Evaluation of GABA-Producing Fermented Whey Formulations: From Strain Selection to Raspberry-Enriched Beverages with Psychobiotic Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Whey: Processing and Physicochemical and Proximate Characterization
2.2. Bacterial Strains and Fermentation Conditions in Whey
2.3. Determination of GABA in Whey Cultures
2.4. Strain Selection and GABA-Based Evaluation for Beverage Formulation
2.5. Raspberry Powder: Processing and Physicochemical, Proximate, and Bioactive Characterization
2.6. Final Beverage Formulation and Proximate Composition
2.7. Shelf-Life Study and In Vitro Gastrointestinal Stability of Functional Fermented Beverages
2.7.1. Physicochemical Parameters
2.7.2. In Vitro Gastrointestinal Digestion
2.7.3. Microbial Viability
2.7.4. GABA Concentration
2.7.5. Total Bioactive Compounds
2.8. Sensory Evaluation of Functional Fermented Beverages
2.9. Statistical Analysis
3. Results
3.1. Physicochemical and Proximate Characterization of Whey
3.2. Fermentation of Whey by Mono-Cultures
3.2.1. Microbial Counts and Acidification
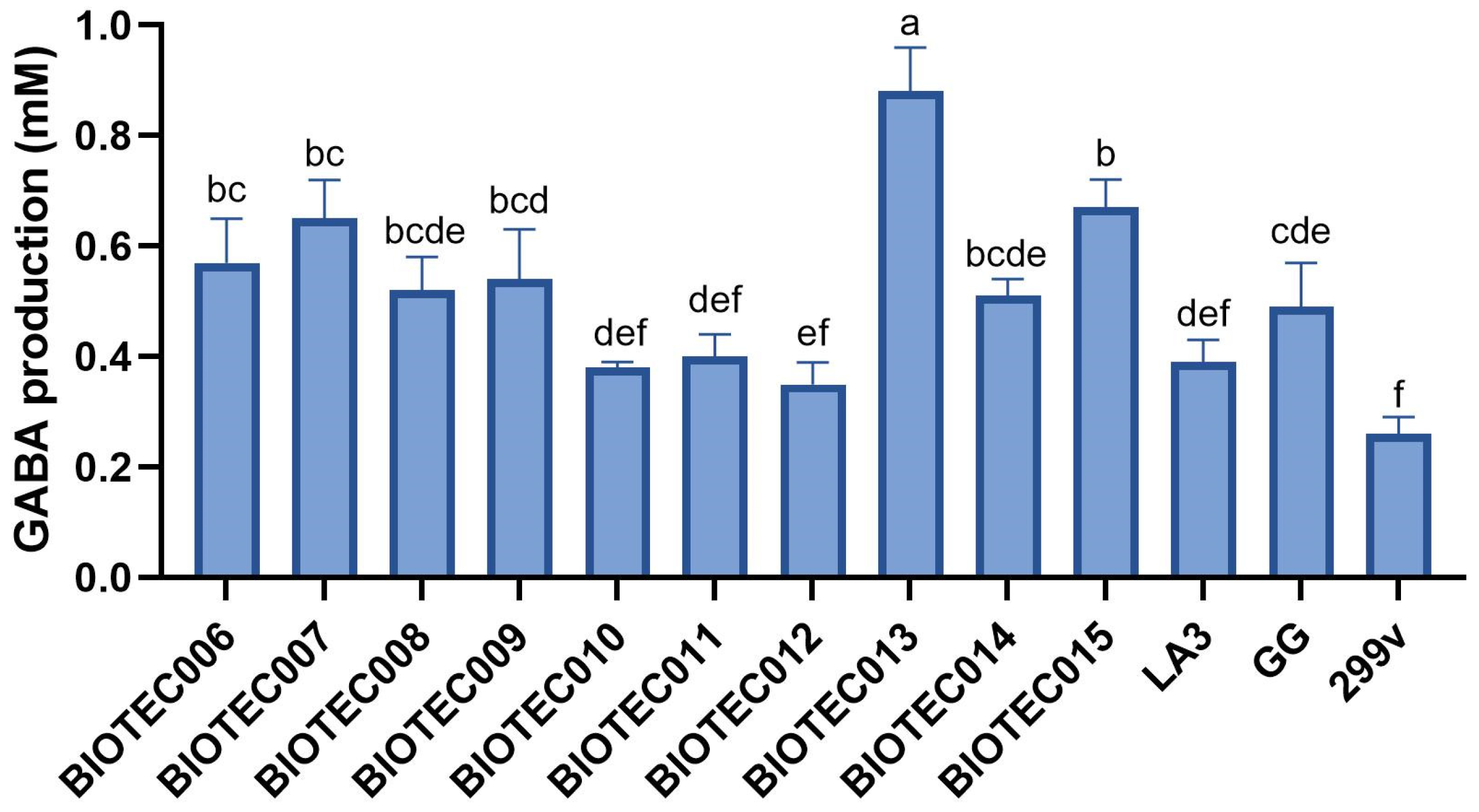
3.2.2. GABA Production by Mono-Cultures
3.3. Strain Selection and Preliminary Formulation Assessment
3.3.1. Sensory Screening of Mono and Mixed Cultures
3.3.2. GABA Production by Mixed Cultures
3.4. Characterization of Raspberry Fruit and Powder
3.5. Proximate Composition and Physicochemical Properties of Functional Fermented Beverages
3.5.1. Proximate Composition of Beverages
3.5.2. Physicochemical Properties During Storage
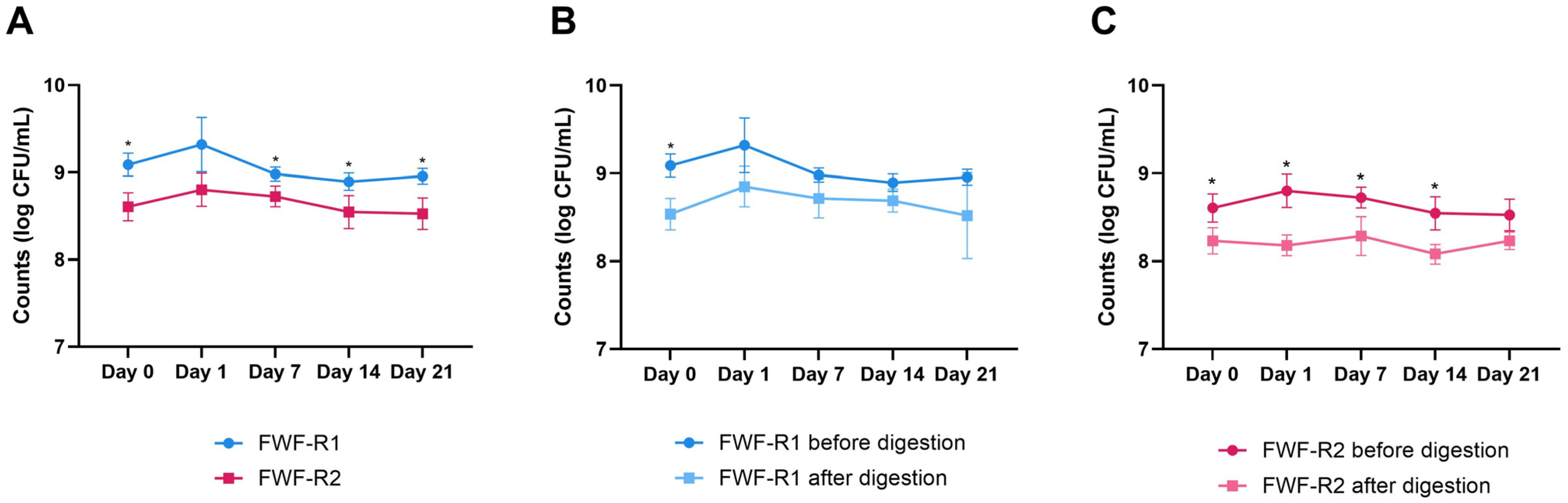
3.6. Microbial Viability During Storage and After Digestion
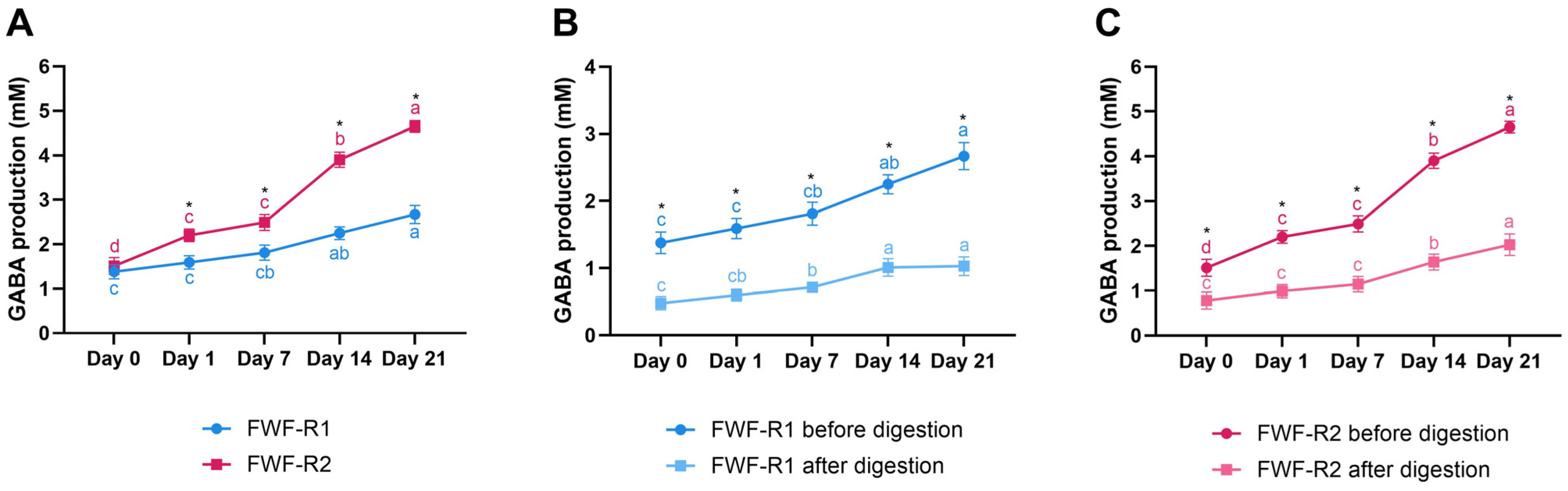
3.7. GABA Content During Storage and After Digestion
3.8. Stability of Bioactive Compounds During Storage and After Digestion
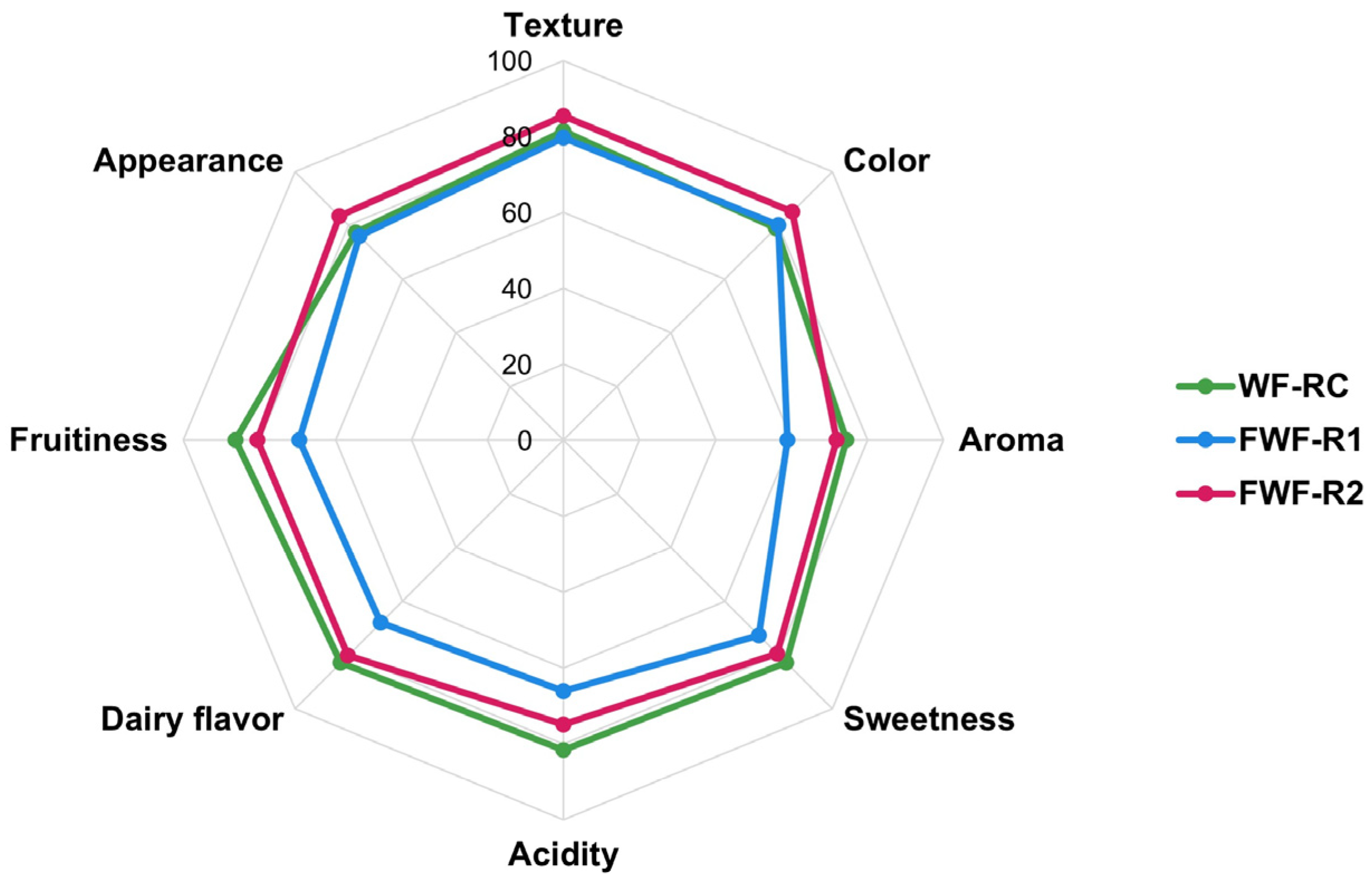
3.9. Consumer Perception and Acceptability of Functional Fermented Beverages
4. Discussion
4.1. Whey as a Fermentation Substrate
4.2. Selection and Characterization of Microbial Strains for GABA-Enriched Fermented Whey Formulations
4.3. Functional Beverage Development, Nutritional Composition, and Physicochemical Stability During Storage
4.4. Microbial Viability and Bioactive Compound Stability in Functional Beverages During Storage and After Digestion
4.5. Sensory Acceptability and Psychobiotic Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| C3G | Cyanidin-3-glucoside equivalents |
| CFU | Colony-forming units |
| CNS | Central nervous system |
| FWF-R | Fermented whey formulation with raspberry |
| GABA | Gamma-aminobutyric acid |
| GAE | Gallic acid equivalents |
| GMBA | Gut–microbiota–brain axis |
| LAB | Lactic acid bacteria |
| LM17 | M17 broth supplemented with 0.5% (w/v) lactose |
| MRS | Man–Rogosa–Sharpe broth |
| SCFA | Short-chain fatty acids |
| TAC | Total anthocyanin content |
| TPC | Total phenolic content |
| WHC | Water-holding capacity |
| YPD | Yeast extract–dextrose–peptone medium |
Appendix A
| Microorganism | Qualitative Sensory Attributes |
|---|---|
| Lactococcus lactis BIOTEC006 | Mild yogurt-like aroma with a pleasant, unsweet clean taste. |
| Lactococcus lactis BIOTEC007 | Light, fruity notes in aroma and a yogurt-like, slightly acidic taste. |
| Lactococcus lactis BIOTEC008 | Pronounced yogurt aroma and noticeable acidic flavor. |
| Kluyveromyces lactis BIOTEC009 | Evident gas formation; alcoholic aroma and dry, non-sweet flavor. |
| Kluyveromyces lactis BIOTEC010 | Gas release and a mild alcoholic flavor profile. |
| Leuconostoc pseudomesenteroides BIOTEC011 | Sweet taste with a characteristic whey-derived flavor. |
| Leuconostoc pseudomesenteroides BIOTEC012 | Slightly fruity aroma with a flavor reminiscent of soft cheese. |
| Lentilactobacillus kefiri BIOTEC013 | Sharp, highly acidic, and noticeably astringent taste. |
| Lentilactobacillus kefiri BIOTEC014 | Mild dairy aroma with a clean, neutral taste. |
| Lentilactobacillus parakefiri BIOTEC015 | Neutral-to-pleasant flavor with low sweetness perception. |
| Lactobacillus acidophilus LA3 | Fruity aroma with intense acidic flavor; slightly astringent. |
| Lacticaseibacillus rhamnosus GG | Yogurt-like aroma; pronounced acidic taste and lingering aftertaste. |
| Lactiplantibacillus plantarum 299v | Faint acidic-like smell; intense acidic flavor. |
| Culture (Mono or Mixed) 1 | Acidity | Sweetness | Texture | Overall Perception |
| BIOTEC007 | 25 | 100 | 63 | 85 |
