Effects of Different Packaging on the Purine Content and Key Enzymes of Refrigerated Yellow Croaker (Larimichthys crocea)
Abstract
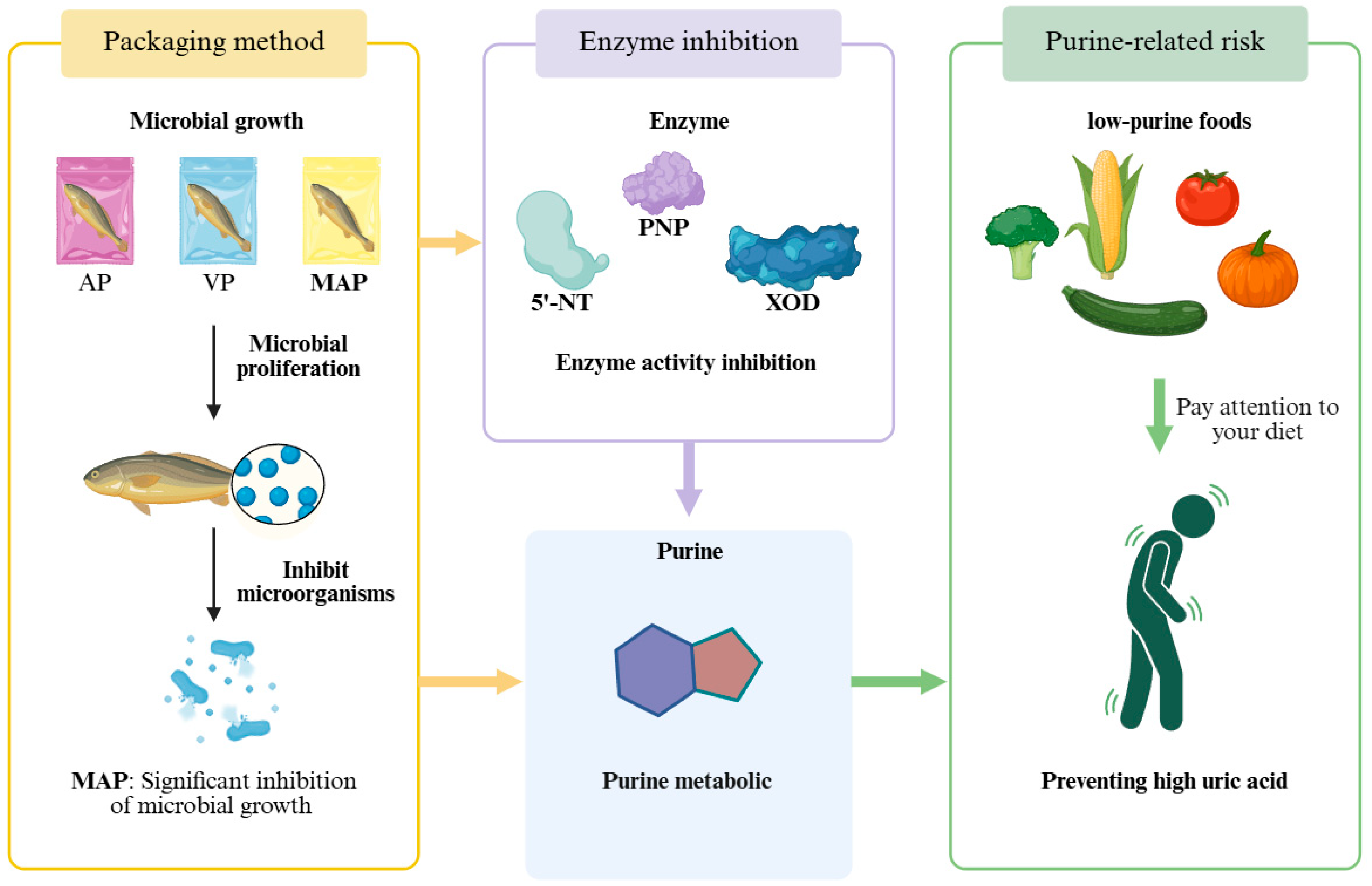
1. Introduction
2. Materials and Methods
2.1. Fish Sample Preparation
2.2. Total Viable Count (TVC)
2.3. The pH and Electrical Conductance (EC)
2.4. Total Volatile Basic Nitrogen (TVB-N)
2.5. Determination of ATP-Related Compounds
2.6. Determination of Purines
2.6.1. Preparation of Purine Standard Solutions
2.6.2. Sample Preparation
2.6.3. Optimization of Detection Methods for Purine Components
2.7. Determination of Enzyme Activities (ACP, AKP, 5′-NT, PNP, and XOD)
2.8. Statistical Analysis
3. Results
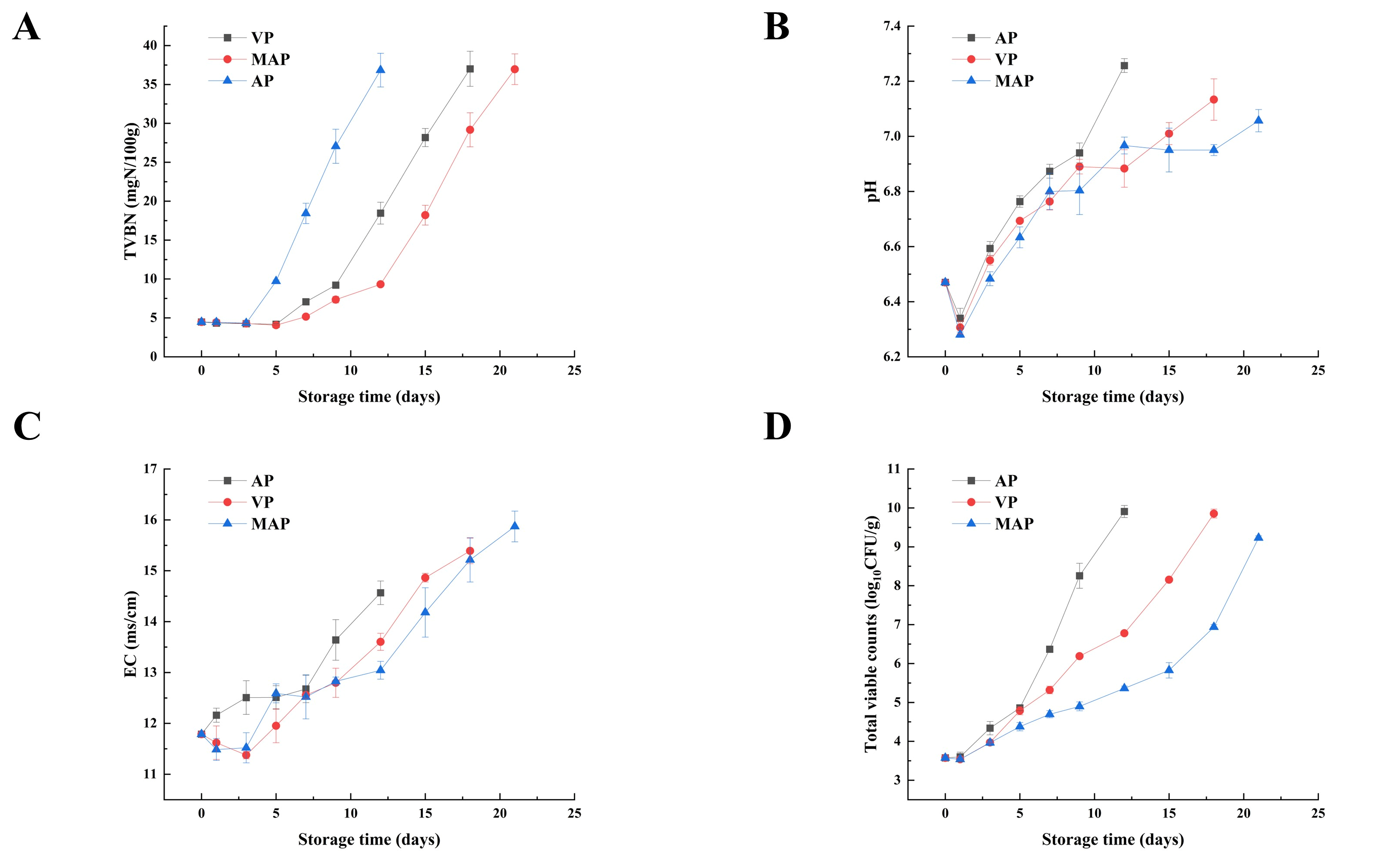
3.1. TVC
3.2. The pH and EC
3.3. TVB-N
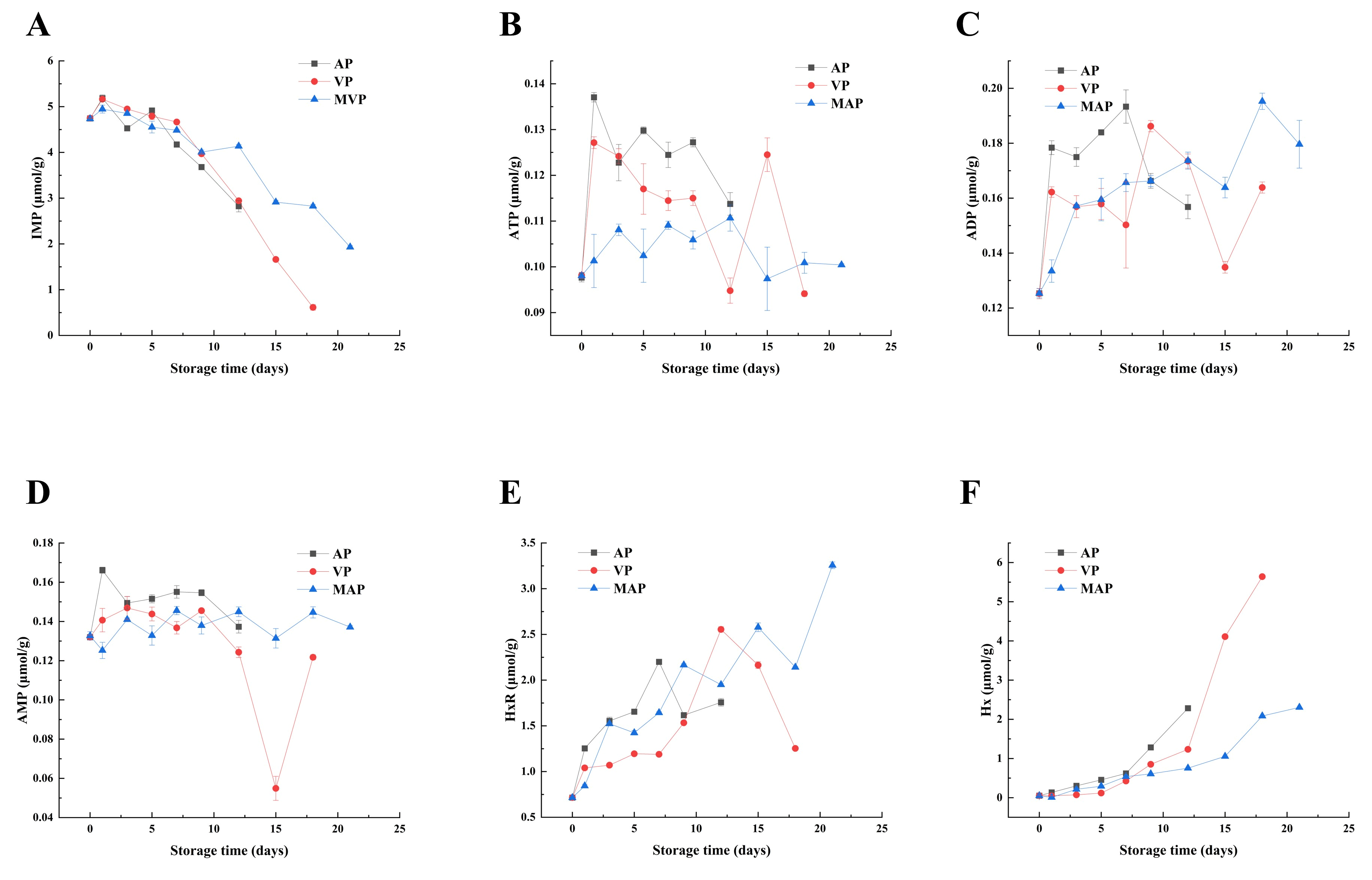
3.4. Changes in ATP-Related Products
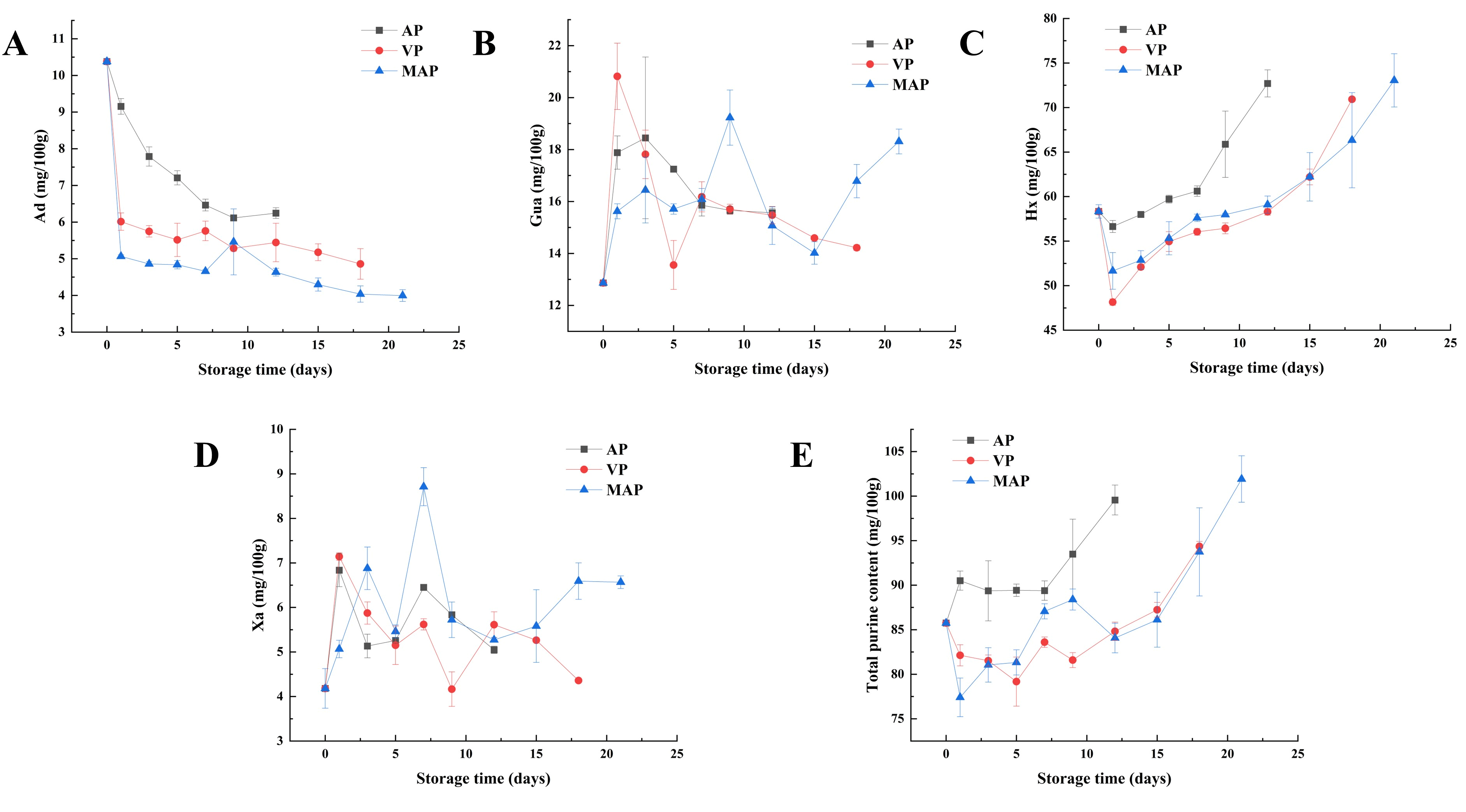
3.5. Changes in Purines
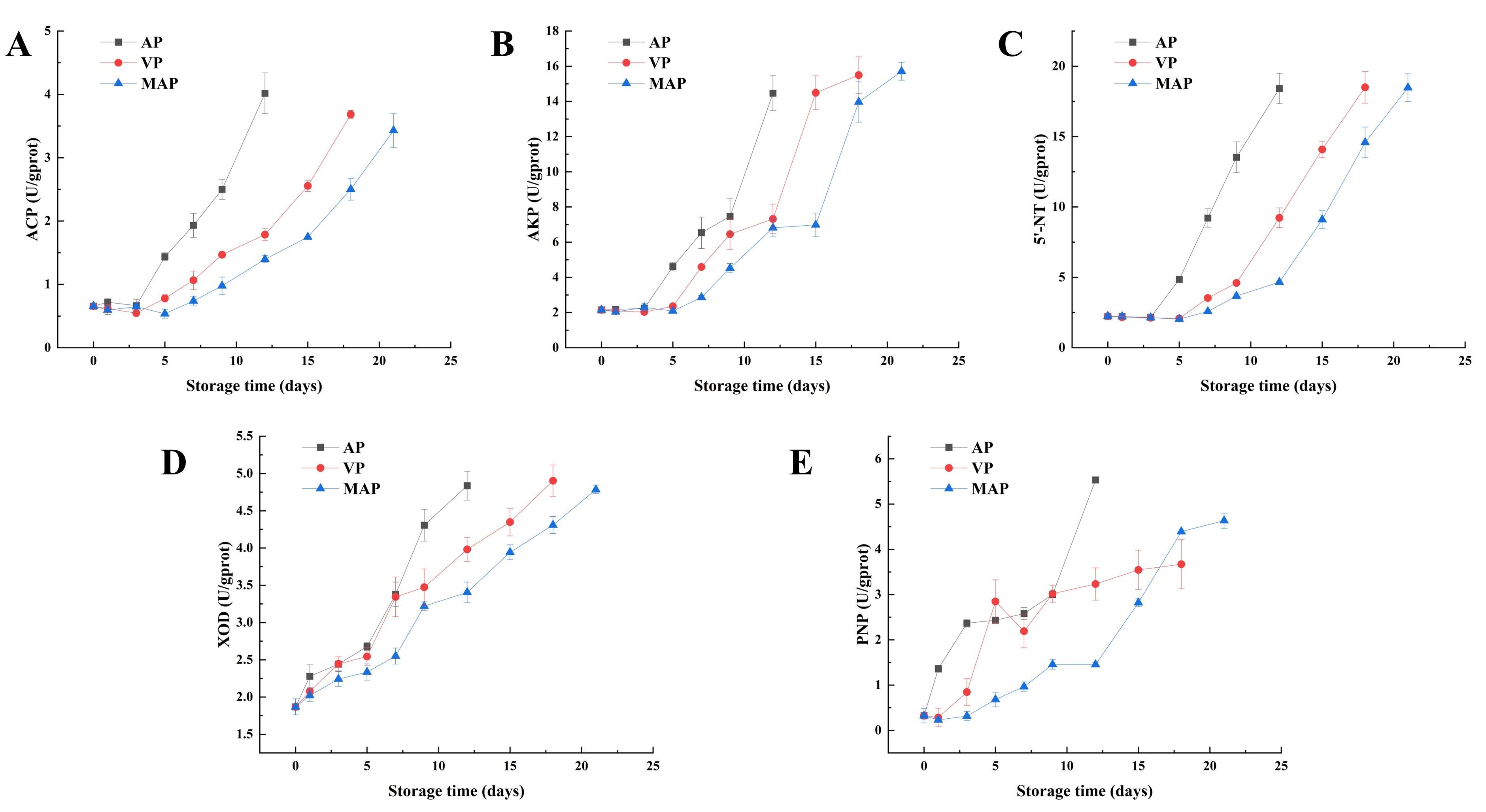
3.6. Changes in Key Enzymes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Zhu, B.; Wang, Y.; Zhou, W.; Jin, S.; Shen, Z.; Zhang, H.; Zhang, X.; Ding, X.; Li, Y. Trend dynamics of gout prevalence among the Chinese population, 1990–2019: A joinpoint and age-period-cohort analysis. Front. Public Health 2022, 10, 1008598. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Yuan, M.; Xu, Y.; Xu, H. Gout and Diet: A Comprehensive Review of Mechanisms and Management. Nutrients 2022, 14, 3525. [Google Scholar] [CrossRef]
