Metabolic Variations in Bamboo Shoot Boiled Liquid During Pediococcus pentosaceus B49 Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of BSBL Broth for Fermentation
2.3. Extraction of Metabolites
2.4. UHPLC-MS/MS Detection
2.5. Metabolite Identification and Quantitative Analysis
2.6. Pathway Analysis
2.7. Statistical Analysis
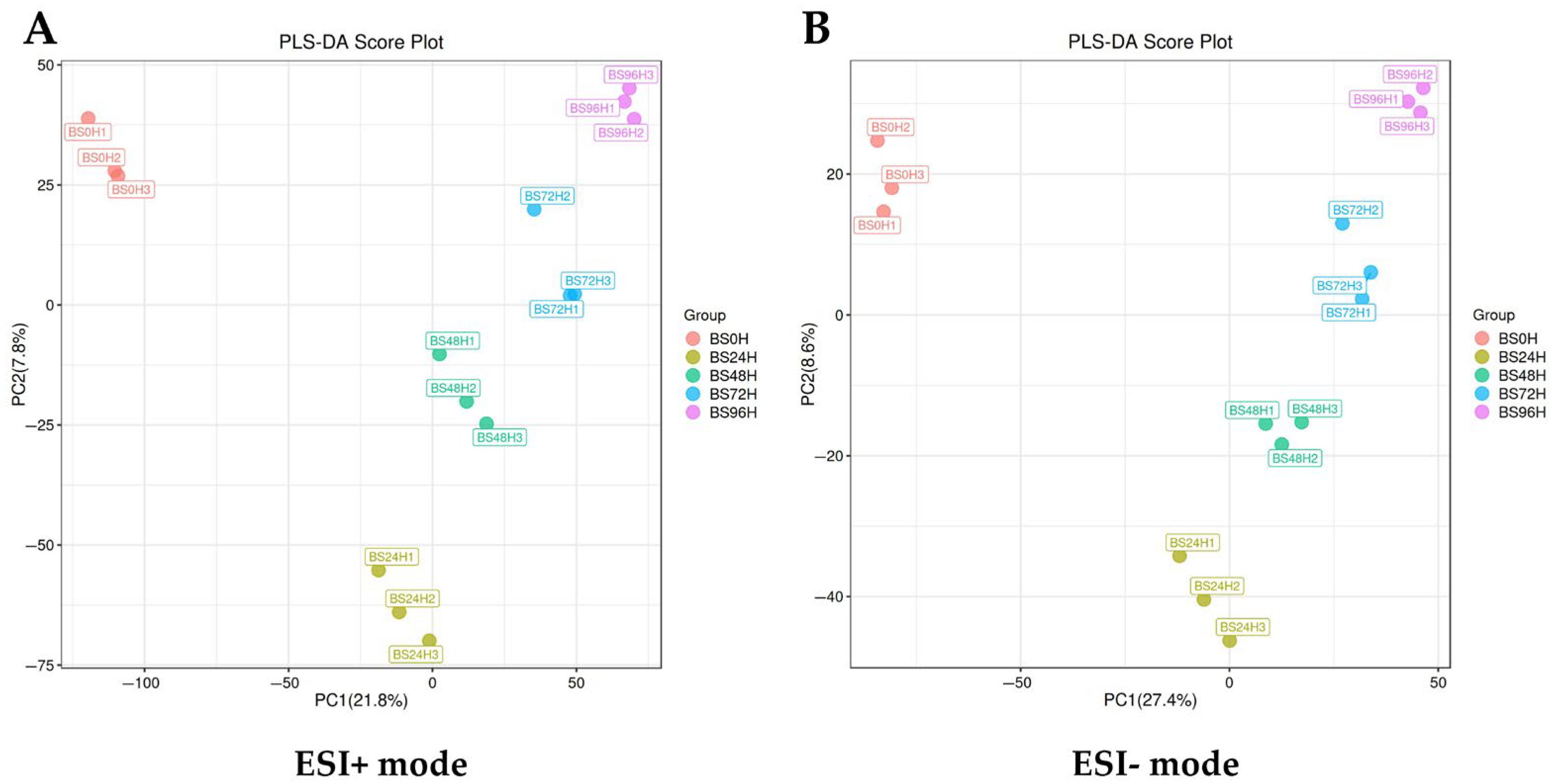
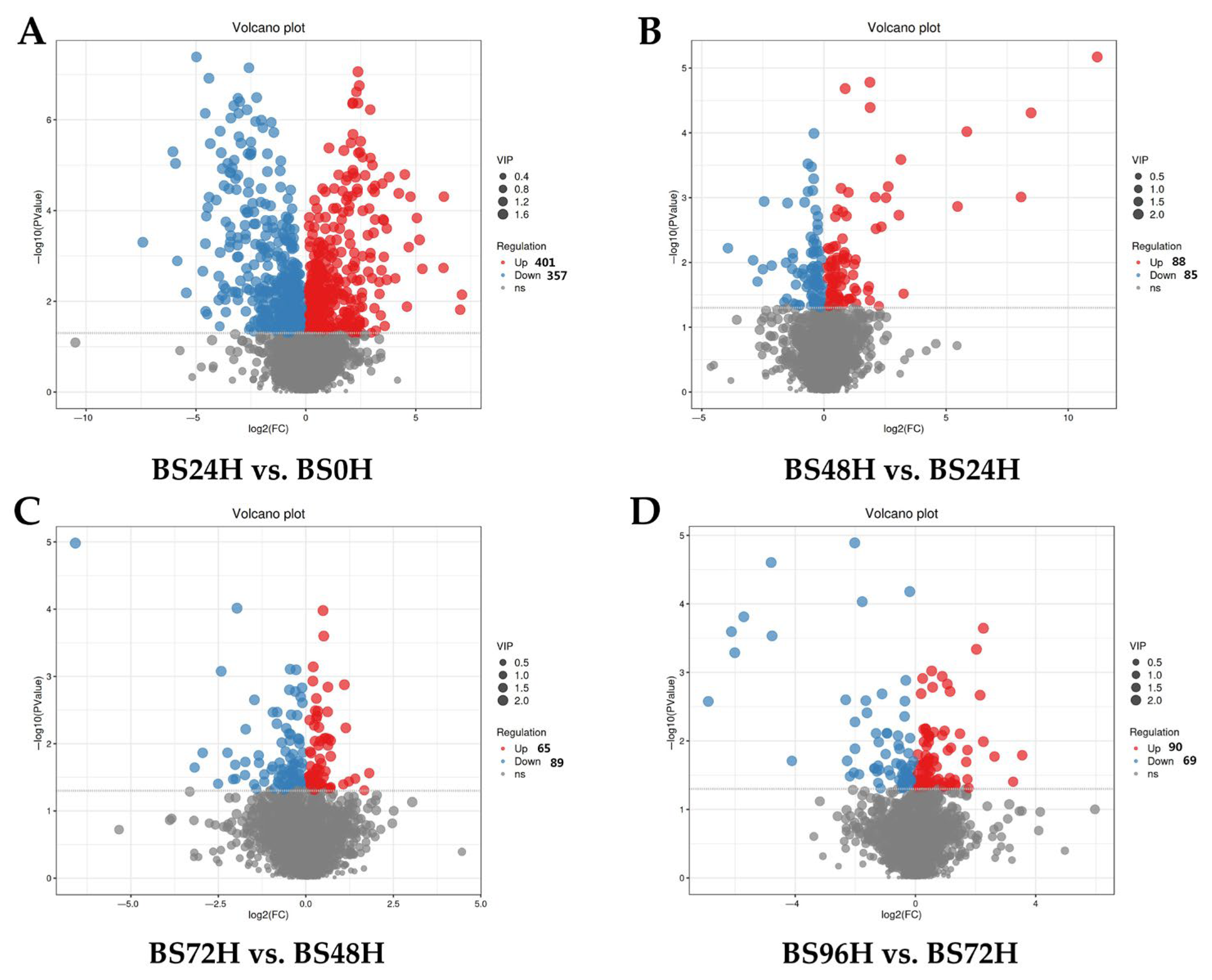
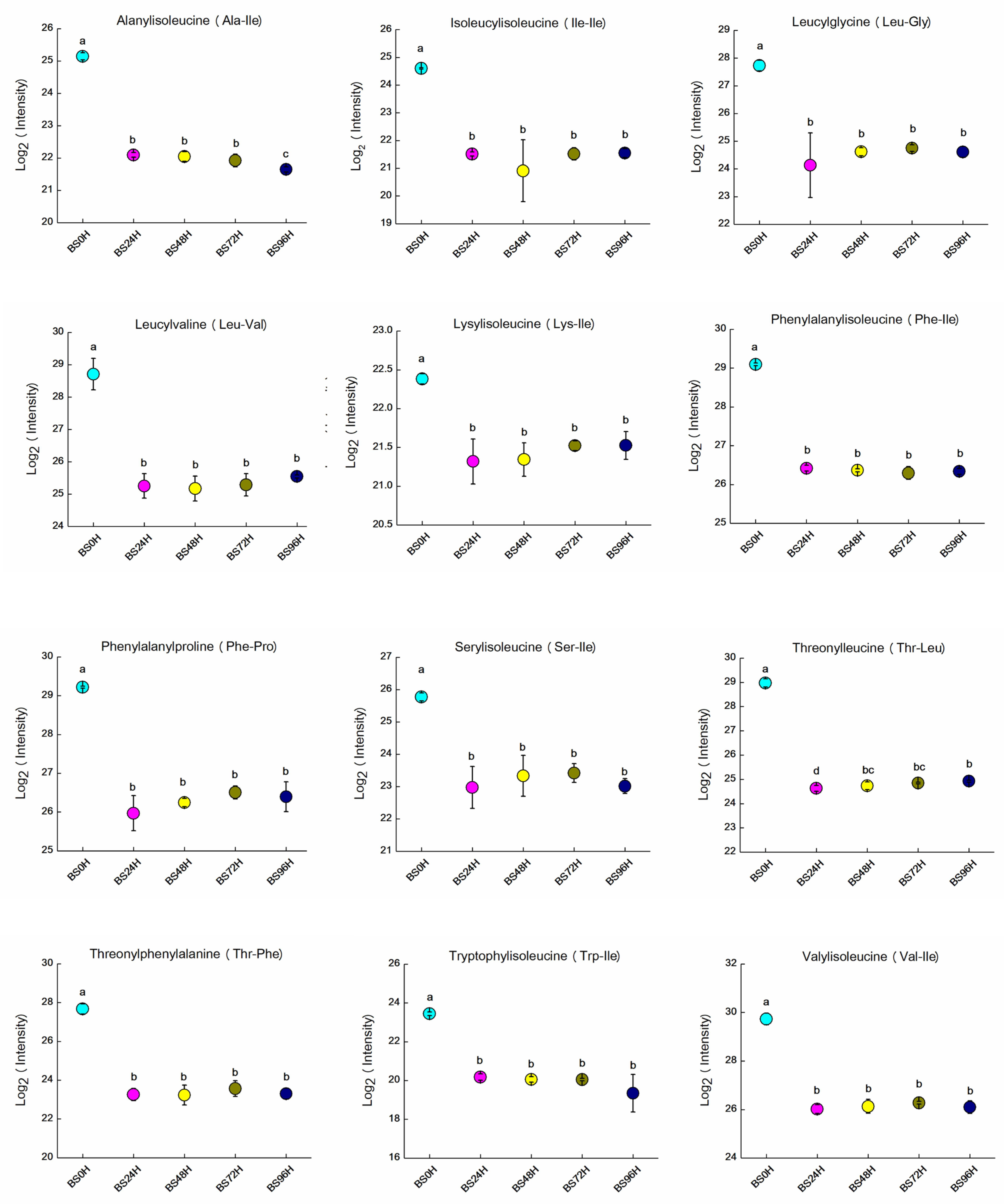
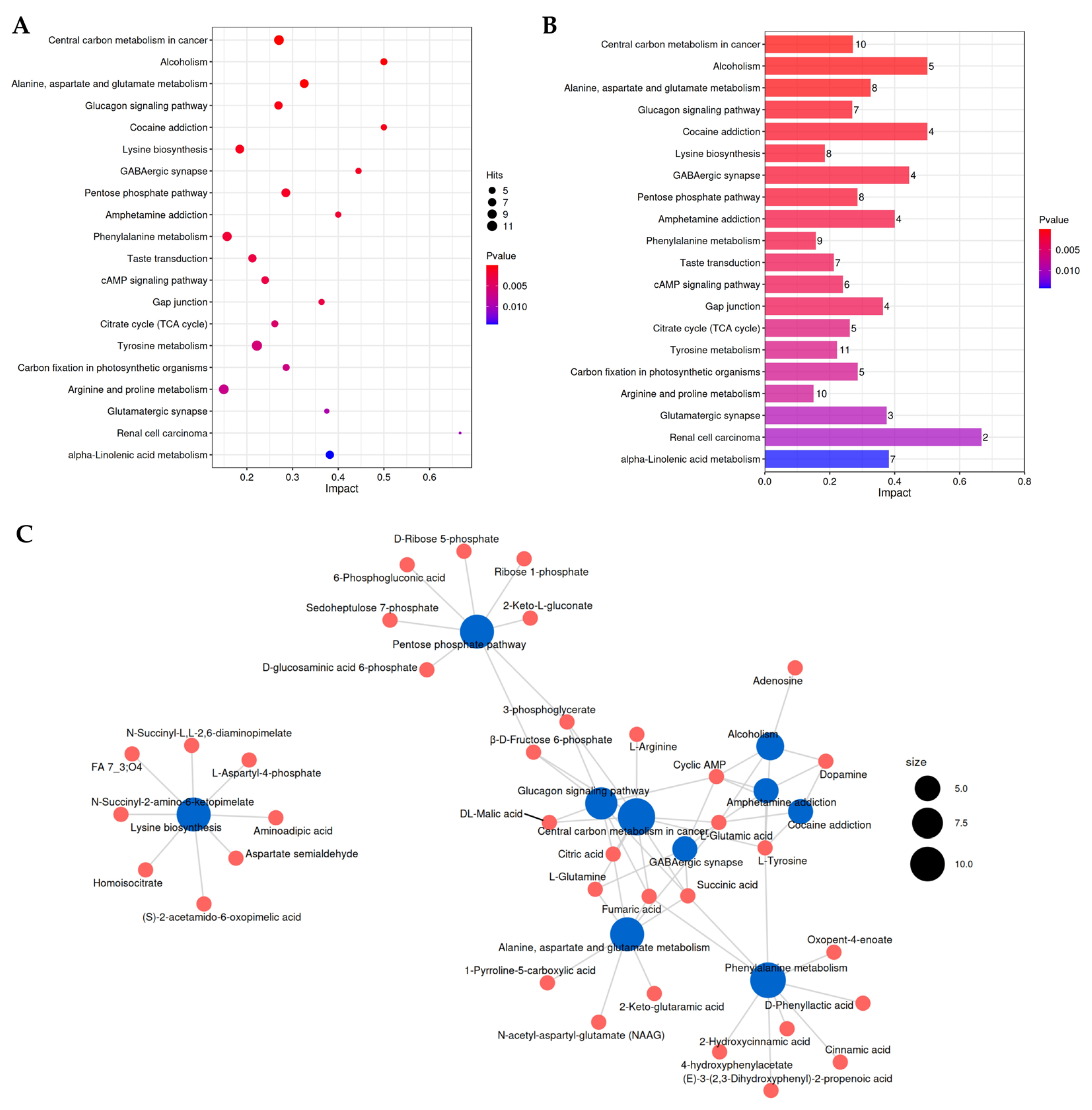
3. Results and Discussion
3.1. Overall Differences in Metabolite Profiles
3.2. Volcano Plot Analysis
3.3. Identification of DMs
3.4. Variations in Selected Phenylpropanoids Metabolites
3.5. Variations in Bitter Peptides
3.6. Overall KEGG Pathway Enrichment Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Wu, L.; Li, Y.; Yang, J.; Yang, H.; Zhao, Y.; Chen, G. Bamboo shoot and its food applications in last decade: An undervalued edible resource from forest to feed future people. Trends. Food Sci. Technol. 2024, 146, 104399. [Google Scholar] [CrossRef]
- Jana, U.K.; Bhardwaj, P.K.; Jeyaram, K.; Shukla, J.K.; Somkuwar, B.G.; Mukherjee, P.K. Bamboo shoots: Comprehensive perspectives on food composition, nutritional value, and therapeutic potential. J. Food Compos. Anal. 2025, 140, 107198. [Google Scholar] [CrossRef]
- NSBC Database. National Bureau of Statistics of China. Available online: http://www.stats.gov.cn/ (accessed on 14 January 2021).
- Ma, T.; Mo, W.; Lv, B.; Wang, W.; He, H.; Jian, C.; Liu, X.; Li, S.; Guo, Y. A Review of the Nutritional Composition, Storage Challenges, Processing Technology and Widespread Use of Bamboo Shoots. Foods 2024, 13, 3539. [Google Scholar] [CrossRef]
- Lin, Z.; Chen, J.; Zhang, J.; Brooks, M.S. Potential for Value-Added Utilization of Bamboo Shoot Processing Waste—Recommendations for a Biorefinery Approach. Food Bioproc. Tech. 2018, 11, 901–912. [Google Scholar] [CrossRef]
