–114. [Google Scholar] [CrossRef]
- Carlo, U.; Savelli, E.; Bravi, L. PDO Labels and Food Preferences: Results from a Sensory Analysis. Br. Food J. 2021, 123, 1170–1189. [Google Scholar] [CrossRef]
- Farrance, I.; Frenkel, R. Uncertainty of Measurement: A Review of the Rules for Calculating Uncertainty Components through Functional Relationships. Clin. Biochem. Rev. 2012, 33, 49–75. [Google Scholar]
- Janssen, R.H.; Vincken, J.P.; Arts, N.J.G.; Fogliano, V.; Lakemond, C.M.M. Effect of Endogenous Phenoloxidase on Protein Solubility and Digestibility after Processing of Tenebrio molitor, Alphitobius diaperinus and Hermetia illucens. Food Res. Int. 2019, 121, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Cacchiarelli, C.; Fratini, F.; Puccini, M.; Vitolo, S.; Paci, G.; Mancini, S. Effects of Different Blanching Treatments on Colour and Microbiological Profile of Tenebrio molitor and Zophobas morio Larvae. LWT 2022, 157, 113112. [Google Scholar] [CrossRef]
- Czerny, M.; Christlbauer, M.; Christlbauer, M.; Fischer, A.; Granvogl, M.; Hammer, M.; Hartl, C.; Hernandez, N.M.; Schieberle, P. Re-Investigation on Odour Thresholds of Key Food Aroma Compounds and Development of an Aroma Language Based on Odour Qualities of Defined Aqueous Odorant Solutions. Eur. Food Res. Technol. 2008, 228, 265–273. [Google Scholar] [CrossRef]
- Feng, M.; Dai, Z.; Yin, Z.; Wang, X.; Chen, S.; Zhang, H. The Volatile Flavor Compounds of Shanghai Smoked Fish as a Special Delicacy. J. Food Biochem. 2021, 45, e13553. [Google Scholar] [CrossRef]
- van Gemert, L.J. Flavour Thresholds: Compilations of Flavour Threshold Values in Water and Other Media, 2nd ed.; Oliemans Punter & Partners: Utretcht, The Netherlands, 2011; Available online: https://www.academia.edu/117947623/Compilations_of_flavour_threshold_values_in_water_and_other_media (accessed on 2 April 2025).
- Khatun, H.; Claes, J.; Smets, R.; De Winne, A.; Akhtaruzzaman, M.; Van Der Borght, M. Characterization of Freeze-Dried, Oven-Dried and Blanched House Crickets (Acheta domesticus) and Jamaican Field Crickets (Gryllus assimilis) by Means of Their Physicochemical Properties and Volatile Compounds. Eur. Food Res. Technol. 2021, 247, 1291–1305. [Google Scholar] [CrossRef]
- Mahajan, S.S.; Goddik, L.; Qian, M.C. Aroma Compounds in Sweet Whey Powder. J. Dairy Sci. 2004, 87, 4057–4063. [Google Scholar] [CrossRef]
- Murnane, S.S.; Lihocky, A.H.; Owens, P.D. Odor Thresholds for Chemicals with Established Occupational Health Hazards, 2nd ed.; AIHA: Falls Church, VA, USA, 2013. [Google Scholar]
- Nagata, Y. Measurement of Odor Threshold by Triangle Odor Bag Method. JAOE J. 2012, 43, 401–407. [Google Scholar] [CrossRef]
