A Study on the Extraction, Fermentation Condition Optimization, and Antioxidant Activity Assessment of Polysaccharides Derived from Kluyveromyces marxianus
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Strains and Media
2.2.1. Strains
2.2.2. Cultivation Conditions for Strain
2.2.3. Culture Media
2.3. Fermentation Optimization
2.3.1. Single-Factor Screening of Fermentation Medium Components
2.3.2. Single-Factor Concentration Screening of Fermentation Medium Components
2.3.3. Box–Behnken Design for Screening the Optimal Fermentation Medium
2.3.4. Single-Factor Screening of Fermentation Conditions
2.3.5. Box–Behnken Design for Screening the Optimal Fermentation Conditions
2.4. Extraction and Purification of Polysaccharides
2.4.1. Extraction of Polysaccharides
2.4.2. DEAE-52 Cellulose Column
2.4.3. Sephadex G-100 Column
2.5. In Vitro Antioxidant Activity of Polysaccharides
2.5.1. Hydroxyl Radical Scavenging Activity
2.5.2. DPPH Radical Scavenging Activity
2.5.3. Fe2+ Reduction Activity
3. Results
3.1. Optimization of Fermentation Medium Components
3.1.1. Single-Factor Screening of Fermentation Medium Components
3.1.2. Effects of Sucrose, Peptone, and CaCl2 Concentrations on the Extracellular Polysaccharide Yield of KM-502
3.1.3. Optimization of Fermentation Medium Using the Box–Behnken Design
3.2. Optimization of Fermentation Conditions
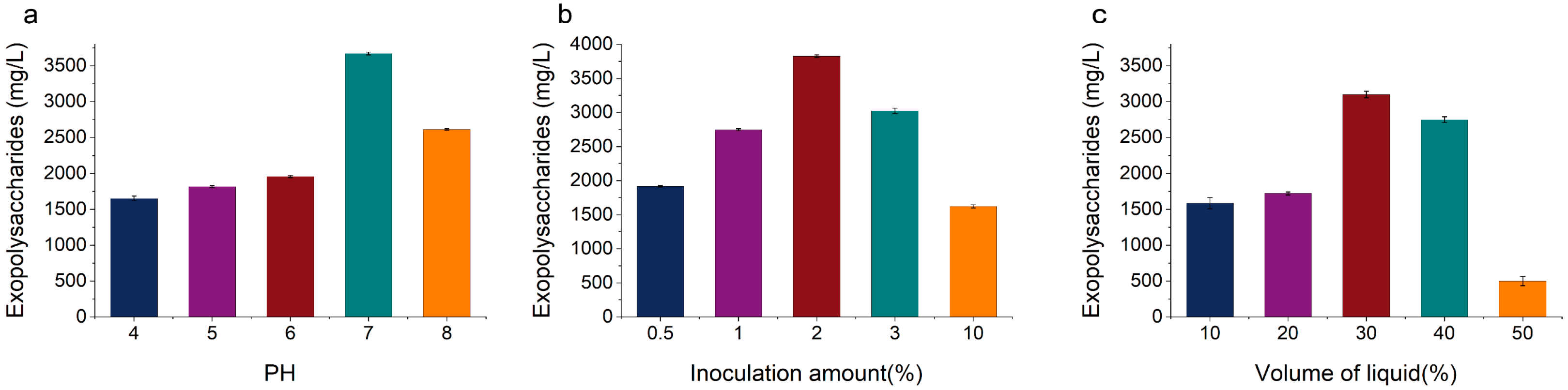
3.2.1. Single-Factor Screening of Fermentation Conditions for EPS Production by KM-502
3.2.2. Optimization of Fermentation Conditions for EPS Production by KM-502 Using the Box–Behnken Design
3.3. Purification of Polysaccharides
3.4. Extracellular Antioxidant Analysis of Polysaccharides
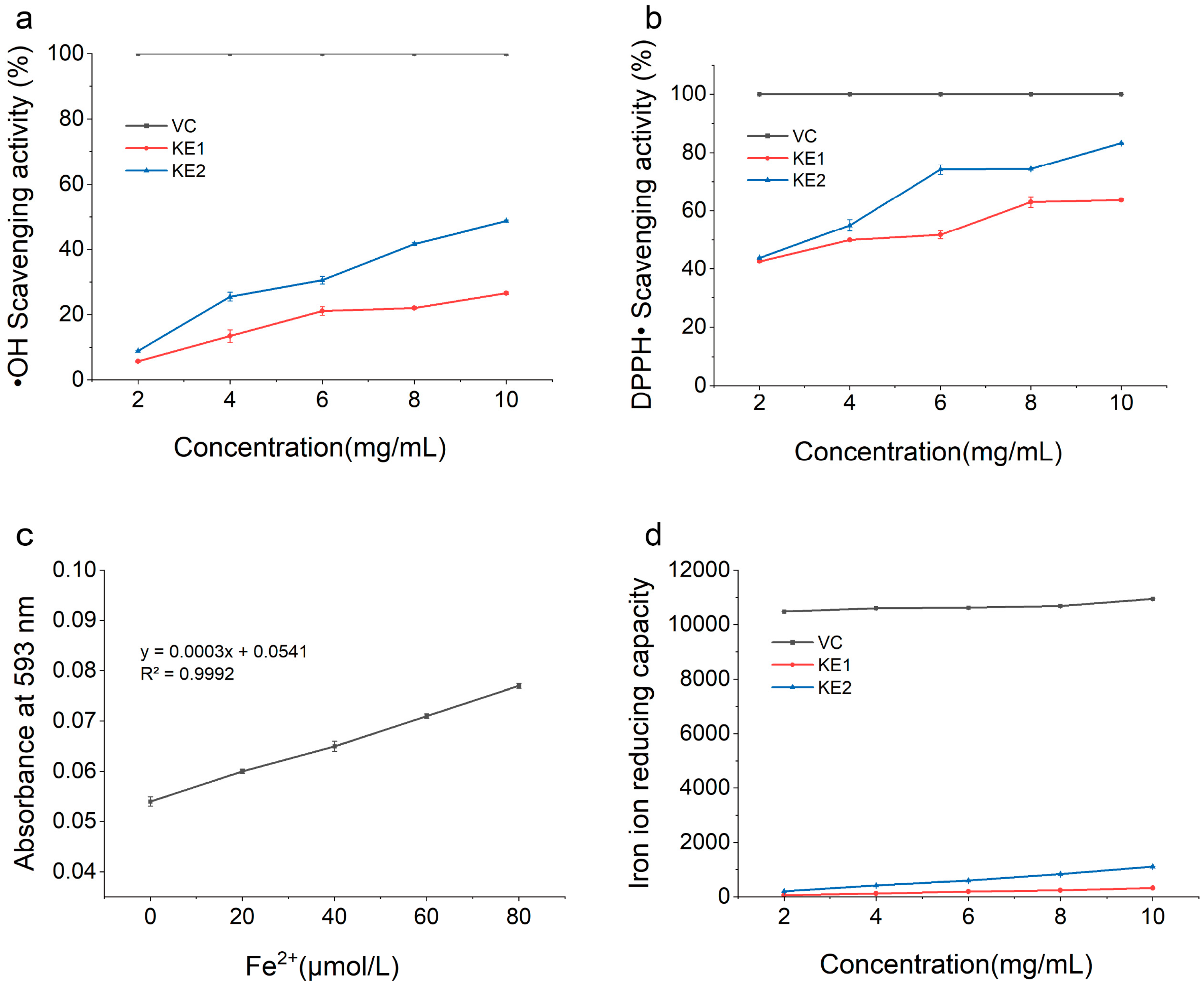
3.4.1. Analysis of Hydroxyl Radical Scavenging Activity of Extracellular Polysaccharides
3.4.2. Analysis of DPPH Scavenging Activity of Extracellular Polysaccharides
3.4.3. Analysis of Fe2+-Reducing Activity of Extracellular Polysaccharides
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sen, S.; Tiwari, O.N.; Arya, R.K.; Bhowmick, T.K.; Gayen, K. New insights on microbial extracellular polysaccharides: Production, biological activity, and applications. Biomass Convers. Biorefin. 2025, 15, 1–15. [Google Scholar] [CrossRef]
- Ma, W.; Chen, X.; Wang, B.; Lou, W.; Chen, X.; Hua, J.; Sun, Y.J.; Zhao, Y.; Peng, T. Characterization, antioxidativity, and anti-carcinoma activity of exopolysaccharide extract from Rhodotorula mucilaginosa CICC 33013. Carbohydr. Polym. 2018, 181, 768–777. [Google Scholar] [CrossRef]
- Chapot-Chartier, M.P. Interactions of the cell-wall glycopolymers of lactic acid bacteria with their bacteriophages. Front. Microbiol. 2014, 5, 236. [Google Scholar] [CrossRef] [PubMed]
