Short-Wavelength Infrared Hyperspectral Imaging and Spectral Unmixing Techniques for Detection and Distribution of Pesticide Residues on Edible Perilla Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
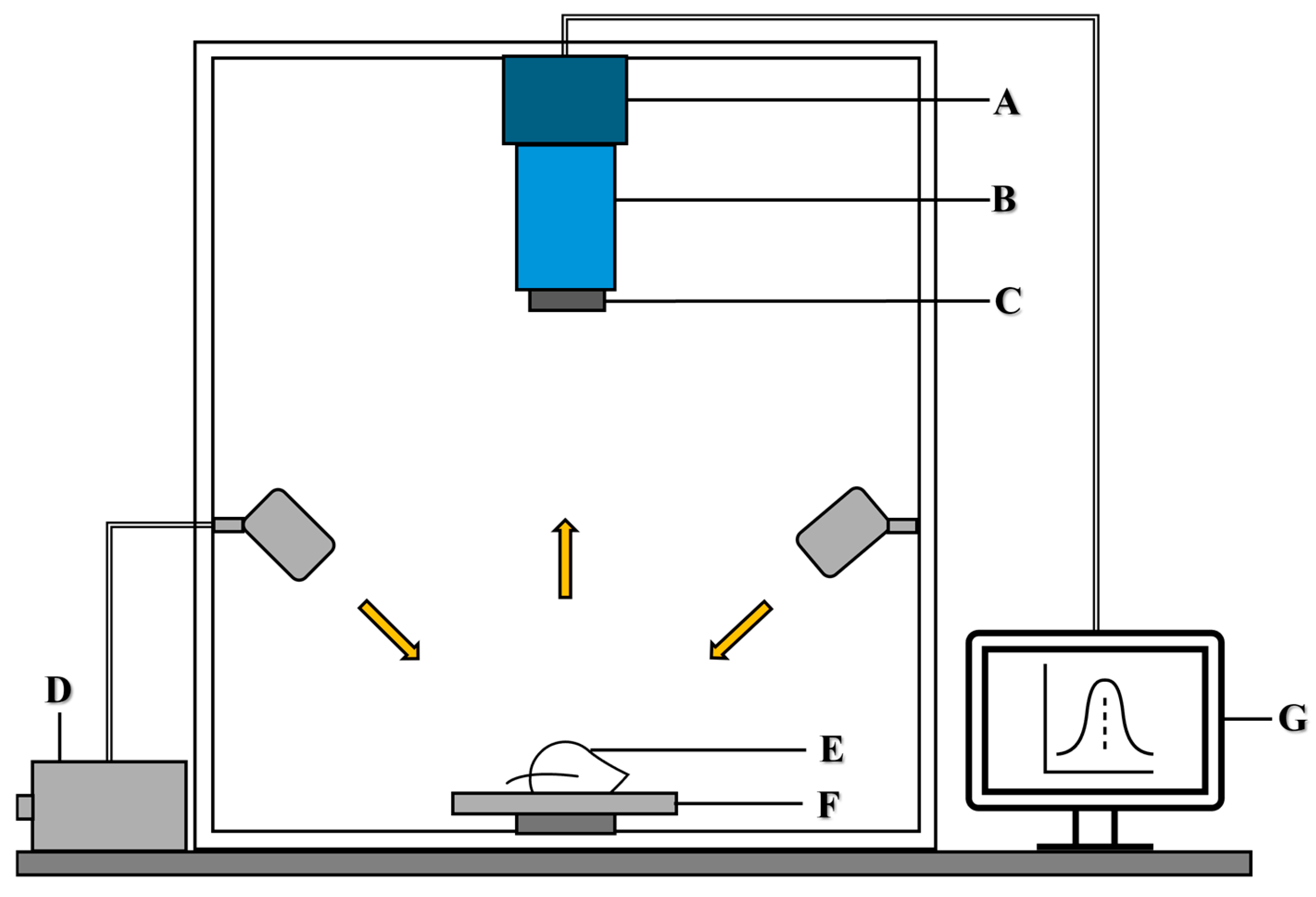
2.2. Hyperspectral Imaging System
2.3. Multivariate Curve Resolution-Alternating Least Squares for Pesticide Residue Analysis
2.4. Quantitative Model Development for Pesticide Residue Estimation
3. Results and Discussion
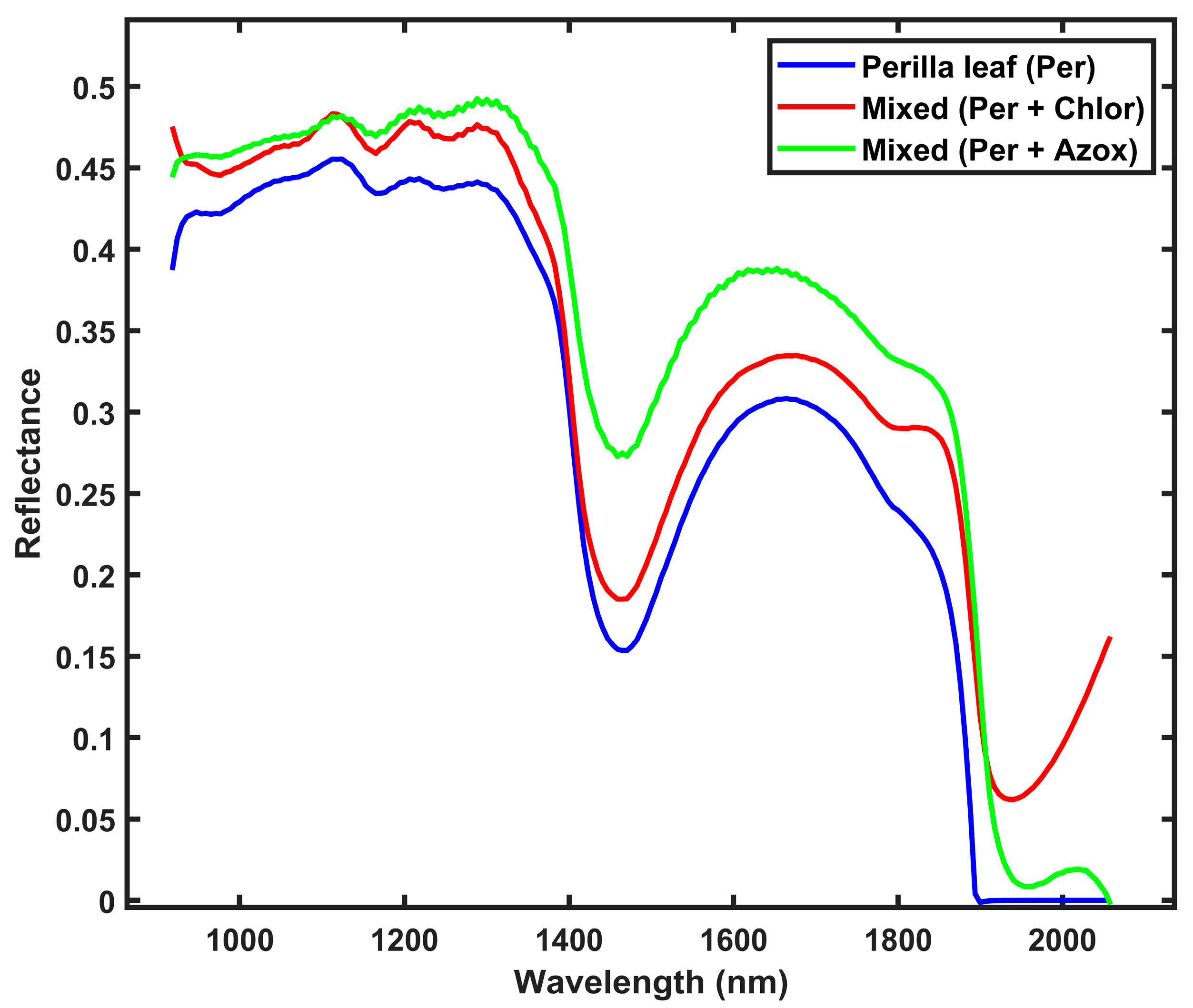
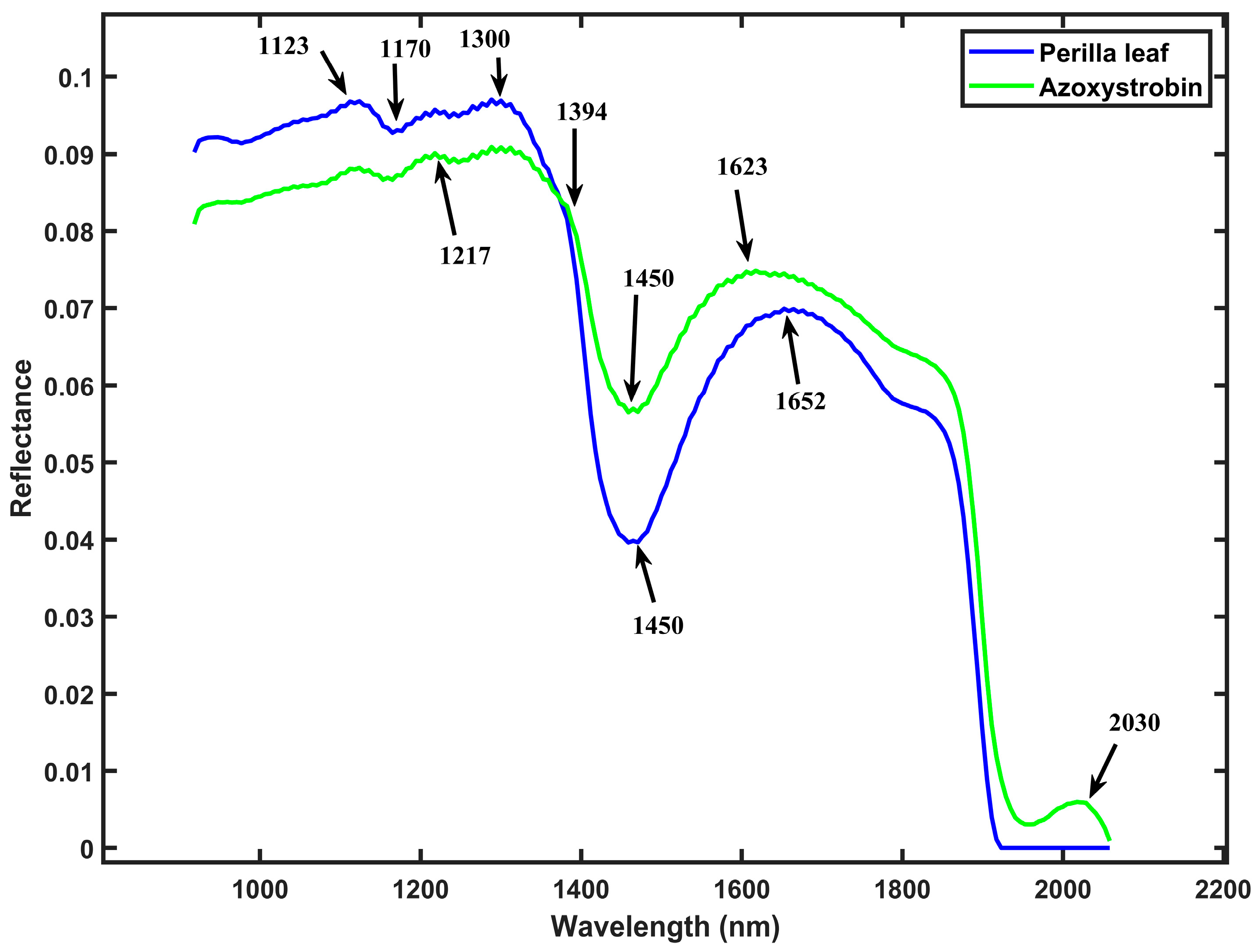
3.1. Spectra of Perilla Leaf and Mixed Samples with Pesticide Residues
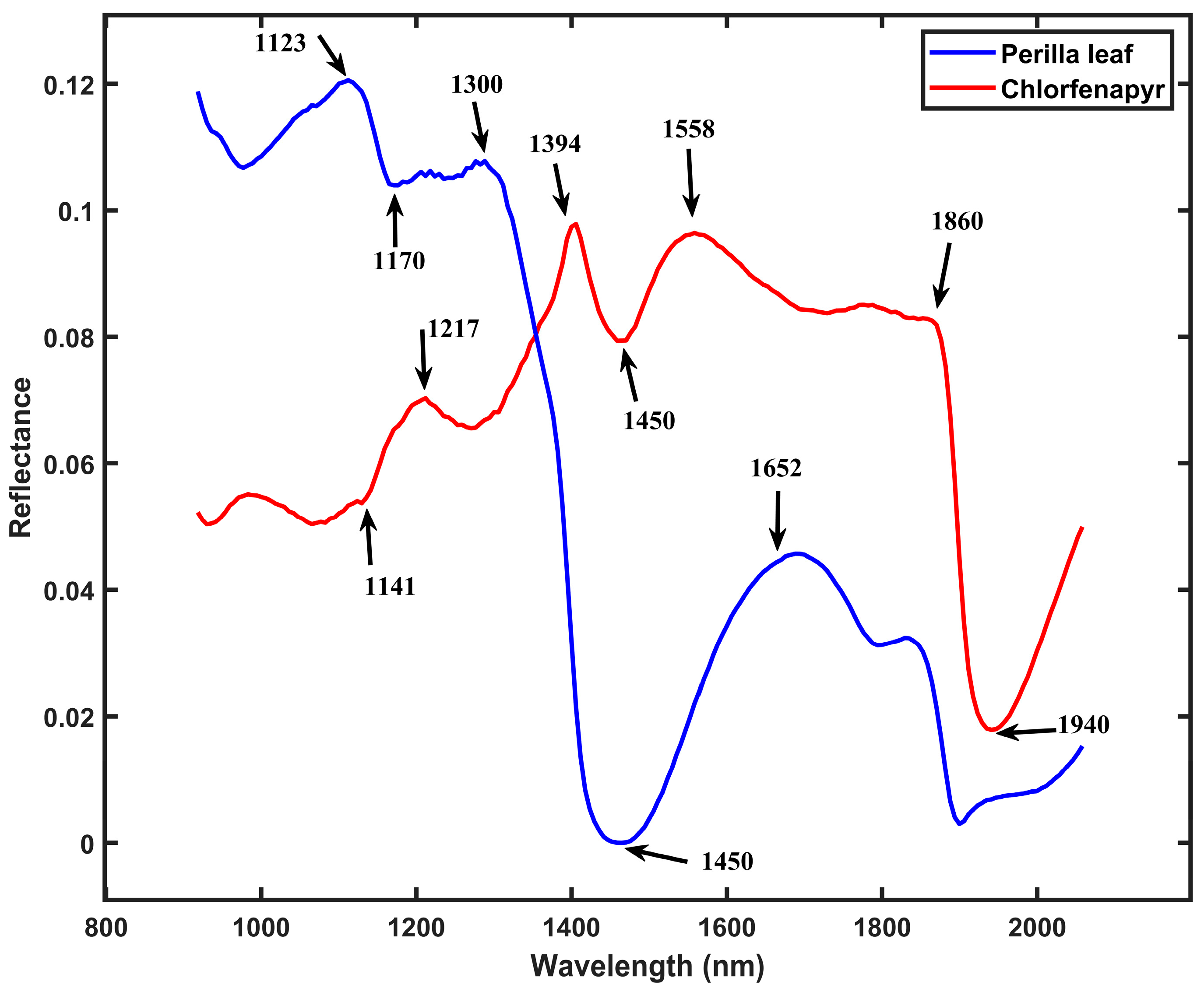
3.2. Detection and Distribution of Chlorfenapyr Residues on Perilla Leaves
3.3. Detection and Distribution of Azoxystrobin Residues on Perilla Leaves
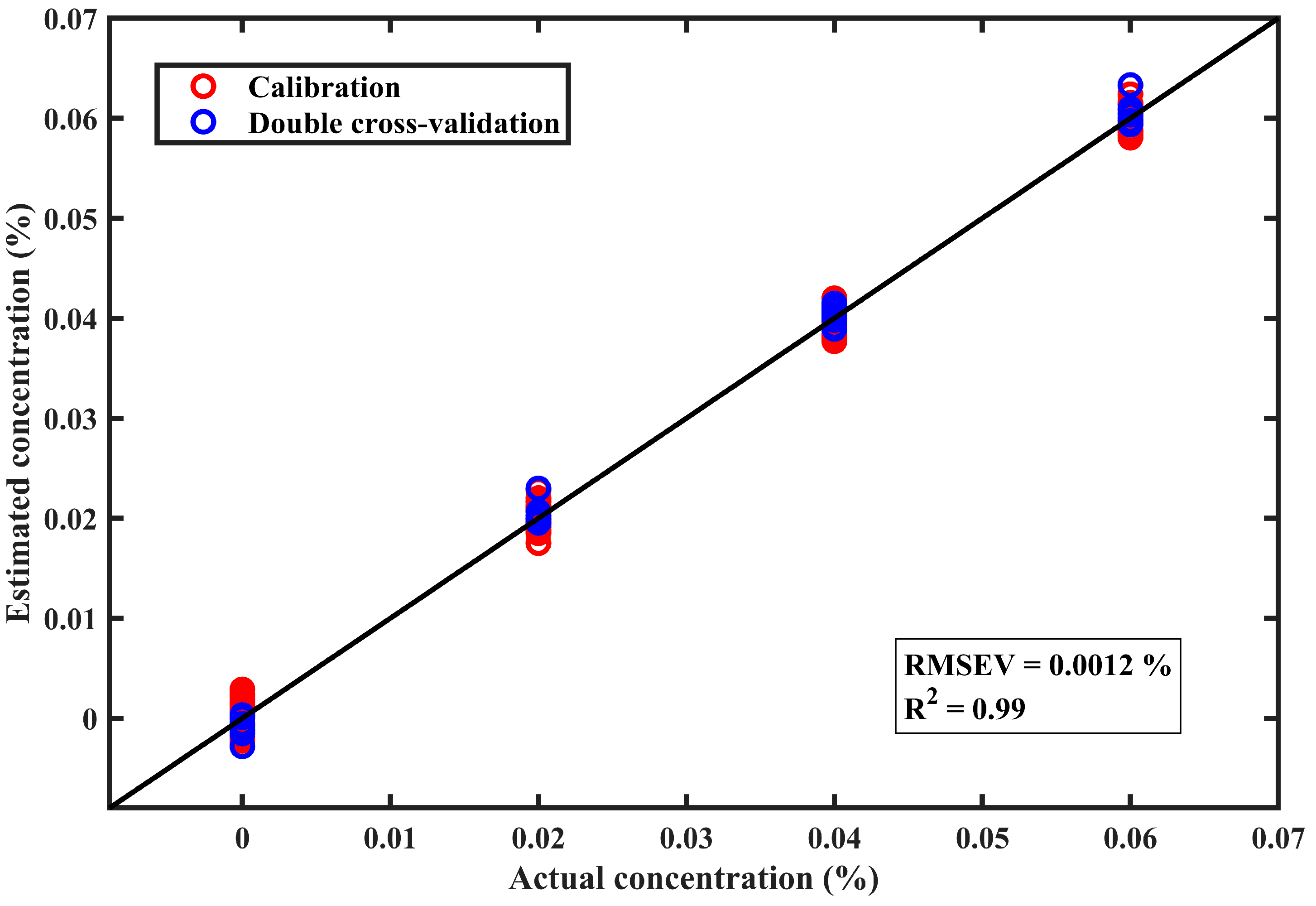
3.4. Estimation Model for Chlorfenapyr Residue Concentration on Perilla Leaves
3.5. Comparison with Other Chemometric Techniques for Chlorfenapyr Residue Concentration on Perilla Leaves
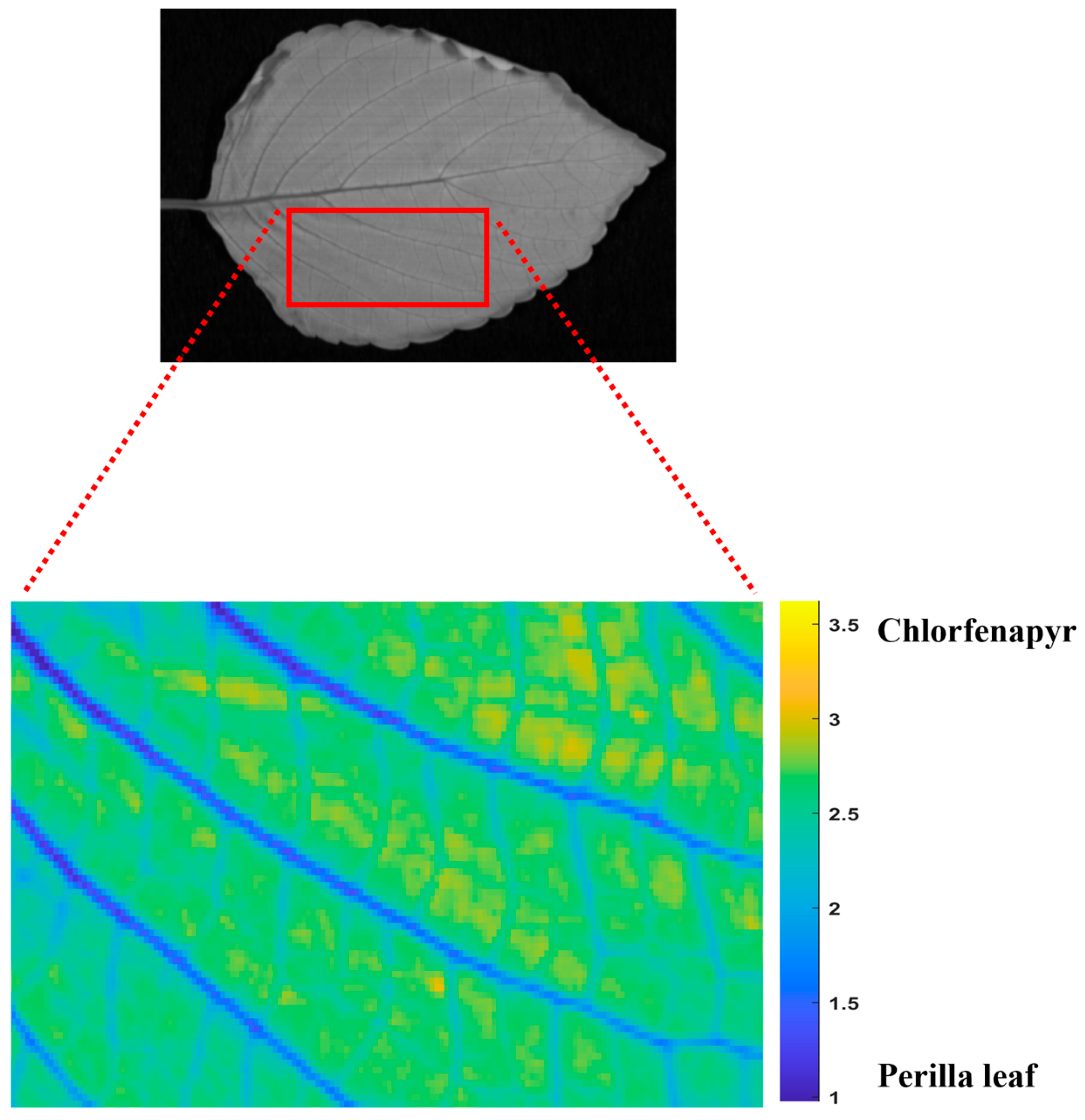
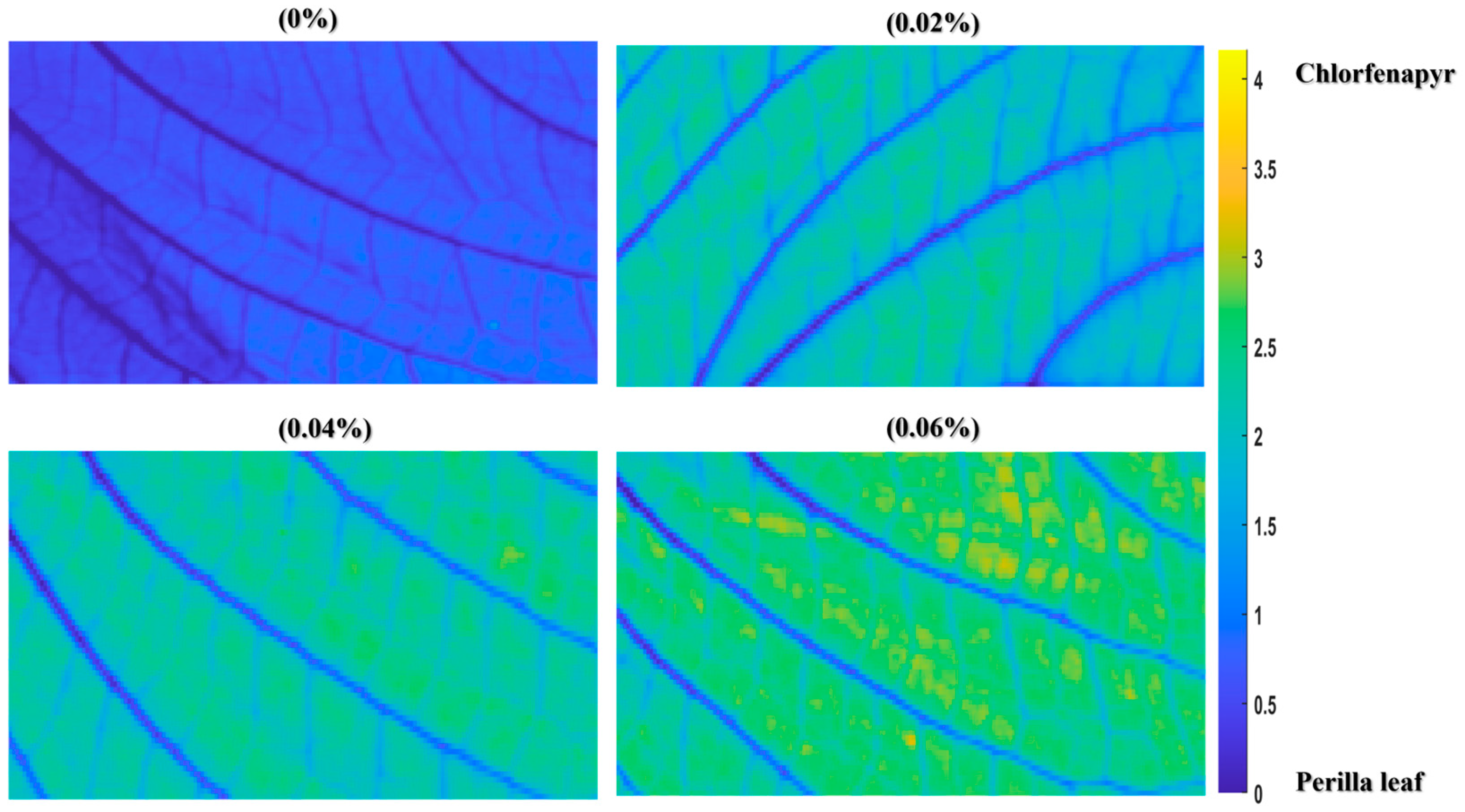
3.6. Visualization of Chlorfenapyr Residue Concentrations on Perilla Leaves