- Perez-Santaescolastica, C.; De Winne, A.; Devaere, J.; Fraeye, I. The Flavor of Edible Insects: A Comprehensive Review on Volatile Compounds and Their Analytical Assessment. Trends Food Sci. Technol. 2022, 127, 352–367. [Google Scholar] [CrossRef]
- Rita de Cássia, R.; Neta, M.T.S.L.; Donizete, R.; Sandes, D.; Narain, N.; de Sousa Galvão, M.; Madruga, M.S.; Costa, R.G. An Insight in Key Volatile Compounds in Goat Milk Based on Their Odor Active Values. J. Food Sci. Nutr. Res. 2019, 2, 49–60. [Google Scholar] [CrossRef]
- Ren, L.; Ma, J.; Lv, Y.; Tong, Q.; Guo, H. Characterization of Key Off-Odor Compounds in Thermal Duck Egg Gels by GC-Olfactometry-MS, Odor Activity Values, and Aroma Recombination. LWT 2021, 143, 111182. [Google Scholar] [CrossRef]
- Szulczýnski, B.; Gębicki, J. Determination of Odor Intensity of Binary Gas Mixtures Using Perceptual Models and an Electronic Nose Combined with Fuzzy Logic. Sensors 2019, 19, 3473. [Google Scholar] [CrossRef]
- Wagner, J.; Schieberle, P.; Granvogl, M. Characterization of the Key Aroma Compounds in Heat-Processed Licorice (Succus liquiritiae) by Means of Molecular Sensory Science. J. Agric. Food Chem. 2017, 65, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.; Czerny, M.; Bielohradsky, J.; Grosch, W. Structure-Odour-Activity Relationships of Alkylpyrazines. Z. Für Leb. Und-Forsch. A 1999, 208, 308–316. [Google Scholar] [CrossRef]
- Zhang, L.; Mi, S.; Liu, R.B.; Sang, Y.X.; Wang, X.H. Evaluation of Volatile Compounds during the Fermentation Process of Yogurts by Streptococcus thermophilus Based on Odor Activity Value and Heat Map Analysis. Int. J. Anal. Chem. 2020, 2020, 3242854. [Google Scholar] [CrossRef]
- Umebara, I.; Akutsu, K.; Kubo, M.; Iijima, A.; Sakurai, R.; Masutomi, H.; Ishihara, K. Analysis of Fatty Acid Composition and Volatile Profile of Powder from Edible Crickets (Acheta domesticus) Reared on Apple by-Products. Foods 2024, 13, 1668. [Google Scholar] [CrossRef]
- Shafat, A.M.; Javeed, I.A.B.; Rouf, A.B.; Bilal, A.B.; Hafiz ul, I.; Shakeel, A.D.; Ishrat, B.; Gowhar, R. Cytogenetic and Bioactive Attributes of Crocus sativus (Saffron): A Tool to Unfold Its Medicinal Mystery. In Medicinal and Aromatic Plants: Expanding Their Horizons Through Omics; Aftab, T., Hakeem, K.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 145–167. [Google Scholar] [CrossRef]
- Ekpa, O.; Fogliano, V.; Linnemann, A. Identification of the Volatile Profiles of 22 Traditional and Newly Bred Maize Varieties and Their Porridges by PTR-QiTOF-MS and HS-SPME GC–MS. J. Sci. Food Agric. 2021, 101, 1618–1628. [Google Scholar] [CrossRef]