- Ji, A.; Tian, Z.; Shi, Y.; Takei, R.; Chang, S.-J.; Yip, R.M.L.; Yin, H.; Li, C. Gout in China. Gout Urate Crystal Depos. Disord. 2025, 3, 1. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2024–Blue Transformation in Action; FAO: Rome, Italy, 2024. [Google Scholar]
- Kaneko, K.; Aoyagi, Y.; Fukuuchi, T.; Inazawa, K.; Yamaoka, N. Total Purine and Purine Base Content of Common Foodstuffs for Facilitating Nutritional Therapy for Gout and Hyperuricemia. Biol. Pharm. Bull. 2014, 37, 709–721. [Google Scholar] [CrossRef]
- Jantasaeng, O.; Duclos, M.J.; Thumanu, K.; Okrathok, S.; Khempaka, S. Effects of low-purine diet supplemented with Sida acuta Burm. f. on growth performance, purine deposition, and biomolecules in slow-growing chickens. Anim. Biosci. 2025, 38, 1756–1772. [Google Scholar] [CrossRef] [PubMed]
- Sabolová, M.; Kulma, M.; Petříčková, D.; Kletečková, K.; Kouřimská, L. Changes in purine and uric acid content in edible insects during culinary processing. Food Chem. 2023, 403, 134349. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Meng, Y.; Zhang, W.; Mai, K. Comparative study on the organoleptic quality of wild and farmed large yellow croaker (Larimichthys crocea). J. Oceanol. Limnol. 2020, 38, 260–274. [Google Scholar] [CrossRef]
