Microalgae-Enriched High-Moisture Meat Analogues: Improved Physicochemical, Functional, and Digestibility Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalgae and Ingredients
2.2. Microalgae Integrated into High-Moisture Extrusion (HME) Experiments
2.3. Physicochemical Properties
2.3.1. Colour Measurements
2.3.2. pH, Moisture Content, and Water Activity
2.3.3. Textural Integrity
2.4. Textural Properties
2.5. Nutritional Properties
2.6. Pigment Determination
2.7. Antioxidant Properties
2.8. Techno-Functional Properties
2.8.1. Rehydration Capacity
2.8.2. Cooking Yield
2.8.3. Water-Holding Capacity
2.8.4. Oil-Holding Capacity
2.9. Characterisation of Extruded Microalgae-Integrated High-Moisture Meat Analogues
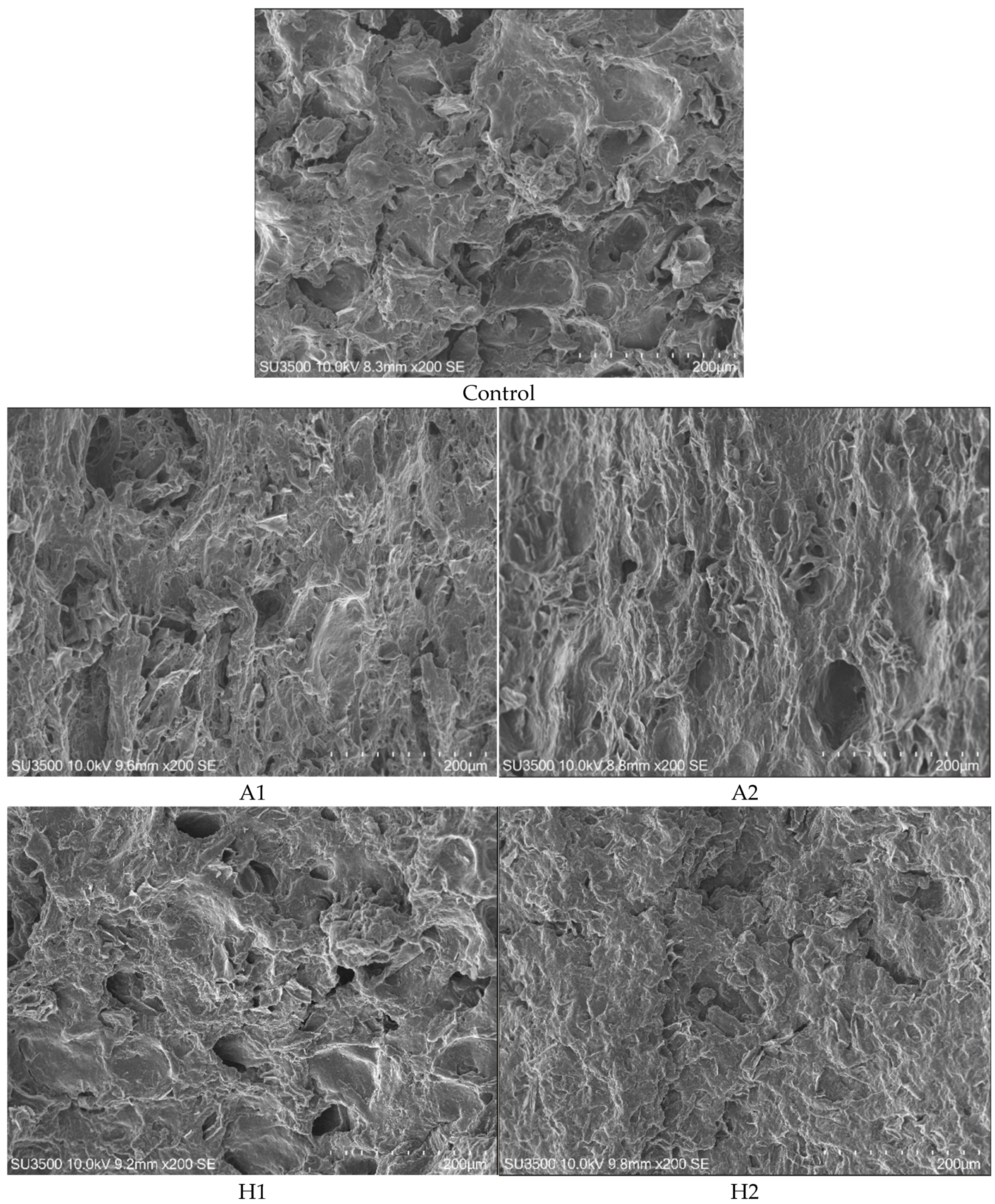
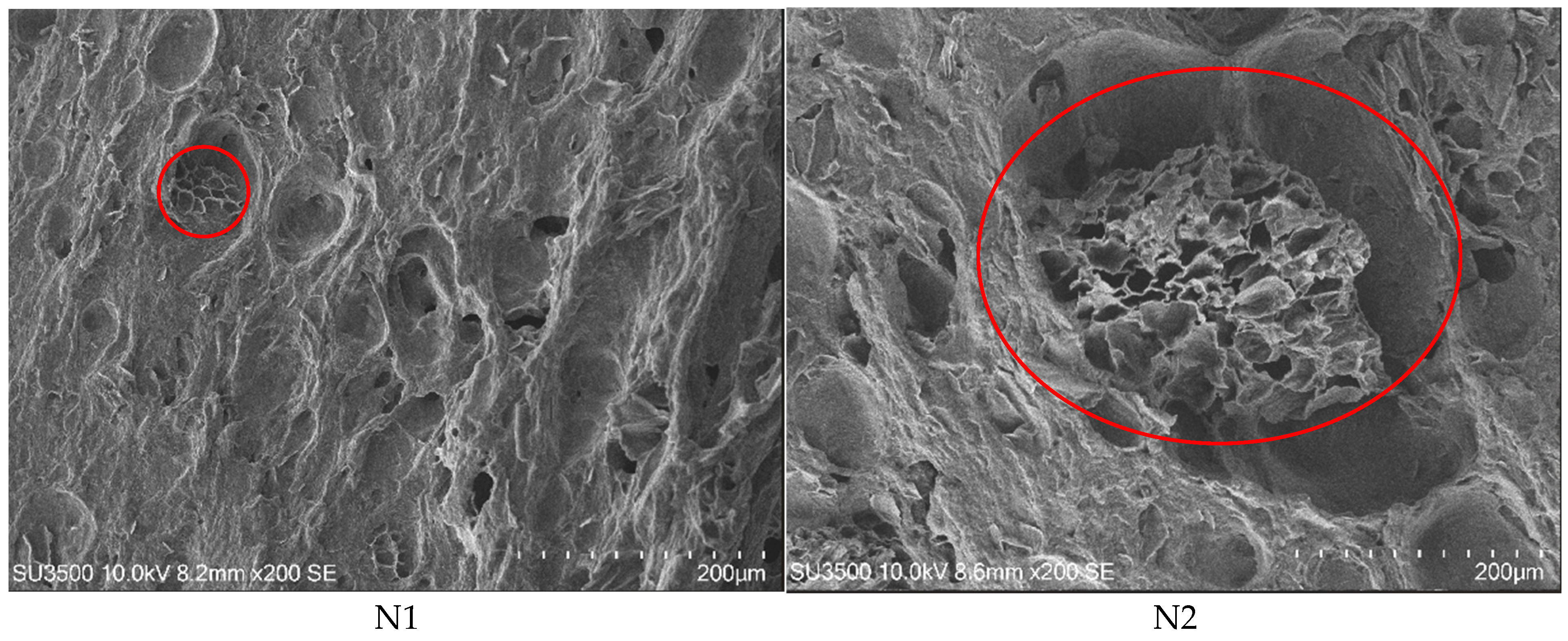
2.9.1. Scanning Electron Microscopy
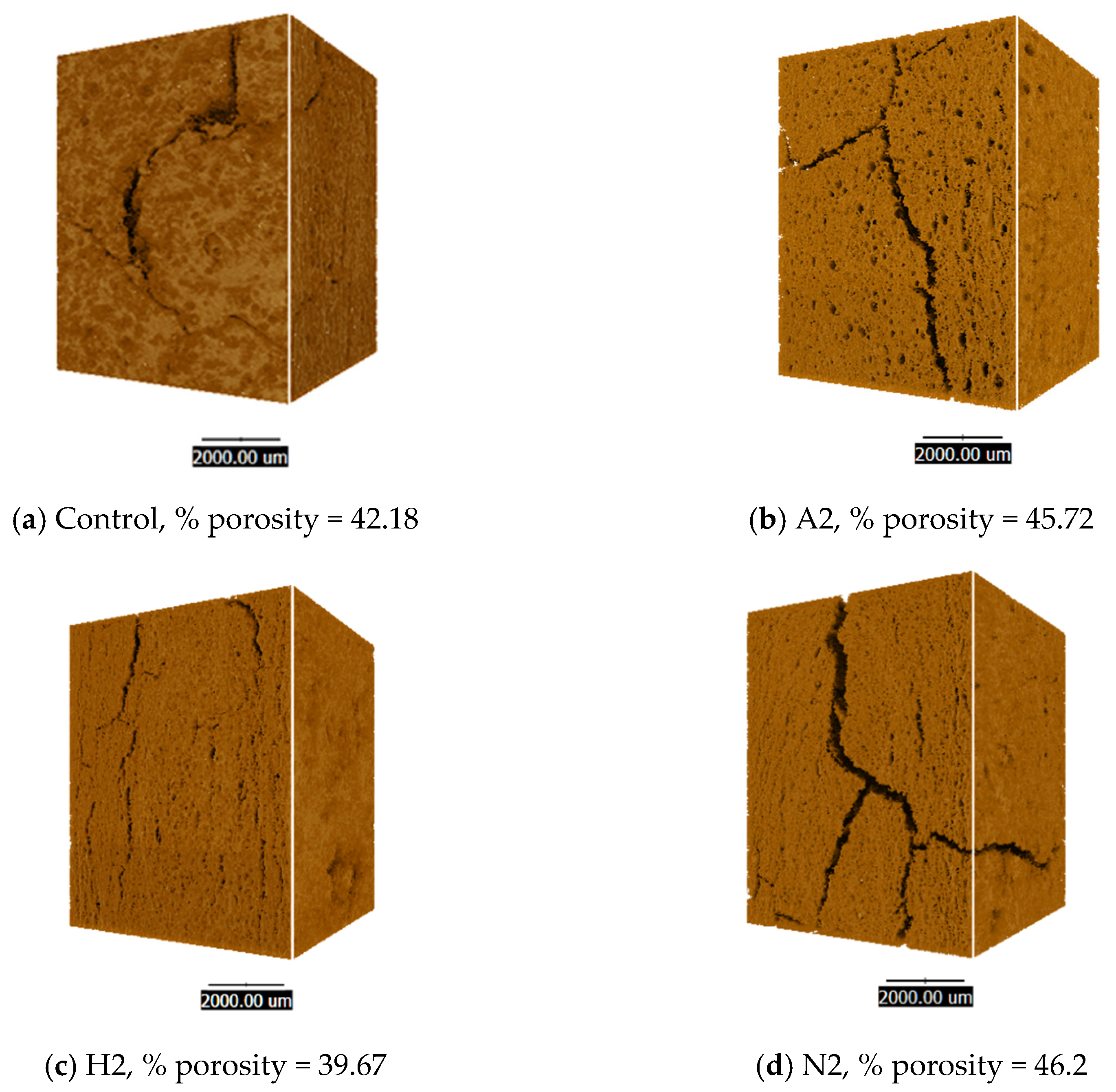
2.9.2. Synchrotron Radiation X-Ray Tomographic Microscopy
2.10. Amino Acid Determination
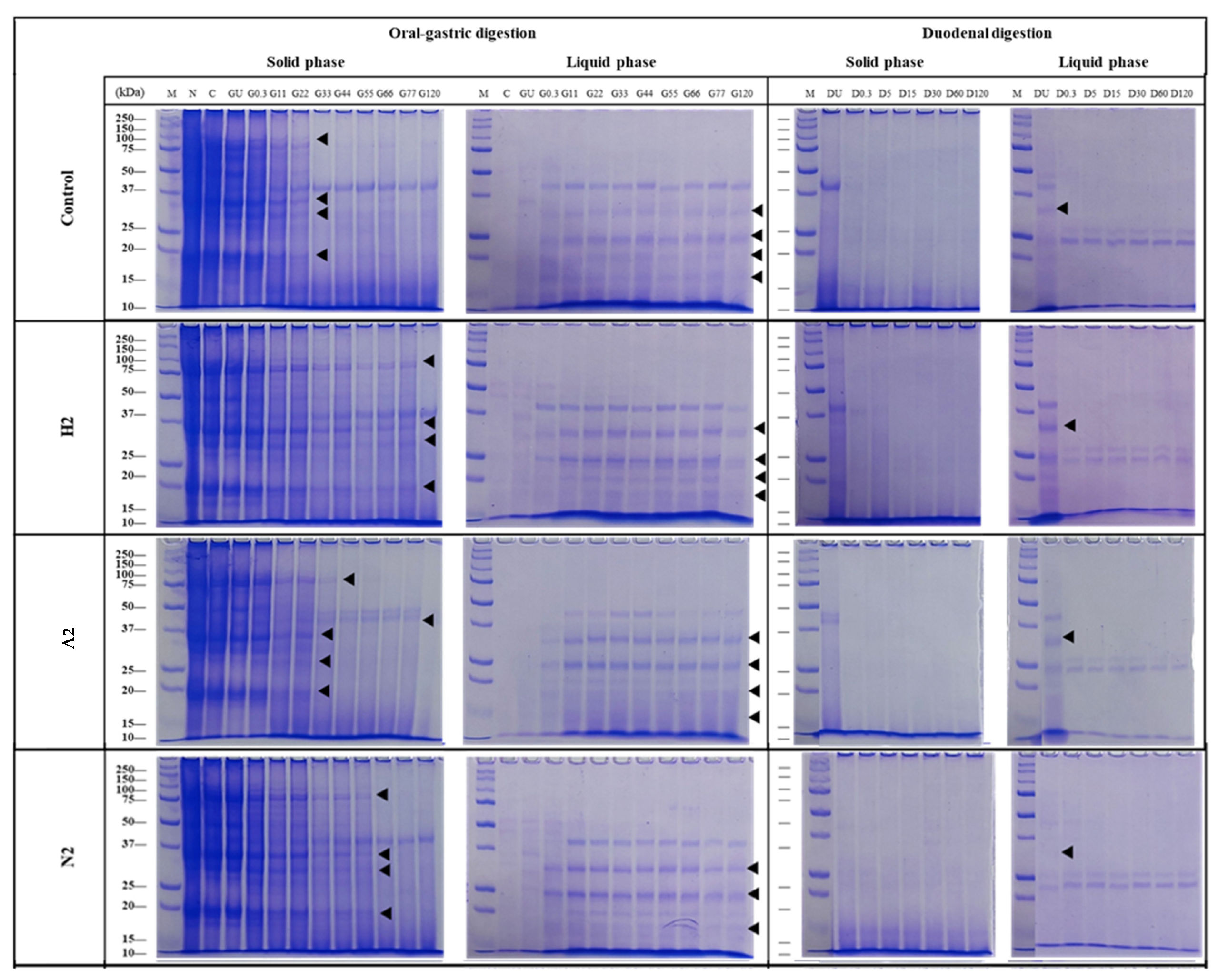
2.11. In Vitro Digestion
- Oral digestion
- Gastric digestion
- Duodenal digestion
- SDS-PAGE
2.12. Statistical Analysis
3. Results
3.1. Physicochemical
3.2. Textural
3.3. Nutritional
3.4. Pigments and Antioxidant
3.5. Techno-Functional
3.6. Characterisation
3.7. Amino Acid Profiles and Digestibility
4. Discussion
4.1. Athrospira-Enriched High-Moisture Meat Analogues
4.2. Haematococcus-Enriched High-Moisture Meat Analogues
4.3. Nannochlropsis-Enriched High-Moisture Meat Analogues
4.4. Properties of Microalgae-Enriched High-Moisture Meat Analogues
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castro, V.; Oliveira, R.; Dias, A.C.P. Microalgae and cyanobacteria as sources of bioactive compounds for cosmetic applications: A systematic review. Algal Res. 2023, 76, 103287. [Google Scholar] [CrossRef]
- Zhou, L.; Li, K.; Duan, X.; Hill, D.; Barrow, C.; Dunshea, F.; Martin, G.; Suleria, H. Bioactive compounds in microalgae and their potential health benefits. Food Biosci. 2022, 49, 101932. [Google Scholar] [CrossRef]
- Eilam, Y.; Khattib, H.; Pintel, N.; Avni, D. Microalgae—Sustainable Source for Alternative Proteins and Functional Ingredients Promoting Gut and Liver Health. Glob. Chall. 2023, 7, 2200177. [Google Scholar] [CrossRef]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for high-value products towards human health and nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef]
- Ampofo, J.; Abbey, L. Microalgae: Bioactive composition, health benefits, safety and prospects as potential high-value ingredients for the functional food industry. Foods 2022, 11, 1744. [Google Scholar] [CrossRef]
- Martínez-Ruiz, F.E.; Andrade-Bustamante, G.; Holguín-Peña, R.J.; Renganathan, P.; Gaysina, L.A.; Sukhanova, N.V.; Puente, E.O.R. Microalgae as Functional Food Ingredients: Nutritional Benefits, Challenges, and Regulatory Considerations for Safe Consumption. Biomass 2025, 5, 25. [Google Scholar] [CrossRef]
- Ayub, A.; Rahayu, F.; Khamidah, A.; Antarlina, S.S.; Iswari, K.; Supriyadi, K.; Mufidah, E.; Singh, A.; Chopra, C.; Wani, A.K. Harnessing microalgae as a bioresource for nutraceuticals: Advancing bioactive compound exploration and shaping the future of health and functional food innovation. Discov. Appl. Sci. 2025, 7, 389. [Google Scholar] [CrossRef]
- Kusmayadi, A.; Leong, Y.K.; Yen, H.-W.; Huang, C.-Y.; Chang, J.-S. Microalgae as sustainable food and feed sources for animals and humans–biotechnological and environmental aspects. Chemosphere 2021, 271, 129800. [Google Scholar] [CrossRef]
- Xu, Y.; Tong, X.; Lu, Y.; Lu, Y.; Wang, X.; Han, J.; Liu, Z.; Ding, J.; Diao, C.; Mumby, W.; et al. Microalgal proteins: Unveiling sustainable alternatives to address the protein challenge. Int. J. Biol. Macromol. 2024, 276, 133747. [Google Scholar] [CrossRef]
- Lucas, B.F.; Brunner, T.A. Attitudes and perceptions towards microalgae as an alternative food: A consumer segmentation in Switzerland. Algal Res. 2024, 78, 103386. [Google Scholar] [CrossRef]
