Analysis and Risk Assessment of Total Iodine Content in Edible Seaweeds in South Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Iodine Analysis
2.3. Method Validation
2.4. Risk Assessment
2.5. Statistical Analysis
3. Results and Discussion
3.1. Sampling Information
3.2. Validation Method for Iodine Content of the Five Major Seaweeds
3.3. Total Iodine Content of Seaweed According to Various Classification Criteria
3.3.1. Variation in Iodine Content According to the Cultivation Method (Wild and Farmed)
3.3.2. Temporal Variation in Iodine Content According to the Collection Year (2021–2024)
3.3.3. Geographical Variation in Iodine Content According to the Collection Area
3.4. Risk Assessment Result
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rogel-Castillo, C.; Latorre-Castañeda, M.; Muñoz-Muñoz, C.; Agurto-Muñoz, C. Seaweeds in Food: Current Trends. Plants 2023, 12, 2287. [Google Scholar] [CrossRef]
- Oh, S. Strategies for Korea–Southeast Asia Cooperation on Securing Seaweed Supply Chains: Focusing on Indonesia and the Philippines. J. Asian Stud. 2025, 28, 59–78. Available online: https://www.kci.go.kr/kciportal/po/search/poSereArtiList.kci?sereId=001671&volIsseId=VOL000183945 (accessed on 29 July 2025). [CrossRef]
- MacArtain, P.; Gill, C.I.R.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional value of edible seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Bito, T.; Watanabe, F. Seaweeds as a Source of Vitamin B12. In Sustainable Global Resources of Seaweeds; Kim, S.-K., Ed.; Springer: Cham, Switzerland, 2022; Volume 2, pp. 339–350. [Google Scholar]
- Brown, E.S.; Allsopp, P.J.; Magee, P.J.; Gill, C.I.R.; Nitecki, S.; Strain, C.R.; McSorley, E.M. Seaweed and human health. Nutr. Rev. 2014, 72, 205–216. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Kim, M.S.; Son, K.T.; Jeong, Y.H.; Jeon, Y.J. Antioxidant activity of marine algal polyphenolic compounds: A mechanistic approach. J. Med. Food 2016, 19, 615–628. [Google Scholar] [CrossRef]
- Shannon, E.; Abu-Ghannam, N. Seaweeds as nutraceuticals for health and nutrition. Phycologia 2019, 58, 563–577. [Google Scholar] [CrossRef]
- Li, Y.X.; Wijesekara, I.; Li, Y.; Kim, S.K. Phlorotannins as bioactive agents from brown algae. Process Biochem. 2011, 46, 2219–2224. [Google Scholar] [CrossRef]
- European Commission. Food from the Ocean (Scientific Opinion No. 3/2017 Publications Office of the European Union: Luxembourg). 2017. Available online: https://ec.europa.eu/research/sam/pdf/sam_food-from-oceans_report.pdf (accessed on 8 December 2022).
- McHugh, D.J. A Guide to the Seaweed Industry. FAO Fisheries Technical Paper; Food and Agriculture Organization: Rome, Italy, 2003; p. 441. Available online: http://www.fao.org/3/y4765e/y4765e00.htm (accessed on 10 May 2025).
- Korea Fisheries Association. 2024 Korean Fisheries Export Performance Report; Ministry of Oceans and Fisheries: Seoul, Republic of Korea, 2024.
- European Commission. EU Seaweed Industry Report 2023; Directorate-General for Maritime Affairs and Fisheries: Brussels, Belgium, 2023; Available online: https://ec.europa.eu/maritimeaffairs (accessed on 20 June 2024).
- Eurostat. EU Trade in Seaweed Products. 2023. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Seaweed_trade_statistics (accessed on 16 December 2024).
- Cherry, P.; O’Hara, C.; Magee, P.J.; McSorley, E.M.; Allsopp, P.J. Risks and benefits of consuming edible seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on the safety of seaweed and iodine content. EFSA J. 2014, 12, 3660–3716. [Google Scholar] [CrossRef]
- Smyth, P.P.A. Iodine, seaweed, and the thyroid. Eur. Thyroid. J. 2021, 10, 101–108. [Google Scholar] [CrossRef] [PubMed]
- ANSES (French Agency for Food, Environmental and Occupational Health and Safety). Annual Report 2018; ANSES: Paris, France, 2018.
- FSANZ (Food Standards Australia New Zealand). Imported Food Risk Statement. Brown Seaweed of the Phaeophyceae Class and Iodinet. 2023. Available online: https://www.foodstandards.gov.au/sites/default/files/2023-11/Brown%20seaweed%20and%20Iodine.pdf (accessed on 10 May 2025).
