Chemical and Nutritional Characterization of Sourdoughs Made with Sprouted and Unsprouted Whole-Wheat Flour and Their Effects on the Technological Quality of Bread †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Sprouting Conditions
2.2.2. Sourdough Preparation
2.2.3. pH and Total Titratable Acidity and Microbial Count
2.2.4. Chemical Analysis of Flour and Freeze-Dried Sourdoughs
α-Amylase Activity
Reducing Sugar Content
Free Amino Acid Content
Water-Extractable Arabinoxylans Content
Total Polyphenol Content and Antioxidant Capacity
Phytic Acid Content
2.2.5. Breadmaking Procedure
2.2.6. Bread Technological Quality
2.2.7. Determination of Volatile Organic Compounds in Breads
2.2.8. Statistical Analysis
3. Results and Discussion
3.1. Effect of Sprouting Conditions on the Degree of Sprouting
3.2. Chemical Composition of Flour and Sourdough
3.3. Selected Nutritional Characteristics of Flour and Sourdough
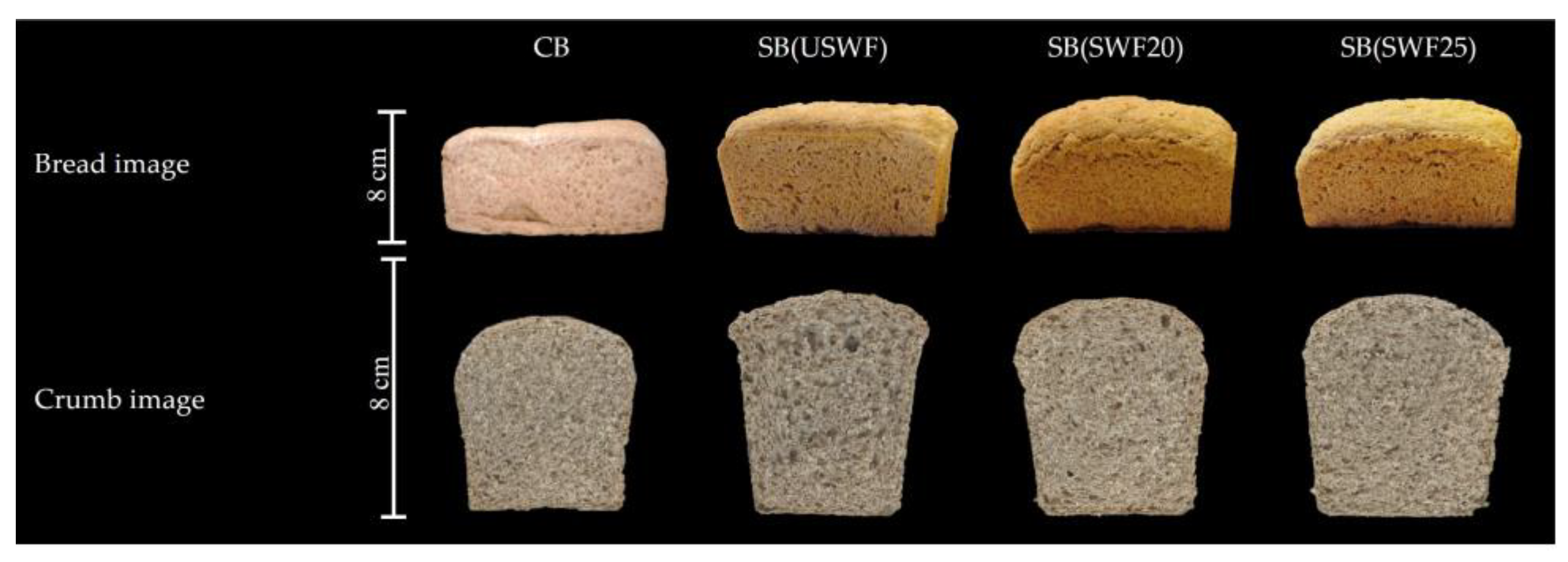
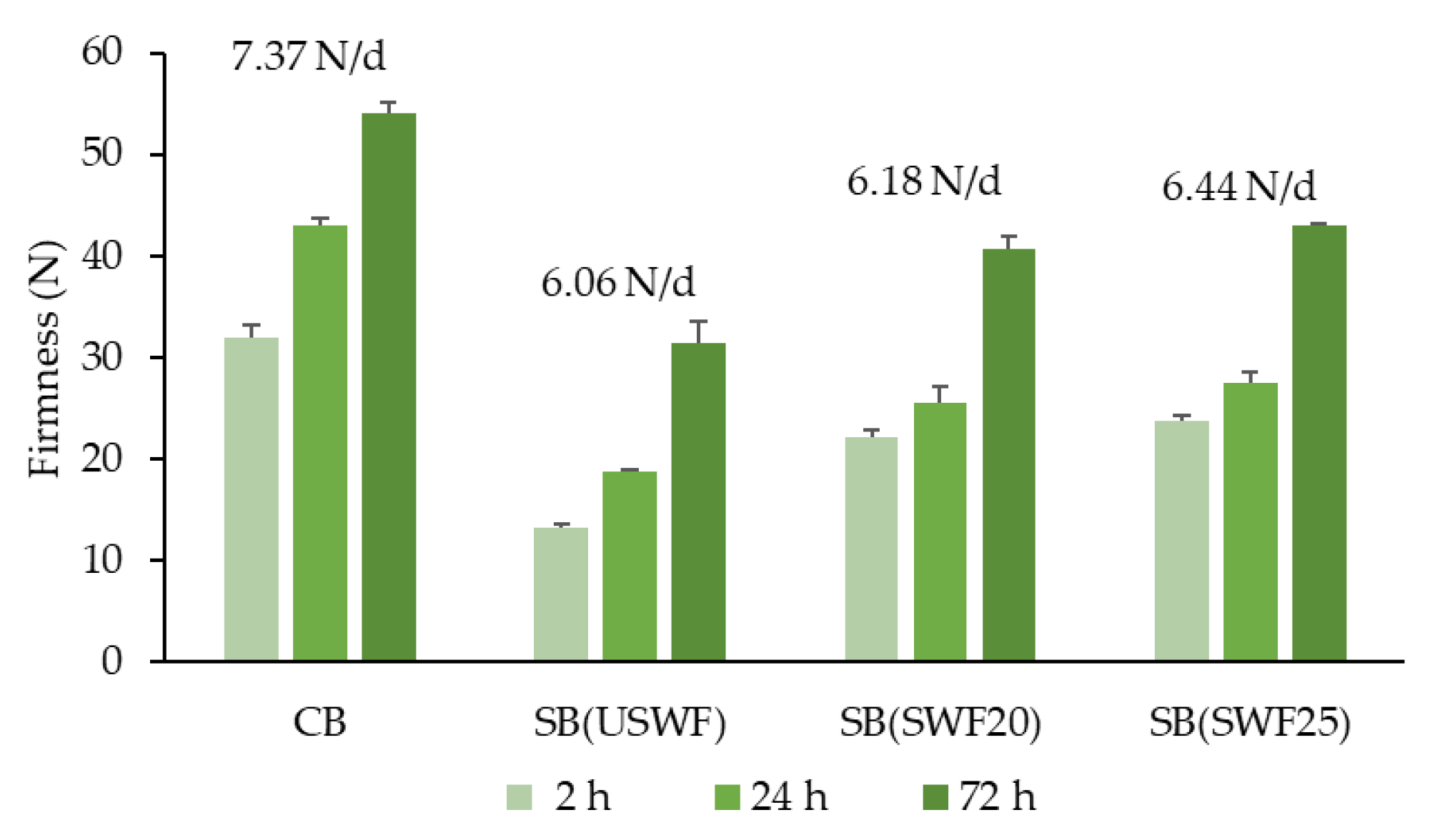
3.4. Effect of Sourdough Fermentation on Leavening and Technological Quality of Breads
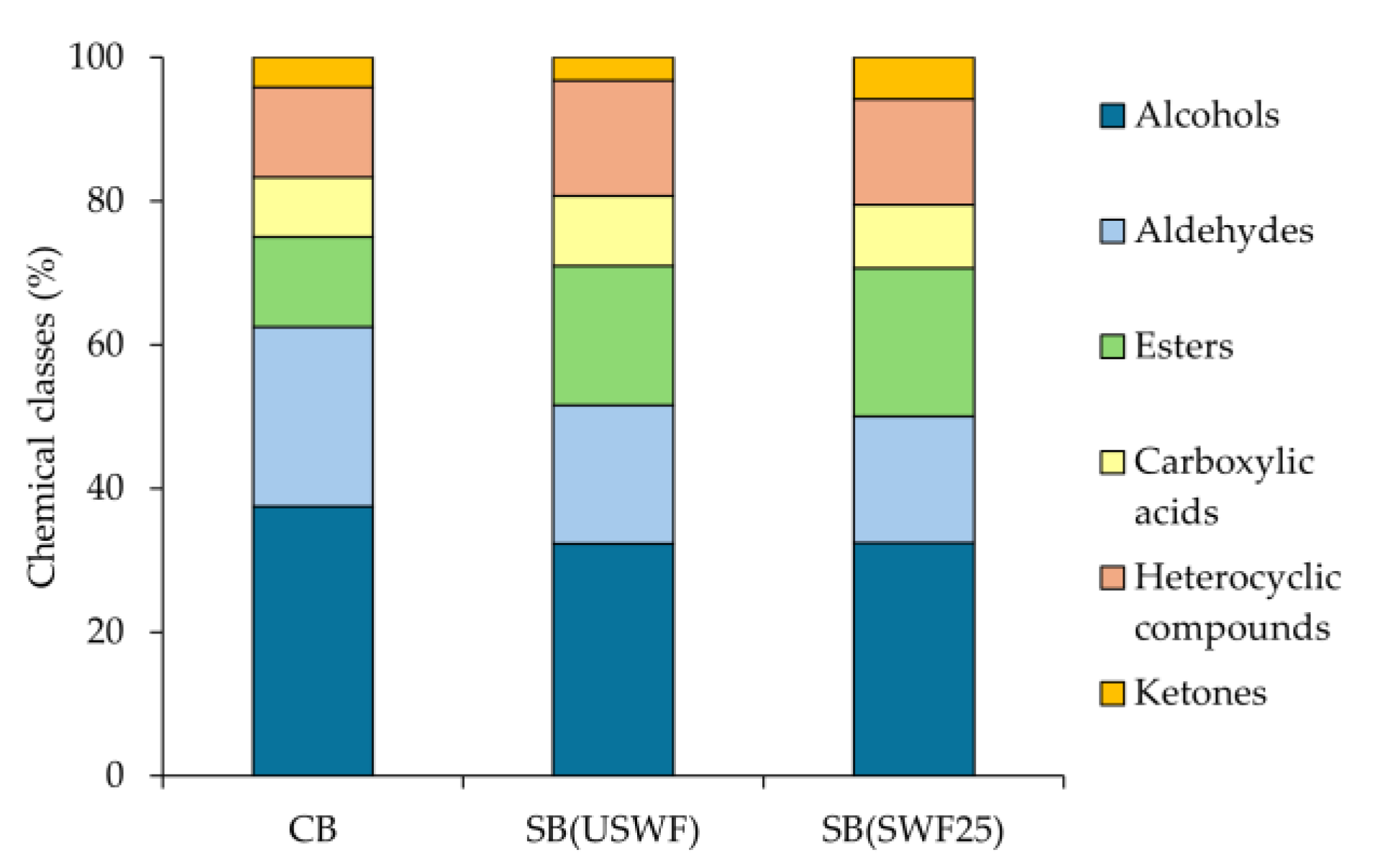
3.5. Volatile Organic Compound Profile of Breads
| Compounds | RT (min) | Odor Description * | Relative Peak Area (%) | ||
|---|---|---|---|---|---|
| CB | SB (USWF) | SB (SWF25) | |||
| Alcohols | 83.11 ± 0.23 a | 81.46 ± 4.75 a | 87.61 ± 0.16 a | ||
| Ethanol | 3.23 | Strong, alcoholic | 70.31 ± 2.56 a | 71.13 ± 7.24 a | 77.32 ± 0.59 a |
| 2-methyl-1-propanol | 6.54 | Alcoholic, wine-like, malty | 2.22 ± 0.07 a | 1.13 ± 0.21 c | 1.76 ± 0.00 b |
| 2-methyl-butanol | 9.87 | Alcoholic, malty | 1.76 ± 0.47 a | 1.14 ± 0.25 a | 1.07 ± 0.29 a |
| 3-methyl-butanol | 9.94 | Balsamic, alcoholic, malty | 5.48 ± 1.37 a | 4.62 ± 1.04 a | 4.77 ± 0.07 a |
| 1-pentanol | 10.9 | Fruity | 0.05 ± 0.01 a | 0.03 ± 0.00 a | 0.04 ± 0.02 a |
| 1-hexanol | 13.52 | Grassy, woody, flowery | 0.66 ± 0.20 a | 0.43 ± 0.01 a | 0.33 ± 0.14 a |
| 1-heptanol | 16.56 | Alcoholic, green, citric | nd | nd | 0.01 ± 0.00 |
| 2-furanmethanol | 20.08 | Alcoholic, honey, sweet | 0.07 ± 0.04 a | 0.09 ± 0.02 a | 0.03 ± 0.01 a |
| z-4-decen-1-ol | 22.42 | Alcoholic, herbal | 0.04 ± 0.02 a | 0.06 ± 0.02 a | 0.03 ± 0.01 a |
| Benzyl alcohol | 23.56 | Alcoholic, pleasant aroma | nd | 0.02 ± 0.00 a | 0.01 ± 0.00 b |
| 2-phenyl-ethanol | 23.94 | Flowery, fermented yeast | 2.52 ± 0.25 a | 2.80 ± 0.97 a | 2.25 ± 0.03 a |
| Aldehydes | 1.38 ± 0.05 a | 0.48 ± 0.17 b | 0.61 ± 0.22 b | ||
| Acetaldehyde | 1.74 | Aldehydic, fruity | 0.29 ± 0.06 a | 0.05 ± 0.04 b | 0.11 ± 0.02 b |
| 2-methyl-propanal | 2.09 | Aldehydic, almond, malty | 0.07 ± 0.00 a | 0.04 ± 0.02 a | 0.06 ± 0.03 a |
