Synergistic Enhancement of Paramylon Production in Edible Microalga Euglena gracilis via Ethanol-Guaiacol Co-Regulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical, Reagent and Algal Strain
2.2. Preparation of Stock Solutions and Experimental Design
2.3. Cell Growth Analysis
2.4. GA Quantification
2.5. Photosynthetic Pigments Analysis
2.6. Paramylon Content and Yield
2.7. Statistical Analysis
3. Results and Discussion
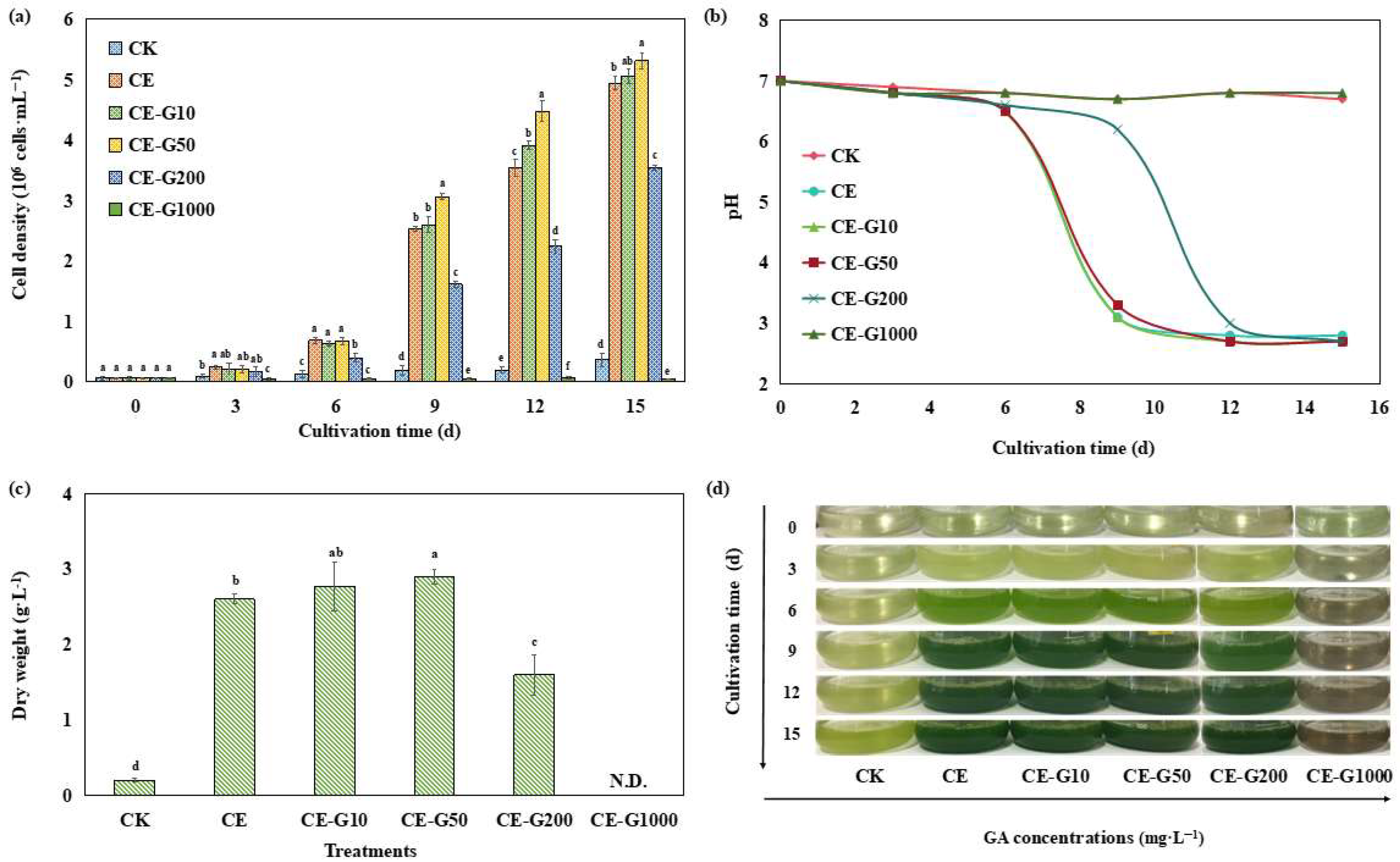
3.1. Changes in Growth Profiles of E. gracilis Under Ethanol-Guaiacol Co-Regulation
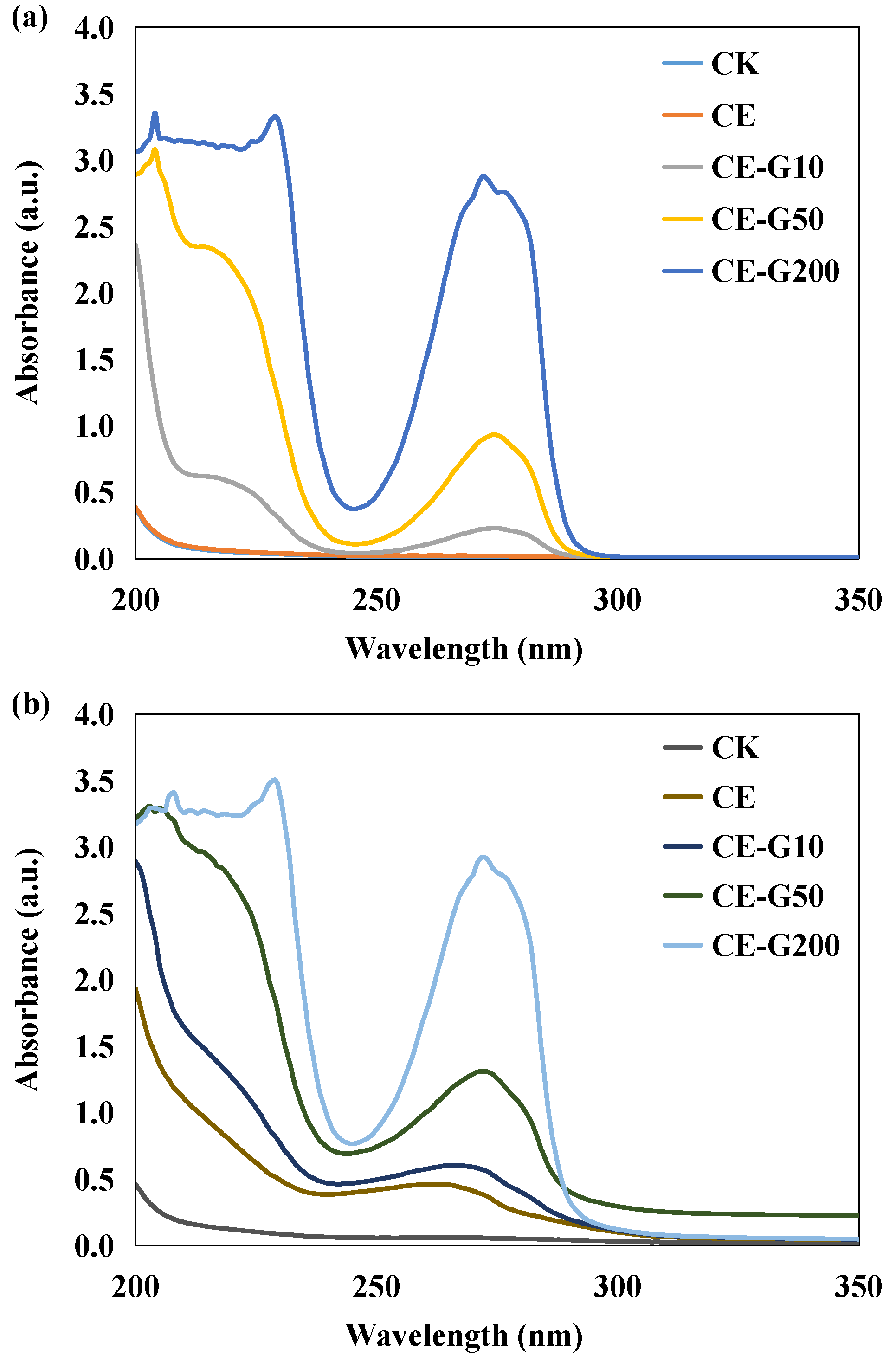
3.2. Changes in UV-Vis Spectra of the Culture Medium
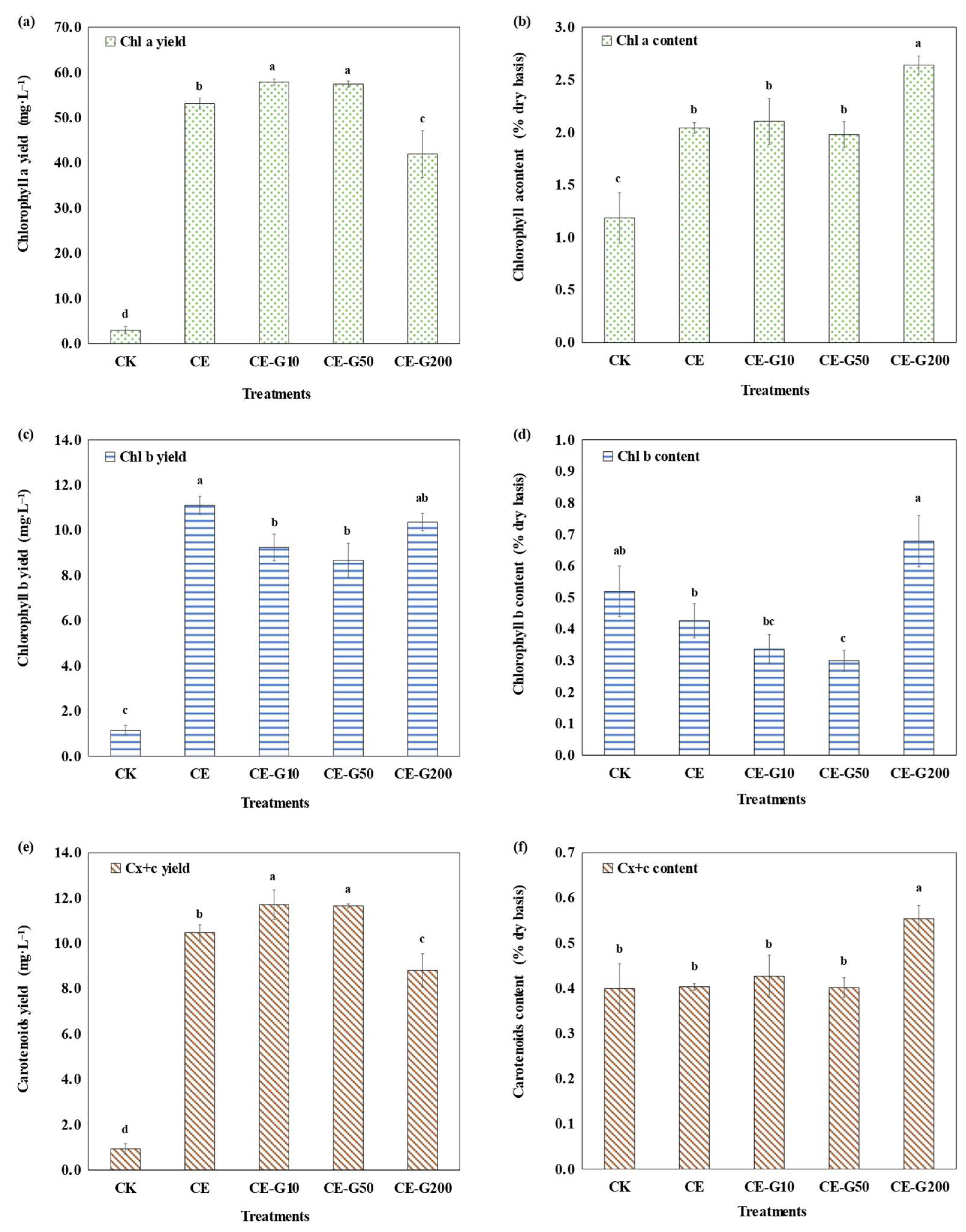
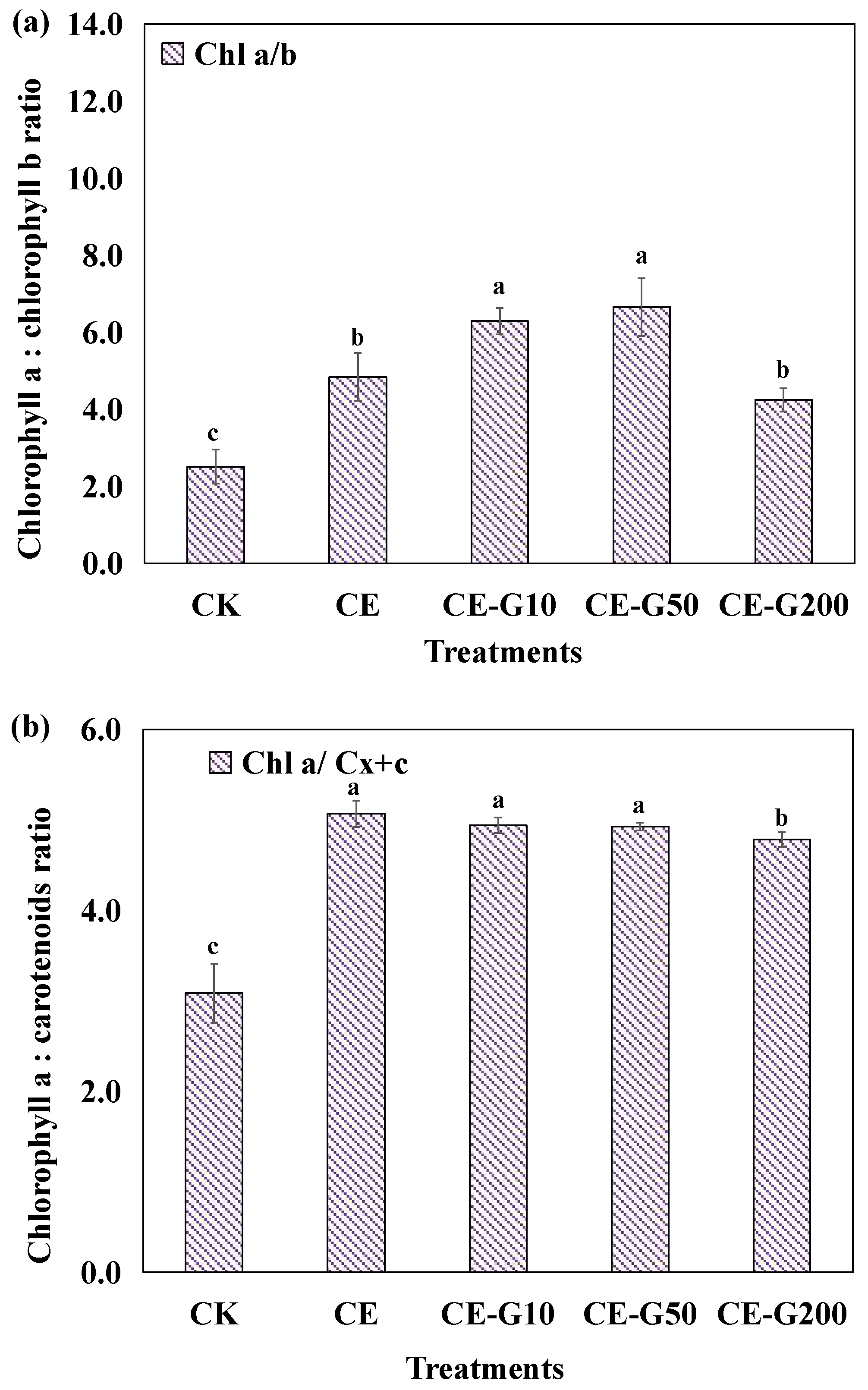
3.3. Photosynthetic Pigment Analysis
3.4. Analysis of Paramylon Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, J.; Gu, X.; Yang, D.; Xu, S.; Wang, S.; Chen, X.; Wang, Z. Bioactive substances and potentiality of marine microalgae. Food Sci. Nutr. 2021, 9, 5279–5292. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liang, H.; Du, H.; Jesumani, V.; He, W.; Cheong, K.L.; Hong, T. Industry chain and challenges of microalgal food industry-a review. Crit. Rev. Food Sci. Nutr. 2022, 64, 4789–4816. [Google Scholar] [CrossRef]
