Parageobacillus and Geobacillus spp.: From Food Spoilage to Beneficial Food Applications
Abstract
1. Introduction
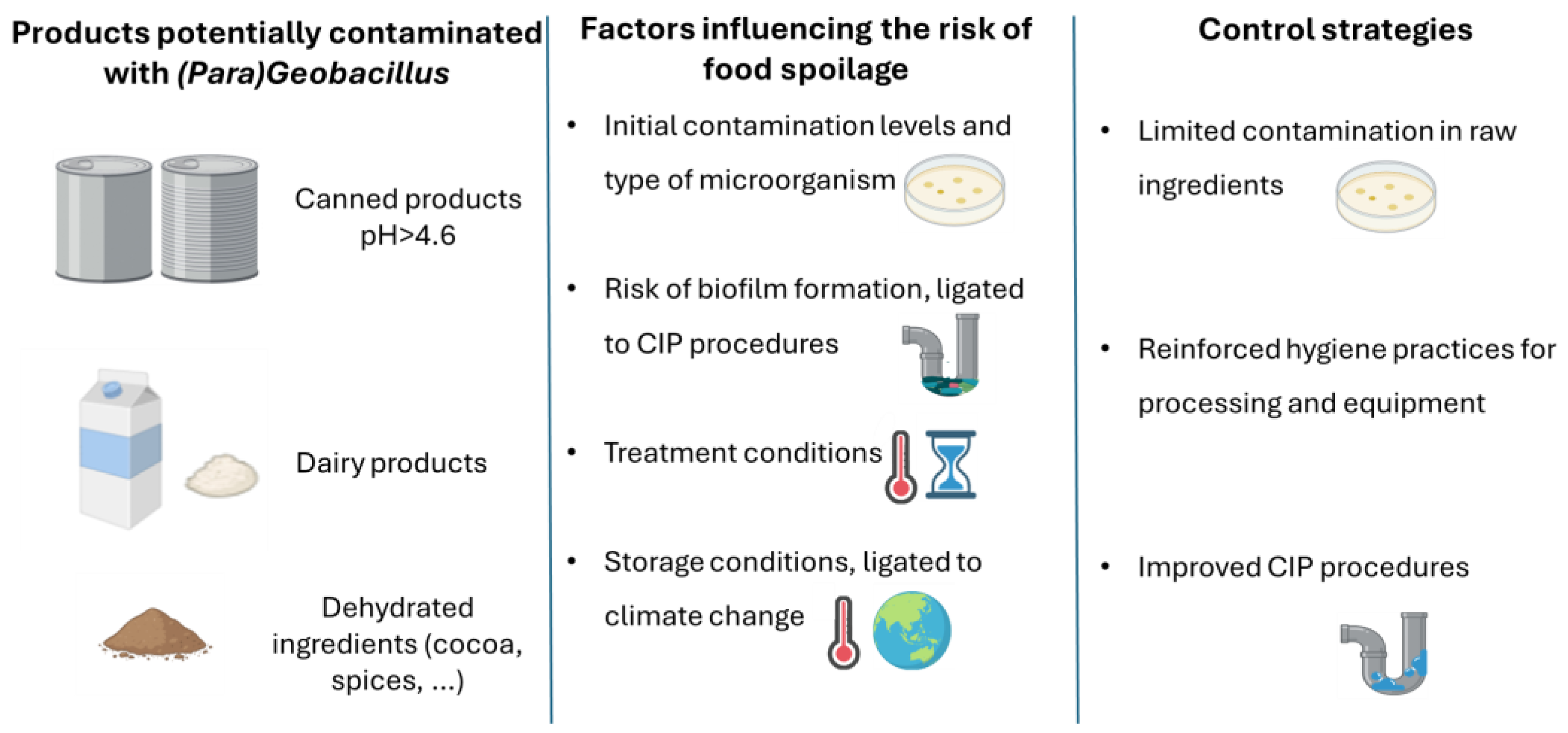
2. Food Spoilage
2.1. Biofilm Formation by (Para)Geobacillus spp. as a Source of Microbial and Enzymatic Contamination in the Dairy Industry
2.2. Strategies to Control (Para)Geobacillus spp. in Food
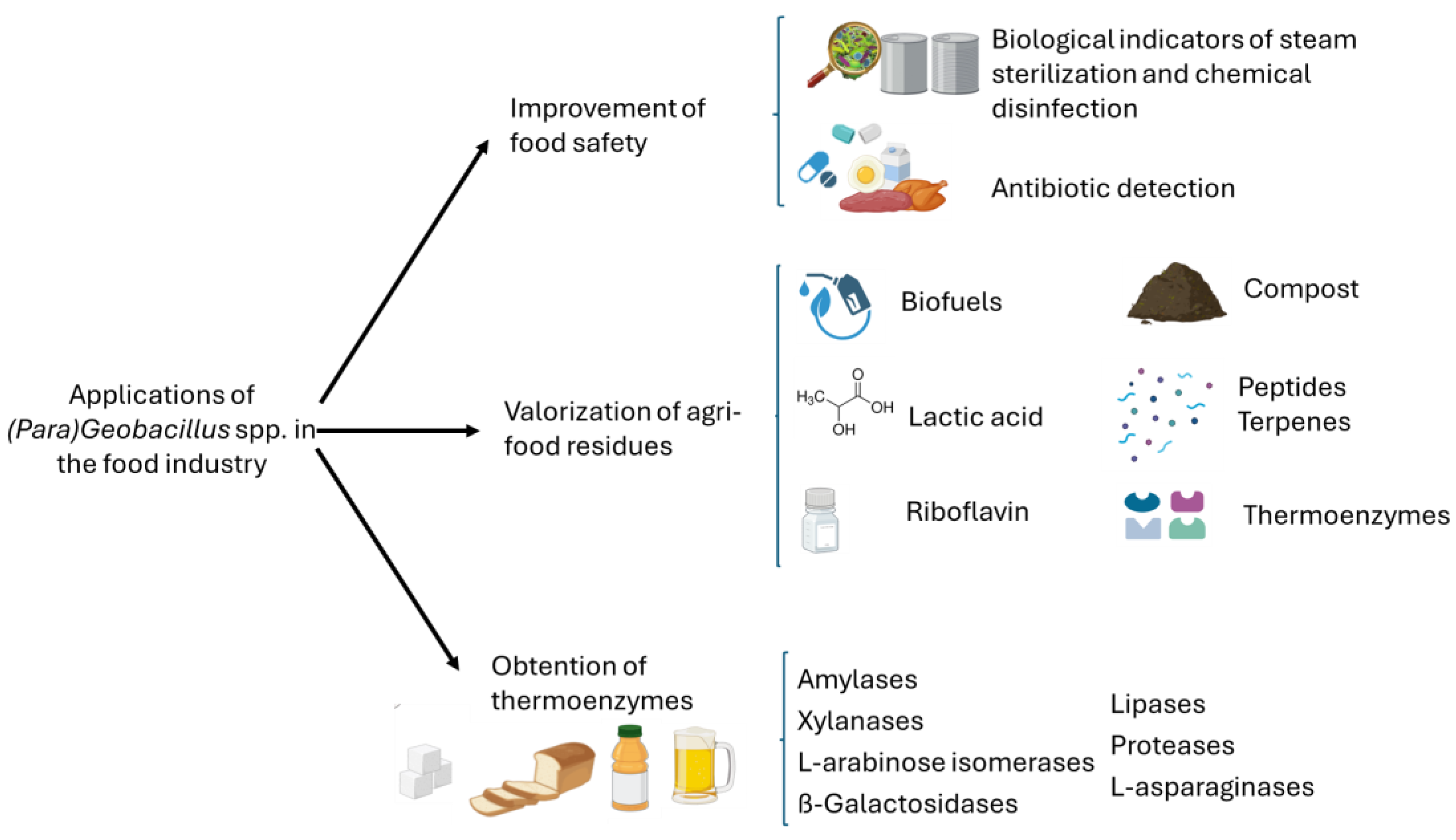
3. Food Safety Applications
4. Valorization of Agri-Food Residues
4.1. Advantages and Applications of (Para)Geobacillus spp. in Fermentation of Agri-Food Residues
4.2. Limitations of (Para)Geobacillus spp. in Fermentation Processes
5. Obtention of Thermostable Enzymes for Food Applications
5.1. Amylases
5.2. Xylanases
5.3. L-Arabinose Isomerase
5.4. β-Galactosidases
5.5. Lipases
5.6. Proteases
5.7. L-Asparaginases
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Studholme, D.J. Some (Bacilli) Like It Hot: Genomics of Geobacillus Species. Microb. Biotechnol. 2015, 8, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Nazina, T.N.; Tourova, T.P.; Poltaraus, A.B.; Novikova, E.V.; Grigoryan, A.A.; Ivanova, A.E.; Lysenko, A.M.; Petrunyaka, V.V.; Osipov, G.A.; Belyaev, S.S.; et al. Taxonomic study of aerobic thermophilic bacilli: Descriptions of Geobacillus subterraneus gen. nov., sp. nov. and Geobacillus uzenensis sp. nov. from petroleum reservoirs and transfer of Bacillus stearothermophilus, Bacillus thermocatenulatus, Bacillus thermoleovorans, Bacillus kaustophilus, Bacillus thermoglucosidasius and Bacillus thermodenitrificans to Geobacillus as the new combinations G. stearothermophilus, G. thermocatenulatus, G. thermoleovorans, G. kaustophilus, G. thermoglucosidasius and G. thermodenitrificans. Int. J. Syst. Evol. Microbiol. 2001, 51, 433–446. [Google Scholar] [PubMed]
- Coorevits, A.; Dinsdale, A.E.; Halket, G.; Lebbe, L.; De Vos, P.; Van Landschoot, A.; Logan, N.A. Taxonomic Revision of the Genus Geobacillus: Emendation of Geobacillus, G. stearothermophilus, G. jurassicus, G. toebii, G. thermodenitrificans and G. thermoglucosidans (nom. corrig., formerly ‘thermoglucosidasius’); transfer of Bacillus thermantarcticus to the genus as G. thermantarcticus comb. nov.; proposal of Caldibacillus debilis gen. nov., comb. nov.; transfer of G. tepidamans to Anoxybacillus as A. tepidamans comb. nov.; and proposal of Anoxybacillus caldiproteolyticus sp. nov. Int. J. Syst. Evol. Microbiol. 2012, 62 Pt 7, 1470–1485. [Google Scholar]
- Aliyu, H.; Lebre, P.; Blom, J.; Cowan, D.; De Maayer, P. Phylogenomic Re-Assessment of the Thermophilic Genus Geobacillus. Syst. Appl. Microbiol. 2016, 39, 527–533. [Google Scholar] [CrossRef]
- Aliyu, H.; Lebre, P.; Blom, J.; Cowan, D.; De Maayer, P. Corrigendum to “Phylogenomic Re-Assessment of the Thermophilic Genus Geobacillus” [Syst. Appl. Microbiol. 39 (2016) 527–533]. Syst. Appl. Microbiol. 2018, 41, 529–530. [Google Scholar] [CrossRef]
- Zeigler, D.R. The Geobacillus Paradox: Why Is a Thermophilic Bacterial Genus So Prevalent on a Mesophilic Planet? Microbiology 2014, 160, 1–11. [Google Scholar] [CrossRef]
- Sadiq, F.A.; Li, Y.; Liu, T.; Flint, S.; Zhang, G.; Yuan, L.; Pei, Z.; He, G. The Heat Resistance and Spoilage Potential of Aerobic Mesophilic and Thermophilic Spore Forming Bacteria Isolated from Chinese Milk Powders. Int. J. Food Microbiol. 2016, 238, 193–201. [Google Scholar] [CrossRef]
- Chen, L.; Coolbear, T.; Daniel, R.M. Characteristics of Proteinases and Lipases Produced by Seven Bacillus Sp. Isolated from Milk Powder Production Lines. Int. Dairy J. 2004, 14, 495–504. [Google Scholar] [CrossRef]
- Lücking, G.; Stoeckel, M.; Atamer, Z.; Hinrichs, J.; Ehling-Schulz, M. Characterization of Aerobic Spore-Forming Bacteria Associated with Industrial Dairy Processing Environments and Product Spoilage. Int. J. Food Microbiol. 2013, 166, 270–279. [Google Scholar] [CrossRef]
- Champidou, C.; Ellouze, M.; Haddad, N.; Membré, J.-M. Modeling Geobacillus stearothermophilus Spores Inactivation in Plant-Based Drinks to Design Uht Processing. Food Res. Int. 2025, 201, 115518. [Google Scholar] [CrossRef]
- Prevost, S.; Andre, S.; Remize, F. Pcr Detection of Thermophilic Spore-Forming Bacteria Involved in Canned Food Spoilage. Curr. Microbiol. 2010, 61, 525–533. [Google Scholar] [CrossRef]
- André, S.; Zuber, F.; Remize, F. Thermophilic Spore-Forming Bacteria Isolated from Spoiled Canned Food and Their Heat Resistance. Results of a French Ten-Year Survey. Int. J. Food Microbiol. 2013, 165, 134–143. [Google Scholar] [CrossRef]
- Burgess, S.A.; Flint, S.H.; Lindsay, D.; Cox, M.P.; Biggs, P.J. Insights into the Geobacillus stearothermophilus Species Based on Phylogenomic Principles. BMC Microbiol. 2017, 17, 140. [Google Scholar] [CrossRef]
- Guizelini, B.P.; Vandenberghe, L.P.S.; Sella, S.R.B.R.; Soccol, C.R. Study of the Influence of Sporulation Conditions on Heat Resistance of Geobacillus stearothermophilus Used in the Development of Biological Indicators for Steam Sterilization. Arch. Microbiol. 2012, 194, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Stier, P.; Maul, S.; Kulozik, U. Effect of Sporulation Conditions Following Solid-State Cultivation on the Resistance of Geobacillus stearothermophilus Spores for Use as Bioindicators Testing Inactivation by H2O2. Lebensm.-Wiss. Technol. 2021, 151, 112078. [Google Scholar] [CrossRef]
- Nagel, O.G.; Beltrán, M.C.; Molina, M.P.; Althaus, R.L. Novel Microbiological System for Antibiotic Detection in Ovine Milk. Small Rumin. Res. 2012, 102, 26–31. [Google Scholar] [CrossRef]
- Wu, Q.; Gao, X.; Shabbir, M.A.B.; Peng, D.; Tao, Y.; Chen, D.; Hao, H.; Cheng, G.; Liu, Z.; Yuan, Z.; et al. Rapid Multi-Residue Screening of Antibiotics in Muscle from Different Animal Species by Microbiological Inhibition Method. Microchem. J. 2020, 152, 104417. [Google Scholar] [CrossRef]
- Fan, W.; Gao, X.-Y.; Zang, M.-W.; Li, H.-N.; Guo, W.-P.; Li, Y.-Y.; Wang, S.-W. Development and Evaluation of a Preliminary Screening Assay for Antibiotic Residues in Meat. Appl. Biochem. Biotechnol. 2021, 193, 1129–1146. [Google Scholar] [CrossRef]
- Mollania, N.; Khajeh, K.; Hosseinkhani, S.; Dabirmanesh, B. Purification and Characterization of a Thermostable Phytate Resistant A-Amylase from Geobacillus Sp. Lh8. Int. J. Biol. Macromol. 2010, 46, 27–36. [Google Scholar] [CrossRef]
- Ezeji, T.C.; Bahl, H. Purification, Characterization, and Synergistic Action of Phytate-Resistant A-Amylase and A-Glucosidase from Geobacillus thermodenitrificans Hro10. J. Biotechnol. 2006, 125, 27–38. [Google Scholar] [CrossRef]
- EFSA Panel on Food Contact Materials, Enzymes, Processing Aids; Lambré, C.; Baviera, J.M.B.; Bolognesi, C.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; Mengelers, M.; et al. Safety Evaluation of the Food Enzyme 1,4-A-Glucan Branching Enzyme from the Non-Genetically Modified Geobacillus thermodenitrificans Strain Trbe14. EFSA J. 2023, 21, e07834. [Google Scholar] [PubMed]
- Kurniawan, D.C.; Rohman, M.S.; Witasari, L.D. Heterologous Expression, Characterization, and Application of Recombinant Thermostable A-Amylase from Geobacillus Sp. Ds3 for Porous Starch Production. BB Rep. 2024, 39, 101784. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Kumar, V.; Satyanarayana, T. Characteristics of Thermostable Endoxylanase and Β-Xylosidase of the Extremely Thermophilic Bacterium Geobacillus thermodenitrificans Tsaa1 and Its Applicability in Generating Xylooligosaccharides and Xylose from Agro-Residues. Extremophiles 2013, 17, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Marcolongo, L.; La Cara, F.; Morana, A.; Di Salle, A.; Del Monaco, G.; Paixão, S.M.; Alves, L.; Ionata, E. Properties of an Alkali-Thermo Stable Xylanase from Geobacillus thermodenitrificans A333 and Applicability in Xylooligosaccharides Generation. World J. Microbiol. Biotechnol. 2015, 31, 633–648. [Google Scholar] [CrossRef]
- Mathew, S.; Aronsson, A.; Karlsson, E.N.; Adlercreutz, P. Xylo- and Arabinoxylooligosaccharides from Wheat Bran by Endoxylanases, Utilisation by Probiotic Bacteria, and Structural Studies of the Enzymes. Appl. Microbiol. Biotechnol. 2018, 102, 3105–3120. [Google Scholar] [CrossRef]
- Chen, W.; Chen, H.; Xia, Y.; Zhao, J.; Tian, F.; Zhang, H. Production, Purification, and Characterization of a Potential Thermostable Galactosidase for Milk Lactose Hydrolysis from Bacillus stearothermophilus. J. Dairy Sci. 2008, 91, 1751–1758. [Google Scholar] [CrossRef]
- Gänzle, M.G. Enzymatic Synthesis of Galacto-Oligosaccharides and Other Lactose Derivatives (Hetero-Oligosaccharides) from Lactose. Int. Dairy J. 2012, 22, 116–122. [Google Scholar] [CrossRef]
