Valorization of Coffee Silverskin via Integrated Biorefinery for the Production of Bioactive Peptides and Xylooligosaccharides: Functional and Prebiotic Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Pretreatment of CS and Protein Extraction
2.3. Microorganism and Enzyme Production
2.3.1. Alkaline Protease (Protease_SE5) Production
2.3.2. Endo-Xylanase Production
2.3.3. Probiotics Preparation
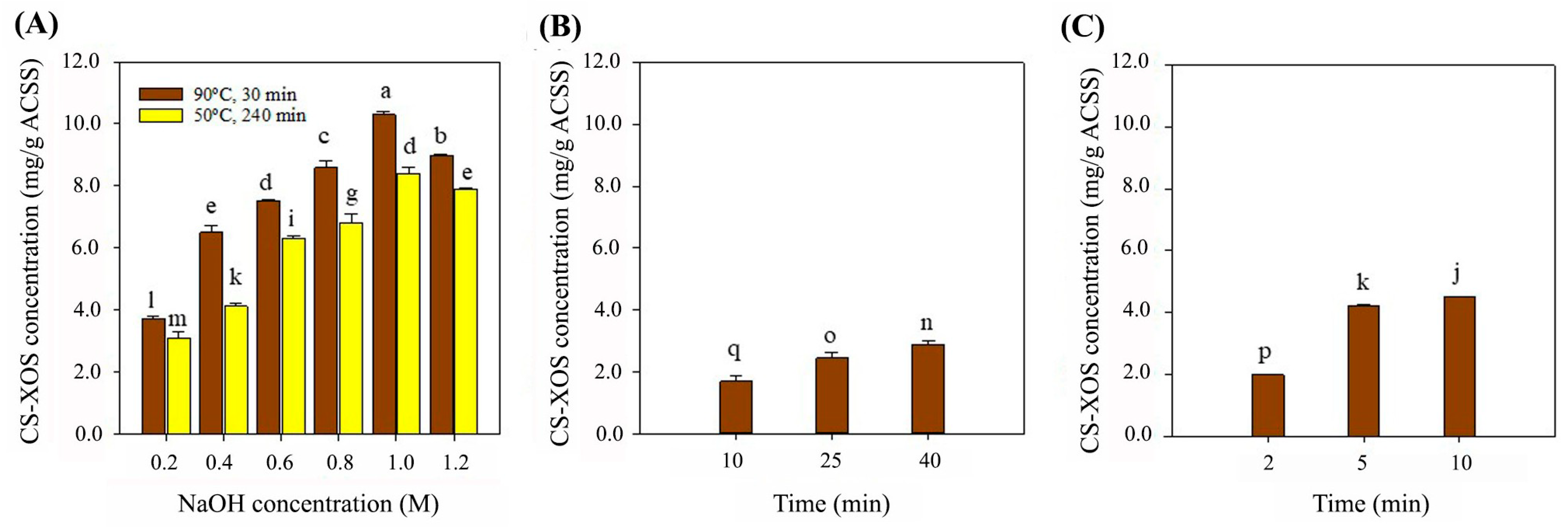
2.4. Effects of MAE, UAE, and CAE Methods on CS-XOS Production
2.5. Enzymatic Production of Bioactive Peptide
2.5.1. Enzymatic Production of Bioactive Peptide from CS-Protein
2.5.2. CS-Peptide Fractionation, Identification, and Synthesis
2.6. Analytical Method
2.6.1. Chemical Composition of CS
2.6.2. Analysis of Biological Activities and Cytotoxicity of Bioactive Peptide
Antioxidant Activity
Total Phenolic Content (TPC)
Angiotensin-I-Converting Enzyme (ACE) Inhibition Activity
Dipeptidyl Peptidase-IV (DPP-IV) Inhibition Activity
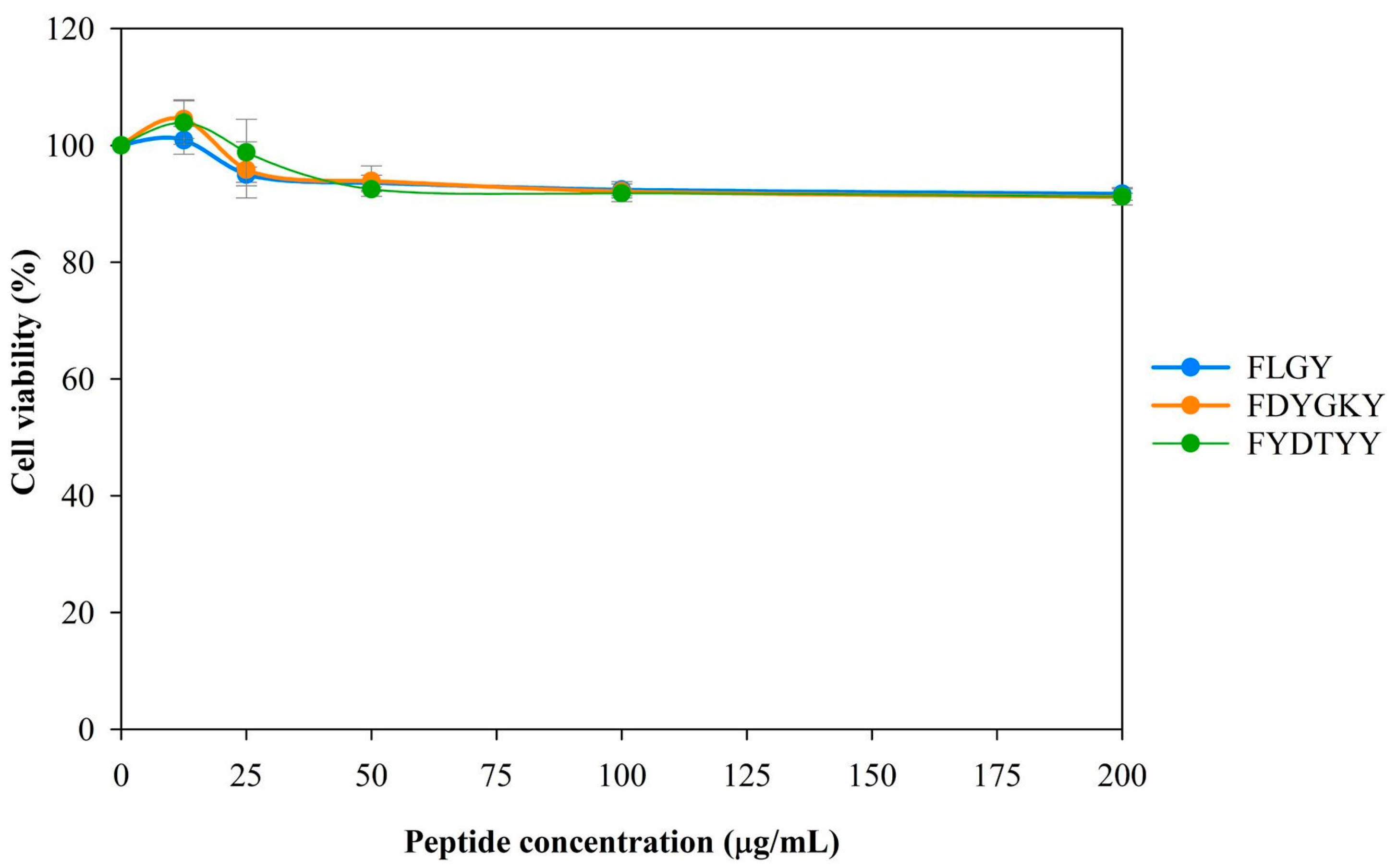
Cytotoxicity Analysis
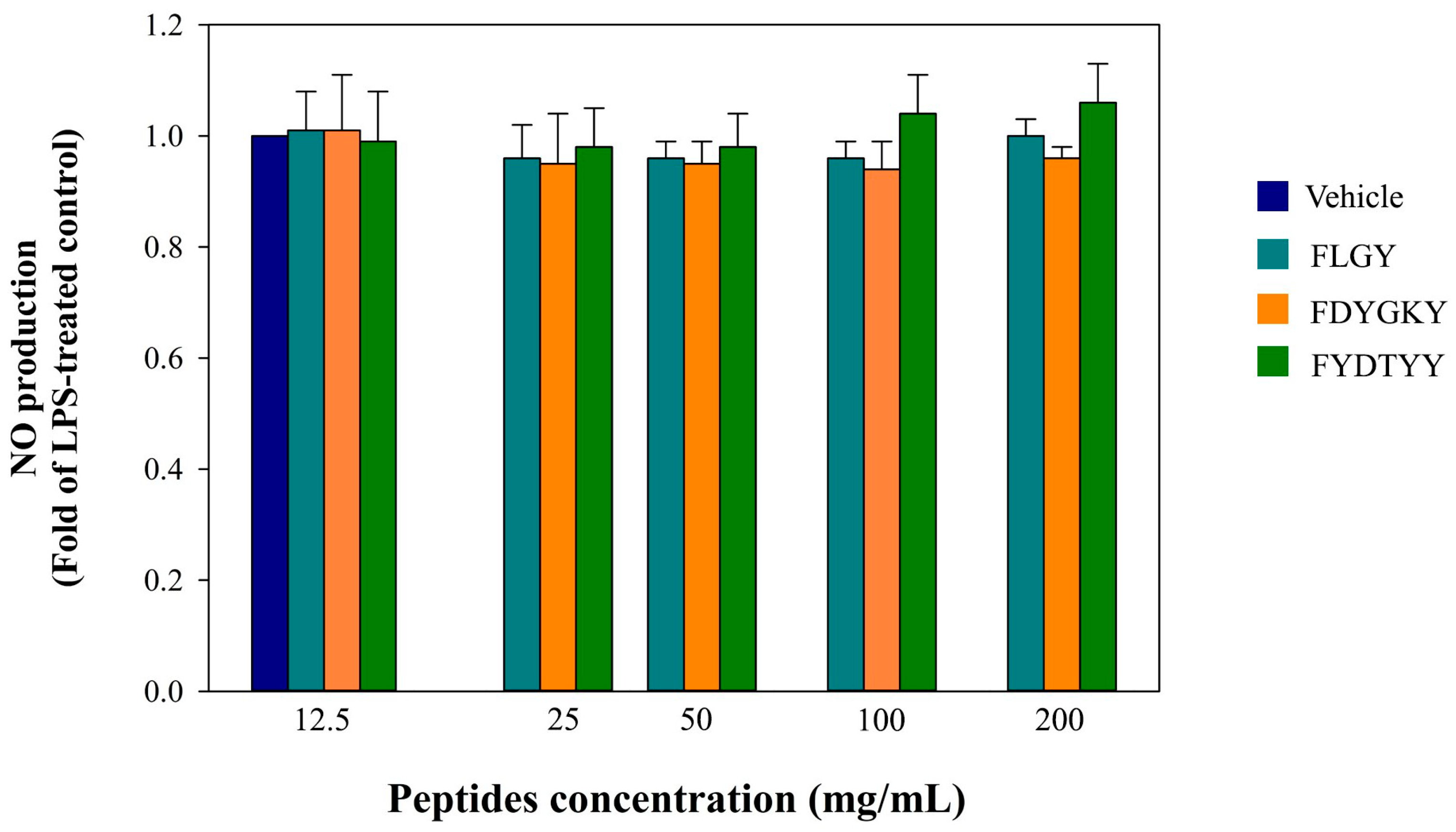
Anti-Inflammatory Activity
2.6.3. XOS, Sugars, and Lactic Acid Analysis
2.6.4. Prebiotic Property of CS-XOS
2.6.5. Statistical Data Analysis
3. Results
3.1. Effects of Extraction Methods on CS-XOS Production
3.2. Bioreactor-Scale Integrated Process of Bioactive Peptide and CS-XOS Production
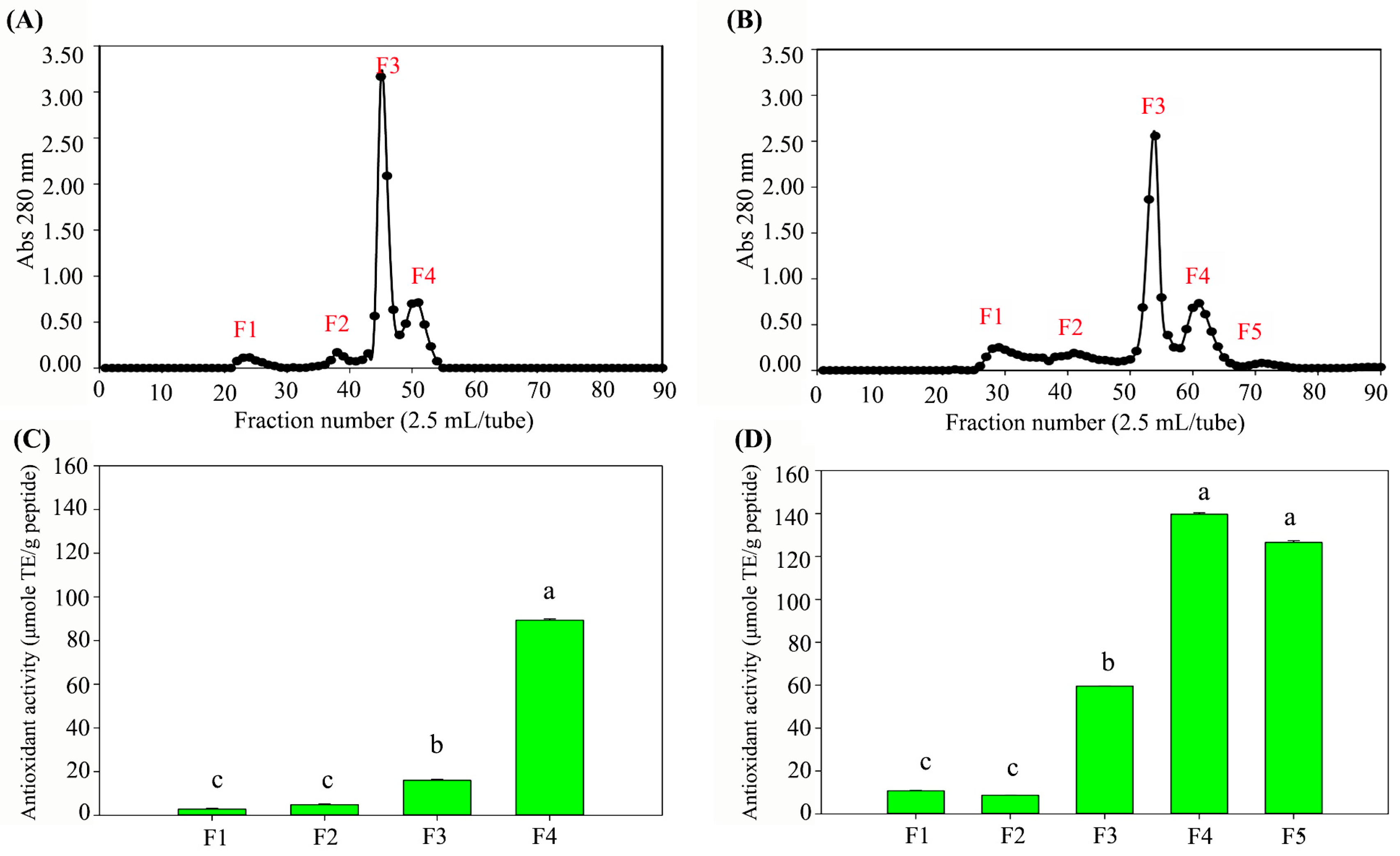
3.3. CS-Peptides Fractionation
3.4. CS-Peptide Identification and Predicted Biological Activity
| No. | Peptide Sequence | BIOPEP * | In Vitro Analysis | ||||
|---|---|---|---|---|---|---|---|
| ACE Inhibitory Activity | DPP-IV Inhibitory Activity | Antioxidant Activity | ACE Inhibition ** (%) | DPP-IV Inhibition *** (%) | Antioxidant Activity ** (%) | ||
| 1 | FLGY | 🗸 | 🗸 | - | 60 ± 1 b | 19 ± 1 a | 48 ± 1 a |
| 2 | FYDTYY | 🗸 | 🗸 | - | 26 ± 7 c | 18 ± 3 a | N.D. |