| 2-methyl-butanal | 2.82 | Roasty, malty | 0.09 ± 0.01 a | 0.03 ± 0.01 a | 0.06 ± 0.04 a |
| 3-methyl-butanal | 2.88 | Roasty, malty | 0.08 ± 0.01 a | 0.08 ± 0.01 a | 0.18 ± 0.13 a |
| Hexanal | 5.90 | Aldehydic, herbal, grassy | 0.38 ± 0.03 a | 0.13 ± 0.04 b | 0.13 ± 0.03 b |
| Benzaldehyde | 18.08 | Aldehydic, caramel, almond | 0.47 ± 0.03 a | 0.14 ± 0.06 b | 0.07 ± 0.01 b |
| Esters | 0.87 ± 0.02 b | 1.63 ± 0.15 a | 1.38 ± 0.04 a | ||
| Ethyl acetate | 2.59 | Fruity, sweet, grassy, green | 0.23 ± 0.04 b | 0.08 ± 0.00 b | 0.57 ± 0.10 a |
| 3-methylbutyl acetate | 6.86 | Fruity | nd | 0.23 ± 0.08 a | 0.06 ± 0.04 b |
| Ethyl hexanoate | 9.54 | Fruity, apple peel-like | nd | 0.08 ± 0.02 a | 0.09 ± 0.03 a |
| Ethyl heptanoate | 12.09 | Fruity, grapes | nd | nd | 0.04 ± 0.01 |
| Ethyl octanoate | 15.40 | Fruity, sweet, fresh | 0.35 ± 0.02 b | 0.57 ± 0.12 a | 0.27 ± 0.10 b |
| Ethyl nonanoate | 18.05 | Fruity | nd | 0.18 ± 0.08 a | 0.09 ± 0.05 a |
| 2-phenylethyl acetate | 22.73 | Flowery | 0.29 ± 0.04 b | 0.50 ± 0.05 a | 0.26 ± 0.03 b |
| Carboxylic acids | 1.97 ± 0.06 b | 4.26 ± 0.77 a | 3.33 ± 1.26 a | ||
| Acetic acid | 17.27 | citric, flowery, pungent | 1.56 ± 0.02 b | 3.58 ± 0.97 a | 3.02 ± 1.28 a |
| Hexanoic acid | 23.29 | Fatty, cheesy | 0.41 ± 0.08 a | 0.64 ± 0.19 a | 0.29 ± 0.01 a |
| Heptanoic acid | 24.46 | Fatty, cheesy | nd | 0.04 ± 0.01 a | 0.02 ± 0.00 b |
| Heterocyclic compounds | 0.40 ± 0.11 a | 0.41 ± 0.07 a | 0.21 ± 0.07 a | ||
| 2-pentylfuran | 9.24 | Flowery, fruity | 0.10 ± 0.07 a | 0.10 ± 0.05 a | 0.04 ± 0.02 a |
| Furfural | 16.92 | Almond, roasty, sweet | 0.07 ± 0.02 a | 0.12 ± 0.01 a | 0.06 ± 0.05 a |
| Maltol | 24.63 | Sweet, caramel, fruity | nd | 0.02 ± 0.00 b | 0.03 ± 0.01 a |
| γ-nonanolactone | 25.21 | Fruity, sweet | 0.23 ± 0.06 a | 0.14 ± 0.05 a | 0.07 ± 0.00 a |
| 2-acetylpirrole | 24.68 | Nuts, cracker-like | nd | 0.04 ± 0.02 a | 0.03 ± 0.00 b |
| Ketones | 0.46 ± 0.15 a | 0.28 ± 0.02 a | 0.22 ± 0.05 a | ||
| Acetoin | 12.18 | Butter, yogurt, cream | 0.46 ± 0.15 a | 0.28 ± 0.02 a | 0.21 ± 0.06 a |
| Geranyl acetone | 23.12 | Flowery | nd | nd | 0.01 ± 0.00 |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| USWF | Unsprouted whole-wheat flour |
| SWF | Sprouted whole-wheat flour |
| SWF20 | Sprouted whole-wheat flour obtained after sprouting at 20 °C |
| SWF25 | Sprouted whole-wheat flour obtained after sprouting at 25 °C |
| SD (USWF) | Sourdough made with USWF |
| SD (SWF20) | Sourdough made with SWF20 |
| SD (SWF25) | Sourdough made with SWF25 |
| CB | Control bread made with USWF |
| SB (USWF) | Sourdough bread made with USWF |
| SB (SWF20) | Sourdough bread made with SWF20 |