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dossou, S.S.K.; Deng, Q.; Li, F.; Wang, L.; Jiang, N.; Kefale, H.; Zhou, R.; Li, D.; Tan, M.; Wang, L. Metabolic Profiling of Perilla Leaves of Different Colors: Insights into Metabolite Variation and Bioactive Compound Distribution. BMC Plant Biol. 2025, 25, 38. [Google Scholar] [CrossRef]
- Wang, M.; Shen, R.; Wang, X.; Zhang, J.; Ma, J.; Shi, Y.; Xu, Q.; Guo, C.; Di, J.; Liu, A.; et al. Evaluation of Edible and Medicinal Varieties Based on Multidimensional Quantitative Data: A Case Study of Perilla Leaf. J. Food Compos. Anal. 2025, 140, 107196. [Google Scholar] [CrossRef]
- Bhattacharya, T.; Joshi, R.; Tufa, L.T.; Goddati, M.; Lee, J.; Tewari, A.; Cho, B.-K. L-Cysteine-Modified Carbon Dots Derived from Hibiscus Rosa-Sinensis for Thiram Pesticides Identification on Edible Perilla Leaves. ACS Omega 2024, 9, 47647–47660. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.R.; Sharma, G.; Nath, S.; Lee, S.-S. Screening the Phytochemicals in Perilla Leaves and Phytosynthesis of Bioactive Silver Nanoparticles for Potential Antioxidant and Wound-Healing Application. Green Process. Synth. 2024, 13, 20240050. [Google Scholar] [CrossRef]
- Ye, W.; Yan, T.; Zhang, C.; Duan, L.; Chen, W.; Song, H.; Zhang, Y.; Xu, W.; Gao, P. Detection of Pesticide Residue Level in Grape Using Hyperspectral Imaging with Machine Learning. Foods 2022, 11, 1609. [Google Scholar] [CrossRef] [PubMed]