- Angelin, J.; Kavitha, M. Exopolysaccharides from probiotic bacteria and their health potential. Int. J. Biol. Macromol. 2020, 162, 853–865. [Google Scholar] [CrossRef]
- Abd El Ghany, K.; Hamouda, R.; Abd Elhafez, E.; Mahrous, H.; Salem-Bekhit, M.; Hamza, H.A. A potential role of LA1 and its exopolysaccharides on cancer cells in male albino mice. Biotechnol. Biotechnol. Equip. 2015, 29, 977–983. [Google Scholar] [CrossRef]
- Yan, C.; Ji, S.; Wu, R.; Li, M.; He, K.; Shi, H.; Wang, C.; Yang, H.; Guo, J.; Wu, J. Structural properties and biological activities of the extracellular polysaccharide of Bacillus subtilis LZ13-4. Int. J. Biol. Macromol. 2024, 259, 129176. [Google Scholar] [CrossRef]
- Kim, Y.; Oh, S.; Yun, H.S.; Oh, S.; Kim, S.H. Cell-bound exopolysaccharide from probiotic bacteria induces autophagic cell death of tumour cells. Lett. Appl. Microbiol. 2010, 51, 123–130. [Google Scholar] [CrossRef]
- Afreen, A.; Ahmed, Z.; Khalid, N.; Ferheen, I.; Ahmed, I. Optimization and cholesterol-lowering activity of exopolysaccharide from Lactiplantibacillus paraplantarum NCCP 962. Appl. Microbiol. Biotechnol. 2023, 107, 1189–1204. [Google Scholar] [CrossRef]
- Nambiar, R.B.; Sellamuthu, P.S.; Perumal, A.B.; Sadiku, E.R.; Phiri, G.; Jayaramudu, J. Characterization of an exopolysaccharide produced by HM47 isolated from human breast milk. Process Biochem. 2018, 73, 15–22. [Google Scholar] [CrossRef]
- Salimi, F.; Farrokh, P. Recent advances in the biological activities of microbial exopolysaccharides. World J. Microbiol. Biotechnol. 2023, 39, 213. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.L.; Rahi, D.K.; Soni, S.K. An indigenous hyperproductive species of Aureobasidium pullulans RYLF-10: Influence of fermentation conditions on exopolysaccharide (EPS) production. Appl. Biochem. Biotechnol. 2014, 172, 1898–1908. [Google Scholar] [CrossRef]
- Saadat, Y.R.; Khosroushahi, A.Y.; Gargari, B.P. Yeast exopolysaccharides and their physiological functions. Folia Microbiol. 2021, 66, 171–182. [Google Scholar] [CrossRef]
- Hamidi, M.; Okoro, O.V.; Milan, P.B.; Khalili, M.R.; Samadian, H.; Nie, L.; Shavandi, A. Fungal exopolysaccharides: Properties, sources, modifications, and biomedical applications. Carbohydr. Polym. 2022, 284, 119152. [Google Scholar] [CrossRef]
- Rajkumar, A.S.; Morrissey, J.P. Protocols for marker-free gene knock-out and knock-down in using CRISPR/Cas9. FEMS Yeast Res. 2022, 22, foab067. [Google Scholar] [CrossRef]