- Panaite, T.D.; Cornescu, G.M.; Predescu, N.C.; Cismileanu, A.; Turcu, R.P.; Saracila, M.; Soica, C. Microalgae (Chlorella vulgaris and Spirulina platensis) as a Protein Alternative and Their Effects on Productive Performances, Blood Parameters, Protein Digestibility, and Nutritional Value of Laying Hens’ Egg. Appl. Sci. 2023, 13, 10451. [Google Scholar] [CrossRef]
- Oslan, S.N.H.; Tan, J.S.; Oslan, S.N.; Matanjun, P.; Mokhtar, R.A.M.; Shapawi, R.; Huda, N. Haematococcus pluvialis as a Potential Source of Astaxanthin with Diverse Applications in Industrial Sectors: Current Research and Future Directions. Molecules 2021, 26, 6470. [Google Scholar] [CrossRef] [PubMed]
- Ha, N.C.; Tam, L.T.; Hien, H.T.M.; Thu, N.T.H.; Hong, D.D.; Thom, L.T. Optimization of Culture Conditions for High Cell Productivity and Astaxanthin Accumulation in Vietnam’s Green Microalgae Haematococcus pluvialis HB and a Neuroprotective Activity of Its Astaxanthin. Bioengineering 2024, 11, 1176. [Google Scholar] [CrossRef] [PubMed]
- Zanella, L.; Vianello, F. Microalgae of the genus Nannochloropsis: Chemical composition and functional implications for human nutrition. J. Funct. Foods 2020, 68, 103919. [Google Scholar] [CrossRef]
- Sathasivam, R.; Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Microalgae metabolites: A rich source for food and medicine. Saudi J. Biol. Sci. 2019, 26, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Godfray, H.C.J.; Aveyard, P.; Garnett, T.; Hall, J.W.; Key, T.J.; Lorimer, J.; Pierrehumbert, R.T.; Scarborough, P.; Springmann, M.; Jebb, S.A. Meat consumption, health, and the environment. Science 2018, 361, eaam5324. [Google Scholar] [CrossRef]
- de Boer, J.; Schösler, H.; Aiking, H. Towards a reduced meat diet: Mindset and motivation of young vegetarians, low, medium and high meat-eaters. Appetite 2017, 113, 387–397. [Google Scholar] [CrossRef]
- Tso, R.; Lim, A.J.; Forde, C.G. A Critical Appraisal of the Evidence Supporting Consumer Motivations for Alternative Proteins. Foods 2021, 10, 24. [Google Scholar] [CrossRef]
- Dekkers, B.L.; Boom, R.M.; van der Goot, A.J. Structuring processes for meat analogues. Trends Food Sci. Technol. 2018, 81, 25–36. [Google Scholar] [CrossRef]
- Pietsch, V.L.; Bühler, J.M.; Karbstein, H.P.; Emin, M.A. High moisture extrusion of soy protein concentrate: Influence of thermomechanical treatment on protein-protein interactions and rheological properties. J. Food Eng. 2019, 251, 11–18. [Google Scholar] [CrossRef]
- Loveday, S.M. Plant protein ingredients with food functionality potential. Nutr. Bull. 2020, 45, 321–327. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, D.; Li, M.; Wen, X.; Ni, Y. The Interactions of Soy Protein and Wheat Gluten for the Development of Meat-like Fibrous Structure. Molecules 2023, 28, 7431. [Google Scholar] [CrossRef] [PubMed]
- Ning, M.; Ji, Y.; Zhang, J.; Pan, H.; Chen, J. The Potential of Soluble Proteins in High-Moisture Soy Protein–Gluten Extrudates Preparation. Polymers 2023, 15, 4686. [Google Scholar] [CrossRef]
- Naik, H.R.; Sekhon, K.S. Influence of defatted soy flour addition on the quality and stability of pretzel type product. J. Food Sci. Technol. 2014, 51, 571–576. [Google Scholar] [CrossRef]
- Pan-utai, W.; Iamtham, S. Chapter 24—Techno-functional properties of microalgae in food products. In Handbook of Food and Feed from Microalgae; Jacob-Lopes, E., Queiroz, M.I., Maroneze, M.M., Zepka, L.Q., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 293–304. [Google Scholar]
- Su, M.; Bastiaens, L.; Verspreet, J.; Hayes, M. Applications of Microalgae in Foods, Pharma and Feeds and Their Use as Fertilizers and Biostimulants: Legislation and Regulatory Aspects for Consideration. Foods 2023, 12, 3878. [Google Scholar] [CrossRef]