| BIOTEC009 | 56 | 75 | 100 | 52 |
| BIOTEC012 | 56 | 70 | 75 | 48 |
| BIOTEC014 | 28 | 60 | 63 | 52 |
| LA3 | 81 | 60 | 100 | 81 |
| BIOTEC007 + BIOTEC009 | 88 | 55 | 88 | 48 |
| BIOTEC007 + BIOTEC012 | 78 | 50 | 88 | 78 |
| BIOTEC007 + BIOTEC014 | 25 | 55 | 69 | 67 |
| BIOTEC007 + LA3 | 100 | 65 | 81 | 89 |
| BIOTEC007 + BIOTEC009 + BIOTEC014 | 66 | 55 | 94 | 67 |
| BIOTEC007 + BIOTEC007 + BIOTEC012 + BIOTEC014 | 44 | 75 | 69 | 81 |
| BIOTEC007 + BIOTEC012 + LA3 | 84 | 55 | 94 | 100 |
| BIOTEC007 + BIOTEC009 + BIOTEC012 + BIOTEC014 + LA3 | 75 | 55 | 94 | 96 |
References
- Errazuriz, A.; Avello-Vega, D.; Ramirez-Mahaluf, J.P.; Torres, R.; Crossley, N.A.; Undurraga, E.A.; Jones, P.B. Prevalence of depressive disorder in the adult population of Latin America: A systematic review and meta-analysis. Lancet Reg. Health Am. 2023, 26, 100587. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. DSM-5 TM Guidebook the Essential Companion to the Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2014. [Google Scholar]
- Jiang, M.; Kang, L.; Wang, Y.-L.; Zhou, B.; Li, H.-Y.; Yan, Q.; Liu, Z.-G. Mechanisms of microbiota-gut-brain axis communication in anxiety disorders. Front. Neurosci. 2024, 18, 1501134. [Google Scholar] [CrossRef]
- Magalhães-Guedes, K.T. Psychobiotic therapy: Method to reinforce the immune system. Clin. Psychopharmacol. Neurosci. 2022, 20, 17–25. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, Y.-K. Understanding the connection between the gut–brain axis and stress/anxiety disorders. Curr. Psychiatry Rep. 2021, 23, 22. [Google Scholar] [CrossRef]
- Casertano, M.; Fogliano, V.; Ercolini, D. Psychobiotics, gut microbiota and fermented foods can help preserving mental health. Food Res. Int. 2022, 152, 110892. [Google Scholar] [CrossRef]
- Devi, P.B.; Rajapuram, D.R.; Jayamanohar, J.; Verma, M.; Kavitake, D.; Meenachi Avany, B.A.; Uma Rani, P.; Ravi, R.; Shetty, P.H. Gamma-aminobutyric acid (GABA) production by potential probiotic strains of indigenous fermented foods origin and RSM based production optimization. LWT–Food Sci. Technol. 2023, 180, 114511. [Google Scholar] [CrossRef]
- Del Toro-Barbosa, M.; Hurtado-Romero, A.; Garcia-Amezquita, L.E.; García-Cayuela, T. Psychobiotics: Mechanisms of action, evaluation methods and effectiveness in applications with food products. Nutrients 2020, 12, 3896. [Google Scholar] [CrossRef] [PubMed]
- Iorizzo, M.; Paventi, G.; Di Martino, C. Biosynthesis of gamma-aminobutyric acid (GABA) by Lactiplantibacillus plantarum in fermented food production. Curr. Issues Mol. Biol. 2024, 46, 200–220. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Tang, J.; Feng, Q.; Niu, Z.; Shen, Q.; Wang, L.; Zhou, S. Gamma-aminobutyric acid (GABA): A comprehensive review of dietary sources, enrichment technologies, processing effects, health benefits, and its applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 8852–8874. [Google Scholar] [CrossRef]
- Hurtado-Romero, A.; Del Toro-Barbosa, M.; Gradilla-Hernández, M.S.; Garcia-Amezquita, L.E.; García-Cayuela, T. Probiotic properties, prebiotic fermentability, and GABA-producing capacity of microorganisms isolated from Mexican milk kefir grains: A clustering evaluation for functional dairy food applications. Foods 2021, 10, 2275. [Google Scholar] [CrossRef]
- Alexandre, E.M.; Aguiar, N.F.; Voss, G.B.; Pintado, M.E. Properties of fermented beverages from food wastes/by-products. Beverages 2023, 9, 45. [Google Scholar] [CrossRef]
- Siddiqui, E.; Rugji, J.; Taşçı, F.; Kahraman, H.A.; Toppi, V.; Musa, L.; Di Giacinto, G.; Bahmid, N.A.; Mehdizadeh, M.; Castro-Muñoz, R. An overview of fermentation in the food industry-looking back from a new perspective. Bioresour. Bioprocess. 2023, 10, 85. [Google Scholar] [CrossRef]
- Rocha-Ibarra, J.E.; Mireles-Arriaga, A.I.; Ruiz-Nieto, J.E.; Maki-Díaz, G. Production and export of berries in Mexico’s agricultural development: A study of competitive. Agrociencia 2024, 1, 1–14. [Google Scholar] [CrossRef]
- Arellano-García, L.; Flores-Payán, V.; McCulligh, C. Cheese whey generation, management and potential for biogas production in Mexico and the State of Jalisco. Int. J. Sustain. Eng. 2024, 17, 995–1007. [Google Scholar] [CrossRef]
- Uribe-Velázquez, T.; Díaz-Vázquez, D.; Barajas-Álvarez, P.; González-López, M.E.; Gradilla-Hernández, M.S.; Garcia-Amezquita, L.E.; García-Cayuela, T. From waste to value: Mitigating the environmental impact of whey in Jalisco, Mexico. J. Clean. Prod. 2025, 501, 145334. [Google Scholar] [CrossRef]