- Liu, L.; Liu, L.; Lu, B.; Chen, M.; Zhang, Y. Evaluation of Bamboo Shoot Peptide Preparation with Angiotensin Converting Enzyme Inhibitory and Antioxidant Abilities from Byproducts of Canned Bamboo Shoots. J. Agric. Food Chem. 2013, 61, 5526–5533. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Sánchez, M.; Carrasco-Navarro, U.; Juárez-Castelán, C.; Lozano-Aguirre Beltrán, L.; Pérez-Chabela, M.L.; Ponce-Alquicira, E. Probiotic Properties and Proteomic Analysis of Pediococcus pentosaceus 1101. Foods 2023, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Wang, Y.; Xie, J.; Xuan, J.; Geng, J.; Liu, G.; Tu, J.; Xiao, H. Pediococcus pentosaceus JS35 improved flavor, metabolic profile of fermentation supernatant of mulberry leaf powder and increased its antioxidant capacity. Front. Nutr. 2025, 12, 1551689. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, S.; Wang, Q.; Guan, X.; Qian, L.; Li, J.; Zheng, Y.; Lin, B. Pediococcus pentosaceus B49 from human colostrum ameliorates constipation in mice. Food Funct. 2020, 11, 5607–5620. [Google Scholar] [CrossRef]
- Demurtas, A.; Pescina, S.; Nicoli, S.; Santi, P.; Ribeiro De Araujo, D.; Padula, C. Validation of a HPLC-UV method for the quantification of budesonide in skin layers. J. Chromatogr. B 2021, 1164, 122512. [Google Scholar] [CrossRef]
- Zelena, E.; Dunn, W.B.; Broadhurst, D.; Francis-McIntyre, S.; Carroll, K.M.; Begley, P.; O’Hagan, S.; Knowles, J.D.; Halsall, A.; Wilson, I.D.; et al. Development of a Robust and Repeatable UPLC−MS Method for the Long-Term Metabolomic Study of Human Serum. Anal. Chem. 2009, 81, 1357–1364. [Google Scholar] [CrossRef]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef]
- Rasmussen, J.A.; Villumsen, K.R.; Ernst, M.; Hansen, M.; Forberg, T.; Gopalakrishnan, S.; Gilbert, M.T.P.; Bojesen, A.M.; Kristiansen, K.; Limborg, M.T. A multi-omics approach unravels metagenomic and metabolic alterations of a probiotic and synbiotic additive in rainbow trout (Oncorhynchus mykiss). Microbiome 2022, 10, 21. [Google Scholar] [CrossRef]
- Navarro-Reig, M.; Jaumot, J.; García-Reiriz, A.; Tauler, R. Evaluation of changes induced in rice metabolome by Cd and Cu exposure using LC-MS with XCMS and MCR-ALS data analysis strategies. Anal. Bioanal. Chem. 2015, 407, 8835–8847. [Google Scholar] [CrossRef] [PubMed]
- Thévenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the Human Adult Urinary Metabolome Variations with Age, Body Mass Index, and Gender by Implementing a Comprehensive Workflow for Univariate and OPLS Statistical Analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wishart, D.S. Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat. Protoc. 2011, 6, 743–760. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Hwang, J. The 5,7-Dimethoxyflavone Suppresses Sarcopenia by Regulating Protein Turnover and Mitochondria Biogenesis-Related Pathways. Nutrients 2020, 12, 1079. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, Y.; Su, X. Antidiabetic and Hypolipidemic Effects of 5,7-Dimethoxyflavone in Streptozotocin-Induced Diabetic Rats. Med. Sci. Monit. Int. Med. J. Exp. Clin. Research 2019, 25, 9893–9901. [Google Scholar] [CrossRef]
- Bae, J.; Kumazoe, M.; Park, S.; Fujimura, Y.; Tachibana, H. The anti-cancer effect of epigallocatechin-3-O-gallate against multiple myeloma cells is potentiated by 5,7-dimethoxyflavone. Febs. Open Bio. 2023, 13, 2147–2156. [Google Scholar] [CrossRef]
- Hong, Z.C.; Duan, X.Y.; Wu, S.T.; Yang, Y.F.; Wu, H.Z. Network Pharmacology Integrated Molecular Docking Reveals the Anti-COVID-19 Mechanism of Qing-Fei-Da-Yuan Granules. Nat. Prod. Commun. 2020, 15, 1–15. [Google Scholar] [CrossRef]
- Kleszczyński, K.; Bilska, B.; Stegemann, A.; Flis, D.J.; Ziolkowski, W.; Pyza, E.; Luger, T.A.; Reiter, R.J.; Böhm, M.; Slominski, A.T. Melatonin and Its Metabolites Ameliorate UVR-Induced Mitochondrial Oxidative Stress in Human MNT-1 Melanoma Cells. Int. J. Mol. Sci. 2018, 19, 3786. [Google Scholar] [CrossRef]
- Cheng, Y.; Park, T.H.; Seong, H.; Kim, T.; Han, N.S. Biological characterization of D-lactate dehydrogenase responsible for high-yield production of D-phenyllactic acid in Sporolactobacillus inulinus. Microb. Biotechnol. 2022, 15, 2717–2729. [Google Scholar] [CrossRef]
- Kandler, O. Carbohydrate metabolism in lactic acid bacteria. Antonie Van Leeuwenhoek 1983, 49, 209–224. [Google Scholar] [CrossRef]
- Yokota, T.; Baba, J.; Koba, S.; Takahashi, N. Purification and Separation of Eight Steroidal Plant-growth Regulators from Dolichos lablab Seed. Agric. Biol. Chem. 1984, 48, 2529–2534. [Google Scholar] [CrossRef]