- Schreuders, F.K.G.; Schlangen, M.; Kyriakopoulou, K.; Boom, R.M.; van der Goot, A.J. Texture methods for evaluating meat and meat analogue structures: A review. Food Control 2021, 127, 108103. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, Y.; An, H.; Kovacs, Z.; Abddollahi, M.; Sun, Z.; Zhang, G.; Li, C. Utilizing Haematococcus pluvialis to simulate animal meat color in high-moisture meat analogues: Texture quality and color stability. Food Res. Int. 2024, 175, 113685. [Google Scholar] [CrossRef]
- Caporgno, M.P.; Böcker, L.; Müssner, C.; Stirnemann, E.; Haberkorn, I.; Adelmann, H.; Handschin, S.; Windhab, E.J.; Mathys, A. Extruded meat analogues based on yellow, heterotrophically cultivated Auxenochlorella protothecoides microalgae. Innov. Food Sci. Emerg. Technol. 2020, 59, 102275. [Google Scholar] [CrossRef]
- Batista, A.P.; Niccolai, A.; Fradinho, P.; Fragoso, S.; Bursic, I.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Microalgae biomass as an alternative ingredient in cookies: Sensory, physical and chemical properties, antioxidant activity and in vitro digestibility. Algal Res. 2017, 26, 161–171. [Google Scholar] [CrossRef]
- Kyriakopoulou, K.; Dekkers, B.; van der Goot, A.J. Chapter 6—Plant-Based Meat Analogues. In Sustainable Meat Production and Processing; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 103–126. [Google Scholar]
- Li, K.; Duan, X.; Zhou, L.; Hill, D.R.A.; Martin, G.J.O.; Suleria, H.A.R. Bioaccessibility and bioactivities of phenolic compounds from microalgae during in vitro digestion and colonic fermentation. Food Funct. 2023, 14, 899–910. [Google Scholar] [CrossRef]
- da Silva, V.T.; Mateus, N.; de Freitas, V.; Fernandes, A. Plant-Based Meat Analogues: Exploring Proteins, Fibers and Polyphenolic Compounds as Functional Ingredients for Future Food Solutions. Foods 2024, 13, 2303. [Google Scholar] [CrossRef]
- Kumar, R.; Hegde, A.S.; Sharma, K.; Parmar, P.; Srivatsan, V. Microalgae as a sustainable source of edible proteins and bioactive peptides—Current trends and future prospects. Food Res. Int. 2022, 157, 111338. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- García-Encinas, J.P.; Ruiz-Cruz, S.; Juárez, J.; Ornelas-Paz, J.d.J.; Del Toro-Sánchez, C.L.; Márquez-Ríos, E. Proteins from Microalgae: Nutritional, Functional and Bioactive Properties. Foods 2025, 14, 921. [Google Scholar] [CrossRef]
- Pan-utai, W.; Iamtham, S.; Boonbumrung, S.; Mookdasanit, J. Improvement in the Sequential Extraction of Phycobiliproteins from Arthrospira platensis Using Green Technologies. Life 2022, 12, 1896. [Google Scholar] [CrossRef]
- Limsangouan, N.; Rodkwan, N.; Pengpinit, W.; Tumpanuvatr, T.; Pengpinit, P.; Paopun, Y.; Kantrong, H. Physical property changes promoting shelf-life extension of soy protein-based high moisture meat analog under high pressure treatment. J. Food Sci. Technol. 2024, 61, 918–927. [Google Scholar] [CrossRef]
- Kantrong, H.; Prasert, W.; Rodkwan, N.; Pengpinit, W. Influence of Sacha inchi (Plukenetia volubilis L.) oil and extrusion process parameters on the quality of soya protein-based meat extender: An optimization approach. J. Food Process. Preserv. 2022, 46, e17140. [Google Scholar] [CrossRef]
- Charlie, E.A.; Angrainy, H.; Kantrong, H. Exploring the use of rice bran and mung bean as soy substitutes in low-moisture extruded plant-based meat. Innov. Food Sci. Emerg. Technol. 2025, 100, 103916. [Google Scholar] [CrossRef]
- Horwitz, W. Official methods of analysis of AOAC International. Volume I, agricultural chemicals, contaminants, drugs/edited by William Horwitz. AOAC Int. 2010, 1. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Pan-utai, W.; Boonpok, S.; Pornpukdeewattana, S. Combination of mechanical and chemical extraction of astaxanthin from Haematococcus pluvialis and its properties of microencapsulation. Biocatal. Agric. Biotechnol. 2021, 33, 101979. [Google Scholar] [CrossRef]
- Park, W.S.; Kim, H.-J.; Li, M.; Lim, D.H.; Kim, J.; Kwak, S.-S.; Kang, C.-M.; Ferruzzi, M.G.; Ahn, M.-J. Two Classes of Pigments, Carotenoids and C-Phycocyanin, in Spirulina Powder and Their Antioxidant Activities. Molecules 2018, 23, 2065. [Google Scholar] [CrossRef]
