Investigation of the Effect of NaCl Concentrations on the Formation of Amyloid Fibrils During the Cooking of Wheat Noodles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Noodles
2.3. Extraction of AFs
2.4. Thioflavin T Fluorescence (ThT)
2.5. Congo Red Staining Observation
2.6. Size Exclusion High-Performance Liquid Chromatography (SE-HPLC)
2.7. Fourier Transform Infrared (FTIR) Spectrum
2.8. Endogenous Fluorescent Light
2.9. Particle Size and ζ-Potential Analysis
2.10. Surface Hydrophobicity (H0)
2.11. Atomic Force Microscope (AFM)
2.12. Statistical Analysis
3. Results and Discussion
3.1. Formation of AFs
3.1.1. Analysis of ThT
3.1.2. Congo Red-Polarized Light Microscope Observation
3.2. Structural Characterization of Fibrils
3.2.1. Secondary Structure
3.2.2. Molecular Weight Distribution
3.2.3. Tertiary Structure
3.3. Physicochemical Properties of Fibrils
3.3.1. ζ-Potential
3.3.2. Average Particle Size
3.3.3. Surface Hydrophobicity
3.4. Morphological Analysis by Atomic Force Microscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFs | Amyloid fibrils |
| NaCl | Sodium chloride |
| ThT | Thioflavin T |
| FTIR | Fourier transform infrared spectroscopy |
| AFM | Atomic force microscopy |
| SE-HPLC | Size exclusion high-performance liquid chromatography |
| H0 | Surface hydrophobicity |
Appendix A
| Sample | A1(FU) | A2 (FU) | t1/2 (min) | Tc (min) | (df/dt) (FU min−1) | R2 |
|---|---|---|---|---|---|---|
| 0% | 9882.48 ± 1997.81 | 34,312.42 ± 998.09 | 0.67 ± 0.06 | 0.19 ± 0.04 | 31,325.25 ± 8359.19 | 0.979 |
| 0.4% | 11,572.59 ± 2183.15 | 39,193.17 ± 1187.69 | 0.72 ± 0.06 | 0.20 ± 0.05 | 35,171.12 ± 9823.06 | 0.978 |
| 1.6% | 15,053.99 ±1597.24 | 40,474.09 ± 1552.08 | 0.77 ± 0.04 | 0.09 ± 0.03 | 67,541.97 ± 25539.60 | 0.95 |
| 2.0% | 16,024.53 ± 2100.52 | 42,057.91 ± 2104.68 | 0.78 ± 0.05 | 0.08 ± 0.04 | 77,535.67 ± 40442.19 | 0.94 |
References
- Feng, Y.; Li, R.; Zhang, H.; Ren, F.; Liu, J.; Wang, J. Formation, structural characteristics and specific peptide identification of gluten amyloid fibrils. Food Chem. 2024, 445, 138648. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ma, C.-M.; Yang, Y.; Bian, X.; Liu, X.-F.; Wang, Y.; Zhang, N. Food-derived protein amyloid-like fibrils: Fibrillation mechanism, structure, and recent advances for the stabilization of emulsions. Food Hydrocoll. 2023, 145, 109146. [Google Scholar] [CrossRef]