| 3 | FDYGKY | 🗸 | - | - | 79 ± 7 a | N.D. | 50 ± 1 a |
3.5. Cytotoxicity of Synthetic Peptides in Murine Macrophage Cells
3.6. Anti-Inflammatory Activity of Synthetic Peptides in Murine Macrophage Cells
3.7. Bioreactor-Scale Production of CS-XOS
3.8. Prebiotic Property of CS-XOS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gil-Gómez, J.A.; Florez-Pardo, L.M.; Leguizamón-Vargas, Y.C. Valorization of coffee by-products in the industry, a vision towards circular economy. Discov. Appl. Sci. 2024, 6, 480. [Google Scholar] [CrossRef]
- Gao, H.; Zhou, Z. Effect of xylo-oligosaccharides supplementation by drinking water on the bone properties and related calcium transporters in growing mice. Nutrients 2020, 12, 3542. [Google Scholar] [CrossRef]
- Chemat, A.; Touraud, D.; Müller, R.; Kunz, W.; Fabiano-Tixier, A.S. Valorization of coffee silverskin using extraction cycles and water as a solvent: Design of process. Molecules 2024, 29, 1318. [Google Scholar] [CrossRef] [PubMed]
- Bessada, S.; Alves, R.; Costa, A.; Nunes, M.; Oliveira, M. Coffea canephora silverskin from different geographical origins: A comparative study. Sci. Total Environ. 2018, 645, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Jethwa, F.; Shetty, P.; Dabade, A. Extraction and functional properties of crude proteins from coffee silver skin and its cost effective application. In Proceedings of the NCIFEH Conference Proceedings, Navi Mumbai, India, 28–29 September 2018; pp. 252–262. [Google Scholar]
- Wen, L.; Álvarez, C.; Zhang, Z.; Poojary, M.M.; Lund, M.N.; Sun, D.W.; Tiwari, B.K. Optimisation and characterisation of protein extraction from coffee silverskin assisted by ultrasound or microwave techniques. Biomass Convers. Biorefinery 2021, 11, 1575–1585. [Google Scholar] [CrossRef]
- Jirarat, W.; Kaewsalud, T.; Yakul, K.; Rachtanapun, P.; Chaiyaso, T. Sustainable valorization of coffee silverskin: Extraction of phenolic compounds and proteins for enzymatic production of bioactive peptides. Foods 2024, 13, 1230. [Google Scholar] [CrossRef]