- Gai, T.; Nie, J.; Ding, Z.; Wu, W.; Liu, X. Progress of Rapid Detection of Pesticides in Fruits and Vegetables. Front. Food Sci. Technol. 2023, 3, 1253227. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, J.; Lei, Y.; Tian, J.; Peng, J.; Chen, M. Classification of Pesticide Residues in Sorghum Based on Hyperspectral and Gradient Boosting Decision Trees. J. Food Saf. 2024, 44, e13166. [Google Scholar] [CrossRef]
- Ridoutt, B.; Baird, D.; Navarro, J.; Hendrie, G.A. Pesticide Toxicity Footprints of Australian Dietary Choices. Nutrients 2021, 13, 4314. [Google Scholar] [CrossRef]
- Pérez-Navarro, J.; Izquierdo-Cañas, P.M.; Mena-Morales, A.; Martínez-Gascueña, J.; Chacón-Vozmediano, J.L.; García-Romero, E.; Hermosín-Gutiérrez, I.; Gómez-Alonso, S. Phenolic Compounds Profile of Different Berry Parts from Novel Vitis vinifera L. Red Grape Genotypes and Tempranillo Using HPLC-DAD-ESI-MS/MS: A Varietal Differentiation Tool. Food Chem. 2019, 295, 350–360. [Google Scholar] [CrossRef]
- Zeng, Y.; Lan, T.; Li, X.; Chen, Y.; Yang, Q.; Qu, B.; Zhang, Y.; Pan, C. A Comparison of the Determination of Multiple Pesticide Residues in Fruits, Vegetables, and Edible Fungi Using Gas Chromatography Combined with Filtration Purification and Solid-Phase Extraction. RSC Adv. 2024, 14, 16898–16911. [Google Scholar] [CrossRef]