- Zimmermann, M.B.; Andersson, M. Update on iodine status worldwide. Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 382–387. [Google Scholar] [CrossRef]
- Sorrenti, S.; Baldini, E.; Pironi, D.; Lauro, A.; D’Orazi, V.; Tartaglia, F.; Tripodi, D.; Lori, E.; Gagliardi, F.; Praticò, M.; et al. Iodine: Its role in thyroid hormone biosynthesis and beyond. Nutrients 2021, 13, 4469. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.Y.; Lee, S.J.; Kim, H.S.; Eom, J.S.; Jo, S.U.; Guan, L.L.; Park, T.S.; Seo, J.K.; Lee, Y.K.; Bae, D.R.; et al. Red seaweed extracts reduce methane production by altering rumen fermentation and microbial composition in vitro. Front. Vet. Sci. 2022, 9, 985824. [Google Scholar] [CrossRef]
- Lee, J.Y.; Yeoh, Y.J.; Seo, M.J.; Lee, G.H.; Kim, C.I. Estimation of dietary iodine intake of Koreans through a total diet study (TDS). Korean J. Community Nutr. 2021, 26, 48–55. [Google Scholar] [CrossRef]
- MoF (Ministry of Oceans and Fisheries). Seaweed Aquaculture Production in South Korea: Ministry of Oceans and Fisheries Report; Ministry of Oceans and Fisheries: Sejong, Republic of Korea, 2018. Available online: https://www.mof.go.kr/doc/ko/selectDoc.do?docSeq=20773&menuSeq=929&bbsSeq=27 (accessed on 10 May 2025).
- MFDS (Ministry of Food and Drug Safety). Common Guidelines for Risk Assessment of Human Applications; Ministry of Food and Drug Safety: Cheongju, Republic of Korea, 2019. Available online: https://www.mfds.go.kr (accessed on 10 May 2025).
- Method. In Association of Official Analytical Communities Official Method of Analysis (OMA), 20th ed.; AOAC International: Rockville, MD, USA, 2016; Volume 2012.
- Kim, M.H.; Cho, M.H.; Kim, S.H.; Lee, Y.M.; Jo, M.R.; Moon, Y.S.; Im, M.H. Monitoring and risk assessment of pesticide residues in fishery products using GC-MS/MS in South Korea. Toxics 2024, 12, 299. [Google Scholar] [CrossRef]
- Schiener, P.; Black, K.D.; Stanley, M.S.; Green, D.H. The seasonal variation in the chemical composition of the kelp species Laminaria digitata, Laminaria hyperborea, Saccharina latissimi and Alaria esculenta. J. Appl. Phycol. 2015, 27, 363–373. [Google Scholar] [CrossRef]
- Roleda, M.Y.; Skjermo, J.; Marfaing, H.; Jónsdóttir, R.; Rebours, C.; Gietl, A.; Stengel, D.B.; Nitschke, U. Iodine content in bulk biomass of wild-harvested and cultivated edible seaweeds: Inherent variations determine species-specific daily allowable consumption. Food Chem. 2018, 254, 333–339. [Google Scholar] [CrossRef]
- Rehder, D. Vanadate-dependent peroxidase in macroalgae: Function, applications, and environmental impact. J. Oceanogr. Mar. Sci. 2014, 2, 2–7. [Google Scholar] [CrossRef]
- Graf, L.; Gong, W.; Murray, D.C.; Huang, Z.; Coleman, M.A.; Jeffs, A.; McKenzie, P.F.; Ross, D.J.; Brown, M.T.; Naughton, S.; et al. A genome-wide investigation of the effect of farming and human-mediated introduction on the ubiquitous seaweed Undaria pinnatifida. Nat. Ecol. Evol. 2021, 5, 360–368. [Google Scholar] [CrossRef]
- Lüning, K. Seaweeds: Their Environment, Biogeography, and Ecophysiology; John Wiley & Sons: New York, NY, USA, 1991. [Google Scholar]
- Gunnarsdóttir, I.; Brantsæter, A.L. Iodine: A scoping review for Nordic Nutrition Recommendations 2023. Food Nutr. Res. 2023, 67, 10369. [Google Scholar] [CrossRef]