- Su, B.C.; Huang, J.K.; Zhang, J.Z.; Cao, L.; Zhang, R.Z.; Wang, H.L.; Xue, X.W.; Wang, X.Y. Development of a novel sterilized thin-film photobioreactor for efficient microalgae cultivation by mixotrophic mode. Aquacult. Eng. 2025, 110, 102539. [Google Scholar] [CrossRef]
- Castro, E.D.M.; Calder, P.C.; Roche, H. Back Cover: β-1,3/1,6-Glucans and Immunity: State of the Art and Future Directions. Mol. Nutr. Food Res. 2020, 65, 1901071. [Google Scholar] [CrossRef]
- Barsanti, L.; Birindelli, L.; Gualtieri, P. Paramylon and other bioactive molecules in micro and macroalgae. Int. J. Mol. Sci. 2022, 23, 8301. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.X.; Mao, X.F.; Xu, X.; Li, Z.X.; Yang, Z.H.; Liu, Z.Y. Molecular cloning and characterization of the β-1,3-glucan recognition protein in Anatolica polita. Gene 2019, 697, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Kawai, T.; Inukai, Y.; Aoki, D.; Feng, Z.H.; Xiao, Y.H.; Fukushima, K.; Lin, X.Y.; Shi, W.M.; Busch, W.; et al. A lignin-derived material improves plant nutrient bioavailability and growth through its metal chelating capacity. Nat. Commun. 2023, 14, 4866. [Google Scholar] [CrossRef]
- Zhu, J.; Tan, X.; Hafid, H.S.; Wakisaka, M. A novel strategy to promote microalgal growth and lipid productivity by supplementation of lignin related phenolic elicitors. Fuel 2023, 334, 126775. [Google Scholar] [CrossRef]
