Biocontrol and Nanotechnology Strategies for Postharvest Disease Management in Fruits and Vegetables: A Comprehensive Review
Abstract
1. Introduction
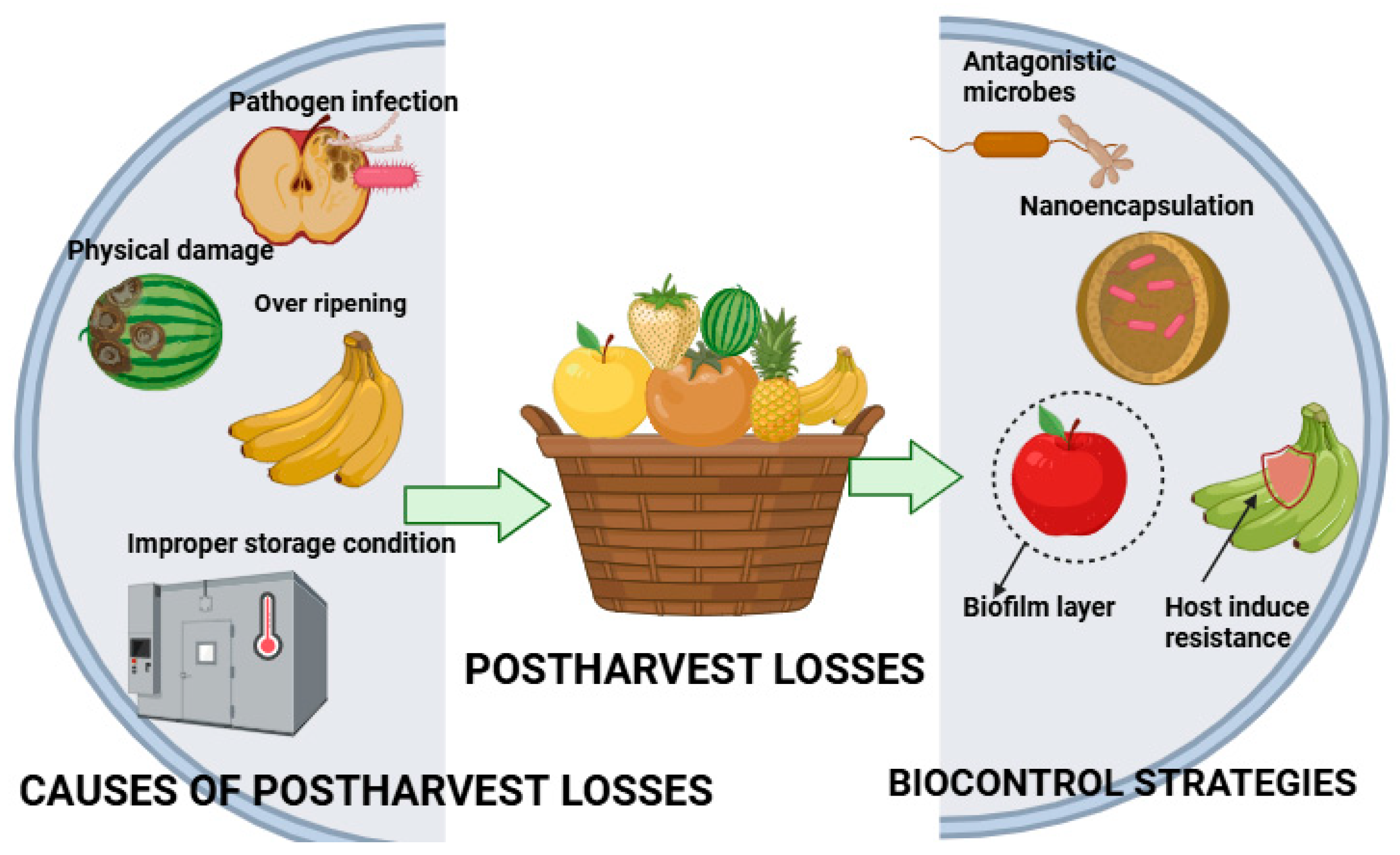
2. Postharvest Challenges in Fruits and Vegetables
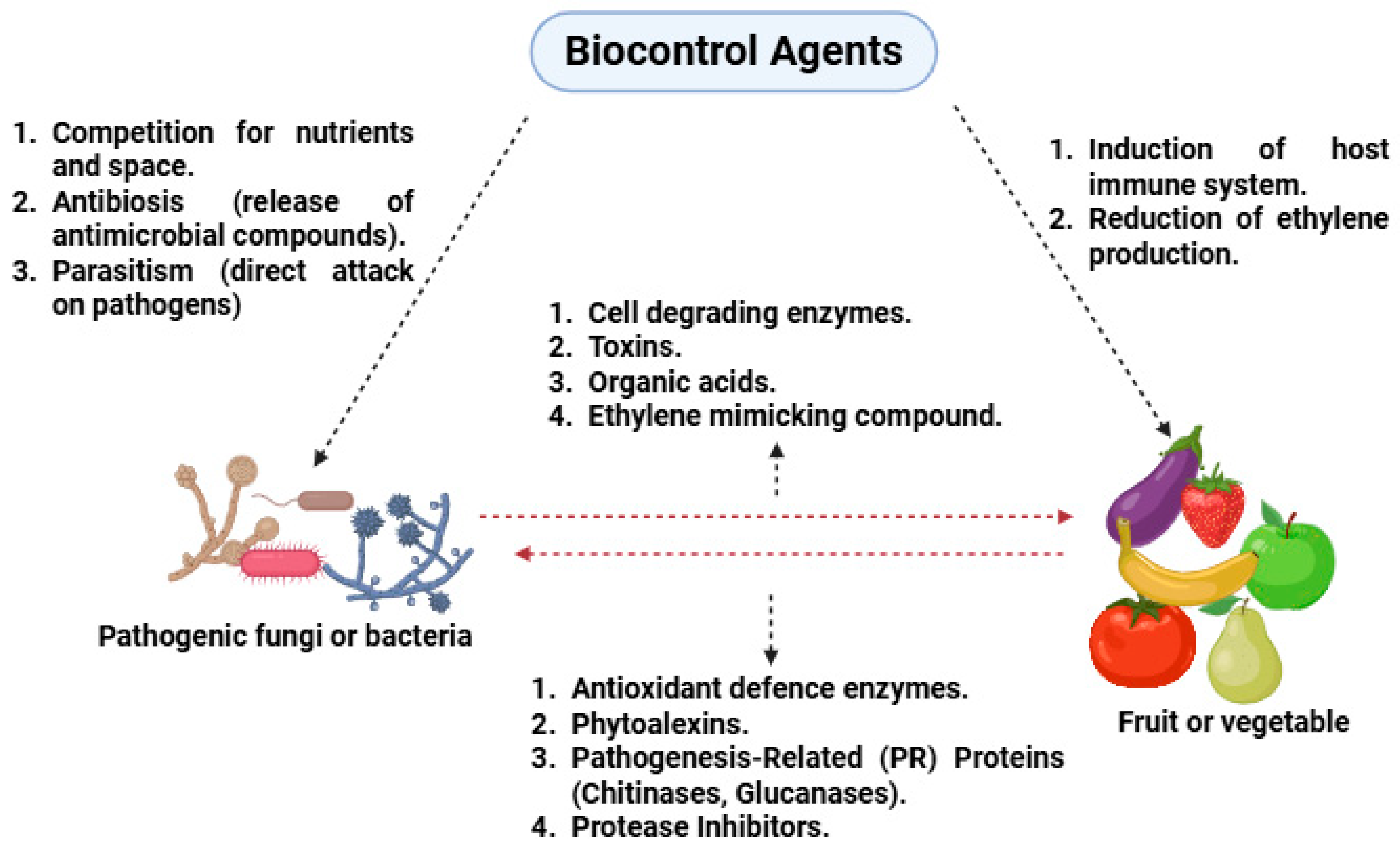
3. Mechanism of Biocontrol in Postharvest Management
3.1. Competition for Nutrients and Space
3.2. Parasitism and Hyper Parasitism
3.3. Antibiosis
3.4. Induction of Host Resistance
3.5. Biofilm Formation and Surface Colonization
4. The Role of Biocontrol Agents in Shelf-Life Extension of Fruits and Vegetables
4.1. Reduction in Postharvest Decay Through Pathogen Suppression
4.2. Maintenance of Postharvest Quality
4.3. Biofilm Formation and Wound Healing
4.4. Environmental Suitability and Consistency
4.5. Safety and Consumer Acceptance
4.6. Case Studies on Major Fruits and Vegetables
4.6.1. Apples and Pears
4.6.2. Citrus Fruits
4.6.3. Tomatoes
4.6.4. Strawberries
4.6.5. Leafy Greens
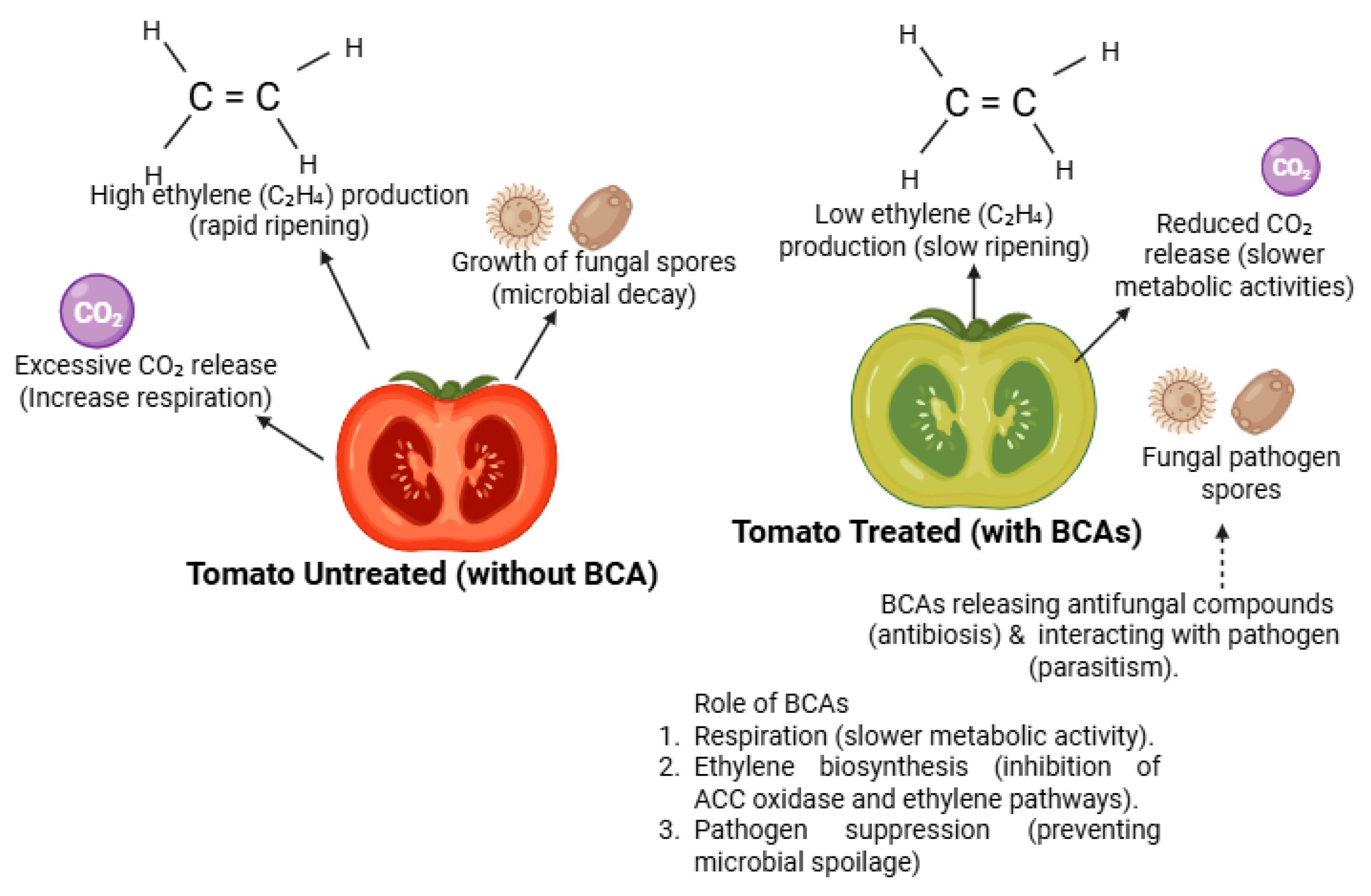
4.7. Impact of Biocontrol Agents on Postharvest Physiology
4.7.1. Delayed Ripening and Reduced Ethylene Production
4.7.2. Reduction in Respiration Rate
4.7.3. Reduction in Oxidative Stress
5. Biocontrol Agents and Food Safety
5.1. Microbial Safety of Biocontrol Agents
5.1.1. Selection and Screening of Non-Pathogenic BCAs
5.1.2. Absence of Toxigenic Compounds
5.1.3. Regulatory Oversight and Compliance
5.2. Residue-Free Benefits of Biocontrol Agents
5.2.1. Chemical Residue Concerns with Synthetic Fungicides
5.2.2. BCAs as a Residue-Free Alternative
5.2.3. Consumer Preferences and Market Demand
6. Nanotechnology in Enhancing Biocontrol Efficacy
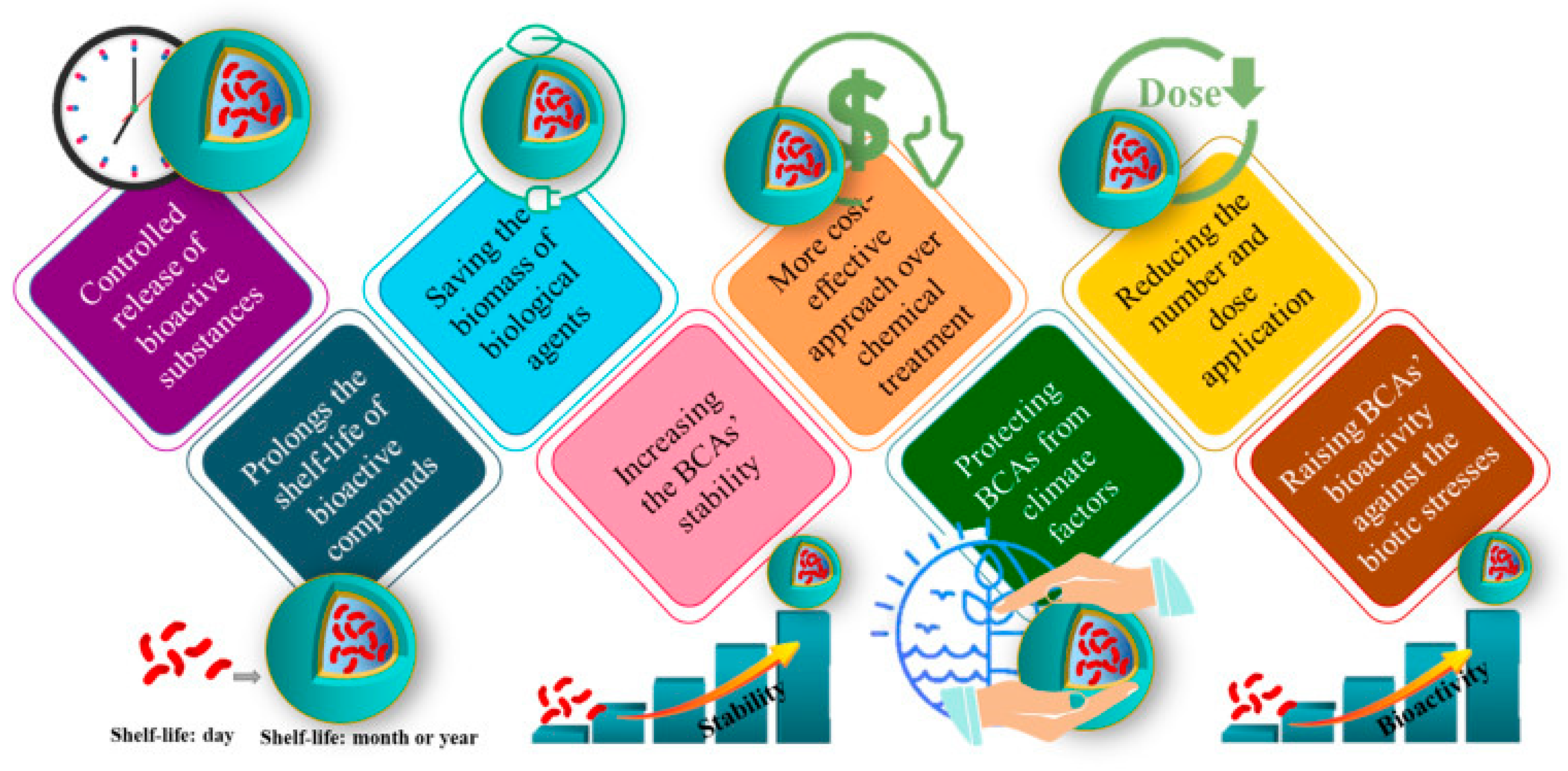
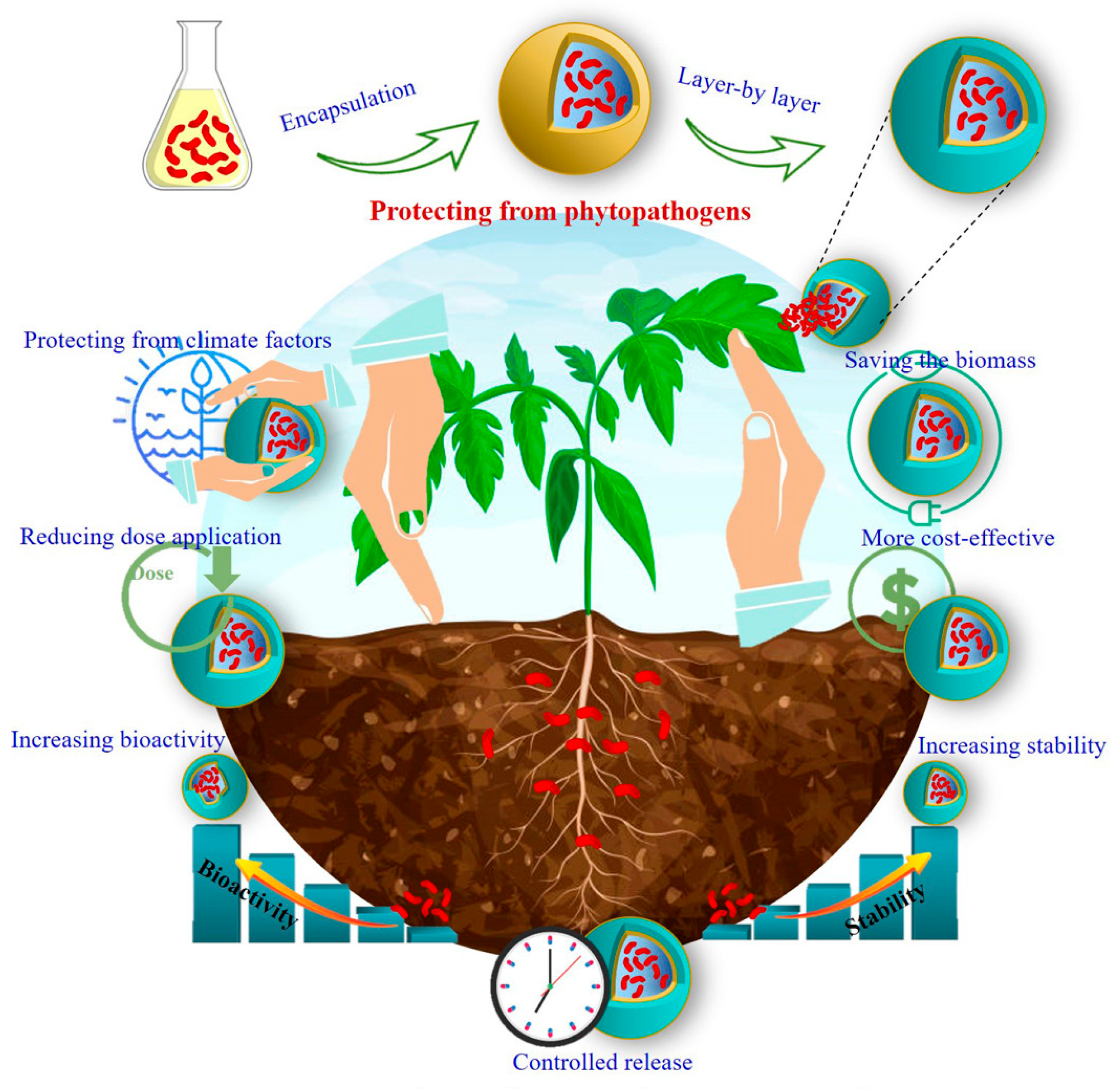
6.1. Nanoencapsulation for Stability and Controlled Release
6.2. Enhanced Adherence and Biofilm Formation on Fruit Surfaces
6.3. Nanoparticle-Mediated Antimicrobial Activity
6.4. Targeted Delivery Systems for Precision Application
| Fruit/Vegetable | Biocontrol Agent | Target Pathogen(s) | Mode of Action | Nanotechnology Enhancement | References |
|---|---|---|---|---|---|
| Apple | Aureobasidium pullulans | Penicillium expansum, Botrytis cinerea | Competitive exclusion, biofilm formation, and nutrient competition | Nano-encapsulation in chitosan nanoparticles for prolonged stability and controlled release | [20,181] |
| Banana | Candida oleophila | Colletotrichum musae, Fusarium oxysporum | Competitive exclusion, quorum sensing interference | Coating with lipid-based nanoparticles for enhanced adhesion and controlled application | [182,183] |
| Tomato | Trichoderma harzianum | Botrytis cinerea, Rhizoctonia solani | Mycoparasitism, enzyme production (chitinase, glucanase), and induction of host resistance | Nano-biofilm technology for improved colonization and pathogen suppression | [184,185,186] |
| Grapes | Pichia guilliermondii | Botrytis cinerea | Competitive exclusion, biofilm formation, and volatile antifungal compound production | Nano-silver coating enhances pathogen suppression and prevents oxidation | [167,187] |
| Citrus (Orange, Lemon) | Bacillus subtilis | Penicillium digitatum, Penicillium italicum | Antibiosis via lipopeptide production, induction of systemic resistance | Encapsulation in pH-responsive nanoparticles for targeted pathogen inhibition | [188,189] |
| Peach | Metschnikowia fructicola | Monilinia laxa, Rhizopus stolonifer | Nutrient competition, host resistance induction, and volatile organic compound (VOC) production | Chitosan nano-coating prolongs BCA activity and reduces fruit respiration | [190,191] |
| Strawberry | Pseudomonas fluorescens | Botrytis cinerea, Alternaria alternata | Siderophore production, hydrogen cyanide (HCN) antifungal activity, and ISR activation | Nanoemulsion-based formulation improves retention and enhances biocontrol efficiency | [192,193] |
| Papaya | Debaryomyces hansenii | Colletotrichum gloeosporioides, Aspergillus spp. | Osmo-tolerance, biofilm formation, and antimicrobial peptide secretion | Nano-chitosan incorporation enhances biofilm formation and adhesion to fruit surfaces | [194,195] |
| Mango | Trichoderma viride | Colletotrichum gloeosporioides | Mycoparasitism, competitive exclusion, and secondary metabolite production | Liposome-mediated delivery improves BCA survival and efficacy under varying storage conditions | [196,197] |
| Blueberry | Bacillus amyloliquefaciens | Alternaria alternata, Botrytis cinerea | Antibiosis (iturin, fengycin production), nutrient competition, and biofilm formation | Zinc oxide nanoparticle synergy enhances antimicrobial action and fruit shelf-life | [198,199] |
| Avocado | Bacillus subtilis | Colletotrichum gloeosporioides | Antibiotic production, biofilm formation, and competition for space | Encapsulation in biodegradable nanogels increases colonization and moisture retention | [200,201] |
| Cherry | Metschnikowia pulcherrima | Botrytis cinerea, Rhizopus stolonifer | Nutrient competition, production of antifungal volatiles, and disruption of pathogen quorum sensing | Nano-coating with essential oil nanoparticles enhances antifungal activity | [202,203] |
| Cucumber | Gliocladium virens | Pythium aphanidermatum, Fusarium solani | Hyperparasitism, nutrient competition, and production of gliotoxin | Biodegradable nano-polysaccharide carriers improve stability and pathogen suppression | [204,205] |
| Bell Pepper | Pseudomonas chlororaphis | Phytophthora capsici | Induced systemic resistance, siderophore production, and competitive exclusion | Electrospun nanofiber delivery system improves adhesion and persistence | [206,207] |
| Pineapple | Pichia kluyveri | Thielaviopsis paradoxa, Ceratocystis paradoxa | Competitive exclusion, disruption of fungal spore germination, and biofilm formation | Nanoencapsulation using alginate nanoparticles improves pathogen suppression | [208,209] |
| Melon | Pseudomonas putida | Fusarium oxysporum, Rhizopus stolonifer | Induced systemic resistance, production of siderophores, and nutrient competition | Smart polymeric nanoparticles for controlled release and pathogen-specific activation | [210,211] |
| Carrot | Bacillus pumilus | Alternaria spp., Penicillium spp. | Antibiosis through antimicrobial peptides, induced systemic resistance, and competitive exclusion | Nano-lipid formulations extend BCA survival under fluctuating storage conditions | [212,213] |
| Cabbage | Pseudomonas syringae | Sclerotinia sclerotiorum, Botrytis cinerea | Siderophore production, nutrient competition, and biofilm formation | Nano-biosensor-integrated application enables precision biocontrol and reduced spoilage | [214,215] |
| Lettuce | Bacillus cereus | Rhizoctonia solani, Botrytis cinerea | Antibiotic production, competition for nutrients, and induction of plant defense responses | Encapsulation in biodegradable nanoparticles enhances BCA adhesion and shelf-life extension | [56,151] |
7. Sustainability and Environmental Impacts of Nanotechnology-Enhanced Biocontrol Agents
8. Challenges and Limitations of Nanotechnology-Enhanced Biocontrol Agents in Agricultural Practices
9. Future Directions of Nanotechnology-Enhanced Biocontrol Agents in Sustainable Agriculture
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Solairaj, D.; Liang, L.; Chen, Y.; Chen, J.; Guo, S.; Zhang, X.; Zhao, L.; Zhang, H. Alginate oligosaccharide induces resistance against Penicillium expansum in pears by priming defense responses. Plant Physiol. Biochem. 2025, 220, 109531. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, K.; Godana, E.; Solairaj, D.; Yang, Q.; Zhang, H. Construction of Composite Microorganisms and Their Physiological Mechanisms of Postharvest Disease Control in Red Grapes. Foods 2025, 14, 408. [Google Scholar] [CrossRef]
- Ahima, J.; Apaliya, M.T.; Osae, R.; Owusu, J.; Agyekum, A.; Zhang, H. Lipid sources in the insect industry, regulatory aspects, and applications. In Insect Oil as a Source of Nutraceuticals; Mariod, A.A., Ed.; Academic Press: Cambridge, MA, USA, 2025; pp. 151–170. [Google Scholar]
- Foku, J.; Godana, E.; Yang, Q.; Zhang, H. Phytic acid promotes the oxidative stress tolerance of Meyerozyma (Pichia) caribbica enhancing its efficacy against natural decay and retaining the quality of table grapes. Plant Physiol. Biochem. 2024, 219, 109463. [Google Scholar] [CrossRef]
- Gurusamy, S.; Solairaj, D.; Liang, L.; Zhang, Y.; Yang, Q.; Li, Y.; Liu, X.; Zhang, H. Edible Fe2ZnO4 nanocomposite for extending shelf-life and preventing blue mold decay in apples. Food Control 2024, 171, 111111. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, L.; Yue, S.; Solairaj, D.; Chen, X.; Zhang, X.; Yang, X.; Song, Y.; Zhang, H.; Wu, M. The molecular networks involve in improving disease resistance in peach fruit induced by Wickerhamomyces anomalus. Sci. Hortic. 2024, 338, 113762. [Google Scholar] [CrossRef]