- Finke, M.D. Complete Nutrient Content of Four Species of Commercially Available Feeder Insects Fed Enhanced Diets during Growth. Zoo Biol. 2015, 34, 554–564. [Google Scholar] [CrossRef]
- Meynier, A.; Novelli, E.; Chizzolini, R.; Zanardi, E.; Gandemer, G. Volatile Compounds of Commercial Milano Salami. Meat Sci. 1999, 51, 175–183. [Google Scholar] [CrossRef]
- Fong, L.C.; Yaylayan, V.A. Model Studies on the Oxygen-Induced Formation of Benzaldehyde from Phenylacetaldehyde Using Pyrolysis GC-MS and FTIR. J. Agric. Food Chem. 2008, 56, 10697–10704. [Google Scholar] [CrossRef]
- Schöpf, A.; Oellig, C.; Granvogl, M. Formation of Furanoic Compounds in Model Systems with Saccharides, Amino Acids, and Fatty Acids, Analyzed with Headspace-Solid Phase Micro-Extraction-Gas Chromatography–Tandem Mass Spectrometry. J. Food Bioact. 2022, 20, 61–71. [Google Scholar] [CrossRef]
- Schöpf, A.; Oellig, C. Formation of Furan and Furan Derivatives in Carotenoid-Containing Model Systems and in Vegetable Puree. ACS Food Sci. Technol. 2024, 4, 668–678. [Google Scholar] [CrossRef]
- Adams, A.; Kitryte, V.; Venskutonis, R.; De Kimpe, N. Model Studies on the Pattern of Volatiles Generated in Mixtures of Amino Acids, Lipid-Oxidation-Derived Aldehydes, and Glucose. J. Agric. Food Chem. 2011, 59, 1449–1456. [Google Scholar] [CrossRef]
- Reale, S.; Biancolillo, A.; Foschi, M.; D’Archivio, A.A. Characterization of the Volatile Profiles of Insect Flours by (HS)-SPME/GC-MS: A Preliminary Study. Molecules 2023, 28, 3075. [Google Scholar] [CrossRef]
- Wang, F.; Shen, H.; Liu, T.; Yang, X.; Yang, Y.; Guo, Y. Formation of Pyrazines in Maillard Model Systems: Effects of Structures of Lysine-Containing Dipeptides/Tripeptides. Foods 2021, 10, 273. [Google Scholar] [CrossRef]
- Park, H.; Seo, H.; Cho, I.H. Effect of Amino Acids in the Maillard Reaction Products Generated from the Reaction Flavors of Tenebrio molitor (Mealworm) Protein and d-Xylose. Food Sci. Biotechnol. 2022, 31, 1647–1660. [Google Scholar] [CrossRef]
- Cerny, C. Savory Flavors. In Handbook of Meat, Poultry and Seafood Quality; Nollet, L.M.L., Ed.; Blackwell Publishing: Ames, IA, USA, 2007; pp. 163–182. [Google Scholar]
- Drijfhout, F.P.; Kather, R.; Martin, S.J. The Role of Cuticular Hydrocarbons in Insects. In Behavioral and Chemical Ecology; Zhang, W., Liu, H., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2010; p. 277. [Google Scholar]
- Shahidi, F.; Oh, W.Y. Lipid-Derived Flavor and off-Flavor of Traditional and Functional Foods: An Overview. J. Food Bioact. 2020, 10, 20–31. [Google Scholar] [CrossRef]