- Abik, F.; Palasingh, C.; Bhattarai, M.; Leivers, S.; Ström, A.; Westereng, B.; Mikkonen, K.S.; Nypelö, T. Potential of wood hemicelluloses and their derivates as food ingredients. J. Agric. Food Chem. 2023, 71, 2667–2683. [Google Scholar] [CrossRef]
- Sonklin, C.; Alashi, M.A.; Laohakunjit, N.; Kerdchoechuen, O.; Aluko, R.E. Identification of antihypertensive peptides from mung bean protein hydrolysate and their effects in spontaneously hypertensive rats. J. Funct. Foods 2020, 64, 103635. [Google Scholar] [CrossRef]
- Haq, I.; Qaisar, K.; Nawaz, A.; Akram, F.; Mukhtar, H.; Zohu, X.; Xu, Y.; Mumtaz, M.; Rashid, U.; Karim, A.; et al. Advances in valorization of lignocellulosic biomass towards energy generation. Catalysts 2021, 11, 309. [Google Scholar] [CrossRef]
- Fernandes, A.; Cruz-Lopes, L.; Esteves, B.; Evtuguin, D.V. Microwaves and ultrasound as emerging techniques for lignocellulosic materials. Materials 2023, 16, 7351. [Google Scholar] [CrossRef]
- Kälkäjä, S.; Lappalainen, K.; Delattre, F.; Lévêque, J.M. Current status of chemical-or enzyme-assisted ultrasonic pre-treatment processes for lignocellulosic biomass to assess industrialization progress: A review. Curr. Opin. Chem. Eng. 2025, 48, 101124. [Google Scholar] [CrossRef]
- Machado, M.; Ferreira, H.; Oliveira, M.B.P.P.; Alves, R.C. Coffee by-products: An underexplored source of prebiotic ingredients. Crit. Rev. Food Sci. Nutr. 2023, 64, 7181–7200. [Google Scholar] [CrossRef]
- Ratnadewi, A.A.I.; Zain, M.H.A.; Kusuma, A.A.N.; Handayani, W.; Nugraha, A.S.; Siswoyo, T.A. Lactobacillus casei fermentation towards xylooligosaccharide (XOS) obtained from coffee peel enzymatic hydrolysate. Biocatal. Agric. Biotechnol. 2020, 23, 101446. [Google Scholar] [CrossRef]