- Falsafi, S.R.; Topuz, F.; Abbasi, Z.; Larki, M.; Rostamabadi, H. β-lactoglobulin amyloid fibrils: Architecture, preparation, characterization, and potential applications. Food Hydrocoll. 2025, 163, 111049. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Li, S.; Li, S.; Fang, Y.; Cao, Y. Formation and morphology of flaxseed protein isolate amyloid fibrils as governed by NaCl concentration. Food Hydrocoll. 2025, 166, 111300. [Google Scholar] [CrossRef]
- Li, T.; Wang, L.; Zhang, X.; Yu, P.; Chen, Z. Effect of ionic strength on assembly behaviors and rheological properties of rice glutelin based fibrils. J. Cereal Sci. 2021, 100, 103224. [Google Scholar] [CrossRef]
- Ji, F.; Xu, J.; Ouyang, Y.; Mu, D.; Li, X.; Luo, S.; Shen, Y.; Zheng, Z. Effects of NaCl concentration and temperature on fibrillation, structure, and functional properties of soy protein isolate fibril dispersions. Lwt 2021, 149, 111862. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Tang, C.-H.; Wen, Q.-B.; Yang, X.-Q.; Li, L.; Deng, W.-L. Thermal aggregation and gelation of kidney bean (Phaseolus vulgaris L.) protein isolate at pH 2.0: Influence of ionic strength. Food Hydrocoll. 2010, 24, 266–274. [Google Scholar] [CrossRef]
- Obadi, M.; Zhang, J.; Xu, B. The role of inorganic salts in dough properties and noodle quality—A review. Food Res. Int. 2022, 157, 111278. [Google Scholar] [CrossRef]
- Fan, H.; Fu, F.; Chen, Y.; Liu, M.; Ai, Z.; Bian, K. Effect of NaCl on rheological properties of dough and noodle quality. J. Cereal Sci. 2020, 93, 102936. [Google Scholar] [CrossRef]
- Rombouts, I.; Jansens, K.J.; Lagrain, B.; Delcour, J.A.; Zhu, K.-X. The impact of salt and alkali on gluten polymerization and quality of fresh wheat noodles. J. Cereal Sci. 2014, 60, 507–513. [Google Scholar] [CrossRef]
- Tan, H.-L.; Tan, T.-C.; Easa, A.M. Comparative study of cooking quality, microstructure, and textural and sensory properties between fresh wheat noodles prepared using sodium chloride and salt substitutes. Lwt 2018, 97, 396–403. [Google Scholar] [CrossRef]
- Wang, J.-R.; Guo, X.-N.; Xing, J.-J.; Zhu, K.-X. Revealing the effect mechanism of NaCl on the rheological properties of dough of Chinese traditional hand-stretched dried noodles. Food Chem. 2020, 320, 126606. [Google Scholar] [CrossRef]