- Zhu, J.; Tan, X.; Hafid, H.S.; Wakisaka, M. Enhancement of biomass yield and lipid accumulation of freshwater microalga Euglena gracilis by phenolic compounds from basic structures of lignin. Bioresour. Technol. 2021, 321, 124441. [Google Scholar] [CrossRef]
- Abate, R.; Bi, Y.; Song, G.; Mi, W.; Cheng, F.; Zhu, Y. Exogenous ethanol induces cell giantism accompanied by enhanced accumulation of lipid and carbohydrates in Chlorella sorokiniana. J. Appl. Phycol. 2024, 36, 1635–1651. [Google Scholar] [CrossRef]
- Zhu, J.; Wakisaka, M. Application of lignosulfonate as the growth promotor for freshwater microalga Euglena gracilis to increase productivity of biomass and lipids. Fuel 2021, 283, 118920. [Google Scholar] [CrossRef]
- Shao, D.; Chu, W.; Li, X.; Yan, W.; Xu, H. Electrochemical oxidation of guaiacol to increase its biodegradability or just remove COD in terms of anodes and electrolytes. RSC Adv. 2016, 6, 4858–4866. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Analysis 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Kavitha, S.; Ravi, Y.K.; Kumar, G.; Tyagi, V.K.; Banu, J.R. Integrated microalgae cultivation and sustainable biofuel production from pretreated food waste. J. Clean. Prod. 2024, 466, 142829.1–142829.10. [Google Scholar] [CrossRef]
- Yamane, Y.I.; Utsunomiya, T.; Watanabe, M.; Sasaki, K. Biomass production in mixotrophic culture of Euglena gracilis under acidic condition and its growth energetics. Biotechnol. Lett. 2001, 13, 1223–1228. [Google Scholar] [CrossRef]
