Adding Value to Brewery Industry By-Products as Novel Ingredients in Non-Alcoholic Malt Beverage Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Proximate Composition
2.3. Production of Protein Hydrolysates
2.4. Water Solubility (WS) and Water Binding Capacity (WBC)
2.5. Emulsion Capacity (EC)
2.6. Amino Acid Composition and Size Exclusion Chromatography
2.7. Preparation of the Beverages
2.8. Color Analyses of the Beverages
2.9. Viscosity of the Beverages
2.10. Turbidity of the Beverages
2.11. Foaming Capacity and Foam Stability of the Beverages
2.12. Statistical Analysis
3. Results and Discussion
3.1. Water Solubility, Water Binding Capacity and Emulsion Capacity
3.2. Amino Acid Composition and Size Exclusion Chromatography Analyses
3.3. Color Analyses
3.4. Kinematic Viscosity
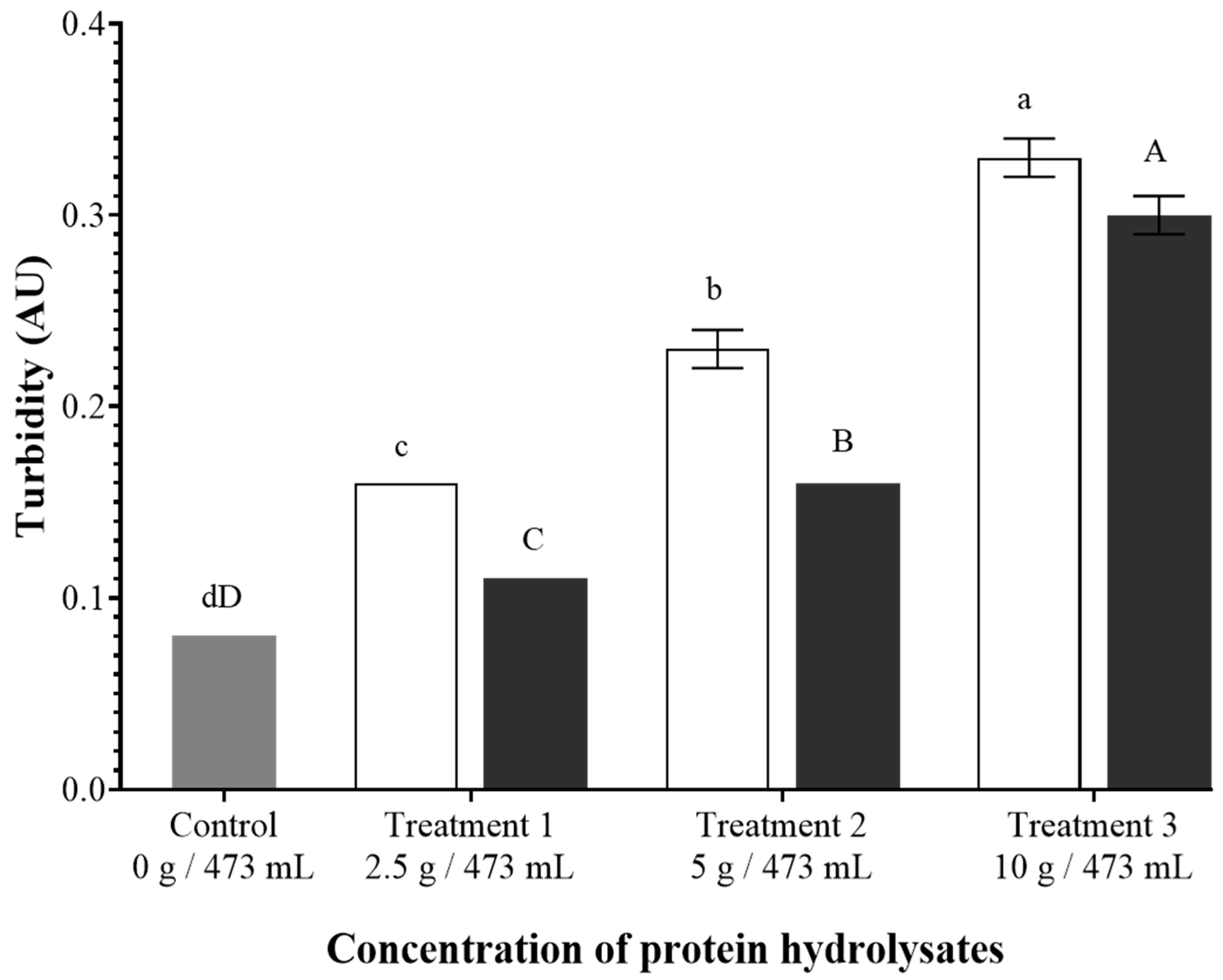
3.5. Turbidity
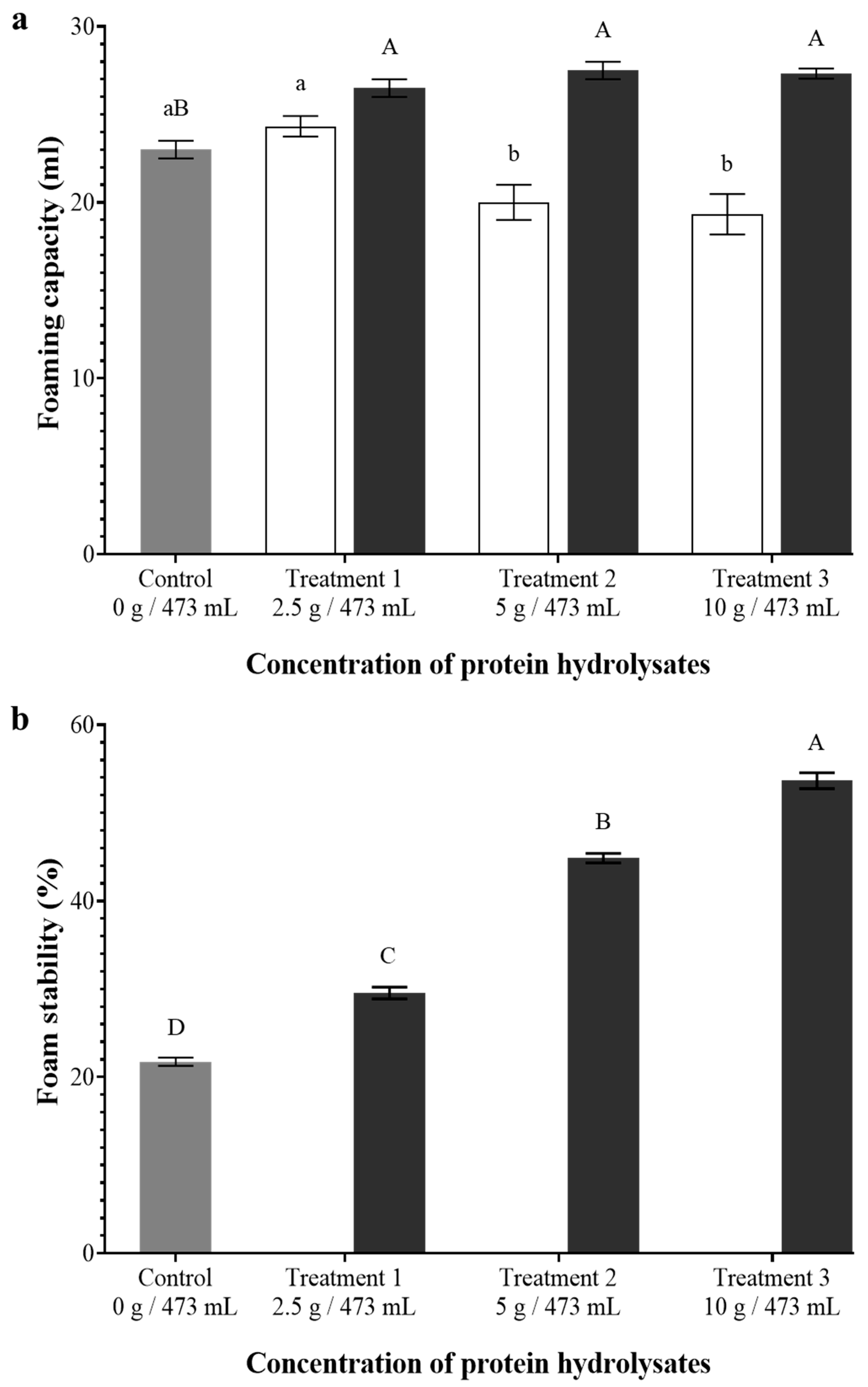
3.6. Foaming Capacity and Foam Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hejna, A.; Barczewski, M.; Kosmela, P.; Aniśko, J.; Szulc, J.; Skórczewska, K.; Piasecki, A.; Kuang, T. More than just a beer–Brewers’ spent grain, spent hops, and spent yeast as potential functional fillers for polymer composites. Waste Manag. 2024, 180, 23–35. [Google Scholar] [CrossRef]
- Casas-Godoy, L.; González-Escobar, J.L.; Mathis, A.G.; Barrera-Martínez, I. Revalorization of untreated Brewer’s spent grain: Novel and versatile feedstock to produce cellulases, lipases, and yeast biomass in a biorefinery approach. Biomass Convers. Biorefin. 2023, 13, 1659–1670. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Duan, Y.; Zhang, H.; Ma, H. A Mini-Review on Brewer’s Spent Grain Protein: Isolation, Physicochemical Properties, Application of Protein, and Functional Properties of Hydrolysates. J. Food Sci. 2019, 84, 3330–3340. [Google Scholar] [CrossRef] [PubMed]
- Puligundla, P.; Mok, C. Recent advances in biotechnological valorization of brewers’ spent grain. Food Sci. Biotechnol. 2021, 30, 341–353. [Google Scholar] [CrossRef]
- Puligundla, P.; Mok, C.; Park, S. Advances in the valorization of spent brewer’s yeast. Innov. Food Sci. Emerg. Technol. 2020, 62, 102350. [Google Scholar] [CrossRef]
- Łukaszewicz, M.; Leszczyński, P.; Jabłoński, S.J.; Kawa-Rygielska, J. Potential Applications of Yeast Biomass Derived from Small-Scale Breweries. Appl. Sci. 2024, 14, 2529. [Google Scholar] [CrossRef]
- Ikram, S.; Huang, L.; Zhang, H.; Wang, J.; Yin, M. Composition and nutrient value proposition of brewers spent grain. J. Food Sci. 2017, 82, 2232–2242. [Google Scholar] [CrossRef]
- Bachmann, S.A.L.; Calvete, T.; Féris, L.A. Potential applications of brewery spent grain: Critical an overview. J. Environ. Chem. Eng. 2022, 10, 106951. [Google Scholar] [CrossRef]