- Yang, Q.; Ji, Q.; Zhang, X.; Solairaj, D.; Ma, J.; Li, Y.; Zhang, H. The transcriptomic and proteomic changes of Hannaella sinensis in response to patulin stress. N. Zealand J. Crop Hortic. Sci. 2025, 53, 523–548. [Google Scholar] [CrossRef]
- Wang, K.; Wang, H.; Xu, M.; Godana, E.; Lu, Y.; Zhang, H. Functional analysis of apple defense protein MdPL and screening of proteins interaction with Penicillium expansum. Postharvest Biol. Technol. 2025, 219, 113289. [Google Scholar] [CrossRef]
- Su, Y.; Wang, K.; Lu, Y.; Zhao, Q.; Ackah, M.; Yang, Q.; Zhang, H. Genome-wide investigation and analysis of U-box E3 ubiquitin-protein ligase gene family in kiwifruit (Actinidia chinensis) in response to the yeast antagonist, Wickerhamomyces anomalus. N. Zealand J. Crop Hortic. Sci. 2025, 53, 502–522. [Google Scholar] [CrossRef]
- Godana, E.; Zhang, H.; Yang, Q.; Wang, K. New Concepts in the Biological Control of Postharvest Diseases of Fruits and Vegetables. In Recent Advances in Postharvest Technologies, Volume 1: Advanced and Novel Technologies; Benkeblia, N., Ed.; Springer International Publishing: Cham, Switzerland, 2024; pp. 181–197. [Google Scholar]
- Zhang, Y.; Zhang, X.; Zhao, Q.; Gurusamy, S.; Lu, Y.; Chen, X.; Yang, Q.; Zeng, K.; Li, Y.; Li, X.; et al. Immobilization of aldo-keto reductase on dopamine/polyethyleneimine functionalized magnetic cellulose nanocrystals to enhance the detoxification of patulin in fresh pear juice. Int. J. Biol. Macromol. 2024, 278, 134689. [Google Scholar] [CrossRef]
- Godana, E.; Sefu, G.; Yang, Q.; Zhang, X.; Zhao, L.; Wang, K.; Legrand, N.; Zhang, H. Wickerhamomyces anomalus: A promising yeast for controlling mold growth and diverse biotechnological applications. Trends Food Sci. Technol. 2024, 151, 104649. [Google Scholar] [CrossRef]
- Yang, Q.; Yin, D.; Zhang, X.; Solairaj, D.; Xi, Y.; Chen, H.; Li, Y.; Zhang, H. Alginate oligosaccharide-driven resistance in Debaryomyces hansenii Y3: A dual omics perspective. N. Zealand J. Crop Hortic. Sci. 2025, 53, 563–586. [Google Scholar] [CrossRef]
- Legrand, N.; Yang, Q.; Meiqiu, X.; Ianiri, G.; Solairaj, D.; Zhang, X.; Bi, Y.; Zhang, H. Revisiting the current and emerging concepts of postharvest fresh fruit and vegetable pathology for next-generation antifungal technologies. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13397. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, K.; Legrand, N.; Godana, E.; Ackah, M.; Solairaj, D.; Zhang, Y.; Su, Y.; Yang, Q.; Zhang, H. Advances in the Multifaceted Functions of Cys2/His2-Type Zinc Finger Proteins in Distinctive Plant Characters. J. Exp. Bot. 2024, 75, 5501–5520. [Google Scholar] [CrossRef]
- Xi, Z.; Yang, Q.; Solairaj, D.; Sallam, N.; Zhu, M.; You, S.; Zhang, H. Volatile Organic Compounds of Wickerhamomyces anomalus Prevent Postharvest Black Spot Disease in Tomato. Foods 2024, 13, 1949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xin, Y.; Yue, Q.; Godana, E.; Gao, L.; Dou, M.; Zhou, H.; Li, J.; Zhao, L.; Zhang, H. Insight into the mechanisms involved in the improved antagonistic efficacy of Pichia caribbica against postharvest black spot of tomato fruits by combined application with oligochitosan. Postharvest Biol. Technol. 2024, 213, 112968. [Google Scholar] [CrossRef]
- Elzein, B. Nano Revolution: “Tiny tech, big impact: How nanotechnology is driving SDGs progress”. Heliyon 2024, 10, e31393. [Google Scholar] [CrossRef]
- Ji, Q.; Yang, Q.; Solairaj, D.; Ma, J.; Zhang, H. Hannaella sinensis, a promising biocontrol agent for combating postharvest pear fruit diseases and patulin degradation. Food Control 2024, 164, 110618. [Google Scholar] [CrossRef]
- Roohallah, S.R.; Hassanisaadi, M.; Vatankhah, M.; Soroush, F.; Varma, R.S. Nano/microencapsulation of plant biocontrol agents by chitosan, alginate, and other important biopolymers as a novel strategy for alleviating plant biotic stresses. Int. J. Biol. Macromol. 2022, 222, 1589–1604. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Godana, E.; Wang, K.; Zhang, H. A proteomic analysis of Wickerhamomyces anomalus incubated with chitosan reveals dynamic changes in protein expression and metabolic pathways. Postharvest Biol. Technol. 2024, 211, 112806. [Google Scholar] [CrossRef]
- Wong, C.K.F. Bioencapsulation of Biocontrol Agents as a Management Strategy for Plant Pathogens. In Sustainable Agrobiology: Design and Development of Microbial Consortia; Maheshwari, D.K., Dheeman, S., Eds.; Springer Nature: Singapore, 2023; pp. 339–358. [Google Scholar]
- Venkidasamy, B.; Shelar, A.; Dhanapal, A.R.; Nile, A.S.; Patil, R.; Zhang, Y.; Kuksal, K.; Nile, S.H. Emerging biopolymer nanocarriers for controlled and protective delivery of food bioactive compounds- current status and future perspective. Food Hydrocoll. 2025, 160, 110769. [Google Scholar] [CrossRef]
- Boateng, N.; Ackah, M.; Wang, K.; Dzah, C.; Zhang, H. Comparative physiological and transcriptomic analysis reveals an improved biological control efficacy of Sporidiobolus pararoseus Y16 enhanced with ascorbic acid against the oxidative stress tolerance caused by Penicillium expansum in pears. Plant Physiol. Biochem. 2024, 210, 108627. [Google Scholar] [CrossRef]
- Feng, P.; Zhang, X.; Godana, E.; Legrand, N.; Solairaj, D.; Gao, L.; Li, J.; Zhao, L.; Zhang, H. Control of postharvest soft rot of green peppers by Bacillus subtilis through regulating ROS metabolism. Physiol. Mol. Plant Pathol. 2024, 131, 102280. [Google Scholar] [CrossRef]
- Ackah, M.; Boateng, N.; Foku, J.; Legrand, N.; Godana, E.; Zhang, H.; Yang, Q. Genome-wide analysis of the PG gene family in Penicillium expansum: Expression during the infection stage in pear fruits (Pyrus bretschneideri). Physiol. Mol. Plant Pathol. 2024, 131, 102270. [Google Scholar] [CrossRef]
- Ping, Y.; Cao, D.; Hu, J.; Lin, Y.; Dang, C.; Xue, D. The application, safety, and challenge of nanomaterials on plant growth and stress tolerance. Ind. Crops Prod. 2024, 222, 119691. [Google Scholar] [CrossRef]
- Huang, X.; Auffan, M.; Eckelman, M.J.; Elimelech, M.; Kim, J.-H.; Rose, J.; Zuo, K.; Li, Q.; Alvarez, P.J.J. Trends, risks and opportunities in environmental nanotechnology. Nat. Rev. Earth Environ. 2024, 5, 572–587. [Google Scholar] [CrossRef]
- Miranda, M.; Bai, J.; Pilon, L.; Torres, R.; Casals, C.; Solsona, C.; Teixidó, N. Fundamentals of Edible Coatings and Combination with Biocontrol Agents: A Strategy to Improve Postharvest Fruit Preservation. Foods 2024, 13, 2980. [Google Scholar] [CrossRef]
- Li, X.; Zeng, S.; Wisniewski, M.; Droby, S.; Yu, L.; An, F.; Leng, Y.; Wang, C.; Li, X.; He, M.; et al. Current and future trends in the biocontrol of postharvest diseases. Crit. Rev. Food Sci. Nutr. 2024, 64, 5672–5684. [Google Scholar] [CrossRef] [PubMed]
- Godana, E.A.; Yang, Q.; Zhang, X.; Zhao, L.; Wang, K.; Dhanasekaran, S.; Mehari, T.G.; Zhang, H. Biotechnological and Biocontrol Approaches for Mitigating Postharvest Diseases Caused by Fungal Pathogens and Their Mycotoxins in Fruits: A Review. J. Agric. Food Chem. 2023, 71, 17584–17596. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, L.; Yue, S.; Shu, Y.; Chen, X.; Solairaj, D.; Zhang, X.; Zhang, H. Assay for transposase accessible-chromatin with high throughput sequencing (ATAC-seq) analysis the molecular responses of postharvest pear during Penicillium expansum infection. Postharvest Biol. Technol. 2024, 209, 112733. [Google Scholar] [CrossRef]
- Wang, K.; Wang, H.; Xu, M.; Legrand, N.; Zhang, H. The proteome of Penicillium expansum during infection of postharvest apple is revealed using Label-Free and Parallel Reaction Monitoring(PRM)Techniques. J. Proteom. 2024, 298, 105142. [Google Scholar] [CrossRef]
- Nawaz, M.; Sun, J.; Shabbir, S.; Khattak, W.A.; Ren, G.; Nie, X.; Bo, Y.; Javed, Q.; Du, D.; Sonne, C. A review of plants strategies to resist biotic and abiotic environmental stressors. Sci. Total Environ. 2023, 900, 165832. [Google Scholar] [CrossRef] [PubMed]
- Maurice, T.A.; Kwaw, E.; Osae, R.; Alolga, R.; Aidoo, P.; Mensah, L.; Zhang, H.; Wilson, C. Effect of different drying methods on the rehydration kinetics, physiochemical and functional properties of unripe plantain (Musa parasidiaca) flour. Food Chem. Adv. 2024, 4, 100610. [Google Scholar] [CrossRef]