- Golovinskaia, O.; Wang, C.-K. Review of functional and pharmacological activities of berries. Molecules 2021, 26, 3904. [Google Scholar] [CrossRef] [PubMed]
- Bandara, T.A.; Munasinghe-Arachchige, S.P.; Gamlath, C.J. Fermented whey beverages: A review of process fundamentals, recent developments and nutritional potential. Int. J. Dairy Technol. 2023, 76, 737–757. [Google Scholar] [CrossRef]
- Rosa, L.S.; Santos, M.L.; Abreu, J.P.; Rocha, R.S.; Esmerino, E.A.; Freitas, M.Q.; Teodoro, A.J. Probiotic fermented whey-milk beverages: Effect of different probiotic strains on the physicochemical characteristics, biological activity, and bioactive peptides. Food Res. Int. 2023, 164, 112396. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Q.; Tan, X.; Zhang, S.; Zeng, L.; Tang, J.; Xiang, W. Characterization of Saccharomyces cerevisiae γ-aminobutyric acid-producing and coculture with Lactobacillus plantarum for mulberry beverage brewing. J. Biosci. Bioeng. 2020, 129, 447–453. [Google Scholar] [CrossRef]
- Secretaría de Economía. Norma Oficial Mexicana NOM-155-SCFI-2012, Leche-Denominaciones, Especificaciones Fisicoquímicas, Información Comercial y Métodos de Prueba. Diario Oficial de la Federación 2012, México. Available online: https://faolex.fao.org/docs/pdf/mex174232.pdf (accessed on 1 July 2025).
- Li, T.; Rui, X.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Water distribution in tofu and application of T2 relaxation measurements in determination of tofu’s water-holding capacity. J. Agric. Food Chem. 2014, 62, 8594–8601. [Google Scholar] [CrossRef] [PubMed]
- Tsukatani, T.; Higuchi, T.; Matsumoto, K. Enzyme-based microtiter plate assay for γ-aminobutyric acid: Application to the screening of γ-aminobutyric acid-producing lactic acid bacteria. Anal. Chim. Acta 2005, 540, 293–297. [Google Scholar] [CrossRef]
- Hurtado-Romero, A.; Zepeda-Hernández, A.; Uribe-Velázquez, T.; Rosales-De la Cruz, M.F.; Raygoza-Murguía, L.V.; Garcia-Amezquita, L.E.; Garcia-Cayuela, T. Utilization of blueberry-based ingredients for formulating a synbiotic Petit Suisse cheese: Physicochemical, microbiological, sensory, and functional characterization during cold storage. LWT 2023, 183, 114955. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Hurtado-Romero, A.; Zepeda-Hernández, A.; Cárdenas-Rangel, J.; Aguilar-Márquez, R.; Garcia-Amezquita, L.E.; Carrillo-Nieves, D.; García-Cayuela, T. Frozen fermented dairy snacks with probiotics and blueberry bagasse: Stability, bioactivity, and digestive viability. Microorganisms 2025, 13, 86. [Google Scholar] [CrossRef]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990. [Google Scholar] [CrossRef]
- Wrolstad, R.; Acree, T.; Decker, E.; Penner, M.; Reid, D.; Schwartz, S.; Shoemaker, C.; Smith, D.; Sporns, P. Handbook of Food Analytical Chemistry: Pigments, Colorants, Flavors, Texture, and Bioactive Food Components; Wiley-Interscience: Hoboken, NJ, USA, 2005; ISBN 978-0-471-71817-8. [Google Scholar]
- Verma, D.K.; Patel, A.R.; Tripathy, S.; Gupta, A.K.; Singh, S.; Shah, N.; Aguilar, C.N. Processing and formulation technology of nutritional and functional food products by utilizing cheese and/or paneer whey: A critical review. J. King Saud Univ.–Sci. 2024, 36, 103508. [Google Scholar] [CrossRef]
- Zayas, J.F. Functionality of Proteins in Food, 1st ed.; Springer Science & Business Media: New York, NY, USA, 1997. [Google Scholar]
- Prazeres, A.R.; Carvalho, F.; Rivas, J. Cheese whey management: A review. J. Environ. Manage. 2012, 110, 48–68. [Google Scholar] [CrossRef] [PubMed]
- Ayed, L.; M’hir, S.; Asses, N. Sustainable whey processing techniques: Innovations in derivative and beverage production. Food Biosci. 2023, 53, 102642. [Google Scholar] [CrossRef]
- de Lima, E.D.L.C.; de Moura Fernandes, J.; Cardarelli, H.R. Optimized fermentation of goat cheese whey with Lactococcus lactis for production of antilisterial bacteriocin-like substances. LWT 2017, 84, 710–716. [Google Scholar] [CrossRef]
- Jurášková, D.; Ribeiro, S.C.; Bastos, R.; Coelho, E.; Coimbra, M.A.; Silva, C.C. Exopolysaccharide (EPS) produced by Leuconostoc mesenteroides SJC113: Characterization of functional and technological properties and application in fat-free cheese. Macromol 2024, 4, 680–696. [Google Scholar] [CrossRef]