- Jin, Z.; Lan, Y.; Ohm, J.B.; Gillespie, J.; Schwarz, P.; Chen, B. Physicochemical composition, fermentable sugars, free amino acids, phenolics, and minerals in brewers’ spent grains obtained from craft brewing operations. J. Cereal Sci. 2022, 104, 103413. [Google Scholar] [CrossRef]
- Yitayew, T.; Moges, D.; Satheesh, N. Effect of Brewery Spent Grain Level and Fermentation Time on the Quality of Bread. Int. J. Food Sci. 2022, 2022, 8704684. [Google Scholar] [CrossRef] [PubMed]
- Vieira, E.; Rocha, M.A.M.; Coelho, E.; Pinho, O.; Saraiva, J.A.; Ferreira, I.M.; Coimbra, M.A. Valuation of brewer’s spent grain using a fully recyclable integrated process for extraction of proteins and arabinoxylans. Ind. Crops Prod. 2014, 52, 136–143. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Ferreira, C.; Pereira, J.O.; Pintado, M.E.; Carvalho, A.P. Spent brewer’s yeast (Saccharomyces cerevisiae) as a potential source of bioactive peptides: An overview. Int. J. Biol. Macromol. 2022, 208, 1116–1126. [Google Scholar] [CrossRef]
- Jeon, Y.H.; Gu, B.J.; Ryu, G.H. Effects of Brewer’s Spent Yeast Content on the Physicochemical Properties of Extruded High-Moisture Meat Analog. J. Korean Soc. Food Sci. Nutr. 2022, 51, 1084–1090. [Google Scholar] [CrossRef]
- Guo, H.; Guo, S.; Liu, H. Antioxidant activity and inhibition of ultraviolet radiation-induced skin damage of Selenium-rich peptide fraction from selenium-rich yeast protein hydrolysate. Bioorg. Chem. 2020, 105, 104431. [Google Scholar] [CrossRef]
- Marson, G.V.; da Costa Machado, M.T.; de Castro, R.J.S.; Hubinger, M.D. Sequential hydrolysis of spent brewer’s yeast improved its physico-chemical characteristics and antioxidant properties: A strategy to transform waste into added-value biomolecules. Process Biochem. 2019, 84, 91–102. [Google Scholar] [CrossRef]
- Kriisa, M.; Taivosalo, A.; Föste, M.; Kütt, M.L.; Viirma, M.; Priidik, R.; Korzeniowska, M.; Tian, Y.; Laaksonen, O.; Yang, B. Effect of enzyme-assisted hydrolysis on brewer’s spent grain protein solubilization–peptide composition and sensory properties. Appl. Food Res. 2022, 2, 100108. [Google Scholar] [CrossRef]
- San Martin, D.; Ibarruri, J.; Iñarra, B.; Luengo, N.; Ferrer, J.; Alvarez-Ossorio, C.; Bald, C.; Gutierrez, M.; Zufía, J. Valorisation of brewer’s spent yeasts’ hydrolysates as high-value bioactive molecules. Sustainability 2021, 13, 6520. [Google Scholar] [CrossRef]
- Kadam, D.; Kadam, A.; Koksel, F.; Aluko, R.E. Plant-derived bioactive peptides: A comprehensive review. Sustain. Food Proteins 2024, 2, 183–214. [Google Scholar] [CrossRef]
- AACC. Approved Methods of Analysis, 11th ed.; Cereals&Grains Association: St. Paul, MN, USA, 2010. [Google Scholar]