- Mafe, A.N.; Edo, G.I.; Makia, R.S.; Joshua, O.A.; Akpoghelie, P.O.; Gaaz, T.S.; Jikah, A.N.; Yousif, E.; Isoje, E.F.; Igbuku, U.A.; et al. A review on food spoilage mechanisms, food borne diseases and commercial aspects of food preservation and processing. Food Chem. Adv. 2024, 5, 100852. [Google Scholar] [CrossRef]
- Raynaldo, F.A.; Ackah, M.; Legrand, N.; Yolandani, Y.; Sheikh, A.; Yang, Q.; Wang, K.; Zhang, X.; Zhang, H. The potentiality of Wickerhamomyces anomalus against postharvest black spot disease in cherry tomatoes and insights into the defense mechanisms involved. Postharvest Biol. Technol. 2023, 209, 112699. [Google Scholar] [CrossRef]
- Ackah, M.; Boateng, N.; Solairaj, D.; Zhang, H.; Yang, Q. Genome wide and comprehensive analysis of the cytochrome P450 (CYPs) gene family in Pyrus bretschneideri: Expression patterns during Sporidiobolus pararoseus Y16 enhanced with ascorbic acid (VC) treatment. Plant Physiol. Biochem. 2023, 206, 108303. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, Y.; Quan, S.; Qiu, J.-E.; Solairaj, D.; Li, B.; Gu, X.; Zhang, X.; Zhang, H. Transcriptome analysis reveals mechanisms of the disease resistance in postharvest kiwifruit induced by Meyerozyma caribbica. Sci. Hortic. 2023, 322, 112452. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, J.; Yang, Q.; Jin, Y.; Zhao, S.; Tan, Z.; Qiu, J.; Zhang, H. The Impact of Mechanical Compression on the Postharvest Quality of ‘Shine Muscat’ Grapes during Short-Term Storage. Agronomy 2023, 13, 2836. [Google Scholar] [CrossRef]
- Zhang, X.; Xin, Y.; Wang, J.; Solairaj, D.; Yue, Q.; Feng, F.; Gu, X.; Li, B.; Zhao, L.; Zhang, H. Characterization of a Bacillus velezensis strain as a potential biocontrol agent against soft rot of eggplant fruits. Int. J. Food Microbiol. 2023, 410, 110480. [Google Scholar] [CrossRef]
- Meiqiu, X.; Godana, E.; Li, J.; Deng, Y.; Ma, Y.; Ya, H.; Zhang, H. Infection of postharvest pear by Penicillium expansum is facilitated by the glycoside hydrolase (eglB) gene. Int. J. Food Microbiol. 2023, 410, 110465. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, K.; Su, Y.; Solairaj, D.; Yang, Q.; Zhang, H. Genome-wide investigation and analysis of C2H2 Zinc Finger Protein gene family in apple: Expression profiles during Penicillium expansum infection process. Physiol. Mol. Plant Pathol. 2023, 128, 102172. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, Q.; Xin, Y.; Yang, Q.; Solairaj, D.; Zhang, X.; Zhang, H. Aureobasidium pullulans S2 controls tomato gray mold and produces volatile organic compounds and biofilms. Postharvest Biol. Technol. 2023, 204, 112450. [Google Scholar] [CrossRef]
- Wang, K.; Huai, S.; Tan, Z.; Legrand, N.; Godana, E.; Shi, J.; Yang, Q.; Zhang, X.; Zhao, L.; Zhang, H. A First Expression, Purification and Characterization of Endo-β-1,3-Glucanase from Penicillium expansum. J. Fungi 2023, 9, 961. [Google Scholar] [CrossRef]
- Marui, Z.; Yang, Q.; Godana, E.; Huo, Y.; Hu, S.; Zhang, H. Efficacy of Wickerhamomyces anomalus in the biocontrol of black spot decay in tomatoes and investigation of the mechanisms involved. Biol. Control 2023, 186, 105356. [Google Scholar] [CrossRef]
- Zhou, H.; Legrand, N.; Godana, E.; Gu, X.; Li, B.; Zhao, L.; Zhang, X.; Zhang, H. Combined application of oligochitosan and Pichia carrbbica improves the disease resistance of postharvest tomato fruits. Biol. Control 2023, 186, 105331. [Google Scholar] [CrossRef]
- Khan, A.A.; Siddiqui, Y.; Siddique, K.H.M.; Bobo, J.A.; Ali, A. Minimizing postharvest food losses: A vital strategy to alleviate food insecurity and malnutrition in developing nations: A review. Discov. Food 2024, 4, 145. [Google Scholar] [CrossRef]
- Qange, S.; Mdoda, L.; Mditshwa, A. Modeling the critical causal factors of postharvest losses in the vegetable supply chain in eThekwini metropolitan municipality: The log-linear regression model. Heliyon 2024, 10, e39565. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, X.; Solairaj, D.; Lin, R.; Wang, K.; Zhang, H. TMT-Based Proteomic Analysis of Hannaella sinensis-Induced Apple Resistance-Related Proteins. Foods 2023, 12, 2637. [Google Scholar] [CrossRef] [PubMed]
- Al-Dairi, M.; Pathare, P.B.; Al-Yahyai, R.; Jayasuriya, H.; Al-Attabi, Z. Postharvest quality, technologies, and strategies to reduce losses along the supply chain of banana: A review. Trends Food Sci. Technol. 2023, 134, 177–191. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, X.; Solairaj, D.; Fu, Y.; Zhang, H. Molecular Response of Meyerozyma guilliermondii to Patulin: Transcriptomic-Based Analysis. J. Fungi 2023, 9, 538. [Google Scholar] [CrossRef]
- Palumbo, M.; Attolico, G.; Capozzi, V.; Cozzolino, R.; Corvino, A.; de Chiara, M.L.V.; Pace, B.; Pelosi, S.; Ricci, I.; Romaniello, R.; et al. Emerging Postharvest Technologies to Enhance the Shelf-Life of Fruit and Vegetables: An Overview. Foods 2022, 11, 3925. [Google Scholar] [CrossRef]
- Palmieri, D.; Ianiri, G.; Del Grosso, C.; Barone, G.; De Curtis, F.; Castoria, R.; Lima, G. Advances and Perspectives in the Use of Biocontrol Agents against Fungal Plant Diseases. Horticulturae 2022, 8, 577. [Google Scholar] [CrossRef]
- Chaudhary, R.; Nawaz, A.; Khattak, Z.; Butt, M.A.; Fouillaud, M.; Dufossé, L.; Munir, M.; Haq, I.u.; Mukhtar, H. Microbial bio-control agents: A comprehensive analysis on sustainable pest management in agriculture. J. Agric. Food Res. 2024, 18, 101421. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, X.; Solairaj, D.; Lin, R.; Ackah, M.; Legrand, N.; Zhang, H. Transcriptomic analyses reveal robust changes in the defense response of apples induced by Hannaella sinensis. Biol. Control 2023, 182, 105237. [Google Scholar] [CrossRef]
- Sellitto, V.M.; Zara, S.; Fracchetti, F.; Capozzi, V.; Nardi, T. Microbial Biocontrol as an Alternative to Synthetic Fungicides: Boundaries between Pre- and Postharvest Applications on Vegetables and Fruits. Fermentation 2021, 7, 60. [Google Scholar] [CrossRef]
- Asad, S.A. Mechanisms of action and biocontrol potential of Trichoderma against fungal plant diseases-A review. Ecol. Complex. 2022, 49, 100978. [Google Scholar] [CrossRef]
- Etesami, H.; Jeong, B.R.; Glick, B.R. Biocontrol of plant diseases by Bacillus spp. Physiol. Mol. Plant Pathol. 2023, 126, 102048. [Google Scholar] [CrossRef]
- Wang, K.; Zheng, X.; Su, Y.; Lu, Y.; Yang, Q.; Shi, Y.; Lanhuang, B.; Zhang, X.; Zhao, L.; Godana, E.; et al. A glycoside hydrolase superfamily gene plays a major role in Penicillium expansum growth and pathogenicity in apples. Postharvest Biol. Technol. 2023, 198, 112228. [Google Scholar] [CrossRef]
- Qianhua, Z.; Shi, Y.; Legrand, N.; Zhang, X.; Yang, Q.; Zhang, Q.; Xu, X.; Zhang, H. Changes of the microbial community in kiwifruit during storage after postharvest application of Wickerhamomyces anomalus. Food Chem. 2023, 404, 134593. [Google Scholar] [CrossRef]
- Wei, M.; Solairaj, D.; Ji, Q.; Yang, Q.; Zhang, H. Sustainable and efficient method utilizing N-acetyl-L-cysteine for complete and enhanced ochratoxin A clearance by antagonistic yeast. J. Hazard. Mater. 2023, 448, 130975. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Q.; Godana, E.; Zhang, Y.; Zhang, H. Ultrastructural observation and transcriptome analysis provide insights into mechanisms of Penicillium expansum invading apple wounds. Food Chem. 2023, 414, 135633. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Li, C.H.; Ali, Q.; Zhao, W.; Chi, Y.K.; Shafiq, M.; Ali, F.; Yu, X.Y.; Yu, Q.; Zhao, J.T.; et al. Bacterial and Fungal Biocontrol Agents for Plant Disease Protection: Journey from Lab to Field, Current Status, Challenges, and Global Perspectives. Molecules 2023, 28, 6735. [Google Scholar] [CrossRef]
- Sheoran, A.R.; Lakra, N.; Saharan, B.S.; Luhach, A.; Kumar, R.; Seth, C.S.; Duhan, J.S. Enhancing Plant Disease Resistance: Insights from Biocontrol Agent Strategies. J. Plant Growth Regul. 2025, 44, 436–459. [Google Scholar] [CrossRef]
- Meiqiu, X.; Godana, E.; Solairaj, D.; Zhang, X.; Yang, Q.; Zhao, L.; Zhang, H. Comparative proteome and transcriptome analyses of the response of postharvest pears to Penicillium expansum infection. Postharvest Biol. Technol. 2023, 196, 112182. [Google Scholar] [CrossRef]
- Ma, J.; Godana, E.; Yang, Q.; Zhang, H. Effect of the antagonistic yeast Hannaella sinensis on the degradation of Patulin. Biol. Control 2022, 178, 105134. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, Q.; Zhang, Q.; Zhao, Q.; Godana, E.; Zhang, X.; Zhou, S.; Zhang, H. The preharvest application of Aureobasidium pullulans S2 remodeled the microbiome of tomato surface and reduced postharvest disease incidence of tomato fruit. Postharvest Biol. Technol. 2022, 194, 112101. [Google Scholar] [CrossRef]