- Rajkumar, A.S.; Varela, J.A.; Juergens, H.; Daran, J.M.G.; Morrissey, J.P. Biological Parts for Synthetic Biology. Front. Bioeng. Biotechnol. 2019, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Löbs, A.K.; Engel, R.; Schwartz, C.; Flores, A.; Wheeldon, I. CRISPR-Cas9-enabled genetic disruptions for understanding ethanol and ethyl acetate biosynthesis in Kluyveromyces marxianus. Biotechnol. Biofuels 2017, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.; Gerliani, N.; Aïder, M. An emerging yeast cell factory for applications in food and biotechnology. Int. J. Food Microbiol. 2020, 333, 108818. [Google Scholar] [CrossRef]
- Hu, Y.B.; Hong, H.L.; Liu, L.Y.; Zhou, J.N.; Wang, Y.; Li, Y.M.; Zhai, L.Y.; Shi, Z.H.; Zhao, J.; Liu, D. Analysis of Structure and Antioxidant Activity of Polysaccharides from. Pharmaceuticals 2022, 15, 1545. [Google Scholar] [CrossRef]
- Yu, M.G.; Chen, M.J.; Gui, J.L.; Huang, S.D.; Liu, Y.M.; Shentu, H.F.; He, J.; Fang, Z.Y.; Wang, W.M.; Zhang, Y.J. Preparation of polysaccharides and their antioxidant activity in vitro and in vivo. Int. J. Biol. Macromol. 2019, 137, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.S.; Lei, X.; Xie, Q.; Zhang, M.J.; Zhang, Y.A.; Xi, J.X.; Duan, J.Y.; Ge, J.; Nian, F.Z. In vitro study on antioxidant and lipid-lowering activities of tobacco polysaccharides. Bioresour. Bioprocess. 2024, 11, 15. [Google Scholar] [CrossRef]
- Kumar, D.; Kastánek, P.; Adhikary, S.P. Exopolysaccharides from cyanobacteria and microalgae and their commercial application. Curr. Sci. India 2018, 115, 234–241. [Google Scholar] [CrossRef]
- Kumari, J.; Kumawat, R.; Prasanna, R.; Jothieswari, D.; Debnath, R.; Ikbal, A.A.; Palit, P.; Rawat, R.; Gopikrishna, K.; Tiwari, O.N. Microbial exopolysaccharides: Classification, biosynthetic pathway, industrial extraction and commercial production to unveil its bioprospection: A comprehensive review. Int. J. Biol. Macromol. 2025, 297, 139917. [Google Scholar] [CrossRef] [PubMed]
- Potocnik, T.; Miklavcic, D.; Macek Lebar, A. Effect of electroporation and recovery medium pH on cell membrane permeabilization, cell survival and gene transfer efficiency in vitro. Bioelectrochemistry 2019, 130, 107342. [Google Scholar] [CrossRef]
- Adesulu-Dahunsi, A.T.; Sanni, A.I.; Jeyaram, K.; Ojediran, J.O.; Ogunsakin, A.O.; Banwo, K. Extracellular polysaccharide from Weissella confusa OF126: Production, optimization, and characterization. Int. J. Biol. Macromol. 2018, 111, 514–525. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, B.; Liu, C.J.; Yang, E. Optimization of Biosynthesis Conditions for the Production of Exopolysaccharides by SP8 and the Exopolysaccharides Antioxidant Activity Test. Indian J. Microbiol. 2020, 60, 334–345. [Google Scholar] [CrossRef]