- Wang, X.; Liang, Y.; Wang, Q.; Zhang, X.; Wang, J. Effect of low-sodium salt on the physicochemical and rheological properties of wheat flour doughs and their respective gluten. J. Cereal Sci. 2021, 102, 103371. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, H.; Jie, Y.; Liu, M.; He, B.; Wang, J. Amyloid-like Aggregation of Wheat Gluten and Its Components during Cooking: Mechanisms and Structural Characterization. J. Agric. Food Chem. 2024, 72, 11080–11093. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Song, J.; Wang, J.; Liu, H.; Wu, X.; He, B.; Zhang, X.; Wang, J. Investigating the effects of NaCl on the formation of AFs from gluten in cooked wheat noodles. Int. J. Mol. Sci. 2023, 24, 9907. [Google Scholar] [CrossRef]
- Liang, Y.; Qu, Z.; Liu, M.; Wang, J.; Zhu, M.; Liu, Z.; Li, J.; Zhan, X.; Jia, F. Effect of curdlan on the quality of frozen-cooked noodles during frozen storage. J. Cereal Sci. 2020, 95, 103019. [Google Scholar] [CrossRef]
- Lambrecht, M.A.; Monge-Morera, M.; Godefroidt, T.; Vluymans, N.; Deleu, L.J.; Goos, P.; Schymkowitz, J.; Rousseau, F.; Delcour, J.A. Hydrothermal treatments cause wheat gluten-derived peptides to form amyloid-like fibrils. J. Agric. Food Chem. 2021, 69, 1963–1974. [Google Scholar] [CrossRef] [PubMed]
- Loveday, S.; Wang, X.; Rao, M.; Anema, S.; Singh, H. β-Lactoglobulin nanofibrils: Effect of temperature on fibril formation kinetics, fibril morphology and the rheological properties of fibril dispersions. Food Hydrocoll. 2012, 27, 242–249. [Google Scholar] [CrossRef]
- Zandomeneghi, G.; Krebs, M.R.; McCammon, M.G.; Fändrich, M. FTIR reveals structural differences between native β-sheet proteins and amyloid fibrils. Protein Sci. 2004, 13, 3314–3321. [Google Scholar] [CrossRef]
- Han, C.; Ma, M.; Li, M.; Sun, Q. Further interpretation of the underlying causes of the strengthening effect of alkali on gluten and noodle quality: Studies on gluten, gliadin, and glutenin. Food Hydrocoll. 2020, 103, 105661. [Google Scholar] [CrossRef]
- Zou, M.; Yang, R.; Gu, Z.; Wang, P. Heat-triggered polymerization of frozen gluten: The micro-morphology and thermal characteristic study. J. Cereal Sci. 2019, 87, 185–193. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, X.; Gao, Y.; Zhang, Y.; Jiang, L.; Sui, X. Structural insights into acidic heating-induced amyloid fibrils derived from soy protein as a function of protein concentration. Food Hydrocoll. 2023, 145, 109085. [Google Scholar] [CrossRef]
- Cao, Y.; Mezzenga, R. Food protein amyloid fibrils: Origin, structure, formation, characterization, applications and health implications. Adv. Colloid Interface Sci. 2019, 269, 334–356. [Google Scholar] [CrossRef]