- Saldaña-Mendoza, S.A.; Pacios-Michelena, S.; Palacios-Ponce, A.S.; Chávez-González, M.L.; Aguilar, C.N. Trichoderma as a biological control agent: Mechanisms of action, benefits for crops and development of formulations. World J. Microbiol. Biotechnol. 2023, 39, 269. [Google Scholar] [CrossRef]
- Pastor, N.; Palacios, S.; Torres, A.M. Microbial consortia containing fungal biocontrol agents, with emphasis on Trichoderma spp.: Current applications for plant protection and effects on soil microbial communities. Eur. J. Plant Pathol. 2023, 167, 593–620. [Google Scholar] [CrossRef]
- Zhao, L.; Shu, Y.; Quan, S.; Solairaj, D.; Zhang, X.; Zhang, H. Screening and Regulation Mechanism of Key Transcription Factors of Penicillium expansum Infecting Postharvest Pears by ATAC-Seq Analysis. Foods 2022, 11, 3855. [Google Scholar] [CrossRef]
- Solairaj, D.; Yang, Q.; Ma, J.; Fu, Y.; Zhang, H. Alterations in the proteome as a regulating mechanism for patulin stress by the antagonistic yeast Meyerozyma guilliermondii. Biol. Control 2022, 177, 105112. [Google Scholar] [CrossRef]
- Ayaz, M.; Zhao, J.T.; Zhao, W.; Chi, Y.K.; Ali, Q.; Ali, F.; Khan, A.R.; Yu, Q.; Yu, J.W.; Wu, W.C.; et al. Biocontrol of plant parasitic nematodes by bacteria and fungi: A multi-omics approach for the exploration of novel nematicides in sustainable agriculture. Front. Microbiol. 2024, 15, 1433716. [Google Scholar] [CrossRef]
- Hashem, A.; Tabassum, B.; Fathi Abd Allah, E. Bacillus subtilis: A plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J. Biol. Sci. 2019, 26, 1291–1297. [Google Scholar] [CrossRef]
- Dimkić, I.; Janakiev, T.; Petrović, M.; Degrassi, G.; Fira, D. Plant-associated Bacillus and Pseudomonas antimicrobial activities in plant disease suppression via biological control mechanisms-A review. Physiol. Mol. Plant Pathol. 2022, 117, 101754. [Google Scholar] [CrossRef]
- Perry, E.K.; Meirelles, L.A.; Newman, D.K. From the soil to the clinic: The impact of microbial secondary metabolites on antibiotic tolerance and resistance. Nat. Rev. Microbiol. 2022, 20, 129–142. [Google Scholar] [CrossRef]
- Yang, Q.; Solairaj, D.; Legrand, N.; Tian, S.; Li, B.; Zhang, H. Unveiling ochratoxin a controlling and biodetoxification molecular mechanisms: Opportunities to secure foodstuffs from OTA contamination. Food Chem. Toxicol. 2022, 169, 113437. [Google Scholar] [CrossRef]
- Lin, R.; Yang, Q.; Xiao, J.; Solairaj, D.; Legrand, N.; Zhang, H. Study on the biocontrol effect and physiological mechanism of Hannaella sinensis on the blue mold decay of apples. Int. J. Food Microbiol. 2022, 382, 109931. [Google Scholar] [CrossRef]
- Castaldi, S.; Masi, M.; Sautua, F.; Cimmino, A.; Isticato, R.; Carmona, M.; Tuzi, A.; Evidente, A. Pseudomonas fluorescens Showing Antifungal Activity against Macrophomina phaseolina, a Severe Pathogenic Fungus of Soybean, Produces Phenazine as the Main Active Metabolite. Biomolecules 2021, 11, 1728. [Google Scholar] [CrossRef] [PubMed]
- Godana, E.; Zhang, X.; Hu, W.; Zhao, L.; Gu, X.; Zhang, H. Transcriptome analysis of asparagus in response to postharvest treatment with Yarrowia lipolytica. Biol. Control 2022, 169, 104906. [Google Scholar] [CrossRef]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of Action of Microbial Biological Control Agents Against Plant Diseases: Relevance Beyond Efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Lanhuang, B.; Yang, Q.; Godana, E.; Zhang, H. Efficacy of the Yeast Wickerhamomyces anomalus in Biocontrol of Gray Mold Decay of Tomatoes and Study of the Mechanisms Involved. Foods 2022, 11, 720. [Google Scholar] [CrossRef]
- Li, X.; Liao, Q.; Zeng, S.; Wang, Y.; Liu, J. The use of Trichoderma species for the biocontrol of postharvest fungal decay in fruits and vegetables: Challenges and opportunities. Postharvest Biol. Technol. 2025, 219, 113236. [Google Scholar] [CrossRef]
- Kour, D.; Negi, R.; Khan, S.S.; Kumar, S.; Kaur, S.; Kaur, T.; Sharma, B.; Dasila, H.; Kour, H.; Ramniwas, S.; et al. Microbes mediated induced systemic response in plants: A review. Plant Stress 2024, 11, 100334. [Google Scholar] [CrossRef]
- Romanazzi, G.; Sanzani, S.M.; Bi, Y.; Tian, S.; Gutiérrez Martínez, P.; Alkan, N. Induced resistance to control postharvest decay of fruit and vegetables. Postharvest Biol. Technol. 2016, 122, 82–94. [Google Scholar] [CrossRef]
- Hussaini, I.M.; Oyewole, O.A.; Sulaiman, M.A.; Dabban, A.I.; Sulaiman, A.N.; Tarek, R. Microbial anti-biofilms: Types and mechanism of action. Res. Microbiol. 2024, 175, 104111. [Google Scholar] [CrossRef]
- Liu, H.Y.; Prentice, E.L.; Webber, M.A. Mechanisms of antimicrobial resistance in biofilms. NPJ Antimicrob. Resist. 2024, 2, 27. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, Q.; Wang, Z.; Sui, Y.; Wang, Q.; Liu, J.; Zhang, H. Analysis of long non-coding RNAs and mRNAs in harvested kiwifruit in response to the yeast antagonist, Wickerhamomyces anomalus. Comput. Struct. Biotechnol. J. 2021, 19, 5589–5599. [Google Scholar] [CrossRef]
- Sui, Y.; Zhao, Q.; Wang, Z.; Liu, J.; Jiang, M.; Yue, J.; Lan, J.; Liu, J.; Liao, Q.; Wang, Q.; et al. A Comparative Analysis of the Microbiome of Kiwifruit at Harvest Under Open-Field and Rain-Shelter Cultivation Systems. Front. Microbiol. 2021, 12, 757719. [Google Scholar] [CrossRef]
- Flemming, H.-C.; van Hullebusch, E.D.; Neu, T.R.; Nielsen, P.H.; Seviour, T.; Stoodley, P.; Wingender, J.; Wuertz, S. The biofilm matrix: Multitasking in a shared space. Nat. Rev. Microbiol. 2023, 21, 70–86. [Google Scholar] [CrossRef]
- Godana, E.; Yang, Q.; Zhao, L.; Zhang, X.; Liu, J.; Zhang, H. Pichia anomala Induced With Chitosan Triggers Defense Response of Table Grapes Against Post-harvest Blue Mold Disease. Front. Microbiol. 2021, 12, 704519. [Google Scholar] [CrossRef]
- Raynaldo, F.A.; Solairaj, D.; Legrand, N.; Yang, Q.; Zhang, X.; Zhang, H. Investigating the biocontrol potentiality of Wickerhamomyces anomalus against postharvest gray mold decay in cherry tomatoes. Sci. Hortic. 2021, 285, 110137. [Google Scholar] [CrossRef]
- Zhang, H.; Boateng, N.; Legrand, N.; Shi, Y.; Lin, H.; Yang, Q.; Wang, K.; Zhang, X.; Zhao, L.; Droby, S. Unravelling the fruit microbiome: The key for developing effective biological control strategies for postharvest diseases. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4906–4930. [Google Scholar] [CrossRef]
- Alegbeleye, O.; Odeyemi, O.A.; Strateva, M.; Stratev, D. Microbial spoilage of vegetables, fruits and cereals. Appl. Food Res. 2022, 2, 100122. [Google Scholar] [CrossRef]
- Zhang, H.; Godana, E.A.; Sui, Y.; Yang, Q.; Zhang, X.; Zhao, L. Biological control as an alternative to synthetic fungicides for the management of grey and blue mould diseases of table grapes: A review. Crit. Rev. Microbiol. 2020, 46, 450–462. [Google Scholar] [CrossRef]
- Wang, L.; Teplitski, M. Microbiological food safety considerations in shelf-life extension of fresh fruits and vegetables. Curr. Opin. Biotechnol. 2023, 80, 102895. [Google Scholar] [CrossRef]
- Rahman, F.U.; Zhu, Q.; Wu, Z.; Li, X.; Chen, W.; Xiong, T.; Zhu, X. Current insights into the biocontrol and biotechnological approaches for postharvest disease management of Botrytis cinerea. Postharvest Biol. Technol. 2024, 216, 113055. [Google Scholar] [CrossRef]
- Ali, A.; Ölmez, F.; Zeshan, M.A.; Mubeen, M.; Iftikhar, Y.; Sajid, A.; Abid, M.; Kumar, A.; Divvela, P.K.; Solanki, M.K. Yeast-based solutions in controlling plant pathogens. Biocatal. Agric. Biotechnol. 2024, 58, 103199. [Google Scholar] [CrossRef]
- Caballero-Flores, G.; Pickard, J.M.; Núñez, G. Microbiota-mediated colonization resistance: Mechanisms and regulation. Nat. Rev. Microbiol. 2023, 21, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Lohita, B.; Srijaya, M. Novel Technologies for Shelf-Life Extension of Food Products as a Competitive Advantage: A Review. In Food Production, Diversity, and Safety Under Climate Change; Chakraborty, R., Mathur, P., Roy, S., Eds.; Springer Nature Switzerland: Cham, Switzerland, 2024; pp. 285–306. [Google Scholar]
- Han, J.; Zhao, L.; Zhu, H.; Solairaj, D.; Zhang, X.; Zhang, H. Study on the effect of alginate oligosaccharide combined with Meyerozyma guilliermondii against Penicillium expansum in pears and the possible mechanisms involved. Physiol. Mol. Plant Pathol. 2021, 115, 101654. [Google Scholar] [CrossRef]
- Karagiannis, E. 1-Postharvest physiology of climacteric and nonclimacteric fruits and vegetables. In Oxygen, Nitrogen and Sulfur Species in Post-Harvest Physiology of Horticultural Crops; Ziogas, V., Corpas, F.J., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 1–21. [Google Scholar]