- Wang, Y.; Du, R.P.; Qiao, X.X.; Zhao, B.; Zhou, Z.J.; Han, Y. Optimization and characterization of exopolysaccharides with a highly branched structure extracted from B-2. Int. J. Biol. Macromol. 2020, 142, 73–84. [Google Scholar] [CrossRef]
- Yang, H.Y.; Meng, H.; Xie, L.M.; Huang, Z.B. Contribution of Quercetin to the Composition and Antioxidant Properties of Exopolysaccharides. Foods 2023, 12, 1004. [Google Scholar] [CrossRef]
- Bai, J.F.; Jia, X.W.; Chen, Y.C.; Ning, Z.X.; Liu, S.H.; Xu, C.P. Effect of Carbon Source on Properties and Bioactivities of Exopolysaccharide Produced by (Agaricomycetes). Int. J. Med. Mushrooms 2020, 22, 289–297. [Google Scholar] [CrossRef]
- Shukla, A.; Mehta, K.; Parmar, J.; Pandya, J.; Saraf, M. Depicting the exemplary knowledge of microbial exopolysaccharides in a nutshell. Eur. Polym. J. 2019, 119, 298–310. [Google Scholar] [CrossRef]
- Feng, J.; Feng, N.; Tang, Q.J.; Liu, Y.F.; Yang, Y.; Liu, F.; Zhang, J.S.; Lin, C.C. Optimization of Polysaccharides Fermentation Process for Large-Scale Production. Appl. Biochem. Biotechnol. 2019, 189, 972–986. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.; Tseng, M.H.; Liu, C.J. Production of polysaccharides from (CCRC 36041) under limitations of nutrients. Enzym. Microb. Technol. 2006, 38, 109–117. [Google Scholar] [CrossRef]
- Tikhomirova, T.S.; Taraskevich, M.R.; Lepekhin, Y.A.; Shevelyova, M.P.; Nemashkalov, V.A. Optimization and scaling up of extracellular polysaccharide production by submerged culture of Ganoderma lucidum on starch-containing medium using response surface methodology and laboratory bioreactors of various designs. Lett. Appl. Microbiol. 2024, 77, ovae115. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.; Tsai, M.J.; Hsu, T.H.; Chang, D.M.; Lo, C.T. Medium optimization for polysaccharide production of Cordyceps sinensis. Appl. Biochem. Biotechnol. 2005, 120, 145–157. [Google Scholar] [CrossRef]
- Wang, L.Y.; Cheong, K.L.; Wu, D.T.; Meng, L.Z.; Zhao, J.; Li, S.P. Fermentation optimization for the production of bioactive polysaccharides from Cordyceps sinensis fungus UM01. Int. J. Biol. Macromol. 2015, 79, 180–185. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Role of free radicals and catalytic metal ions in human disease: An overview. Methods Enzymol. 1990, 186, 1–85. [Google Scholar] [CrossRef]
- Wang, Z.B.; Pei, J.J.; Ma, H.L.; Cai, P.F.; Yan, J.K. Effect of extraction media on preliminary characterizations and antioxidant activities of Phellinus linteus polysaccharides. Carbohydr. Polym. 2014, 109, 49–55. [Google Scholar] [CrossRef]