- Lu, B.; Ren, Y.; Zhang, Y.; Gong, J. Effects of genetic variability, parts and seasons on the sterol content and composition in bamboo shoots. Food Chem. 2009, 112, 1016–1021. [Google Scholar] [CrossRef]
- Jiang, W.; Ye, C.; Hu, M.; Liu, Y.; Zhang, Y.; Liang, J.; Chen, Y. Mechanism of Alisma orientale triterpenes on anti-liver fibrosis effect based on network pharmacology and molecular docking. Chin. Tradit. Herb. Drugs 2022, 53, 1100–1111. [Google Scholar]
- Zhang, Y.; Yao, X.; Bao, B.; Zhang, Y. Anti-fatigue activity of a triterpenoid-rich extract from Chinese bamboo shavings (Caulis bamfusae in taeniam). Phytother. Res. 2006, 20, 872–876. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, C.; Urios, A.; González, M.C.; Moya, P.; Blanco, M. Screening for metabolites from Penicillium novae-zeelandiae displaying radical-scavenging activity and oxidative mutagenicity: Isolation of gentisyl alcohol. Mutat. Res./Genet. Toxicol. Environ. Mutagenesis. 2003, 539, 187–194. [Google Scholar] [CrossRef]
- Kaleta, M.; Kolitha, B.S.; Novák, O.; Rad, F.M.; Bergquist, J.; Ubhayasekera, S.J.K.A. Targeted analysis of seven selected tryptophan-melatonin metabolites: Simultaneous quantification of plasma analytes using fast and sensitive UHPLC—MS/MS. J. Chromatogr. B 2025, 1256, 124520. [Google Scholar] [CrossRef]
- Shin, M.; Umezawa, C.; Shin, T. Natural Anti-Microbial Systems Antimicrobial Compounds in Plants. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press Oxford: Oxford, UK, 2014; pp. 920–929. ISBN 978-0-12-384733-1. [Google Scholar]
- Kozlowska, L.; Viegas, S.; Scheepers, P.T.J.; Duca, R.C.; Godderis, L.; Martins, C.; Ciura, K.; Jagiello, K.; João Silva, M.; Mahiout, S.; et al. HBM4EU E-waste study—An untargeted metabolomics approach to characterize metabolic changes during E-waste recycling. Environ. Int. 2025, 196, 109281. [Google Scholar] [CrossRef]
- Jana, A.; Halder, S.K.; Banerjee, A.; Paul, T.; Pati, B.R.; Mondal, K.C.; Das Mohapatra, P.K. Biosynthesis, structural architecture and biotechnological potential of bacterial tannase: A molecular advancement. Bioresour. Technol. 2014, 157, 327–340. [Google Scholar] [CrossRef]