- Arias, A.; Feijoo, G.; Moreira, M.T. Exploring the potential of antioxidants from fruits and vegetables and strategies for their recovery. Innov. Food Sci. Emerg. Technol. 2022, 77, 102974. [Google Scholar] [CrossRef]
- Wang, T.; Song, Y.; Lai, L.; Fang, D.; Li, W.; Cao, F.; Su, E. Sustaining freshness: Critical review of physiological and biochemical transformations and storage techniques in postharvest bananas. Food Packag. Shelf Life 2024, 46, 101386. [Google Scholar] [CrossRef]
- Droby, S.; Zhimo, V.Y.; Wisniewski, M.; Freilich, S. The pathobiome concept applied to postharvest pathology and its implication on biocontrol strategies. Postharvest Biol. Technol. 2022, 189, 111911. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, Q.; Solairaj, D.; Godana, E.; Routledge, M.; Zhang, H. Biodegradation of mycotoxin patulin by the yeast Meyerozyma guilliermondii. Biol. Control 2021, 160, 104692. [Google Scholar] [CrossRef]
- Hu, W.; Godana, E.; Meiqiu, X.; Yang, Q.; Solairaj, D.; Zhang, H. Transcriptome Characterization and Expression Profiles of Disease Defense-Related Genes of Table Grapes in Response to Pichia anomala Induced with Chitosan. Foods 2021, 10, 1451. [Google Scholar] [CrossRef]
- Legrand, N.; Qian, X.; Yang, Q.; Solairaj, D.; Ianiri, G.; Ballester, A.-R.; Zhang, X.; Castoria, R.; Zhang, H. Securing fruit production: Opportunities from the elucidation of the molecular mechanisms of postharvest fungal infections. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2508–2533. [Google Scholar] [CrossRef]
- Elkhairy, B.M.; Salama, N.M.; Desouki, A.M.; Abdelrazek, A.B.; Soliman, K.A.; Ibrahim, S.A.; Khalil, H.B. Towards unlocking the biocontrol potential of Pichia kudriavzevii for plant fungal diseases: In vitro and in vivo assessments with candidate secreted protein prediction. BMC Microbiol. 2023, 23, 356. [Google Scholar] [CrossRef]
- Fenta, L.; Mekonnen, H. Microbial Biofungicides as a Substitute for Chemical Fungicides in the Control of Phytopathogens: Current Perspectives and Research Directions. Scientifica 2024, 2024, 5322696. [Google Scholar] [CrossRef] [PubMed]
- Remolif, G.; Schiavon, G.; Garello, M.; Spadaro, D. Efficacy of Postharvest Application of Aureobasidium pullulans to Control White Haze on Apples and Effect on the Fruit Mycobiome. Horticulturae 2024, 10, 927. [Google Scholar] [CrossRef]
- Rueda-Mejia, M.P.; Nägeli, L.; Lutz, S.; Hayes, R.D.; Varadarajan, A.R.; Grigoriev, I.V.; Ahrens, C.H.; Freimoser, F.M. Genome, transcriptome and secretome analyses of the antagonistic, yeast-like fungus Aureobasidium pullulans to identify potential biocontrol genes. Microb. Cell 2021, 8, 184–202. [Google Scholar] [CrossRef]
- Wilson, M.D.; Stanley, R.A.; Eyles, A.; Ross, T. Innovative processes and technologies for modified atmosphere packaging of fresh and fresh-cut fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2019, 59, 411–422. [Google Scholar] [CrossRef]
- Buchmüller, K.; Bearth, A.; Siegrist, M. Consumers’ perceptions of chemical household products and the associated risks. Food Chem. Toxicol. 2020, 143, 111511. [Google Scholar] [CrossRef] [PubMed]
- Khursheed, A.; Rather, M.A.; Jain, V.; Wani, A.R.; Rasool, S.; Nazir, R.; Malik, N.A.; Majid, S.A. Plant based natural products as potential ecofriendly and safer biopesticides: A comprehensive overview of their advantages over conventional pesticides, limitations and regulatory aspects. Microb. Pathog. 2022, 173, 105854. [Google Scholar] [CrossRef]
- Qiru, Z.; Zhao, L.; Li, B.; Gu, X.; Zhang, X.; Boateng, N.; Zhang, H. Molecular Dissection of Defense Response of Pears Induced by the Biocontrol Yeast, Wickerhamomyces anomalusUsing Transcriptomics and Proteomics Approaches. Biol. Control 2020, 148, 104305. [Google Scholar] [CrossRef]
- Zheng, X.; Yang, Q.; Zhao, L.; Apaliya, M.T.; Zhang, X.; Zhang, H. Author Correction: Crosstalk between proteins expression and lysine acetylation in response to patulin stress in Rhodotorula mucilaginosa. Sci. Rep. 2020, 10, 9843. [Google Scholar] [CrossRef]
- Cheng, Y.; Lin, Y.; Cao, H.; Li, Z. Citrus Postharvest Green Mold: Recent Advances in Fungal Pathogenicity and Fruit Resistance. Microorganisms 2020, 8, 449. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Gao, R.; Zhang, F.; Ren, Y.; Li, W.; He, B. Postharvest biocontrol of green mold (Penicillium digitatum) in citrus by Bacillus velezensis strain S161 and its mode of action. Biol. Control 2023, 187, 105392. [Google Scholar] [CrossRef]
- Poveda, J.; Eugui, D. Combined use of Trichoderma and beneficial bacteria (mainly Bacillus and Pseudomonas): Development of microbial synergistic bio-inoculants in sustainable agriculture. Biol. Control 2022, 176, 105100. [Google Scholar] [CrossRef]
- Petrasch, S.; Knapp, S.J.; van Kan, J.A.L.; Blanco-Ulate, B. Grey mould of strawberry, a devastating disease caused by the ubiquitous necrotrophic fungal pathogen Botrytis cinerea. Mol. Plant Pathol. 2019, 20, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Moura, G.G.D.; Barros, A.V.; Machado, F.; Martins, A.D.; Silva, C.M.D.; Durango, L.G.C.; Forim, M.; Alves, E.; Pasqual, M.; Doria, J. Endophytic bacteria from strawberry plants control gray mold in fruits via production of antifungal compounds against Botrytis cinerea L. Microbiol. Res. 2021, 251, 126793. [Google Scholar] [CrossRef]
- Knez, M.; Mattas, K.; Gurinovic, M.; Gkotzamani, A.; Koukounaras, A. Revealing the power of green leafy vegetables: Cultivating diversity for health, environmental benefits, and sustainability. Glob. Food Secur. 2024, 43, 100816. [Google Scholar] [CrossRef]
- Luo, L.; Zhao, C.; Wang, E.; Raza, A.; Yin, C. Bacillus amyloliquefaciens as an excellent agent for biofertilizer and biocontrol in agriculture: An overview for its mechanisms. Microbiol. Res. 2022, 259, 127016. [Google Scholar] [CrossRef]
- He, Y.; Degraeve, P.; Oulahal, N. Bioprotective yeasts: Potential to limit postharvest spoilage and to extend shelf life or improve microbial safety of processed foods. Heliyon 2024, 10, e24929. [Google Scholar] [CrossRef]
- Zhang, H.; Mahunu, G.K.; Castoria, R.; Apaliya, M.T.; Yang, Q. Augmentation of biocontrol agents with physical methods against postharvest diseases of fruits and vegetables. Trends Food Sci. Technol. 2017, 69, 36–45. [Google Scholar] [CrossRef]
- Alonso-Salinas, R.; López-Miranda, S.; Pérez-López, A.J.; Acosta-Motos, J.R. Strategies to Delay Ethylene-Mediated Ripening in Climacteric Fruits: Implications for Shelf Life Extension and Postharvest Quality. Horticulturae 2024, 10, 840. [Google Scholar] [CrossRef]
- Oliveira, A.N.; Martins de Oliveira, C.; José Ulhoa, C.; de Carvalho Barros Côrtes, M.V.; Lobo Júnior, M.; Rúbia da Rocha, M. Trichoderma harzianum and Trichoderma asperellum are potential biocontrol agents of Meloidogyne javanica in banana cv. Grande Naine. Biol. Control 2022, 175, 105054. [Google Scholar] [CrossRef]
- Umeohia, U.; Olapade, A. Physiological Processes Affecting Postharvest Quality of Fresh Fruits and Vegetables. Asian Food Sci. J. 2024, 23, 1–14. [Google Scholar] [CrossRef]
- Tang, Y.; Li, R.; Jiang, Z.; Cheng, Z.; Li, W.; Shao, Y. Combined effect of Debaryomyces hansenii and Bacillus atrophaeus on the physicochemical attributes, defense-related enzyme activity, and transcriptomic profile of stored litchi fruit. Biol. Control 2022, 172, 104975. [Google Scholar] [CrossRef]
- Rosman, N.; Malek, N.S.A.; Omar, H.; Hajar, N.; Buniyamin, I.; Abdullah, S.; Razak, A.R.A.; Rusop Mahmood, M.; Asli, N.A. Impact of Zinc Oxide-Corn Starch Coating on Mango Postharvest to Extend Shelf Life. Food Biophys. 2024, 20, 5. [Google Scholar] [CrossRef]
- Sang, Y.; Yang, W.; Liu, Y.; Zhang, W.; Guo, T.; Shen, P.; Tang, Y.; Guo, M.; Chen, G. Influences of low temperature on the postharvest quality and antioxidant capacity of winter jujube (Zizyphus jujuba Mill. cv. Dongzao). LWT 2022, 154, 112876. [Google Scholar] [CrossRef]
- Pearson, A.J.; Mukherjee, K.; Fattori, V.; Lipp, M. Opportunities and challenges for global food safety in advancing circular policies and practices in agrifood systems. NPJ Sci. Food 2024, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- King, T.; Cole, M.; Farber, J.M.; Eisenbrand, G.; Zabaras, D.; Fox, E.M.; Hill, J.P. Food safety for food security: Relationship between global megatrends and developments in food safety. Trends Food Sci. Technol. 2017, 68, 160–175. [Google Scholar] [CrossRef]
