Unveiling Adulterated Cheese: A 1H-NMR-Based Lipidomic Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
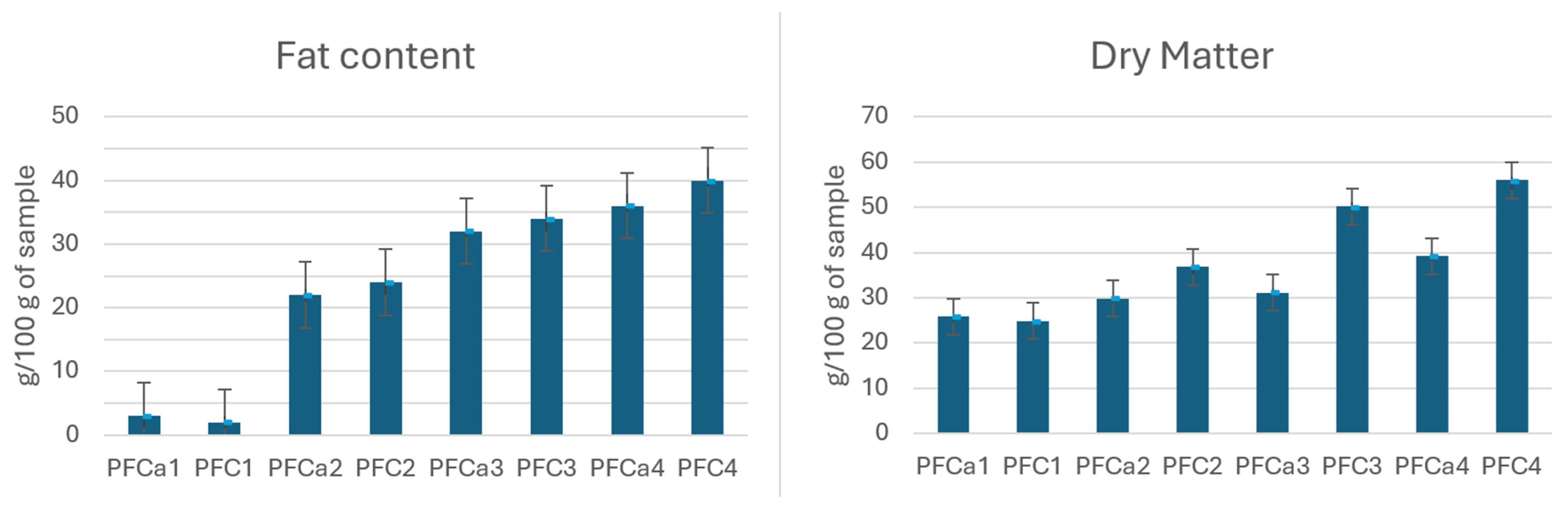
2.2. Dry Matter Content
2.3. Total Fat Content
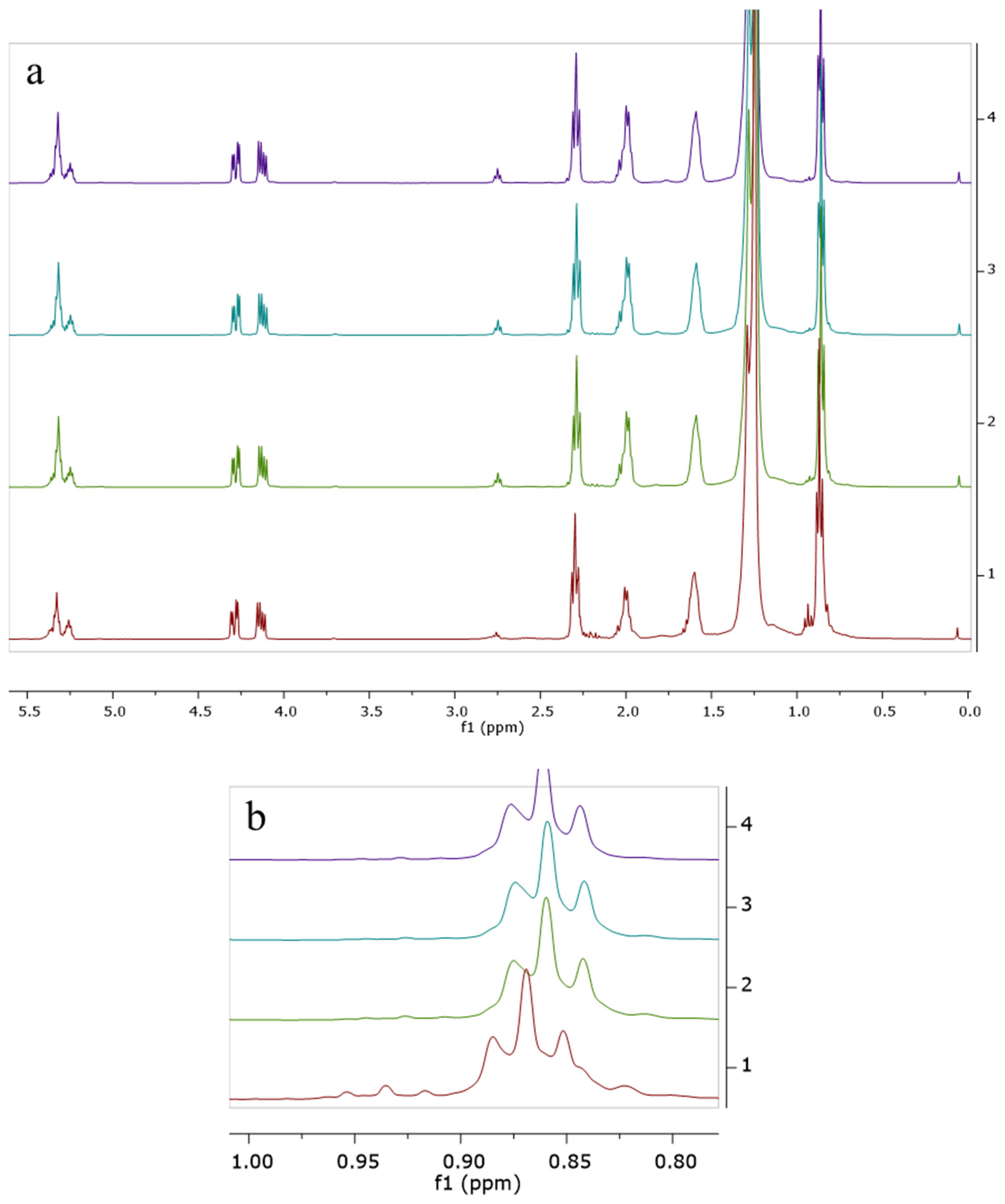
2.4. 1H-NMR Spectroscopy
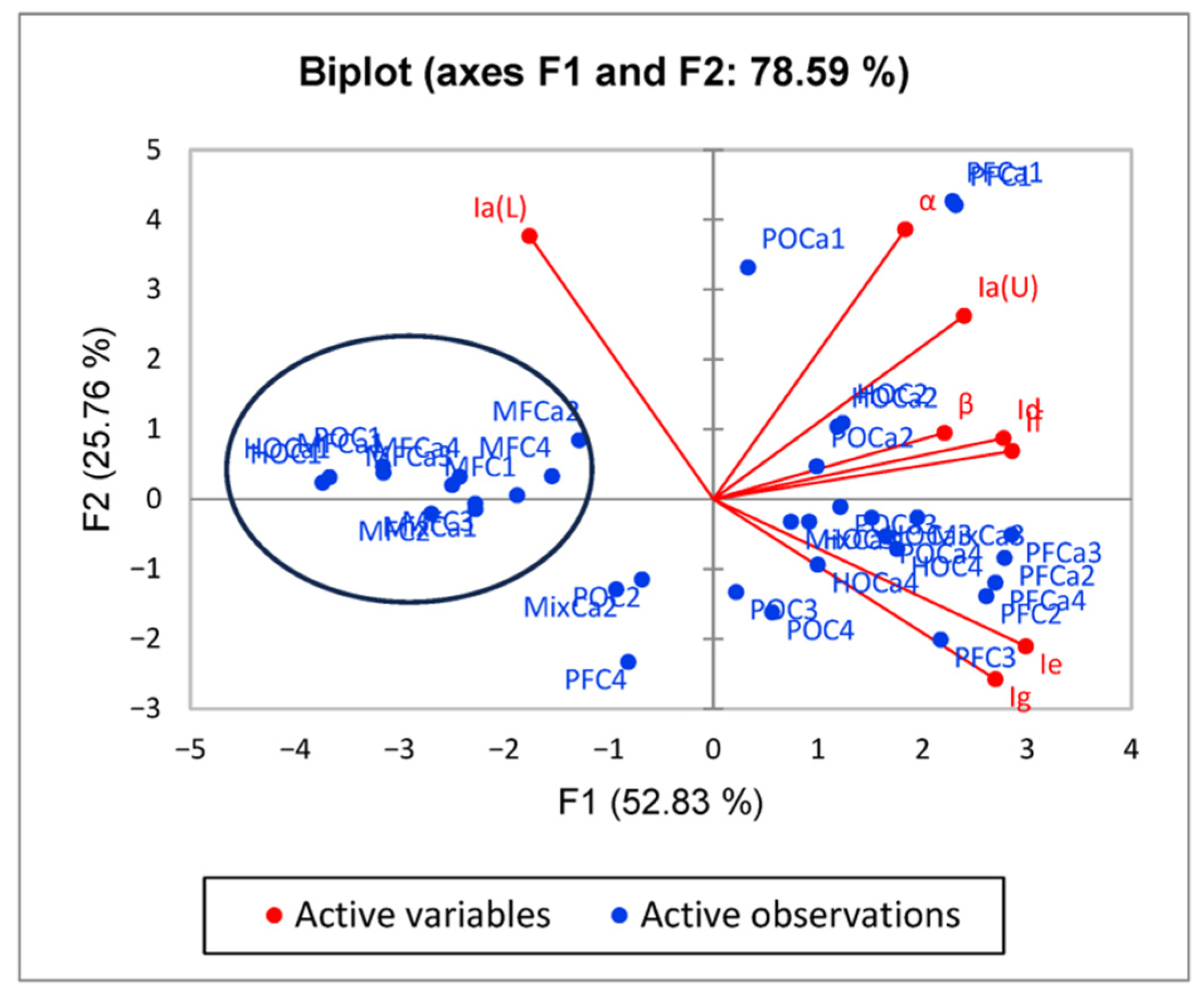
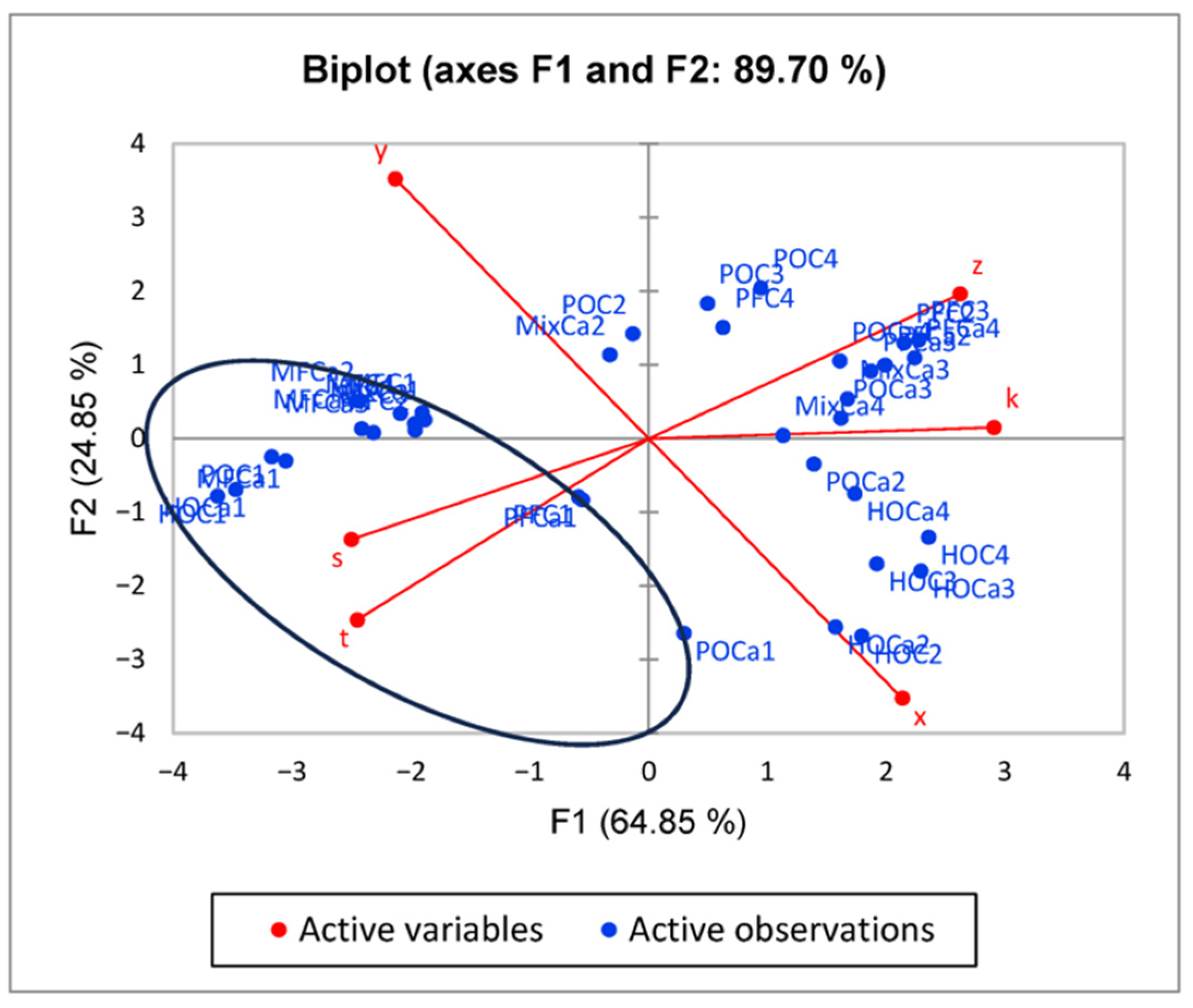
2.5. PCA
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, H.J.; Park, J.M.; Lee, J.H.; Kim, J.M. Detection for Non-Milk Fat in Dairy Product by Gas Chromatography. Korean J. Food Sci. Anim. Resour. 2016, 36, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Bratu, A.; Mihalache, M.; Hanganu, A.; Chira, N.A.; Todasca, M.C.; Rosca, S. Gas Chromatography Coupled with Chemometric Method for Authentication of Romanian Cheese. Rev. Chim. 2012, 63, 1099–1102. [Google Scholar]
- Santiago-López, L.; Aguilar-Toalá, J.E.; Hernández-Mendoza, A.; Vallejo-Cordoba, B.; Liceaga, A.M.; González-Córdova, A.F. Invited Review: Bioactive Compounds Produced during Cheese Ripening and Health Effects Associated with Aged Cheese Consumption. J. Dairy Sci. 2018, 101, 3742–3757. [Google Scholar] [CrossRef]
- Haddad, L.; Francis, J.; Rizk, T.; Akoka, S.; Remaud, G.S.; Bejjani, J. Cheese Characterization and Authentication through Lipid Biomarkers Obtained by High-Resolution 1H NMR Profiling. Food Chem. 2022, 383, 132434. [Google Scholar] [CrossRef] [PubMed]