- Tayyab, M.; Rashid, N.; Akhtar, M. Isolation and Identification of Lipase Producing Thermophilic Geobacillus Sp. Sbs-4s: Cloning and Characterization of the Lipase. J. Biosci. Bioeng. 2011, 111, 272–278. [Google Scholar] [CrossRef]
- Novik, G.; Savich, V.; Meerovskaya, O. Geobacillus Bacteria: Potential Commercial Applications in Industry, Bioremediation, and Bioenergy Production; IntechOpen: London, UK, 2019. [Google Scholar]
- Zhang, J.; Tian, M.; Lv, P.; Luo, W.; Wang, Z.; Xu, J.; Wang, Z. High-Efficiency Expression of the Thermophilic Lipase from Geobacillus thermocatenulatus in Escherichia coli and Its Application in the Enzymatic Hydrolysis of Rapeseed Oil. 3 Biotech 2020, 10, 523. [Google Scholar] [CrossRef]
- Hussein, A.H.; Lisowska, B.K.; Leak, D.J. Chapter One—the Genus Geobacillus and Their Biotechnological Potential. Adv. Appl. Microbiol. 2015, 92, 1–48. [Google Scholar]
- Wu, P.; Guo, Y.; Golly, M.K.; Ma, H.; He, R.; Luo, S.; Zhang, C.; Zhang, L.; Zhu, J. Feasibility Study on Direct Fermentation of Soybean Meal by Bacillus stearothermophilus under Non-Sterile Conditions. J. Sci. Food Agric. 2019, 99, 3291–3298. [Google Scholar] [CrossRef] [PubMed]
- Alonso, V.P.P.; de Oliveira Morais, J.; Kabuki, D.Y. Incidence of Bacillus cereus, Bacillus sporothermodurans and Geobacillus stearothermophilus in Ultra-High Temperature Milk and Biofilm Formation Capacity of Isolates. Int. J. Food Microbiol. 2021, 354, 109318. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.A.; Flint, S.H.; Lindsay, D. Characterization of Thermophilic Bacilli from a Milk Powder Processing Plant. J. Appl. Microbiol. 2014, 116, 350–359. [Google Scholar] [CrossRef] [PubMed]
- André, S.; Vallaeys, T.; Planchon, S. Spore-Forming Bacteria Responsible for Food Spoilage. Res. Microbiol. 2017, 168, 379–387. [Google Scholar] [CrossRef]
- Nakano, M. Multiplex Pcr for Rapid Detection of Thermophilic Moorella thermoacetica and Geobacillus stearothermophilus from Canned Foods and Beverages. Int. J. Food Sci. Technol. 2018, 53, 1352–1362. [Google Scholar] [CrossRef]
- Heckler, C.; Vale, M.G.; Canales, H.D.S.; Stradiotto, G.C.; Giordano, A.L.P.L.; Schreiber, A.Z.; Anderson, S. Sant’Ana. Spore-Forming Bacteria in Gelatin: Characterization, Identification by 16S rRNA and Maldi-Tof Mass Spectrometry (Ms), and Presence of Heat Resistance and Virulence Genes. Int. J. Food Microbiol. 2024, 422, 110813. [Google Scholar] [CrossRef]
- Misiou, O.; Koutsoumanis, K.; Membré, J.-M. Quantitative Microbial Spoilage Risk Assessment of Plant-Based Milk Alternatives by Geobacillus stearothermophilus in Europe. Food Res. Int. 2023, 166, 112638. [Google Scholar] [CrossRef]
- Kyrylenko, A.; Eijlander, R.T.; Alliney, G.; de Bos, E.L.-V.; Wells-Bennik, M.H.J. Levels and Types of Microbial Contaminants in Different Plant-Based Ingredients Used in Dairy Alternatives. Int. J. Food Microbiol. 2023, 407, 110392. [Google Scholar] [CrossRef]
- Misiou, O.; Kasiouras, G.; Koutsoumanis, K. Development and Validation of an Extended Predictive Model for the Effect of Ph and Water Activity on the Growth Kinetics of Geobacillus stearothermophilus in Plant-Based Milk Alternatives. Food Res. Int. 2021, 145, 110407. [Google Scholar] [CrossRef]
- Wells-Bennik, M.H.J.; Janssen, P.W.M.; Klaus, V.; Yang, C.; Zwietering, M.H.; Den Besten, H.M.W. Heat Resistance of Spores of 18 Strains of Geobacillus stearothermophilus and Impact of Culturing Conditions. Int. J. Food Microbiol. 2019, 291, 161–172. [Google Scholar] [CrossRef]
- Kakagianni, M.; Gougouli, M.; Koutsoumanis, K.P. Development and Application of Geobacillus stearothermophilus Growth Model for Predicting Spoilage of Evaporated Milk. Food Microbiol. 2016, 57, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Misiou, O.; Koutsoumanis, K. Climate Change and Its Implications for Food Safety and Spoilage. Trends Food Sci. Technol. 2022, 126, 142–152. [Google Scholar] [CrossRef]
- Koutsoumanis, K.P.; Misiou, O.D.; Kakagianni, M.N. Climate Change Threatens the Microbiological Stability of Non-Refrigerated Foods. Food Res. Int. 2022, 162, 111990. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.A.; Brooks, J.D.; Rakonjac, J.; Walker, K.M.R.; Flint, S.H. The Formation of Thermophilic Spores During the Manufacture of Whole Milk Powder. Int. J. Dairy Technol. 2007, 60, 109–117. [Google Scholar] [CrossRef]
- Burgess, S.A.; Lindsay, D.; Flint, S.H. Thermophilic Bacilli and Their Importance in Dairy Processing. Int. J. Food Microbiol. 2010, 144, 215–225. [Google Scholar] [CrossRef]
- Miller, R.A.; Kent, D.J.; Watterson, M.J.; Boor, K.J.; Martin, N.H.; Wiedmann, M. Spore Populations among Bulk Tank Raw Milk and Dairy Powders Are Significantly Different. J. Dairy Sci. 2015, 98, 8492–8504. [Google Scholar] [CrossRef]
- Dettling, A.; Doll, E.; Wedel, C.; Hinrichs, J.; Scherer, S.; Wenning, M. Accurate Quantification of Thermophilic Spores in Dairy Powders. Int. Dairy J. 2019, 98, 64–71. [Google Scholar] [CrossRef]
- McHugh, A.J.; Feehily, C.; Fenelon, M.A.; Gleeson, D.; Hill, C.; Cotter, P.D. Tracking the Dairy Microbiota from Farm Bulk Tank to Skimmed Milk Powder. mSystems 2020, 5, e00226-20. [Google Scholar] [CrossRef]
- Sadiq, F.A.; Flint, S.; Yuan, L.; Li, Y.; Liu, T.; He, G. Propensity for Biofilm Formation by Aerobic Mesophilic and Thermophilic Spore Forming Bacteria Isolated from Chinese Milk Powders. Int. J. Food Microbiol. 2017, 262, 89–98. [Google Scholar] [CrossRef]
- Rückert, A.; Ronimus, R.S.; Morgan, H.W. A Rapd-Based Survey of Thermophilic Bacilli in Milk Powders from Different Countries. Int. J. Food Microbiol. 2004, 96, 263–272. [Google Scholar] [CrossRef]
- Delaunay, L.; Cozien, E.; Gehannin, P.; Mouhali, N.; Mace, S.; Postollec, F.; Leguerinel, I.; Mathot, A.-G. Occurrence and Diversity of Thermophilic Sporeformers in French Dairy Powders. Int. Dairy J. 2021, 113, 104889. [Google Scholar] [CrossRef]
- Sadiq, F.A.; Flint, S.; He, G. Microbiota of Milk Powders and the Heat Resistance and Spoilage Potential of Aerobic Spore-Forming Bacteria. Int. Dairy J. 2018, 85, 159–168. [Google Scholar] [CrossRef]
- Wedel, C.; Atamer, Z.; Dettling, A.; Wenning, M.; Scherer, S.; Hinrichs, J. Towards Low-Spore Milk Powders: A Review on Microbiological Challenges of Dairy Powder Production with Focus on Aerobic Mesophilic and Thermophilic Spores. Int. Dairy J. 2022, 126, 105252. [Google Scholar] [CrossRef]
- Schwan, R.F.; Wheals, A.E. The Microbiology of Cocoa Fermentation and Its Role in Chocolate Quality. Crit. Rev. Food Sci. Nutr. 2004, 44, 205–221. [Google Scholar] [CrossRef]
- Eijlander, R.T.; Breitenwieser, F.; De Groot, R.; Hoornstra, E.; Kamphuis, H.; Kokken, M.; Kuijpers, A.; De Mello, I.G.; Van De Rijdt, G.; Vadier, C.; et al. Wells-Bennik. Enumeration and Identification of Bacterial Spores in Cocoa Powders. J. Food Prot. 2020, 83, 1530–1539. [Google Scholar] [CrossRef]
- Gabis, D.A.; Langlois, B.E.; Rudnick, A.W. Microbiological Examination of Cocoa Powder. Appl. Microbiol. 1970, 20, 644–645. [Google Scholar] [CrossRef]
- Lima, L.J.R.; Kamphuis, H.J.; Nout, M.J.R.; Zwietering, M.H. Microbiota of Cocoa Powder with Particular Reference to Aerobic Thermoresistant Spore-Formers. Food Microbiol. 2011, 28, 573–582. [Google Scholar] [CrossRef]
- Lima, L.J.R.; van der Velpen, V.; Wolkers-Rooijackers, J.; Kamphuis, H.J.; Zwietering, M.H.; Nout, M.J.R. Microbiota Dynamics and Diversity at Different Stages of Industrial Processing of Cocoa Beans into Cocoa Powder. Appl. Environ. Microbiol. 2012, 78, 2904–2913. [Google Scholar] [CrossRef]
- Pereira, A.P.M.; Stelari, H.A.; Carlin, F.; Sant’Ana, A.S. Inactivation Kinetics of Bacillus cereus and Geobacillus stearothermophilus Spores through Roasting of Cocoa Beans and Nibs. Lebensm.-Wiss. Technol. 2019, 111, 394–400. [Google Scholar] [CrossRef]
- Pereira, A.P.M.; Stellari, H.A.; Vilela, L.F.; Schwan, R.F.; Sant’Ana, A.S. Dynamics of Geobacillus stearothermophilus and Bacillus cereus Spores Inoculated in Different Time Intervals During Simulated Cocoa Beans Fermentation. Lebensm.-Wiss. Technol. 2020, 120, 108941. [Google Scholar] [CrossRef]
- Karaca, B.; Buzrul, S.; Cihan, A.C. Anoxybacillus and Geobacillus Biofilms in the Dairy Industry: Effects of Surface Material, Incubation Temperature and Milk Type. Biofouling 2019, 35, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Flint, S.; Palmer, J.; Bloemen, K.; Brooks, J.; Crawford, R. The Growth of Bacillus stearothermophilus on Stainless Steel. J. Appl. Microbiol. 2001, 90, 151–157. [Google Scholar] [CrossRef]