- Campos Assumpção de Amarante, M.; Cavalcante Braga, A.R.; Sala, L.; Juliano Kalil, S. Colour stability and antioxidant activity of C-phycocyanin-added ice creams after in vitro digestion. Food Res. Int. 2020, 137, 109602. [Google Scholar] [CrossRef] [PubMed]
- Renugadevi, K.; Valli Nachiyar, C.; Sowmiya, P.; Sunkar, S. Antioxidant activity of phycocyanin pigment extracted from marine filamentous cyanobacteria Geitlerinema sp TRV57. Biocatal. Agric. Biotechnol. 2018, 16, 237–242. [Google Scholar] [CrossRef]
- Gulzar, S.; Tagrida, M.; Martín-Belloso, O.; Soliva-Fortuny, R. Optimizing high-moisture meat analogue textures through Artificial Intelligence: The effect of sorbitol in soy protein concentrate blends. LWT 2025, 217, 117416. [Google Scholar] [CrossRef]
- Limaye, A. Drishti, A Volume Exploration and Presentation Tool. In Proceedings of the SPIE Optical Engineering + Applications, San Diego, CA, USA, 17 October 2012; Volume 8506, p. 85060X. [Google Scholar]
- Dai, Z.; Wu, Z.; Jia, S.; Wu, G. Analysis of amino acid composition in proteins of animal tissues and foods as pre-column o-phthaldialdehyde derivatives by HPLC with fluorescence detection. J. Chromatogr. B 2014, 964, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Pantoa, T.; Baricevic-Jones, I.; Suwannaporn, P.; Kadowaki, M.; Kubota, M.; Roytrakul, S.; Mills, E.N.C. Young rice protein as a new source of low allergenic plant-base protein. J. Cereal Sci. 2020, 93, 102970. [Google Scholar] [CrossRef]
- Pan-utai, W.; Pantoa, T.; Roytrakul, S.; Praiboon, J.; Kosawatpat, P.; Tamtin, M.; Thongdang, B. Ultrasonic-Assisted Extraction and Antioxidant Potential of Valuable Protein from Ulva rigida Macroalgae. Life 2023, 13, 86. [Google Scholar] [CrossRef] [PubMed]
- Chini Zittelli, G.; Lauceri, R.; Faraloni, C.; Silva Benavides, A.M.; Torzillo, G. Valuable pigments from microalgae: Phycobiliproteins, primary carotenoids, and fucoxanthin. Photochem. Photobiol. Sci. 2023, 22, 1733–1789. [Google Scholar] [CrossRef]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.-T.; Show, P.-L. Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Dolganyuk, V.; Sukhikh, S.; Kalashnikova, O.; Ivanova, S.; Kashirskikh, E.; Prosekov, A.; Michaud, P.; Babich, O. Food proteins: Potential resources. Sustainability 2023, 15, 5863. [Google Scholar] [CrossRef]
- Lestingi, A.; Alagawany, M.; Di Cerbo, A.; Crescenzo, G.; Zizzadoro, C. Spirulina (Arthrospira platensis) Used as Functional Feed Supplement or Alternative Protein Source: A Review of the Effects of Different Dietary Inclusion Levels on Production Performance, Health Status, and Meat Quality of Broiler Chickens. Life 2024, 14, 1537. [Google Scholar] [CrossRef] [PubMed]
- Podgórska-Kryszczuk, I. Spirulina—An Invaluable Source of Macro- and Micronutrients with Broad Biological Activity and Application Potential. Molecules 2024, 29, 5387. [Google Scholar] [CrossRef]
- Benković, M.; Jurinjak Tušek, A.; Sokač Cvetnić, T.; Jurina, T.; Valinger, D.; Gajdoš Kljusurić, J. An Overview of Ingredients Used for Plant-Based Meat Analogue Production and Their Influence on Structural and Textural Properties of the Final Product. Gels 2023, 9, 921. [Google Scholar] [CrossRef]
- Bashir, S.; Sharif, M.K.; Butt, M.S.; Rizvi, S.S.H.; Paraman, I.; Ejaz, R. Preparation of Micronutrients Fortified Spirulina Supplemented Rice-Soy Crisps Processed Through Novel Supercritical Fluid Extrusion. J. Food Process. Preserv. 2017, 41, e12986. [Google Scholar] [CrossRef]
- Franco Lucas, B.; Morais, M.; Duarte Santos, T.; Costa, J.A. Effect of Spirulina addition on the physicochemical and structural properties of extruded snacks. Food Sci. Technol. 2017, 37, 16–23. [Google Scholar] [CrossRef]
- Silva, P.C.d.; Toledo, T.; Brião, V.; Bertolin, T.E.; Costa, J.A.V. Development of extruded snacks enriched by bioactive peptides from microalga Spirulina sp. LEB 18. Food Biosci. 2021, 42, 101031. [Google Scholar] [CrossRef]