- Tociu, M.; Todasca, M.C.; Bratu, A.; Mihalache, M.; Manolache, F. Fast Approach for Fatty Acid Profiling of Dairy Products Fats Using 1H-NMR Spectroscopy. Int. Dairy J. 2018, 83, 52–57. [Google Scholar] [CrossRef]
- Cardin, M.; Cardazzo, B.; Mounier, J.; Novelli, E.; Coton, M.; Coton, E. Authenticity and Typicity of Traditional Cheeses: A Review on Geographical Origin Authentication Methods. Foods 2022, 11, 3379. [Google Scholar] [CrossRef]
- Cuibus, L.; Maggio, R.; Mureşan, V.; Diaconeasa, Z.; Fetea, F.; Socaciu, C. Preliminary Discrimination of Cheese Adulteration by FT-IR Spectroscopy. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2014, 71, 142–146. [Google Scholar] [CrossRef][Green Version]
- Ciampa, A.; Danesi, F.; Picone, G. NMR-Based Metabolomics for a More Holistic and Sustainable Research in Food Quality Assessment: A Narrative Review. Appl. Sci. 2023, 13, 372. [Google Scholar] [CrossRef]
- Ungureanu, C.; Zgârian, R.; Tihan, G.; Fadeev, V. Exploring Pathogenic Bacteria in Cheese: Insights from Microbial Isolation Studies. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2024, 86, 49–64. [Google Scholar][Green Version]
- Mengucci, C.; Rabiti, D.; Urbinati, E.; Picone, G.; Romano, R.; Aiello, A.; Ferranti, P.; Capozzi, F. Spotting Frozen Curd in PDO Buffalo Mozzarella Cheese through Insights on Its Supramolecular Structure Acquired by 1H TD-NMR Relaxation Experiments. Appl. Sci. 2021, 11, 1466. [Google Scholar] [CrossRef]