- Zhao, Y.; Caspers, M.P.M.; Metselaar, K.I.; De Boer, P.; Roeselers, G.; Moezelaar, R.; Groot, M.N.; Montijn, R.C.; Abee, T.; Kort, R. Abiotic and Microbiotic Factors Controlling Biofilm Formation by Thermophilic Sporeformers. Appl. Environ. Microbiol. 2013, 79, 5652–5660. [Google Scholar] [CrossRef] [PubMed]
- Somerton, B.; Lindsay, D.; Palmer, J.; Brooks, J.; Flint, S. Changes in Sodium, Calcium, and Magnesium Ion Concentrations That Inhibit Geobacillus Biofilms Have No Effect on Anoxybacillus flavithermus Biofilms. Appl. Environ. Microbiol. 2015, 81, 5115–5122. [Google Scholar] [CrossRef] [PubMed]
- Flint, S.; Bremer, P.; Brooks, J.; Palmer, J.; Sadiq, F.A.; Seale, B.; Teh, K.H.; Wu, S.; Zain, S.N.M. Bacterial Fouling in Dairy Processing. Int. Dairy J. 2020, 101, 104593. [Google Scholar] [CrossRef]
- Hebishy, E.; Yerlikaya, O.; Reen, F.J.; Mahony, J.; Akpinar, A.; Saygili, D.; Datta, N. Microbiological Aspects and Challenges of Dairy Powders—Ii: Biofilm/Biofouling. Int. J. Dairy Technol. 2024, 77, 691–712. [Google Scholar] [CrossRef]
- Hinton, A.R.; Trinh, K.T.; Brooks, J.D.; Manderson, G.J. Thermophile Survival in Milk Fouling and on Stainless Steel During Cleaning. Food Bioprod. Process. 2002, 80, 299–304. [Google Scholar] [CrossRef]
- Wedel, C.; Konschelle, T.; Dettling, A.; Wenning, M.; Scherer, S.; Hinichs, J. Thermally Induced Milk Fouling: Survival of Thermophilic Spore Formers and Potential of Contamination. Int. Dairy J. 2020, 101, 104582. [Google Scholar] [CrossRef]
- Brent Seale, R.; Flint, S.H.; McQuillan, A.J.; Bremer, P.J. Effect of Naoh (Caustic Wash) on the Viability, Surface Characteristics and Adhesion of Spores of a Geobacillus Sp. Isolated from a Milk Powder Production Line. Lett. Appl. Microbiol. 2011, 52, 104–108. [Google Scholar] [CrossRef]
- Seale, R.B.; Flint, S.H.; McQuillan, A.J.; Bremer, P.J. Recovery of Spores from Thermophilic Dairy Bacilli and Effects of Their Surface Characteristics on Attachment to Different Surfaces. Appl. Environ. Microbiol. 2008, 74, 731–737. [Google Scholar] [CrossRef]
- Wang, T.; Flint, S.; Palmer, J. Heterogeneous Response of Geobacillus stearothermophilus Biofilms to Calcium. Int. Dairy J. 2021, 116, 104961. [Google Scholar] [CrossRef]
- Pant, K.; Palmer, J.; Flint, S. Multispecies Biofilm Cities and the Importance of the Order of Colonization. Food Control. 2025, 175, 111319. [Google Scholar] [CrossRef]
- Zhao, Y.; Kumar, M.; Caspers, M.P.M.; Groot, M.N.N.; Van Der Vossen, J.M.B.M.; Abee, T. Short Communication: Growth of Dairy Isolates of Geobacillus thermoglucosidans in Skim Milk Depends on Lactose Degradation Products Supplied by Anoxybacillus flavithermus as Secondary Species. J. Dairy Sci. 2018, 101, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Jin, Y.; He, G.; Yuan, L. Development of Multi-Species Biofilm Formed by Thermophilic Bacteria on Stainless Steel Immerged in Skimmed Milk. Food Res. Int. 2021, 150, 110754. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.-A.; Hardin, M.T.; Chen, X.D. The Influence of Milk Composition on the Growth of Bacillus stearothermophilus. J. Financ. Econ. 2006, 77, 96–102. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Wehaidy, H.R.; Ibrahim, O.A.; El Ghani, S.A.; El-Hofi, M.A. Novel Milk-Clotting Enzyme from Bacillus stearothermophilus as a Coagulant in Uf-White Soft Cheese. Biocatal. Agric. Biotechnol. 2016, 7, 241–249. [Google Scholar] [CrossRef]
- Najm, T.A.; Walsh, M.K. Characterization of Lipases from Geobacillus stearothermophilus and Anoxybacillus flavithermus cell Lysates. Front. Nat. Sci. 2022, 13, 238–251. [Google Scholar]
- Chen, X.G.; Stabnikova, O.; Tay, J.H.; Wang, J.Y.; Tay, S.T. Thermoactive Extracellular Proteases of Geobacillus caldoproteolyticus, sp. nov., from Sewage Sludge. Extremophiles 2004, 8, 489–498. [Google Scholar] [CrossRef]
- Stoeckel, M.; Lücking, G.; Ehling-Schulz, M.; Atamer, Z.; Hinrichs, J. Bacterial Spores Isolated from Ingredients, Intermediate and Final Products Obtained from Dairies: Thermal Resistance in Milk. J. Digit. Sci. Technol. 2016, 96, 569–577. [Google Scholar] [CrossRef]
- Rigaux, C.; André, S.; Albert, I.; Carlin, F. Quantitative Assessment of the Risk of Microbial Spoilage in Foods. Prediction of Non-Stability at 55 °C Caused by Geobacillus stearothermophilus in Canned Green Beans. Int. J. Food Microbiol. 2014, 171, 119–128. [Google Scholar] [CrossRef]
- Gao, Y.-L.; Ju, X.-R.; Jiang, H.-H. Analysis of Reduction of Geobacillus stearothermophilus Spores Treated with High Hydrostatic Pressure and Mild Heat in Milk Buffer. J. Biotechnol. 2006, 125, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Espejo, G.G.A.; Hernández-Herrero, M.M.; Juan, B.; Trujillo, A.J. Inactivation of Bacillus Spores Inoculated in Milk by Ultra High Pressure Homogenization. Food Microbiol. 2014, 44, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, J.D.; Drews, M.J.; LaBerge, M.; Michael, A. Matthews. Sterilization of Bacterial Spores by Using Supercritical Carbon Dioxide and Hydrogen Peroxide. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 80B, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Dettling, A.; Wedel, C.; Huptas, C.; Hinrichs, J.; Scherer, S.; Wenning, M. High Counts of Thermophilic Spore Formers in Dairy Powders Originate from Persisting Strains in Processing Lines. Int. J. Food Microbiol. 2020, 335, 108888. [Google Scholar] [CrossRef]
- Parkar, S.G.; Flint, S.H.; Brooks, J.D. Evaluation of the Effect of Cleaning Regimes on Biofilms of Thermophilic Bacilli on Stainless Steel. J. Appl. Microbiol. 2004, 96, 110–116. [Google Scholar] [CrossRef]
- Hayrapetyan, H.; Nederhoff, L.; Vollebregt, M.; Mastwijk, H.; Groot, M.N. Inactivation Kinetics of Geobacillus stearothermophilus Spores by a Peracetic Acid or Hydrogen Peroxide Fog in Comparison to the Liquid Form. Int. J. Food Microbiol. 2020, 316, 108418. [Google Scholar] [CrossRef]
- Nam, Y.; Barnebey, A.; Kim, H.K.; Yannone, S.M.; Flint, S. Novel Hyperthermoacidic Archaeal Enzymes for Removal of Thermophilic Biofilms from Stainless Steel. J. Appl. Microbiol. 2023, 134, lxad106. [Google Scholar] [CrossRef]
- Almalki, T.; Anand, S. Ultrasound-Assisted Cavitation Effect on the Biofilm-Forming Ability of Common Dairy Sporeformers. Dairy 2023, 4, 100–107. [Google Scholar] [CrossRef]
- van Zyl, L.J.; Sunda, F.; Taylor, M.P.; Cowan, D.A.; Trindade, M.I. Identification and Characterization of a Novel Geobacillus thermoglucosidasius Bacteriophage, Gve3. Arch. Virol. 2015, 160, 2269–2282. [Google Scholar] [CrossRef]
- Zebrowska, J.; Witkowska, M.; Struck, A.; Laszuk, P.E.; Raczuk, E.; Ponikowska, M.; Skowron, P.M.; Zylicz-Stachula, A. Antimicrobial Potential of the Genera Geobacillus and Parageobacillus, as Well as Endolysins Biosynthesized by Their Bacteriophages. Antibiotics 2022, 11, 242. [Google Scholar] [CrossRef]
- Schlisselberg, D.B.; Yaron, S. The Effects of Stainless Steel Finish on Salmonella Typhimurium Attachment, Biofilm Formation and Sensitivity to Chlorine. Food Microbiol. 2013, 35, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Podtburg, T.C.; Zhao, P.; Chen, D.; Baker, D.A.; Cords, B.; Doyle, M.P. Reduction by Competitive Bacteria of Listeria monocytogenes in Biofilms and Listeria Bacteria in Floor Drains in a Ready-to-Eat Poultry Processing Plant. J. Food Prot. 2013, 76, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Kakagianni, M.; Koutsoumanis, K.P. Mapping the Risk of Evaporated Milk Spoilage in the Mediterranean Region Based on the Effect of Temperature Conditions on Geobacillus stearothermophilus Growth. Food Res. Int. 2018, 111, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Griep, E.R.; Cheng, Y.; Moraru, C.I. Efficient Removal of Spores from Skim Milk Using Cold Microfiltration: Spore Size and Surface Property Considerations. J. Dairy Sci. 2018, 101, 9703–9713. [Google Scholar] [CrossRef]
- Huesca-Espitia, L.C.; Suvira, M.; Rosenbeck, K.; Korza, G.; Setlow, B.; Li, W.; Wang, S.; Li, Y.Q.; Setlow, P. Effects of Steam Autoclave Treatment on Geobacillus stearothermophilus Spores. J. Appl. Microbiol. 2016, 121, 1300–1311. [Google Scholar] [CrossRef]
- Salvador, M.; Condón, S.; Gayán, E. Germination and Heat Resistance of Parageobacillus and Geobacillus spp. Spores. Foods 2025, 14, 2061. [Google Scholar] [CrossRef]