- Machado, M.; Galrinho, M.F.; Passos, C.P.; Espírito Santo, L.; Chiș, M.S.; Ranga, F.; Puga, H.; Palmeira, J.D.; Coimbra, M.A.; Oliveira, M.B.P.P.; et al. Prebiotic potential of a coffee silverskin extract obtained by ultrasound-assisted extraction on Lacticaseibacillus paracasei subsp. paracasei. J. Funct. Foods 2024, 120, 106378. [Google Scholar] [CrossRef]
- Ramírez, K.; Pineda-Hidalgo, K.V.; Rochín-Medina, J.J. Fermentation of Spent Coffee Grounds by Bacillus clausii Induces Release of Potentially Bioactive Peptides. LWT 2021, 138, 110685. [Google Scholar] [CrossRef]
- Yakul, K.; Kaewsalud, T.; Techapun, C.; Seesuriyachan, P.; Jantanasakulwong, K.; Watanabe, M.; Takenaka, S.; Chaiyaso, T. Enzymatic valorization process of yellow cocoon waste for production of antioxidative sericin and fibroin film. J. Chem. Technol. Biotechnol. 2021, 96, 953–962. [Google Scholar] [CrossRef]
- Chaiyaso, T.; Boonchuay, P.; Takenaka, S.; Techapun, C.; Rachtanapun, P.; Jantanasakulwong, K.; Watanabe, M. Efficient enzymatic process for mulberry paper production: An approach for xylooligosaccharide production coupled with minimizing bleaching agent doses. Waste Biomass Valoriz. 2021, 12, 5347–5360. [Google Scholar] [CrossRef]
- Boonchuay, P.; Wongpoomchai, R.; Jaturasitha, S.; Mahatheeranont, S.; Watanabe, M.; Chaiyaso, T. Prebiotic properties, antioxidant activity, and acute oral toxicity of xylooligosaccharides derived enzymatically from corncob. Food Biosci. 2021, 40, 100895. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; Method 935.14 and 992.24; Association of Official Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Yakul, K.; Takenaka, S.; Peterbauer, C.; Haltrich, D.; Techapun, C.; Seesuriyachan, P.; Leksawasdi, N.; Chaiyaso, T. Functional modification of thermostable alkaline protease from Bacillus halodurans SE5 for efficient production of antioxidative and ACE-inhibitory peptides from sericin. Biocatal. Agric. Biotechnol. 2023, 54, 102943. [Google Scholar] [CrossRef]