- Wu, G.S.; Nabih, T.; Youel, L.; Peczyńska Czoch, W.; Rosazza, J.P.N. Microbial Transformations of Natural Antitumor Agents: O-Demethylation of Vindoline by Sepedonium chrysospermum. Antimicrob. Agents Chemother. 1978, 14, 601–604. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Wu, P. 5′-Methylthioadenosine and Cancer: Old molecules, new understanding. J. Cancer 2019, 10, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Chávez, D.; Mata, R. Purpuracenin: A new cytotoxic adjacent bis-tetrahydrofuran annonaceous acetogenin from the seeds of Annona purpurea. Phytochemistry 1999, 50, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Vangaveti, V.N.; Jansen, H.; Kennedy, R.L.; Malabu, U.H. Hydroxyoctadecadienoic acids: Oxidised derivatives of linoleic acid and their role in inflammation associated with metabolic syndrome and cancer. Eur. J. Pharmacol. 2016, 785, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, M.; Shimizu, M.; Lu, P.; Takahashi, Y.; Yamauchi, Y.; Sato, S.; Kiyono, H.; Kishino, S.; Ogawa, J.; Nagata, K.; et al. Lactic acid bacteria–derived γ-linolenic acid metabolites are PPARδ ligands that reduce lipid accumulation in human intestinal organoids. J. Biol. Chem. 2022, 298, 102534. [Google Scholar] [CrossRef]
- Nicolella, H.D.; Ribeiro, A.B.; Melo, M.R.S.D.; Ozelin, S.D.; Domingos Da Silva, L.H.; Sola Veneziani, R.C.; Crispim Tavares, D. Antitumor Effect of Manool in a Murine Melanoma Model. J. Nat. Prod. 2022, 85, 426–432. [Google Scholar] [CrossRef]
- Giossi, C.; Cartaxana, P.; Cruz, S. Photoprotective Role of Neoxanthin in Plants and Algae. Molecules 2020, 25, 4617. [Google Scholar] [CrossRef]
- Fang, J.; Guo, Y.; Yin, W.; Zhang, L.; Li, G.; Ma, J.; Xu, L.; Xiong, Y.; Liu, L.; Zhang, W.; et al. Neoxanthin alleviates the chronic renal failure-induced aging and fibrosis by regulating inflammatory process. Int. Immunopharmacol. 2023, 114, 109429. [Google Scholar] [CrossRef]
- Kirby, J.; Nishimoto, M.; Park, J.G.; Withers, S.T.; Nowroozi, F.; Behrendt, D.; Rutledge, E.J.G.; Fortman, J.L.; Johnson, H.E.; Anderson, J.V.; et al. Cloning of casbene and neocembrene synthases from Euphorbiaceae plants and expression in Saccharomyces cerevisiae. Phytochemistry 2010, 71, 1466–1473. [Google Scholar] [CrossRef]