- McEvoy, B.; Maksimovic, A.; Rowan, N.J. Geobacillus stearothermophilus and Bacillus atrophaeus Spores Exhibit Linear Inactivation Kinetic Performance When Treated with an Industrial Scale Vaporized Hydrogen Peroxide (VH2O2) Sterilization Process. J. Appl. Microbiol. 2022, 134, lxac028. [Google Scholar]
- Wu, Q.; Peng, D.; Liu, Q.; Shabbir, M.A.B.; Sajid, A.; Liu, Z.; Wang, Y.; Yuan, Z. A Novel Microbiological Method in Microtiter Plates for Screening Seven Kinds of Widely Used Antibiotics Residues in Milk, Chicken Egg and Honey. Front Microbiol. 2019, 10, 436. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Wang, X.; Wang, X.; Ye, D.; Chen, P.; Ren, D. Hypersensitive Colorimetric Assay Based on Microbial Inhibition for the Detection of Multiple Antibiotics. J. Food Compos. Anal. 2024, 134, 106579. [Google Scholar] [CrossRef]
- Singh, S.; Shukla, S.; Tandia, N.; Kumar, N.; Paliwal, R. Antibiotic Residues: A Global Challenge. Pharma Sci. Monit. 2014, 5, 184–197. [Google Scholar]
- Vishnuraj, M.R.; Kandeepan, G.; Rao, K.H.; Chand, S.; Kumbhar, V. Occurrence, Public Health Hazards and Detection Methods of Antibiotic Residues in Foods of Animal Origin: A Comprehensive Review. Cogent Food Agric. 2016, 2, 1235458. [Google Scholar] [CrossRef]
- Nisha, A.R. Antibiotic Residues—A Global Health Hazard. Vet. World 2008, 1, 375–377. [Google Scholar] [CrossRef]
- CAFA (Commission on Antimicrobial Feed Additives). Antimicrobial Feed Additive; Ministry of Agriculture, Ed.; SOU: Stockholm, Sweden, 1997; Volume 132. [Google Scholar]
- European Commission. Regulation (Ec) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on Additives for Use in Animal Nutrition. Off. J. Eur. Union L 2003, 268, 29. [Google Scholar]
- U.S. Food and Drug Administration. Guidance for Industry #213: New Animal Drugs and New Animal Drug Combination Products Administered in or on Medicated Feed or Drinking Water of Food-Producing Animals—Recommendations for Drug Sponsors for Voluntarily Aligning Product Use Conditions with Gfi #209; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2013. [Google Scholar]
- Maron, D.F.; Smith, T.J.S.; Keeve, E. Nachman. Restrictions on Antimicrobial Use in Food Animal Production: An International Regulatory and Economic Survey. Global Health 2013, 9, 48. [Google Scholar] [CrossRef]
- Da Silva, R.A.; Arenas, N.E.; Luiza, V.L.; Bermudez, J.A.Z.; Clarke, S.E. Regulations on the Use of Antibiotics in Livestock Production in South America: A Comparative Literature Analysis. Antibiotics 2023, 12, 1303. [Google Scholar] [CrossRef]
- World Helth Organization. Antibiotic Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed on 1 June 2025).
- Baynes, R.E.; Dedonder, K.; Kissell, L.; Mzyk, D.; Marmulak, T.; Smith, G.; Tell, L.; Gehring, R.; Davis, J.; Riviere, J.E. Health Concerns and Management of Select Veterinary Drug Residues. Food Chem. Toxicol. 2016, 88, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Berruga, M.I.; Molina, A.; Althaus, R.L.; Molina, M.P. Control and Prevention of Antibiotic Residues and Contaminants in Sheep and Goat’s Milk. Small Rumin. Res. 2016, 142, 38–43. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (Eu) No 37/2010 of 22 December 2009 on Pharmacologically Active Substances and Their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin. Off. J. Eur. Union 2010, L 15, 1. [Google Scholar]
- FAO/WHO (Food and Agricultures of the United Nations/World Health Organization). Maximum Residue Limits (Mrls) and Risk Management Recommendations (Rmrs) for Residues of Veterinary Drugs in Foods; CX/MRL 2-2018; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Serrano, M.J.; Elorduy, J.; Zabaleta, I.; Istamboulie, G.; González-Fandos, E.; Bousquet-Mélou, A.; Mata, L.; Aymard, C.; Martínez-Laorden, A.; Da Silva-Guedes, J.; et al. Antimicrobial Residue Assessment in 5,357 Commercialized Meat Samples from the Spain-France Cross-Border Area: A New Approach for Effective Monitoring. Food Control 2022, 138, 109033. [Google Scholar] [CrossRef]
- Nagel, O.; Molina, M.P.; Althaus, R. Microbiological System in Microtitre Plates for Detection and Classification of Antibiotic Residues in Milk. Int. Dairy J. 2013, 32, 150–155. [Google Scholar] [CrossRef]
- Ahmed, S.; Ning, J.; Peng, D.; Chen, T.; Ahmad, I.; Ali, A.; Lei, Z.; Abu bakr Shabbir, M.; Cheng, G.; Yuan, Z. Current Advances in Immunoassays for the Detection of Antibiotics Residues: A Review. Food Agric. Immunol. 2020, 31, 268–290. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (Eu) 2021/808 of 22 March 2021 on the Performance of Analytical Methods for Residues of Pharmacologically Active Substances Used in Food-Producing Animals and on the in-Terpretation of Results as Well as on the Methods to Be Used for Sampling and Repealing Decisions 2002/657/Ec and 98/179/Ec (Text with Eea Relevance). Off. J. Eur. Union 2021, L 180, 84. [Google Scholar]
- Wu, Q.; Zhu, Q.; Liu, Y.; Shabbir, M.A.B.; Sattar, A.; Peng, D.; Tao, Y.; Chen, D.; Wang, Y.; Yuan, Z. A Microbiological Inhibition Method for the Rapid, Broad-Spectrum, and High-Throughput Screening of 34 Antibiotic Residues in Milk. J. Dairy Sci. 2019, 102, 10825–10837. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, X.; Dun, X.; Shabbir, M.A.B.; Peng, D.; Yuan, Z.; Wang, Y. The Screening and Identification of Six Commonly Used Antibiotics in Swine Kidney by a Microbiological Inhibition Method. Microchem. J. 2021, 161, 105796. [Google Scholar] [CrossRef]
- Gaudin, V.; Juhel-Gaugain, M.; Morétain, J.-P.; Sanders, P. Afnor Validation of Premi®Test, a Microbiological-Based Screening Tube-Test for the Detection of Antimicrobial Residues in Animal Muscle Tissue. Food Addit. Contam. Part A 2008, 25, 1451–1464. [Google Scholar] [CrossRef] [PubMed]
- Communities, Commission of the European. Veterinary Drug Residues Residues in Food Producing Animals and Their Products: Reference Materials and Methods, 2nd ed.; Heitzman, R.J., Ed.; Blackwell Scientific Publications: Oxford, UK, 1994. [Google Scholar]
- Schneider, M.J.; Lehotay, S.J. A Comparison of the Fast, Premi® and Kis™ Tests for Screening Antibiotic Residues in Beef Kidney Juice and Serum. Anal. Bioanal. Chem. 2008, 390, 1775–1779. [Google Scholar] [CrossRef]
- Cháfer-Pericás, C.; Maquieira, Á.; Puchades, R. Fast Screening Methods to Detect Antibiotic Residues in Food Samples. TrAC Trends Anal. Chem. 2010, 29, 1038–1049. [Google Scholar] [CrossRef]
- Gaudin, V.; Hedou, C.; Verdon, E. Validation of a Wide-Spectrum Microbiological Tube Test, the Explorer® Test, for the Detection of Antimicrobials in Muscle from Different Animal Species. Food Addit. Contam. 2009, 26, 1162–1171. [Google Scholar] [CrossRef][Green Version]
- CHR Hansen. Brt Mrl Screening Test. Available online: https://www.chr-hansen.com/en/food-cultures-and-enzymes/test-and-equipment/cards/product-cards/brt-mrl-screening-test (accessed on 1 June 2025).
- BTR AiM. Brt Detection Sensitivities. Available online: https://www.aim-bayern.de/html/e_km_validierungs_zertifikat.html (accessed on 1 June 2025).
- Brown, A. Cmt—Copan Milk Test. Available online: https://silo.tips/download/cmt-copan-milk-test# (accessed on 1 June 2025).
- DSM. Delvotest® the Gold Standard for Detecting Antibiotics in Milk. Available online: https://www.dsm.com/food-specialties/en_US/products/dairy/delvotest.html (accessed on 1 June 2025).
- Hennart, S.L.A.; Faragher, J. Validation of the Delvotest Sp Nt Da. J. AOAC Int. 2012, 95, 252–260. [Google Scholar] [CrossRef]
- Zeulab. Residuos Antibióticos Sector Lácteo. Available online: https://www.zeulab.com/kit-para-deteccion-de-antibioticos-en-leche-sector-lacteo/ (accessed on 1 June 2025).
- Charm Sciences. Charm Blue Yellow Ii Test. Available online: https://www.charm.com/products/test-and-kits/antibiotic-tests/inhibition-tests/charm-blue-yellow-ii-test/ (accessed on 15 December 2021).
- Stead, S.; Sharman, M.; Tarbin, J.A.; Gibson, E.; Richmond, S.; Stark, J.; Geijp, E. Meeting Maximum Residue Limits: An Improved Screening Technique for the Rapid Detection of Antimicrobial Residues in Animal Food Products. Food Addit. Contam. 2004, 21, 216–221. [Google Scholar] [CrossRef]
- R-Biopharm. Premi ® Test Tips and Tricks. Available online: https://food.r-biopharm.com/analytes/residues/ (accessed on 1 June 2025).
- Zeulab. Residuos Antibióticos Otros Sectores. Available online: https://www.zeulab.com/residuos-antibioticos-otros-sectores/ (accessed on 1 June 2025).
- Charm Sciences. Charm Kidney Inhibition Swab Test (Kis). Available online: https://www.charm.com/products/test-and-kits/antibiotic-tests/inhibition-tests/charm-kidney-inhibition-swab-test/ (accessed on 1 June 2025).