- Grahl, S.; Palanisamy, M.; Strack, M.; Meier-Dinkel, L.; Toepfl, S.; Mörlein, D. Towards more sustainable meat alternatives: How technical parameters affect the sensory properties of extrusion products derived from soy and algae. J. Clean. Prod. 2018, 198, 962–971. [Google Scholar] [CrossRef]
- Grahl, S.; Strack, M.; Mensching, A.; Mörlein, D. Alternative protein sources in Western diets: Food product development and consumer acceptance of spirulina-filled pasta. Food Qual. Prefer. 2020, 84, 103933. [Google Scholar] [CrossRef]
- Nakib, D.; Ibrahim, M.; Mahmoud, N.; Abd, E.; Rahman, E.; Ghaly, A.; Preti, R. Incorporation of Spirulina (Athrospira platensis) in Traditional Egyptian Cookies as a Source of Natural Bioactive Molecules and Functional Ingredients: Preparation and Sensory Evaluation of Nutrition Snack for School Children. Eur. J. Nutr. Food Saf. 2019, 9, 372–397. [Google Scholar] [CrossRef]
- Šárka, E.; Sluková, M.; Henke, S. Changes in Phenolics during Cooking Extrusion: A Review. Foods 2021, 10, 2100. [Google Scholar] [CrossRef]
- Chiang, J.H.; Loveday, S.M.; Hardacre, A.K.; Parker, M.E. Effects of soy protein to wheat gluten ratio on the physicochemical properties of extruded meat analogues. Food Struct. 2019, 19, 100102. [Google Scholar] [CrossRef]
- Ramos-Enríquez, J.R.; Ramírez-Wong, B.; Robles-Sánchez, R.M.; Robles-Zepeda, R.E.; González-Aguilar, G.A.; Gutiérrez-Dorado, R. Effect of Extrusion Conditions and the Optimization of Phenolic Compound Content and Antioxidant Activity of Wheat Bran Using Response Surface Methodology. Plant Foods Hum Nutr 2018, 73, 228–234. [Google Scholar] [CrossRef]
- Pogorzelska, E.; Godziszewska, J.; Brodowska, M.; Wierzbicka, A. Antioxidant potential of Haematococcus pluvialis extract rich in astaxanthin on colour and oxidative stability of raw ground pork meat during refrigerated storage. Meat Sci. 2018, 135, 54–61. [Google Scholar] [CrossRef]
- Carballo, D.E.; Giráldez, F.J.; Andrés, S.; Caro, I.; Fernández-Gutiérrez, M.; Mateo, J. Effects of dietary astaxanthin supplementation on the oxidative stability of meat from suckling lambs fed a commercial milk-replacer containing butylated hydroxytoluene. Meat Sci. 2019, 156, 68–74. [Google Scholar] [CrossRef]
- Seo, J.-K.; Parvin, R.; Park, J.; Yang, H.-S. Utilization of Astaxanthin as a Synthetic Antioxidant Replacement for Emulsified Sausages. Antioxidants 2021, 10, 407. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, Y.; Zhu, L.; Zhao, X. Effects of Haematococcus pluvialis Addition on the Sensory Properties of Plant-Based Meat Analogues. Foods 2023, 12, 3435. [Google Scholar] [CrossRef]
- Zhou, Q.; Xu, J.; Yang, L.; Gu, C.; Xue, C. Thermal stability and oral absorbability of astaxanthin esters from Haematococcus pluvialis in Balb/c mice. J. Sci. Food Agric. 2019, 99, 3662–3671. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Sakai, K.; Zhang, J.; McClements, D.J. Plant-based meat analogs: Color challenges and coloring agents. Food Nutr. Health 2024, 1, 4. [Google Scholar] [CrossRef]
- Zhao, L.; Khang, H.M.; Du, J. Incorporation of microalgae (Nannochloropsis oceanica) into plant-based fishcake analogue: Physical property characterisation and in vitro digestion analysis. Food Hydrocoll. 2024, 146, 109212. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kesavan, R.k.; Babu, P.A.S.; Sivarajan, M.; Sukumar, M. Functional Foods Enriched with Marine Microalga Nannochloropsis oculata as a Source of ω-3 Fatty Acids. Food Technol. Biotechnol. 2014, 52, 292–299. [Google Scholar]
- Piyatilleke, S.; Thevarajah, B.; Nimarshana, P.H.V.; Ariyadasa, T.U. Large-scale production of Nannochloropsis-derived EPA: Current status and perspectives via a biorefinery context. Food Bioprod. Process. 2024, 148, 255–268. [Google Scholar] [CrossRef]