- Pintado, A.I.; Barbosa, C.C.; Pintado, M.E.; Malcata, F.X.; Gomes, A.M. Efficient screening and enhanced exopolysaccharide production by functional lactic acid bacteria (LAB) in lactose supplemented media. Appl. Microbiol. Theory Technol. 2024, 5, 37–50. [Google Scholar] [CrossRef]
- D’Alessandro, M.; Gottardi, D.; Franceschini, S.; Braschi, G.; Siroli, L.; Nissen, L.; Patrignani, F. Impact of cheese whey enriched in lactobionic acid on the characteristics of fermented milks prepared with or without the probiotic Lacticaseibacillus rhamnosus GG. Int. Dairy J. 2025, 139, 106327. [Google Scholar] [CrossRef]
- Pires, A.; Bożek, A.; Pietruszka, H.; Szkolnicka, K.; Gomes, D.; Díaz, O.; Pereira, C. Whey cheeses containing probiotic and bioprotective cultures produced with ultrafiltrated cow’s whey. Foods 2024, 13, 1214. [Google Scholar] [CrossRef] [PubMed]
- Marcus, J.F.; DeMarsh, T.A.; Alcaine, S.D. Upcycling of whey permeate through yeast- and mold-driven fermentations under anoxic and oxic conditions. Fermentation 2021, 7, 16. [Google Scholar] [CrossRef]
- Gordillo-Andia, C.; Almirón, J.; Barreda-Del-Carpio, J.E.; Roudet, F.; Tupayachy-Quispe, D.; Vargas, M. Influence of Kluyveromyces lactis and Enterococcus faecalis on obtaining lactic acid by cheese whey fermentation. Appl. Sci. 2024, 14, 4649. [Google Scholar] [CrossRef]
- Linares, D.M.; Gómez, C.; Renes, E.; Fresno, J.M.; Tornadijo, M.E.; Ross, R.P.; Stanton, C. Lactic acid bacteria and bifidobacteria with potential to design natural biofunctional health-promoting dairy foods. Front. Microbiol. 2017, 8, 846. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Miao, K.; Niyaphorn, S.; Qu, X. Production of gamma-aminobutyric acid from lactic acid bacteria: A systematic review. Int. J. Mol. Sci. 2020, 21, 995. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, B.; Gao, L.; Ge, R.; Cui, X.; Zhou, J.; Li, Z. Gamma-aminobutyric acid (GABA) promotes characteristics of Levilactobacillus sp. LB-2. LWT 2023, 184, 115014. [Google Scholar] [CrossRef]
- Wicaksono, S.; Nuraida, L.; Faridah, D.N. Tapping the potential of lactic acid bacteria: Optimizing gamma-aminobutyric acid production for enhanced health benefits in fermented milk. Braz. J. Food Technol. 2024, 27, e2024015. [Google Scholar] [CrossRef]
- Yu, X.; Sun, Y.; Shen, X.; Li, W.; Cai, H.; Guo, S.; Sun, Z. Effect of different isolation sources of Lactococcus lactis subsp. lactis on volatile metabolites in fermented milk. Food Chem. X 2024, 21, 101224. [Google Scholar] [CrossRef]
- Guo, X.; Yu, L.; Liu, Y.; Xiao, M.; Zhang, C.; Zhao, J.; Zhai, Q. Metabolic interactions between Lactococcus lactis and commercial starter cultures enhances the quality and flavor of fermented milk. Food Res. Int. 2025, 180, 116403. [Google Scholar] [CrossRef]
- Abd El-Fattah, A.; Sakr, S.; El-Dieb, S.; Elkashef, H. Developing functional yogurt rich in bioactive peptides and gamma-aminobutyric acid related to cardiovascular health. LWT 2018, 98, 390–397. [Google Scholar] [CrossRef]
- Tan, X.; Zhang, Q.; Liu, J.; Shang, Y.; Min, Y.; Sun, X.; Tang, J. Enhanced γ-aminobutyric acid production by co-culture fermentation with Enterococcus faecium AB157 and Saccharomyces cerevisiae SC125. LWT 2024, 208, 116739. [Google Scholar] [CrossRef]
- Shukla, V.; Villarreal, M.; Padilla-Zakour, O.I. Consumer acceptance and physicochemical properties of a yogurt beverage formulated with upcycled yogurt acid whey. Beverages 2024, 10, 18. [Google Scholar] [CrossRef]
- Herrera-Ponce, A.L.; Salmeron-Ochoa, I.; Rodriguez-Figueroa, J.C.; Santellano-Estrada, E.; Garcia-Galicia, I.A.; Vargas-Bello-Pérez, E.; Alarcon-Rojo, A.D. Functional properties and consumer acceptance of whey–oat beverages under different ultrasonication times and inulin concentration. J. Food Process. Preserv. 2022, 46, e16907. [Google Scholar] [CrossRef]
- Kheto, A.; Bist, Y.; Awana, A.; Kaur, S.; Kumar, Y.; Sehrawat, R. Utilization of inulin as a functional ingredient in food: Processing, physicochemical characteristics, food applications, and future research directions. Food Chem. Adv. 2023, 3, 100443. [Google Scholar] [CrossRef]
- Zepeda-Hernández, A.; Rosales-De la Cruz, M.F.; Antunes-Ricardo, M.; Ortega-Hernández, E.; García-García, J.D.; Garcia-Amezquita, L.E.; García-Cayuela, T. Probiotic fermentation enhances the technological properties and health-promoting potential of skyr-type yogurts. Int. Dairy J. 2025, 139, 106276. [Google Scholar] [CrossRef]