| SB (SWF25) | Sourdough bread made with SWF25 |
| DoS | Degree of sprouting |
| db | Dry basis |
| LAB | Lactic acid bacteria |
| TTA | Total titratable acidity |
| DNS | 3,5-dinitrosalicylic acid |
| AU | α-amylase unit |
| OPA | o-phthalaldehyde |
| PAP | Available phosphorus phytate |
| GAE | Gallic acid equivalent |
| TE | Trolox equivalent |
| SBV | Specific bread volume |
| VOC | Volatile organic compound |
| TPA | Texture profile analysis |
| OAV | Odor activity value |
| SPME | Solid phase extraction |
| ANOVA | Analysis of variance |
References
- Naumenko, N.; Potoroko, I.; Kalinina, I.; Fatkullin, R.; Ivanisova, E. The Influence of the Use of Whole Grain Flour from Sprouted Wheat Grain on the Rheological and Microstructural Properties of Dough and Bread. Int. J. Food Sci. 2021, 2021, 7548759. [Google Scholar] [CrossRef]
- Navarro, J.L.; Losano Richard, P.; Moiraghi, M.; Bustos, M.; León, A.E.; Steffolani, M.E. Effect of Different Wheat Sprouting Conditions on the Characteristics of Whole-Wheat Flour. Food Technol. Biotechnol. 2024, 62, 264–274. [Google Scholar] [CrossRef]
- Cardone, G.; Incecco, P.D.; Casiraghi, M.C.; Marti, A. Exploiting Milling By-Products in Bread-Making: The Case of Sprouted Wheat. Foods 2020, 9, 260. [Google Scholar] [CrossRef]
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted grains: A comprehensive review. Nutrients 2019, 11, 421. [Google Scholar] [CrossRef]
- Cizeikiene, D.; Jagelaviciute, J.; Stankevicius, M.; Maruska, A. Thermophilic lactic acid bacteria affect the characteristics of sourdough and whole-grain wheat bread. Food Biosci. 2020, 38, 100791. [Google Scholar] [CrossRef]
- Gänzle, M.G. Enzymatic and bacterial conversions during sourdough fermentation. Food Microbiol. 2014, 37, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Lancetti, R.; Sciarini, L.; Pérez, G.T.; Salvucci, E. Technological Performance and Selection of Lactic Acid Bacteria Isolated from Argentinian Grains as Starters for Wheat Sourdough. Curr. Microbiol. 2021, 78, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Peláez, J.; Paesani, C.; Gómez, M. Sourdough technology as a tool for the development of healthier grain-based products: An update. Agronomy 2020, 10, 1962. [Google Scholar] [CrossRef]
- Gobbetti, M.; De Angelis, M.; Di Cagno, R.; Calasso, M.; Archetti, G.; Rizzello, C.G. Novel insights on the functional/nutritional features of the sourdough fermentation. Int. J. Food Microbiol. 2019, 302, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Quattrini, M.; Liang, N.; Fortina, M.G.; Xiang, S.; Curtis, J.M.; Gänzle, M. Exploiting synergies of sourdough and antifungal organic acids to delay fungal spoilage of bread. Int. J. Food Microbiol. 2019, 302, 8–14. [Google Scholar] [CrossRef]