- Gutiérrez, P.M. Diseño Y Evaluación De Test Rápidos Para La Detección De Antibióticos Y Sulfamidas En Carne. Ph.D. Dissertation, University of Zaragoza, Aragon, Spain, 2013. [Google Scholar]
- Althaus, R.; Torres, A.; Peris, C.; Beltran, M.C.; Fernandez, N.; Molina, M.P. Accuracy of Brt and Delvotest Microbial Inhibition Tests as Affected by Composition of Ewe’s Milk. J. Food Prot. 2003, 66, 473–478. [Google Scholar] [CrossRef]
- Beltrán, M.C.; Berruga, M.I.; Molina, A.; Althaus, R.L.; Molina, M.P. Performance of Current Microbial Tests for Screening Antibiotics in Sheep and Goat Milk. Int. Dairy J. 2015, 41, 13–15. [Google Scholar] [CrossRef]
- Molina, M.P.; Althaus, R.; Molina, A.; Fernández, N. Antimicrobial Agent Detection in Ewes’ Milk by the Microbial Inhibitor Test Brilliant Black Reduction Test-Brt Aİm®. Int. Dairy J. 2003, 13, 821–826. [Google Scholar] [CrossRef]
- Tumini, M.; Nagel, O.G.; Althaus, R.L. Five-Assay Microbiological System for the Screening of Antibiotic Residues. Rev. Argent. Microbiol. 2019, 51, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Nouws, J.F.M.; Loeffen, G.; Schouten, J.; Van Egmond, H.; Keukens, H.; Stegeman, H. Testing of Raw Milk for Tetracycline Residues. J. Dairy Sci. 1998, 81, 2341–2345. [Google Scholar] [CrossRef]
- FAO (Food and Agricultures of the United Nations). Global Food Losses and Food Waste—Extent, Causes and Prevention; FAO: Rome, Italy, 2011. [Google Scholar]
- Carrillo-Nieves, D.; Alanís, M.J.R.; De La Cruz Quiroz, R.; Ruiz, H.A.; Iqbal, H.M.N.; Parra-Saldívar, R. Current Status and Future Trends of Bioethanol Production from Agro-Industrial Wastes in Mexico. Renew. Sust. Energ. 2019, 102, 63–74. [Google Scholar] [CrossRef]
- Ibenegbu, C.C.; Leak, D.J. Simultaneous Saccharification and Ethanologenic Fermentation (SSF) of Waste Bread by an Amylolytic Parageobacillus thermoglucosidasius Strain TM333. Microb. Cell Factories 2022, 21, 251. [Google Scholar] [CrossRef]
- Yukesh Kannah, R.; Merrylin, J.; Devi, T.P.; Kavitha, S.; Sivashanmugam, P.; Kumar, G.; Banu, J.R. Food Waste Valorization: Biofuels and Value Added Product Recovery. Bioresour. Technol. 2020, 11, 100524. [Google Scholar] [CrossRef]
- Ahmad, W.; Tayyab, M.; Aftab, M.N.; Hashmi, A.S.; Ahmad, M.D.; Firyal, S.; Wasim, M.; Awan, A.R. Optimization of Conditions for the Higher Level Production of Protease: Characterization of Protease from Geobacillus Sbs-4s. Waste Biomass Valori. 2020, 11, 6613–6623. [Google Scholar] [CrossRef]
- Verma, D.; Anand, A.; Satyanarayana, T. Thermostable and Alkalistable Endoxylanase of the Extremely Thermophilic Bacterium Geobacillus thermodenitrificans TSAA1: Cloning, Expression, Characteristics and Its Applicability in Generating Xylooligosaccharides and Fermentable Sugars. Appl. Biochem. Biotechnol. 2013, 170, 119–130. [Google Scholar] [CrossRef]
- Nagarajan, S.; Ramasamy, B.; Natarajan, H. Bioconversion of Chicken Feather Wastes into Value Added Bioactive Peptide by Geobacillus thermodenitrificans PS41 Strain. Process Biochem. 2023, 133, 49–58. [Google Scholar] [CrossRef]
- Kunasundari, B.; Zulkeple, M.F.; Teoh, Y.P. Screening for Direct Production of Lactic Acid from Rice Starch Waste by Geobacillus stearothermophilus. MATEC Web Conf. 2017, 97, 01049. [Google Scholar] [CrossRef]
- Bashir, Z.; Sheng, L.; Anil, A.; Lali, A.; Minton, N.P.; Zhang, Y. Engineering Geobacillus thermoglucosidasius for Direct Utilisation of Holocellulose from Wheat Straw. Biotechnol. Biofuels 2019, 12, 199. [Google Scholar] [CrossRef] [PubMed]
- Raita, M.; Ibenegbu, C.; Champreda, V.; Leak, D.J. Production of Ethanol by Thermophilic Oligosaccharide Utilising Geobacillus thermoglucosidasius TM242 Using Palm Kernel Cake as a Renewable Feedstock. Biomass Bioenerg. 2016, 95, 45–54. [Google Scholar] [CrossRef]
- Bibra, M.; Kunreddy, V.; Sani, R. Thermostable Xylanase Production by Geobacillus sp. Strain DUSELR13, and Its Application in Ethanol Production with Lignocellulosic Biomass. Microorganisms 2018, 6, 93. [Google Scholar] [CrossRef]
- Cripps, R.E.; Eley, K.; Leak, D.J.; Rudd, B.; Taylor, M.; Todd, M.; Boakes, S.; Martin, S.; Atkinson, T. Metabolic Engineering of Geobacillus thermoglucosidasius for High Yield Ethanol Production. Metab. Eng. 2009, 11, 398–408. [Google Scholar] [CrossRef]
- Abdel-Banat, B.M.A.; Hoshida, H.; Ano, A.; Nonklang, S.; Akada, R. High-Temperature Fermentation: How Can Processes for Ethanol Production at High Temperatures Become Superior to the Traditional Process Using Mesophilic Yeast? Appl. Microbiol. Biotechnol. 2010, 85, 861–867. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, J.; Han, X.; Meng, X.; Li, M.; Wang, J.; Xue, H.; Yang, Y.; Xu, P.; Tao, F. Eliminating Host-Guest Incompatibility Via Enzyme Mining Enables the High-Temperature Production of N-Acetylglucosamine. iScience 2023, 26, 105774. [Google Scholar] [CrossRef]
- Rai, R.; Bibra, M.; Chadha, B.S.; Sani, R.K. Enhanced Hydrolysis of Lignocellulosic Biomass with Doping of a Highly Thermostable Recombinant Laccase. Int. J. Biol. Macromol. 2019, 137, 232–237. [Google Scholar] [CrossRef]
- Dror, A.; Kanteev, M.; Kagan, I.; Gihaz, S.; Shahar, A.; Fishman, A. Structural Insights into Methanol-Stable Variants of Lipase T6 from Geobacillus stearothermophilus. Appl. Microbiol. Biotechnol. 2015, 99, 9449–9461. [Google Scholar] [CrossRef]
- Christopher, L.P.; Zambare, V.P.; Zambare, A.; Kumar, H.; Malek, L. A Thermo-Alkaline Lipase from a New Thermophile Geobacillus thermodenitrificans AV-5 with Potential Application in Biodiesel Production. J. Chem. Technol. Biotechnol. 2015, 90, 2007–2016. [Google Scholar] [CrossRef]
- Samoylova, Y.V.; Piligaev, A.V.; Sorokina, K.N.; Rozanov, A.S.; Peltek, S.E.; Novikov, A.A.; Almyasheva, N.R.; Parmon, V.N. Application of the Immobilized Bacterial Recombinant Lipase from Geobacillus stearothermophilus G3 for the Production of Fatty Acid Methyl Esters. Catal. Ind. 2016, 8, 187–193. [Google Scholar] [CrossRef]
- Correa, D.F.; Beyer, H.L.; Fargione, J.E.; Hill, J.D.; Possingham, H.P.; Thomas-Hall, S.R.; Peer, M. Schenk. Towards the Implementation of Sustainable Biofuel Production Systems. Renew. Sustain. Energy Rev. 2019, 107, 250–263. [Google Scholar] [CrossRef]
- Ayodele, B.V.; Alsaffar, M.A.; Mustapa, S.I. An Overview of Integration Opportunities for Sustainable Bioethanol Production from First- and Second-Generation Sugar-Based Feedstocks. J. Clean. Prod. 2020, 245, 118857. [Google Scholar] [CrossRef]
- Sharma, P.; Gaur, V.K.; Sirohi, R.; Varjani, S.; Kim, S.H.; Wong, J.W.C. Sustainable Processing of Food Waste for Production of Bio-Based Products for Circular Bioeconomy. Bioresour. Technol. 2021, 325, 124684. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, K.; Irbis, C.; Takada, J.; Matsuura, A. An Ability of Isolated Strains to Efficiently Cooperate in Ethanolic Fermentation of Agricultural Plant Refuse under Initially Aerobic Thermophilic Conditions: Oxygen Deletion Process Appended to Consolidated Bioprocessing (CBP). Bioresour. Technol. 2008, 99, 1768–1775. [Google Scholar] [CrossRef]
- Jiang, Y.; Xin, F.; Lu, J.; Dong, W.; Zhang, W.; Zhang, M.; Wu, H.; Ma, J.; Jiang, M. State of the Art Review of Biofuels Production from Lignocellulose by Thermophilic Bacteria. Bioresour. Technol. 2017, 245, 1498–1506. [Google Scholar] [CrossRef]
- Paredes-Barrada, M.; Kopsiaftis, P.; Claassens, N.J.; van Kranenburg, R. Parageobacillus thermoglucosidasius as an Emerging Thermophilic Cell Factory. Metab. Eng. 2024, 83, 39–51. [Google Scholar] [CrossRef]
- Bibra, M.; Rathinam, N.K.; Johnson, G.R.; Sani, R.K. Single Pot Biovalorization of Food Waste to Ethanol by Geobacillus and Thermoanaerobacter spp. Renew. Energy 2020, 155, 1032–1041. [Google Scholar]
- Van Zyl, L.J.; Taylor, M.P.; Eley, K.; Tuffin, M.; Cowan, D.A. Engineering Pyruvate Decarboxylase-Mediated Ethanol Production in the Thermophilic Host Geobacillus thermoglucosidasius. Appl. Microbiol. Biotechnol. 2014, 98, 1247–1259. [Google Scholar] [CrossRef]
- Olson, D.G.; Sparling, R.; Lynd, L.R. Ethanol Production by Engineered Thermophiles. Curr. Opin. Biotechnol. 2015, 33, 130–141. [Google Scholar] [CrossRef]
- Taylor, M.P.; Eley, K.L.; Martin, S.; Tuffin, M.I.; Burton, S.G.; Cowan, D.A. Thermophilic Ethanologenesis: Future Prospects for Second-Generation Bioethanol Production. Trends Biotechnol. 2009, 27, 398–405. [Google Scholar] [CrossRef]
- Bartosiak-Jentys, J.; Hussein, A.H.; Lewis, C.J.; Leak, D.J. Modular System for Assessment of Glycosyl Hydrolase Secretion in Geobacillus thermoglucosidasius. Microbiol. 2013, 159, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Kovács, K.; Winzer, K.; Zhang, Y.; Minton, N.P. Development and Implementation of Rapid Metabolic Engineering Tools for Chemical and Fuel Production in Geobacillus thermoglucosidasius NCIMB 11955. Biotechnol Biofuels 2017, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, A.; Cripps, R.E.; Eley, K. Sporulation-deficient Thermophilic Microorganisms for the Production of Ethanol. US Patent number US8486687B2, 8 September 2011. [Google Scholar]
- Atkinson, A.; Cripps, R.E.; Eley, K.; Rudd, B.; Todd, M. Thermophilic Micro-Organisms for Ethanol Production. US Patent number US8852906, 7 October 2014. [Google Scholar]
- Ortenzi, M.V. Strain Improvement of Parageobacillus thermoglucosidasius—Continuous Mutagenesis and Selection to Elicit Complex Phenotypes. Ph.D. Dissertation, University of Bath, Bath, UK, 2021. [Google Scholar]
- Calverley, J.; Ibenegbu, C.; Hussein-Sheik, A.; Bandulasena, H.C.H.; Leak, D.J. Ethanologenic Fermentation by Parageobacillus thermoglucosidasius with Continuous Hot Microbubble Gas-Stripping. Microb. Cell Fact. 2024, 23, 165. [Google Scholar] [CrossRef] [PubMed]
- Singhvi, M.; Maharjan, A.; Thapa, A.; Jun, H.-B.; Kim, B.S. Nanoparticle-Associated Single Step Hydrogen Fermentation for the Conversion of Starch Potato Waste Biomass by Thermophilic Parageobacillus thermoglucosidasius. Bioresour. Technol. 2021, 337, 125490. [Google Scholar] [CrossRef]