- Li, K.; Wei, W.; Xu, C.; Lian, X.; Bao, J.; Yang, S.; Zhong, S. Prebiotic inulin alleviates anxiety and depression-like behavior in alcohol withdrawal mice by modulating the gut microbiota and 5-HT metabolism. Phytomedicine 2024, 135, 156181. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, W.; Jiang, Y.; Xiao, X.; Zou, Q.; Liang, J.; Liu, Z. A synbiotic formulation of Lactobacillus reuteri and inulin alleviates ASD-like behaviors in a mouse model: The mediating role of the gut–brain axis. Food Funct. 2024, 15, 387–400. [Google Scholar] [CrossRef]
- Frías-Moreno, M.N.; Parra-Quezada, R.Á.; Ruíz-Carrizales, J.; González-Aguilar, G.A.; Sepulveda, D.; Molina-Corral, F.J.; Olivas, G.I. Quality, bioactive compounds and antioxidant capacity of raspberries cultivated in northern Mexico. Int. J. Food Prop. 2021, 24, 603–614. [Google Scholar] [CrossRef]
- Vahapoglu, B.; Erskine, E.; Gultekin Subasi, B.; Capanoglu, E. Recent studies on berry bioactives and their health-promoting roles. Molecules 2021, 27, 108. [Google Scholar] [CrossRef]
- Cao, Y.; Fang, Y.; Hekmat, S. Impact of fruit powders incorporation on probiotic viability and sensory properties of yogurt. Nutr. Food Sci. 2025, 55, 248–261. [Google Scholar] [CrossRef]
- Savas, B.S.; Akan, E. Oat bran fortified raspberry probiotic dairy drinks: Physicochemical, textural, microbiologic properties, in vitro bioaccessibility of antioxidants and polyphenols. Food Biosci. 2021, 43, 101223. [Google Scholar] [CrossRef]
- León-López, A.; Pérez-Marroquín, X.A.; Campos-Lozada, G.; Campos-Montiel, R.G.; Aguirre-Álvarez, G. Characterization of whey-based fermented beverages supplemented with hydrolyzed collagen: Antioxidant activity and bioavailability. Foods 2020, 9, 1106. [Google Scholar] [CrossRef]
- Liutkevičius, A.; Speičienė, V.; Kaminskas, A.; Jablonskienė, V.; Alenčikienė, G.; Mieželienė, A.; Garmienė, G. Development of a functional whey beverage, containing calcium, vitamin D, and prebiotic dietary fiber, and its influence on human health. CyTA-J. Food 2016, 14, 309–316. [Google Scholar] [CrossRef]
- Elkot, W.F.; Elmahdy, A.; Talaat, H.; Alghamdia, O.A.; Alhag, S.K.; Al-Shahari, E.A.; Ismail, H.A. Development and characterization of a novel flavored functional fermented whey-based sports beverage fortified with Spirulina platensis. Int. J. Biol. Macromol. 2024, 258, 128999. [Google Scholar] [CrossRef]
- Hernández, T.; Vélez-Ruiz, J.F. Development, characterization, and stability of a functional beverage from whey. MOJ Food Process. Technol. 2024, 12, 140–147. [Google Scholar] [CrossRef]
- Bulatović, M.L.; Krunić, T.Ž.; Vukašinović-Sekulić, M.S.; Zarić, D.B.; Rakin, M.B. Quality attributes of a fermented whey-based beverage enriched with milk and a probiotic strain. RSC Adv. 2014, 4, 55503–55510. [Google Scholar] [CrossRef]
- Aly, E.; Darwish, A.A.; Tawfek, M. Quality characteristics of sweet whey-based fruits beverages fermented with Lactobacillus plantarum. Egypt. J. Food Sci. 2019, 47, 141–254. [Google Scholar] [CrossRef]
- Montero-Zamora, J.; Cortés-Muñoz, M.; Esquivel, P.; Mora-Villalobos, J.A.; Velázquez, C. Growth conditions and survival kinetics during storage of Lactobacillus rhamnosus GG for the design of a sustainable probiotic whey-based beverage containing Costa Rican guava fruit pulp. J. Food Sci. 2020, 85, 3478–3486. [Google Scholar] [CrossRef] [PubMed]
- Kotsaki, P.; Aspri, M.; Papademas, P. Novel whey fermented beverage enriched with a mixture of juice concentrates: Evaluation of antimicrobial, antioxidant, and angiotensin I converting enzyme inhibitory (ACE) activities before and after simulated gastrointestinal digestion. Microorganisms 2025, 13, 1490. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.N.; Tagliapietra, B.L.; do Amaral Flores, V.; dos Santos Richards, N.S.P. In vitro test to evaluate survival in the gastrointestinal tract of commercial probiotics. Curr. Res. Food Sci. 2021, 4, 320–325. [Google Scholar] [CrossRef]
- Oliveira, D.R.; Lopes, A.C.A.; Pereira, R.A.; Cardoso, P.G.; Duarte, W.F. Selection of potentially probiotic Kluyveromyces lactis for the fermentation of cheese whey–based beverage. Ann. Microbiol. 2019, 69, 1361–1372. [Google Scholar] [CrossRef]
- Martínez, F.G.; Moreno-Martín, G.; Mohamed, F.; Pescuma, M.; Madrid-Albarrán, Y.; Mozzi, F. Selenium-enriched fermented beverage with improved sensorial properties using lactic acid bacteria. J. Food Sci. Technol. 2024, 61, 2133–2144. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Shah, B.R.; Li, J.; Liang, H.; Zhan, F.; Geng, F.; Li, B. A critical review on interplay between dietary fibers and gut microbiota. Trends Food Sci. Technol. 2022, 124, 237–249. [Google Scholar] [CrossRef]