- Fan, X.; Li, C.; Luo, J.; Wang, R.; Zhang, H. Lipid composition and its molecular classes of milk fat globule membranes derived from yak, buffalo, and holstein cow milk were characterized based on UHPLC-MS/MS and untargeted-lipidomics. LWT 2025, 219, 117563. [Google Scholar] [CrossRef]
- Leonard, W.; Zhang, P.; Ying, D.; Adhikari, B.; Fang, Z. Fermentation transforms the phenolic profiles and bioactivities of plant-based foods. Biotechnol. Adv. 2021, 49, 107763. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Y.; Wu, Y.; Liu, Y.; Wu, Z. Fermentation and complex enzyme hydrolysis for improving the total soluble phenolic contents, flavonoid aglycones contents and bio-activities of guava leaves tea. Food Chem. 2018, 264, 189–198. [Google Scholar] [CrossRef]
- Abbasi-Parizad, P.; De Nisi, P.; Pepè Sciarria, T.; Scarafoni, A.; Squillace, P.; Adani, F.; Scaglia, B. Polyphenol bioactivity evolution during the spontaneous fermentation of vegetal by-products. Food Chem. 2022, 374, 131791. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, J.; Li, L.; Gu, T.; Chen, S.; Wang, J.; Gao, M. The Release of Bound Phenolics to Enhance the Antioxidant Activity of Cornmeal by Liquid Fermentation with Bacillus subtilis. Foods 2025, 14, 499. [Google Scholar] [CrossRef]
- Li, J.; Qin, C.; Chen, N. Evaluation of antioxidant, antidiabetic and antiobesity potential of phenylpropanoids (PPs): Structure-activity relationship and insight into action mechanisms against dual digestive enzymes by comprehensive technologies. Bioorg. Chem. 2024, 146, 107290. [Google Scholar] [CrossRef]
- Sultan, M.F.; Karim, T.; Hossain Shaon, M.S.; Ali, M.M.; Ibrahim, S.M.; Akter, M.S.; Ahmed, K.; Bui, F.M.; Moni, M.A. BitterEN: A novel ensemble model for the identification of bitter peptide. Comput. Biol. Med. 2025, 195, 110528. [Google Scholar] [CrossRef]

| Name | log2FC (BS24H vs. BS0H) | Name | log2FC (BS48H vs. BS24H) |
|---|---|---|---|
| 5,7-dimethoxyflavone | 4.50 | homodolicholide | 11.19 |
