Potential of Plant-Based Oil Processing Wastes/By-Products as an Alternative Source of Bioactive Compounds in the Food Industry
Abstract
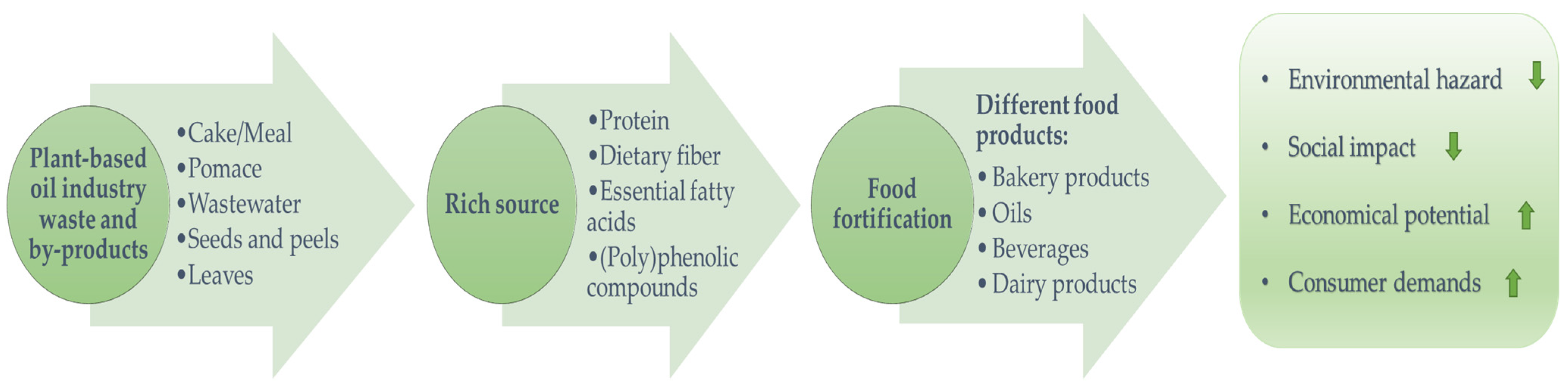
1. Introduction
2. Valorization of Waste/By-Products from Fruit-Based Oil Processing
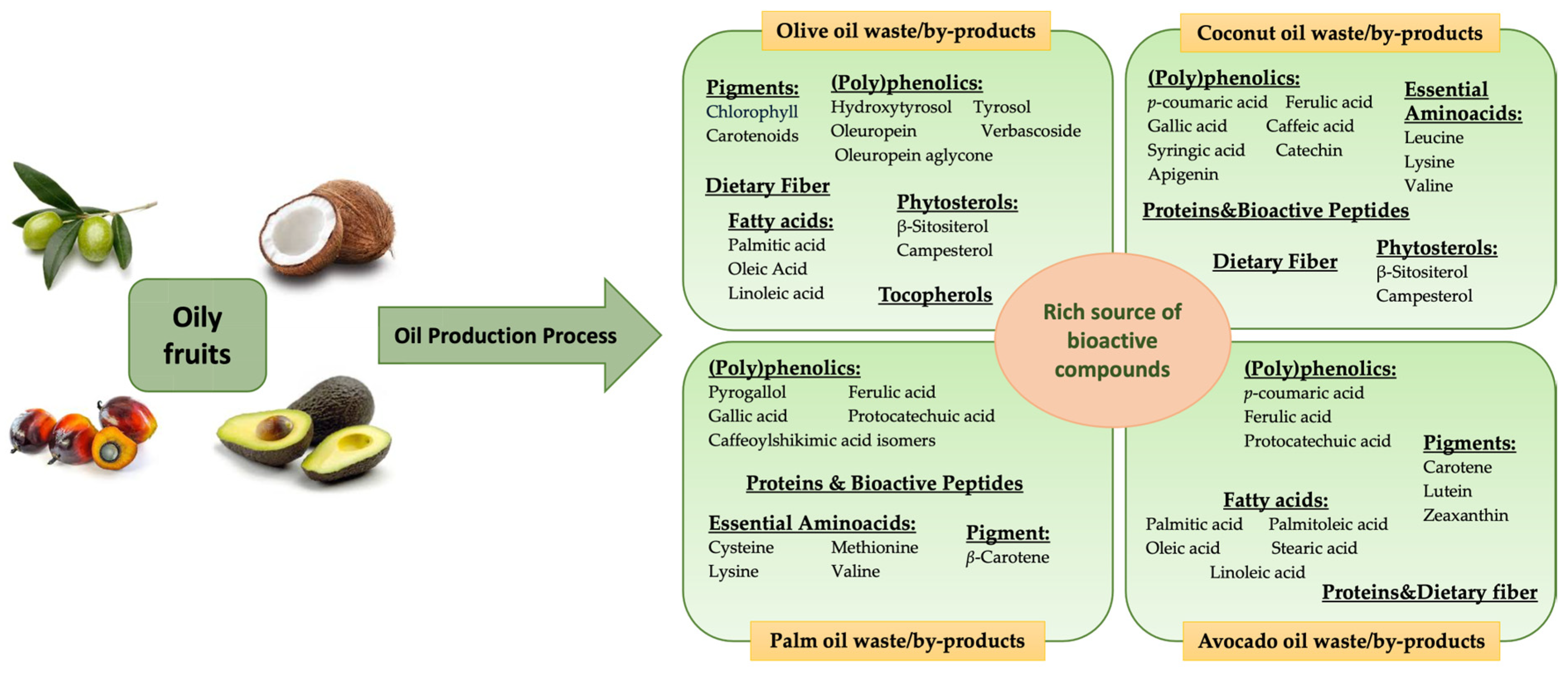
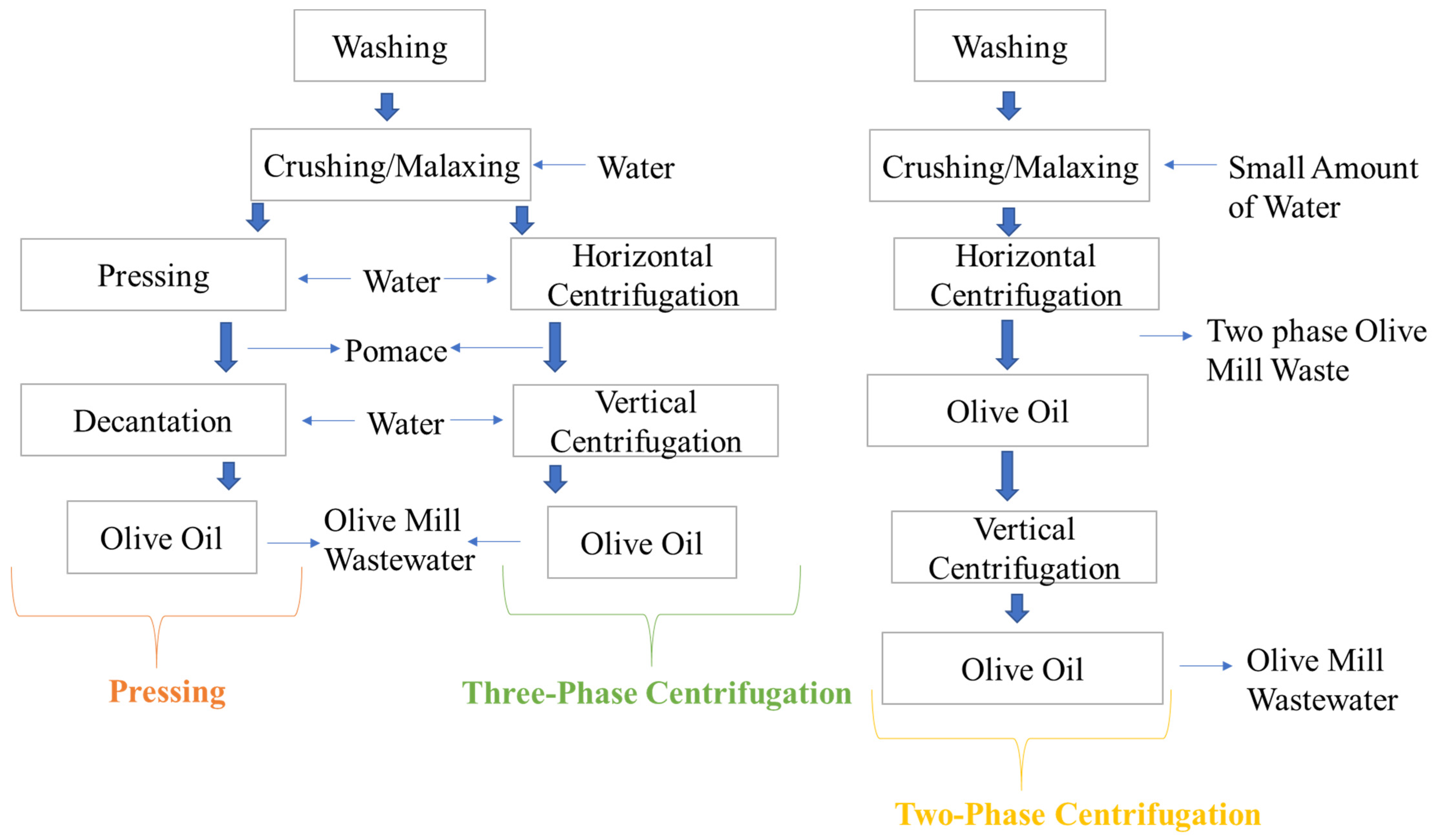
2.1. Olive Oil Processing Waste/By-Products
2.2. Palm Oil Processing Waste/By-Products
2.3. Avocado Oil Processing Waste/By-Products
2.4. Coconut Oil Processing Waste/By-Products
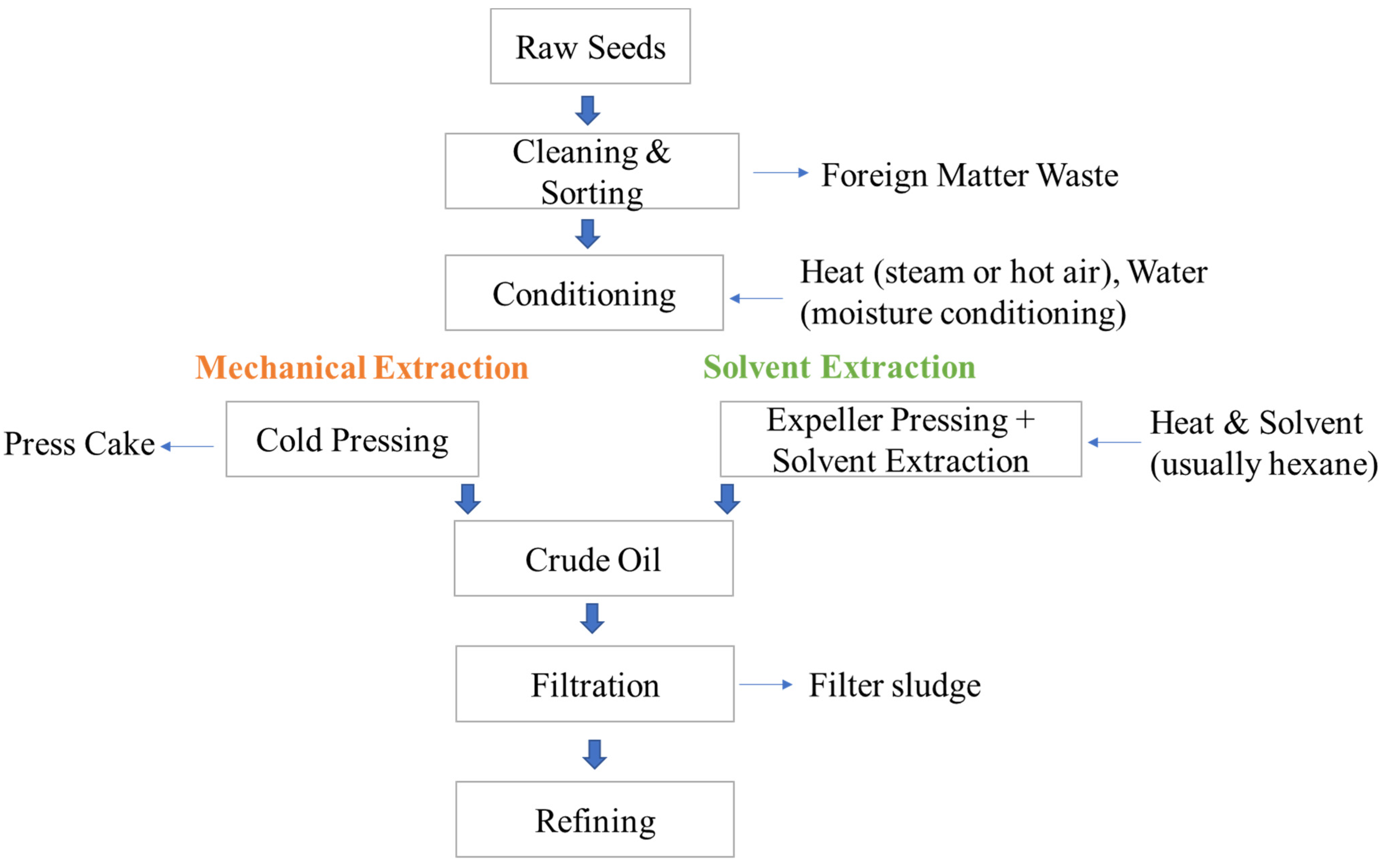
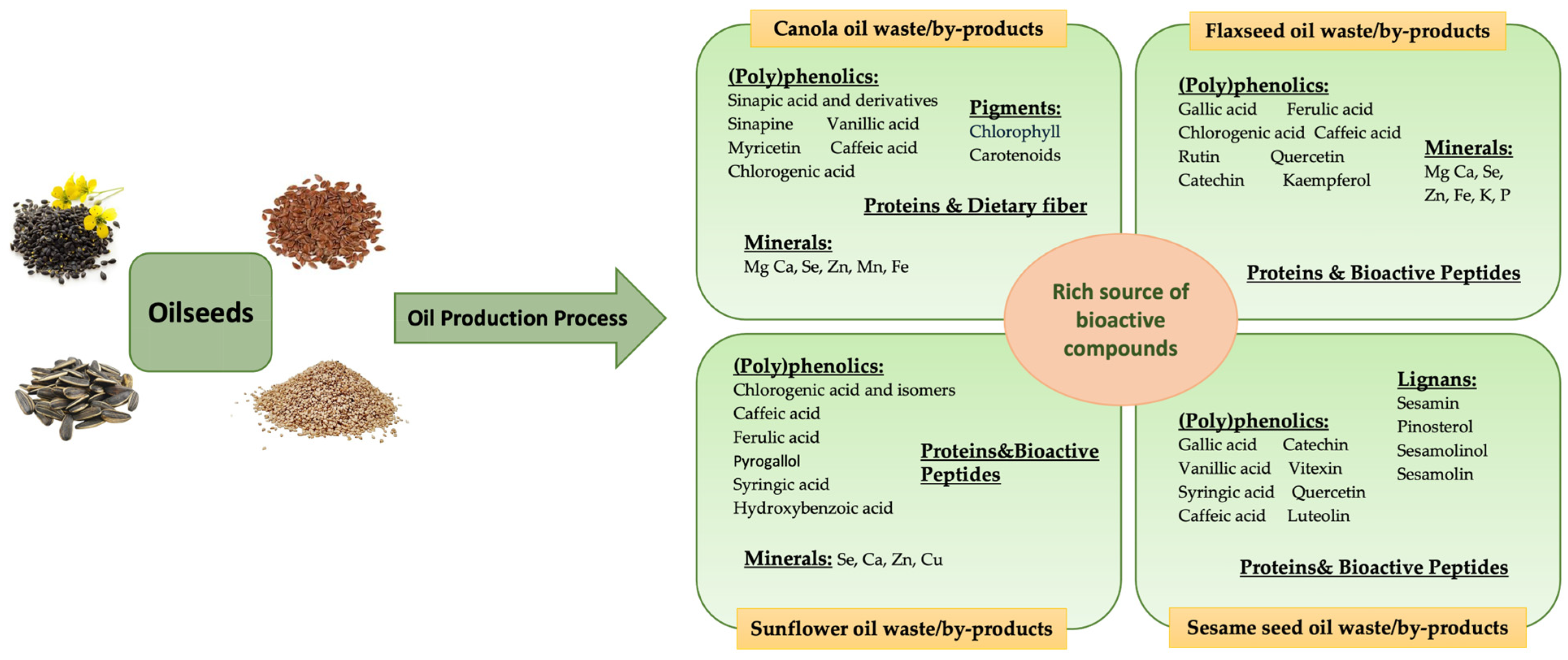
3. Valorization of Waste and By-Products from Oilseed-Based Oil Processing
3.1. Canola/Rapeseed Oil Processing Waste/By-Products
3.2. Sunflower Oil Processing Waste/By-Products
3.3. Flaxseed Oil Processing Waste/By-Products
3.4. Sesame Seed Oil Processing Waste/By-Products
4. Limitations and Future Perspectives
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) assay |
| BHA | Butylated hydroxyanisole |
| BHT | Butylated hydroxytoluene |
| CAGR | Compound annual growth rate |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl assay |
| dw | Dry weight |
| FAO | Food and Drug Organisation |
| FRAP | Ferric reducing antioxidant power assay |
| GAE | Gallic acid equivalent |
| HPMC | Hydroxypropyl methylcellulose |
| ELISA | Enzyme-linked immunosorbent assay |
| NADES | Natural deep eutectic Ssolvents |
| TBARS | Thiobarbituric acid-reactive substances |
| TBHQ | Tert-butylhydroquinone |
References
- Sharma, P.; Gaur, V.K.; Gupta, S.; Varjani, S.; Pandey, A.; Gnansounou, E.; You, S.; Ngo, H.H.; Wong, J.W.C. Trends in Mitigation of Industrial Waste: Global Health Hazards, Environmental Implications and Waste Derived Economy for Environmental Sustainability. Sci. Total Environ. 2022, 811, 152357. [Google Scholar] [CrossRef]
- Capanoglu, E.; Nemli, E.; Tomas-Barberan, F. Novel Approaches in the Valorization of Agricultural Wastes and Their Applications. J. Agric. Food Chem. 2022, 70, 6787–6804. [Google Scholar] [CrossRef]
- Martins, S.H.F.; Pontes, K.V.; Fialho, R.L.; Fakhouri, F.M. Extraction and Characterization of the Starch Present in the Avocado Seed (Persea Americana Mill) for Future Applications. J. Agric. Food Res. 2022, 8, 100303. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Álvarez-Rivera, G.; Valdés, A.; Ibáñez, E.; Cifuentes, A. Food By-Products and Food Wastes: Are They Safe Enough for Their Valorization? Trends Food Sci. Technol. 2021, 114, 133–147. [Google Scholar] [CrossRef]
- Rao, P.; Rathod, V. Valorization of Food and Agricultural Waste: A Step towards Greener Future. Chem. Rec. 2019, 19, 1858–1871. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data. (accessed on 15 June 2025).
- Gunstone, F.D. Production and Trade of Vegetable Oils. In Vegetable Oils in Food Technology: Composition, Properties and Uses, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2011; pp. 1–24. [Google Scholar] [CrossRef]
- Pilorgé, E. Sunflower in the Global Vegetable Oil System: Situation, Specificities and Perspectives. OCL 2020, 27, 34. [Google Scholar] [CrossRef]
- Kahar, P.; Rachmadona, N.; Pangestu, R.; Palar, R.; Triyono Nugroho Adi, D.; Betha Juanssilfero, A.; Yopi; Manurung, I.; Hama, S.; Ogino, C. An Integrated Biorefinery Strategy for the Utilization of Palm-Oil Wastes. Bioresour. Technol. 2022, 344, 126266. [Google Scholar] [CrossRef]
- Romero-García, J.M.; Niño, L.; Martínez-Patiño, C.; Álvarez, C.; Castro, E.; Negro, M.J. Biorefinery Based on Olive Biomass. State of the Art and Future Trends. Bioresour. Technol. 2014, 159, 421–432. [Google Scholar] [CrossRef]
- Vieira, F.; Santana, H.E.P.; Jesus, M.; Santos, J.; Pires, P.; Vaz-Velho, M.; Silva, D.P.; Ruzene, D.S. Coconut Waste: Discovering Sustainable Approaches to Advance a Circular Economy. Sustainability 2024, 16, 3066. [Google Scholar] [CrossRef]
- FAO; IFAD; UNICEF; WHO. The State of Food Security and Nutrition in the World 2022: Repurposing Food and Agricultural Policies to Make Healthy Diets More Affordable; FAO; IFAD; UNICEF; WFP; WHO: Rome, Italy, 2022. [Google Scholar]
- Ortega, M.L.S.; Orellana-Palacios, J.C.; Garcia, S.R.; Rabanal-Ruiz, Y.; Moreno, A.; Hadidi, M. Olive Leaf Protein: Extraction Optimization, in Vitro Digestibility, Structural and Techno-Functional Properties. Int. J. Biol. Macromol. 2024, 256, 128273. [Google Scholar] [CrossRef]
- Mihai, A.L.; Negoiță, M.; Horneț, G.A.; Belc, N. Valorization Potential of Oil Industry By-Products as Sources of Essential Fatty Acids. Processes 2022, 10, 2373. [Google Scholar] [CrossRef]
- Del Castillo-Llamosas, A.; Rodríguez-Martínez, B.; del Río, P.G.; Eibes, G.; Garrote, G.; Gullón, B. Hydrothermal Treatment of Avocado Peel Waste for the Simultaneous Recovery of Oligosaccharides and Antioxidant Phenolics. Bioresour. Technol. 2021, 342, 125981. [Google Scholar] [CrossRef] [PubMed]
- Şen, F.B.; Nemli, E.; Bekdeşer, B.; Çelik, S.E.; Lalikoglu, M.; Aşçı, Y.S.; Capanoglu, E.; Bener, M.; Apak, R. Microwave-Assisted Extraction of Valuable Phenolics from Sunflower Pomace with Natural Deep Eutectic Solvents and Food Applications of the Extracts. Biomass Convers. Biorefinery 2024, 15, 9915–9930. [Google Scholar] [CrossRef]
- Bouizgma, K.; Rabbah, N.; Abbas, Z.; Abourriche, A. Unlocking Sustainable Extraction of Natural Antioxidants: Green Solvents, Smart Technologies, Scalability and Future Directions. Sep. Sci. Technol. 2025, 60, 657–683. [Google Scholar] [CrossRef]
- Švarc-Gajić, J.; Morais, S. Recent Advances in the Development and Application of Green Extraction Techniques. Appl. Sci. 2022, 12, 10510. [Google Scholar] [CrossRef]
- Günal-Köroğlu, D.; Erskine, E.; Ozkan, G.; Capanoglu, E.; Esatbeyoglu, T. Applications and Safety Aspects of Bioactives Obtained from By-Products/Wastes. In Advances in Food and Nutrition Research; Capanoglu, E., Navarro-Hortal, M.D., Forbes-Hernández, T.Y., Battino, M., Eds.; Academic Press: Cambridge, MA, USA, 2023; Volumn 107, pp. 213–261. [Google Scholar]
- Rifna, E.J.; Misra, N.N.; Dwivedi, M. Recent Advances in Extraction Technologies for Recovery of Bioactive Compounds Derived from Fruit and Vegetable Waste Peels: A Review. Crit. Rev. Food Sci. Nutr. 2021, 63, 719–752. [Google Scholar] [CrossRef]
- Dahdouh, A.; Khay, I.; Le Brech, Y.; El Maakoul, A.; Bakhouya, M. Olive Oil Industry: A Review of Waste Stream Composition, Environmental Impacts, and Energy Valorization Paths. Environ. Sci. Pollut. Res. 2023, 30, 45473–45497. [Google Scholar] [CrossRef]
- Soares, T.F.; Alves, R.C.; Oliveira, M.B.P.P. From Olive Oil Production to By-Products: Emergent Technologies to Extract Bioactive Compounds. Food Rev. Int. 2024, 40, 3342–3369. [Google Scholar] [CrossRef]
- Jafarian Asl, P.; Niazmand, R. Bioactive Phytochemicals from Rapeseed (Brassica Napus) Oil Processing By-Products. In Bioactive Phytochemicals from Vegetable Oil and Oilseed Processing By-Products; Ramadan Hassanien, M.F., Ed.; Springer: Cham, Switzerland; Berlin/Heidelberg, Germany, 2022; pp. 1–22. [Google Scholar] [CrossRef]
- Calabriso, N.; Scoditti, E.; Pellegrino, M.; Annunziata Carluccio, M. Olive Oil. In The Mediterranean Diet: An Evidence-Based Approach; Academic Press: London, UK, 2015; pp. 135–142. [Google Scholar] [CrossRef]
- Madureira, J.; Margaça, F.M.A.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Verde, S.C.; Barros, L. Applications of Bioactive Compounds Extracted from Olive Industry Wastes: A Review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 453–476. [Google Scholar] [CrossRef]
- Mili, S.; Bouhaddane, M. Forecasting Global Developments and Challenges in Olive Oil Supply and Demand: A Delphi Survey from Spain. Agriculture 2021, 11, 191. [Google Scholar] [CrossRef]
- Delgado, A.; Chammem, N.; Issaoui, M.; Ammar, E. Bioactive Phytochemicals from Olive (Olea Europaea) Processing By-Products. In Bioactive Phytochemicals from Vegetable Oil and Oilseed Processing By-Products; Ramadan Hassanien, M.F., Ed.; Springer: Cham, Switzerland; Berlin/Heidelberg, Germany, 2022; pp. 1–37. [Google Scholar] [CrossRef]
- Gavahian, M.; Mousavi Khaneghah, A.; Lorenzo, J.M.; Munekata, P.E.S.; Garcia-Mantrana, I.; Collado, M.C.; Meléndez-Martínez, A.J.; Barba, F.J. Health Benefits of Olive Oil and Its Components: Impacts on Gut Microbiota Antioxidant Activities, and Prevention of Noncommunicable Diseases. Trends Food Sci. Technol. 2019, 88, 220–227. [Google Scholar] [CrossRef]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Gammazza, A.M.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef] [PubMed]