- Machado, I.; Gérez, N.; Pistón, M.; Heinzen, H.; Cesio, M.V. Determination of Pesticide Residues in Globe Artichoke Leaves and Fruits by GC–MS and LC–MS/MS Using the Same QuEChERS Procedure. Food Chem. 2017, 227, 227–236. [Google Scholar] [CrossRef]
- Cutillas, V.; García-Gallego, G.; Murcia-Morales, M.; Ferrer, C.; Fernández-Alba, A.R. Beyond Helium: Hydrogen as a Carrier Gas in Multiresidue Pesticide Analysis in Fruits and Vegetables by GC-MS/MS. Anal. Methods 2024, 16, 1564–1569. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Zheng, Z.; Yu, Y.; Xu, J.; Liu, X.; Wu, X.; Dong, F.; Zheng, Y. Supercritical Fluid Chromatography–Tandem Mass Spectrometry-Assisted Methodology for Rapid Enantiomeric Analysis of Fenbuconazole and Its Chiral Metabolites in Fruits, Vegetables, Cereals, and Soil. Food Chem. 2018, 241, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Narenderan, S.T.; Meyyanathan, S.N.; Babu, B. Review of Pesticide Residue Analysis in Fruits and Vegetables. Pre-Treatment, Extraction and Detection Techniques. Food Res. Int. 2020, 133, 109141. [Google Scholar] [CrossRef]