- Hong, H.; Regenstein, J.M.; Luo, Y. The Importance of ATP-related Compounds for the Freshness and Flavor of Post-mortem Fish and Shellfish Muscle: A Review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1787–1798. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, G.; Xue, P.; Dong, X.; Xia, Y.; Regenstein, J.; Du, M.; Sun, L. Spoilage microbes’ effect on freshness and IMP degradation in sturgeon fillets during chilled storage. Food Biosci. 2021, 41, 101008. [Google Scholar] [CrossRef]
- Chen, B.; Xu, T.; Yan, Q.; Karsli, B.; Li, D.; Xie, J. Effect of temperature fluctuations on large yellow croaker fillets (Larimichthys crocea) in cold chain logistics: A microbiological and metabolomic analysis. J. Food Eng. 2025, 386, 112290. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, P.; Fang, S.; Mei, J.; Xie, J. Preservative Effects of Gelatin Active Coating Containing Eugenol and Higher CO2 Concentration Modified Atmosphere Packaging on Chinese Sea bass (Lateolabrax maculatus) during Superchilling (−0.9 °C) Storage. Molecules 2020, 25, 871. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, C.; Chen, S.; Xue, Y.; Wang, Y.; Wu, Y. Effects of Modified Atmosphere Packaging with Different Gas Ratios on the Quality Changes of Golden Pompano (Trachinotus ovatus) Fillets during Superchilling Storage. Foods 2022, 11, 1943. [Google Scholar] [CrossRef]