- Escobar-Ramírez, M.C.; Jaimez-Ordaz, J.; Escorza-Iglesias, V.A.; Rodríguez-Serrano, G.M.; Contreras López, E.; Ramírez-Godínez, J.; Castañeda-Ovando, A.; Morales-Estrada, A.I.; Felix-Reyes, N.; González-Olivares, L.G. Lactobacillus pentosus ABHEAU-05: An in vitro digestion resistant lactic acid bacterium isolated from a traditional fermented Mexican beverage. Rev. Argent. Microbiol. 2020, 52, 305–314. [Google Scholar] [CrossRef]
- Hinojosa-Avila, C.R.; García-Cayuela, T. Enhancing probiotic viability in beer fermentation: Selection of stress-resistant lactic acid bacteria and alternative approaches. ACS Food Sci. Technol. 2024, 4, 2575–2584. [Google Scholar] [CrossRef]
- Hussin, F.S.; Chay, S.Y.; Hussin, A.S.M.; Wan Ibadullah, W.Z.; Muhialdin, B.J.; Abd Ghani, M.S.; Saari, N. GABA enhancement by simple carbohydrates in yoghurt fermented using novel, self-cloned Lactobacillus plantarum Taj-Apis362 and metabolomics profiling. Sci. Rep. 2021, 11, 9417. [Google Scholar] [CrossRef]
- Zarei, F.; Nateghi, L.; Eshaghi, M.R.; Abadi, M.E.T. Production of gamma-aminobutyric acid (GABA) in whey protein drink during fermentation by Lactobacillus plantarum. J. Microbiol. Biotechnol. Food Sci. 2020, 9, 1087–1092. Available online: https://office2.jmbfs.org/index.php/JMBFS/article/view/4493 (accessed on 1 July 2025). [CrossRef]
- Garzón, A.G.; Van de Velde, F.; Drago, S.R. Gastrointestinal and colonic in vitro bioaccessibility of γ-aminobutyric acid (GABA) and phenolic compounds from novel fermented sorghum food. LWT 2020, 130, 109664. [Google Scholar] [CrossRef]
- Guzmán-Ortiz, F.A.; Muñoz-Llandes, C.B.; Martínez-Villaluenga, C. Time matters: Exploring the dynamics of bioactive compounds content, bioaccessibility and antioxidant activity during Lupinus angustifolius germination. Food Res. Int. 2024, 187, 114426. [Google Scholar] [CrossRef]
- Diep, T.T.; Yoo, M.J.Y.; Rush, E. Effect of in vitro gastrointestinal digestion on amino acids, polyphenols and antioxidant capacity of tamarillo yoghurts. Int. J. Mol. Sci. 2022, 23, 2526. [Google Scholar] [CrossRef]
- Wanyo, P.; Chamsai, T.; Chomnawang, C. Enhancing bioactivity and bioaccessibility of mulberry leaf tea: The influence of pretreatment and kombucha fermentation. ACS Food Sci. Technol. 2025, 5, 999–1009. [Google Scholar] [CrossRef]
- Sahab, N.R.; Subroto, E.; Balia, R.L.; Utama, G.L. γ-Aminobutyric acid found in fermented foods and beverages: Current trends. Heliyon 2020, 6, e05526. [Google Scholar] [CrossRef] [PubMed]
- Oketch-Rabah, H.A.; Madden, E.F.; Roe, A.L.; Betz, J.M. United States Pharmacopeia (USP) safety review of gamma-aminobutyric acid (GABA). Nutrients 2021, 13, 2742. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, S.; Sivadas, A.; Kwakowsky, A. The Effect of Oral GABA on the Nervous System: Potential for Therapeutic Intervention. Nutraceuticals 2024, 4, 241–259. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, P.; Pan, D.; Zeng, X.; Guo, Y.; Zhao, G. Effect of adzuki bean sprout fermented milk enriched in γ-aminobutyric acid on mild depression in a mouse model. J. Dairy Sci. 2021, 104, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Takishima, T.; Kometani, T.; Yokogoshi, H. Psychological stress-reducing effect of chocolate enriched with γ-aminobutyric acid (GABA) in humans: Assessment of stress using heart rate variability and salivary chromogranin A. Int. J. Food Sci. Nutr. 2009, 60, 106–113. [Google Scholar] [CrossRef]
- Hinton, T.; Jelinek, H.F.; Viengkhou, V.; Johnston, G.A.; Matthews, S. Effect of GABA-Fortified Oolong Tea on Reducing Stress in a University Student Cohort. Front. Nutr. 2019, 6, 27. [Google Scholar] [CrossRef]
- Bortolini, D.G.; Barros, L.; Maciel, G.M.; Peralta, R.M.; Corrêa, V.G.; Finimundy, T.C.; Isidoro Haminiuk, C.W. Bioaccessibility performance of phenolic compounds from red fruits during simulated gastrointestinal digestion and colonic fermentation. ACS Food Sci. Technol. 2025, 5, 569–577. [Google Scholar] [CrossRef]
- Du, X.; Myracle, A.D. Fermentation alters the bioaccessible phenolic compounds and increases the alpha-glucosidase inhibitory effects of aronia juice in a dairy matrix following in vitro digestion. Food Funct. 2018, 9, 2998–3007. [Google Scholar] [CrossRef]