| 1,3-nonanediol | 3.79 | alisol C monoacetate | 5.47 |
| (3Z,6Z)-3,6-nonadienal | 2.93 | 7-methylxanthosine | 3.15 |
| medicocarpin | 2.70 | neoxanthin | 2.54 |
| N-succinyl-2-amino-6-ketopimelate | 2.49 | edetic acid | 2.13 |
| 1-methoxy-2-hydroxyanthracene | 2.46 | norvaline | 1.88 |
| 6-hydroxymelatonin | 2.43 | PG (16:0/16:0) | 1.80 |
| D-phenyllactic acid | 2.39 | gentisyl alcohol | 1.30 |
| N-succinyl-L,L-2,6-diaminopimelate | 2.37 | TG(24_2) | 1.19 |
| 10-hydroxydecanoic acid | 2.30 | bruceine | 1.00 |
| (E)-3-heptenyl acetate | 2.14 | 6-hydroxymelatonin | 0.87 |
| toluene-cis-dihydrodiol | 2.14 | suberylglycine | 0.86 |
| citramalate | 2.12 | 2,3,5-hexanetrione | 0.84 |
| 2-inosose | 2.05 | Pro-Met | 0.82 |
| N-acetyl-serylaspartic acid | 1.89 | farnesyl acetate | 0.77 |
| ornithine | 1.73 | 1-octadecanethiol | 0.75 |
| β-alanine | 1.49 | phaseollin | 0.70 |
| Ile-Val-Gln | −2.03 | 5-methoxyindoleacetate | 0.67 |
| Ile-Val-Gly | −2.24 | vanylglycol | 0.47 |
| DL-malic acid | −2.59 | tartaric acid | −0.52 |
| 3-phosphoglycerate | −2.63 | 7-methylxanthine | −0.53 |
| Phe-Ile | −2.68 | (+)-gallocatechin | −0.65 |
| Ala-Ile | −3.05 | N-acetylanthranilate | −0.66 |
| Ile-Ile | −3.09 | acetyl-L-carnitine | −0.79 |
| Trp-Ile | −3.26 | spermine | −0.89 |
| Val-Val-Ala | −3.35 | phosphoribosyl pyrophosphate | −1.08 |
| Val-Ile-Thr | −3.80 | O-demethylmetoprolol | −1.30 |
| Thr-Leu | −4.35 | N-acetylprocainamide | −1.55 |
| Ile-Val-Val | −4.41 | leucocyanidin | −2.90 |
| 6-phosphogluconic acid | −4.98 | PG (18:1/18:1) | −3.93 |
| Name | log2FC (BS72H vs. BS48H) | Name | log2FC (BS96H vs. BS72H) |
|---|---|---|---|
| N-demethylvindolidine | 1.10 | citramalate | 2.26 |
| 5-methylthioadenosine | 0.71 | Gly-Val | 2.03 |
| cyclic melatonin | 0.71 | manool | 1.69 |
| acetyl-L-carnitine | 0.65 | 3-hydroxy-3-methylglutarate | 1.48 |
| Val-Ile-Val-Leu-Leu | 0.63 | neoxanthin | 1.15 |
| purpuritenin B | 0.63 | neocembrene | 1.08 |
| 6-hydroxymelatonin | 0.62 | Gly-Pro-Arg | 0.97 |
| DG(15_0) | 0.56 | 3-oxalomalic acid | 0.89 |
| canavaninosuccinate | 0.51 | ethyl tetradecanoate | 0.61 |
| Gly-Pro-Hyp | 0.49 | (S)-styrene oxide | 0.60 |