- Olsen, M.L.; Olsen, K.; Jensen, P.E. Consumer acceptance of microalgae as a novel food—Where are we now? And how to get further. Physiol. Plant. 2024, 176, e14337. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Qazanfarzadeh, Z.; Majzoobi, M.; Sheiband, S.; Oladzadabbasabad, N.; Esmaeili, Y.; Barrow, C.J.; Timms, W. Alternative proteins; A path to sustainable diets and environment. Curr. Res. Food Sci. 2024, 9, 100882. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-R.; Peng, N.; Li, Y.-Q.; Liang, Y.; Guo, Z.-W.; Wang, C.-Y.; Wang, Z.-Y.; Wang, C.; Ren, X. Physicochemical properties, structural characteristics and protein digestibility of pea protein-wheat gluten composited meat analogues prepared via high-moisture extrusion. Food Hydrocoll. 2024, 156, 110283. [Google Scholar] [CrossRef]
- Lee, J.-S.; Oh, H.; Choi, I.; Yoon, C.S.; Han, J. Physico-chemical characteristics of rice protein-based novel textured vegetable proteins as meat analogues produced by low-moisture extrusion cooking technology. LWT 2022, 157, 113056. [Google Scholar] [CrossRef]
- Pöri, P.; Nisov, A.; Nordlund, E. Enzymatic modification of oat protein concentrate with trans- and protein-glutaminase for increased fibrous structure formation during high-moisture extrusion processing. LWT 2022, 156, 113035. [Google Scholar] [CrossRef]
- Palanisamy, M.; Franke, K.; Berger, R.G.; Heinz, V.; Töpfl, S. High moisture extrusion of lupin protein: Influence of extrusion parameters on extruder responses and product properties. J. Sci. Food Agric. 2019, 99, 2175–2185. [Google Scholar] [CrossRef] [PubMed]
- Saldanha do Carmo, C.; Knutsen, S.H.; Malizia, G.; Dessev, T.; Geny, A.; Zobel, H.; Myhrer, K.S.; Varela, P.; Sahlstrøm, S. Meat analogues from a faba bean concentrate can be generated by high moisture extrusion. Future Foods 2021, 3, 100014. [Google Scholar] [CrossRef]
- Yu, J.; Wang, L.; Zhang, Z. Plant-based meat proteins: Processing, nutrition composition, and future prospects. Foods 2023, 12, 4180. [Google Scholar] [CrossRef]
- Aghagholizadeh, R.; Rigi, A.A. High-Moisture Extrusion in Plant-Based Meat: Challenges and Emerging Trends. J. Food Process Eng. 2025, 48, e70107. [Google Scholar] [CrossRef]
- Wang, Y.; Tibbetts, S.M.; McGinn, P.J. Microalgae as Sources of High-Quality Protein for Human Food and Protein Supplements. Foods 2021, 10, 3002. [Google Scholar] [CrossRef] [PubMed]
- Joint WHO/FAO/UNU Expert Consultation. Protein and amino acid requirements in human nutrition. In World Health Organization Technical Report Series; World Health Organization: Genève, Switzerland, 2007; p. 1. [Google Scholar]
- Irvani, N.; Leong, S.Y.; Carne, A.; Agyei, D.; Oey, I. Impact of High-Speed Homogenisation Followed by pH Treatment of Arthrospira platensis on Protein Accessibility and In Vitro Protein Digestibility. Food Bioprocess Technol. 2024, 17, 2421–2434. [Google Scholar] [CrossRef]
- Aydin, S. Enhancement of microbial diversity and methane yield by bacterial bioaugmentation through the anaerobic digestion of Haematococcus pluvialis. Appl. Microbiol. Biotechnol. 2016, 100, 5631–5637. [Google Scholar] [CrossRef]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications--a review. Mar Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- García-Vaquero, M.; Brunton, N.; Lafarga, T. Chapter 7—Microalgae as a source of pigments for food applications. In Cultured Microalgae for the Food Industry; Lafarga, T., Acién, G., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 177–198. [Google Scholar]
- Bakhsh, A.; Park, J.; Baritugo, K.A.; Kim, B.; Sil Moon, S.; Rahman, A.; Park, S. A holistic approach toward development of plant-based meat alternatives through incorporation of novel microalgae-based ingredients. Front. Nutr. 2023, 10, 1110613. [Google Scholar] [CrossRef] [PubMed]
- Uribe-Wandurraga, Z.N.; Martínez-Sánchez, I.; Savall, C.; García-Segovia, P.; Martínez-Monzó, J. Microalgae fortification of low-fat oil-in-water food emulsions: An evaluation of the physicochemical and rheological properties. J. Food Sci. Technol. 2021, 58, 3701–3711. [Google Scholar] [CrossRef] [PubMed]