- Sæther, M.; Diehl, N.; Monteiro, C.; Li, H.; Niedzwiedz, S.; Burgunter-Delamare, B.; Scheschonk, L.; Bischof, K.; Forbord, S. The sugar kelp Saccharina latissima II: Recent advances in farming and applications. J. Appl. Phycol. 2024, 36, 1953–1985. [Google Scholar] [CrossRef]
- Aakre, I.; Solli, D.D.; Markhus, M.W.; Mæhre, H.K.; Dahl, L.; Henjum, S.; Alexander, J.; Korneliussen, P.-A.; Madsen, L.; Kjellevold, M. Commercially available kelp and seaweed products—Valuable iodine source or risk of excess intake? Food Nutr. Res. 2021, 65, 7584–7600. [Google Scholar] [CrossRef]
- Küpper, F.C.; Carpenter, L.J.; McFiggans, G.B.; Palmer, C.J.; Waite, T.J.; Boneberg, E.M.; Woitsch, S.; Weiller, M.; Abela, R.; Grolimund, D.; et al. Iodine accumulation provides kelp with an inorganic antioxidant impacting atmospheric chemistry. Proc. Natl Acad. Sci. USA. 2008, 105, 6954–6958. [Google Scholar] [CrossRef]
- Teas, J.; Pino, S.; Critchley, A.; Braverman, L.E. Variability of iodine content in common commercially available edible seaweeds. Thyroid 2004, 14, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Milinovic, J.; Rodrigues, C.; Diniz, M.; Noronha, J.P. Determination of total iodine content in edible seaweeds: Application of inductively coupled plasma-atomic emission spectroscopy. Algal Res. 2021, 53, 102149. [Google Scholar] [CrossRef]
- Allen, L.H.; Carriquiry, A.L.; Murphy, S.P. Perspective: Proposed harmonized nutrient reference values for populations. Adv. Nutr. 2020, 11, 469–483. [Google Scholar] [CrossRef]
- EFSA. Dietary reference values for nutrients. Summ. Rep. EFSA Support. Publ. 2017, 2017, e15121. Available online: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.e15121/epdf (accessed on 24 July 2019).
- EFSA, Scientific Committee on Food and Scientific Panel on Dietetic Products, Nutrition and Allergies. Summary of Tolerable Upper Intake Levels, 2nd ed.; EFSA: Parma, Italy, 2017; Available online: https://www.efsa.europa.eu/sites/default/files/assets/UL_Summary_tables.pdf (accessed on 13 August 2025).
- Aakre, I.; Tveit, I.B.; Myrmel, L.S.; Fjære, E.; Ballance, S.; Rosendal-Riise, H. Bioavailability of iodine from a meal consisting of sushi and a wakame seaweed salad—A Randomized Crossover Trial. Food Sci. Nutr. 2023, 11, 7707–7717. [Google Scholar] [CrossRef]
- González, A.; Paz, S.; Rubio, C.; Gutiérrez, Á.J.; Hardisson, A. Human exposure to iodine from the consumption of edible seaweeds. Biol. Trace Elem. Res. 2020, 197, 361–366. [Google Scholar] [CrossRef]
- Park, S.J.; Chen, L.; Wallace, T.; Lee, H.J. The association between iodine intake and thyroid disease in iodine-replete regions: The Korean genome and epidemiology study. Nutr. Res. Pract. 2025, 19, 554–565. Available online: https://e-nrp.org/pdf/10.4162/nrp.2025.19.4.554 (accessed on 13 August 2025). [CrossRef] [PubMed]
- World Health Organization (WHO)/Europe; Iodine Global Network (IGN). People in the WHO European Region at Greater Risk of Iodine Deficiency Due to Changing Diets: WHO/Europe and the Iodine Global Network Urgently Call for Iodine Fortification of Salt and Plant-Based Dairy Alternatives. 2024. Available online: https://www.who.int/europe/news/item/28-06-2024-people-in-the-who-european-region-at-greater-risk-of-iodine-deficiency-due-to-changing-diets (accessed on 28 June 2024).