- Sun, Y.; Miao, R.; Guan, L. Effect of germinated brown rice flour on volatile compounds and sensory evaluation of germinated brown rice steamed bread. J. Food Process. Preserv. 2021, 45, e14994. [Google Scholar] [CrossRef]
- Montemurro, M.; Pontonio, E.; Gobbetti, M.; Rizzello, C.G. Investigation of the nutritional, functional and technological effects of the sourdough fermentation of sprouted flours. Int. J. Food Microbiol. 2019, 302, 47–58. [Google Scholar] [CrossRef]
- Perri, G.; Calabrese, F.M.; Rizzello, C.G.; De Angelis, M.; Gobbetti, M.; Calasso, M. Sprouting process affects the lactic acid bacteria and yeasts of cereal, pseudocereal and legume flours. LWT 2020, 126, 109314. [Google Scholar] [CrossRef]
- American Association of Cereal Chemists. Approved Methods of the American Association of Cereal Chemistry, 11th ed.; AACC: St. Paul, MN, USA, 2010. [Google Scholar]
- Krapf, J.; Arysanto, A.; Walther, G.; Flöter, E. Effect of sprouting conditions on the properties of direct expanded extruded wheat. J. Food Process Eng. 2019, 42, e13123. [Google Scholar] [CrossRef]
- Calasso, M.; Ressa, A.; Calabrese, F.M.; Minervini, F.; De Angelis, M. Microbial ecology, biochemical and nutritional features in sprouted composite type I sourdough made of wheat and blend flours. LWT 2023, 187, 115285. [Google Scholar] [CrossRef]
- Bustos, M.C.; Vignola, M.B.; Paesani, C.; León, A.E. Berry fruits-enriched pasta: Effect of processing and in vitro digestion on phenolics and its antioxidant activity, bioaccessibility and potential bioavailability. Int. J. Food Sci. Technol. 2020, 55, 2104–2112. [Google Scholar] [CrossRef]
- Podio, N.S.; Baroni, M.V.; Wunderlin, D.A. Relation between polyphenol profile and antioxidant capacity of different Argentinean wheat varieties. A Boosted Regression Trees study. Food Chem. 2017, 232, 79–88. [Google Scholar] [CrossRef]
- Raboy, V.; Johnson, A.; Bilyeu, K.; Brinch-Pedersen, H.; Cichy, K.; Hurrell, R.F.; Zeder, C.; Rasmussen, S.K.; Warkentin, T.D.; Thavarajah, P.; et al. Evaluation of Simple and Inexpensive High-Throughput Methods for Phytic Acid Determination. J. Am. Oil Chem. Soc. 2017, 94, 353–362. [Google Scholar] [CrossRef]
- Steffolani, M.E.; Ribotta, P.D.; Pérez, G.T.; León, A.E. Combinations of glucose oxidase, α-amylase and xylanase affect dough properties and bread quality. Int. J. Food Sci. Technol. 2012, 47, 525–534. [Google Scholar] [CrossRef]
- Lancetti, R.P.; Salvucci, E.; Paesani, C.; Pérez, G.T.; Sciarini, L.S. Sourdough on quinoa and buckwheat gluten-free breads: Evaluation of autochthonous starter fermentation on bread nutritional and technological properties. Int. J. Food Sci. Technol. 2022, 57, 4804–4815. [Google Scholar] [CrossRef]