- Rehman, A.; Farooq, M.; Lee, D.-J.; Siddique, K.H.M. Sustainable agricultural practices for food security and ecosystem services. Environ. Sci. Pollut. Res. 2022, 29, 84076–84095. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Cassan, F.; Kostić, T.; Johnson, L.; Brader, G.; Trognitz, F.; Sessitsch, A. Harnessing the plant microbiome for sustainable crop production. Nat. Rev. Microbiol. 2025, 23, 9–23. [Google Scholar] [CrossRef]
- Kurokawa, M.; Nakano, M.; Kitahata, N.; Kuchitsu, K.; Furuya, T. An efficient direct screening system for microorganisms that activate plant immune responses based on plant–microbe interactions using cultured plant cells. Sci. Rep. 2021, 11, 7396. [Google Scholar] [CrossRef]
- Moussa, S.; Iasur, K.L. Balancing Nature and Nurture: The Role of Biocontrol Agents in Shaping Plant Microbiomes for Sustainable Agriculture. Microorganisms 2025, 13, 323. [Google Scholar] [CrossRef]
- Chemla, Y.; Sweeney, C.J.; Wozniak, C.A.; Voigt, C.A. Design and regulation of engineered bacteria for environmental release. Nat. Microbiol. 2025, 10, 281–300. [Google Scholar] [CrossRef] [PubMed]
- Matrose, N.A.; Obikeze, K.; Belay, Z.A.; Caleb, O.J. Plant extracts and other natural compounds as alternatives for post-harvest management of fruit fungal pathogens: A review. Food Biosci. 2021, 41, 100840. [Google Scholar] [CrossRef]
- Fares, N.V.; Hassan, Y.A.A.; Hussein, L.A.; Ayad, M.F. Determination of fungicides’ residues and their degradation kinetics in orange tree fruits using liquid chromatography–Tandem mass spectrometry coupled with QuEChERS method. Microchem. J. 2021, 168, 106376. [Google Scholar] [CrossRef]
- Beyuo, J.; Sackey, L.N.A.; Yeboah, C.; Kayoung, P.Y.; Koudadje, D. The implications of pesticide residue in food crops on human health: A critical review. Discov. Agric. 2024, 2, 123. [Google Scholar] [CrossRef]
- Cao, L.; Kang, Q.; Tian, Y. Pesticide residues: Bridging the gap between environmental exposure and chronic disease through omics. Ecotoxicol. Environ. Saf. 2024, 287, 117335. [Google Scholar] [CrossRef]
- Kachhawa, D. Microorganisms as a biopesticides. J. Entomol. Zool. Stud. 2017, 5, 468–473. [Google Scholar]
- Greppi, A.; Saubade, F.; Botta, C.; Humblot, C.; Guyot, J.-P.; Cocolin, L. Potential probiotic Pichia kudriavzevii strains and their ability to enhance folate content of traditional cereal-based African fermented food. Food Microbiol. 2017, 62, 169–177. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Yin, J. Effects of hot air treatment in combination with Pichia guilliermondii on postharvest preservation of peach fruit. J. Sci. Food Agric. 2019, 99, 647–655. [Google Scholar] [CrossRef]
- Gamage, A.; Gangahagedara, R.; Gamage, J.; Jayasinghe, N.; Kodikara, N.; Suraweera, P.; Merah, O. Role of organic farming for achieving sustainability in agriculture. Farming Syst. 2023, 1, 100005. [Google Scholar] [CrossRef]
- Panday, D.; Bhusal, N.; Das, S.; Ghalehgolabbehbahani, A. Rooted in Nature: The Rise, Challenges, and Potential of Organic Farming and Fertilizers in Agroecosystems. Sustainability 2024, 16, 1530. [Google Scholar] [CrossRef]
- Nohara, K. Willingness to pay for pesticide-free vegetables in Hokkaido, Japan: The relationship between appearance and pesticide use. Humanit. Soc. Sci. Commun. 2024, 11, 12. [Google Scholar] [CrossRef]
- Li, S.; Kallas, Z. Meta-analysis of consumers’ willingness to pay for sustainable food products. Appetite 2021, 163, 105239. [Google Scholar] [CrossRef]
- Smith, E.K.; Kolcava, D.; Bernauer, T. Stringent sustainability regulations for global supply chains are supported across middle-income democracies. Nat. Commun. 2024, 15, 1049. [Google Scholar] [CrossRef]
- Yadav, D.; Dutta, G.; Kumar, S. Food safety standards adoption and its impact on firms’ export performance: A systematic literature review. J. Clean. Prod. 2021, 329, 129708. [Google Scholar] [CrossRef]
- Shankar, S.; Mohanty, A.K.; DeEll, J.R.; Carter, K.; Lenz, R.; Misra, M. Advances in antimicrobial techniques to reduce postharvest loss of fresh fruit by microbial reduction. NPJ Sustain. Agric. 2024, 2, 25. [Google Scholar] [CrossRef]
- Katouzian, I.; Jafari, S.M. Nano-encapsulation as a promising approach for targeted delivery and controlled release of vitamins. Trends Food Sci. Technol. 2016, 53, 34–48. [Google Scholar] [CrossRef]
- Pateiro, M.; Gómez, B.; Munekata, P.E.S.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Lorenzo, J.M. Nanoencapsulation of Promising Bioactive Compounds to Improve Their Absorption, Stability, Functionality and the Appearance of the Final Food Products. Molecules 2021, 26, 1547. [Google Scholar] [CrossRef]
- Rezagholizade-shirvan, A.; Soltani, M.; Shokri, S.; Radfar, R.; Arab, M.; Shamloo, E. Bioactive compound encapsulation: Characteristics, applications in food systems, and implications for human health. Food Chem. X 2024, 24, 101953. [Google Scholar] [CrossRef]
- Maruyama, C.; Bilesky-José, N.; Lima, R.; Fraceto, L. Encapsulation of Trichoderma harzianum Preserves Enzymatic Activity and Enhances the Potential for Biological Control. Front. Bioeng. Biotechnol. 2020, 8, 225. [Google Scholar] [CrossRef]
- Vejan, P.; Khadiran, T.; Abdullah, R.; Ahmad, N. Controlled release fertilizer: A review on developments, applications and potential in agriculture. J. Control. Release 2021, 339, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Dawoud, M. Chitosan coated solid lipid nanoparticles as promising carriers for docetaxel. J. Drug Deliv. Sci. Technol. 2021, 62, 102409. [Google Scholar] [CrossRef]
- Jafernik, K.; Ładniak, A.; Blicharska, E.; Czarnek, K.; Ekiert, H.; Wiącek, A.E.; Szopa, A. Chitosan-Based Nanoparticles as Effective Drug Delivery Systems–A review. Molecules 2023, 28, 1963. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, A.; Sharma, A.; Thakur, M.; Sharma, S.; Sharma, H.; Thakur, R.; Thakur, D.; Suhag, R. Nano-edible coatings for quality enhancement and shelf-life extension of fruits and vegetables. J. Food Sci. Technol. 2025, 62, 397–412. [Google Scholar] [CrossRef]
- Karnwal, A.; Kumar, G.; Singh, R.; Selvaraj, M.; Malik, T.; Al Tawaha, A.R.M. Natural biopolymers in edible coatings: Applications in food preservation. Food Chem. X 2025, 25, 102171. [Google Scholar] [CrossRef]
- Saharan, B.S.; Beniwal, N.; Duhan, J.S. From formulation to function: A detailed review of microbial biofilms and their polymer-based extracellular substances. Microbe 2024, 5, 100194. [Google Scholar] [CrossRef]
- Bamford, N.C.; MacPhee, C.E.; Stanley-Wall, N.R. Microbial Primer: An introduction to biofilms-what they are, why they form and their impact on built and natural environments. Microbiology 2023, 169, 001338. [Google Scholar] [CrossRef]
- Mohsin, M.Z.; Omer, R.; Huang, J.; Mohsin, A.; Guo, M.; Qian, J.; Zhuang, Y. Advances in engineered Bacillus subtilis biofilms and spores, and their applications in bioremediation, biocatalysis, and biomaterials. Synth. Syst. Biotechnol. 2021, 6, 180–191. [Google Scholar] [CrossRef]
- Pan, Y.; Cao, L.; Chen, L.; Gao, L.; Wei, X.; Lin, H.; Jiang, L.; Wang, Y.; Cheng, H. Enhanced Bacterial and Biofilm Adhesion Resistance of ALD Nano-TiO(2) Coatings Compared to AO Coatings on Titanium Abutments. Int. J. Nanomed. 2024, 19, 11143–11159. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Deka, K.; Nongbet, R.D.; Das, K.; Saikia, P.; Kaur, S.; Talukder, A.; Thakuria, B. Understanding the mechanism underlying the green synthesis of metallic nanoparticles using plant extract(s) with special reference to Silver, Gold, Copper and Zinc oxide nanoparticles. Hybrid Adv. 2025, 9, 100399. [Google Scholar] [CrossRef]
- El-Gebaly, A.S.; Sofy, A.R.; Hmed, A.A.; Youssef, A.M. Green synthesis, characterization and medicinal uses of silver nanoparticles (Ag-NPs), copper nanoparticles (Cu-NPs) and zinc oxide nanoparticles (ZnO-NPs) and their mechanism of action: A review. Biocatal. Agric. Biotechnol. 2024, 55, 103006. [Google Scholar] [CrossRef]
- Mondal, S.K.; Chakraborty, S.; Manna, S.; Mandal, S.M. Antimicrobial nanoparticles: Current landscape and future challenges. RSC Pharm. 2024, 1, 388–402. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Urnukhsaikhan, E.; Bold, B.-E.; Gunbileg, A.; Sukhbaatar, N.; Mishig-Ochir, T. Antibacterial activity and characteristics of silver nanoparticles biosynthesized from Carduus crispus. Sci. Rep. 2021, 11, 21047. [Google Scholar] [CrossRef]
- Dey, S.; Mohanty, D.l.; Divya, N.; Bakshi, V.; Mohanty, A.; Rath, D.; Das, S.; Mondal, A.; Roy, S.; Sabui, R. A critical review on zinc oxide nanoparticles: Synthesis, properties and biomedical applications. Intell. Pharm. 2024, 3, 53–70. [Google Scholar] [CrossRef]
- Naz, S.; Gul, A.; Zia, M.; Javed, R. Synthesis, biomedical applications, and toxicity of CuO nanoparticles. Appl. Microbiol. Biotechnol. 2023, 107, 1039–1061. [Google Scholar] [CrossRef]