- Yu, Z.; Li, N.; Liu, Y.; Zhang, B.; Zhang, M.; Wang, X.; Wang, X. Formation, structure and functional characteristics of amyloid fibrils formed based on soy protein isolates. Int. J. Biol. Macromol. 2024, 254, 127956. [Google Scholar] [CrossRef]
- Abelein, A.; Jarvet, J.; Barth, A.; Graslund, A.; Danielsson, J. Ionic strength modulation of the free energy landscape of Aβ40 peptide fibril formation. J. Am. Chem. Soc. 2016, 138, 6893–6902. [Google Scholar] [CrossRef]
- Li, T.; Wang, D.; Zhang, X.; Chen, Z.; Wang, L. Specific ions effect on aggregation behaviors and structural changes of amyloid fibrils from rice glutelin. Food Chem. 2024, 441, 138351. [Google Scholar] [CrossRef]
- Wei, Z.; Huang, Q. Assembly of iron-bound ovotransferrin amyloid fibrils. Food Hydrocoll. 2019, 89, 579–589. [Google Scholar] [CrossRef]
- Jansens, K.J.; Lambrecht, M.A.; Rombouts, I.; Monge Morera, M.; Brijs, K.; Rousseau, F.; Schymkowitz, J.; Delcour, J.A. Conditions governing food protein amyloid fibril formation—Part I: Egg and cereal proteins. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1256–1276. [Google Scholar] [CrossRef] [PubMed]
- Wawer, J.; Szociński, M.; Olszewski, M.; Piątek, R.; Naczk, M.; Krakowiak, J. Influence of the ionic strength on the amyloid fibrillogenesis of hen egg white lysozyme. Int. J. Biol. Macromol. 2019, 121, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Adachi, M.; Muta, H.; So, M. Salt-induced formations of partially folded intermediates and amyloid fibrils suggests a common underlying mechanism. Biophys. Rev. 2018, 10, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Mikalauskaite, K.; Ziaunys, M.; Sneideris, T.; Smirnovas, V. Effect of ionic strength on thioflavin-T affinity to amyloid fibrils and its fluorescence intensity. Int. J. Mol. Sci. 2020, 21, 8916. [Google Scholar] [CrossRef]
- Feng, Y.; Li, R.; Zhang, H.; Wang, J. Investigation of self-assembly mechanism of gluten protein amyloid fibrils and molecular characterization of structure units. Food Chem. 2025, 479, 143637. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, S.; Jin, C.; Ren, F.; Wang, J. Wheat gluten amyloid fibrils: Conditions, mechanism, characterization, application, and future perspectives. Int. J. Biol. Macromol. 2023, 253, 126435. [Google Scholar] [CrossRef]
- Monge-Morera, M.; Lambrecht, M.A.; Deleu, L.J.; Louros, N.N.; Rousseau, F.; Schymkowitz, J.; Delcour, J.A. Heating wheat gluten promotes the formation of amyloid-like fibrils. ACS Omega 2021, 6, 1823–1833. [Google Scholar] [CrossRef]