| sinuatol | 0.44 | suberoyl-L-carnitine | 0.54 |
| andrographic acid | 0.41 | Lys-Phe | 0.46 |
| 3-phenylcatechol | 0.40 | 5-methyltricosanoylcarnitine | 0.46 |
| obacunone | 0.39 | 6-hydroxymelatonin | 0.43 |
| magnoflorine | 0.34 | N-hexanoyl-L-homoserine lactone | 0.29 |
| lysionotin | −0.52 | dihydrovaltrate | 0.28 |
| 9-methylhenicosanoylcarnitine | −0.58 | myristoleate | 0.28 |
| 9-cis-Retinoic acid | −0.69 | 7-methylxanthine | 0.27 |
| 13-OxoODE | −0.72 | Val-Arg-Ser | 0.25 |
| 3-oxalomalic acid | −0.75 | Thr-Arg-Glu | 0.24 |
| Ile-Gly-Lys | −0.95 | Gly-Pro-Hyp | −0.36 |
| palmitic acid | −0.98 | matairesinoside | −0.47 |
| γ-glutamyl-L-putrescine | −1.34 | carviolin | −0.47 |
| geranylhydroquinone | −1.35 | andrographic acid | −0.56 |
| Leu-Leu-Asp-Leu-Leu | −1.47 | N-acetylanthranilate | −0.58 |
| citramalate | −1.97 | ethyl 3-indoleacetate | −0.64 |
| peonidin 3-rhamnoside 5-glucoside | −2.24 | DG (15:0/15:0) | −0.83 |
| sorbose | −2.95 | methylphosphatidylcholine (42:9) | −1.20 |
| alisol C monoacetate | −3.18 | 13(S)-hydroperoxy-9,11-octadecadienoic acid | −1.77 |
| benzoylcholine | −6.59 | 1-hydroxy-γ-carotene glucoside | −2.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Sun, M.; Guan, X.; Zhong, L.; Li, J.; Wang, Q.; Zhang, S. Metabolic Variations in Bamboo Shoot Boiled Liquid During Pediococcus pentosaceus B49 Fermentation. Foods 2025, 14, 2731. https://doi.org/10.3390/foods14152731
Huang J, Sun M, Guan X, Zhong L, Li J, Wang Q, Zhang S. Metabolic Variations in Bamboo Shoot Boiled Liquid During Pediococcus pentosaceus B49 Fermentation. Foods. 2025; 14(15):2731. https://doi.org/10.3390/foods14152731
Chicago/Turabian StyleHuang, Juqing, Meng Sun, Xuefang Guan, Lingyue Zhong, Jie Li, Qi Wang, and Shizhong Zhang. 2025. "Metabolic Variations in Bamboo Shoot Boiled Liquid During Pediococcus pentosaceus B49 Fermentation" Foods 14, no. 15: 2731. https://doi.org/10.3390/foods14152731
APA StyleHuang, J., Sun, M., Guan, X., Zhong, L., Li, J., Wang, Q., & Zhang, S. (2025). Metabolic Variations in Bamboo Shoot Boiled Liquid During Pediococcus pentosaceus B49 Fermentation. Foods, 14(15), 2731. https://doi.org/10.3390/foods14152731