- Fleyfel, L.M.; Leitner, N.K.V.; Deborde, M.; Matta, J.; El Najjar, N.H. Olive Oil Liquid Wastes–Characteristics and Treatments: A Literature Review. Process Saf. Environ. Prot. 2022, 168, 1031–1048. [Google Scholar] [CrossRef]
- Malekjani, N.; Jafari, S.M. Valorization of Olive Processing By-Products via Drying Technologies: A Case Study on the Recovery of Bioactive Phenolic Compounds from Olive Leaves, Pomace, and Wastewater. Crit. Rev. Food Sci. Nutr. 2023, 63, 9797–9815. [Google Scholar] [CrossRef]
- Nunes, M.A.; Pimentel, F.B.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P. Olive By-Products for Functional and Food Applications: Challenging Opportunities to Face Environmental Constraints. Innov. Food Sci. Emerg. Technol. 2016, 35, 139–148. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Koubaa, M.; Moubarik, A.; Lopes, R.P.; Saraiva, J.A.; Boussetta, N.; Grimi, N.; Barba, F.J. Emerging Opportunities for the Effective Valorization of Wastes and By-Products Generated during Olive Oil Production Process: Non-Conventional Methods for the Recovery of High-Added Value Compounds. Trends Food Sci. Technol. 2015, 45, 296–310. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Kotsiou, K. Recovery of Bioactive Compounds from Olive Mill Waste. In Olive Mill Waste: Recent Advances for Sustainable Management; Academic Press: London, UK, 2017; pp. 205–229. ISBN 9780128053140. [Google Scholar]
- Galanakis, C.M. Phenols Recovered from Olive Mill Wastewater as Additives in Meat Products. Trends Food Sci. Technol. 2018, 79, 98–105. [Google Scholar] [CrossRef]
- Simsek, M.; Süfer, Ö. Olive Pomace from Olive Oil Processing as Partial Flour Substitute in Breadsticks: Bioactive, Textural, Sensorial and Nutritional Properties. J. Food Process. Preserv. 2022, 46, e15705. [Google Scholar] [CrossRef]
- Jahanbakhshi, R.; Ansari, S. Physicochemical Properties of Sponge Cake Fortified by Olive Stone Powder. J. Food Qual. 2020, 2020, 1493638. [Google Scholar] [CrossRef]
- Abbattista, R.; Ventura, G.; Calvano, C.D.; Cataldi, T.R.I.; Losito, I. Bioactive Compounds in Waste By-Products from Olive Oil Production: Applications and Structural Characterization by Mass Spectrometry Techniques. Foods 2021, 10, 1236. [Google Scholar] [CrossRef]
- Ronca, C.L.; Marques, S.S.; Ritieni, A.; Giménez-Martínez, R.; Barreiros, L.; Segundo, M.A. Olive Oil Waste as a Source of Functional Food Ingredients: Assessing Polyphenolic Content and Antioxidant Activity in Olive Leaves. Foods 2024, 13, 189. [Google Scholar] [CrossRef]
- Antónia Nunes, M.; Costa, A.S.G.; Bessada, S.; Santos, J.; Puga, H.; Alves, R.C.; Freitas, V.; Oliveira, M.B.P.P. Olive Pomace as a Valuable Source of Bioactive Compounds: A Study Regarding Its Lipid- and Water-Soluble Components. Sci. Total Environ. 2018, 644, 229–236. [Google Scholar] [CrossRef]
- Pavez, I.C.; Lozano-Sánchez, J.; Borrás-Linares, I.; Nuñez, H.; Robert, P.; Segura-Carretero, A. Obtaining an Extract Rich in Phenolic Compounds from Olive Pomace by Pressurized Liquid Extraction. Molecules 2019, 24, 3108. [Google Scholar] [CrossRef]
- Gueboudji, Z.; Kadi, K.; Mahmoudi, M.; Hannachi, H.; Nagaz, K.; Addad, D.; Yahya, L.B.; Lachehib, B.; Hessini, K. Maceration and Liquid–Liquid Extractions of Phenolic Compounds and Antioxidants from Algerian Olive Oil Mill Wastewater. Environ. Sci. Pollut. Res. 2023, 30, 3432–3439. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, L.; Xavier, L.; Zecchi, B. Extraction of Phenolic Compounds with Antioxidant Activity from Olive Pomace Using Natural Deep Eutectic Solvents: Modelling and Optimization by Response Surface Methodology. Discov. Food 2024, 4, 29. [Google Scholar] [CrossRef]
- Chanioti, S.; Katsouli, M.; Tzia, C.; Tsimidou, M.Z.; Escribano-Bailón, T. Novel Processes for the Extraction of Phenolic Compounds from Olive Pomace and Their Protection by Encapsulation. Molecules 2021, 26, 1781. [Google Scholar] [CrossRef]
- Mir-Cerdà, A.; Granados, M.; Saurina, J.; Sentellas, S. Olive Tree Leaves as a Great Source of Phenolic Compounds: Comprehensive Profiling of NaDES Extracts. Food Chem. 2024, 456, 140042. [Google Scholar] [CrossRef]
- Hrelia, S.; Angeloni, C.; Barbalace, M.C. Agri-Food Wastes as Natural Source of Bioactive Antioxidants. Antioxidants 2023, 12, 351. [Google Scholar] [CrossRef]
- Alwazeer, D.; Elnasanelkasim, M.A.; Engin, T.; Çiğdem, A. Use of Hydrogen-Rich Water as a Green Solvent for the Extraction of Phytochemicals: Case of Olive Leaves. J. Appl. Res. Med. Aromat. Plants 2023, 35, 100472. [Google Scholar] [CrossRef]
- Pasten, A.; Uribe, E.; Stucken, K.; Rodríguez, A.; Vega-Gálvez, A. Influence of Drying on the Recoverable High-Value Products from Olive (Cv. Arbequina) Waste Cake. Waste and Biomass Valorization 2019, 10, 1627–1638. [Google Scholar] [CrossRef]
- Ribeiro, T.B.; Oliveira, A.L.; Costa, C.; Nunes, J.; Vicente, A.A.; Pintado, M. Total and Sustainable Valorisation of Olive Pomace Using a Fractionation Approach. Appl. Sci. 2020, 10, 6785. [Google Scholar] [CrossRef]
- Rubio-Senent, F.; Rodríguez-Gutiérrez, G.; Lama-Muñoz, A.; Fernández-Bolaños, J. Pectin Extracted from Thermally Treated Olive Oil By-Products: Characterization, Physico-Chemical Properties, in Vitro Bile Acid and Glucose Binding. Food Hydrocoll. 2015, 43, 311–321. [Google Scholar] [CrossRef]
- Speroni, C.S.; Bender, A.B.B.; Stiebe, J.; Ballus, C.A.; Ávila, P.F.; Goldbeck, R.; Morisso, F.D.P.; da Silva, L.P.; Emanuelli, T. Granulometric Fractionation and Micronization: A Process for Increasing Soluble Dietary Fiber Content and Improving Technological and Functional Properties of Olive Pomace. LWT 2020, 130, 109526. [Google Scholar] [CrossRef]
- Aslan Türker, D.; Işçimen, E.M. Insoluble Dietary Fiber and Microparticle Formation from Olive Pomace: Effects on Emulsification and Interfacial Behavior in Pickering Emulsions. J. Food Meas. Charact. 2025, 19, 2684–2699. [Google Scholar] [CrossRef]
- Nunes, M.A.; Palmeira, J.D.; Melo, D.; Machado, S.; Lobo, J.C.; Costa, A.S.G.; Alves, R.C.; Ferreira, H.; Oliveira, M.B.P.P. Chemical Composition and Antimicrobial Activity of a New Olive Pomace Functional Ingredient. Pharmaceuticals 2021, 14, 913. [Google Scholar] [CrossRef] [PubMed]
- Vergara-Barberán, M.; Lerma-García, M.J.; Herrero-Martínez, J.M.; Simó-Alfonso, E.F. Use of an Enzyme-Assisted Method to Improve Protein Extraction from Olive Leaves. Food Chem. 2015, 169, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, H.; Gultekin Subasi, B. Distinctive Processing Effects on Recovered Protein Isolates from Laurel (Bay) and Olive Leaves: A Comparative Study. ACS Omega 2023, 8, 36179–36187. [Google Scholar] [CrossRef]
- Simonato, B.; Trevisan, S.; Tolve, R.; Favati, F.; Pasini, G. Pasta Fortification with Olive Pomace: Effects on the Technological Characteristics and Nutritional Properties. LWT 2019, 114, 108368. [Google Scholar] [CrossRef]
- Panza, O.; Lacivita, V.; Palermo, C.; Conte, A.; Del Nobile, M.A. Food By-Products to Extend Shelf Life: The Case of Cod Sticks Breaded with Dried Olive Paste. Foods 2020, 9, 1902. [Google Scholar] [CrossRef]
- Karadag, A.; Kayacan Cakmakoglu, S.; Metin Yildirim, R.; Karasu, S.; Avci, E.; Ozer, H.; Sagdic, O. Enrichment of Lecithin with Phenolics from Olive Mill Wastewater by Cloud Point Extraction and Its Application in Vegan Salad Dressing. J. Food Process. Preserv. 2022, 46, e16645. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Voulgaris, A.; Katsoulis, K.; Lalas, S.I.; Roussis, I.G.; Gortzi, O. Development of Enriched Oil with Polyphenols Extracted from Olive Mill Wastewater. Foods 2023, 12, 497. [Google Scholar] [CrossRef]
- Durante, M.; Bleve, G.; Selvaggini, R.; Veneziani, G.; Servili, M.; Mita, G. Bioactive Compounds and Stability of a Typical Italian Bakery Products “Taralli” Enriched with Fermented Olive Paste. Molecules 2019, 24, 3258. [Google Scholar] [CrossRef]
- El Moudden, H.; El Idrissi, Y.; Belmaghraoui, W.; Belhoussaine, O.; El Guezzane, C.; Bouayoun, T.; Harhar, H.; Tabyaoui, M. Olive Mill Wastewater Polyphenol-Based Extract as a Vegetable Oil Shelf Life Extending Additive. J. Food Process. Preserv. 2020, 44, e14990. [Google Scholar] [CrossRef]