- Morel, B.; Varela, L.; Azuaga, A.I.; Conejero-Lara, F. Environmental conditions affect the kinetics of nucleation of amyloid fibrils and determine their morphology. Biophys. J. 2010, 99, 3801–3810. [Google Scholar] [CrossRef]
- Huraskin, D.; Horn, A.H. Alkali ion influence on structure and stability of fibrillar amyloid-β oligomers. J. Mol. Model. 2019, 25, 37. [Google Scholar] [CrossRef]
- Juarez, J.; López, S.G.; Cambon, A.; Taboada, P.; Mosquera, V. Influence of electrostatic interactions on the fibrillation process of human serum albumin. J. Phys. Chem. B 2009, 113, 10521–10529. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Lin, Y.; Yagi, H.; Lee, Y.-H.; Kitayama, H.; Sakurai, K.; So, M.; Ogi, H.; Naiki, H.; Goto, Y. Distinguishing crystal-like amyloid fibrils and glass-like amorphous aggregates from their kinetics of formation. Proc. Natl. Acad. Sci. USA 2012, 109, 14446–14451. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, T.; Zhang, X.; Wang, L. Investigating the effects of ion strength on amyloid fibril formation of rice proteins. Food Biosci. 2023, 51, 102068. [Google Scholar] [CrossRef]
- Muzaffar, M.; Ahmad, A. The mechanism of enhanced insulin amyloid fibril formation by NaCl is better explained by a conformational change model. PLoS ONE 2011, 6, e27906. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Huang, L.-H. Effect of heat-induced formation of rice bran protein fibrils on morphological structure and physicochemical properties in solutions and gels. Food Sci. Biotechnol. 2014, 23, 1417–1423. [Google Scholar] [CrossRef]
- Wang, K.; Luo, S.; Cai, J.; Sun, Q.; Zhao, Y.; Zhong, X.; Jiang, S.; Zheng, Z. Effects of partial hydrolysis and subsequent cross-linking on wheat gluten physicochemical properties and structure. Food Chem. 2016, 197, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Wang, X.; Zhao, J.; Liang, X.; Zhang, Y.; Jiang, L.; Xu, Z.; Sui, X. Elucidating the modulatory influence of Hofmeister divalent ions on the structural dynamics and rheological properties of soy protein amyloid fibrils. Food Hydrocoll. 2024, 151, 109871. [Google Scholar] [CrossRef]
- Han, C.; Ma, M.; Yang, T.; Li, M.; Sun, Q. Heat mediated physicochemical and structural changes of wheat gluten in the presence of salt and alkali. Food Hydrocoll. 2021, 120, 106971. [Google Scholar] [CrossRef]