- Babic Milijasevic, J.; Milijasevic, M.; Lilic, S.; Djinovic-Stojanovic, J.; Nastasijevic, I.; Geric, T. Effect of Vacuum and Modified Atmosphere Packaging on the Shelf Life and Quality of Gutted Rainbow Trout (Oncorhynchus mykiss) during Refrigerated Storage. Foods 2023, 12, 3015. [Google Scholar] [CrossRef]
- Ramadhan, A.H.; Yu, D.; Hlaing, K.S.S.; Jiang, Q.; Xu, Y.; Xia, W. Effects of packaging and storage time on lipid and protein oxidation and modifications in texture characteristics of refrigerated grass carp (Ctenopharyngodon idellus) fish muscles. Int. J. Food Sci. Technol. 2025, 60, vvaf082. [Google Scholar] [CrossRef]
- Feng, S.; Wu, S.; Xie, F.; Yang, C.S.; Shao, P. Natural compounds lower uric acid levels and hyperuricemia: Molecular mechanisms and prospective. Trends Food Sci. Technol. 2022, 123, 87–102. [Google Scholar] [CrossRef]
- Yan, Q.; Guo, M.; Chen, B.; Zhang, C.; Li, D.; Xie, J. Molecular characterization of spoilage microbiota in high CO2 refrigerated large yellow croaker (Larimichthys crocea) fillets using metagenomic and metabolomic approaches. Food Biosci. 2023, 56, 103227. [Google Scholar] [CrossRef]
- Chen, B.; Xu, T.; Yan, Q.; Li, D.; Xie, J. Specific spoilage bacteria in cold chain logistics: A study on ATP-related compounds degradation in large yellow croaker (Larimichthys crocea). Food Biosci. 2024, 61, 104745. [Google Scholar] [CrossRef]
- Krata, A.A.; Domagała, J.; Głowacki, R. Hydrophilic interaction liquid chromatography based method for simultaneous determination of purines and their derivatives in food spices. Food Chem. 2024, 441, 138285. [Google Scholar] [CrossRef]