- Farrag, A.F.; Zahran, H.A.; Al-Okbay, M.F.; El-Sheikh, M.M.; Soliman, T.N. Physicochemical Properties of White Soft Cheese Supplemented With Encapsulated Olive Phenolic Compounds. Egypt. J. Chem. 2020, 63, 2921–2931. [Google Scholar] [CrossRef]
- Cedola, A.; Palermo, C.; Centonze, D.; Del Nobile, M.A.; Conte, A. Characterization and Bio-Accessibility Evaluation of Olive Leaf Extract-Enriched “Taralli”. Foods 2020, 9, 1268. [Google Scholar] [CrossRef] [PubMed]
- Conte, P.; Pulina, S.; Del Caro, A.; Fadda, C.; Urgeghe, P.P.; De Bruno, A.; Difonzo, G.; Caponio, F.; Romeo, R.; Piga, A. Gluten-Free Breadsticks Fortified with Phenolic-Rich Extracts from Olive Leaves and Olive Mill Wastewater. Foods 2021, 10, 923. [Google Scholar] [CrossRef] [PubMed]
- Spizzirri, U.G.; Carullo, G.; Aiello, F.; Paolino, D.; Restuccia, D. Valorisation of Olive Oil Pomace Extracts for a Functional Pear Beverage Formulation. Int. J. Food Sci. Technol. 2021, 56, 5497–5505. [Google Scholar] [CrossRef]
- Trindade, P.C.O.; Dalfolo, A.d.C.; Monteiro, C.S.; Wagner, R.; Dos Santos, B.A.; Nora, F.M.D.; Verruck, S.; da ROSA, C.S. Development and Characterization of Biscuits with Olive Pomace. Food Sci. Technol. 2023, 43, e99922. [Google Scholar] [CrossRef]
- Chew, S.C.; How, Y.H.; Hasan, Z.A.B.A.; Chu, C.C. Recovery of Bioactive Compounds from Oil Palm Waste Using Green Extraction Techniques and Its Applications. Int. J. Food Sci. Technol. 2024, 59, 8101–8113. [Google Scholar] [CrossRef]
- Ofori-Boateng, C.; Lee, K.T. Sustainable Utilization of Oil Palm Wastes for Bioactive Phytochemicals for the Benefit of the Oil Palm and Nutraceutical Industries. Phytochem. Rev. 2013, 12, 173–190. [Google Scholar] [CrossRef]
- Gozan, M.; Abd-Aziz, S.; Jenol, M.A. Utilization of Palm Oil Waste as a Sustainable Food Resource. In Handbook of Biorefinery Research and Technology: Production of Biofuels and Biochemicals; Springer: Singapore, 2024; pp. 573–592. [Google Scholar] [CrossRef]
- Awoh, E.T.; Kiplagat, J.; Kimutai, S.K.; Mecha, A.C. Current Trends in Palm Oil Waste Management: A Comparative Review of Cameroon and Malaysia. Heliyon 2023, 9, e21410. [Google Scholar] [CrossRef]
- Ayyildiz, H.F.; Shoaib, H.; Kara, H. Bioactive Phytochemicals from Palm Oil Processing By-Products. In Bioactive Phytochemicals from Vegetable Oil and Oilseed Processing By-Products; Ramadan Hassanien, M.F., Ed.; Springer: Cham, Switzerland; Berlin/Heidelberg, Germany, 2023; pp. 235–268. [Google Scholar] [CrossRef]
- Liew, W.L.; Kassim, M.; Muda, K.; Loh, S. Feasibility Study on Palm Oil Processing Wastes towards Achieving Zero Discharge. ARPN J. Eng. Appl. Sci. 2016, 11, 2400–2405. [Google Scholar]
- Chang, S.K.; Ismail, A.; Yanagita, T.; Esa, N.M.; Baharuldin, M.T.H. Biochemical Characterisation of the Soluble Proteins, Protein Isolates and Hydrolysates from Oil Palm (Elaeis Guineensis) Kernel. Food Biosci. 2014, 7, 1–10. [Google Scholar] [CrossRef]
- Binti Bahari, N.A.; Ahmadi, R.; Muhialdin, B.J.; Saari, N.; Feng, Y.; Zarei, M. Palm Kernel Cake- A Potential Natural Source of Protein, Hydrolysates, and Bioactive Peptides. JAOCS, J. Am. Oil Chem. Soc. 2025. [Google Scholar] [CrossRef]
- Zarei, M.; Forghani, B.; Ebrahimpour, A.; Abdul-Hamid, A.; Anwar, F.; Saari, N. In Vitro and in Vivo Antihypertensive Activity of Palm Kernel Cake Protein Hydrolysates: Sequencing and Characterization of Potent Bioactive Peptides. Ind. Crops Prod. 2015, 76, 112–120. [Google Scholar] [CrossRef]
- Surangkulwattana, K.; Hlosrıchok, A.; Aunpad, R. Antioxidant Activity of Palm Kernel Meal Protein Hydrolysate and Characterization of Its Peptide Profile. Food Sci. Technol. 2023, 43, e18923. [Google Scholar] [CrossRef]
- Ramachandran, V.; Ismail, F.S.; Noor, M.J.M.M.; Akhir, F.N.M.D.; Othman, N.; Zakaria, Z.; Hara, H. Extraction and Intensive Conversion of Lignocellulose from Oil Palm Solid Waste into Lignin Monomer by the Combination of Hydrothermal Pretreatment and Biological Treatment. Bioresour. Technol. Reports 2020, 11, 100456. [Google Scholar] [CrossRef]
- Bello, B.; Mustafa, S.; Tan, J.S.; Ibrahim, T.A.T.; Tam, Y.J.; Ariff, A.B.; Manap, M.Y.; Abbasiliasi, S. Evaluation of the Effect of Soluble Polysaccharides of Palm Kernel Cake as a Potential Prebiotic on the Growth of Probiotics. 3 Biotech 2018, 8, 346. [Google Scholar] [CrossRef]
- Jahromi, M.F.; Liang, J.B.; Abdullah, N.; Goh, Y.M.; Ebrahimi, R.; Shokryazdan, P. Extraction and Characterization of Oligosaccharides from Palm Kernel Cake as Prebiotic. BioResources 2016, 11, 674–695. [Google Scholar] [CrossRef]
- TEH, S.S. Nutritional Potential of Supercritical Fluid-Extracted Palm Fruit. J. Oil Palm Res. 2022, 34, 475–487. [Google Scholar] [CrossRef]
- Tsouko, E.; Alexandri, M.; Fernandes, K.V.; Freire, D.M.G.; Mallouchos, A.; Koutinas, A.A. Extraction of Phenolic Compounds from Palm Oil Processing Residues and Their Application as Antioxidants. Food Technol. Biotechnol. 2019, 57, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Sambanthamurthi, R.; Tan, Y.; Sundram, K.; Abeywardena, M.; Sambandan, T.G.; Rha, C.; Sinskey, A.J.; Subramaniam, K.; Leow, S.S.; Hayes, K.C.; et al. Oil Palm Vegetation Liquor: A New Source of Phenolic Bioactives. Br. J. Nutr. 2011, 106, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Kua, S.F.; Ibrahim, J.; Ooi, C.K.W.; Nan, K.I.; Hashim, N.; Mohd Yusof, H. Optimisation of Phenolic Extraction and Quantification of Phenolics in Palm Kernel Cake. Renew. Bioresour. 2015, 3, 2. [Google Scholar] [CrossRef]
- Nomanbhay, S.; Uskenbayeva, S.A.; Ong, M.Y. On Increase of the Efficiency of Extracting Phenolic Compounds from Palm Oil Mill Effluent. J. Chem. Technol. Metall. 2018, 53, 101–111. [Google Scholar]
- Dal Prá, V.; Lunelli, F.C.; Vendruscolo, R.G.; Martins, R.; Wagner, R.; Lazzaretti, A.P.; Freire, D.M.G.; Alexandri, M.; Koutinas, A.; Mazutti, M.A.; et al. Ultrasound-Assisted Extraction of Bioactive Compounds from Palm Pressed Fiber with High Antioxidant and Photoprotective Activities. Ultrason. Sonochem. 2017, 36, 362–366. [Google Scholar] [CrossRef]
- Tang, P.L.; Hong, W.L.; Yue, C.S.; Harun, S. Recovery of Lignin and Phenolics via One-Pot Pretreatment of Oil Palm Empty Fruit Bunch Fiber and Palm Oil Mill Effluent. Biomass Convers. Biorefinery 2023, 13, 4705–4715. [Google Scholar] [CrossRef]
- Dal Prá, V.; Soares, J.F.; Monego, D.L.; Vendruscolo, R.G.; Freire, D.M.G.; Alexandri, M.; Koutinas, A.; Wagner, R.; Mazutti, M.A.; Da Rosa, M.B. Extraction of Bioactive Compounds from Palm (Elaeis Guineensis) Pressed Fiber Using Different Compressed Fluids. J. Supercrit. Fluids 2016, 112, 51–56. [Google Scholar] [CrossRef]
- Teh, S.S.; Ong, A.S.H.; Mah, S.H. Recovery and Utilization of Palm Oil Mill Effluent Source as Value-Added Food Products. J. Oleo Sci. 2017, 66, 1183–1191. [Google Scholar] [CrossRef][Green Version]
- Ngatirah, N.; Ruswanto, A.; Sunardi, S. Effect of Hydroxypropyl Methylcellulose from Oil Palm Empty Fruit Bunch on Oil Uptake and Physical Properties of French Fries. Food Sci. Technol. 2022, 42, e110421. [Google Scholar] [CrossRef]
- Maulidna; Wirjosentono, B.; Tamrin; Marpaung, L. Microencapsulation of Ginger-Based Essential Oil (Zingiber Cassumunar Roxb) with Chitosan and Oil Palm Trunk Waste Fiber Prepared by Spray-Drying Method. Case Stud. Therm. Eng. 2020, 18, 100606. [Google Scholar] [CrossRef]
- Ajayi, S.M.; Olusanya, S.O.; Sodeinde, K.O.; Didunyemi, A.E.; Atunde, M.O.; Fapojuwo, D.P.; Olumayede, E.G.; Lawal, O.S. Hydrophobic Modification of Cellulose from Oil Palm Empty Fruit Bunch: Characterization and Application in Pickering Emulsions Stabilization. Carbohydr. Polym. Technol. Appl. 2023, 5, 100282. [Google Scholar] [CrossRef]
- Cárdenas-Castro, A.P.; Fernández-Ochoa, Á.; Cádiz-Gurrea, M.d.l.L.; Segura Carretero, A.; Sáyago-Ayerdi, S.G. Bioactive Phytochemicals from Avocado Oil Processing By-Products. In Bioactive Phytochemicals from Vegetable Oil and Oilseed Processing By-Products; Ramadan Hassanien, M.F., Ed.; Springer: Berlin/Heidelberg, Germany, 2023; pp. 403–430. [Google Scholar] [CrossRef]
- Cervantes-Paz, B.; Yahia, E.M. Avocado Oil: Production and Market Demand, Bioactive Components, Implications in Health, and Tendencies and Potential Uses. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4120–4158. [Google Scholar] [CrossRef]