- Liu, D.; Xin-An, Z.; Sun, D.-W. Recent Developments and Applications of Hyperspectral Imaging for Quality Evaluation of Agricultural Products: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1744–1757. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Yoon, H.I.; Lee, H.; Kim, M.; Yang, J.; Jung, D.; Ahn, J.Y.; Park, S.H. Evaluating the Accuracy of Machine Learning Classification Models for Similar Herbal Medicine Using Hyperspectral Imaging. J. Biosyst. Eng. 2024, 49, 156–166. [Google Scholar] [CrossRef]
- Aline, U.; Bhattacharya, T.; Faqeerzada, M.A.; Kim, M.S.; Baek, I.; Cho, B.-K. Advancement of Non-Destructive Spectral Measurements for the Quality of Major Tropical Fruits and Vegetables: A Review. Front. Plant Sci. 2023, 14, 1240361. [Google Scholar] [CrossRef]
- Huang, H.; Liu, L.; Ngadi, M.O. Recent Developments in Hyperspectral Imaging for Assessment of Food Quality and Safety. Sensors 2014, 14, 7248–7276. [Google Scholar] [CrossRef]
- Hu, Y.; Ma, B.; Wang, H.; Li, Y.; Zhang, Y.; Yu, G. Non-Destructive Detection of Different Pesticide Residues on the Surface of Hami Melon Classification Based on THBA-ELM Algorithm and SWIR Hyperspectral Imaging. Foods 2023, 12, 1773. [Google Scholar] [CrossRef]
- Abou Fadel, M.; de Juan, A.; Touati, N.; Vezin, H.; Duponchel, L. New Chemometric Approach MCR-ALS to Unmix EPR Spectroscopic Data from Complex Mixtures. J. Magn. Reson. 2014, 248, 27–35. [Google Scholar] [CrossRef]