- Selwal, N.; Rahayu, F.; Herwati, A.; Latifah, E.; Supriyono; Suhara, C.; Kade Suastika, I.B.; Mahayu, W.M.; Wani, A.K. Enhancing secondary metabolite production in plants: Exploring traditional and modern strategies. J. Agric. Food Res. 2023, 14, 100702. [Google Scholar] [CrossRef]
- Chen, L.; Hong, W.; Ren, W.; Xu, T.; Qian, Z.; He, Z. Recent progress in targeted delivery vectors based on biomimetic nanoparticles. Signal Transduct. Target. Ther. 2021, 6, 225. [Google Scholar] [CrossRef]
- Madlhophe, S.; Ogugua, U.V.; Makhubu, F.N.; Figlan, S. Use of biological control agents for managing fungal pathogens in Solanaceae crops: Progress and future perspectives—A review. Discov. Appl. Sci. 2025, 7, 83. [Google Scholar] [CrossRef]
- Gressler, S.; Hipfinger, C.; Part, F.; Pavlicek, A.; Zafiu, C.; Giese, B. A systematic review of nanocarriers used in medicine and beyond — definition and categorization framework. J. Nanobiotechnol. 2025, 23, 90. [Google Scholar] [CrossRef]
- Bolla, P.K.; Rodriguez, V.A.; Kalhapure, R.S.; Kolli, C.S.; Andrews, S.; Renukuntla, J. A review on pH and temperature responsive gels and other less explored drug delivery systems. J. Drug Deliv. Sci. Technol. 2018, 46, 416–435. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, W.; Yang, Q. Transcriptome Analysis Reveals the Regulation of Aureobasidium pullulans under Different pH Stress. Int. J. Mol. Sci. 2023, 24, 16103. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Sahandi Zangabad, P.; Ghasemi, A.; Amiri, M.; Bahrami, M.; Malekzad, H.; Ghahramanzadeh Asl, H.; Mahdieh, Z.; Bozorgomid, M.; Ghasemi, A.; et al. Temperature-Responsive Smart Nanocarriers for Delivery Of Therapeutic Agents: Applications and Recent Advances. ACS Appl. Mater. Interfaces 2016, 8, 21107–21133. [Google Scholar] [CrossRef]
- Singh, K.; Singhal, S.; Pahwa, S.; Sethi, V.A.; Sharma, S.; Singh, P.; Kale, R.D.; Ali, S.W.; Sagadevan, S. Nanomedicine and drug delivery: A comprehensive review of applications and challenges. Nano-Struct. Nano-Objects 2024, 40, 101403. [Google Scholar] [CrossRef]
- El-Baky, N.A.; Amara, A. Recent Approaches towards Control of Fungal Diseases in Plants: An Updated Review. J. Fungi 2021, 7, 900. [Google Scholar] [CrossRef]
- RodrÍGuez-Seijo, A.; SantÁS-Miguel, V.; Arenas-Lago, D.; Arias-EstÉVez, M.; PÉRez-RodrÍGuez, P. Use of nanotechnology for safe agriculture and food production: Challenges and limitations. Pedosphere 2025, 35, 20–32. [Google Scholar] [CrossRef]
- Sare, A.R.; Jijakli, M.H.; Massart, S. Microbial ecology to support integrative efficacy improvement of biocontrol agents for postharvest diseases management. Postharvest Biol. Technol. 2021, 179, 111572. [Google Scholar] [CrossRef]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; Rehman, H.u.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef] [PubMed]
- Yanat, M.; Schroën, K. Preparation methods and applications of chitosan nanoparticles; with an outlook toward reinforcement of biodegradable packaging. React. Funct. Polym. 2021, 161, 104849. [Google Scholar] [CrossRef]
- Mena-Giraldo, P.; Pérez-Buitrago, S.; Londoño-Berrío, M.; Ortiz-Trujillo, I.C.; Hoyos-Palacio, L.M.; Orozco, J. Photosensitive nanocarriers for specific delivery of cargo into cells. Sci. Rep. 2020, 10, 2110. [Google Scholar] [CrossRef]
- Brunelle, T.; Chakir, R.; Carpentier, A.; Dorin, B.; Goll, D.; Guilpart, N.; Maggi, F.; Makowski, D.; Nesme, T.; Roosen, J.; et al. Reducing chemical inputs in agriculture requires a system change. Commun. Earth Environ. 2024, 5, 369. [Google Scholar] [CrossRef]
- Arora, P.K.; Tripathi, S.; Omar, R.A.; Chauhan, P.; Sinhal, V.K.; Singh, A.; Srivastava, A.; Garg, S.K.; Singh, V.P. Next-generation fertilizers: The impact of bionanofertilizers on sustainable agriculture. Microb. Cell Factories 2024, 23, 254. [Google Scholar] [CrossRef]
- Gotor-Vila, A.; Usall, J.; Torres, R.; Solsona, C.; Teixidó, N. Enhanced shelf-life of the formulated biocontrol agent Bacillus amyloliquefaciens CPA-8 combining diverse packaging strategies and storage conditions. Int. J. Food Microbiol. 2019, 290, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Mu, J.; Liang, L. Nanocarriers for intracellular delivery of proteins in biomedical applications: Strategies and recent advances. J. Nanobiotechnology 2024, 22, 688. [Google Scholar] [CrossRef]
- Reshmy, R.; Philip, E.; Madhavan, A.; Sindhu, R.; Pugazhendhi, A.; Binod, P.; Sirohi, R.; Awasthi, M.K.; Tarafdar, A.; Pandey, A. Advanced biomaterials for sustainable applications in the food industry: Updates and challenges. Environ. Pollut. 2021, 283, 117071. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Chakrabartty, I.; Mishra, A.K.; Chopra, H.; Mahanta, S.; Avula, S.K.; Patowary, K.; Ahmed, R.; Mishra, B.; Mohanta, T.K.; et al. Nanotechnology in combating biofilm: A smart and promising therapeutic strategy. Front. Microbiol. 2022, 13, 1028086. [Google Scholar] [CrossRef]
- He, H.-M.; Liu, L.-N.; Munir, S.; Bashir, N.H.; Wang, Y.; Yang, J.; Li, C.-Y. Crop diversity and pest management in sustainable agriculture. J. Integr. Agric. 2019, 18, 1945–1952. [Google Scholar] [CrossRef]
- Campa, M.F.; Brown, C.M.; Byrley, P.; Delborne, J.; Glavin, N.; Green, C.; Griep, M.; Kaarsberg, T.; Linkov, I.; Miller, J.B.; et al. Nanotechnology solutions for the climate crisis. Nat. Nanotechnol. 2024, 19, 1422–1426. [Google Scholar] [CrossRef]
- Alqahtani, A.S.; Elbeltagi, S. Advancing chemistry sustainably: From synthesis to benefits and applications of green synthesis. J. Organomet. Chem. 2025, 1027, 123508. [Google Scholar] [CrossRef]
- Manikandan, V.; Min, S.C. Roles of polysaccharides-based nanomaterials in food preservation and extension of shelf-life of food products: A review. Int. J. Biol. Macromol. 2023, 252, 126381. [Google Scholar] [CrossRef]
- Nile, S.H.; Baskar, V.; Selvaraj, D.; Nile, A.; Xiao, J.; Kai, G. Nanotechnologies in Food Science: Applications, Recent Trends, and Future Perspectives. Nano-Micro Lett. 2020, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Ferreira, M.D.; Correa, D.S.; Teodoro, K.B.R.; Procopio, F.R.; Brexó, R.P.; Sarkhosh, A.; Brecht, J.K. Advances in postharvest nanotechnology: Enhancing fresh produce shelf life and quality to reduce losses and waste. Postharvest Biol. Technol. 2025, 222, 113397. [Google Scholar] [CrossRef]
- Keller, A.A.; Ehrens, A.; Zheng, Y.; Nowack, B. Developing trends in nanomaterials and their environmental implications. Nat. Nanotechnol. 2023, 18, 834–837. [Google Scholar] [CrossRef]
- Thapa, R.K.; Kim, J.O. Nanomedicine-based commercial formulations: Current developments and future prospects. J. Pharm. Investig. 2023, 53, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Wahab, Y.A.; Al-Ani, L.A.; Khalil, I.; Schmidt, S.; Tran, N.N.; Escribà-Gelonch, M.; Woo, M.W.; Davey, K.; Gras, S.; Hessel, V.; et al. Nanomaterials: A critical review of impact on food quality control and packaging. Food Control 2024, 163, 110466. [Google Scholar] [CrossRef]
- Gupta, D.; Boora, A.; Thakur, A.; Gupta, T.K. Green and sustainable synthesis of nanomaterials: Recent advancements and limitations. Environ. Res. 2023, 231, 116316. [Google Scholar] [CrossRef]
- Biswas, R.; Alam, M.; Sarkar, A.; Haque, M.I.; Hasan, M.M.; Hoque, M. Application of nanotechnology in food: Processing, preservation, packaging and safety assessment. Heliyon 2022, 8, e11795. [Google Scholar] [CrossRef]
- Saha, B.; Biswas, S.; Datta, S.; Mojumdar, A.; Pal, S.; Mohanty, P.S.; Giri, M.K. Sustainable Nano solutions for global food security and biotic stress management. Plant Nano Biol. 2024, 9, 100090. [Google Scholar] [CrossRef]
- Sharma, B.; Tiwari, S.; Kumawat, K.C.; Cardinale, M. Nano-biofertilizers as bio-emerging strategies for sustainable agriculture development: Potentiality and their limitations. Sci. Total Environ. 2023, 860, 160476. [Google Scholar] [CrossRef]
- Ambikapathi, R.; Schneider, K.R.; Davis, B.; Herrero, M.; Winters, P.; Fanzo, J.C. Global food systems transitions have enabled affordable diets but had less favourable outcomes for nutrition, environmental health, inclusion and equity. Nat. Food 2022, 3, 764–779. [Google Scholar] [CrossRef] [PubMed]
- Teixidó, N.; Usall, J.; Torres, R. Insight into a Successful Development of Biocontrol Agents: Production, Formulation, Packaging, and Shelf Life as Key Aspects. Horticulturae 2022, 8, 305. [Google Scholar] [CrossRef]
- Yunxia, J.; Wang, Y.; Wang, X.; Lv, C.; Zhou, Q.; Jiang, G.; Yan, B.; Chen, L. Beyond the promise: Exploring the complex interactions of nanoparticles within biological systems. J. Hazard. Mater. 2024, 468, 133800. [Google Scholar] [CrossRef]