- Zhu, J.; Wakisaka, M. Effect of two lignocellulose related sugar alcohols on the growth and metabolites biosynthesis of Euglena gracilis. Bioresour. Technol. 2020, 303, 122950. [Google Scholar] [CrossRef]
- Sivaramakrishnan, R.; Suresh, S.; Incharoensakdi, A. Development of Chlamydomonas sp. biorefinery for sustainable methyl ester and malic acid production. Fuel 2024, 371, 132017.1–132017.11. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, H.; Cheng, J.; Song, W.; Wang, H.; Zhang, Y.; Wang, X. Gas-Phase Oxidation of Guaiacol by NO3 Radicals: Kinetic Measurements and Implications. ACS ES&T Air 2025, 2, 903–910. [Google Scholar] [CrossRef]
- Sun, Y.X.; Jiang, Y.; Pang, J.H.; Zhang, Y.X.; Deng, Y.Y.; Zhao, T.C.; Shen, P.H.; Huang, L.D. Low-cost cocultivation of vischeria stellata and tribonema sp. biomass using molasses vinasse: A novel wastewater reuse model for functional aquatic feed production. Algal Res. 2025, 89, 104051. [Google Scholar] [CrossRef]
- Wang, S.; Mukhambet, Y.; Esakkimuthu, S.; Abomohra, E.F. Integrated microalgal biorefinery-routes, energy, economic and environmental perspectives. J. Clean. Prod. 2022, 348, 131245. [Google Scholar] [CrossRef]