- Lacroix, I.M.; Li-Chan, E.C. Dipeptidyl peptidase-IV inhibitory activity of dairy protein hydrolysates. Int. Dairy J. 2012, 25, 97–102. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef]
- Singai, C.; Pitchakarn, P.; Taya, S.; Wongpoomchai, R.; Wongnoppavich, A. Genotoxic and Anti-Genotoxic Assessments of Fermented Houttuynia cordata Thunb. Leaf Ethanolic Extract and Its Anti-Cancer Effect in a Dual-Organ Carcinogenesis Model of Colon and Liver in Rats. Foods 2024, 13, 3645. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Jahandideh, F.; Wu, J. Food-derived bioactive peptides on inflammation and oxidative stress. Biomed. Res. Int. 2014, 1, 608979. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Gottstein, V.; Bernhardt, M.; Dilger, E.; Keller, J.; Breitling-Utzmann, C.M.; Schwarz, S.; Kuballa, T.; Lachenmeier, D.W.; Bunzel, M. Coffee silverskin: Chemical characterization with special consideration of dietary fiber and heat-induced contaminants. Foods 2021, 10, 1705. [Google Scholar] [CrossRef]
- Jiménez-Zamora, A.; Pastoriza, S.; Rufián-Henares, J.A. Revalorization of coffee by-products: Prebiotic, antimicrobial and antioxidant properties. LWT Food Sci. Technol. 2015, 61, 12–18. [Google Scholar] [CrossRef]
- Niglio, S.; Procentese, A.; Russo, M.E.; Sannia, G.; Marzocchella, A. Investigation of enzymatic hydrolysis of coffee silverskin aimed at the production of butanol and succinic acid by fermentative processes. Bioenergy Res. 2019, 12, 312–324. [Google Scholar] [CrossRef]
- Flores, E.M.; Cravotto, G.; Bizzi, C.A.; Santos, D.; Iop, G.D. Ultrasound-assisted biomass valorization to industrial interesting products: State-of-the-art, perspectives and challenges. Ultrason. Sonochem. 2021, 12, 105455. [Google Scholar] [CrossRef]