- Markwell, M.A.K.; Haas, S.M.; Bieber, L.; Tolbert, N. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 1978, 87, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Asen, N.D.; Aluko, R.E. Acetylcholinesterase and butyrylcholinesterase inhibitory activities of antioxidant peptides obtained from enzymatic pea protein hydrolysates and their ultrafiltration peptide fractions. J. Food Biochem. 2022, 46, e14289. [Google Scholar] [CrossRef]
- Müller, J. Dumas or Kjeldahl for Reference Analysis; FOSS: Hilleroed, Denmark, 2017. [Google Scholar]
- Mathias, T.R.d.S.; Alexandre, V.M.F.; Cammarota, M.C.; de Mello, P.P.M.; Sérvulo, E.F.C. Characterization and determination of brewer’s solid wastes composition. J. Inst. Brew. 2015, 121, 400–404. [Google Scholar] [CrossRef]
- Li, X.; Masatcioglu, M.T.; Koksel, F. Physical and functional properties of wheat flour extrudates produced by nitrogen injection assisted extrusion cooking. J. Cereal Sci. 2019, 89, 102811. [Google Scholar] [CrossRef]
- Luo, S.; Koksel, F. Physical and technofunctional properties of yellow pea flour and bread crumb mixtures processed with low moisture extrusion cooking. J. Food Sci. 2020, 85, 2688–2698. [Google Scholar] [CrossRef] [PubMed]
- AOAC International. Official Methods of Analysis of AOAC International, 19th ed.; 2012; Volume 45, pp. 75–76. [Google Scholar]
- Astephen, N. Waters AccQ Tag method for hydrolysate amino acid analysis. In Waters Application Notebook; Waters Corporation: Milford, MA, USA, 2018. [Google Scholar]
- ISO 13904:2016; Animal Feeding Stuffs–Determination of Tryptophan Content. ISO (International Organization for Standardization): Geneva, Switzerland, 2016.
- Alashi, A.M.; Wu, H.; Aluko, R.E. Indigestible cowpea proteins reduced plasma cholesterol after long-term oral administration to Sprague-Dawley rats. Food Prod. Process. Nutr. 2021, 3, 16. [Google Scholar] [CrossRef]
- Li, Y.; Maurice, M.J.S. Development of a fast and reliable microwave-based assay for measurement of malt color. J. Am. Soc. Brew. Chem. 2013, 71, 144–148. [Google Scholar] [CrossRef]
- Koksel, F.; Masatcioglu, M.T. Physical properties of puffed yellow pea snacks produced by nitrogen gas assisted extrusion cooking. LWT 2018, 93, 592–598. [Google Scholar] [CrossRef]
- Sae-Leaw, T.; Aluko, R.E.; Chantakun, K.; Benjakul, S. Physicochemical, antioxidant and sensory properties of ready-to-drink chrysanthemum tea fortified with hydrolyzed collagen from salmon scale ossein. J. Aquat. Food Prod. Technol. 2021, 30, 1159–1172. [Google Scholar] [CrossRef]
- Aluko, R.E.; Mofolasayo, O.A.; Watts, B.M. Emulsifying and foaming properties of commercial yellow pea (Pisum sativum L.) seed flours. J. Agric. Sci. Technol. 2009, 57, 9793–9800. [Google Scholar]
- Chin, Y.L.; Keppler, J.K.; Dinani, S.T.; Chen, W.N.; Boom, R. Brewers’ spent grain proteins: The extraction method determines the functional properties. Innov. Food Sci. Emerg. Technol. 2024, 94, 103666. [Google Scholar] [CrossRef]