- Li, P.; Mei, J.; Xie, J. Antibacterial mechanism of CO2 combined with low temperature against Shewanella putrefaciens by biochemical and metabolomics analysis. Food Chem. 2024, 460, 140555. [Google Scholar] [CrossRef] [PubMed]
- Kimbuathong, N.; Leelaphiwat, P.; Harnkarnsujarit, N. Inhibition of melanosis and microbial growth in Pacific white shrimp (Litopenaeus vannamei) using high CO2 modified atmosphere packaging. Food Chem. 2020, 312, 126114. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, S.; Wu, G. Nutrition and metabolism of glutamate and glutamine in fish. Amino Acids. 2020, 52, 671–691. [Google Scholar] [CrossRef]
- Chen, B.; Yan, Q.; Li, D.; Xie, J. Degradation mechanism and development of detection technologies of ATP-related compounds in aquatic products: Recent advances and remaining challenges. Crit. Rev. Food Sci. Nutr. 2025, 65, 101–122. [Google Scholar] [CrossRef]
- Yavuzer, E.; Kose, M. Prediction of fish quality level with machine learning. Int. J. Food Sci. Technol. 2022, 57, 5250–5255. [Google Scholar] [CrossRef]
- Park, E.J.; Jung, S.Y.; Kim, S.C.; Lee, D.S.; An, D.S. Modified atmosphere packaging of flounder fillet: Modelling of package conditions and comparison of different flushing atmospheres for quality preservation. Int. Food Res. J. 2023, 30, 1519–1527. [Google Scholar] [CrossRef]