- Tociu, M.; Todasca, M.C.; Stanescu, M.D. Authentication and Nutritional Benefits of Cheeses Based on Vegetable Oils. Rev. Chim. 2017, 68, 2002–2005. [Google Scholar] [CrossRef]
- Andueza, D.; Agabriel, C.; Constant, I.; Lucas, A.; Martin, B. Using Visible or near Infrared Spectroscopy (NIRS) on Cheese to Authenticate Cow Feeding Regimes. Food Chem. 2013, 141, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Vlasiou, M.C. Cheese and Milk Adulteration: Detection with Spectroscopic Techniques and HPLC: Advantages and Disadvantages. Dairy 2023, 4, 509–514. [Google Scholar] [CrossRef]
- Zhang, L.G.; Zhang, X.; Ni, L.J.; Xue, Z.B.; Gu, X.; Huang, S.X. Rapid Identification of Adulterated Cow Milk by Non-Linear Pattern Recognition Methods Based on near Infrared Spectroscopy. Food Chem. 2014, 145, 342–348. [Google Scholar] [CrossRef]
- Jirankalgikar, N.M.; De, S. Detection of Tallow Adulteration in Cow Ghee by Derivative Spectrophotometry. J. Nat. Sci. Biol. Med. 2014, 5, 317–319. [Google Scholar] [CrossRef]
- Dankowska, A.; Małecka, M.; Kowalewski, W. Detection of Plant Oil Addition to Cheese by Synchronous Fluorescence Spectroscopy. Dairy Sci. Technol. 2015, 95, 413–424. [Google Scholar] [CrossRef]