- Marson, G.V.; de Castro, R.J.S.; Machado, M.T.d.C.; da Silva Zandonadi, F.; Barros, H.D.d.F.Q.; Maróstica Júnior, M.R.; Sussulini, A.; Hubinger, M.D. Proteolytic enzymes positively modulated the physicochemical and antioxidant properties of spent yeast protein hydrolysates. Process Biochem. 2020, 91, 34–45. [Google Scholar] [CrossRef]
- Pérez-Gálvez, R.; Maldonado-Valderrama, J.; Jones, N.C.; Hoffmann, S.V.; Guadix, E.; García-Moreno, P.J. Influence of the enzymatic treatment and pH on the interfacial and emulsifying properties of sunflower and olive protein hydrolysates. Food Hydrocoll. 2024, 154, 110135. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Owolabi, I.O.; Fadairo, O.S.; Ghosal, A.; Coker, O.J.; Soladoye, O.P.; Aluko, R.E.; Bandara, N. Enzymatic Modification of Plant Proteins for Improved Functional and Bioactive Properties. Food Bioproc. Technol. 2023, 16, 1216–1234. [Google Scholar] [CrossRef]
- de Castro, R.J.S.; Sato, H.H. Comparison and synergistic effects of intact proteins and their hydrolysates on the functional properties and antioxidant activities in a simultaneous process of enzymatic hydrolysis. Food Bioprod. Process. 2014, 92, 80–88. [Google Scholar] [CrossRef]
- Wagoner, T.B.; Ward, L.; Foegeding, E.A. Using state diagrams for predicting colloidal stability of whey protein beverages. J. Agric. Sci. Technol. 2015, 63, 4335–4344. [Google Scholar] [CrossRef] [PubMed]
- Abeynayake, R.; Zhang, S.; Yang, W.; Chen, L. Development of antioxidant peptides from brewers’ spent grain proteins. LWT 2022, 158, 113162. [Google Scholar] [CrossRef]
- Grosso, A.L.; Cestonaro, G.; Scampicchio, M.; Ferrentino, G.; Costanzo, E. Development of a protein-enriched oat beverage: Enhancing antioxidant activity, functional properties and stability with soy protein hydrolysates. LWT 2025, 222, 117644. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, D.; Huang, Y.; Chen, L.; Bao, P.; Fang, H.; Xu, B.; Zhou, C. Effects of basic amino acid on the tenderness, water binding capacity and texture of cooked marinated chicken breast. LWT 2020, 129, 109524. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Q.; Liu, Z.; Zhi, L.; Jiao, B.; Hu, H.; Ma, X.; Agyei, D.; Shi, A. Plant protein-based emulsifiers: Mechanisms, techniques for emulsification enhancement and applications. Food Hydrocoll. 2023, 144, 109008. [Google Scholar] [CrossRef]
- Arteaga, V.G.; Guardia, M.A.; Muranyi, I.; Eisner, P.; Schweiggert-Weisz, U. Effect of enzymatic hydrolysis on molecular weight distribution, techno-functional properties and sensory perception of pea protein isolates. Innov. Food Sci. Emerg. Technol. 2020, 65, 102449. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Ferreira, C.; Pereira, J.O.; Pintado, M.E.; Carvalho, A.P. Valorisation of protein-rich extracts from spent brewer’s yeast (Saccharomyces cerevisiae): An overview. Biomass Convers. Biorefin. 2025, 15, 1771–1793. [Google Scholar] [CrossRef]