- Doménech, P.; Pogrebnyakov, I.; Jensen, S.I.; Driessen, J.; Riisager, A.; Nielsen, A.T. Metabolic Engineering of Parageobacillus thermoglucosidasius for Thermophilic Production of 1-Butanol. AMB Express 2025, 15, 75. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Wang, W.; Pang, S.; Yao, Y.; Yuan, F.; Wang, H.; Xu, Z.; Pan, G.; Liu, Z.; et al. Dynamic Control Strategy to Produce Riboflavin with Lignocellulose Hydrolysate in the Thermophile Geobacillus thermoglucosidasius. ACS Synth. Biol. 2022, 11, 2163–2174. [Google Scholar] [CrossRef]
- Averianova, L.A.; Balabanova, L.A.; Son, O.M.; Podvolotskaya, A.B.; Tekutyeva, L.A. Production of Vitamin B2 (Riboflavin) by Microorganisms: An Overview. Front. Bioeng. Biotechnol. 2020, 8, 570828. [Google Scholar] [CrossRef]
- Kunasundari, B.; Naresh, S.; Safie, M.F.M. Optimization of Lactic Acid Production from Glucose Using Geobacillus stearothermophilus Strain 15. AIP Conf. Proc. 2017, 1885, 020181. [Google Scholar] [CrossRef]
- Smerilli, M.; Neureiter, M.; Wurz, S.; Haas, C.; Frühauf, S.; Fuchs, W. Direct Fermentation of Potato Starch and Potato Residues to Lactic Acid by Geobacillus stearothermophilus under Non-Sterile Conditions. J. Chem. Technol. Biotechnol. 2015, 90, 648–657. [Google Scholar] [CrossRef]
- Liu, J.; Han, X.; Tao, F.; Xu, P. Metabolic Engineering of Geobacillus thermoglucosidasius for Polymer-Grade Lactic Acid Production at High Temperature. Bioresour. Technol. 2024, 393, 130164. [Google Scholar] [CrossRef]
- Styles, M.Q.; Nesbitt, E.A.; Hoffmann, T.D.; Queen, J.; Ortenzi, M.V.; Leak, D.J. The Heterologous Production of Terpenes by the Thermophile Parageobacillus thermoglucosidasius in a Consolidated Bioprocess Using Waste Bread. Metab. Eng. 2021, 65, 146–155. [Google Scholar] [CrossRef]
- Martin, V.J.J.; Pitera, D.J.; Withers, S.T.; Newman, J.D.; Keasling, J.D. Engineering a Mevalonate Pathway in Escherichia Coli for Production of Terpenoids. Nat. Biotechnol. 2003, 21, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.; Petrides, D. Production of Farnesene (a Terpene) via Fermentation-Process Modeling and Techno-Economic Assessment (Tea) Using Superpro Designer; Intelligen Inc.: Tucson, AZ, USA, 2020. [Google Scholar] [CrossRef]
- Siddharthan, N.; Balagurunathan, R.; Raguvaran, K.; Ragavendran, C.; Khan, S.U.; Jannat, S.; Ullah, I.; Kamaraj, C.; Maheswaran, R.; Hemalatha, N.; et al. Valorization of Chick Feather Wastes by Geobacillus thermodenitrificans PS41 to Enhance the Growth of Vigna Unguiculata Plant and Cyprinus Carpio Fish. Arch. Microbiol. 2023, 205, 100. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Wang, X.; Cong, C.; Li, J.; Xu, Y.; Li, X.; Hou, F.; Wu, Y.; Wang, L. Effect of Inoculating Microorganisms in Chicken Manure Composting with Maize Straw. Bioresour. Technol. 2020, 301, 122730. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Yang, B.; Jahng, D. Spent Coffee Ground as a New Bulking Agent for Accelerated Biodrying of Dewatered Sludge. Water Res. 2018, 138, 250–263. [Google Scholar] [CrossRef]
- Sarkar, S.; Banerjee, R.; Chanda, S.; Das, P.; Ganguly, S.; Pal, S. Effectiveness of Inoculation with Isolated Geobacillus Strains in the Thermophilic Stage of Vegetable Waste Composting. Bioresour. Technol. 2010, 101, 2892–2895. [Google Scholar] [CrossRef]
- Xu, J.; Lu, Y.; Shan, G.; He, X.-S.; Huang, J.; Li, Q. Inoculation with Compost-Born Thermophilic Complex Microbial Consortium Induced Organic Matters Degradation While Reduced Nitrogen Loss During Co-Composting of Dairy Manure and Sugarcane Leaves. Waste Biomass Valoriz. 2019, 10, 2467–2477. [Google Scholar] [CrossRef]
- Papale, M.; Romano, I.; Finore, I.; Giudice, A.L.; Piccolo, A.; Cangemi, S.; Di Meo, V.; Nicolaus, B.; Poli, A. Prokaryotic Diversity of the Composting Thermophilic Phase: The Case of Ground Coffee Compost. Micoorganisms 2021, 9, 218. [Google Scholar] [CrossRef]
- Takaku, H.; Kodaira, S.; Kimoto, A.; Nashimoto, M.; Takagi, M. Microbial Communities in the Garbage Composting with Rice Hull as an Amendment Revealed by Culture-Dependent and -Independent Approaches. J. Biosci. Bioeng. 2006, 101, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, G.; Bhalla, A.; Adhikari, A.; Bischoff, K.M.; Hughes, S.R.; Christopher, L.P.; Sani, R.K. Characterization of Thermostable Cellulases Produced by Bacillus and Geobacillus Strains. Bioresour. Technol. 2010, 101, 8798–8806. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Wang, N.; Yang, N.; Chen, X.; Liu, Q.; Wang, Z.; Shi, J.; Liu, L. Multivariate Insights into the Effects of Inoculating Thermophilic Aerobic Bacteria on the Biodegradation of Food Waste: Process Properties, Organic Degradation and Bacterial Communities. Environ. Technol. Innov. 2023, 29, 102968. [Google Scholar] [CrossRef]
- Vavitsas, K.; Glekas, P.D.; Hatzinikolaou, D.G. Synthetic Biology of Thermophiles: Taking Bioengineering to the Extremes? Appl. Microbiol. 2022, 2, 165–174. [Google Scholar] [CrossRef]
- Pavlostathis, S.G.; Marchant, R.; Banat, I.M.; Ternan, N.G.; McMullan, G. High Growth Rate and Substrate Exhaustion Results in Rapid Cell Death and Lysis in the Thermophilic Bacterium Geobacillus thermoleovorans. Biotechnol. Bioeng. 2006, 95, 84–95. [Google Scholar] [CrossRef]
- Holland, A. Optimisation of Feedstock Utilisation by Geobacillus thermoglucosidasius. Ph.D. Dissertation, University of Bath, Bath, UK, 2017. [Google Scholar]
- Zhou, J.; Wu, K.; Rao, C.V. Evolutionary Engineering of Geobacillus thermoglucosidasius for Improved Ethanol Production. Biotechnol. Bioeng. 2016, 113, 2156–2167. [Google Scholar] [CrossRef]
- Chen, G.; Kumar, A.; Wyman, T.H.; Moran, C.P., Jr. Spo0a-Dependent Activation of an Extended -10 Region Promoter in Bacillus subtilis. J. Bacteriol. 2006, 188, 1411–1418. [Google Scholar] [CrossRef][Green Version]
- Fujita, M.; González-Pastor, J.E.; Losick, R. High- and Low-Threshold Genes in the Spo0a Regulon of Bacillus subtilis. J. Bacteriol. 2005, 187, 1357–1368. [Google Scholar] [CrossRef]
- González-Pastor, J.E. Cannibalism: A Social Behavior in Sporulating Bacillus subtilis. FEMS Microbiol. Rev. 2011, 35, 415–424. [Google Scholar] [CrossRef]
- González-Pastor, J.E.; Hobbs, E.C.; Losick, R. Cannibalism by Sporulating Bacteria. Science 2003, 301, 510–513. [Google Scholar] [CrossRef]
- Ellermeier, C.D.; Hobbs, E.C.; Gonzalez-Pastor, J.E.; Losick, R. A Three-Protein Signaling Pathway Governing Immunity to a Bacterial Cannibalism Toxin. Cell 2006, 124, 549–559. [Google Scholar] [CrossRef]
- Kunst, F.; Ogasawara, N.; Moszer, I.; Albertini, A.M.; Alloni, G.; Azevedo, V.; Bertero, M.G.; Bessières, P.; Bolotin, A.; Borchert, S.; et al. The Complete Genome Sequence of the Gram-Positive Bacterium Bacillus subtilis. Nature 1997, 390, 249–256. [Google Scholar] [CrossRef]
- Smith, T.J.; Blackman, S.A.; Foster, S.J. Autolysins of Bacillus subtilis: Multiple Enzymes with Multiple Functions. Microbiology 2000, 146 Pt 2, 249–262. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Zhao, R.; Jin, T.; Zhang, X.; Chen, X. Deleting Multiple Lytic Genes Enhances Biomass Yield and Production of Recombinant Proteins by Bacillus subtilis. Microb. Cell Fact. 2014, 13, 129. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Enzymes. Available online: https://ec.europa.eu/food/safety/food_improvement_agents/enzymes_en (accessed on 1 June 2025).
- Guerrand, D. Chapter 26—Economics of Food and Feed Enzymes: Status and Prospectives. In Enzymes in Human and Animal Nutrition; Nunes, C.S., Kumar, V., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 487–514. [Google Scholar]
- Sutay Kocabaş, D.; Grumet, R. Evolving Regulatory Policies Regarding Food Enzymes Produced by Recombinant Microorganisms. GM Crops Food 2019, 10, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Atalah, J.; Cáceres-Moreno, P.; Espina, G.; Blamey, J.M. Thermophiles and the Applications of Their Enzymes as New Biocatalysts. Bioresour. Technol. 2019, 280, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-H.; Zhang, K.-C.; Tao, W.-Y.; Wang, D.; Li, S. Geobacillus Strains That Have Potential Value in Microbial Enhanced Oil Recovery. Appl. Microbiol. Biotechnol. 2019, 103, 8339–8350. [Google Scholar] [CrossRef]
- Vieille, C.; Zeikus, G.J. Hyperthermophilic Enzymes: Sources, Uses, and Molecular Mechanisms for Thermostability. Microbiol. Mol. Biol. Rev. 2001, 65, 1–43. [Google Scholar] [CrossRef]
- Rigoldi, F.; Donini, S.; Redaelli, A.; Parisini, E.; Gautieri, A. Review: Engineering of Thermostable Enzymes for Industrial Applications. APL Bioeng. 2018, 2, 011501. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Gras Notice No. 975: Maltogenic Alpha-Amylase Enzyme Preparation Produced by Bacillus licheniformis Carrying the Gene Coding for Maltogenic Alpha-Amylase from Geobacillus stearothermophilus; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2025. [Google Scholar]
- Novozymes. Novamyl®: A Maltogenic A-Amylase with Excellent Anti-Staling Properties; Novozymes: Bagsvaerd, Denmark, 2022. [Google Scholar]
- Zhu, M.; Zhai, W.; Song, R.; Lin, L.; Wei, W.; Wei, D. Enhanced Thermostability of Geobacillus stearothermophilus A-Amylase by Rational Design of Disulfide Bond and Application in Corn Starch Liquefaction and Bread Quality Improvement. J. Agric. Food Chem. 2023, 71, 18928–18942. [Google Scholar] [CrossRef]
- Nisha, M.; Satyanarayana, T. Characterization and Multiple Applications of a Highly Thermostable and Ca2+-Independent Amylopullulanase of the Extreme Thermophile Geobacillus thermoleovorans. Appl. Biochem. Biotechnol. 2014, 174, 2594–2615. [Google Scholar] [CrossRef]
- Cakmak, U.; Ertunga, N.S. Gene Cloning, Expression, Immobilization and Characterization of Endo-Xylanase from Geobacillus sp. TF16 and Investigation of Its Industrial Applications. J. Mol. Catal. B Enzym. 2016, 133, S288–S298. [Google Scholar] [CrossRef]
- Algan, M.; Sürmeli, Y.; Şanlı-Mohamed, G. A Novel Thermostable Xylanase from Geobacillus vulcani GS90: Production, Biochemical Characterization, and Its Comparative Application in Fruit Juice Enrichment. J. Food Biochem. 2021, 45, e13716. [Google Scholar] [CrossRef] [PubMed]
- Sari, B.; Faiz, O.; Genc, B.; Sisecioglu, M.; Adiguzel, A.; Adiguzel, G. New Xylanolytic Enzyme from Geobacillus galactosidasius BS61 from a Geothermal Resource in Turkey. Int. J. Biol. Macromol. 2018, 119, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Gong, S.; Liu, Z.; Yan, Q.; Jiang, Z. High Level Expression and Biochemical Characterization of an Alkaline Serine Protease from Geobacillus stearothermophilus to Prepare Antihypertensive Whey Protein Hydrolysate. BMC Biotechnol. 2021, 21, 21. [Google Scholar] [CrossRef]