- Ramezani, M.; Aminlari, M.; Majzoobi, M. Alkaline Extraction of Proteins from Oilseed Meals: A Review. J. Food Biochem. 2021, 45, e13666. [Google Scholar] [CrossRef]
- Yılmaz Celebioglu, H.; Cekmecelioglu, D.; Dervisoglu, M.; Kahyaoglu, T. Effect of extraction conditions on hemicellulose yields and optimisation for industrial processes. Int. J. Food Sci. Technol. 2012, 47, 2597–2605. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, F.; Wang, X.; Qi, J.; Shi, Y. Optimization of Alkaline Extraction Conditions for Protein from Rapeseed Meal and the Functional Properties of the Protein Isolates. Molecules 2019, 24, 3191. [Google Scholar] [CrossRef]
- Ng, J.W.; Teh, T.M.; Tan, C.F.; Bi, X.; Low, Z.E.; Talukder, M.M.R. Alkaline solubilization of microalgal protein and its impact on the functional properties of protein extract. Future Foods 2024, 9, 100368. [Google Scholar] [CrossRef]
- Guedes, D.; Duarte, M.O.A.; Rivero-Pino, M.; Pérez-Gregorio, R.; Recio, I. Strengths and Limitations of In Silico Tools to Assess Physicochemical Properties, Bioactivity, and Bioavailability of Food-Derived Peptides. Trends Food Sci. Technol. 2023, 138, 433–447. [Google Scholar] [CrossRef]
- Senadheera, T.R.L.; Hossain, A.; Dave, D.; Shahidi, F. In Silico Analysis of Bioactive Peptides Produced from Underutilized Sea Cucumber By-Products—A Bioinformatics Approach. Mar. Drugs 2022, 20, 610. [Google Scholar] [CrossRef]
- Sheng, Y.; Qiu, Y.T.; Wang, Y.M.; Chi, C.F.; Wang, B. Novel antioxidant collagen peptides of Siberian sturgeon (Acipenser baerii) cartilages: The preparation, characterization, and cytoprotection of H2O2-damaged human umbilical vein endothelial cells (HUVECs). Mar. Drugs 2022, 20, 325. [Google Scholar] [CrossRef]
- Koh, J.A.; Ong, J.H.; Abd Manan, F.; Ee, K.Y.; Wong, F.C.; Chai, T.T. Discovery of bifunctional anti-DPP-IV and anti-ACE peptides from housefly larval proteins after in silico gastrointestinal digestion. Biointerface Res. Appl. Chem. 2022, 12, 4929–4944. [Google Scholar] [CrossRef]