- Alfaro-Diaz, A.; Escobedo, A.; Luna-Vital, D.A.; Castillo-Herrera, G.; Mojica, L. Common beans as a source of food ingredients: Techno-functional and biological potential. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2910–2944. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Sarkar, R. Site-selective cleavage of peptides and proteins targeting aromatic amino acid residues. RSC Adv. 2025, 15, 9159–9179. [Google Scholar] [CrossRef]
- Delphine, N.; Micaël, B.E.; Christelle, A.I.; Charlotte, E.A. Nutritional Potential of Spent Brewer’s Yeast, A Residual By-Product of Beer Production in Breweries for Future Applications. J. Adv. Biol. Biotechnol. 2023, 26, 30–39. [Google Scholar] [CrossRef]
- Egerton, S.; Culloty, S.; Whooley, J.; Stanton, C.; Ross, R.P. Characterization of protein hydrolysates from blue whiting (Micromesistius poutassou) and their application in beverage fortification. Food Chem. 2018, 245, 698–706. [Google Scholar] [CrossRef]
- Lee, H.M.; Thai, T.D.; Lim, W.; Ren, J.; Na, D. Functional small peptides for enhanced protein delivery, solubility, and secretion in microbial biotechnology. J. Biotechnol. 2023, 375, 40–48. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, F.; Zhao, M.; Ning, Z.; Sun-Waterhouse, D.; Sun, B. Soy peptide aggregates formed during hydrolysis reduced protein extraction without decreasing their nutritional value. Food Funct. 2017, 8, 4384–4395. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.L.; Chai, K.F.; Chen, W.N. Upcycling of brewers’ spent grains via solid-state fermentation for the production of protein hydrolysates with antioxidant and techno-functional properties. Food Chem. X 2022, 13, 100184. [Google Scholar] [CrossRef] [PubMed]
- Devi, M.K.; Das, S.K. Kinetics of color changes of popped rice during microwave popping: Effect of salt and moisture content. J. Food Process Eng. 2017, 40, e12560. [Google Scholar] [CrossRef]
- Bazsefidpar, N.; Ghandehari Yazdi, A.P.; Karimi, A.; Yahyavi, M.; Amini, M.; Ahmadi Gavlighi, H.; Simal-Gandara, J. Brewers spent grain protein hydrolysate as a functional ingredient for muffins: Antioxidant, antidiabetic, and sensory evaluation. Food Chem. 2024, 435, 137565. [Google Scholar] [CrossRef]
- Kotlar, C.E.; Ponce, A.G.; Roura, S.I. Improvement of functional and antimicrobial properties of brewery byproduct hydrolysed enzymatically. LWT-Food Sci. Technol. 2013, 50, 378–385. [Google Scholar] [CrossRef]
- Dridi, C.; Millette, M.; Aguilar, B.; Salmieri, S.; Lacroix, M. Storage Stability of a Fermented Probiotic Beverage Enriched with Cricket Protein Hydrolysates. Food Bioproc. Technol. 2022, 15, 2587–2600. [Google Scholar] [CrossRef]