- Cheptoo, A.; Ebere, R.; Arimi, J. Avocado Pulp: A Review of Nutritional Profile, Functional Attributes, Drying Techniques, and Avocado Pulp Products. J. Food Process. Preserv. 2025, 2025, 4810929. [Google Scholar] [CrossRef]
- Lin, X.; Li, Z. Key Components and Multiple Health Functions of Avocado Oil: A Review. J. Funct. Foods 2024, 122, 106494. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, B.; Romaní, A.; Eibes, G.; Garrote, G.; Gullón, B.; del Río, P.G. Potential and Prospects for Utilization of Avocado By-Products in Integrated Biorefineries. Bioresour. Technol. 2022, 364, 128034. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vallejo, M.C.; Agudelo Patiño, T.; Poveda-Giraldo, J.A.; Piedrahita-Rodríguez, S.; Cardona Alzate, C.A. Alternatives for the Valorization of Avocado Waste Generated in the Different Links of the Value Chain Based on a Life-Cycle Analysis Approach. Agronomy 2023, 13, 2229. [Google Scholar] [CrossRef]
- Tesfaye, T.; Ayele, M.; Gibril, M.; Ferede, E.; Limeneh, D.Y.; Kong, F. Beneficiation of Avocado Processing Industry By-Product: A Review on Future Prospect. Curr. Res. Green Sustain. Chem. 2022, 5, 100253. [Google Scholar] [CrossRef]
- Permal, R.; Leong Chang, W.; Seale, B.; Hamid, N.; Kam, R. Converting Industrial Organic Waste from the Cold-Pressed Avocado Oil Production Line into a Potential Food Preservative. Food Chem. 2020, 306, 125635. [Google Scholar] [CrossRef]
- Marra, A.; Manousakis, V.; Zervas, G.P.; Koutis, N.; Finos, M.A.; Adamantidi, T.; Panoutsopoulou, E.; Ofrydopoulou, A.; Tsoupras, A. Avocado and Its By-Products as Natural Sources of Valuable Anti-Inflammatory and Antioxidant Bioactives for Functional Foods and Cosmetics with Health-Promoting Properties. Appl. Sci. 2024, 14, 5978. [Google Scholar] [CrossRef]
- Velderrain-Rodríguez, G.R.; Salvia-Trujillo, L.; González-Aguilar, G.A.; Martín-Belloso, O. Interfacial Activity of Phenolic-Rich Extracts from Avocado Fruit Waste: Influence on the Colloidal and Oxidative Stability of Emulsions and Nanoemulsions. Innov. Food Sci. Emerg. Technol. 2021, 69, 102665. [Google Scholar] [CrossRef]
- Zuñiga-Martínez, B.S.; Domínguez-Avila, J.A.; Wall-Medrano, A.; Ayala-Zavala, J.F.; Hernández-Paredes, J.; Salazar-López, N.J.; Villegas-Ochoa, M.A.; González-Aguilar, G.A. Avocado Paste from Industrial Byproducts as an Unconventional Source of Bioactive Compounds: Characterization, in Vitro Digestion and in Silico Interactions of Its Main Phenolics with Cholesterol. J. Food Meas. Charact. 2021, 15, 5460–5476. [Google Scholar] [CrossRef]
- Figueroa, J.G.; Borrás-Linares, I.; Del Pino-García, R.; Curiel, J.A.; Lozano-Sánchez, J.; Segura-Carretero, A. Functional Ingredient from Avocado Peel: Microwave-Assisted Extraction, Characterization and Potential Applications for the Food Industry. Food Chem. 2021, 352, 129300. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Mayol, I.; Céspedes-Acuña, C.; Silva, F.L.; Alarcón-Enos, J. Improvement of the Polyphenol Extraction from Avocado Peel by Assisted Ultrasound and Microwaves. J. Food Process Eng. 2019, 42, e13197. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, B.; Ferreira-Santos, P.; Alfonso, I.M.; Martínez, S.; Genisheva, Z.; Gullón, B. Deep Eutectic Solvents as a Green Tool for the Extraction of Bioactive Phenolic Compounds from Avocado Peels. Molecules 2022, 27, 6646. [Google Scholar] [CrossRef]
- Della Posta, S.; Gallo, V.; Ascrizzi, A.M.; Gentili, A.; De Gara, L.; Dugo, L.; Fanali, C. Development of a Green Ultrasound-Assisted Procedure for the Extraction of Phenolic Compounds from Avocado Peel with Deep Eutectic Solvents. Green Anal. Chem. 2023, 7, 100083. [Google Scholar] [CrossRef]
- Dainton, A.N.; He, F.; Bingham, T.W.; Sarlah, D.; Detweiler, K.B.; Mangian, H.J.; De Godoy, M.R.C. Nutritional and Physico-Chemical Implications of Avocado Meal as a Novel Dietary Fiber Source in an Extruded Canine Diet. J. Anim. Sci. 2022, 100, 1–10. [Google Scholar] [CrossRef]
- Neves, B.B.; Pinto, S.; Pais, A.R.; Batista, J.; Pinho, M.; Goracci, L.; Domingues, P.; Domingues, M.R.; Melo, T. Avocado By-Products: Recycling Low-Cost Lipids towards High-End Applications. LWT 2025, 216, 117376. [Google Scholar] [CrossRef]
- Marović, R.; Badanjak Sabolović, M.; Brnčić, M.; Ninčević Grassino, A.; Kljak, K.; Voća, S.; Karlović, S.; Rimac Brnčić, S. The Nutritional Potential of Avocado By-Products: A Focus on Fatty Acid Content and Drying Processes. Foods 2024, 13, 2003. [Google Scholar] [CrossRef]
- Barbosa-Martín, E.; Chel-Guerrero, L.; González-Mondragón, E.; Betancur-Ancona, D. Chemical and Technological Properties of Avocado (Persea Americana Mill.) Seed Fibrous Residues. Food Bioprod. Process. 2016, 100, 457–463. [Google Scholar] [CrossRef]
- Pires, J.B.; dos Santos, F.N.; da Cruz, E.P.; Fonseca, L.M.; Siebeneichler, T.J.; Lemos, G.S.; Gandra, E.A.; da Rosa Zavareze, E.; Dias, A.R.G. Starch Extraction from Avocado By-Product and Its Use for Encapsulation of Ginger Essential Oil by Electrospinning. Int. J. Biol. Macromol. 2024, 254, 127617. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Wang, A.B.; Zang, X.P.; Tan, L.; Xu, B.Y.; Chen, H.H.; Jin, Z.Q.; Ma, W.H. Physicochemical, Functional and Emulsion Properties of Edible Protein from Avocado (Persea Americana Mill.) Oil Processing by-Products. Food Chem. 2019, 288, 146–153. [Google Scholar] [CrossRef]
- Zuñiga-Martínez, B.S.; Domínguez-Avila, J.A.; Robles-Sánchez, R.M.; Ayala-Zavala, J.F.; Viuda-Martos, M.; López-Díaz, J.A.; Villegas-Ochoa, M.A.; Torres-García, G.; González-Aguilar, G.A. Lyophilized Avocado Paste Improves Corn Chips’ Nutritional Properties and Sensory Acceptability. Foods 2024, 13, 1220. [Google Scholar] [CrossRef]
- Campos-González, N.; Gómez-Salazar, J.A.; Cerón-García, A.; Ozuna, C.; Saldaña-Robles, A.; Sáyago-Ayerdi, S.G.; Sosa-Morales, M.E. Valorization of Avocado (Persea Americana) Residual Paste: Microwave-Assisted Extraction, Optimization and Addition to an Artisanal Pork Ham. CyTA-J. Food 2024, 22, 2333889. [Google Scholar] [CrossRef]
- Permal, R.; Chia, T.; Arena, G.; Fleming, C.; Chen, J.; Chen, T.; Chang, W.L.; Seale, B.; Hamid, N.; Kam, R. Converting Avocado Seeds into a Ready to Eat Snack and Analysing for Persin and Amygdalin. Food Chem. 2023, 399, 134011. [Google Scholar] [CrossRef] [PubMed]
- Permal, R.; Chang, W.L.; Chen, T.; Seale, B.; Hamid, N.; Kam, R. Optimising the Spray Drying of Avocado Wastewater and Use of the Powder as a Food Preservative for Preventing Lipid Peroxidation. Foods 2020, 9, 1187. [Google Scholar] [CrossRef] [PubMed]
- Siol, M.; Sadowska, A. Chemical Composition, Physicochemical and Bioactive Properties of Avocado (Persea Americana) Seed and Its Potential Use in Functional Food Design. Agriculture 2023, 13, 316. [Google Scholar] [CrossRef]
- Trujillo-Mayol, I.; M Madalena C, S.M.C.; Viegas, O.; Cunha, S.C.; Alarcón-Enos, J.; Pinho, O.; Ferreira, I.M.P.L.V.O. Incorporation of Avocado Peel Extract to Reduce Cooking-Induced Hazards in Beef and Soy Burgers: A Clean Label Ingredient. Food Res. Int. 2021, 147, 110434. [Google Scholar] [CrossRef]
- Ferreira, S.M.; Santos, L. From By-Product to Functional Ingredient: Incorporation of Avocado Peel Extract as an Antioxidant and Antibacterial Agent. Innov. Food Sci. Emerg. Technol. 2022, 80, 103116. [Google Scholar] [CrossRef]
- Fufa, D.D.; Bekele, T.; Bultosa, G.; Tamene, A. Avocado Seeds Valorization for Sustainable Food Ingredient Use: Quality Attributes Evaluation in the Traditional Fermented Teff Injera. Discov. Sustain. 2025, 6, 288. [Google Scholar] [CrossRef]
- Viola, E.; Buzzanca, C.; Tinebra, I.; Settanni, L.; Farina, V.; Gaglio, R.; Di Stefano, V. A Functional End-Use of Avocado (Cv. Hass) Waste through Traditional Semolina Sourdough Bread Production. Foods 2023, 12, 3743. [Google Scholar] [CrossRef]
- Oluba, O.M.; Ojeaburu, S.I.; Bayo-Olorunmeke, O.A.; Erifeta, G.; Josiah, S.J. Quality Attributes and Shelf-Life of Freshly Cut Beef Coated with Waste Feather Keratin-Ginger Starch Composite Enriched with Avocado Peel Polyphenolic-Rich Extract. Food Sci. Preserv. 2024, 31, 1–14. [Google Scholar] [CrossRef]
- da Silva Lima, R.; Block, J.M. Coconut Oil: What Do We Really Know about It so Far? Food Qual. Saf. 2019, 3, 61–72. [Google Scholar] [CrossRef]