- Cavallini, N.; Strani, L.; Becchi, P.P.; Pizzamiglio, V.; Michelini, S.; Savorani, F.; Cocchi, M.; Durante, C. Tracing the Identity of Parmigiano Reggiano “Prodotto Di Montagna—Progetto Territorio” Cheese Using NMR Spectroscopy and Multivariate Data Analysis. Anal. Chim. Acta 2023, 1278, 341761. [Google Scholar] [CrossRef]
- Schievano, E.; Pasini, G.; Cozzi, G.; Mammi, S. Identification of the Production Chain of Asiago d’Allevo Cheese by Nuclear Magnetic Resonance Spectroscopy and Principal Component Analysis. J. Agric. Food Chem. 2008, 56, 7208–7214. [Google Scholar] [CrossRef]
- Vítová, E.; Loupancová, B.; Sklenářová, K.; Divišová, R.; Buňka, F. Identification of Volatile Aroma Compounds in Processed Cheese Analogues Based on Different Types of Fat. Chem. Pap. 2012, 66, 907–913. [Google Scholar] [CrossRef]
- Prema, D.; Turner, T.D.; Jensen, J.; Pilfold, J.L.; Church, J.S.; Donkor, K.K.; Cinel, B. Rapid Determination of Total Conjugated Linoleic Acid Concentrations in Beef by 1H NMR Spectroscopy. J. Food Compos. Anal. 2015, 41, 54–57. [Google Scholar] [CrossRef]
- Corsaro, C.; Mallamace, D.; Vasi, S.; Ferrantelli, V.; Dugo, G.; Cicero, N. 1H HR-MAS NMR Spectroscopy and the Metabolite Determination of Typical Foods in Mediterranean Diet. J. Anal. Methods Chem. 2015, 2015, 175696. [Google Scholar] [CrossRef] [PubMed]