- Jaumot, J.; de Juan, A.; Tauler, R. MCR-ALS GUI 2.0: New Features and Applications. Chemom. Intell. Lab. Syst. 2015, 140, 1–12. [Google Scholar] [CrossRef]
- Laborde, A.; Puig-Castellví, F.; Jouan-Rimbaud Bouveresse, D.; Eveleigh, L.; Cordella, C.; Jaillais, B. Detection of Chocolate Powder Adulteration with Peanut Using Near-Infrared Hyperspectral Imaging and Multivariate Curve Resolution. Food Control 2021, 119, 107454. [Google Scholar] [CrossRef]
- Badaró, A.T.; Amigo, J.M.; Blasco, J.; Aleixos, N.; Ferreira, A.R.; Clerici, M.T.P.S.; Barbin, D.F. Near Infrared Hyperspectral Imaging and Spectral Unmixing Methods for Evaluation of Fiber Distribution in Enriched Pasta. Food Chem. 2021, 343, 128517. [Google Scholar] [CrossRef] [PubMed]
- Bayat, M.; Marín-García, M.; Ghasemi, J.B.; Tauler, R. Application of the Area Correlation Constraint in the MCR-ALS Quantitative Analysis of Complex Mixture Samples. Anal. Chim. Acta 2020, 1113, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Park, B.K.; Kwon, S.H.; Yeom, M.S.; Joo, K.S.; Heo, M.J. Detection of Pesticide Residues and Risk Assessment from the Local Fruits and Vegetables in Incheon, Korea. Sci. Rep. 2022, 12, 9613. [Google Scholar] [CrossRef]
- Taejun Agrotech Chlorfenapyr Product Introduction. Available online: http://www.taejun.co.kr/shop/item.php?it_id=1654753637 (accessed on 1 May 2025).