- Ramesh, S.V.; Pandiselvam, R.; Shameena Beegum, P.P.; Saravana Kumar, R.M.; Manikantan, M.R.; Hebbar, K.B. Review of Cocos Nucifera L. Testa-Derived Phytonutrients with Special Reference to Phenolics and Its Potential for Encapsulation. J. Food Sci. Technol. 2023, 60, 1–10. [Google Scholar] [CrossRef]
- Abeysekara, M.G.D.; Prasada, D.V.P.; Pathiraja, P.M.E.K. Equilibrium Relations in the Coconut Sector: An Analysis of Fresh Nut, Oil and Desiccated Coconut Market in Sri Lanka for the Period 1956-2017. Trop. Agric. Res. 2020, 31, 1–12. [Google Scholar] [CrossRef]
- Kaur, K.; Chhikara, N.; Sharma, P.; Garg, M.K.; Panghal, A. Coconut Meal: Nutraceutical Importance and Food Industry Application. Foods Raw Mater. 2019, 7, 419–427. [Google Scholar] [CrossRef]
- Samarasinghe, H.G.A.S.; Samaranayake, U.C.; Dharmaprema, G.A.D.B.S. Exploring the Quality and Safety Issues in the Coconut Oil Processing Industry in Sri Lanka: A Review. J. Sci. Univ. Kelaniya 2023, 16, 63–76. [Google Scholar] [CrossRef]
- Yin, J.-J.; Huang, H.; Jiang, X.-M.; Guo, X.-Y.; Pan, B.-H.; Gao, P.; Zhong, W.; Hu, C.-R.; He, D.-P. Coconut Oil Research: Past, Present, and Future Directions. Food Heal. 2024, 6, 5. [Google Scholar] [CrossRef]
- Deen, A.; Visvanathan, R.; Wickramarachchi, D.; Marikkar, N.; Nammi, S.; Jayawardana, B.C.; Liyanage, R. Chemical Composition and Health Benefits of Coconut Oil: An Overview. J. Sci. Food Agric. 2021, 101, 2182–2193. [Google Scholar] [CrossRef]
- Duranova, H.; Kuzelova, L.; Fialkova, V.; Simora, V.; Kovacikova, E.; Joanidis, P.; Borotova, P.; Straka, D.; Hoskin, R.T.; Moncada, M.; et al. Coconut-Sourced MCT Oil: Its Potential Health Benefits beyond Traditional Coconut Oil. Phytochem. Rev. 2024, 24, 659–700. [Google Scholar] [CrossRef]
- Shakeela, H.; Mohan, K.; Nisha, P. Unlocking a Nutritional Treasure: Health Benefits and Sustainable Applications of Spent Coconut Meal. Sustain. Food Technol. 2024, 2, 497–505. [Google Scholar] [CrossRef]
- Wallace, T.C. Health Effects of Coconut Oil—A Narrative Review of Current Evidence. J. Am. Coll. Nutr. 2019, 38, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.J.; Tham, P.E.; Khoo, K.S.; Cheng, C.K.; Chew, K.W.; Show, P.L. A Comprehensive Review on the Techniques for Coconut Oil Extraction and Its Application. Bioprocess Biosyst. Eng. 2021, 44, 1807–1818. [Google Scholar] [CrossRef] [PubMed]
- Yani, M.; Toruan, D.P.M.L.; Puspaningrum, T.; Sarfat, M.S.; Indrawanto, C. Life Cycle Assessment of Coconut Oil Product. IOP Conf. Ser. Earth Environ. Sci. 2022, 1063, 012017. [Google Scholar] [CrossRef]
- Sirodj, D.A.N.; Gunawan, I. Recycling Solid Waste of Coconut Oil Industry: A Response Surface-Goal Programming Approach. J. Optimasi Sist. Ind. 2020, 19, 111–121. [Google Scholar] [CrossRef]
- Mariano, A.P.B.; Unpaprom, Y.; Ramaraj, R. Hydrothermal Pretreatment and Acid Hydrolysis of Coconut Pulp Residue for Fermentable Sugar Production. Food Bioprod. Process. 2020, 122, 31–40. [Google Scholar] [CrossRef]
- Fonseca-Bustos, V.; Madera-Santana, T.J.; Valenzuela-Melendres, M.; Islas-Rubio, A.R.; Montoya-Ballesteros, L.d.C. Effect of the Incorporation of Virgin Coconut Oil Byproduct in the Optimization Process of a Baked Snack. J. Food Process. Preserv. 2023, 2023, 4851416. [Google Scholar] [CrossRef]
- Singh, R.; Langyan, S.; Sangwan, S.; Rohtagi, B.; Khandelwal, A.; Shrivastava, M. Protein for Human Consumption From Oilseed Cakes: A Review. Front. Sustain. Food Syst. 2022, 6, 856401. [Google Scholar] [CrossRef]
- Thaiphanit, S.; Anprung, P. Physicochemical and Emulsion Properties of Edible Protein Concentrate from Coconut (Cocos Nucifera L.) Processing by-Products and the Influence of Heat Treatment. Food Hydrocoll. 2016, 52, 756–765. [Google Scholar] [CrossRef]
- Rodsamran, P.; Sothornvit, R. Physicochemical and Functional Properties of Protein Concentrate from By-Product of Coconut Processing. Food Chem. 2018, 241, 364–371. [Google Scholar] [CrossRef]
- Patil, U.; Benjakul, S. Characteristics of Albumin and Globulin from Coconut Meat and Their Role in Emulsion Stability without and with Proteolysis. Food Hydrocoll. 2017, 69, 220–228. [Google Scholar] [CrossRef]
- Liu, X.Y.; Yang, D.W.; Liu, W.T.; Kan, J.T.; Yang, K.L.; Zhang, J.G.; Wang, Y.Y.; Zhu, K.X.; Zhang, Y.F. Structural and Techno-Functional Characteristics of Protein from the by-Product of Virgin Coconut Oil Produced by Centrifugation Using Coconut Milk. Food Chem. X 2025, 28, 102561. [Google Scholar] [CrossRef]
- Liu, X.; Yang, D.; Liu, W.; Kan, J.; Zhang, Y. Effect of Dry Processing of Coconut Oil on the Structure and Physicochemical Properties of Coconut Isolate Proteins. Foods 2024, 13, 2496. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, Y.; Zhang, Y.; Xu, J.; Gao, G. Antioxidant Activity of Coconut (Cocos Nucifera L.) Protein Fractions. Molecules 2018, 23, 707. [Google Scholar] [CrossRef] [PubMed]
- Gunathilake, K.; Yalegama, C.; Kumara, A. Use of Coconut Flour as a Source of Protein and Dietary Fibre in Wheat Bread. Asian J. ofFood Agro-Industry 2009, 2, 386–395. [Google Scholar]
- Du, X.; Wang, L.; Huang, X.; Jing, H.; Ye, X.; Gao, W.; Bai, X.; Wang, H. Effects of Different Extraction Methods on Structure and Properties of Soluble Dietary Fiber from Defatted Coconut Flour. LWT 2021, 143, 111031. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y.; Xu, J.; Gao, G.; Niu, F. Adsorption Activity of Coconut (Cocos Nucifera L.) Cake Dietary Fibers: Effect of Acidic Treatment, Cellulase Hydrolysis, Particle Size and PH. RSC Adv. 2018, 8, 2844–2850. [Google Scholar] [CrossRef]
- Yan, J.; Li, Y.; Bai, S.; Zheng, J.; Hassan, N.A.; Lu, B.; Hu, A. Comparison of Structural, Physicochemical and Functional Properties of Dried Coconut Dietary Fiber by Steam Explosion and Extrusion Modification. Ind. Crops Prod. 2024, 218, 118916. [Google Scholar] [CrossRef]
- Kasapoglu, M.Z.; Sagdic, O.; Avci, E.; Tekin-Cakmak, Z.H.; Karasu, S.; Turker, R.S. The Potential Use of Cold-Pressed Coconut Oil By-Product as an Alternative Source in the Production of Plant-Based Drink and Plant-Based Low-Fat Ice Cream: The Rheological, Thermal, and Sensory Properties of Plant-Based Ice Cream. Foods 2023, 12, 650. [Google Scholar] [CrossRef]
- Tekin-Cakmak, Z.H.; Karasu, S.; Kayacan-Cakmakoglu, S.; Akman, P.K. Investigation of Potential Use of By-Products from Cold-Press Industry as Natural Fat Replacers and Functional Ingredients in a Low-Fat Salad Dressing. J. Food Process. Preserv. 2021, 45, e15388. [Google Scholar] [CrossRef]
- Seneviratne, K.N.; Prasadani, W.C.; Jayawardena, B. Phenolic Extracts of Coconut Oil Cake: A Potential Alternative for Synthetic Antioxidants. Food Sci. Technol. 2016, 36, 591–597. [Google Scholar] [CrossRef][Green Version]
- Wirkijowska, A.; Sobota, A.; Zarzycki, P.; Nawrocka, A.; Blicharz-Kania, A.; Andrejko, D. Chemical, Technological, and Sensory Evaluation of the Suitability of Coconut by-Products in White Rolls. J. Sci. Food Agric. 2022, 102, 3370–3378. [Google Scholar] [CrossRef]
- Sarabandi, K.; Dashipour, A.; Akbarbaglu, Z.; Peighambardoust, S.H.; Ayaseh, A.; Kafil, H.S.; Jafari, S.M.; Mousavi Khaneghah, A. Incorporation of Spray-Dried Encapsulated Bioactive Peptides from Coconut (Cocos Nucifera L.) Meal by-Product in Bread Formulation. Food Sci. Nutr. 2024, 12, 4723–4734. [Google Scholar] [CrossRef]
- Adeloye, J.B.; Osho, H.; Idris, L.O. Defatted Coconut Flour Improved the Bioactive Components, Dietary Fibre, Antioxidant and Sensory Properties of Nixtamalized Maize Flour. J. Agric. Food Res. 2020, 2, 100042. [Google Scholar] [CrossRef]
- Sykut-Domańska, E.; Zarzycki, P.; Sobota, A.; Teterycz, D.; Wirkijowska, A.; Blicharz-Kania, A.; Andrejko, D.; Mazurkiewicz, J. The Potential Use of By-Products from Coconut Industry for Production of Pasta. J. Food Process. Preserv. 2020, 44, e14490. [Google Scholar] [CrossRef]
- Srivastava, Y.; Semwal, A.D. Effect of Virgin Coconut Meal (VCM) on the Rheological, Micro-Structure and Baking Properties of Cake and Batter. J. Food Sci. Technol. 2015, 52, 8122–8130. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, T.; Subramaniam, S.; Chinnapa, V.; Rajoo, B. Effect of Defatted Coconut Flour on Functional, Nutritional, Textural and Sensory Attributes of Rice Noodles. Int. J. Food Sci. Technol. 2023, 58, 5077–5088. [Google Scholar] [CrossRef]