- Raza, A.; Uddin, J.; Akbar, S.; Alarfaj, F.K.; Zou, Q.; Ahmad, A. Comprehensive analysis of computational methods for predicting anti-inflammatory peptides. Arch. Comput. Methods Eng. 2024, 31, 3211–3229. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, J.; Shi, H.; Yu, L.L. Isolation and characterization of anti-inflammatory peptides derived from whey protein. J. Dairy Sci. 2016, 99, 6902–6912. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, S.; Yuan, L.; Yang, Y.; Lin, S. Glutamine and methionine targeted pulsed electric field treatment for enhanced immune activity in pine nut Gln-Trp-Phe-Met peptides. Int. J. Food Sci. Technol. 2020, 55, 2954–2961. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Cardelle-Cobas, A.; Alonso, J.L.; Pintado, M.; Gomes, A.M. Effects of hemicellulose-derived saccharides on behavior of Lactobacilli under simulated gastrointestinal conditions. Food Res. Int. 2014, 64, 880–888. [Google Scholar] [CrossRef] [PubMed]
| CS-Protein Extraction | CS-Protein Hydrolysis | ||||
|---|---|---|---|---|---|
| Samples | Volume (mL) | Protein Recovery (mg/g CS) | Volume (mL) | Peptide Concentration (mg/mL) | Antioxidant Activity (µmole TE/L) |
| 250 mL Duran bottle | 100 | 83.12 ± 2.66 | 100 | 0.323 ± 0.001 | 1501 ± 21 |
| 5 L Stirred-tank bioreactor | 3000 | 80.64 ± 4.82 | 3000 | 0.302 ± 0.014 | 1511 ± 58 |
| Production Scale | XOS Concentration (mg/g ACSS) | Antioxidant Activity (µmole TE/ mg XOS) | TPC (mg GAE/mg) | ||
|---|---|---|---|---|---|
| ABTS | DPPH | FRAP | |||
| 250 mL Duran bottle | 20.30 ± 0.10 | 85.01 ± 2.94 | 36.00 ± 0.75 | 39.42 ± 3.29 | 71.67 ± 2.04 |
| 5.0 L Stirred-tank bioreactor | 52.5 ± 0.08 * | 89.43 ± 1.31 | 32.05 ± 1.24 | 35.77 ± 2.11 | 74.56 ± 2.91 |
| Strains | Prebiotics | µmax (h−1) | Sugar Consumption (g/L) | Lactic Acid (g/L) |
|---|---|---|---|---|