- Monakhova, Y.B.; Godelmann, R.; Andlauer, C.; Kuballa, T.; Lachenmeier, D.W. Identification of Imitation Cheese and Imitation Ice Cream Based on Vegetable Fat Using NMR Spectroscopy and Chemometrics. Int. J. Food Sci. 2013, 2013, 367841. [Google Scholar] [CrossRef] [PubMed]
- Picone, G.; Mengucci, C.; Capozzi, F. The NMR Added Value to the Green Foodomics Perspective: Advances by Machine Learning to the Holistic View on Food and Nutrition. Magn. Reson. Chem. 2022, 60, 590–596. [Google Scholar] [CrossRef]
- Ray, C.L.; Gawenis, J.A.; Bylo, M.P.; Pescaglia, J.; Greenlief, C.M. Detection of Vegetable Oil Adulteration in Pre-Grated Bovine Hard Cheeses via 1H NMR Spectroscopy. Molecules 2023, 28, 920. [Google Scholar] [CrossRef]
- Joolaei Ahranjani, P.; Joolaei Ahranjani, P.; Dehghan, K.; Esfandiari, Z.; Ferrentino, G. Advances in Spectroscopic Techniques for the Detection of Cheese Adulteration: A Systematic Review. Food Chem. X 2025, 29, 102685. [Google Scholar] [CrossRef]
- Fang, G.; Goh, J.Y.; Tay, M.; Lau, H.F.; Li, S.F.Y. Characterization of Oils and Fats by 1H NMR and GC/MS Fingerprinting: Classification, Prediction and Detection of Adulteration. Food Chem. 2013, 138, 1461–1469. [Google Scholar] [CrossRef]
- Picone, G. The 1H HR-NMR Methods for the Evaluation of the Stability, Quality, Authenticity, and Shelf Life of Foods. Encyclopedia 2024, 4, 1617–1628. [Google Scholar] [CrossRef]