- Kwon, S.O.; Kwon, K.I.; Lee, M.Y.; Lee, H.Y.; Kim, C.I. Iodine intake from brown seaweed and the related nutritional risk assessment in Koreans. Nutr. Res. Pract. 2024, 18, 412–424. [Google Scholar] [CrossRef] [PubMed]
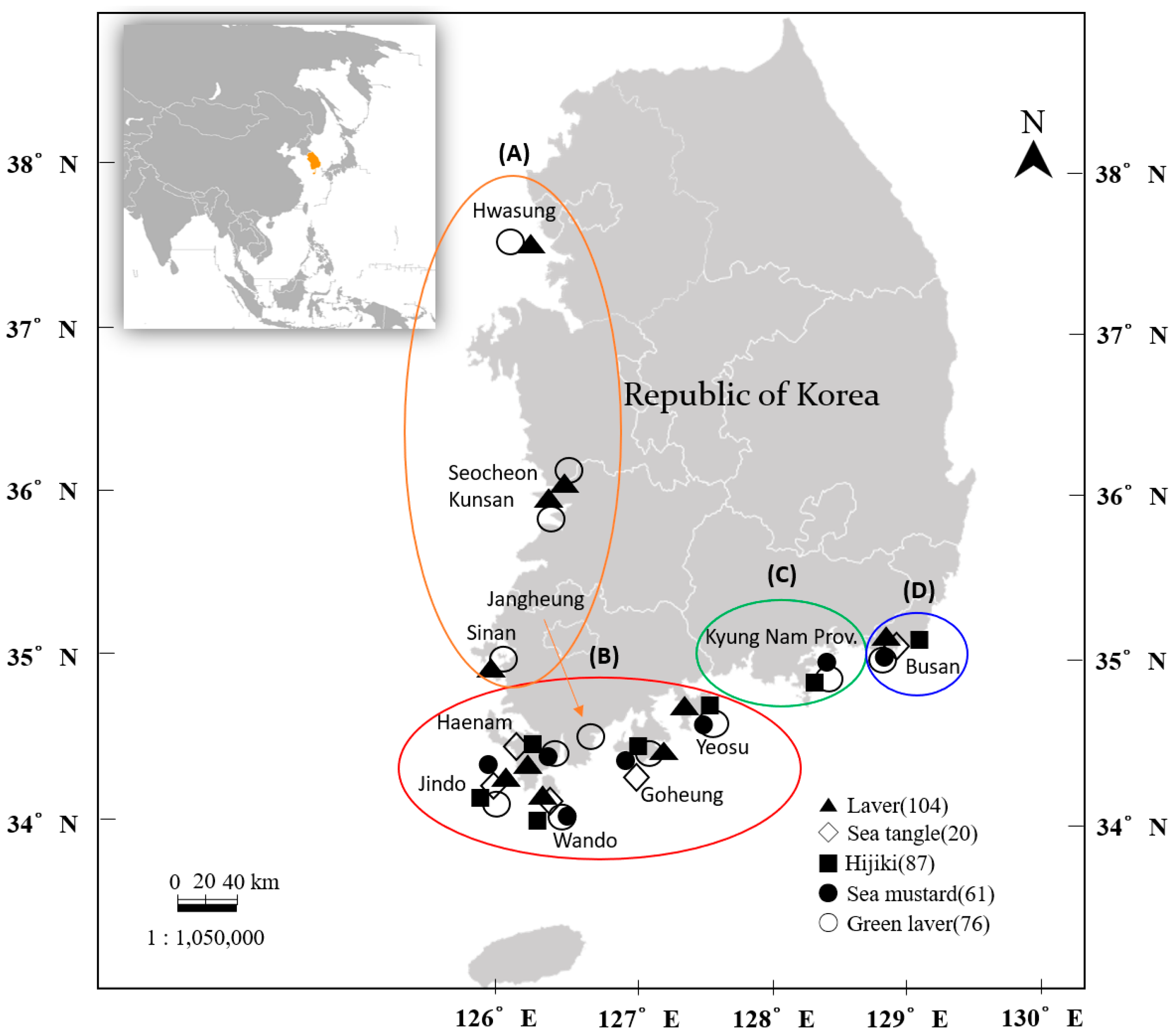
| KN | BS | JN | Others | Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| GH | YS | WD | JD | HN | |||||
| Laver | 0 | 4 | 9 | 1 | 10 | 35 | 26 | 19 | 104 |
| Sea tangle | 0 | 5 | 3 | 0 | 7 | 2 | 3 | 0 | 20 |
| Sea mustard | 2 | 8 | 15 | 2 | 22 | 4 | 8 | 0 | 61 |
| Hijiki | 1 | 3 | 21 | 6 | 35 | 18 | 3 | 0 | 87 |
| Green laver | 2 | 3 | 8 | 2 | 30 | 13 | 15 | 3 | 76 |
| Total | 5 | 23 | 56 | 11 | 104 | 72 | 55 | 22 | 348 |
| CRM (SRM 3232, 944 ± 88 mg/kg) | LOD a | LOQ b | Linearity | Recovery |
| (µg/kg) | (µg/kg) | (R2) | (%) | |
| 0.92 | 2.75 | 0.9996 | 87.44 ± 2.74 |
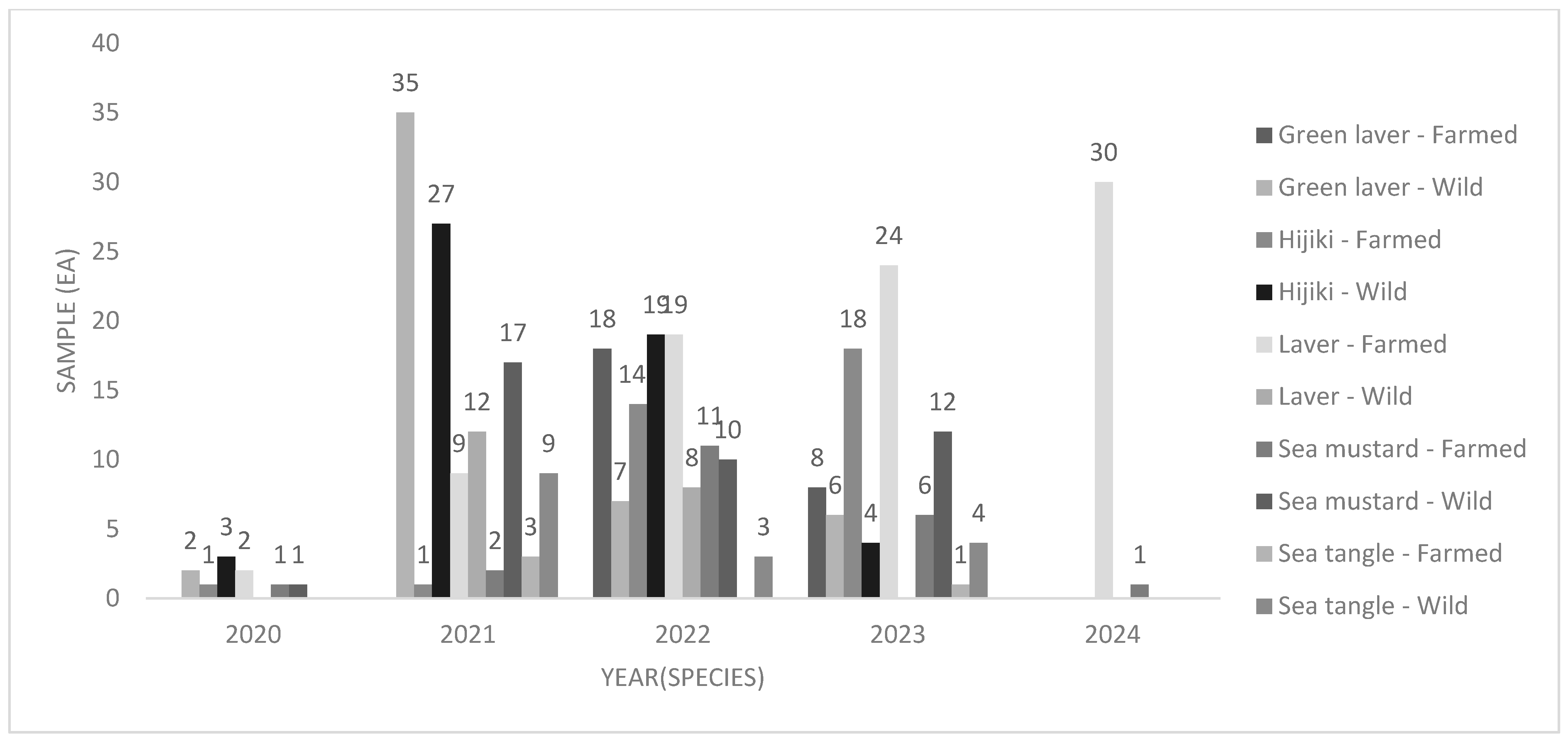
| Species | Growth Method | Harvest Year | Sampling Region | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wild | Farmed | 2020–21 | 2022 | 2023 | 2024 | KN | BS | JN | Others | |||||||||||||||||||
| GH | YS | WD | JD | HN | ||||||||||||||||||||||||
| W.W | D.W | W.W | D.W | W.W | D.W | W.W | D.W | W.W | D.W | W.W | D.W | W.W | D.W | W.W | D.W | W.W | D.W | W.W | D.W | W.W | D.W | W.W | D.W | W.W | D.W | W.W | D.W | |