- Li, M.; Sun, Q.-J.; Han, C.-W.; Chen, H.-H.; Tang, W.-T. Comparative study of the quality characteristics of fresh noodles with regular salt and alkali and the underlying mechanisms. Food Chem. 2018, 246, 335–342. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Liu, M.; Zhang, X.; Liang, Y.; Wang, J. Effect of thermal treatment on the self-assembly of wheat gluten polypeptide. Molecules 2023, 28, 834. [Google Scholar] [CrossRef]
- Zheng, X.; Ren, C.; Wei, Y.; Wang, J.; Xu, X.; Du, M.; Wu, C. Soy protein particles with enhanced anti-aggregation behaviors under various heating temperatures, pH, and ionic strengths. Food Res. Int. 2023, 170, 112924. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, T.; Zhang, X.; Wang, L. Effect of different valence metal ions on rice protein fibrillation: Binding mechanism, structural characterization and rheology. Food Biophys. 2023, 18, 570–579. [Google Scholar] [CrossRef]
- Xu, J.; Tang, M.; Xu, X. Effect of ultrasound pretreatment on the fibrillization of oat globulins: Aggregation kinetics, structural evolution, and core composition. Food Hydrocoll. 2025, 165, 111233. [Google Scholar] [CrossRef]
- Tang, C.-H.; Wang, S.-S.; Huang, Q. Improvement of heat-induced fibril assembly of soy β-conglycinin (7S Globulins) at pH 2.0 through electrostatic screening. Food Res. Int. 2012, 46, 229–236. [Google Scholar] [CrossRef]
- Li, T.; Wang, L.; Geng, H.; Zhang, X.; Chen, Z. Formation, structural characteristics, foaming and emulsifying properties of rice glutelin fibrils. Food Chem. 2021, 354, 129554. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, Z.; Wang, F.; Wu, J.; Liu, Y.; Li, X. Characterization of rice glutelin fibrils and their effect on in vitro rice starch digestibility. Food Hydrocoll. 2020, 106, 105918. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Y.; Qi, G.; Li, Y.; Sun, X.S.; Qiu, D.; Li, Y. Formation and physicochemical properties of amyloid fibrils from soy protein. Int. J. Biol. Macromol. 2020, 149, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tang, C.-H. Heat-induced fibril assembly of vicilin at pH 2.0: Reaction kinetics, influence of ionic strength and protein concentration, and molecular mechanism. Food Res. Int. 2013, 51, 621–632. [Google Scholar] [CrossRef]
- McCann, T.H.; Day, L. Effect of sodium chloride on gluten network formation, dough microstructure and rheology in relation to breadmaking. J. Cereal Sci. 2013, 57, 444–452. [Google Scholar] [CrossRef]