- Beran, F.; Köllner, T.G.; Gershenzon, J.; Tholl, D. Chemical Convergence between Plants and Insects: Biosynthetic Origins and Functions of Common Secondary Metabolites. New Phytol. 2019, 223, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Keeling, P.J.; Palmer, J.D. Horizontal Gene Transfer in Eukaryotic Evolution. Nat. Rev. Genet. 2008, 9, 605–618. [Google Scholar] [CrossRef]
- Köllner, T.G.; Schnee, C.; Gershenzon, J.; Degenhardt, J. The Sesquiterpene Hydrocarbons of Maize (Zea mays) Form Five Groups with Distinct Developmental and Organ-Specific Distributions. Phytochemistry 2004, 65, 1895–1902. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Tian, B.; Li, J.; Zhang, W.; Bi, S.; Fu, B.; Jing, Y. Mechanisms Underlying the Formation of Main Volatile Odor Sulfur Compounds in Foods during Thermal Processing. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13389. [Google Scholar] [CrossRef]
- Ssepuuya, G.; Nakimbugwe, D.; Van Campenhout, L.; De Winne, A.; Claes, J.; Van Der Borght, M. Towards Establishing the Spoilage Mechanisms of the Long-Horned Grasshopper Ruspolia differens Serville. Eur. Food Res. Technol. 2021, 247, 2915–2926. [Google Scholar] [CrossRef]
- Kumar, P.; Rudra, S.G.; Varghese, E.; Kaur, C. Extrusion Conditions Effects Functional and Pasting Properties of Finger Millet. Vegetos 2016, 29, 21–30. [Google Scholar]
- Patil, S.; Kaur, C.; Puniya, M.K.; Mahapatra, A.; Dhakane-Lad, J.; Jalgaonkar, K.; Mahawar, M.K. Functional Properties of Extruded Corn Flour. Turk. J. Agri. Eng. Res. 2021, 2, 167–174. [Google Scholar] [CrossRef]
- Kaushal, P.; Kumar, V.; Sharma, H.K. Comparative Study of Physicochemical, Functional, Antinutritional and Pasting Properties of Taro (Colocasia esculenta), Rice (Oryza sativa) Flour, Pigeonpea (Cajanus cajan) Flour and Their Blends. LWT 2012, 48, 59–68. [Google Scholar] [CrossRef]
- Yuan, T.Z.; Liu, S.; Reimer, M.; Isaak, C.; Ai, Y. Evaluation of Pasting and Gelling Properties of Commercial Flours under High Heating Temperatures Using Rapid Visco Analyzer 4800. Food Chem. 2021, 344, 128616. [Google Scholar] [CrossRef]
- Ragaee, S.; Abdel-Aal, E.S.M. Pasting Properties of Starch and Protein in Selected Cereals and Quality of Their Food Products. Food Chem. 2006, 95, 9–18. [Google Scholar] [CrossRef]
- Imoisi, C.; Iyasele, J.U.; Imhontu, E.E.; Okpebho, A.O. Pasting Properties of Composite of Cassava and Wheat Flours. J. Chem. Soc. Nigeria 2020, 45, 1157–1163. [Google Scholar] [CrossRef]
- Xiao, W.; Shen, M.; Ren, Y.; Wen, H.; Li, J.; Rong, L.; Liu, W.; Xie, J. Controlling the Pasting, Rheological, Gel, and Structural Properties of Corn Starch by Incorporation of Debranched Waxy Corn Starch. Food Hydrocoll. 2022, 123, 107136. [Google Scholar] [CrossRef]