- Chan, S.S.; Rotabakk, B.T.; Løvdal, T.; Sone, I.; Roth, B. Sub-chilling methods for Atlantic salmon with 7 days in refrigerated seawater and subsequent sub-chilled storage. Sci Rep. 2025, 15, 24382. [Google Scholar] [CrossRef]
- Xu, T.; Yan, Q.; Chen, B.; Li, D.; Xie, J. Novel perspective on spoilage metabolism of refrigerated large yellow croaker (Larimichthys crocea) under air-packaging and modified atmosphere-packaging: Insights into adenosine triphosphate degradation. Food Sci. Hum. Wellness 2025. [Google Scholar] [CrossRef]
- Almeida, C.; Neves, M.C.; Freire, M.G. Towards the use of adsorption methods for the removal of purines from beer. Molecules 2021, 26, 6460. [Google Scholar] [CrossRef]
- Amaral, R.A.; Pinto, C.A.; Lima, V.; Tavares, J.; Martins, A.P.; Fidalgo, L.G.; Silva, A.M.; Gil, M.M.; Teixeira, P.; Barbosa, J.; et al. Chemical-Based Methodologies to Extend the Shelf Life of Fresh Fish—A Review. Foods 2021, 10, 2300. [Google Scholar] [CrossRef]
- Gopi, N.; Rekha, R.; Vijayakumar, S.; Liu, G.; Monserrat, J.M.; Faggio, C.; Nor, S.A.M.; Vaseeharan, B. Interactive effects of freshwater acidification and selenium pollution on biochemical changes and neurotoxicity in Oreochromis mossambicus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 250, 109161. [Google Scholar] [CrossRef]
- Hou, C.; Xiao, G.; Amakye, W.K.; Sun, J.; Xu, Z.; Ren, J. Guidelines for purine extraction and determination in foods. Food Front. 2021, 2, 557–573. [Google Scholar] [CrossRef]