- Teuling, E.; Schrama, J.W.; Gruppen, H.; Wierenga, P.A. Characterizing emulsion properties of microalgal and cyanobacterial protein isolates. Algal Res. 2019, 39, 101471. [Google Scholar] [CrossRef]
- Ubbink, J.; Muhialdin, B.J. Protein physical state in meat analogue processing. Curr. Opin. Food Sci. 2022, 45, 100822. [Google Scholar] [CrossRef]
- De Angelis, D.; van der Goot, A.J.; Pasqualone, A.; Summo, C. Advancements in texturization processes for the development of plant-based meat analogs: A review. Curr. Opin. Food Sci. 2024, 58, 101192. [Google Scholar] [CrossRef]



| Composition | Protein | Lipid | Fibre | Ash | Carbohydrate |
|---|---|---|---|---|---|
| A. platensis | 52.99 ± 3.78 c | 1.12 ± 0.07 a | 9.76 ± 0.08 c | 5.51 ± 0.18 b | 30.63 ± 3.45 a |
| H. pluvialis | 15.41 ± 1.51 a | 3.91 ± 0.06 b | 6.79 ± 0.28 b | 2.37 ± 0.21 a | 71.52 ± 1.93 c |
| N. oculata | 33.85 ± 0.25 b | 6.87 ± 0.10 c | 4.58 ± 0.66 a | 16.14 ± 0.01 c | 38.56 ± 0.30 b |
| Experiment | L* | a* | b* |
|---|---|---|---|
| Control | 74.21 ± 0.36 f | 2.06 ± 0.12 c | 21.91 ± 0.42 cd |
| A1 | 67.58 ± 0.99 e | −0.71 ± 0.05 b | 18.76 ± 0.25 b |
| A2 | 59.58 ± 0.37 b | −3.34 ± 0.04 a | 17.03 ± 0.19 a |
| H1 | 61.77 ± 0.77 c | 18.16 ± 0.61 d | 18.67 ± 0.33 b |
| H2 | 43.76 ± 0.85 a | 26.73 ± 0.24 e | 16.76 ± 0.31 a |
| N1 | 73.46 ± 0.52 f | 1.85 ± 0.10 c | 21.48 ± 0.29 c |
| N2 | 66.02 ± 0.53 d | 1.80 ± 0.11 c | 22.13 ± 0.25 d |
| Experiment | pH ns | Moisture Content (% Wet Basis) | Water Activity (aw) | Textural Integrity (%) |
|---|---|---|---|---|
| Control | 5.67 ± 0.05 | 62.38 ± 0.16 b | 0.89 ± 0.00 a | 28.72 ± 1.21 a |
| A1 | 6.12 ± 0.06 | 64.82 ± 0.07 d | 0.89 ± 0.00 a | 30.01 ± 0.67 a |
| A2 | 4.68 ± 2.19 | 65.58 ± 0.14 e | 0.89 ± 0.00 a | 29.47 ± 2.59 a |
| H1 | 6.71 ± 0.10 | 63.17 ± 0.16 c | 0.89 ± 0.00 a | 29.60 ± 1.46 a |
| H2 | 6.65 ± 0.01 | 55.51 ± 0.31 a | 0.89 ± 0.00 a | 28.47 ± 0.37 a |
| N1 | 5.35 ± 0.01 | 62.22 ± 0.24 b | 0.90 ± 0.00 b | 37.15 ± 0.44 b |
| N2 | 5.94 ± 0.01 | 67.51 ± 0.02 f | 0.91 ± 0.01 b | 39.16 ± 4.20 b |
| Experiment | Cutting Force (N) | Hardness (N) | Chewiness (N) |
|---|---|---|---|
| Control | 19.29 ± 1.87 a | 137.06 ± 8.53 c | 85.85 ± 4.38 c |
| A1 | 26.72 ± 6.42 b | 128.35 ± 9.48 bc | 87.02 ± 6.47 c |
| A2 | 24.43 ± 2.72 b | 86.68 ± 7.08 a | 60.35 ± 6.85 b |
| H1 | 25.13 ± 1.69 b | 132.86 ± 8.48 c | 84.69 ± 5.99 c |
| H2 | 38.91 ± 2.75 c | 120.08 ± 6.17 b | 75.48 ± 5.33 b |
| N1 | 25.13 ± 2.74 b | 93.20 ± 7.27 a | 67.87 ± 5.12 ab |
| N2 | 27.94 ± 5.87 b | 133.25 ± 9.57 c | 97.06 ± 7.40 d |
| Experiment | Protein ns | Lipid | Fibre | Ash ns | Carbohydrate ns |
|---|---|---|---|---|---|
| Control | 62.13 ± 0.35 | 0.18 ± 0.04 ab | 1.34 ± 0.17 abc | 4.08 ± 0.62 | 32.28 ± 0.14 |
| A1 | 58.88 ± 4.69 | 0.59 ± 0.21 c | 1.49 ± 0.03 c | 4.29 ± 0.08 | 32.75 ± 4.79 |
| A2 | 59.06 ± 1.70 | 0.18 ± 0.06 ab | 1.17 ± 0.11 ab | 4.31 ± 0.07 | 35.28 ± 1.80 |
| H1 | 56.24 ± 0.43 | 0.36 ± 0.06 b | 1.13 ± 0.11 a | 4.27 ± 0.13 | 37.99 ± 0.51 |
| H2 | 58.58 ± 1.97 | 0.10 ± 0.00 a | 1.39 ± 0.07 bc | 3.95 ± 0.19 | 35.98 ± 2.08 |
| N1 | 61.05 ± 3.61 | 0.34 ± 0.02 b | 1.38 ± 0.03 bc | 3.98 ± 0.03 | 33.25 ± 3.54 |
| N2 | 61.19 ± 0.37 | 0.28 ± 0.06 ab | 1.16 ± 0.04 ab | 3.93 ± 0.04 | 33.44 ± 0.43 |
| Experiment | Total Chlorophyll (mg/mg) | Total Carotenoids (µg/mg) |
|---|---|---|
| Control | ND | ND |
| A1 | 7.78 ± 0.38 ab | 4.11 ± 0.30 b |
| A2 | 19.91 ± 1.05 d | 7.01 ± 0.28 c |
| H1 | 6.52 ± 1.11 ab | 13.43 ± 0.99 d |
| H2 | 9.45 ± 1.22 bc | 34.59 ± 1.80 e |
| N1 | 5.60 ± 0.75 a | 2.01 ± 0.19 a |
| N2 | 12.11 ± 2.31 c | 3.02 ± 0.36 ab |
| Experiment | TPC | ABTS | FRAP |
|---|---|---|---|
| (mg GAE/g) | (mg AAE/g) | (mg AAE/g) | |
| Control | 2.29 ± 0.02 b | 0.47 ± 0.00 a | 0.48 ± 0.04 a |
| A1 | 1.09 ± 0.07 a | 0.65 ± 0.08 b | 0.87 ± 0.06 c |
| A2 | 2.40 ± 0.19 bc | 0.68 ± 0.02 b | 0.81 ± 0.08 c |
| H1 | 2.60 ± 0.07 d | 0.89 ± 0.08 c | 0.68 ± 0.01 b |
| H2 | 2.23 ± 0.09 b | 0.84 ± 0.03 c | 0.98 ± 0.05 d |
| N1 | 2.86 ± 0.13 e | 0.39 ± 0.01 a | 0.68 ± 0.01 b |
| N2 | 2.56 ± 0.06 cd | 0.46 ± 0.06 a | 0.67 ± 0.04 b |