- Beegum, S.; Sharma, M.; Manikantan, M.R.; Gupta, R.K. Effect of Virgin Coconut Oil Cake on Physical, Textural, Microbial and Sensory Attributes of Muffins. Int. J. Food Sci. Technol. 2017, 52, 540–549. [Google Scholar] [CrossRef]
- Shameena Beegum, P.P.; Manikantan, M.R.; Sharma, M.; Pandiselvam, R.; Gupta, R.K.; Hebbar, K.B. Optimization of Processing Variables for the Development of Virgin Coconut Oil Cake Based Extruded Snacks. J. Food Process Eng. 2019, 42, e13048. [Google Scholar] [CrossRef]
- Petraru, A.; Amariei, S. Rapeseed—An Important Oleaginous Plant in the Oil Industry and the Resulting Meal a Valuable Source of Bioactive Compounds. Plants 2024, 13, 3085. [Google Scholar] [CrossRef]
- Renzyaeva, T.V.; Renzyaev, A.O.; Kravtchenko, S.N.; Reznichenko, I.Y. Capabilities of Rapeseed Oilcake as Food Raw Materials. Storage Process. Farm Prod. 2020, 2, 143–160. [Google Scholar] [CrossRef]
- Karabulut, G.; Subasi, B.G.; Ivanova, P.; Goksen, G.; Chalova, V.; Capanoglu, E. Towards Sustainable and Nutritional-Based Plant Protein Sources: A Review on the Role of Rapeseed. Food Res. Int. 2025, 202, 115553. [Google Scholar] [CrossRef]
- James, G.C.; Euston, S.R. Molecular Dynamics Simulation Allows Mechanistic Understanding of Natural Deep Eutectic Solvents Action on Rapeseed Proteins. Food Hydrocoll. 2025, 166, 111328. [Google Scholar] [CrossRef]
- Multescu, M.; Marinas, I.C.; Susman, I.E.; Belc, N. Byproducts (Flour, Meals, and Groats) from the Vegetable Oil Industry as a Potential Source of Antioxidants. Foods 2022, 11, 253. [Google Scholar] [CrossRef] [PubMed]
- Apak, R. Current Issues in Antioxidant Measurement. J. Agric. Food Chem. 2019, 67, 9187–9202. [Google Scholar] [CrossRef] [PubMed]
- Rabiej-Kozioł, D.; Szydłowska-Czerniak, A. Antioxidant Potential Evaluation at Various Stages of Black Cumin Oil Production. Foods 2024, 13, 3518. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Rehman, A.U.; Obied, H.K.; Luckett, D.J.; Blanchard, C.L. Extraction, Chemical Characterization, In Vitro Antioxidant, and Antidiabetic Activity of Canola (Brassica Napus L.) Meal. Separations 2022, 9, 38. [Google Scholar] [CrossRef]
- Cisneros-Yupanqui, M.; Chalova, V.I.; Kalaydzhiev, H.R.; Mihaylova, D.; Krastanov, A.I.; Lante, A. Preliminary Characterisation of Wastes Generated from the Rapeseed and Sunflower Protein Isolation Process and Their Valorisation in Delaying Oil Oxidation. Food Bioprocess Technol. 2021, 14, 1962–1971. [Google Scholar] [CrossRef]
- Zadbashkhanshir, K.; Fadaei, V.; Fahimdanesh, M. Canola Meal Phenolic Compounds Electrosprayed into Capsules to Increase the Oxidative Stability of Canola Oil. Chem. Biol. Technol. Agric. 2023, 10, 4. [Google Scholar] [CrossRef]
- Rabiej-Kozioł, D.; Szydłowska-Czerniak, A. The Valorization of Rapeseed Meal as Hydrolyzed and Lyophilized Extract to Improve the Antioxidant Properties of Refined Rapeseed Oil During Frying and Fried French Fries. Foods 2025, 14, 1444. [Google Scholar] [CrossRef]
- Włodarczyk, K.; Czaplicki, S.; Tańska, M.; Szydłowska-Czerniak, A. Microwave Pre-Treatment as a Promising Strategy to Develop Functional Milk Alternatives Obtained from Oil Industry by-Products. Innov. Food Sci. Emerg. Technol. 2023, 88, 103443. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A.; Poliński, S.; Momot, M. Optimization of Ingredients for Biscuits Enriched with Rapeseed Press Cake—Changes in Their Antioxidant and Sensory Properties. Appl. Sci. 2021, 11, 1558. [Google Scholar] [CrossRef]
- Hadidi, M.; Aghababaei, F.; McClements, D.J. Sunflower Meal/Cake as a Sustainable Protein Source for Global Food Demand: Towards a Zero-Hunger World. Food Hydrocoll. 2024, 147, 109329. [Google Scholar] [CrossRef]
- Petraru, A.; Ursachi, F.; Amariei, S. Nutritional Characteristics Assessment of Sunflower Seeds, Oil and Cake. Perspective of Using Sunflower Oilcakes as a Functional Ingredient. Plants 2021, 10, 2487. [Google Scholar] [CrossRef] [PubMed]
- Buranelo Egea, M.; Gonçalves De Oliveira Filho, J.; Romanelli, M.; Bertolo, V.; Castelo De Araú, J.; Gautério, G.V.; Lemes, A.C. Bioactive Phytochemicals from Sunflower (Helianthus Annuus L.) Oil Processing Byproducts. In Bioactive Phytochemicals from Vegetable Oil and Oilseed Processing By-Products; Ramadan Hassanien, M.F., Ed.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–16. [Google Scholar] [CrossRef]
- Ali, M.; Khalil, M.; Badawy, W.Z.; Hellwig, M. Ultrasonic Treatment as a Modern Technique to Facilitate the Extraction of Phenolic Compounds from Organic Sunflower Seed Cakes. J. Sci. Food Agric. 2024, 104, 2245–2251. [Google Scholar] [CrossRef] [PubMed]
- Gültekin Subaşı, B.; Vahapoğlu, B.; Capanoglu, E.; Mohammadifar, M.A. A Review on Protein Extracts from Sunflower Cake: Techno-Functional Properties and Promising Modification Methods. Crit. Rev. Food Sci. Nutr. 2022, 62, 6682–6697. [Google Scholar] [CrossRef] [PubMed]
- Girotto, F.; Merlino, M.; Giovanelli, G.; Condurso, C.; Piazza, L. Unveiling the Potential of Micronized Dehulled Sunflower Press-Cake: A Breakthrough in Sustainable Plant-Based Protein-Rich Sport Beverages. Int. J. Food Sci. Technol. 2024, 59, 4784–4796. [Google Scholar] [CrossRef]
- Francesca, G.; Costanza, C.; Federica, N.; Laura, P. Investigating the Suitability of Sunflower Press-Cake Proteins in Formulated Sports Beverages. Food Funct. 2025, 16, 1992–2003. [Google Scholar] [CrossRef]
- Michalska-Ciechanowska, A.; Brzezowska, J.; Lech, K.; Masztalerz, K.; Korzeniowska, M.; Zambrowicz, A.; Szoltysik, M. Exploiting the Potential of Powdered Blends of Recovered Sunflower Seed Cake Phenolics and Whey—Development of Sustainable Food Additives. Foods 2024, 13, 1433. [Google Scholar] [CrossRef]
- Blicharz-Kania, A.; Pecyna, A.; Zdybel, B.; Andrejko, D.; Marczuk, A. Sunflower Seed Cake as a Source of Nutrients in Gluten-Free Bread. Sci. Rep. 2023, 13, 10864. [Google Scholar] [CrossRef]
- Grasso, S.; Pintado, T.; Pérez-Jiménez, J.; Ruiz-Capillas, C.; Herrero, A.M. Potential of a Sunflower Seed By-Product as Animal Fat Replacer in Healthier Frankfurters. Foods 2020, 9, 445. [Google Scholar] [CrossRef]
- de Sousa Bezerra, F.; Ramos, G.S.M.; de Oliveira Carvalho, M.G.; Koblitz, M.G.B. Natural Deep Eutectic Solvents Characteristics Determine Their Extracting and Protective Power on Chlorogenic Acids from Sunflower Meal. Sustain. Chem. Pharm. 2024, 37, 101430. [Google Scholar] [CrossRef]
- de Sousa Bezerra, F.; Ramos, G.M.S.; de Oliveira Carvalho, M.G.; Carvalho, H.S.; de Souza, J.P.; de Carvalho Neto, S.L.; de Souza, S.M.A.G.U.; da Costa Ferraz, D.C.; Koblitz, M.G.B. Cytotoxic Potential of Sunflower Meal NaDES and Liquid-Liquid Extracts. Food Chem. 2025, 474, 143148. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Liu, X.; Ma, Y.; Huang, X.; Zhang, X.; Liu, J.; Song, L.; Qiao, M.; Li, T.; Wang, T. Effects of Different Processing Methods on Phenolic Compounds in Flaxseed Meal. Food Chem. X 2024, 24, 101934. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, M.K.; Selim, K.A.H.; Mahmoud, A.A.T.; Ali, R.A. Effect of Bioactive Compounds of Defatted Flaxseed Meal on Rheological and Sensorial Properties of Toast and Cake. SDRP J. Food Sci. Technol. 2019, 4, 707–719. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, X.; Zhou, S. Effect of Flaxseed Marc Flour on High-Yield Wheat Bread Production: Comparison in Baking, Staling, Antioxidant and Digestion Properties. LWT 2022, 169, 113979. [Google Scholar] [CrossRef]
- Pinton, M.B.; dos Santos, B.A.; Correa, L.P.; Cargnin, G.; Cichoski, A.J.; da Silva, L.P.; Lorenzo, J.M.; Campagnol, P.C.B. Replacement of Alkaline Phosphate with Flaxseed Cake and Its Impact on Mortadella’s Technological, Oxidative, Microbiological, and Sensory Aspects. Int. J. Food Sci. Technol. 2024, 59, 4851–4865. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, B.; Kaur, A. Microwave Processing Effects on Physico-Chemical, Functional Properties, Phenolic Profile, and Maillard Products of Flaxseed Flour and Flaxseed Press Cake Flour. Ind. Crops Prod. 2024, 218, 118900. [Google Scholar] [CrossRef]
- Talwar, B.; Chopra, R.; Taneja, N.K.; Chand, M.; Homroy, S.; Dhiman, A.; Singh, P.K.; Chaudhary, S. Use of Flaxseed Cake as a Source of Nutrients in the Food Industry and Possible Health Benefits—A Review. Food Prod. Process. Nutr. 2025, 7, 22. [Google Scholar] [CrossRef]