- Polachini, T.C.; Norwood, E.A.; Le-Bail, P.; Le-Bail, A. Post-sprouting thermal treatment of green barley malt to produce functional clean-label ingredients: Impact on fermentation, bread-making properties and bread quality. Food Res. Int. 2023, 167, 112696. [Google Scholar] [CrossRef] [PubMed]
- López, M.S.; Sciarini, L.S.; Salvucci, E.J.; Pérez, G.T. Autochthonous lactic acid bacteria in gluten-free sourdoughs produce nutritional and technological improvements in quinoa and buckwheat breads. Int. J. Gastron. Food Sci. 2024, 37, 100970. [Google Scholar] [CrossRef]
- Whole Grains Council Working Group on Sprouted Grains. Multiple Criteria for a Sprouted Whole Grain First: Establish Viability, Then Use Intentional Processes. Whole Grains Council: Boston, MA, USA, 2017. [Google Scholar]
- Guzmán-Ortiz, F.A.; Castro-Rosas, J.; Gómez-Aldapa, C.A.; Mora-Escobedo, R.; Rojas-León, A.; Rodríguez-Marín, M.L.; Falfán-Cortés, R.N.; Román-Gutiérrez, A.D. Enzyme activity during germination of different cereals: A review. Food Rev. Int. 2019, 35, 177–200. [Google Scholar] [CrossRef]
- Lemmens, E.; De Brier, N.; Goos, P.; Smolders, E.; Delcour, J.A. Steeping and germination of wheat (Triticum aestivum L.). I. Unlocking the impact of phytate and cell wall hydrolysis on bio-accessibility of iron and zinc elements. J. Cereal Sci. 2019, 90, 102847. [Google Scholar] [CrossRef]
- Cornejo, F.; Rosell, C.M. Influence of germination time of brown rice in relation to flour and gluten free bread quality. J. Food Sci. Technol. 2015, 52, 6591–6598. [Google Scholar] [CrossRef]
- Simonson, L.; Salovaara, H.; Korhola, M. Response of wheat sourdough parameters to temperature, NaCl and sucrose variations. Food Microbiol. 2003, 20, 193–199. [Google Scholar] [CrossRef]
- Manini, F.; Brasca, M.; Plumed-Ferrer, C.; Morandi, S.; Erba, D.; Casiraghi, M.C. Study of the chemical changes and evolution of microbiota during sourdoughlike fermentation of wheat bran. Cereal Chem. 2014, 91, 342–349. [Google Scholar] [CrossRef]
- Gebruers, K.; Dornez, E.; Bedõ, Z.; Rakszegi, M.; Courtin, C.M.; Delcour, J.A. Variability in xylanase and xylanase inhibition activities in different cereals in the HEALTHGRAIN diversity screen and contribution of environment and genotype to this variability in common wheat. J. Agric. Food Chem. 2010, 58, 9362–9371. [Google Scholar] [CrossRef]
- Nikinmaa, M.; Mattila, O.; Holopainen-Mantila, U.; Heiniö, R.L.; Nordlund, E. Impact of lactic acid bacteria starter cultures and hydrolytic enzymes on the characteristics of wholegrain crackers. J. Cereal Sci. 2019, 88, 1–8. [Google Scholar] [CrossRef]