- Ssepuuya, G.; Jjoloba, W.; Nakamya, L.; Musalima, J.H.; Nakimbugwe, D.; Ssendagala, G.W. Impact of Food-to-Food Fortification by House Cricket Powder on the Sensory, Functional, and Nutritional Properties of Climate-Smart Cassava Flour. Food Biophys. 2025, 20, 22. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, S.; Shen, M.; Jiang, L.; Ren, Y.; Luo, Y.; Xie, J. Effect of Different Mesona chinensis Polysaccharides on Pasting, Gelation, Structural Properties and in Vitro Digestibility of Tapioca Starch-Mesona chinensis Polysaccharides Gels. Food Hydrocoll. 2020, 99, 105327. [Google Scholar] [CrossRef]
- Manley, S.; Davidovitch, B.; Davies, N.R.; Cipelletti, L.; Bailey, A.E.; Christianson, R.J.; Gasser, U.; Prasad, V.; Segre, P.N.; Doherty, M.P.; et al. Time-Dependent Strength of Colloidal Gels. Phys. Rev. Lett. 2005, 95, 048302. [Google Scholar] [CrossRef]
- Gordon, M.B.; Kloxin, C.J.; Wagner, N.J. Structural and Rheological Aging in Model Attraction-Driven Glasses by Rheo-SANS. J. Soft Matter 2021, 17, 924–935. [Google Scholar] [CrossRef] [PubMed]
- Anvari, M.; Joyner, H.S. Effect of Fish Gelatin and Gum Arabic Interactions on Concentrated Emulsion Large Amplitude Oscillatory Shear Behavior and Tribological Properties. Food Hydrocoll. 2018, 79, 518–525. [Google Scholar] [CrossRef]
- Su, C.; Li, D.; Wang, L.; Wang, Y. Development of Corn Starch-Sodium Alginate Emulsion Gels as Animal Fat Substitute: Effect of Oil Concentration. Food Hydrocoll. 2024, 157, 110439. [Google Scholar] [CrossRef]
- Petrova, V.A.; Elokhovskiy, V.Y.; Raik, S.V.; Poshina, D.N.; Romanov, D.P.; Skorik, Y.A. Alginate Gel Reinforcement with Chitin Nanowhiskers Modulates Rheological Properties and Drug Release Profile. Biomolecules 2019, 9, 291. [Google Scholar] [CrossRef]
- Hu, X.; Tang, Y.; Wang, Q.; Li, Y.; Yang, J.; Du, Y.; Kennedy, J.F. Rheological Behaviour of Chitin in NaOH/Urea Aqueous Solution. Carbohydr. Polym. 2011, 83, 1128–1133. [Google Scholar] [CrossRef]
- Lombardi, A.; Vecchio, R.; Borrello, M.; Caracciolo, F.; Cembalo, L. Willingness to Pay for Insect-Based Food: The Role of Information and Carrier. Food Qual. Prefer. 2019, 72, 177–187. [Google Scholar] [CrossRef]
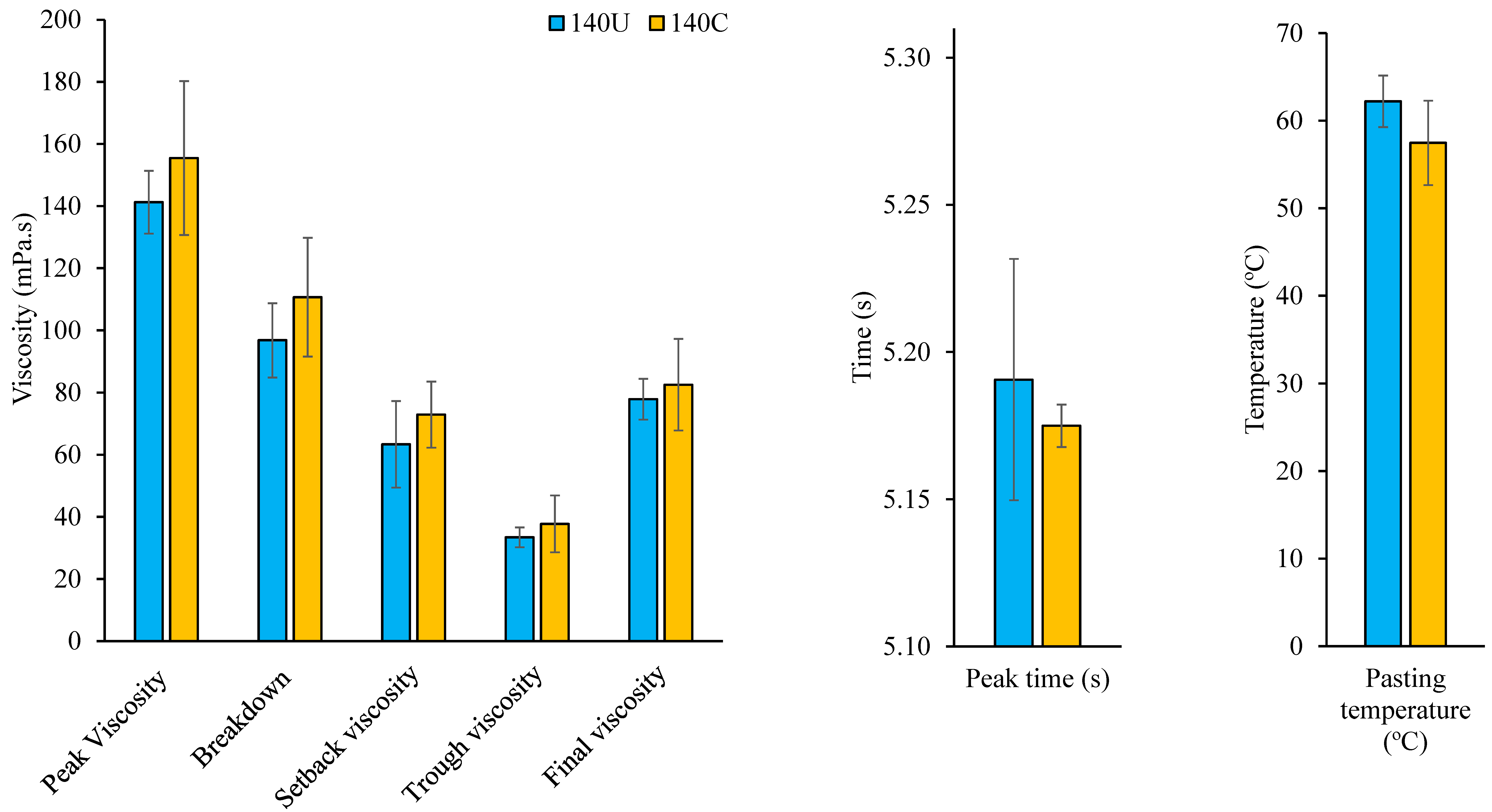
| Color Parameter | Sample Treatments | |
|---|---|---|
| 140U | 140C | |
| Lightness (L*) | 59.99 ± 0.13 a | 61.28 ± 0.33 b |
| Redness (a*) | 6.31 ± 0.00 a | 6.24 ± 0.06 a |
| Yellowness (b*) | 22.90 ± 0.09 a | 23.01 ± 0.19 a |
| Browning index (BI) | 54.85 ± 0.01 a | 53.62 ± 0.02 a |
| Chroma (C) | 23.75 ± 0.09 a | 23.84 ± 0.19 a |
| ΔC | −0.09 ± 0.20 | |