| Laver | 3.92 | 69.6 | 6.67 | 107 | 4.48 | 78 | 4.85 | 71.5 | 5.41 | 90.1 | 7.95 | 130 | - a | - | 3.96 | 43.94 | 5.88 | 97.02 | 1.81 | 32.59 | 4.87 | 89.60 | 7.21 | 102.32 | 6.7 | 110.78 | 4.85 | 104.18 |
| Sea tangle | 219 | 2308 | 264 | 2929 | 235 | 2540 | 254 | 2079 | 140 | 1888 | 229 | 3329 | - | - | 268 | 3102 | 83 | 1195 | - | - | 241 | 2531 | 318 | 2023 | 215 | 2594 | - | - |
| Sea mustard | 11 | 157 | 15.9 | 205 | 10.72 | 143 | 10.8 | 177 | 16.2 | 185 | 12.4 | 196 | 16.7 | 235 | 17.8 | 201.1 | 12.1 | 185 | 12.9 | 170.8 | 10.6 | 144.1 | 14.5 | 207.5 | 13.5 | 199.3 | - | - |
| Hijiki | 58.1 | 505 | 64.2 | 561 | 72.2 | 620 | 51.3 | 438 | 67.1 | 599 | - | - | 77.3 | 557 | 98.4 | 770 | 52.5 | 461 | 50.5 | 557 | 67.2 | 558 | 49.5 | 453 | 80.0 | 752 | - | - |
| Green laver | 5.54 | 70.3 | 7.04 | 116 | 5.41 | 60.2 | 5.57 | 76.3 | 6.5 | 104 | 19.5 | 366 | 6.23 | 132 | 9.84 | 165 | 5.75 | 74 | 9.43 | 130 | 6.58 | 86 | 4.98 | 70 | 5.06 | 74 | 3.92 | 100 |
| Species | ACDI (mg/kg dw) | SC (g/day) a | EDI 1 (µg/day/person) c | EDI 2 (µg/kg bw/day) d | Scenario 1 e | Scenario 2 f | Scenario 3 g | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HI (Korean MFDS) | HI (EFSA) | HI (PMTDI) | ||||||||||
| Avg. b | Avg. | Act. | Avg. | Act. | Avg. | Act. | Avg. | Act. | Avg. | Act. | ||
| Laver | 100.2 | 1.02 | 102.44 | 294.69 | 1.69 | 4.85 | 0.04 | 0.12 | 0.17 | 0.49 | 0.10 | 0.29 |