- Hu, Y.; Ma, B.; Wang, H.; Zhang, Y.; Li, Y.; Yu, G. Detecting Different Pesticide Residues on Hami Melon Surface Using Hyperspectral Imaging Combined with 1D-CNN and Information Fusion. Front. Plant Sci. 2023, 14, 1105601. [Google Scholar] [CrossRef]
- King Son Instrument Tech. Co., Ltd. Korean Perilla Leaves. Available online: https://www.kingson-foodtech.com/en/a3-2255/Korean-perilla-leaves.html (accessed on 24 July 2025).
- Viršilė, A.; Gudžinskaitė, I.; Laužikė, K.; Kudirka, G.; Pukalskas, A.; Samuolienė, G. Light Intensity Effects on Productivity and Post-Harvest Quality in Perilla Frutescens Cultivated in CEA. Agriculture 2024, 14, 2079. [Google Scholar] [CrossRef]
- Semyalo, D.; Kim, Y.; Omia, E.; Arief, M.A.; Kim, H.; Sim, E.-Y.; Kim, M.S.; Baek, I.; Cho, B.-K. Nondestructive Identification of Internal Potato Defects Using Visible and Short-Wavelength Near-Infrared Spectral Analysis. Agriculture 2024, 14, 2014. [Google Scholar] [CrossRef]
- Yu, X.; Lu, H.; Wu, D. Development of Deep Learning Method for Predicting Firmness and Soluble Solid Content of Postharvest Korla Fragrant Pear Using Vis/NIR Hyperspectral Reflectance Imaging. Postharvest Biol. Technol. 2018, 141, 39–49. [Google Scholar] [CrossRef]
- He, M.; Li, C.; Cai, Z.; Qi, H.; Zhou, L.; Zhang, C. Leafy Vegetable Freshness Identification Using Hyperspectral Imaging with Deep Learning Approaches. Infrared Phys. Technol. 2024, 138, 105216. [Google Scholar] [CrossRef]
- Olmos, V.; Benítez, L.; Marro, M.; Loza-Alvarez, P.; Piña, B.; Tauler, R.; de Juan, A. Relevant Aspects of Unmixing/Resolution Analysis for the Interpretation of Biological Vibrational Hyperspectral Images. TrAC Trends Anal. Chem. 2017, 94, 130–140. [Google Scholar] [CrossRef]
- Ghaffari, M.; Hugelier, S.; Duponchel, L.; Abdollahi, H.; Ruckebusch, C. Effect of Image Processing Constraints on the Extent of Rotational Ambiguity in MCR-ALS of Hyperspectral Images. Anal. Chim. Acta 2019, 1052, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Lin, C.; Dong, Z.; Xu, J.-L.; He, Y. Early Yield Prediction of Oilseed Rape Using UAV-Based Hyperspectral Imaging Combined with Machine Learning Algorithms. Agriculture 2025, 15, 1100. [Google Scholar] [CrossRef]
- Ghosh, S.S.; Dey, S.; Bhogapurapu, N.; Homayouni, S.; Bhattacharya, A.; McNairn, H. Gaussian Process Regression Model for Crop Biophysical Parameter Retrieval from Multi-Polarized C-Band SAR Data. Remote Sens. 2022, 14, 934. [Google Scholar] [CrossRef]
- Li, Y.; Huang, J. Leaf Anthocyanin Content Retrieval with Partial Least Squares and Gaussian Process Regression from Spectral Reflectance Data. Sensors 2021, 21, 3078. [Google Scholar] [CrossRef]
- Xie, R.; Darvishzadeh, R.; Skidmore, A.; van der Meer, F. Characterizing Foliar Phenolic Compounds and Their Absorption Features in Temperate Forests Using Leaf Spectroscopy. ISPRS J. Photogramm. Remote Sens. 2024, 212, 338–356. [Google Scholar] [CrossRef]
- Lambarki, L.Z.; Jhilal, F.; Slimani, L.; El Hajji, R.; Bakkali, F.; Iskandar, S.; El Jemli, M.; Ihssane, B.; El Ghali, W.; Saffaj, T. Comparison of Approaches for Assessing Detection and Quantitation Limits in Bioanalytical Methods Using HPLC for Sotalol in Plasma. Sci. Rep. 2025, 15, 5472. [Google Scholar] [CrossRef]
- Ermer, J. ICH Q2 (R2): Validation of Analytical Procedures. In Method Validation in Pharmaceutical Analysis: A Guide to Best Practice; Wiley Online Library: Hoboken, NJ, USA, 2025; pp. 351–372. [Google Scholar]
- ICH. ICH Guideline Q2(R2) on Validation of Analytical Procedures—Step 2b; ICH: Geneva, Switzerland, 2022. [Google Scholar]
- ICH. ICH Q2(R2) Guideline on Validation of Analytical Procedures—Step 5; ICH: Geneva, Switzerland, 2024. [Google Scholar]
- Siedliska, A.; Baranowski, P.; Zubik, M.; Mazurek, W.; Sosnowska, B. Detection of Fungal Infections in Strawberry Fruit by VNIR/SWIR Hyperspectral Imaging. Postharvest Biol. Technol. 2018, 139, 115–126. [Google Scholar] [CrossRef]
- Aenugu, H.P.R.; Sathis Kumar, D.; Srisudharson, N.P.; Ghosh, S.S.; Banji, D. Near Infra Red Spectroscopy—An Overview. Int. J. ChemTech Res. 2011, 3, 825–836. [Google Scholar]
- Li, H.; Yang, W.; Lei, J.; She, J.; Zhou, X. Estimation of Leaf Water Content from Hyperspectral Data of Different Plant Species by Using Three New Spectral Absorption Indices. PLoS ONE 2021, 16, e0249351. [Google Scholar] [CrossRef]