- Li, X.; Liu, Z.; Li, Z.; Xiong, X.; Zhang, X.; Yang, C.; Zhao, L.; Zhao, R. A simple, rapid and sensitive HILIC LC-MS/MS method for simultaneous determination of 16 purine metabolites in plasma and urine. Talanta 2024, 267, 125171. [Google Scholar] [CrossRef]
- Dehlin, M.; Jacobsson, L.; Roddy, E. Global epidemiology of gout: Prevalence, incidence, treatment patterns and risk factors. Nat. Rev. Rheumatol. 2020, 16, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wang, Z.; Zhou, L.; Niu, Y.; Yuan, C.; Tian, Y.; Takaki, K. Ultrastructural and biochemical changes of sarcoplasmic reticulum in spotted mackerel (Scomber australasicus Cuvier, 1832) muscle during cold storage at 5 °C. Int. J. Food Sci. Technol. 2023, 58, 3810–3818. [Google Scholar] [CrossRef]
- Wu, G.; Liao, J.; Zhu, X.; Zhang, Y.; Lin, Y.; Zeng, Y.; Zhao, J.; Zhang, J.; Yao, T.; Shen, X.; et al. Shexiang Baoxin Pill enriches Lactobacillus to regulate purine metabolism in patients with stable coronary artery disease. Phytomedicine 2024, 130, 155727. [Google Scholar] [CrossRef]
- Sadeghi, S.; Khodanazary, A.; Hosseini, S.M. The influence of chitosan-carboxymethyl cellulose composite and bi-layer film and coatings on flavor quality and volatile profile of Asian sea bass during storage at refrigerator. J. Food Meas. Charact. 2021, 15, 5495–5506. [Google Scholar] [CrossRef]