- Montemurro, M.; Celano, G.; De Angelis, M.; Gobbetti, M.; Rizzello, C.G.; Pontonio, E. Selection of non-Lactobacillus strains to be used as starters for sourdough fermentation. Food Microbiol. 2020, 90, 103491. [Google Scholar] [CrossRef]
- Ohm, J.B.; Lee, C.W.; Cho, K. Germinated wheat: Phytochemical composition and mixing characteristics. Cereal Chem. 2016, 93, 612–617. [Google Scholar] [CrossRef]
- Świeca, M.; Dziki, D. Improvement in sprouted wheat flour functionality: Effect of time, temperature and elicitation. Int. J. Food Sci. Technol. 2015, 50, 2135–2142. [Google Scholar] [CrossRef]
- Arora, K.; Ameur, H.; Polo, A.; Di Cagno, R.; Rizzello, C.G.; Gobbetti, M. Thirty years of knowledge on sourdough fermentation: A systematic review. Trends Food Sci. Technol. 2021, 108, 71–83. [Google Scholar] [CrossRef]
- Koj, K.; Pejcz, E. Rye Dietary Fiber Components upon the Influence of Fermentation Inoculated with Probiotic Microorganisms. Molecules 2023, 28, 1910. [Google Scholar] [CrossRef]
- Perri, G.; Rizzello, C.G.; Ampollini, M.; Celano, G.; Coda, R.; Gobbetti, M.; De Angelis, M.; Calasso, M. Bioprocessing of barley and lentil grains to obtain in situ synthesis of exopolysaccharides and composite wheat bread with improved texture and health properties. Foods 2021, 10, 1489. [Google Scholar] [CrossRef] [PubMed]
- Naji-Tabasi, S.; Shahidi-Noghabi, M.; Davtalab, M. Optimization of fermentation conditions in Barbari bread based on mixed whole flour (barley and sprouted wheat) and sourdough. Food Sci. Technol. Int. 2021, 29, 126–137. [Google Scholar] [CrossRef]
- Corona, O.; Alfonzo, A.; Ventimiglia, G.; Nasca, A.; Francesca, N.; Martorana, A.; Moschetti, G.; Settanni, L. Industrial application of selected lactic acid bacteria isolated from local semolinas for typical sourdough bread production. Food Microbiol. 2016, 59, 43–56. [Google Scholar] [CrossRef]
- Pico, J.; Bernal, J.; Gómez, M. Wheat bread aroma compounds in crumb and crust: A review. Food Res. Int. 2015, 75, 200–215. [Google Scholar] [CrossRef]
- Perri, G.; Coda, R.; Rizzello, C.G.; Celano, G.; Ampollini, M.; Gobbetti, M.; De Angelis, M.; Calasso, M. Sourdough fermentation of whole and sprouted lentil flours: In situ formation of dextran and effects on the nutritional, texture and sensory characteristics of white bread. Food Chem. 2021, 355, 129638. [Google Scholar] [CrossRef]
- Settanni, L.; Ventimiglia, G.; Alfonzo, A.; Corona, O.; Miceli, A.; Moschetti, G. An integrated technological approach to the selection of lactic acid bacteria of flour origin for sourdough production. Food Res. Int. 2013, 54, 1569–1578. [Google Scholar] [CrossRef]