| Sea tangle | 2432.1 | 0.67 | 1632.61 | 38,393.31 | 26.88 | 632.01 | 0.68 | 16.00 | 2.72 | 63.99 | 1.58 | 37.18 |
| Sea mustard | 176.9 | 0.72 | 127.12 | 854.19 | 2.09 | 14.06 | 0.05 | 0.36 | 0.21 | 1.42 | 0.12 | 0.83 |
| Hijiki | 526.6 | 0.09 | 47.50 | 9636.62 | 0.78 | 158.63 | 0.02 | 4.02 | 0.08 | 16.06 | 0.05 | 9.33 |
| Green laver | 86.1 | 0.38 | 32.86 | 2100.32 | 0.54 | 34.57 | 0.01 | 0.88 | 0.05 | 0.88 | 0.03 | 2.03 |
| Total | 0.81 | 21.37 | 3.24 | 85.47 | 1.88 | 49.31 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.; Park, H.J.; Jo, M.; Ha, K.S.; Mok, J.S. Analysis and Risk Assessment of Total Iodine Content in Edible Seaweeds in South Korea. Foods 2025, 14, 2865. https://doi.org/10.3390/foods14162865
Lee Y, Park HJ, Jo M, Ha KS, Mok JS. Analysis and Risk Assessment of Total Iodine Content in Edible Seaweeds in South Korea. Foods. 2025; 14(16):2865. https://doi.org/10.3390/foods14162865
Chicago/Turabian StyleLee, YoonMi, Hyung June Park, Mira Jo, Kwang Soo Ha, and Jong Soo Mok. 2025. "Analysis and Risk Assessment of Total Iodine Content in Edible Seaweeds in South Korea" Foods 14, no. 16: 2865. https://doi.org/10.3390/foods14162865
APA StyleLee, Y., Park, H. J., Jo, M., Ha, K. S., & Mok, J. S. (2025). Analysis and Risk Assessment of Total Iodine Content in Edible Seaweeds in South Korea. Foods, 14(16), 2865. https://doi.org/10.3390/foods14162865