- Reddy, N.S.; Nimmagadda, A.; Rao, K.R.S.S. An Overview of the Microbial A-Amylase Family. Afr. J. Biotechnol. 2003, 2, 645–648. [Google Scholar]
- Margaryan, A.; Shahinyan, G.; Hovhannisyan, P.; Panosyan, H.; Birkeland, N.; Trchounian, A. Geobacillus and Anoxybacillus spp. From Terrestrial Geothermal Springs Worldwide: Diversity and Biotechnological Applications. Microorg. Sustain. 2018, 8, 119–166. [Google Scholar]
- Naik, B.; Kumar, V.; Goyal, S.K.; Tripathi, A.D.; Mishra, S.; Saris, P.E.J.; Kumar, A.; Rizwanuddin, S.; Kumar, V.; Rustagi, S. Pullulanase: Unleashing the Power of Enzyme with a Promising Future in the Food Industry. Front. Bioeng. Biotechnol. 2023, 11, 1139611. [Google Scholar] [CrossRef]
- Elyasi Far, B.; Ahmadi, Y.; Khosroshahi, A.Y.; Dilmaghani, A. Microbial Alpha-Amylase Production: Progress, Challenges and Perspectives. Adv. Pharm. Bull. 2020, 10, 350–358. [Google Scholar] [CrossRef]
- Al-Qodah, Z. Production and Characterization of Thermostable A-Amylase by Thermophilic Geobacillus stearothermophilus. Biotechnol. J. 2006, 1, 850–857. [Google Scholar] [CrossRef]
- Reichenberger, K.; Luz, A.; Seitl, I.; Fischer, L. Determination of the Direct Activity of the Maltogenic Amylase from Geobacillus stearothermophilus in White Bread. Food Anal. Methods 2020, 13, 496–502. [Google Scholar] [CrossRef]
- Rebholz, G.F.; Sebald, K.; Dirndorfer, S.; Dawid, C.; Hofmann, T.; Scherf, K.A. Impact of Exogenous Maltogenic A-Amylase and Maltotetraogenic Amylase on Sugar Release in Wheat Bread. Eur. Food Res. Technol. 2021, 247, 1425–1436. [Google Scholar] [CrossRef]
- Souza, P.M.D.; De Oliveira, E.P. Application of Microbial A-Amylase in Industry—A Review. Braz. J. Microbiol. 2010, 41, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Hossain, K.M.; Khan, U.; Rahman, S.M.M.; Khan, M.S. Potential Antimicrobial and Fruit Juice Clarification Activity of Amylase Enzyme from Bacillus Strains. Biotechnol. Rep. 2024, 44, e00861. [Google Scholar] [CrossRef]
- Wang, F.; Xu, H.; Wang, M.; Yu, X.; Cui, Y.; Xu, L.; Ma, A.; Ding, Z.; Huo, S.; Zou, B.; et al. Application of Immobilized Enzymes in Juice Clarification. Foods 2023, 12, 4258. [Google Scholar] [CrossRef]
- De Schepper, C.F.; Buvé, C.; Van Loey, A.M.; Courtin, C.M. A Kinetic Study on the Thermal Inactivation of Barley Malt A-Amylase and Β-Amylase During the Mashing Process. Food Res. Int. 2022, 157, 111201. [Google Scholar] [CrossRef]
- Dheeran, P.; Kumar, S.; Jaiswal, Y.K.; Adhikari, D.K. Characterization of Hyperthermostable A-Amylase from Geobacillus sp. IIPTN. Appl. Microbiol. Biotechnol. 2010, 86, 1857–1866. [Google Scholar] [CrossRef]
- Kotresh, K.R.; Neelagund, S.E.; Gurumurthy, D.M. Novel Geobacillus thermoleovorans KNG 112 Thermophilic Bacteria from Bandaru Hot Spring: A Potential Producer of Thermostable Enzymes. Asian J. Pharm. Clin. Res. 2019, 13, 134–141. [Google Scholar] [CrossRef][Green Version]
- Burhanoğlu, T.; Sürmeli, Y.; Şanlı-Mohamed, G. Identification and Characterization of Novel Thermostable A-Amylase from Geobacillus sp. GS33. Int. J. Biol. Macromol. 2020, 164, 578–585. [Google Scholar] [CrossRef]
- Abbas Bukhari, D.; Bibi, Z.; Ullah, A.; Rehman, A. Isolation, Characterization, and Cloning of Thermostable Pullulanase from Geobacillus stearothermophilus ADM-11. Saudi J. Biol. Sci. 2024, 31, 103901. [Google Scholar] [CrossRef]
- Jia, X.; Ye, X.; Chen, J.; Lin, X.; Vasseur, L.; You, M. Purification and Biochemical Characterization of a Cyclodextrin Glycosyltransferase From Geobacillus thermoglucosidans CHB1. Starch-Stärke 2018, 70, 1700016. [Google Scholar] [CrossRef]
- Sonnendecker, C.; Zimmermann, W. Domain Shuffling of Cyclodextrin Glucanotransferases for Tailored Product Specificity and Thermal Stability. FEBS Open Bio 2019, 9, 384–395. [Google Scholar] [CrossRef]
- Tao, X.; Su, L.; Wang, L.; Chen, X.; Wu, J. Improved Production of Cyclodextrin Glycosyltransferase from Bacillus stearothermophilus NO2 in Escherichia coli Via Directed Evolution. Appl. Microbiol. Biotechnol. 2020, 104, 173–185. [Google Scholar] [CrossRef]
- Butt, M.S.; Tahir-Nadeem, M.; Ahmad, Z.; Sultan, M.T. Xylanases and Their Applications in Baking Industry. Food Technol. Biotechnol. 2008, 46, 22–31. [Google Scholar]
- Xu, Z.; Li, S.; Feng, X.; Liang, J.; Xu, H. L-Arabinose Isomerase and Its Use for Biotechnological Production of Rare Sugars. Appl. Microbiol. Biotechnol. 2014, 98, 8869–8878. [Google Scholar] [CrossRef]
- Ravikumar, Y.; Ponpandian, L.N.; Zhang, G.; Yun, J.; Qi, X. Harnessing -Arabinose Isomerase for Biological Production of -Tagatose: Recent Advances and Its Applications. Trends Food Sci. Technol. 2021, 107, 16–30. [Google Scholar] [CrossRef]
- Oh, D.-K. Tagatose: Properties, Applications, and Biotechnological Processes. Appl. Microbiol. Biotechnol. 2007, 76, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Levin, G.V. Tagatose, the New Gras Sweetener and Health Product. J. Med. Food 2002, 5, 23–26. [Google Scholar] [CrossRef]
- European Commission. List of Authorisations under the Former Novel Food Regulation. Available online: https://ec.europa.eu/food/safety/novel_food/authorisations/list_authorisations_en (accessed on 1 December 2024).
- Roh, H.J.; Kim, P.; Park, Y.C.; Choi, J.H. Bioconversion of D-Galactose into D-Tagatose by Expression of L-Arabinose Isomerase. Biotechnol. Appl. Biochem. 2000, 31, 1–4. [Google Scholar] [CrossRef]
- Ryu, S.A.; Kim, C.S.; Kim, H.J.; Baek, D.H.; Oh, D.K. Continuous D-Tagatose Production by Immobilized Thermostable L-Arabinose Isomerase in a Packed-Bed Bioreactor. Biotechnol. Prog. 2003, 19, 1643–1647. [Google Scholar] [CrossRef]
- Hong, Y.-H.; Lee, D.-W.; Pyun, Y.-R.; Lee, S.H. Creation of Metal-Independent Hyperthermophilicl-Arabinose Isomerase by Homologous Recombination. J. Agric. Food Chem. 2011, 59, 12939–12947. [Google Scholar] [CrossRef]
- Kim, B.-J.; Hong, S.-H.; Shin, K.-C.; Jo, Y.-S.; Oh, D.-K. Characterization of a F280n Variant of L-Arabinose Isomerase from Geobacillus thermodenitrificans Identified as a D-Galactose Isomerase. Appl. Microbiol. Biotechnol. 2014, 98, 9271–9281. [Google Scholar] [CrossRef]
- Seo, M.-J. Characterization of an L-arabinose isomerase from Bacillus thermoglucosidasius for D-tagatose production. Biosci. Biotechnol. Biochem. 2013, 77, 385–388. [Google Scholar] [CrossRef]
- Rhimi, M.; Bejar, S. Cloning, Purification and Biochemical Characterization of Metallic-Ions Independent and Thermoactive L-Arabinose Isomerase from the Bacillus stearothermophilus US100 Strain. BBA-General. Subjects 2006, 1760, 191–199. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, J.H.; Oh, H.J.; Oh, D.K. Characterization of a Mutated Geobacillus stearothermophilus L-Arabinose Isomerase That Increases the Production Rate of D-Tagatose. J. Appl. Microbiol. 2006, 101, 213–221. [Google Scholar] [CrossRef]
- Cheng, L.; Mu, W.; Jiang, B. Thermostable L-Arabinose Isomerase from Bacillus stearothermophilus IAM 11001 for D-Tagatose Production: Gene Cloning, Purification and Characterisation. J. Sci. Food Agric. 2010, 90, 1327–1333. [Google Scholar] [CrossRef]
- Lee, D.-W.; Choe, E.-A.; Kim, S.-B.; Eom, S.-H.; Hong, Y.-H.; Lee, S.-J.; Lee, H.-S.; Lee, D.-Y.; Pyun, Y.-R. Distinct Metal Dependence for Catalytic and Structural Functions in the L-Arabinose Isomerases from the Mesophilic Bacillus halodurans and the Thermophilic Geobacillus stearothermophilus. Arch. Biochem. Biophys. 2005, 434, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.-C.; Sim, D.-H.; Seo, M.-J.; Oh, D.-K. Increased Production of Food-Grade D-Tagatose from D-Galactose by Permeabilized and Immobilized Cells of Corynebacterium Glutamicum, a Gras Host, Expressing D-Galactose Isomerase from Geobacillus thermodenitrificans. J. Agric. Food Chem. 2016, 64, 8146–8153. [Google Scholar] [CrossRef] [PubMed]
- Rhimi, M.; Aghajari, N.; Juy, M.; Chouayekh, H.; Maguin, E.; Haser, R.; Bejar, S. Rational Design of Bacillus stearothermophilus US100 L-Arabinose Isomerase: Potential Applications for D-Tagatose Production. Biochimie 2009, 91, 650–653. [Google Scholar] [CrossRef] [PubMed]
- Laksmi, F.A.; Arai, S.; Arakawa, T.; Tsurumaru, H.; Nakamura, Y.; Saksono, B.; Tokunaga, M.; Ishibashi, M. Expression and Characterization of L-Arabinose Isomerase from Geobacillus stearothermophilus for Improved Activity under Acidic Condition. Protein Expr. Purif. 2020, 175, 105692. [Google Scholar] [CrossRef]
- Moss, M.M.; Taylor, B.J.; Griffitts, J.S.; Kenealey, J.D. Simultaneous Production of D-Allulose and D-Tagatose from Lactose. Int. Dairy J. 2024, 157, 106022. [Google Scholar] [CrossRef]
- Harju, M.; Kallioinen, H.; Tossavainen, O. Lactose Hydrolysis and Other Conversions in Dairy Products: Technological Aspects. Int. Dairy J. 2012, 22, 104–109. [Google Scholar] [CrossRef]
- Neves, L.N.D.O.; De Oliveira, M.A.L. Assessment of Enzymatic Hydrolysis of Lactose in Lactose-Free Milk Production—A Comparative Study Using Capillary Zone Electrophoresis and Cryoscopy. Lebensm.-Wiss. Technol. 2021, 138, 110585. [Google Scholar] [CrossRef]
- Jensen, T.Ø.; Pogrebnyakov, I.; Falkenberg, K.B.; Redl, S.; Nielsen, A.T. Application of the Thermostable Β-Galactosidase, Bgab, from Geobacillus stearothermophilus as a Versatile Reporter under Anaerobic and Aerobic Conditions. AMB Express 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Yuan, J.; Li, D. Biological Activity of Galacto-Oligosaccharides: A Review. Front. Microbiol. 2022, 13, 993052. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Park, S.Y.; Lee, J.K.; Oh, T.K. Gene Cloning and Characterization of Thermostable Lipase from Bacillus stearothermophilus L1. Biosci. Biotechnol. Biochem. 1998, 62, 66–71. [Google Scholar] [CrossRef]
- Leow, T.C.; Rahman, R.N.Z.R.A.; Basri, M.; Salleh, A.B. A Thermoalkaliphilic Lipase of Geobacillus sp. T1. Extremophiles 2007, 11, 527–535. [Google Scholar] [CrossRef]
- Baykara, S.G.; Sürmeli, Y.; Şanlı-Mohamed, G. Purification and Biochemical Characterization of a Novel Thermostable Serine Protease from Geobacillus sp. GS53. Appl. Biochem. Biotechnol. 2021, 193, 1574–1584. [Google Scholar] [CrossRef]