| ΔE | 1.30 ± 0.35 | |
| Compound | 140U | 140C | Sensory Attribute | Recognition Thresholds |
|---|---|---|---|---|
| Mean ± StDev | ||||
| Total aldehydes | 8067 ± 332 a | 7042 ± 760 a | ||
| Total saturated aldehydes | 6082 ± 273 a | 5489 ± 454 a | ||
| Hexanal | 4088 ± 228 a | 4021 ± 384 a | Green, apple, grassy, aldehydic, fresh, fruit, oil | 10 µg/L |
| Heptanal | 1092 ± 95 a | 495 ± 41 b | Citrus, fat, green, nut, floral, dry fish | 0.01 mg/kg |
| Nonanal | 353 ± 75 a | 423 ± 49 a | Citrus, fatty, green, aldehydic | 8 µg/L |
| Pentanal | 337 ± 49 a | 334 ± 28 a | 0.008 mg/kg | |
| Octanal | 138 ± 16 b | 184 ± 18 a | Citrus, grassy, green, fat, soap, lemon, mushroom, moldy | 6.9 µg/L |
| Total aromatic aldehydes | 1185 ± 82 a | 880 ± 137 b | ||
| Phenylacetaldehyde | 623 ± 79 a | 401 ± 97 b | Berry, geranium, honey, nut, pungent | 5.2 µg/L |
| Benzaldehyde | 544 ± 2 a | 468 ± 37 b | Fruity, sweet, bitter almond, burnt sugar, cherry, malt, roasted, pepper | 350 µg/kg |
| Safranal | 17.64 ± 1.06 a | 11.05 ± 2.39 b | ||
| Total unsaturated aldehydes | 569 ± 67 a | 577 ± 180 a | ||
| (E,E)-2,4-Decadienal | 280 ± 36 a | 309 ± 125 a | Baked, grease and oil | 0.07 µg/kg |
| 2-Heptenal | 55.67 ± 7.78 a | 69.11 ± 5.27 a | Grease and fruity | 13 µg/kg |
| 2-Octenal | 64.77 ± 17.35 a | 54.50 ± 12.91 a | Roasted pea nuts, fatty | 3 µg/kg |
| 2-Nonenal | 65.27 ± 13.06 a | 45.75 ± 23.94 a | Fatty, pungent | 0.69 µg/L |
| (E,Z)-2,4-Decadienal | 38.70 ± 1.76 a | 42.40 ± 18.49 a | Fatty, cooked grain, deep-fried | 0.07 µg/kg |
| 4-Heptenal | 36.08 ± 1.96 a | 21.84 ± 2.32 b | Fishy, fish-oil-like | 0.025 µg/L |
| 2-Decenal | 16.10 ± 1.70 a | 21.67 ± 7.54 a | ||
| 2-Butyl-2-octenal | 11.87 ± 4.96 a | 12.31 ± 3.42 a | ||
| Total branched aldehydes | 727 ± 129 a | 325 ± 101 b | ||
| 3-Methylbutanal | 446 ± 71 a | 180 ± 54 b | Aldehydic, ethereal, acrid, almond, chocolate, malty, pungent | 0.5 µg/L |
| 2-Methylbutanal | 281 ± 58 a | 145 ± 48 b | Chocolate, musty, nutty, malty, almond, fermented | 1.5 µg/L |
| 2-Methylpropanal | 73.35 ± 17.56 a | 32.00 ± 12.46 b | Aldehydic, caramel, cocoa, malt, nut | |
| Total furan derivatives | 6633 ± 323 a | 5174 ± 910 b | ||
| 2-Pentylfuran | 5601 ± 339 a | 4222 ± 836 b | Fruity, green, earthy, bean, buttery, fishy, grassy | 6 µg/kg |
| Furfural | 483 ± 12 a | 470 ± 26 a | Almond, baked potatoes, bread, burnt, spice, bready | 0.002–0.713 ppm |
| 2-Ethylfuran | 300 ± 13 a | 254 ± 19 b | ||
| 2-Butylfuran | 115 ± 17 a | 113 ± 30 a | ||
| 2-Methylfuran | 83.22 ± 5.48 a | 74.61 ± 13.64 b | Sweet, green, fruity | |
| 3-Methylfuran | 50.72 ± 0.26 a | 38.90 ± 5.53 b | ||