- Hanganu, A.; Todaşca, M.C.; Chira, N.A.; Maganu, M.; Roşca, S. The Compositional Characterisation of Romanian Grape Seed Oils Using Spectroscopic Methods. Food Chem. 2012, 134, 2453–2458. [Google Scholar] [CrossRef]
- Schripsema, J. Comprehensive Analysis of Polar and Apolar Constituents of Butter and Margarine by Nuclear Magnetic Resonance, Reflecting Quality and Production Processes. J. Agric. Food Chem. 2008, 56, 2547–2552. [Google Scholar] [CrossRef]
- Fadzillah, N.A.; Man, Y.B.C.; Rohman, A.; Rosman, A.S.; Ismail, A.; Mustafa, S.; Khatib, A. Detection of Butter Adulteration with Lard by Employing 1H-NMR Spectroscopy and Multivariate Data Analysis. J. Oleo Sci. 2015, 64, 697–703. [Google Scholar] [CrossRef]
- Spyros, A. Application of NMR in Food Analysis. In Nuclear Magnetic Resonance; Ramesh, V., Ed.; The Royal Society of Chemistry location: London, UK, 2016; pp. 269–308. [Google Scholar]
- Ralli, E.; Spyros, A. A Study of Greek Graviera Cheese by NMR-Based Metabolomics. Molecules 2023, 28, 5488. [Google Scholar] [CrossRef] [PubMed]
- Maestrello, V.; Solovyev, P.; Franceschi, P.; Stroppa, A.; Bontempo, L. 1H-NMR Approach for the Discrimination of PDO Grana Padano Cheese from Non-PDO Cheeses. Foods 2024, 13, 358. [Google Scholar] [CrossRef] [PubMed]
- ISO 659:2009; Oilseeds—Determination of Oil Content (Reference Method). ISO: Geneva, Switzerland, 2009.