- Lozano, J.E. Color, turbidity, and other sensorial and structural properties of fruits and fruit products. In Fruit Manufacturing: Scientific Basis, Engineering Properties, and Deteriorative Reactions of Technological Importance; Springer: New York, NY, USA, 2006; pp. 99–132. [Google Scholar]
- Olšovská, J.; Kyselová, L.; Kubizniaková, P.; Slabý, M. Non-microbiological turbidity of beer: Part 1–semireview. Kvasny Prum. 2021, 67, 484–497. [Google Scholar] [CrossRef]
- Hosseini, E.; Kadivar, M.; Shahedi, M. Physicochemical Properties and Storability of Non-alcoholic Malt Drinks Prepared from Oat and Barley Malts. J. Agric. Sci. Technol. 2012, 14, 173–182. [Google Scholar]
- Yu, J.; Ahmedna, M.; Goktepe, I. Peanut protein concentrate: Production and functional properties as affected by processing. Food Chem. 2007, 103, 121–129. [Google Scholar] [CrossRef]
- Bao, Z.J.; Zhao, Y.; Wang, X.Y.; Chi, Y.J. Effects of degree of hydrolysis (DH) on the functional properties of egg yolk hydrolysate with alcalase. J. Food Sci. Technol. 2017, 54, 669–678. [Google Scholar] [CrossRef]
- Odelli, D.; Sarigiannidou, K.; Soliani, A.; Marie, R.; Mohammadifar, M.A.; Jessen, F.; Spigno, G.; Vall-Llosera, M.; de Carvalho, A.F.; Verni, M. Interaction between fish skin gelatin and pea protein at air-water interface after ultrasound treatment. Foods 2022, 11, 659. [Google Scholar] [CrossRef]
- Wouters, A.G.; Rombouts, I.; Legein, M.; Fierens, E.; Brijs, K.; Blecker, C.; Delcour, J.A. Air–water interfacial properties of enzymatic wheat gluten hydrolyzates determine their foaming behavior. Food Hydrocoll. 2016, 55, 155–162. [Google Scholar] [CrossRef]
- Vieira, M.C.; Brandelli, A.; Thys, R.C.S. Evaluation of the technological functional properties and antioxidant activity of protein hydrolysate obtained from brewers’ spent grain. J. Food Process. Preserv. 2022, 46, e16638. [Google Scholar] [CrossRef]
- Connolly, A.; Piggott, C.O.; FitzGerald, R.J. Technofunctional properties of a brewers’ spent grain protein-enriched isolate and its associated enzymatic hydrolysates. LWT-Food Sci. Technol. 2014, 59, 1061–1067. [Google Scholar] [CrossRef]
- Yalçın, E.; Çelik, S.; İbanoğlu, E. Foaming properties of barley protein isolates and hydrolysates. Eur. Food Res. Technol. 2008, 226, 967–974. [Google Scholar] [CrossRef]
- Allegretti, C.; Bellinetto, E.; D’Arrigo, P.; Griffini, G.; Marzorati, S.; Rossato, L.A.M.; Ruffini, E.; Schiavi, L.; Serra, S.; Strini, A. Towards a complete exploitation of brewers’ spent grain from a circular economy perspective. Fermentation 2022, 8, 151. [Google Scholar] [CrossRef]