| Total benzene derivatives | 2402 ± 147 a | 2538 ± 443 a | ||
| Toluene | 1764 ± 72 a | 1932 ± 308 a | Sweet, pungent, benzene-like | 0.33 ppm |
| m-Xylene | 218 ± 42 a | 226 ± 48 a | 0.041 ppm | |
| o-Xylene | 182 ± 25 a | 185 ± 45 a | Sweet | 0.38 ppm |
| Ethylbenzene | 81.43 ± 7.98 a | 70.39 ± 18.93 a | Gasoline | 0.2 mg/kg |
| 1,2,3-Trimethylbenzene | 87.88 ± 7.27 a | 57.05 ± 7.63 b | Aromatic | 0.006–2.4 ppm |
| p-Xylene | 69.96 ± 6.15 a | 68.06 ± 19.50 a | Cold meat fat, metal | 0.058 ppm |
| Total alcohols | 1280 ± 191 a | 1260 ± 280 a | ||
| Total aliphatic alcohols | 797 ± 115 a | 920 ± 188 a | ||
| 1-Octene-3-ol | 267 ± 45 a | 259 ± 59 a | Earthy, fishy, fat, mould, mushroom | 1 µg/kg |
| 1-Hexanol | 194 ± 8 b | 285 ± 35 a | Herbal, flower, fruit, green, wood | 0.7 mg/kg |
| 2-Ethyl-1-hexanol | 177 ± 53 a | 185 ± 59 a | Citrus, green, flowery | |
| 1-Pentanol | 92.91 ± 2.14 a | 99.32 ± 14.96 a | Fermented, oily, sweet, vinegar | 5.0 mg/kg |
| 1-Heptanol | 41.12 ± 6.26 a | 58.79 ± 12.31 a | 0.2 mg/kg | |
| 1-Octanol | 24.91 ± 6.85 a | 33.57 ± 9.86 a | Fatty, waxy | 120 µg/kg |
| Total phenols | 483 ± 76 a | 340 ± 96 a | ||
| 2,4-Di-tert-butylphenol | 187 ± 24 a | 136 ± 37 a | ||
| 2-Methoxy-4-vinylphenol | 132 ± 47 a | 84.75 ± 51.56 a | Clove-like, smoky | 19 µg/L |
| 4-Vinylphenol | 99.81 ± 17.48 a | 83.86 ± 31.50 a | ||
| Phenol | 46.60 ± 5.49 a | 11.78 ± 1.51 b | Tarry | 0.0045–1.95 ppm |
| 4-(1-Methylpropyl) phenol | 16.99 ± 1.68 a | 23.33 ± 5.86 a | ||
| Total ketones | 1119 ± 39 a | 718 ± 101 b | ||
| 2-Heptanone | 462 ± 7 a | 246 ± 28 b | Cheesy, fruity, spicy, sweet | 0.14 mg/kg |
| 2-Nonanone | 201 ± 21 a | 144 ± 27 b | Fragrant, fruit, green, hot milk, cheese, coconut | 0.08 mg/kg |
| 2,3-Pentanedione | 91.01 ± 8.55 a | 74.37 ± 8.12 b | Buttery | |
| 6-Methyl-5-heptane-2-one | 103 ± 12 a | 54 ± 1 b | ||
| 2-Octanone | 78.20 ± 6.53 a | 46.90 ± 9.07 b | 0.04 mg/kg | |
| 2-Undecanone | 62.09 ± 20.66 a | 49.73 ± 22.03 a | Orange, grassy, fresh | 0.08 mg/kg |
| 2-Propanone | 56.03 ± 8.57 a | 40.94 ± 7.31 b | ||
| 2,3-Octanedione | 33.29 ± 7.69 a | 30.80 ± 5.83 a | Dill and earthy | |
| 3-Octene-2-one | 31.81 ± 0.65 a | 32.38 ± 3.88 a | Rose | |
| Total pyrazines and pyrroles | 1558 ± 34 a | 590 ± 23 b | ||
| 2,6-Dimethylpyrazine | 530 ± 26 a | 174 ± 11 b | Cooked meat | 1720 ng/L air |
| 2-Methylpyrazine | 361 ± 13 a | 135 ± 1 b | ||
| 2,5-Dimethyl-3-ethylpyrazine | 326 ± 21 a | 126 ± 16 b | 3.6 ng/L air | |
| 2,5-Dimethylpyrazine | 191 ± 13 a | 90 ± 6 b | Cocoa, roast beef, roasted nut, burnt, chocolate | 1820 ng/L air |
| Pyrazine | 70.70 ± 11.08 a | 37.00 ± 2.73 b | Bitter taste | 0.16 mg/kg |