- Zhou, Z.; Ji, Z.; Liu, S.; Han, X.; Zheng, F.; Mao, J. Characterization of the volatile compounds of huangjiu using comprehensive two-dimensional gas chromatography coupled to time of flight mass spectrometry (GC × GC-TOFMS). J. Food Process. Preserv. 2019, 43. [Google Scholar] [CrossRef]
- Birch, A.N.; Petersen, M.A.; Hansen, Å.S. The aroma profile of wheat bread crumb influenced by yeast concentration and fermentation temperature. LWT 2013, 50, 480–488. [Google Scholar] [CrossRef]
- Boeswetter, A.R.; Scherf, K.A.; Schieberle, P.; Koehler, P. Identification of the Key Aroma Compounds in Gluten-Free Rice Bread. J. Agric. Food Chem. 2019, 67, 2963–2972. [Google Scholar] [CrossRef]
- Cavallo, N.; De Angelis, M.; Calasso, M.; Quinto, M.; Mentana, A.; Minervini, F.; Cappelle, S.; Gobbetti, M. Microbial cell-free extracts affect the biochemical characteristics and sensorial quality of sourdough bread. Food Chem. 2017, 237, 159–168. [Google Scholar] [CrossRef]
- Johnston, R.; Martin, J.M.; Vetch, J.M.; Byker-Shanks, C.; Finnie, S.; Giroux, M.J. Controlled sprouting in wheat increases quality and consumer acceptability of whole-wheat bread. Cereal Chem. 2019, 96, 866–877. [Google Scholar] [CrossRef]
- Plessas, S.; Bekatorou, A.; Gallanagh, J.; Koutinas, A.A.; Soupioni, M.; Psarianos, C. Application of novel starter cultures for sourdough bread production. Anaerobe 2011, 17, 486–489. [Google Scholar] [CrossRef] [PubMed]
| Samples | α-Amylase Activity (AU/g db) | Reducing Sugar (g/100 g db) | Water-Extractable Arabinoxylans (g/100 g db) | Free Amino Acid Groups (µmol Serine/mg of Protein db) | |
|---|---|---|---|---|---|
| USWF | 0.45 ± 0.03 c | 1.28 ± 0.50 b | 0.86 ± 0.02 b | 0.21 ± 0.00 c | |
| SWF20 | 11.81 ± 0.01 a | 5.37 ± 0.89 a | 1.48 ± 0.20 a | 0.39 ± 0.01 a | |
| SWF25 | 12.19 ± 0.55 a | 5.27 ± 0.43 a | 1.79 ± 0.08 a | 0.36 ± 0.01 b | |
| SD (USWF) | 0.14 ± 0.01 A | 5.75 ± 0.52 B | 1.38 ± 0.07 B | 0.82 ± 0.01 C | |
| SD (SWF20) | 0.16 ± 0.02 A | 6.44 ± 0.61 B | 1.95 ± 0.09 A | 0.92 ± 0.03 B | |
| SD (SWF25) | 0.12 ± 0.01 A | 8.72 ± 0.52 A | 2.04 ± 0.09 A | 1.11 ± 0.01 A |
| Time | Sample | pH | TTA (mL NaOH/g) | ΔpH | Yeast (log CFU/mL) | LAB (log CFU/mL) |
|---|---|---|---|---|---|---|
| t0 | SD (USWF) | 6.02 ± 0.12 a | 3.75 ± 0.07 b | - | 2.36 ± 0.08 b | 3.15 ± 0.07 b |
| SD (SWF20) | 5.50 ± 0.04 b | 12.15 ± 0.07 a | - | 4.13 ± 0.17 a | 6.21 ± 0.19 a | |
| SD (SWF25) | 5.45 ± 0.02 b | 10.00 ± 0.14 a | - | 3.96 ± 0.11 a | 6.24 ± 0.24 a | |
| t7 | SD (USWF) | 3.74 ± 0.01 B | 17.00 ± 0.00 A | 2.27 ± 0.11 A | 6.69 ± 0.06 B | 8.25 ± 0.07 B |
| SD (SWF20) | 3.80 ± 0.01 A | 17.50 ± 0.71 A | 1.71 ± 0.02 B | 6.90 ± 0.03 A | 8.39 ± 0.04 A | |