- Jansens, K.J.; Lagrain, B.; Rombouts, I.; Brijs, K.; Smet, M.; Delcour, J.A. Effect of temperature, time and wheat gluten moisture content on wheat gluten network formation during thermomolding. J. Cereal Sci. 2011, 54, 434–441. [Google Scholar] [CrossRef]
- Meng, Y.; Wei, Z.; Xue, C. Protein fibrils from different food sources: A review of fibrillation conditions, properties, applications and research trends. Trends Food Sci. Technol. 2022, 121, 59–75. [Google Scholar] [CrossRef]
- Liu, G.; Zhong, Q. Dispersible and thermal stable nanofibrils derived from glycated whey protein. Biomacromolecules 2013, 14, 2146–2153. [Google Scholar] [CrossRef]
- Aubrey, L.D.; Blakeman, B.J.; Lutter, L.; Serpell, C.J.; Tuite, M.F.; Serpell, L.C.; Xue, W.-F. Quantification of amyloid fibril polymorphism by nano-morphometry reveals the individuality of filament assembly. Commun. Chem. 2020, 3, 125. [Google Scholar] [CrossRef]
- Misra, G.S. Introductory Polymer Chemistry; New Age International: Hong Kong, China, 1993. [Google Scholar]
- Loveday, S.M.; Anema, S.G.; Singh, H. β-Lactoglobulin nanofibrils: The long and the short of it. Int. Dairy J. 2017, 67, 35–45. [Google Scholar] [CrossRef]
- Loveday, S.; Wang, X.; Rao, M.; Anema, S.; Creamer, L.; Singh, H. Tuning the properties of β-lactoglobulin nanofibrils with pH, NaCl and CaCl2. Int. Dairy J. 2010, 20, 571–579. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, Y.; Li, H. Self-assembly of rice bran globulin fibrils in electrostatic screening: Nanostructure and gels. J. Nanomater. 2014, 2014, 951240. [Google Scholar] [CrossRef]


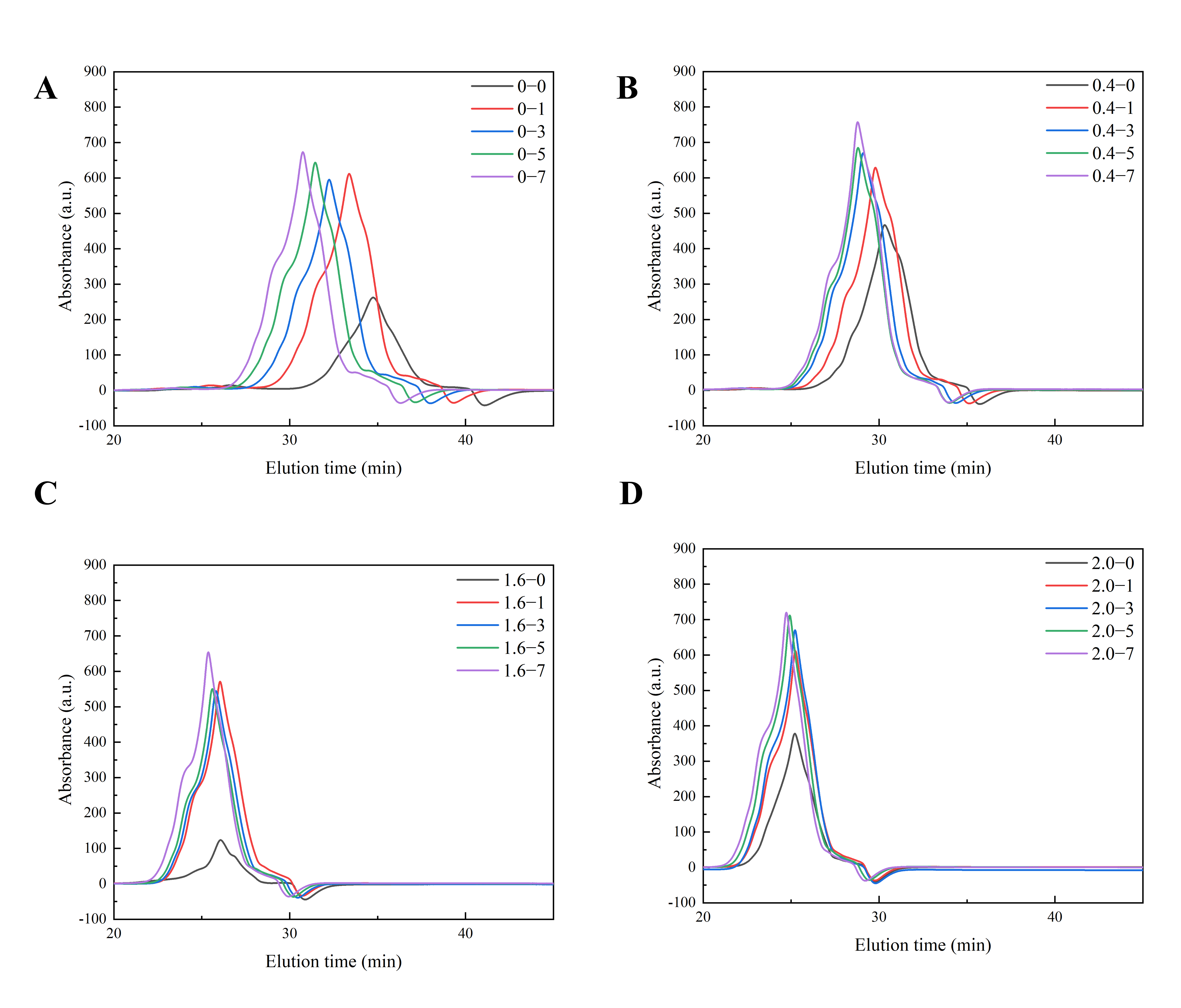
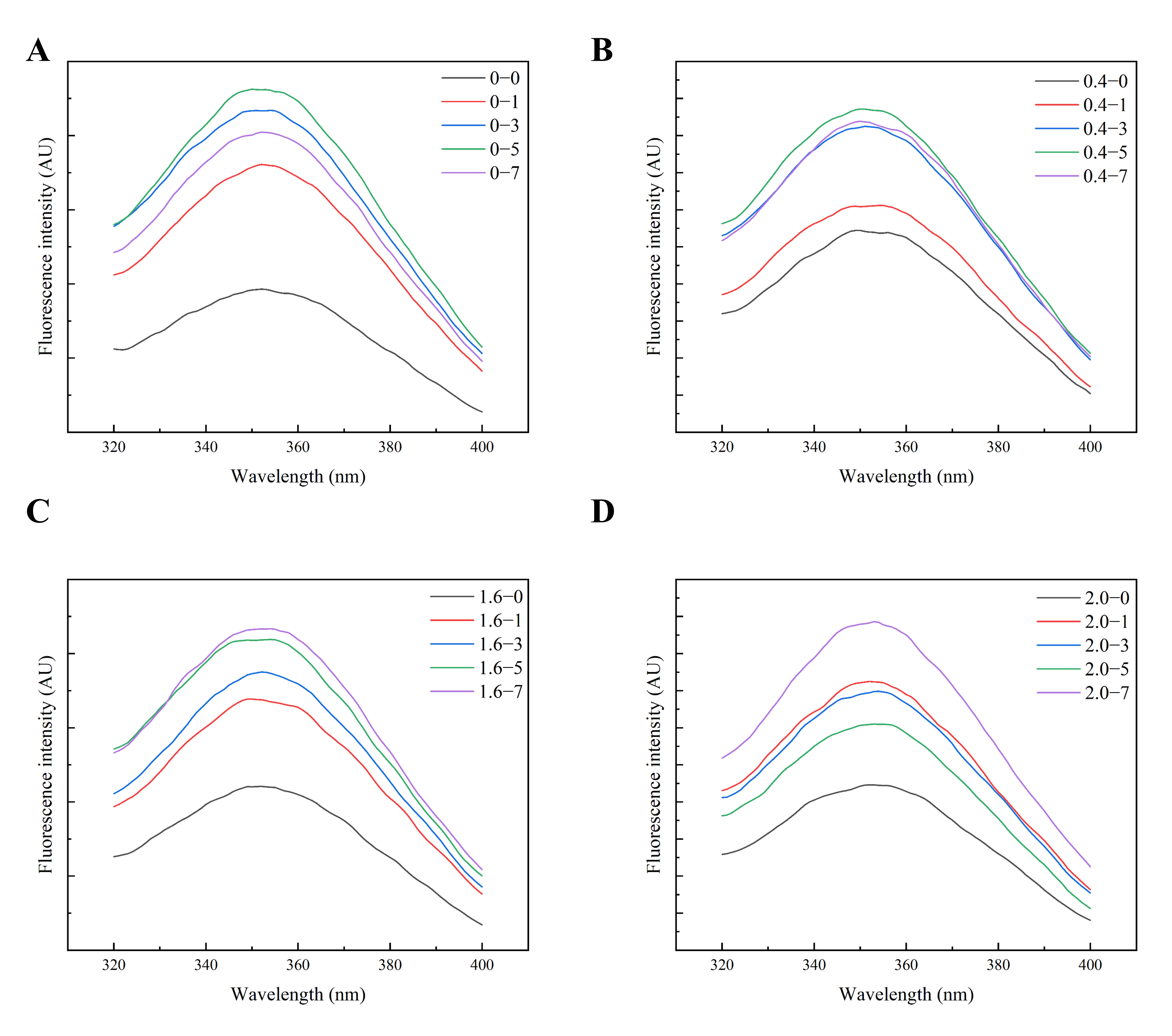


| Salt Concentration/% | Cooking Time/min | β-Sheet/% | Random Coil/% | Alpha-Helix/% | β-Turning Angle/% |
|---|---|---|---|---|---|
| 0 | 0 | 29.8 ± 0.07 Db | 13.6 ± 0.14 Aa | 18.0 ± 0.04 Aa | 38.6 ± 0.07 Aa |
| 1 | 31.2 ± 0.14 Cb | 12.3 ± 0.12 Ab | 18.8 ± 0.03 Aa | 37.8 ± 0.07 ABa | |
| 3 | 31.5 ± 0.35 Cc | 11.1 ± 0.25 Ac | 19.7 ± 0.22 Aa | 37.8 ± 0.28 ABa | |
| 5 | 33.3 ± 0.57 Bb | 13.0 ± 3.21 Ab | 17.3 ± 2.56 Aa | 36.4 ± 0.07 ABb | |
| 7 | 37.2 ± 0.85 Ab | 11.3 ± 2.60 Ab | 20.8 ± 2.74 Aa | 30.8 ± 6.15 Bab | |
| 0.4 | 0 | 36.6 ± 0.07 Da | 14.5 ± 0.05 Da | 15.8 ± 0.05 Ab | 33.1 ± 0.07 Aa |
| 1 | 39.1 ± 0.00 Ca | 17.7 ± 0.00 Ca | 17.4 ± 0.00 Aa | 25.8 ± 0.00 Bb | |
| 3 | 39.5 ± 0.28 BCa | 17.7 ± 0.56 Ca | 20.3 ± 4.55 Aa | 22.5 ± 4.28 Bc | |
| 5 | 39.9 ± 0.41 Ba | 19.7 ± 0.59 Ba | 16.1 ± 1.39 Aa | 24.3 ± 0.41 Bc | |