- Jiang, D.; Yang, X.P.; Babadi, A.A.; Cheng, X.X.; Ling, Q.F.; Li, H.P.; He, S.R.; Cao, B.; Hu, X.; Wang, S. Study of the catalytic pyrolysis mechanism of guaiacol over seaweed-derived carbon catalyst: Based on density function theory and machine learning. Fuel 2024, 369, 131529. [Google Scholar] [CrossRef]
- Fr, C.B.C.I.C. Oxidation of guaiacol by myeloperoxidase: A two-electron-oxidized guaiacol transient species as a mediator of NADPH oxidation. Biochem. J. 1998, 336, 395–404. [Google Scholar] [CrossRef]
- Xiong, Q.; Hu, L.; Liu, Y.; Zhao, J.; He, L.; Ying, G. Microalgae-based technology for antibiotics removal: From mechanisms to application of innovational hybrid systems. Environ. Int. 2021, 155, 106594. [Google Scholar] [CrossRef]
- Chen, S.; Wang, L.; Feng, W.; Yuan, M.; Zhang, W. Sulfonamides-induced oxidative stress in freshwater microalga chlorella vulgaris: Evaluation of growth, photosynthesis, antioxidants, ultrastructure, and nucleic acids. Sci. Rep. 2020, 10, 8243. [Google Scholar] [CrossRef] [PubMed]
- Simkin, A.J.; Kapoor, L.; Doss, C.G.P.; Hofmann, T.A.; Lawson, T.; Ramamoorthy, S. The role of photosynthesis related pigments in light harvesting, photoprotection and enhancement of photosynthetic yield in planta. Photosynth. Res. 2022, 152, 23–42. [Google Scholar] [CrossRef]