- Gharib, G.; Chohan, S.M.; Rashid, N.; Akhtar, M. Heterologous Gene Expression and Characterization of Recombinant Aspartate Aminotransferase from Geobacillus thermopakistaniensis. Protein Expr. Purif. 2020, 175, 105709. [Google Scholar] [CrossRef]
- Lopes, W.; Santos, B.; Sampaio, A.L.F.; Fontão, A.P.G.A.; Nascimento, H.J.; Jurgilas, P.B.; Torres, F.A.G.; Bon, E.; Almeida, R.V.; Ferrara, M.A. Expression, Purification, and Characterization of Asparaginase Ii from Saccharomyces cerevisiae in Escherichia coli. Protein Expr. Purif. 2019, 159, 21–26. [Google Scholar] [CrossRef]
- Sajed, M.; Ahmad, N.; Rashid, N. Temperature Dependent Autocleavage and Applications of Recombinant L-Asparaginase from Thermococcus kodakarensis for Acrylamide Mitigation. 3 Biotech. 2022, 12, 129. [Google Scholar] [CrossRef]
- Medeiros Vinci, R.; Mestdagh, F.; De Meulenaer, B. Acrylamide Formation in Fried Potato Products—Present and Future, a Critical Review on Mitigation Strategies. Food Chemistry 2012, 133, 1138–1154. [Google Scholar] [CrossRef]
- Maan, A.A.; Anjum, M.A.; Khan, M.K.I.; Nazir, A.; Saeed, F.; Afzaal, M.; Aadil, R.M. Acrylamide Formation and Different Mitigation Strategies During Food Processing—A Review. Food Rev. Int. 2022, 38, 70–87. [Google Scholar] [CrossRef]
- Özdemir, F.İ.; Karaaslan, B.; Tülek, A.; Yucebilgic, G.; Yildirim, D. Immobilization of Recombinant L-Asparaginase from Geobacillus kaustophilus on Magnetic Mwcnt-Nickel Composites. Process Biochem. 2023, 127, 10–20. [Google Scholar] [CrossRef]
- Xu, F.; Oruna-Concha, M.J.; Elmore, J.S. The Use of Asparaginase to Reduce Acrylamide Levels in Cooked Food. Food Chem. 2016, 210, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, F.İ.; Karaaslan, B.; Tülek, A.; Yildirim, D. Covalent Immobilization of Recombinant L-Asparaginase from Geobacillus kaustophilus on Relizyme Supports for Mitigation of Acrylamide. Biocatal. Biotransform. 2024, 42, 426–439. [Google Scholar] [CrossRef]
- Nadeem, M.; Al-Ghamdi, M.; Khan, J. Studies on the Recombinant Production in E. coli and Characterization of Pharmaceutically Important Thermostable L-Asparaginase from Geobacillus thermodenitrificans. Pak. J. Zool. 2019, 51, 1203–1598. [Google Scholar] [CrossRef]
- Sania, A.; Muhammad, M.A.; Sajed, M.; Azim, N.; Ahmad, N.; Aslam, M.; Tang, X.-F.; Rashid, N. Structural and Functional Analyses of an L-Asparaginase from Geobacillus thermopakistaniensis. Int. J. Biol. Macromol. 2024, 263, 130438. [Google Scholar] [CrossRef]
| Commercial Test | Microorganism | Target Foods | Antibiotics Detection | References |
|---|---|---|---|---|
| BRT AiM, BR-Test, BR-AS, BR-Blue Star (AiM-Analytik in Milch Produktions-und Vertriebs GmbH, München, Germany) | G. stearothermophilus calidolactis C953 | Raw cow, sheep, and goat milk | Mainly β-lactams (penicillin and cephalosporin) at or below EU MRLs. Aminoglycosides, macrolides, sulfonamides, tetracyclines, and chloramphenicol above- EU MRLs. | [125,126] |
| Copan milk test (Copan Italia SpA, Brescia, Italy) | G. stearothermophilus calidolactis * | Raw, heat-treated, and powdered milk from cow, sheep, and goat | β-lactams and sulfonamides at or below EU MRLs. Tetracyclines, aminoglycosides, macrolides, and others above EU MRLs | [127] |
| Delvotest SP-NT and Delvotest T (DSM Food Specialties, Delf, The Netherlands) | G. stearothermophilus calidolactis * | Cow, sheep, buffalo, and goat milk and milk products | 40–65 antibiotics (β-lactams, tetracyclines, sulfonamides, macrolides, glycopeptides, aminoglycosides, and others) at or above EU MRLs | [128,129] |
| Eclipse FARM 3G (ZEU-Inmunotec SL, Zaragoza, Spain) | G. stearothermophilus calidolactis * | Raw, heat-treated, skim milk, and powdered milk from cow, sheep, goat, and buffalo | More than 50 antibiotics of 8 groups (β-lactams, tetracyclines, sulfonamides, macrolides, aminoglycosides, lincosamides, anasamycins, and sulfones) at or below EU MRLs | [130] |
| Eclipse FARM 4G (ZEU-Inmunotec SL, Zaragoza, Spain) | Raw milk | [130] | ||
| Charm Blue-Yellow II (Charm Sciences, Lawrence, MA, USA) | G. stearothermophilus calidolactis * | Raw and ultra-pasteurized cow milk. Goat and sheep milk using longer incubation times | 29 antibiotics (β-lactams, sulfonamides, aminoglycosides, and specially tetracyclines) at or below EU MRLs | [131] |
| Charm Cowside II (Charm Sciences, Lawrence, MA, USA) | G. stearothermophilus calidolactis * | Raw commingled and ultra-pasteurized cow milk. | 11 antibiotics (β-lactams, sulfonamides, tetracyclines, macrolides, and aminoglycosides) at or below US MRLs and 30 antibiotics (β-lactams, sulfonamides, tetracyclines, macrolides, and aminoglycosides) at or below EU MRLs | [131] |
| PremiTest (R-Biopharm AG, Darmstadt, Alemania) | G. stearothermophilus calidolactis * | Eggs, meat (beef, pork, chicken), fish, shrimps, feed, kidney, and liver | β-lactams, cephalosporines, macrolides, tetracyclines, sulfonamides, aminoglycosides, quinolones, amphenicols, and polypeptides for beef, pork, and poultry at or below EU MRLs. Fish, shrimps, eggs, kidney, liver, and feed matrices require customer validation | [122,132,133] |
| Explorer 2.0 (ZEULAB S.L., Zaragoza, Spain) | G. stearothermophilus * | Meat (pork, chicken, ovine, bovine, etc.), liver, kidney, eggs, feed, and blood | More than 50 antibiotics of 8 classes of antibiotics (β-lactams, tetracyclines, sulfonamides, macrolides, aminoglycosides, lincosamides, anasamycins, and sulfones) at or below EU MRLs | [124,134] |
| Charm KIS (Charm Sciences, Lawrence, MA, USA) | G. stearothermophilus * | Fresh or frozen/thawed kidney tissue and muscle tissue (bovine, porcine, caprine, poultry, and ovine). Adaptable to water, feed extracts, poultry serum, and live animal urine | 5 classes of antibiotics at or near kidney US or EU MRLs | [122,135] |
| No | G. stearothermophilus ATCC 12980 | Muscle (porcine, bovine, poultry, and fish) | β-lactams, tetracyclines, macrolides, and sulfonamides in fish, porcine, bovine, and poultry muscle, and minoglycosides and lincosamides in fish muscle at or below EU MRLs | [17] |
| No | G. stearothermophilus C953 | Meat | β-lactams, tetracyclines, aminoglycosides, and macrolides at or below EU MRLs | [18] |
| No | G. stearothermophilus C953 | Milk, eggs, and honey with previous heating treatment | β-lactams, aminoglycosides, macrolides, and lincosamides in milk and eggs at or below EU MRLs. Tetracyclines, quinolones, and sulfonamides in milk above EU MRLs | [99] |
| No | G. stearothermophilus ATCC 12980 | Milk | β-lactams, aminoglycosides (gentamicin, neomycin), macrolides (tylosin, tilmicosin), and sulfonamides at or below EU MRLs. Tetracyclines, streptomycin, dihydrostreptomycin, kanamycin, spectinomycin, erythromycin, spiramycin, sulfadimidine, and lincomycin above EU MRLs. | [118] |
| No | G. stearothermophilus ATCC 7953 | Milk | β-lactams, tetracyclines, sulfonamides, and lincosamides (penicillin G, lincomycin, tylosin, neomyxin, and gentamicin) at or below Chinese MRLs. Kanamycin, streptomycin, and enrofloxacin at concentrations higher than Chinese MRLs | [100] |
| Agri-Food Residue | % Carbohydrates and Type | Ethanol Production (g/L) | Productivity (g/L/h) | Microorganisms | References |
|---|---|---|---|---|---|
| Palm kernel cake hydrolysate (5%) | 42–57% (hexoses: mannose and glucose; trace amounts of pentoses) | 9.9 (48 h) | 0.21 | P. thermoglucosidasius TM242 | [151] |
| Wheat straw (1%) | 67–90% (cellulose, hemicellulose, lignin) | 1.8 (24 h) | 0.08 | P. thermoglucosidasius LS242 | [150] |
| 3.9 (24 h) | 0.16 | P. thermoglucosidasius BZ243 | |||
| 3.4 (24 h) | 0.14 | P. thermoglucosidasius BZ244 | |||
| Cafeteria food waste (20%) | 56% (mainly starch sugars and cellulose and hemicellulose) | 9.7 (48 h) | 0.20 | P. thermoglucosidasius ATCC 43742 | [166] |
| 18.4 (120 h) | 0.15 | P. thermoglucosidasius ATCC 43742 and T. ethanolicus ATCC 31938 | |||
| Corn stover (1%) | 79.9% (mainly cellulose and hemicellulose) | 3.7 (72 h) | 0.05 | Geobacillus sp. DUSELR13 and P. thermoglucosidasius ATCC 43742 | [152] |
| Bean curd refuse (1%) | 35% (cellulose, hemicellulose, and pectin) | 1.2 (48 h) | 0.02 | Geobacillus kpuB3 and Thermoanaerobacterium kpu04 | [163] |
| Enzyme | Strains | Food Industry Applications | References |
|---|---|---|---|
| α-amylase | G. thermodenitrificans | Reduction in staling, extension of shelf life, and/or retardation of retrogradation in cereal-based dough, rice, or pasta processing | [21] |
| G. stearothermophilus | Starch processing, baking, brewing, and production of other cereal-based beverages | [214] | |
| G. stearothermophilus | Retardation of starch retrogradation, preservation of crumb softness, and preservation of elasticity | [215] | |
| G. stearothermophilus | Starch saccharification and improvement of bread shelf life and quality | [216] | |
| Amylopullulanase | G. thermoleovorans NP33 | Starch saccharification and improvement of bread shelf life, texture, and volume | [217] |
| Xylanase | P. galactosidasius BS61, G. vulcani GS90, and Geobacillus sp. TF16 | Clarification of juices | [218,219,220] |
| Geobacillus sp. TF16 | Increase in rise rate of dough | [218] | |
| β-Galactosidase | G. kaustophilus ATCC 8005 | Production of low-lactose and lactose-free milk products | [26] |
| Metalloendopeptidase | G. stearothermophilus | Synthesis of phenylalanine (precursor of aspartame) | [31] |
| Protease | G. stearothermophilus CAU209 | Production of antihypertensive whey protein hydrolysates | [221] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvador, M.; Condón, S.; Gayán, E. Parageobacillus and Geobacillus spp.: From Food Spoilage to Beneficial Food Applications. Foods 2025, 14, 2775. https://doi.org/10.3390/foods14162775
Salvador M, Condón S, Gayán E. Parageobacillus and Geobacillus spp.: From Food Spoilage to Beneficial Food Applications. Foods. 2025; 14(16):2775. https://doi.org/10.3390/foods14162775
Chicago/Turabian StyleSalvador, Maika, Santiago Condón, and Elisa Gayán. 2025. "Parageobacillus and Geobacillus spp.: From Food Spoilage to Beneficial Food Applications" Foods 14, no. 16: 2775. https://doi.org/10.3390/foods14162775
APA StyleSalvador, M., Condón, S., & Gayán, E. (2025). Parageobacillus and Geobacillus spp.: From Food Spoilage to Beneficial Food Applications. Foods, 14(16), 2775. https://doi.org/10.3390/foods14162775