- Singh, S.; Shahi, N.C.; Lohani, U.C.; Bhat, M.I.; Sirohi, R.; Singh, S. Process Optimization for the Extraction of Bioactive Compounds from Defatted Flaxseed Cake (Linum Usitatissimu) Using Ultrasound-Assisted Extraction Method and Its Characterization. J. Food Process Eng. 2023, 46, e14217. [Google Scholar] [CrossRef]
- Guo, X.; Shi, L.; Yang, S.; Yang, R.; Dai, X.; Zhang, T.; Liu, R.; Chang, M.; Jin, Q.; Wang, X. Effect of Sea-Buckthorn Pulp and Flaxseed Residues on Quality and Shelf Life of Bread. Food Funct. 2019, 10, 4220–4230. [Google Scholar] [CrossRef]
- Waszkowiak, K.; Makowska, A.; Mikołajczak, B.; Myszka, K.; Barthet, V.J.; Zielińska-Dawidziak, M.; Kmiecik, D.; Truszkowska, M. Fermenting of Flaxseed Cake with Lactiplantibacillus Plantarum K06 to Increase Its Application as Food Ingredient—The Effect on Changes in Protein and Phenolic Profiles, Cyanogenic Glycoside Degradation, and Functional Properties. LWT 2025, 217, 117419. [Google Scholar] [CrossRef]
- Mashanova, N.; Satayeva, Z.; Smagulova, M.; Kundyzbayeva, N.; Karimova, G. Nutritional and Structural Evaluation of Gluten-Free Flour Mixtures Incorporating Various Oilseed Cakes. Processes 2024, 12, 1616. [Google Scholar] [CrossRef]
- Farzanfar, F.; Mahoonak, A.S.; Ghorbani, M.; Qaboos, S.H.H.; Kaveh, S. The Effect of Ultrasound Pretreatment on the Antioxidant Properties of Hydrolyzed Protein from Flaxseed Meal Using Alcalase and Pancreatin Enzymes by Response Surface Methodology. J. food Sci. Technol. 2024, 21, 187–205. [Google Scholar] [CrossRef]
- Farzanfar, F.; Mahoonak, A.S.; Ghorbani, M.; Qaboos, S.H.H.; Kaveh, S. Production of Bioactive Peptides from Flaxseed Meal: The Effect of Protease Type and Concentration, Hydrolysis Time, and Microwave Pretreatment. J. food Sci. Technol. 2025, 21, 191–204. [Google Scholar] [CrossRef]
- Wei, K.; Wan, Y.; Wei, C.; Liu, W.; Wu, H.; Leng, Y.; Xu, M.; Li, Y.; Chen, Z.; Wang, J.; et al. Ultrasound-Assisted Preparation of Antioxidant Peptides in Flaxseed Meal: Purification, Characterization and Molecular Docking Analysis. Food Chem. 2025, 487, 144724. [Google Scholar] [CrossRef] [PubMed]
- Taglieri, I.; Sanmartin, C.; Venturi, F.; Macaluso, M.; Zinnai, A.; Tavarini, S.; Serra, A.; Conte, G.; Flamini, G.; Angelini, L.G. Effect of the Leavening Agent on the Compositional and Sensorial Characteristics of Bread Fortified with Flaxseed Cake. Appl. Sci. 2020, 10, 5235. [Google Scholar] [CrossRef]
- Sanmartin, C.; Taglieri, I.; Venturi, F.; Macaluso, M.; Zinnai, A.; Tavarini, S.; Botto, A.; Serra, A.; Conte, G.; Flamini, G.; et al. Flaxseed Cake as a Tool for the Improvement of Nutraceutical and Sensorial Features of Sourdough Bread. Foods 2020, 9, 204. [Google Scholar] [CrossRef]
- Krupa-Kozak, U.; Baczek˛, N.; Capriles, V.D.; Łopusiewicz, Ł. Novel Gluten-Free Bread with an Extract from Flaxseed By-Product: The Relationship between Water Replacement Level and Nutritional Value, Antioxidant Properties, and Sensory Quality. Molecules 2022, 27, 2690. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Drozłowska, E.; Siedlecka, P.; Mężyńska, M.; Bartkowiak, A.; Sienkiewicz, M.; Zielińska-Bliźniewska, H.; Kwiatkowski, P. Development, Characterization, and Bioactivity of Non-Dairy Kefir-Like Fermented Beverage Based on Flaxseed Oil Cake. Foods 2019, 8, 544. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Drozłowska, E.; Tarnowiecka-Kuca, A.; Bartkowiak, A.; Mazurkiewicz-Zapałowicz, K.; Salachna, P. Biotransformation of Flaxseed Oil Cake into Bioactive Camembert-Analogue Using Lactic Acid Bacteria, Penicillium Camemberti and Geotrichum Candidum. Microorganisms 2020, 8, 1266. [Google Scholar] [CrossRef]
- Farhan, M.; Ali, S.F.; Sadiq, M.B. Optimized Extraction and Characterization of Sesame Seed Press Cake Protein and Its Application in the Formulation of Meatball Analog. Food Biophys. 2025, 20, 62. [Google Scholar] [CrossRef]
- Noptana, R.; McClements, D.J.; McLandsborough, L.A.; Onsaard, E. Comparison of Characteristics and Antioxidant Activities of Sesame Protein Hydrolysates and Their Fractions. Heliyon 2024, 10, e27891. [Google Scholar] [CrossRef]
- Gaipova, S.; Ruzibayev, A.; Khakimova, Z.; Salijanova, S.; Fayzullayev, A. Formulation of Mayonnaise Recipe Enriched with Biological Active Compounds of Sesame Cake. IOP Conf. Ser. Earth Environ. Sci. 2021, 939, 012085. [Google Scholar] [CrossRef]
- El-Enzi, S.M.; Andigani, N.M.; Al-Tamimi, N.A.; Gabr, G.A. Physico Chemical and Sensory Evaluation of the Fortified Biscuits with Sesame Cake Flour. Asian Food Sci. J. 2018, 5, 1–8. [Google Scholar] [CrossRef]
- Mohammady Assous, M.T.; Abd Elaziz, H.M.; Ahmed, M.F. Effect Of Partial Substitution Of Date Paste By Sesame Seed Cake On The Nutritional Value Of School Meal (Biscuit-Date). New Val. J. Agric. Sci. 2023, 3, 1016–1027. [Google Scholar] [CrossRef]
- Sundar, S.; Singh, B.; Kaur, A. Evaluating the Synergistic Effects of Sesame Cake Powder and Soy Protein Isolate on Rheological, Textural, Nutritional, and Phenolic Profiles of High-Moisture Extrusion Processed Meat Analogs. J. Food Sci. 2024, 89, 9433–9455. [Google Scholar] [CrossRef]
- Lucini Mas, A.; Brigante, F.I.; Salvucci, E.; Ribotta, P.; Martinez, M.L.; Wunderlin, D.A.; Baroni, M.V. Novel Cookie Formulation with Defatted Sesame Flour: Evaluation of Its Technological and Sensory Properties. Changes in Phenolic Profile, Antioxidant Activity, and Gut Microbiota after Simulated Gastrointestinal Digestion. Food Chem. 2022, 389, 133122. [Google Scholar] [CrossRef]
- Idowu, A.O.; Famuwagun, A.A.; Fagbemi, T.N.; Aluko, R.E. Antioxidant and Enzyme-Inhibitory Properties of Sesame Seed Protein Fractions and Their Isolate and Hydrolyzate. Int. J. Food Prop. 2021, 24, 780–795. [Google Scholar] [CrossRef]
- Norouzi, Z.; Nateghi, L.; Rashidi, L.; Movahhed, S. Increasing the Bioactive Properties of Sesame Seed Meal as a Valuable Protein Source through the Enzymatic Hydrolysis. Food Biosci. 2025, 68, 106790. [Google Scholar] [CrossRef]
- Cao, P.H.; Zhang, C.X.; Ma, Y.X.; Yu, Y.M.; Liu, H.M.; Wang, X.D.; Zheng, Y.Z. Extraction of Protein from Sesame Meal: Impact of Deep Eutectic Solvents on Protein Structure and Functionality. LWT 2023, 187, 115366. [Google Scholar] [CrossRef]
- Sengupta, S.; Basu, S.; Bhowal, J. Biochemical Characterization and Hypocholesterolemic Properties of Sesame Yogurt Made from Deoiled Edible Quality Sesame Flour (DEQSF) Supplemented with Rice Bran Oil. Food Prod. Process. Nutr. 2023, 5, 56. [Google Scholar] [CrossRef]
- Zubair, A.B.; Femi, F.A.; Azeez, S.O.; Maxwell, Y.M.O.; Isah, L.R.; Jiya, M.J.; Owheruo, J.O. Proximate, Mineral and Sensory Evaluation of Cake Baked from Wheat and Sesame Seed Flour Blends. Niger. J. Nutr. Sci. 2021, 42, 124–129. [Google Scholar]
- Nouska, C.; Irakli, M.; Palakas, P.; Lytou, A.E.; Bouloumpasi, E.; Biliaderis, C.G.; Lazaridou, A. Influence of Sesame Cake on Physicochemical, Antioxidant and Sensorial Characteristics of Fortified Wheat Breads. Food Res. Int. 2024, 178, 113980. [Google Scholar] [CrossRef] [PubMed]
- Yaver, E. Impact of Defatted Sesame Meal on the Technological and Sensory Quality and Antioxidant Activity of Gluten-Free Buckwheat Crackers, ID: 385. In Proceedings of the IV. International Congresses of Turkish Journal of Agriculture-Food Science and Technology, Konya, Türkiye, 21 May 2025; pp. 282–285. [Google Scholar]
- Smeu, I.; Dobre, A.A.; Cucu, E.M.; Mustățea, G.; Belc, N.; Ungureanu, E.L. Byproducts from the Vegetable Oil Industry: The Challenges of Safety and Sustainability. Sustainability 2022, 14, 2039. [Google Scholar] [CrossRef]
- Torres-León, C.; Ramírez-Guzman, N.; Londoño-Hernandez, L.; Martinez-Medina, G.A.; Díaz-Herrera, R.; Navarro-Macias, V.; Alvarez-Pérez, O.B.; Picazo, B.; Villarreal-Vázquez, M.; Ascacio-Valdes, J.; et al. Food Waste and Byproducts: An Opportunity to Minimize Malnutrition and Hunger in Developing Countries. Front. Sustain. Food Syst. 2018, 2, 52. [Google Scholar] [CrossRef]
- Tomas, M.; Wen, Y.; Liao, W.; Zhang, L.; Zhao, C.; Julian McClements, D.; Nemli, E.; Bener, M.; Apak, R.; Capanoglu, E.; et al. Recent Progress in Promoting the Bioavailability of Polyphenols in Plant-Based Foods. Crit. Rev. Food Sci. Nutr. 2025, 65, 2343–2364. [Google Scholar] [CrossRef]
- Vilas-Boas, A.A.; Pintado, M.; Oliveira, A.L.S. Natural Bioactive Compounds from Food Waste: Toxicity and Safety Concerns. Foods 2021, 10, 1564. [Google Scholar] [CrossRef]