- Berkaloff, C.; Caron, L.; Rousseau, B. Subunit organization of PSI particles from brown algae and diatoms: Polypeptide and pigment analysis. Photosynth. Res. 1990, 23, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Kepekci, R.A.; Saygldeger, S.D. Enhancement of phenolic compound productlon in Spirulina platensis by two-step batch mode cultivation. J. Appl. Phycol. 2012, 24, 897–905. [Google Scholar] [CrossRef]
- Sheng, X.; Watanabe, A.; Li, A.; Kim, E.; Liu, Z. Structural insight into light harvesting for photosystem II in green algae. Nat. Plants 2019, 5, 1320–1330. [Google Scholar] [CrossRef]
- Orosa, M.; Franqueira, D.; Cid, A.; Abalde, J. Analysis and enhancement of astaxanthin accumulation in Haematococcus pluvialis. Bioresour. Technol. 2005, 96, 373–378. [Google Scholar] [CrossRef]
- Solovchenko, A.E.; Khozin-Goldberg, I.; Cohen, Z.; Merzlyak, M.N. Carotenoid-tochlorophyll ratio as a proxy for assay of total fatty acids and arachidonic acidcontent in the green microalga Parietochloris incisa. J. Appl. Phycol. 2009, 21, 361–366. [Google Scholar] [CrossRef]
- Kitajima, K.; Hogan, K.P. Increases of chlorophyll a/b ratios during acclimation of tropical woody seedlings to nitrogen limitation and high light. Plant Cell Environ. 2003, 26, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.C.; Qin, H.; Deng, J.Q.; Liu, Y.Q.; Lei, A.P.; Zhu, H.; Hu, Z.L.; Wang, J.X. A new pilot-scale fermentation mode enhances Euglena gracilis biomass and paramylon (β-1, 3-glucan) production. J. Clean. Prod. 2021, 321, 128996. [Google Scholar] [CrossRef]