| Lacticaseibacillus casei TISTR1463 | Commercial-XOS | 0.103 ± 0.001 b | 5.59 ± 0.05 d | 9.19 ± 0.55 b,c |
| CS-XOS | 0.100 ± 0.021 b | 5.16 ± 0.17 c | 8.49 ± 0.45 c | |
| Lactobacillus delbrueckii subsp. lactis TISTR1464 | Commercial-XOS | 0.103 ± 0.003 b | 2.41 ± 0.10 a | 7.03 ± 0.25 a |
| CS-XOS | 0.112 ± 0.001 ab | 2.51 ± 0.04 a | 7.65 ± 0.07 b,c | |
| Lactiplantibacillus plantarum TISTR1465 | Commercial-XOS | 0.104 ± 0.001 b | 4.22 ± 0.14 b | 7.45 ± 0.26 a,b |
| CS-XOS | 0.122 ± 0.002 a | 4.23 ± 0.17 b | 7.47 ± 0.51 b,c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaiyaso, T.; Yakul, K.; Jirarat, W.; Tapingkae, W.; Leksawasdi, N.; Rachtanapun, P. Valorization of Coffee Silverskin via Integrated Biorefinery for the Production of Bioactive Peptides and Xylooligosaccharides: Functional and Prebiotic Properties. Foods 2025, 14, 2745. https://doi.org/10.3390/foods14152745
Chaiyaso T, Yakul K, Jirarat W, Tapingkae W, Leksawasdi N, Rachtanapun P. Valorization of Coffee Silverskin via Integrated Biorefinery for the Production of Bioactive Peptides and Xylooligosaccharides: Functional and Prebiotic Properties. Foods. 2025; 14(15):2745. https://doi.org/10.3390/foods14152745
Chicago/Turabian StyleChaiyaso, Thanongsak, Kamon Yakul, Wilasinee Jirarat, Wanaporn Tapingkae, Noppol Leksawasdi, and Pornchai Rachtanapun. 2025. "Valorization of Coffee Silverskin via Integrated Biorefinery for the Production of Bioactive Peptides and Xylooligosaccharides: Functional and Prebiotic Properties" Foods 14, no. 15: 2745. https://doi.org/10.3390/foods14152745
APA StyleChaiyaso, T., Yakul, K., Jirarat, W., Tapingkae, W., Leksawasdi, N., & Rachtanapun, P. (2025). Valorization of Coffee Silverskin via Integrated Biorefinery for the Production of Bioactive Peptides and Xylooligosaccharides: Functional and Prebiotic Properties. Foods, 14(15), 2745. https://doi.org/10.3390/foods14152745