- Hou, S.J.; Cheng, K.C.; Lin, S.H.; Hsiao, I.L.; Santoso, S.P.; Singajaya, S.; Chou, Y.C.; Lin, S.P. Improvement of extracellular polysaccharides production from Cordyceps militaris immobilized alginate beads in repeated-batch fermentation. Lwt-Food Sci. Technol. 2024, 193, 115752. [Google Scholar] [CrossRef]
- Wang, Z.C.; Jia, S.T.; Cui, J.W.; Qu, J.H.; Yue, Y.Y.; Sun, Q.; Zhang, H.R. Antioxidant activity of a polysaccharide produced by CGMCC 6882. Int. J. Biol. Macromol. 2019, 141, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.B.; Seo, E.W.; Jeong, H.J. Effect of extracts from pine needle against oxidative DNA damage and apoptosis induced by hydroxyl radical via antioxidant activity. Food Chem. Toxicol. 2009, 47, 2135–2141. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Saravanakumar, K.; Park, S.; Han, K.S.; Wang, M.H. Isolation, Characterization, Antioxidant, and Wound Healing Activities of Extracellular Polysaccharide from Endophytic Fungus Talaromyces purpureogenus. Appl. Biochem. Biotechnol. 2023, 195, 3822–3839. [Google Scholar] [CrossRef]
- Li, X.B.; Sun, Q.Y.; Li, S.; Chen, W.C.; Shi, Z.M.; Xu, Z.Y.; Xu, L.; Chen, M.; Li, Z.H. Production with Fermentation Culture and Antioxidant Activity of Polysaccharides from. Fermentation 2024, 10, 46. [Google Scholar] [CrossRef]
- Diment, D.; Musl, O.; Balakshin, M.; Rigo, D. Guidelines for Evaluating the Antioxidant Activity of Lignin via the 2,2-diphenyl-1-picrylhydrazyl (DPPH) Assay. ChemSusChem 2025, 18, e202402383. [Google Scholar] [CrossRef] [PubMed]
- Zhuansun, W.; Xu, J.; Liu, H.; Zhao, Y.; Chen, L.; Shan, S.; Song, S.; Zhang, H.; Dong, T.; Zeng, H.; et al. Optimisation of the production of a selenium-enriched polysaccharide from Cordyceps cicadae S1 and its structure and antioxidant activity. Front. Nutr. 2022, 9, 1032289. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, B.; Ibrahim, S.A.; Gao, S.S.; Yang, H.; Huang, W. Purification, characterization and antioxidant activity of polysaccharides from Flammulina velutipes residue. Carbohydr. Polym. 2016, 145, 71–77. [Google Scholar] [CrossRef]
- Chen, B.J.; Shi, M.J.; Cui, S.; Hao, S.X.; Hider, R.C.; Zhou, T. Improved antioxidant and anti-tyrosinase activity of polysaccharide from by degradation. Int. J. Biol. Macromol. 2016, 92, 715–722. [Google Scholar] [CrossRef]
- Xue, T.; Ruan, K.; Tang, Z.; Duan, J.; Xu, H. Isolation, structural properties, and bioactivities of polysaccharides from Althaea officinalis Linn.: A review. Int. J. Biol. Macromol. 2023, 242, 125098. [Google Scholar] [CrossRef]
- Chen, H.L.; Ju, Y.; Li, J.J.; Yu, M. Antioxidant activities of polysaccharides from and their significance for disease prevention. Int. J. Biol. Macromol. 2012, 50, 214–218. [Google Scholar] [CrossRef]