- Kim, S.; Lee, D.; Lim, D.; Lim, S.; Park, S.; Kang, C.; Yu, J.; Lee, T. Paramylon production from heterotrophic cultivation of Euglena gracilis in two different industrial byproducts: Corn steep liquor and brewer’s spent grain. Algal Res. 2020, 47, 101826. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Xu, H.; Yang, Z.; Yin, Y.; Fang, W.; Wakisaka, M.; Zhu, J. Synergistic Enhancement of Paramylon Production in Edible Microalga Euglena gracilis via Ethanol-Guaiacol Co-Regulation. Foods 2025, 14, 2457. https://doi.org/10.3390/foods14142457
Yan X, Xu H, Yang Z, Yin Y, Fang W, Wakisaka M, Zhu J. Synergistic Enhancement of Paramylon Production in Edible Microalga Euglena gracilis via Ethanol-Guaiacol Co-Regulation. Foods. 2025; 14(14):2457. https://doi.org/10.3390/foods14142457
Chicago/Turabian StyleYan, Xinyi, Hao Xu, Zhengfei Yang, Yongqi Yin, Weiming Fang, Minato Wakisaka, and Jiangyu Zhu. 2025. "Synergistic Enhancement of Paramylon Production in Edible Microalga Euglena gracilis via Ethanol-Guaiacol Co-Regulation" Foods 14, no. 14: 2457. https://doi.org/10.3390/foods14142457
APA StyleYan, X., Xu, H., Yang, Z., Yin, Y., Fang, W., Wakisaka, M., & Zhu, J. (2025). Synergistic Enhancement of Paramylon Production in Edible Microalga Euglena gracilis via Ethanol-Guaiacol Co-Regulation. Foods, 14(14), 2457. https://doi.org/10.3390/foods14142457