| 2-Ethyl-5-methylpyrazine | 52.65 ± 4.97 a | 15.43 ± 2.38 b | ||
| 2-Formylpyrrole | 26.13 ± 4.94 a | 11.31 ± 2.23 b | ||
| Total aliphatic hydrocarbons | 1104 ± 77 a | 440 ± 104 b | ||
| 3-Ethyl-2-methyl-1,3-hexadiene | 974 ± 50 a | 324 ± 60 b | ||
| Pentane | 76.52 ± 27.01 a | 57.25 ± 30.67 a | Sweet | 1.29–1147 ppm |
| Octane | 38.80 ± 3.06 a | 41.42 ± 8.21 a | Gasoline, alkane | 0.66–235 ppm |
| Heptane | 14.55 ± 2.53 a | 16.74 ± 6.08 a | ||
| Total terpenes | 536 ± 52 a | 46 ± 21 b | ||
| Total sesquiterpenes | 502 ± 46a | 20 ± 16b | ||
| Ar-curcumene | 233 ± 23 a | 20.11 ± 15.87 b | ||
| a-Zingiberene | 203 ± 16 | n.d | ||
| b-Sesquiphellandrene | 55.91 ± 6.38 | n.d | ||
| b-Bisabolene | 10.18 ± 3.29 | n.d | ||
| Total monoterpene ketones | 33.0 ± 6.2 a | 26.2 ± 6.9 a | ||
| Geranyl acetone | 33.01 ± 6.19 a | 26.18 ± 6.88 a | ||
| Total lactones | 101 ± 7 a | 119 ± 25 a | ||
| g-Nonalactone | 101 ± 7 a | 119 ± 25 a | ||
| Total sulfur compounds | 144 ± 16 a | 48.6 ± 7.2 b | ||
| Dimethyl disulfide | 86.16 ± 5.32 a | 17.26 ± 2.14 b | Garlic, putrid, asparagus | 0.0022 ppm |
| Dimethyl sulfide | 57.56 ± 10.64 a | 31.34 ± 5.09 b | Sulfurous, onion, sweet | 0.0030 ppm |
| Total acids | 12.7 ± 5.2 a | 7.2 ± 0.7 a | ||
| Nonanoic acid | 12.68 ± 5.02 a | 7.18 ± 0.70 a | ||
| Total volatiles | 23450 ± 801 a | 18210 ± 2518 b | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bbosa, T.; Nakimbugwe, D.; Matthys, C.; Devaere, J.; De Winne, A.; Gunes, D.Z.; Van Der Borght, M. Physico-Chemical and Sensory Characteristics of Extruded Cereal Composite Flour Porridge Enriched with House Crickets (Acheta domesticus). Foods 2025, 14, 2893. https://doi.org/10.3390/foods14162893
Bbosa T, Nakimbugwe D, Matthys C, Devaere J, De Winne A, Gunes DZ, Van Der Borght M. Physico-Chemical and Sensory Characteristics of Extruded Cereal Composite Flour Porridge Enriched with House Crickets (Acheta domesticus). Foods. 2025; 14(16):2893. https://doi.org/10.3390/foods14162893
Chicago/Turabian StyleBbosa, Tom, Dorothy Nakimbugwe, Christophe Matthys, Jolien Devaere, Ann De Winne, Deniz Zeynel Gunes, and Mik Van Der Borght. 2025. "Physico-Chemical and Sensory Characteristics of Extruded Cereal Composite Flour Porridge Enriched with House Crickets (Acheta domesticus)" Foods 14, no. 16: 2893. https://doi.org/10.3390/foods14162893
APA StyleBbosa, T., Nakimbugwe, D., Matthys, C., Devaere, J., De Winne, A., Gunes, D. Z., & Van Der Borght, M. (2025). Physico-Chemical and Sensory Characteristics of Extruded Cereal Composite Flour Porridge Enriched with House Crickets (Acheta domesticus). Foods, 14(16), 2893. https://doi.org/10.3390/foods14162893