- Zeko-Pivač, A.; Tišma, M.; Žnidaršič-Plazl, P.; Kulisic, B.; Sakellaris, G.; Hao, J.; Planinić, M. The potential of brewer’s spent grain in the circular bioeconomy: State of the art and future perspectives. Front. Bioeng. Biotechnol. 2022, 10, 870744. [Google Scholar] [CrossRef] [PubMed]
- Schäufele, I.; Hamm, U. Consumers’ perceptions, preferences and willingness-to-pay for wine with sustainability characteristics: A review. J. Clean. Prod. 2017, 147, 379–394. [Google Scholar] [CrossRef]
- Powell, P.A.; Jones, C.R.; Consedine, N.S. It’s not queasy being green: The role of disgust in willingness-to-pay for more sustainable product alternatives. Food Qual. Prefer. 2019, 78, 103737. [Google Scholar] [CrossRef]



| Composition | BSG | BSY | BSGH | BSYH |
|---|---|---|---|---|
| Moisture (g/100 g) | 73.36 ± 0.23 | 91.14 ± 1.71 | 8.81 ± 0.01 | 13.25 ± 0.09 |
| Protein (g/100 g db) | 23.41 ± 0.18 | 43.59 ± 0.07 | 50.69 ± 0.45 | 50.11 ± 0.11 |
| Fat (g/100 g db) | 10.10 ± 0.23 | 2.86 ± 0.10 | n.d. | n.d. |
| Ash (g/100 g db) | 3.44 ± 0.14 | 4.4 ± 0.01 | 8.53 ± 0.03 | 14.35 ± 0.04 |
| Total carbohydrates (g/100 g db) | 62.94 ± 0.21 | 49.13 ± 0.59 | 40.78 ± 0.46 | 35.54 ± 0.14 |
| Samples | WS (%) | WBC (%) | EC (%) |
|---|---|---|---|
| BSG | 12.06 ± 0.38 | 359.44 ± 3.12 | 2.12 ± 0.07 |
| BSY | 17.57 ± 0.42 | 308.77 ± 4.11 | 14.95 ± 0.51 |
| BSGH | 97.98 ± 0.15 | 14.01 ± 0.34 | 2.63 ± 0.00 |
| BSYH | 96.81 ± 0.17 | 61.70 ± 0.82 | 16.28 ± 017 |
| Amino Acids (AA) | BSG | BSY | BSGH | BSYH |
|---|---|---|---|---|
| Essential AA (%) | ||||
| Histidine | 1.99 ± 0.16 | 2.21 ± 0.06 | 1.87 ± 0.03 | 2.19 ± 0.03 |
| Threonine | 3.49 ± 0.16 | 5.48 ± 0.00 | 4.02 ± 0.05 | 4.78 ± 0.03 |
| Lysine | 4.59 ± 0.18 | 6.65 ± 0.09 | 4.34 ± 0.03 | 6.03 ± 0.04 |
| Valine | 5.36 ± 0.27 | 5.87 ± 0.01 | 5.56 ± 0.06 | 5.98 ± 0.04 |
| Leucine | 7.82 ± 0.41 | 7.36 ± 0.02 | 7.77 ± 0.01 | 7.36 ± 0.03 |
| Isoleucine | 4.24 ± 0.25 | 4.73 ± 0.04 | 4.16 ± 0.03 | 4.34 ± 004 |
| Tyrosine | 3.46 ± 0.24 | 3.97 ± 0.12 | 3.62 ± 0.02 | 4.03 ± 0.02 |
| Phenylalanine | 5.78 ± 0.35 | 4.70 ± 0.00 | 5.62 ± 0.04 | 4.57 ± 0.01 |
| Cysteine | 2.19 ± 0.01 | 2.30 ± 0.00 | 2.15 ± 0.01 | 2.86 ± 0.08 |
| Methionine | 1.84 ± 0.02 | 1.83 ± 0.02 | 1.53 ± 0.07 | 1.58 ± 0.03 |
| Tryptophan | 1.57 ± 0.03 | 1.66 ± 0.01 | 1.83 ± 0.00 | 1.97 ± 0.02 |
| Total | 42.32 | 46.76 | 42.47 | 45.69 |
| Non-essential AA (%) | ||||
| Serine | 4.30 ± 0.23 | 6.22 ± 0.04 | 4.74 ± 0.01 | 5.06 ± 0.04 |
| Arginine | 4.85 ± 0.36 | 4.34 ± 0.07 | 4.26 ± 0.03 | 4.07 ± 0.01 |
| Glycine | 3.79 ± 0.19 | 4.50 ± 0.02 | 3.58 ± 0.04 | 4.82 ± 0.04 |
| Asx * | 7.01 ± 0.25 | 10.02 ± 0.06 | 7.22 ± 0.01 | 10.18 ± 0.12 |
| Glx * | 22.40 ± 1.14 | 15.22 ± 0.01 | 22.55 ± 0.06 | 16.42 ± 0.18 |
| Alanine | 4.58 ± 0.18 | 6.12 ± 0.02 | 4.54 ± 0.01 | 5.86 ± 0.05 |
| Proline | 10.74 ± 0.67 | 6.82 ± 0.02 | 10.64 ± 0.06 | 7.89 ± 0.07 |