- Tomas, M. Effect of dietary fiber addition on the content and in vitro bioaccessibility of antioxidants in red raspberry purée. Food Chem. 2022, 375, 131897. [Google Scholar] [CrossRef] [PubMed]
- AbdulAlim, T.S.; Zayan, A.F.; Campelo, P.H.; Bakry, A.M. Development of new functional fermented product: Mulberry-whey beverage. J. Nutr. Food Technol. 2018, 1, 64–69. [Google Scholar] [CrossRef]
- Nascimento, A.L.A.A.; de Souza, M.S.D.S.; Borges, L.L.R.; Eller, M.R.; de Barros, F.A.R.; Mendonça, A.C.; Stringheta, P.C. Influence of spontaneous and inoculated fermentation of açaí on simulated digestion, antioxidant capacity and cytotoxic activity. Food Res. Int. 2023, 173, 113222. [Google Scholar] [CrossRef]
- Wróblewska, B.; Kuliga, A.; Wnorowska, K. Bioactive dairy-fermented products and phenolic compounds: Together or apart. Molecules 2023, 28, 8081. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Antioxidant Activity of Anthocyanins and Anthocyanidins: A Critical Review. Int. J. Mol. Sci. 2024, 25, 12001. [Google Scholar] [CrossRef]
- Rangseekajee, P.; Piyavhatkul, N.; Wattanathorn, J.; Thukham-Mee, W.; Paholpak, P. Positive effects of anthocyanin-rich mulberry milk on mental health problems in the working population: An open-label study. Nutr. Res. Pract. 2024, 18, 110. [Google Scholar] [CrossRef]
- Méndez-Galarraga, M.P.; Hurtado-Romero, A.; Antunes-Ricardo, M.; Garcia-Amezquita, L.E.; Pirovani, M.É.; Vinderola, G.; Van de Velde, F.; García-Cayuela, T. Enhancing safety and bioactivity of blueberry-watermelon smoothies through combined ultrasound and lactic acid fermentation with potential probiotics. Food Biosci. 2025, 69, 106991. [Google Scholar] [CrossRef]
- Bertelsen, A.S.; Mielby, L.A.; Alexi, N.; Byrne, D.V.; Kidmose, U. Sweetness enhancement by aromas: Measured by descriptive sensory analysis and relative to reference scaling. Chem. Senses 2020, 45, 293–301. [Google Scholar] [CrossRef]
- de Morais, E.C.; Lima, G.C.; de Morais, A.R.; André Bolini, H.M. Prebiotic and diet/light chocolate dairy dessert: Chemical composition, sensory profiling and relationship with consumer expectation. LWT-Food Sci. Technol. 2015, 62 Pt 2, 424–430. [Google Scholar] [CrossRef]
- Janiaski, D.R.; Pimentel, T.C.; Cruz, A.G.; Prudencio, S.H. Strawberry-flavored yogurts and whey beverages: What is the sensory profile of the ideal product? J. Dairy Sci. 2016, 99, 5273–5283. [Google Scholar] [CrossRef] [PubMed]
- Bertelsen, A.S.; Zeng, Y.; Mielby, L.A.; Sun, Y.-X.; Byrne, D.V.; Kidmose, U. Cross-modal effect of vanilla aroma on sweetness of different sweeteners among Chinese and Danish consumers. Food Qual. Prefer. 2021, 87, 104036. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Teasdale, S.; Cowan, C.; Opie, R.; Jacka, F.N.; Rocks, T. Diet interventions for depression: Review and recommendations for practice. Aust. N. Z. J. Psychiatry 2025, 59, 115–127. [Google Scholar] [CrossRef] [PubMed]



| Microorganism | Mix 1 | Mix 2 | Mix 3 |
|---|---|---|---|
| Lactococcus lactis BIOTEC007 | 2% | 2% | 2% |
| Kluyveromyces lactis BIOTEC009 | — | — | 0.5% |
| Leuconostoc pseudomesenteroides BIOTEC012 | — | 1% | 0.5% |
| Lentilactobacillus kefiri BIOTEC014 | — | — | 0.5% |
| Lactobacillus acidophilus LA3 | 2% | 1% | 0.5% |
| Parameter | Value 1 |
|---|---|
| Physicochemical properties | |
| pH | 5.70 ± 0.02 |
| Titratable acidity (g/L) | 1.68 ± 0.07 |
| Viscosity (mPa·s) | 42.75 ± 1.07 |
| Water-holding capacity (%) | 1.33 ± 0.10 |
| Proximate composition (g/100 g fresh whey) | |
| Protein | 0.67 ± 0.03 |
| Fat | 0.07 ± 0.03 |
| Carbohydrates | 4.59 ± 0.04 |
| Dietary fiber | 0.00 ± 0.00 |
| Ash | 0.51 ± 0.00 |
| Microorganism | Initial Count (Log CFU/mL) | Final Count (Log CFU/mL) | pH After Fermentation |
|---|---|---|---|
| Pasteurized whey (non-inoculated control) | <1 | <1 | 5.70 ± 0.02 a |
| Lactococcus lactis BIOTEC006 | 6.68 ± 0.16 | 6.91 ± 0.12 | 5.48 ± 0.06 b |
| Lactococcus lactis BIOTEC007 | 6.91 ± 0.40 | 8.88 ± 0.27 * | 4.62 ± 0.01 e |
| Lactococcus lactis BIOTEC008 | 6.87 ± 0.06 | 8.85 ± 0.51 * | 5.00 ± 0.01 c |
| Kluyveromyces lactis BIOTEC009 | 6.92 ± 0.28 | 7.72 ± 0.28 * | 5.02 ± 0.01 c |
| Kluyveromyces lactis BIOTEC010 | 6.69 ± 0.38 | 6.35 ± 0.17 | 5.71 ± 0.00 a |
| Leuconostoc pseudomesenteroides BIOTEC011 | 6.32 ± 0.12 | 6.10 ± 0.58 | 5.73 ± 0.03 a |
| Leuconostoc pseudomesenteroides BIOTEC012 | 7.61 ± 0.03 | 8.55 ± 0.16 * | 4.60 ± 0.01 e |
| Lentilactobacillus kefiri BIOTEC013 | 6.84 ± 0.49 | 8.51 ± 0.10 * | 4.64 ± 0.04 e |