| SD (SWF25) | 3.78 ± 0.01 A | 18.00 ± 0.00 A | 1.67 ± 0.03 B | 6.27 ± 0.08 C | 8.51 ± 0.05 A |
| Sample | TPC (mg GAE/100 g db) | FRAP (µmol TE/g db) | ABTS˙+ (µmol TE/g db) | PA (mg PAP/g db) |
|---|---|---|---|---|
| USWF | 50.28 ± 1.70 c | 20.63 ± 0.73 b | 6.16 ± 0.73 b | 19.93 ± 0.07 a |
| SWF20 | 86.47 ± 0.96 b | 27.74 ± 0.10 a | 9.66 ± 1.67 a | 18.25 ± 0.17 b |
| SWF25 | 137.78 ± 0.46 a | 26.51 ± 0.53 a | 11.12 ± 0.18 a | 18.29 ± 0.26 b |
| SD (USWF) | 129.67 ± 0.16 B | 25.85 ± 1.45 B | 17.10 ± 0.64 A | 0.92 ± 0.06 A |
| SD (SWF20) | 160.15 ± 17.83 A | 31.50 ± 0.93 A | 21.11 ± 1.49 A | 0.96 ± 0.09 A |
| SD (SWF25) | 149.40 ± 5.42 A | 30.43 ± 0.53 A | 19.77 ± 0.47 A | 1.00 ± 0.09 A |
| Parameters | CB | SB (USWF) | SB (SWF20) | SB (SWF25) |
|---|---|---|---|---|
| Specific bread volume (mL/g) | 1.69 ± 0.04 b | 2.34 ± 0.09 a | 2.22 ± 0.02 a | 2.23 ± 0.01 a |
| Crust | ||||
| Browning (100-L*) | 38.56 ± 0.34 b | 41.09 ± 0.81 b | 43.25 ± 1.48 a | 44.57 ± 1.67 a |
| a* | 10.84 ± 1.05 a | 12.77 ± 0.89 a | 11.72 ± 0.83 a | 11.29 ± 1.43 a |
| b* | 24.91 ± 0.72 a | 22.64 ± 2.17 a | 25.53 ± 2.18 a | 24.82 ± 1.53 a |
| Crumb | ||||
| Browning (100-L*) | 43.57 ± 0.05 a | 45.01 ± 1.73 a | 42.96 ± 2.31 a | 43.42 ± 1.92 a |
| a* | 7.40 ± 0.10 a | 7.05 ± 0.22 a | 6.79 ± 0.42 a | 8.00 ± 0.37 a |
| b* | 21.94 ± 0.21 a | 22.17 ± 0.78 a | 21.86 ± 0.49 a | 22.72 ± 0.16 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro, J.L.; López, M.S.; Salvucci, E.; León, A.E.; Steffolani, M.E. Chemical and Nutritional Characterization of Sourdoughs Made with Sprouted and Unsprouted Whole-Wheat Flour and Their Effects on the Technological Quality of Bread . Foods 2025, 14, 2805. https://doi.org/10.3390/foods14162805
Navarro JL, López MS, Salvucci E, León AE, Steffolani ME. Chemical and Nutritional Characterization of Sourdoughs Made with Sprouted and Unsprouted Whole-Wheat Flour and Their Effects on the Technological Quality of Bread . Foods. 2025; 14(16):2805. https://doi.org/10.3390/foods14162805
Chicago/Turabian StyleNavarro, José Luis, María Soledad López, Emiliano Salvucci, Alberto Edel León, and María Eugenia Steffolani. 2025. "Chemical and Nutritional Characterization of Sourdoughs Made with Sprouted and Unsprouted Whole-Wheat Flour and Their Effects on the Technological Quality of Bread " Foods 14, no. 16: 2805. https://doi.org/10.3390/foods14162805
APA StyleNavarro, J. L., López, M. S., Salvucci, E., León, A. E., & Steffolani, M. E. (2025). Chemical and Nutritional Characterization of Sourdoughs Made with Sprouted and Unsprouted Whole-Wheat Flour and Their Effects on the Technological Quality of Bread . Foods, 14(16), 2805. https://doi.org/10.3390/foods14162805