| Total | 57.68 | 53.24 | 57.53 | 54.31 |
| Acidic AAs * | 29.42 | 25.24 | 29.77 | 26.60 |
| Basic AAs ** | 11.42 | 13.20 | 10.48 | 12.28 |
| Acidic/Basic AAs | 2.57 | 1.91 | 2.84 | 2.17 |
| CIELab | ||||||
|---|---|---|---|---|---|---|
| L* | a* | b* | C | h | ΔE | |
| BSG | 63.7 ± 0.3 | 4.3 ± 0.1 | 19.7 ± 0.3 | 20.1 ± 0.3 | 77.6 ± 0.1 | - |
| BSGH | 72.9 ± 0.3 | 4.4 ± 0.2 | 17.8 ± 0.6 | 18.3 ± 0.6 | 76.1 ± 0.1 | 9.2 ± 0.3 |
| BSY | 73.5 ± 0.4 | 4.5 ± 0.1 | 19.0 ± 0.3 | 19.5 ±0.3 | 76.8 ± 0.2 | - |
| BSYH | 60.8 ± 0.4 | 7.1 ± 0.3 | 22.3 ± 0.2 | 23.4 ± 0.3 | 72.2 ± 0.5 | 13.4 ± 0.5 |
| ASBC | CIELab | ||||||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | C | h | ΔE | ||
| Control beverage | |||||||
| Control 0 g/473 mL | 7.7 ± 0.1 dD | 69.6 ± 0.2 aA | −0.6 ± 0.0 dD | 22.4 ± 0.2 dD | 22.4 ± 0.2 dD | 91.5 ± 0.1 aA | - |
| BSGH incorporated beverages | |||||||
| Treatment 1 2.5 g/473 mL | 10.5 ± 0.3 c | 67.6 ± 0.2 b | 0.2 ± 0.1 c | 25.4 ± 0.3 c | 25.4 ± 0.3 c | 89.6 ± 0.2 b | 3.7 ± 0.3 c |
| Treatment 2 5 g/473 mL | 13.0 ± 0.1 b | 65.6 ± 0.1 c | 1.1 ± 0.0 b | 28.2 ± 0.2 b | 28.2 ± 0.2 b | 87.7 ± 0.0 c | 7.2 ± 0.2 b |
| Treatment 3 10 g/473 mL | 17.0 ± 0.3 a | 62.5 ± 0.2 d | 2.7 ± 0.1 a | 32.4 ± 0.3 a | 32.5 ± 0.3 a | 85.2 ± 0.1 d | 12.7 ± 0.4 a |
| BSYH incorporated beverages | |||||||
| Treatment 1 2.5 g/473 mL | 9.9 ± 0.1 C | 67.6 ± 0.1 B | 0.4 ± 0.0 C | 26.7 ± 0.2 C | 26.7 ± 0.2 C | 89.2 ± 0.1 B | 4.8 ± 0.2 C |
| Treatment 2 5 g/473 mL | 12.6 ± 0.1 B | 65.4 ± 0.1 C | 1.6 ± 0.1 B | 30.9 ± 0.2 B | 30.9 ± 0.2 B | 87.0 ± 0.1 C | 9.7 ± 0.3 B |
| Treatment 3 10 g/473 mL | 18.4 ± 0.6 A | 61.4 ± 0.2 D | 4.2 ± 0.2 A | 37.1 ± 0.6 A | 37.3 ± 0.6 A | 83.6 ± 0.2 D | 17.5 ± 0.6 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akram, M.U.; Agunbiade, H.O.; Kadam, D.; Aluko, R.E.; Koksel, F. Adding Value to Brewery Industry By-Products as Novel Ingredients in Non-Alcoholic Malt Beverage Applications. Foods 2025, 14, 2882. https://doi.org/10.3390/foods14162882
Akram MU, Agunbiade HO, Kadam D, Aluko RE, Koksel F. Adding Value to Brewery Industry By-Products as Novel Ingredients in Non-Alcoholic Malt Beverage Applications. Foods. 2025; 14(16):2882. https://doi.org/10.3390/foods14162882
Chicago/Turabian StyleAkram, Muhammad Usman, Helen Oluwaseun Agunbiade, Deepak Kadam, Rotimi Emmanuel Aluko, and Filiz Koksel. 2025. "Adding Value to Brewery Industry By-Products as Novel Ingredients in Non-Alcoholic Malt Beverage Applications" Foods 14, no. 16: 2882. https://doi.org/10.3390/foods14162882
APA StyleAkram, M. U., Agunbiade, H. O., Kadam, D., Aluko, R. E., & Koksel, F. (2025). Adding Value to Brewery Industry By-Products as Novel Ingredients in Non-Alcoholic Malt Beverage Applications. Foods, 14(16), 2882. https://doi.org/10.3390/foods14162882