| 7 | 41.5 ± 0.04 Aa | 21.8 ± 0.24 Aa | 14.7 ± 0.15 Ab | 22.0 ± 0.13 Bb | |
| 1.6 | 0 | 27.8 ± 2.28 Cb | 16.2 ± 4.82 Aa | 12.3 ± 1.42 Cc | 39.4 ± 5.30 Aa |
| 1 | 38.6 ± 1.41 Aa | 11.6 ± 1.29 Ab | 20.5 ± 0.27 ABa | 29.4 ± 0.42 Aab | |
| 3 | 34.0 ± 0.28 Bb | 12.6 ± 0.61 Ab | 23.2 ± 0.11 Aa | 30.2 ± 0.99 Ab | |
| 5 | 33.3 ± 0.57 Bb | 12.1 ± 0.77 Ab | 19.1 ± 0.53 Ba | 35.5 ± 0.78 Ab | |
| 7 | 33.2 ± 1.84 Bc | 12.9 ± 2.07 Ab | 21.1 ± 2.46 ABa | 32.8 ± 6.36 Aab | |
| 2.0 | 0 | 28.9 ± 1.34 Bb | 13.1 ± 0.68 ABa | 18.1 ± 0.62 Aa | 39.9 ± 1.27 Aa |
| 1 | 32.5 ± 1.48 Ab | 12.9 ± 1.84 ABb | 21.3 ± 2.91 Aa | 33.4 ± 6.22 Aab | |
| 3 | 30.0 ± 0.42 ABd | 12.3 ± 0.24 ABbc | 18.9 ± 0.19 Aa | 38.8 ± 0.42 Aa | |
| 5 | 30.7 ± 0.36 ABc | 11.0 ± 0.56 Bb | 20.0 ± 0.42 Aa | 38.2 ± 0.47 Aa | |
| 7 | 29.2 ± 0.35 Bd | 14.2 ± 0.31 Ab | 17.9 ± 0.03 Aab | 38.7 ± 0.07 Aa |
| Heating Time/min | 0% | 0.4% | 1.6% | 2.0% |
|---|---|---|---|---|
| 0 | 1398 ± 193 cB | 3796 ± 366 cA | 1099 ± 282 bB | 1670 ± 321 cB |
| 1 | 6219 ± 32 bA | 4971 ± 248 cB | 6246 ± 468 aA | 4976 ± 676 bB |
| 3 | 6412 ± 208 bA | 6456 ± 223 bA | 6432 ± 534 aA | 5854 ± 381 abA |
| 5 | 6861 ± 398 bB | 8726 ± 536 aA | 5818 ± 377 aC | 6166 ± 341 aBC |
| 7 | 8553 ± 788 aA | 9980 ± 935 aA | 5472 ± 472 aB | 5923 ± 295 abB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Zheng, C.; Yang, L.; Huang, M.; Liu, J.; Liu, H.; He, B.; Wang, J. Investigation of the Effect of NaCl Concentrations on the Formation of Amyloid Fibrils During the Cooking of Wheat Noodles. Foods 2025, 14, 2892. https://doi.org/10.3390/foods14162892
Liang Y, Zheng C, Yang L, Huang M, Liu J, Liu H, He B, Wang J. Investigation of the Effect of NaCl Concentrations on the Formation of Amyloid Fibrils During the Cooking of Wheat Noodles. Foods. 2025; 14(16):2892. https://doi.org/10.3390/foods14162892
Chicago/Turabian StyleLiang, Ying, Chunlei Zheng, Liu Yang, Minqian Huang, Jiajia Liu, Hao Liu, Baoshan He, and Jinshui Wang. 2025. "Investigation of the Effect of NaCl Concentrations on the Formation of Amyloid Fibrils During the Cooking of Wheat Noodles" Foods 14, no. 16: 2892. https://doi.org/10.3390/foods14162892
APA StyleLiang, Y., Zheng, C., Yang, L., Huang, M., Liu, J., Liu, H., He, B., & Wang, J. (2025). Investigation of the Effect of NaCl Concentrations on the Formation of Amyloid Fibrils During the Cooking of Wheat Noodles. Foods, 14(16), 2892. https://doi.org/10.3390/foods14162892