- Yu, P.; Wang, J.; Liu, J.; Zhou, Y.; Luo, F.; Yang, M.; Ai, X. Preparation techniques, structural features, and bioactivities of Eucommia ulmoides polysaccharides: A review. Int. J. Biol. Macromol. 2024, 275, 133686. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; He, R.; Sun, P.; Zhang, F.; Linhardt, R.J.; Zhang, A. Molecular mechanisms of bioactive polysaccharides from Ganoderma lucidum (Lingzhi), a review. Int. J. Biol. Macromol. 2020, 150, 765–774. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, X.; Yu, J.; Li, Z.; Shi, H.; Zhang, G.; Ling, J. Structural basis of immunomodulation by edible fungal polysaccharides: From molecular characteristics to action mechanisms. Carbohydr. Res. 2025, 555, 109591. [Google Scholar] [CrossRef]
- Ding, L.; Shangguan, H.; Wang, X.; Liu, J.; Shi, Y.; Xu, X.; Xie, Y. Extraction, purification, structural characterization, biological activity, mechanism of action and application of polysaccharides from Ganoderma lucidum: A review. Int. J. Biol. Macromol. 2025, 288, 138575. [Google Scholar] [CrossRef] [PubMed]





| Std. | Run | Factor 1: Source% | Factor 2: Peptone% | Factor 3: CaCl2% | Response 1: Exopolysaccharide Concentration mg/L |
|---|---|---|---|---|---|
| 1 | 4 | 4 | 1 | 0.125 | 1367.74 |
| 2 | 13 | 8 | 1 | 0.125 | 820.32 |
| 3 | 12 | 4 | 3 | 0.125 | 328.88 |
| 4 | 7 | 8 | 3 | 0.125 | 2400.08 |
| 5 | 3 | 4 | 2 | 0.05 | 1684.31 |
| 6 | 2 | 8 | 2 | 0.05 | 2833.88 |
| 7 | 11 | 4 | 2 | 0.2 | 1079.57 |
| 8 | 6 | 8 | 2 | 0.2 | 2139.89 |
| 9 | 14 | 6 | 1 | 0.05 | 1267.42 |
| 10 | 17 | 6 | 3 | 0.05 | 1082.27 |
| 11 | 9 | 6 | 1 | 0.2 | 592.37 |
| 12 | 10 | 6 | 3 | 0.2 | 597.687 |
| 13 | 1 | 6 | 2 | 0.125 | 2741.52 |
| 14 | 15 | 6 | 2 | 0.125 | 2833.59 |
| 15 | 8 | 6 | 2 | 0.125 | 2703.4 |
| 16 | 16 | 6 | 2 | 0.125 | 2597.55 |
| 17 | 5 | 6 | 2 | 0.125 | 2626.28 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 1.297 × 107 | 9 | 1.441 × 106 | 62.39 | <0.0001 | significant |
| A-Sucrose | 1.743 × 106 | 1 | 1.743 × 106 | 75.43 | <0.0001 | |
| B-Peptone | 16,296.14 | 1 | 16,296.14 | 0.7054 | 0.4287 | |
| C-CaCl2 | 7.554 × 105 | 1 | 7.554 × 105 | 32.70 | 0.0007 | |
| AB | 1.714 × 106 | 1 | 1.714 × 106 | 74.21 | <0.0001 | |
| AC | 1991.09 | 1 | 1991.09 | 0.0862 | 0.7776 | |
| BC | 9069.71 | 1 | 9069.71 | 0.3926 | 0.5508 | |
| A2 | 1.872 × 105 | 1 | 1.872 × 105 | 8.10 | 0.0248 | |
| B2 | 6.688 × 106 | 1 | 6.688 × 106 | 289.52 | <0.0001 | |
| C2 | 1.298 × 106 | 1 | 1.298 × 106 | 56.18 | 0.0001 | |
| Residual | 1.617 × 105 | 7 | 23,100.98 | |||
| Lack of Fit | 1.262 × 105 | 3 | 42,065.19 | 4.74 | 0.0835 | not significant |
| Pure Error | 35,511.30 | 4 | 8877.83 | |||
| Cor Total | 1.313 × 107 | 16 |