- Kang, D.; Zhang, Y.; Yu, D.-G.; Kim, I.; Song, W. Integrating synthetic polypeptides with innovative material forming techniques for advanced biomedical applications. J. Nanobiotechnology 2025, 23, 101. [Google Scholar] [CrossRef]
- Simão, S.A.V.; Rohden, S.F.; Pinto, D.C. Natural claims and sustainability: The role of perceived efficacy and sensorial expectations. Sustain. Prod. Consum. 2022, 34, 505–517. [Google Scholar] [CrossRef]
- Barrett, B. Health and sustainability co-benefits of eating behaviors: Towards a science of dietary eco-wellness. Prev. Med. Rep. 2022, 28, 101878. [Google Scholar] [CrossRef]
- Viroli, G.; Kalmpourtzidou, A.; Cena, H. Exploring Benefits and Barriers of Plant-Based Diets: Health, Environmental Impact, Food Accessibility and Acceptability. Nutrients 2023, 15, 4723. [Google Scholar] [CrossRef] [PubMed]
- Allan, J.; Belz, S.; Hoeveler, A.; Hugas, M.; Okuda, H.; Patri, A.; Rauscher, H.; Silva, P.; Slikker, W.; Sokull-Kluettgen, B.; et al. Regulatory landscape of nanotechnology and nanoplastics from a global perspective. Regul. Toxicol. Pharmacol. 2021, 122, 104885. [Google Scholar] [CrossRef]
- Shakeran, Z.; Keyhanfar, M.; Varshosaz, J.; Sutherland, D.S. Biodegradable nanocarriers based on chitosan-modified mesoporous silica nanoparticles for delivery of methotrexate for application in breast cancer treatment. Mater. Sci. Eng. C 2021, 118, 111526. [Google Scholar] [CrossRef] [PubMed]
- Khezerlou, A.; Alizadeh-Sani, M.; Azizi-Lalabadi, M.; Ehsani, A. Nanoparticles and their antimicrobial properties against pathogens including bacteria, fungi, parasites and viruses. Microb. Pathog. 2018, 123, 505–526. [Google Scholar] [CrossRef]
- Onyeaka, H.; Passaretti, P.; Miri, T.; Al-Sharify, Z.T. The safety of nanomaterials in food production and packaging. Curr. Res. Food Sci. 2022, 5, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Frewer, L.J.; Gupta, N.; George, S.; Fischer, A.R.H.; Giles, E.L.; Coles, D. Consumer attitudes towards nanotechnologies applied to food production. Trends Food Sci. Technol. 2014, 40, 211–225. [Google Scholar] [CrossRef]
- Fadel, T.R.; Steevens, J.A.; Thomas, T.A.; Linkov, I. The challenges of nanotechnology risk management. Nano Today 2015, 10, 6–10. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Luo, Y.; Liang, Y. A comprehensive review of conventional and stimuli-responsive delivery systems for bioactive peptides: From food to biomedical applications. Adv. Compos. Hybrid Mater. 2024, 8, 12. [Google Scholar] [CrossRef]
- Xu, D.; Ma, Y.; Jin, G.; Cao, L. Intelligent Photonics: A Disruptive Technology to Shape the Present and Redefine the Future. Engineering 2025, 46, 186–213. [Google Scholar] [CrossRef]
- Saifullah, M.; Shishir, M.R.I.; Ferdowsi, R.; Tanver Rahman, M.R.; Van Vuong, Q. Micro and nano encapsulation, retention and controlled release of flavor and aroma compounds: A critical review. Trends Food Sci. Technol. 2019, 86, 230–251. [Google Scholar] [CrossRef]
- Panda, S.; Hajra, S.; Kaushik, A.; Rubahn, H.G.; Mishra, Y.K.; Kim, H.J. Smart nanomaterials as the foundation of a combination approach for efficient cancer theranostics. Mater. Today Chem. 2022, 26, 101182. [Google Scholar] [CrossRef]
- Cappellano, G.; Abreu, H.; Casale, C.; Dianzani, U.; Chiocchetti, A. Nano-Microparticle Platforms in Developing Next-Generation Vaccines. Vaccines 2021, 9, 606. [Google Scholar] [CrossRef]
- Islam, A.; Rahat, I.; Anurag; Rejeeth, C.; Sharma, D.; Sharma, A. Recent advcances on plant-based bioengineered nanoparticles using secondary metabolites and their potential in lung cancer management. J. Future Foods 2025, 5, 1–20. [Google Scholar] [CrossRef]
- Bhushan, D.; Shoran, S.; Kumar, R.; Gupta, R. Plant biomass-based nanoparticles for remediation of contaminants from water ecosystems: Recent trends, challenges, and future perspectives. Chemosphere 2024, 365, 143340. [Google Scholar] [CrossRef] [PubMed]
- Zain, M.; Ma, H.; Ur Rahman, S.; Nuruzzaman, M.; Chaudhary, S.; Azeem, I.; Mehmood, F.; Duan, A.; Sun, C. Nanotechnology in precision agriculture: Advancing towards sustainable crop production. Plant Physiol. Biochem. 2024, 206, 108244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Feng, G.; Sun, X. Advanced technologies of soil moisture monitoring in precision agriculture: A Review. J. Agric. Food Res. 2024, 18, 101473. [Google Scholar] [CrossRef]
- Chavan, N.; Dharmaraj, D.; Sarap, S.; Surve, C. Magnetic nanoparticle –A new era in nanotechnology. J. Drug Deliv. Sci. Technol. 2022, 77, 103899. [Google Scholar] [CrossRef]
- Csóka, I.; Ismail, R.; Jójárt-Laczkovich, O.; Pallagi, E. Regulatory Considerations, Challenges and Risk-based Approach in Nanomedicine Development. Curr. Med. Chem. 2021, 28, 7461–7476. [Google Scholar] [CrossRef]
- Teixeira, T.; Kweder, S.L.; Saint-Raymond, A. Are the European Medicines Agency, US Food and Drug Administration, and Other International Regulators Talking to Each Other? Clin. Pharmacol. Ther. 2020, 107, 507–513. [Google Scholar] [CrossRef]
- Ali, F.; Neha, K.; Parveen, S. Current regulatory landscape of nanomaterials and nanomedicines: A global perspective. J. Drug Deliv. Sci. Technol. 2023, 80, 104118. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Turcuş, V.; Predoi, G.; Iordache, F. Nanoencapsulation techniques for compounds and products with antioxidant and antimicrobial activity-A critical view. Eur. J. Med. Chem. 2018, 157, 1326–1345. [Google Scholar] [CrossRef] [PubMed]
- Chai, S.; Shih, W. Bridging science and technology through academic–industry partnerships. Res. Policy 2016, 45, 148–158. [Google Scholar] [CrossRef]
- Pokrajac, L.; Abbas, A.; Chrzanowski, W.; Dias, G.M.; Eggleton, B.J.; Maguire, S.; Maine, E.; Malloy, T.; Nathwani, J.; Nazar, L.; et al. Nanotechnology for a Sustainable Future: Addressing Global Challenges with the International Network4Sustainable Nanotechnology. ACS Nano 2021, 15, 18608–18623. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Tyagi, P.K.; Tyagi, S.; Ghorbanpour, M. Integrating green nanotechnology with sustainable development goals: A pathway to sustainable innovation. Discov. Sustain. 2024, 5, 364. [Google Scholar] [CrossRef]
- Puri, A.; Mohite, P.; Maitra, S.; Subramaniyan, V.; Kumarasamy, V.; Uti, D.E.; Sayed, A.A.; El-Demerdash, F.M.; Algahtani, M.; El-kott, A.F.; et al. From nature to nanotechnology: The interplay of traditional medicine, green chemistry, and biogenic metallic phytonanoparticles in modern healthcare innovation and sustainability. Biomed. Pharmacother. 2024, 170, 116083. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Niyazi, S.; Firdoos, A.; Wang, C.; Manzoor, M.A.; Ramakrishnan, M.; Upadhyay, A.; Ding, Y. Enhancing plant resilience: Nanotech solutions for sustainable agriculture. Heliyon 2024, 10, e40735. [Google Scholar] [CrossRef]
- Ashraf, S.A.; Siddiqui, A.J.; Elkhalifa, A.E.O.; Khan, M.I.; Patel, M.; Alreshidi, M.; Moin, A.; Singh, R.; Snoussi, M.; Adnan, M. Innovations in nanoscience for the sustainable development of food and agriculture with implications on health and environment. Sci. Total Environ. 2021, 768, 144990. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawal, H.; Gaddafi, M.S.; Jamiu, A.M.; Edo, G.S.; Fremah, O.G.; El-yakub, A.U.; Mahunu, G.K.; Wang, K.; Zhang, H.; Yang, Q. Biocontrol and Nanotechnology Strategies for Postharvest Disease Management in Fruits and Vegetables: A Comprehensive Review. Foods 2025, 14, 2782. https://doi.org/10.3390/foods14162782
Lawal H, Gaddafi MS, Jamiu AM, Edo GS, Fremah OG, El-yakub AU, Mahunu GK, Wang K, Zhang H, Yang Q. Biocontrol and Nanotechnology Strategies for Postharvest Disease Management in Fruits and Vegetables: A Comprehensive Review. Foods. 2025; 14(16):2782. https://doi.org/10.3390/foods14162782
Chicago/Turabian StyleLawal, Habiba, Mohammed Sani Gaddafi, Aasia Muhammed Jamiu, Gerefa Sefu Edo, Opoku Genevieve Fremah, Abdulgaffar Usman El-yakub, Gustav Komla Mahunu, Kaili Wang, Hongyin Zhang, and Qiya Yang. 2025. "Biocontrol and Nanotechnology Strategies for Postharvest Disease Management in Fruits and Vegetables: A Comprehensive Review" Foods 14, no. 16: 2782. https://doi.org/10.3390/foods14162782
APA StyleLawal, H., Gaddafi, M. S., Jamiu, A. M., Edo, G. S., Fremah, O. G., El-yakub, A. U., Mahunu, G. K., Wang, K., Zhang, H., & Yang, Q. (2025). Biocontrol and Nanotechnology Strategies for Postharvest Disease Management in Fruits and Vegetables: A Comprehensive Review. Foods, 14(16), 2782. https://doi.org/10.3390/foods14162782