- Huang, C.; Zheng, M.; Huang, Y.; Cai, L.; Zou, X.; Yao, T.; Xie, X.; Yang, B.; Xiao, S.; Ma, J.; et al. Unraveling genetic underpinnings of purine content in pork. J. Integr. Agric. 2024; in press. [Google Scholar]
- Zhou, X.; Liu, K.; Shi, C.; Zhang, M.; Liu, S.; Hou, C.; Di, B. Estimation of the spatial pattern of gout prevalence across China by wastewater-based epidemiology. Sci. Total Environ. 2024, 924, 171565. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, T.; Lu, W.; Chen, B.; Li, D.; Xie, J. Effects of Different Packaging on the Purine Content and Key Enzymes of Refrigerated Yellow Croaker (Larimichthys crocea). Foods 2025, 14, 2732. https://doi.org/10.3390/foods14152732
Xu T, Lu W, Chen B, Li D, Xie J. Effects of Different Packaging on the Purine Content and Key Enzymes of Refrigerated Yellow Croaker (Larimichthys crocea). Foods. 2025; 14(15):2732. https://doi.org/10.3390/foods14152732
Chicago/Turabian StyleXu, Tiansheng, Wenxuan Lu, Bohan Chen, Dapeng Li, and Jing Xie. 2025. "Effects of Different Packaging on the Purine Content and Key Enzymes of Refrigerated Yellow Croaker (Larimichthys crocea)" Foods 14, no. 15: 2732. https://doi.org/10.3390/foods14152732
APA StyleXu, T., Lu, W., Chen, B., Li, D., & Xie, J. (2025). Effects of Different Packaging on the Purine Content and Key Enzymes of Refrigerated Yellow Croaker (Larimichthys crocea). Foods, 14(15), 2732. https://doi.org/10.3390/foods14152732