| Lentilactobacillus kefiri BIOTEC014 | 7.11 ± 0.16 | 7.96 ± 0.10 * | 4.99 ± 0.01 c,d |
| Lentilactobacillus parakefiri BIOTEC015 | 5.64 ± 0.40 | 7.98 ± 0.09 * | 4.94 ± 0.01 d |
| Lactobacillus acidophilus LA3 | 7.82 ± 0.11 | 8.89 ± 0.19 * | 4.44 ± 0.02 f |
| Lacticaseibacillus rhamnosus GG | 7.79 ± 0.35 | 8.86 ± 0.06 * | 4.03 ± 0.01 g |
| Lactiplantibacillus plantarum 299v | 7.88 ± 0.14 | 8.86 ± 0.19 * | 4.04 ± 0.04 g |
| Parameter | Value 1 |
|---|---|
| Physicochemical properties | |
| Apical caliber (cm) | 2.38 ± 0.35 |
| Equatorial caliber (cm) | 2.19 ± 0.18 |
| Weight (g) | 5.39 ± 1.60 |
| Moisture content (g/100 g fresh weight) | 84.27 ± 0.53 |
| Soluble solids (°Brix at 25 °C) | 10.10 ± 0.10 |
| pH | 2.81 ± 0.01 |
| Titratable acidity (g citric acid/100 g fresh weight) | 0.51 ± 0.09 |
| Proximate composition (g/100 g dry weight) | |
| Protein | 7.67 ± 0.21 |
| Fat | 2.53 ± 0.05 |
| Carbohydrates | 65.51 ± 0.31 |
| Dietary fiber | 20.82 ± 0.18 |
| Insoluble dietary fiber | 16.80 ± 0.21 |
| Soluble dietary fiber | 4.02 ± 0.10 |
| Ash | 3.47 ± 0.41 |
| Total bioactive compounds (mg/g dry weight) | |
| Total phenolic content 2 | 9.21 ± 0.61 |
| Total monomeric anthocyanin content 3 | 2.64 ± 0.14 |
| Parameter (g/100 g Fresh Weight) | FWF-R1 1 | FWF-R2 1 |
|---|---|---|
| Protein | 1.24 ± 0.08 | 1.24 ± 0.01 |
| Fat | 0.13 ± 0.00 | 0.13 ± 0.02 |
| Carbohydrates | 8.98 ± 0.05 | 9.12 ± 0.06 |
| Dietary fiber | 1.12 ± 0.09 | 0.97 ± 0.08 |
| Insoluble dietary fiber | 0.68 ± 0.06 | 0.56 ± 0.06 |
| Soluble dietary fiber | 0.44 ± 0.03 | 0.41 ± 0.02 |
| Ash | 0.57 ± 0.04 | 0.66 ± 0.09 |
| Property | Formulation | Day 0 | Day 1 | Day 7 | Day 14 | Day 21 |
|---|---|---|---|---|---|---|
| pH | FWF-R1 | 3.71 ± 0.01 b,B | 3.74 ± 0.01 b,A | 3.75 ± 0.01 b,A | 3.74 ± 0.02 b,A | 3.74 ± 0.01 b,A |
| FWF-R2 | 3.78 ± 0.01 a,B | 3.82 ± 0.01 a,A,B | 3.86 ± 0.02 a,A | 3.85 ± 0.03 a,A | 3.84 ± 0.03 a,A | |
| Titratable acidity (% lactic acid) | FWF-R1 | 1.25 ± 0.02 a,A | 1.27 ± 0.06 a,A | 1.25 ± 0.01 a,A | 1.24 ± 0.02 a,A | 1.26 ± 0.01 a,A |
| FWF-R2 | 1.19 ± 0.02 b,A | 1.21 ± 0.02 a,A | 1.19 ± 0.02 b,A | 1.19 ± 0.09 a,A | 1.20 ± 0.07 a,A | |
| Soluble solids (°Brix at 25 °C) | FWF-R1 | 9.90 ± 0.10 b,B | 10.33 ± 0.06 b,A | 10.34 ± 0.06 b,A | 10.47 ± 0.15 b,A | 10.37 ± 0.25 b,A |
| FWF-R2 | 10.50 ± 0.03 a,B | 10.60 ± 0.10 a,B | 10.63 ± 0.06 a,B | 10.90 ± 0.02 a,A | 10.83 ± 0.05 a,A | |
| Viscosity (mPa·s) | FWF-R1 | 266.93 ± 11.30 a,C | 347.10 ± 37.43 a,B | 384.43 ± 2.40 a,B | 405.17 ± 1.14 a,A | 408.50 ± 3.27 a,A |
| FWF-R2 | 226.30 ± 3.42 b,D | 301.23 ± 6.80 b,C | 301.57 ± 5.03 b,C | 321.00 ± 3.25 b,B | 344.27 ± 3.52 b,A | |
| Water-holding capacity (%) | FWF-R1 | 28.75 ± 0.71 a,B | 33.49 ± 2.96 a,A | 36.59 ± 2.53 a,A | 34.48 ± 2.84 a,A | 34.37 ± 0.88 a,A |
| FWF-R2 | 29.82 ± 0.26 a,B | 33.71 ± 0.43 a,A | 35.27 ± 1.46 a,A | 34.50 ± 0.73 a,A | 33.73 ± 0.56 a,A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Toro-Barbosa, M.; Uribe-Velázquez, T.; Hurtado-Romero, A.; Rosales-De la Cruz, M.F.; Carrillo-Nieves, D.; Garcia-Amezquita, L.E.; García-Cayuela, T. Evaluation of GABA-Producing Fermented Whey Formulations: From Strain Selection to Raspberry-Enriched Beverages with Psychobiotic Potential. Foods 2025, 14, 2762. https://doi.org/10.3390/foods14162762
Del Toro-Barbosa M, Uribe-Velázquez T, Hurtado-Romero A, Rosales-De la Cruz MF, Carrillo-Nieves D, Garcia-Amezquita LE, García-Cayuela T. Evaluation of GABA-Producing Fermented Whey Formulations: From Strain Selection to Raspberry-Enriched Beverages with Psychobiotic Potential. Foods. 2025; 14(16):2762. https://doi.org/10.3390/foods14162762
Chicago/Turabian StyleDel Toro-Barbosa, Mariano, Tlalli Uribe-Velázquez, Alejandra Hurtado-Romero, María Fernanda Rosales-De la Cruz, Danay Carrillo-Nieves, Luis Eduardo Garcia-Amezquita, and Tomás García-Cayuela. 2025. "Evaluation of GABA-Producing Fermented Whey Formulations: From Strain Selection to Raspberry-Enriched Beverages with Psychobiotic Potential" Foods 14, no. 16: 2762. https://doi.org/10.3390/foods14162762
APA StyleDel Toro-Barbosa, M., Uribe-Velázquez, T., Hurtado-Romero, A., Rosales-De la Cruz, M. F., Carrillo-Nieves, D., Garcia-Amezquita, L. E., & García-Cayuela, T. (2025). Evaluation of GABA-Producing Fermented Whey Formulations: From Strain Selection to Raspberry-Enriched Beverages with Psychobiotic Potential. Foods, 14(16), 2762. https://doi.org/10.3390/foods14162762