- Kagawa, N.; Iguchi, H.; Henzan, M.; Hanaoka, M. Drying the Leaves of Perilla Frutescens Increases Their Content of Anticancer Nutraceuticals. Food Sci. Nutr. 2019, 7, 1494–1501. [Google Scholar] [CrossRef]
- Yang, L.; Gao, H.; Meng, L.; Fu, X.; Du, X.; Wu, D.; Huang, L. Nondestructive Measurement of Pectin Polysaccharides Using Hyperspectral Imaging in Mulberry Fruit. Food Chem. 2021, 334, 127614. [Google Scholar] [CrossRef]
- Dong, J.; Guo, W. Nondestructive Determination of Apple Internal Qualities Using Near-Infrared Hyperspectral Reflectance Imaging. Food Anal. Methods 2015, 8, 2635–2646. [Google Scholar] [CrossRef]
- Wu, X.; Dong, S.; Chen, H.; Guo, M.; Sun, Z.; Luo, H. Perilla Frutescens: A Traditional Medicine and Food Homologous Plant. Chin. Herb. Med. 2023, 15, 369. [Google Scholar] [CrossRef] [PubMed]
- Abdel Ghani, S.B.; Abdallah, O.I. Method Validation and Dissipation Dynamics of Chlorfenapyr in Squash and Okra. Food Chem. 2016, 194, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Aronin, R.; Brázda, P.; Smith, L.N.; Zhang, C.J.; Benedict, J.B.; Marr, Z.Y.; Rybtchinski, B.; Weissman, H.; Shtukenberg, A.G.; Kahr, B. Chlorfenapyr Crystal Polymorphism and Insecticidal Activity. Cryst. Growth Des. 2024, 24, 1284–1292. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, W.; Pang, S.; Lin, Z.; Zhang, Y.; Huang, Y.; Bhatt, P.; Chen, S. Kinetics and New Mechanism of Azoxystrobin Biodegradation by an Ochrobactrum anthropi Strain SH14. Microorganisms 2020, 8, 625. [Google Scholar] [CrossRef]
- Singh, N.; Singh, S.B. Effect of Moisture and Compost on Fate of Azoxystrobin in Soils. J. Environ. Sci. Health Part B 2010, 45, 676–681. [Google Scholar] [CrossRef]
- Corrias, F.; Arru, N.; Atzei, A.; Milia, M.; Scano, E.; Angioni, A. Determination of Pesticide Residues in IV Range Artichoke (Cynara cardunculus L.) and Its Industrial Wastes. Foods 2023, 12, 1807. [Google Scholar] [CrossRef]
- Ministry of Food and Drug Safety. Pesticide MRLs in Food. 2017. Available online: https://www.mfds.go.kr/eng/brd/m_15/view.do?seq=72123&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=3 (accessed on 23 July 2025).
- Hinton, P.R. Mann–Whitney U Test. In Encyclopedia of Research Design; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 2010; pp. 748–750. [Google Scholar]
- Park, Y. Optimal Two-Stage Group Sequential Designs Based on Mann-Whitney-Wilcoxon Test. PLoS ONE 2025, 20, e0318211. [Google Scholar] [CrossRef]


| Datasets | Mean | Min 1 | Max 2 | SD 3 |
|---|---|---|---|---|
| (%) | ||||
| Dataset before split | 0.030 | 0 | 0.060 | 0.020 |
| Calibration set | 0.030 | 0 | 0.060 | 0.022 |
| Test set | 0.030 | 0 | 0.060 | 0.023 |
| Pesticide Residue | Lack of Fit (%) | Variance Explained (%) |
|---|---|---|
| chlorfenapyr | 1.03 | 99 |
| azoxystrobin | 1.78 | 99 |
| Technique | R2c 1 | RMSEC (%) 2 | R2v 3 | RMSEV (%) 4 |
|---|---|---|---|---|
| MCR-ALS-GPR 5 | 0.99 | 0.0013 | 0.99 | 0.0012 |
| PLSR 6 | 0.98 | 0.0033 | 0.97 | 0.0037 |
| SVR 7 | 0.97 | 0.0039 | 0.97 | 0.0037 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semyalo, D.; Joshi, R.; Kim, Y.; Omia, E.; Alal, L.B.; Kim, M.S.; Baek, I.; Cho, B.-K. Short-Wavelength Infrared Hyperspectral Imaging and Spectral Unmixing Techniques for Detection and Distribution of Pesticide Residues on Edible Perilla Leaves. Foods 2025, 14, 2864. https://doi.org/10.3390/foods14162864
Semyalo D, Joshi R, Kim Y, Omia E, Alal LB, Kim MS, Baek I, Cho B-K. Short-Wavelength Infrared Hyperspectral Imaging and Spectral Unmixing Techniques for Detection and Distribution of Pesticide Residues on Edible Perilla Leaves. Foods. 2025; 14(16):2864. https://doi.org/10.3390/foods14162864
Chicago/Turabian StyleSemyalo, Dennis, Rahul Joshi, Yena Kim, Emmanuel Omia, Lorna Bridget Alal, Moon S. Kim, Insuck Baek, and Byoung-Kwan Cho. 2025. "Short-Wavelength Infrared Hyperspectral Imaging and Spectral Unmixing Techniques for Detection and Distribution of Pesticide Residues on Edible Perilla Leaves" Foods 14, no. 16: 2864. https://doi.org/10.3390/foods14162864
APA StyleSemyalo, D., Joshi, R., Kim, Y., Omia, E., Alal, L. B., Kim, M. S., Baek, I., & Cho, B.-K. (2025). Short-Wavelength Infrared Hyperspectral Imaging and Spectral Unmixing Techniques for Detection and Distribution of Pesticide Residues on Edible Perilla Leaves. Foods, 14(16), 2864. https://doi.org/10.3390/foods14162864