| Std. | Run | Factor 1: Volume of Liquid % | Factor 2: pH | Factor 3: Inoculation Amount % | Response 1: Exopolysaccharide Concentration mg/L |
|---|---|---|---|---|---|
| 1 | 1 | 20 | 6 | 2 | 5180.06 |
| 2 | 9 | 40 | 6 | 2 | 2483.62 |
| 3 | 8 | 20 | 8 | 2 | 3739.54 |
| 4 | 7 | 40 | 8 | 2 | 4014.76 |
| 5 | 10 | 20 | 7 | 1 | 3741.93 |
| 6 | 13 | 40 | 7 | 1 | 2745.87 |
| 7 | 16 | 20 | 7 | 3 | 4474.89 |
| 8 | 6 | 40 | 7 | 3 | 1482.35 |
| 9 | 17 | 60 | 6 | 1 | 4124.54 |
| 10 | 3 | 60 | 8 | 1 | 3041.3 |
| 11 | 12 | 60 | 6 | 3 | 2926.94 |
| 12 | 15 | 60 | 8 | 3 | 3296.03 |
| 13 | 14 | 60 | 7 | 2 | 5980.57 |
| 14 | 2 | 60 | 7 | 2 | 5233.54 |
| 15 | 11 | 60 | 7 | 2 | 5545.68 |
| 16 | 5 | 60 | 7 | 2 | 5585.22 |
| 17 | 4 | 60 | 7 | 2 | 5586.25 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 2.663 × 107 | 9 | 2.959 × 106 | 29.99 | <0.0001 | significant |
| A-Volume of liquid | 5.136 × 106 | 1 | 5.136 × 106 | 52.04 | 0.0002 | |
| B-PH | 48,598.71 | 1 | 48,598.71 | 0.4924 | 0.5055 | |
| C-Inoculation amount | 2.714 × 105 | 1 | 2.714 × 105 | 2.75 | 0.1412 | |
| AB | 2.208 × 106 | 1 | 2.208 × 106 | 22.37 | 0.0021 | |
| AC | 9.965 × 105 | 1 | 9.965 × 105 | 10.10 | 0.0155 | |
| BC | 5.273 × 105 | 1 | 5.273 × 105 | 5.34 | 0.0541 | |
| A2 | 4.076 × 106 | 1 | 4.076 × 106 | 41.30 | 0.0004 | |
| B2 | 2.355 × 106 | 1 | 2.355 × 106 | 23.86 | 0.0018 | |
| C2 | 9.362 × 106 | 1 | 9.362 × 106 | 94.86 | <0.0001 | |
| Residual | 6.908 × 105 | 7 | 98,690.20 | |||
| Lack of Fit | 4.093 × 105 | 3 | 1.364 × 105 | 1.94 | 0.2652 | not significant |
| Pure Error | 2.815 × 105 | 4 | 70,384.90 | |||
| Cor Total | 2.733 × 107 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Xu, L.; Chen, M.; Li, Z. A Study on the Extraction, Fermentation Condition Optimization, and Antioxidant Activity Assessment of Polysaccharides Derived from Kluyveromyces marxianus. Foods 2025, 14, 2796. https://doi.org/10.3390/foods14162796
Xu Z, Xu L, Chen M, Li Z. A Study on the Extraction, Fermentation Condition Optimization, and Antioxidant Activity Assessment of Polysaccharides Derived from Kluyveromyces marxianus. Foods. 2025; 14(16):2796. https://doi.org/10.3390/foods14162796
Chicago/Turabian StyleXu, Ziyin, Lin Xu, Mei Chen, and Zhonghai Li. 2025. "A Study on the Extraction, Fermentation Condition Optimization, and Antioxidant Activity Assessment of Polysaccharides Derived from Kluyveromyces marxianus" Foods 14, no. 16: 2796. https://doi.org/10.3390/foods14162796
APA StyleXu, Z., Xu, L., Chen, M., & Li, Z. (2025). A Study on the Extraction, Fermentation Condition Optimization, and Antioxidant Activity Assessment of Polysaccharides Derived from Kluyveromyces marxianus. Foods, 14(16), 2796. https://doi.org/10.3390/foods14162796