- Siudem, P.; Zielińska, A.; Paradowska, K. Application of 1H NMR in the Study of Fatty Acids Composition of Vegetable Oils. J. Pharm. Biomed. Anal. 2022, 212, 114658. [Google Scholar] [CrossRef] [PubMed]
- Abedini, A.; Salimi, M.; Mazaheri, Y.; Sadighara, P.; Alizadeh Sani, M.; Assadpour, E.; Jafari, S.M. Assessment of Cheese Frauds, and Relevant Detection Methods: A Systematic Review. Food Chem. X 2023, 19, 100825. [Google Scholar] [CrossRef]
- Patel, H.; Aru, V.; Sørensen, K.M.; Engelsen, S.B. Towards On-Line Cheese Monitoring: Exploration of Semi-Hard Cheeses Using NIR and 1H NMR Spectroscopy. Food Chem. 2024, 454, 139786. [Google Scholar] [CrossRef]




| Proportion of the Added Fat | Milk Fat | Hydrogenated Oil | Palm Oil | Pork Fat | Mixture of Exogenous Fats | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0% | MFCa1 | MFC1 | HOCa1 | HOC1 | POCa1 | POC1 | PFCa1 | PFC1 | MixCa1 | MixC1 |
| 5% | MFCa2 | MFC2 | HOCa2 | HOC2 | POCa2 | POC2 | PFCa2 | PFC2 | MixCa2 | MixC2 |
| 12% | MFCa3 | MFC3 | HOCa3 | HOC3 | POCa3 | POC3 | PFCa3 | PFC3 | MixCa3 | MixC3 |
| 15% | MFCa4 | MFC4 | HOCa4 | HOC4 | POCa4 | POC4 | PFCa4 | PFC4 | MixCa4 | MixC4 |
| Precipitation agent | Calcium chloride | Citric acid | Calcium chloride | Citric acid | Calcium chloride | Citric acid | Calcium chloride | Citric acid | Calcium chloride | Citric acid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todașcă, M.-C.; Tociu, M.; Manolache, F.-A. Unveiling Adulterated Cheese: A 1H-NMR-Based Lipidomic Approach. Foods 2025, 14, 2789. https://doi.org/10.3390/foods14162789
Todașcă M-C, Tociu M, Manolache F-A. Unveiling Adulterated Cheese: A 1H-NMR-Based Lipidomic Approach. Foods. 2025; 14(16):2789. https://doi.org/10.3390/foods14162789
Chicago/Turabian StyleTodașcă, Maria-Cristina, Mihaela Tociu, and Fulvia-Ancuța Manolache. 2025. "Unveiling Adulterated Cheese: A 1H-NMR-Based Lipidomic Approach" Foods 14, no. 16: 2789. https://doi.org/10.3390/foods14162789
APA StyleTodașcă, M.-C., Tociu, M., & Manolache, F.-A. (2025). Unveiling Adulterated Cheese: A 1H-NMR-Based Lipidomic Approach. Foods, 14(16), 2789. https://doi.org/10.3390/foods14162789