| Experiment | Rehydration Capacity (%) | Cooking Yield (%) |
|---|---|---|
| Control | 22.38 ± 1.02 ab | 122.96 ± 0.11 bc |
| A1 | 31.95 ± 0.40 d | 124.76 ± 0.09 d |
| A2 | 28.20 ± 1.59 c | 125.58 ± 0.80 d |
| H1 | 21.39 ± 0.71 ab | 122.52 ± 0.01 b |
| H2 | 20.37 ± 0.29 a | 123.92 ± 0.00 cd |
| N1 | 26.44 ± 1.64 c | 119.42 ± 0.82 a |
| N2 | 24.99 ± 3.08 bc | 124.39 ± 0.20 a |
| Amino Acid | Control | A2 | H2 | N2 |
|---|---|---|---|---|
| Aspartic acid * | 4929.95 ± 181.57 | 5582.65 ± 453.68 | 4961.99 ± 466.57 | 5647.08 ± 498.53 |
| Glutamic acid * | 13,508.61 ± 490.81 | 14,862.75 ± 1406.91 | 13,418.75 ± 1319.40 | 15,323.39 ± 1223.74 |
| Serine * | 2705.74 ± 119.46 | 3088.08 ± 334.52 | 2748.53 ± 263.70 | 3123.60 ± 242.23 |
| Histidine * | 1010.50 ± 41.41 | 1140.27 ± 78.46 | 1010.99 ± 94.33 | 1133.40 ± 92.68 |
| Glycine * | 2248.64 ± 66.46 | 2614.27 ± 291.09 | 2329.51 ± 198.72 | 2570.41 ± 159.58 |
| Threonine * | 1183.85 ± 75.86 | 1312.34 ± 130.88 | 1170.69 ± 116.70 | 1361.08 ± 115.71 |
| Arginine * | 2257.15 ± 119.86 | 2429.65 ± 151.94 | 2341.98 ± 235.93 | 2659.62 ± 240.47 |
| Alanine * | 1896.25 ± 71.96 | 2176.26 ± 239.38 | 1920.97 ± 171.75 | 2189.91 ± 157.72 |
| Tyrosine * | 1351.16 ± 63.12 | 1523.61 ± 104.25 | 1339.85 ± 135.63 | 1564.79 ± 133.28 |
| Cysteine | 5311.31 ± 144.66 b | 4410.39 ± 275.73 a | 4274.20 ± 542.86 a | 5834.98 ± 57.36 b |
| Valine * | 777.03 ± 61.73 | 860.14 ± 77.87 | 773.98 ± 78.41 | 888.15 ± 76.66 |
| Methionine | 453.56 ± 24.00 a | 544.82 ± 31.18 ab | 476.14 ± 41.99 ab | 565.20 ± 45.44 b |
| Phenylalanine * | 2066.80 ± 103.87 | 2269.81 ± 201.21 | 2053.52 ± 202.70 | 2360.23 ± 191.64 |
| Isoleucine * | 646.84 ± 58.52 | 712.03 ± 63.99 | 642.14 ± 66.27 | 751.04 ± 69.15 |
| Leucine * | 2869.74 ± 160.77 | 3186.19 ± 281.31 | 2867.85 ± 283.23 | 3297.15 ± 272.44 |
| Lysine * | 1818.85 ± 70.72 | 1987.37 ± 150.05 | 1801.92 ± 173.20 | 2061.38 ± 153.50 |
| Tryptophan * | 190.42 ± 16.96 | 194.94 ± 16.98 | 196.77 ± 17.41 | 195.83 ± 3.26 |
| Proline | 2739.28 ± 34.15d | 1188.55 ± 113.23 a | 1677.74 ± 40.57 b | 2255.17 ± 45.05 c |
| Microalgae Species | Key Nutrients and Properties | Main Functional Effects | Optimal Inclusion Level in HMMA | Potential Limitations |
|---|---|---|---|---|
| A. platensis | High protein | Improved textural integrity, high protein digestibility, enhanced antioxidant capacity | 1.5% | Earthy/musty odour at higher levels |
| H. pluvialis | High carotenoids (astaxanthin) | Stable red pigmentation after cooking, antioxidant activity | 1.5% | Slower gastric digestion due to the cell wall structure |
| N. oculata | High lipid (EPA), carotenoids, chlorophyll | Stable green colour | 1.5% | May impart a fishy odour, slower gastric digestibility |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan-utai, W.; Pantoa, T.; Prasert, W.; Sangkhiaw, J.; Rojviriya, C.; Phoovasawat, C.; Kantrong, H. Microalgae-Enriched High-Moisture Meat Analogues: Improved Physicochemical, Functional, and Digestibility Properties. Foods 2025, 14, 2838. https://doi.org/10.3390/foods14162838
Pan-utai W, Pantoa T, Prasert W, Sangkhiaw J, Rojviriya C, Phoovasawat C, Kantrong H. Microalgae-Enriched High-Moisture Meat Analogues: Improved Physicochemical, Functional, and Digestibility Properties. Foods. 2025; 14(16):2838. https://doi.org/10.3390/foods14162838
Chicago/Turabian StylePan-utai, Wanida, Thidarat Pantoa, Waraporn Prasert, Janya Sangkhiaw, Catleya Rojviriya, Chalermluck Phoovasawat, and Hataichanok Kantrong. 2025. "Microalgae-Enriched High-Moisture Meat Analogues: Improved Physicochemical, Functional, and Digestibility Properties" Foods 14, no. 16: 2838. https://doi.org/10.3390/foods14162838
APA StylePan-utai, W., Pantoa, T., Prasert, W., Sangkhiaw, J., Rojviriya, C., Phoovasawat, C., & Kantrong, H. (2025). Microalgae-Enriched High-Moisture Meat Analogues: Improved Physicochemical, Functional, and Digestibility Properties. Foods, 14(16), 2838. https://doi.org/10.3390/foods14162838