- Minervini, F.; Comitini, F.; De Boni, A.; Fiorino, G.M.; Rodrigues, F.; Tlais, A.Z.A.; Carafa, I.; De Angelis, M. Sustainable and Health-Protecting Food Ingredients from Bioprocessed Food by-Products and Wastes. Sustainability 2022, 14, 15283. [Google Scholar] [CrossRef]
- Rao, M.; Bast, A.; de Boer, A. Valorized Food Processing By-Products in the EU: Finding the Balance between Safety, Nutrition, and Sustainability. Sustainability 2021, 13, 4428. [Google Scholar] [CrossRef]
| Waste/By-Products | Form of the Additive | Food Product | The Final Quality of the Fortified Product | References |
|---|---|---|---|---|
| Olive pomace | Flour: 5 and 10% flour substitution | Pasta | ↑ TPC: 2.28 and 0.69 mg GAE/g dw for uncooked and cooked, respectively ↑ Antioxidant potential (DPPH and ABTS) ↓ Rapidly digestible starch and ↑ slow digestible starch and resistant starch | [56] |
| Olive pomace | Fermented (Saccharomyces cerevisiae) flour: 20% flour substitution | “Taralli” (bakery products) | ↑ TPC: up to 1377 μg/g dw (mainly hydroxytyrosol, tyrosol, verbascoside, oleacin, oleocanthal) ↑ Polyphenols (hydroxytyrosol, verbascoside, oleacin), triterpenic acids, tocochromanols (α-tocopherol, β-tocotrienol), carotenoids (mainly lutein) Storage at 25 °C for 90 days: ≈ bioactive compounds | [60] |
| Olive mill wastewater | Phenolic extract: 100 ppm | Sunflower oil and rapeseed oil | ↓ Oxidative deterioration: acid value, PV, and extinction coefficient (K270) Accelerated storage at 60 °C for 4 weeks: ↓ the loss of phytosterols and tocopherols | [61] |
| Olive mill solid waste | Encapsulated phenolic extract: 100 mg/100 g | White soft cheese | ↑ TPC and antioxidant capacity (DPPH) ↑ Total solids and protein Storage at 5 °C for 30 days: ≈ antioxidant capacity | [62] |
| Olive leaves | Phenolic extract: 75 g in 250 g flour | “Taralli” (bakery product) | ↑ TPC, TFC, and antioxidant capacity (FRAP) for uncooked and cooked pasta and after in vitro digestion | [63] |
| Olive leaves and olive mill wastewater | Phenolic extract: 500 and 1000 mg/kg | Gluten-free breadstick | ↑ TPC (mg GAE/100 g): from (control) 162.87 up to 139.68 with olive leave extract and 130.64 with olive mill wastewater extract ↑ Antioxidant activity (DPPH) ↑ Soluble and ↓ insoluble polyphenol fraction ↑ Bioaccessibility of polyphenols: up to 23.0 and 15.1% for fortification with olive leaves and olive mill wastewater, respectively ↑ Induction period via Oxitest | [64] |
| Olive pomace | Phenolic extract conjugate with inulin: 50 and 100 mg/mL | Pear beverage | ↑ TPC: up to 28.59 GAE/L ↑ Antioxidant potential (DPPH and ABTS) Storage at 4 °C for 20 days: ≈ TPC, and antioxidant potential | [65] |
| Olive pomace | Flour: 10 and 20% flour substitution | Biscuit | ↑ Dietary fiber, mineral, and lipid contents ↓ Total carbohydrates ↓ Better results with 10% addition | [66] |
| Waste/By-Products | Form of the Additive | Food Product | The Final Quality of the Fortified Product | References |
|---|---|---|---|---|
| Avocado seed | Flour: 6, 12, and 18% | Cereal snacks | ↑ TPC: up to 167.78 mg GAE/100 g dw ↑ Antioxidant capacity (ABTS): up to 14.62 mmol TE/100 g dw ↑ Fiber content and ↓ carbohydrate and protein content | [117] |
| Avocado peel | Phenolic extract: 0.5% and 1% | Beef and soy-based burgers | ↑ Protein, fat, and ash contents During cooking and storage: ↓ TBARS value, hexanal, and carbonyl content ↓ Heterocyclic aromatic amines and acrylamide after cooking | [118] |
| Avocado peel | Phenolic extract: 0.5% and 1.0% | Mayonnaise | Antimicrobial on Escherichia coli and Staphylococcus aureus ≈ Emulsion stability, and free fatty acids Refrigerated storage for 6 weeks: ↓ PV, p-AV, TOTOX value | [119] |
| Avocado seed | Flour: 5%, 10%, 15%, and 20% | Injera (fermented food) | ↑ TPC: up to 265.76 mgGAE/100 g ↑ TFC: up to 72.37 mg QE/100 g ↑ Antioxidant potential (DPPH, FRAP, ABTS) ↑ Vitamin C and vitamin A: up to 8.28 and 26.01 mg/100 g, respectively ↑ β-carotene: up to 155.74 μg/100 g ↓ Anti-nutritional factor: phytic acid ↑ Protein, fat, and fiber contents and ↓ total carbohydrate content ↓ Rapidly digestible starch, and slowly digestible starch and ↑ resistant starch | [120] |
| Avocado pulp, seed, and peel | Flour: 5% and 10% | Semolina sourdough bread | ↑ TPC: up to 23.882 mgGAE/g ↑ Antioxidant potential (DPPH assay: 9.234 mmol TE/100 g and ABTS: up to 6.656 mmol TE/100 g) | [121] |
| Avocado peel | Phenolic extract: keratin-starch composites functionalized with 0.0, 0.2, 0.6, and 1.0 mL extract | Freshly cut beef | ↓ TBARS value, carbonyl content, and metmyoglobin level Storage at 4° for 12 days: ↓ yeast and mold count | [122] |
| Waste/By-Products | Form of the Additive | Food Product | The Final Quality of the Fortified Product | References |
|---|---|---|---|---|
| Defatted coconut flour | Flour: 10, 20, 30, 40, and 50% flour substitution | Nixtamalized maize flour | ↑ TPC: up to 116.40 mg GAE/100 g ↑ TFC: up to 94.81 mg/100 g ↑ Antioxidant potential (DPPH and FRAP assays) ↑ Protein and fat contents and ↓ carbohydrate content ↑ Dietary fiber content: up to 45.95 and 5.12% for insoluble and soluble dietary fiber, respectively | [154] |
| Coconut residue | Flour: 5, 10, 15, 20, and 25% flour substitution | Pasta | ↑ Protein, fat, and total dietary fiber contents and ↓ carbohydrate content | [155] |
| Virgin coconut meal | Flour: 5, 10, 15, and 20% flour substitution | Cake | ↑ Moisture, fat, protein, and ash contents, and ↓ carbohydrate content | [156] |
| Defatted coconut flour | Flour: 10, 20, 30, 40, and 50% flour substitution | Rice noodles | ↑ TPC: up to 2.35 g/1000 g ↑ TFC: up to 4.62 g/1000 g ↑ Antioxidant activity: up to 14.71% for DPPH assay ↑ Mineral content: up to 21.40, 326.47, 9.17, 0.36, 1.15, and 1.26 ppm for Na, K, Ca, Cu, Fe, and Zn | [157] |
| Virgin coconut oil cake | Flour: 10, 20, 30, 40 and 50% flour substitution | Muffin | ↑ Protein, fat, crude fiber, and total mineral contents Storage at 4 °C and 35 °C for 16 days: no change in the quality | [158] |
| Virgin coconut oil cake | Flour: 20, 25, 30% flour substitution | Extruded snacks | The optimized conditions: 28.7% virgin oil cake, 14% feed moisture, and 300 rpm screw speed Optimized products: moisture, carbohydrate, protein, fat, ash, and crude fiber content: 5.1, 74.19, 11.14, 5.07, 2.3, and 1.58 g/100 g | [159] |
| Waste/By-Products | Form of the Additive | Food Product | The Final Quality of the Fortified Product | References |
|---|---|---|---|---|
| Rapeseed meal | Phenolic-rich washout: 5, 15% | Soybean, rapeseed, and sunflower oil | ↑ Induction period by Rancimat method Comparable antioxidant activity index at 15% level to BHT (0.02%) | [168] |
| Canola meal | Nano-encapsulated phenolic extract: 200, 400, and 800 ppm | Canola oil, storage at 30 °C for 60 days | ↓ PV, TBARS level ↑ Antioxidant activity (DPPH), iodine value, ↑ Induction period by Rancimat method: highest at 800 ppm: 12.9 h Comparable to TBHQ at 200 ppm; superior effect at 400–800 ppm | [169] |
| Rapeseed meal | Acid-hydrolyzed + lyophilized Extract: 200 ppm | Oil collected after 24 h of deep-frying French fries | ↓ PV, p-AV, K232, K268 values ↓ TOTOX index: 221.10 (control) →196.8 (with extract) ↓ INTOX index: 390.72 (control) → 343.16 (with extract) ↑ TPC and antioxidant potential (ABTS, FRAP, DPPH) | [170] |
| Rapeseed cake, with/without black cumin cake | Flour: 1:6 ratio | Plant-based beverage, microwave pre-treated | ↑ TPC, tocopherol, carotenoid, and chlorophyll content Optimum conditions: 800 W, 3 sec, black cumin/rapeseed meal ratio: 0.1144 | [171] |
| Rapeseed press cake | Flour: 20, 40% flour substitution | Biscuit | Antioxidant activity: from 535–80 to 8375–10 088 µmol TE/100 g Optimized formula: 40% rapeseed cake + 2.3% saturated fat | [172] |
| Waste/By-Products | Form of the Additive | Food Product | The Final Quality of the Fortified Product | References |
|---|---|---|---|---|
| Sunflower meal | Phenolic-rich washout: 5, 15% | Soybean, rapeseed, and sunflower oil | ↑ Induction period by Rancimat method Comparable antioxidant activity index at both levels to BHT (0.02%) | [168] |
| Dehulled sunflower press-cake | Protein concentrate: 10% | Protein-rich sport beverages | ↑ TPC compared to whey and pea protein concentrate ↓ Total phenolic bioaccessibility: 84.3% reduction | [179] |
| Sunflower meal | Phenolic-rich washout: 10, 20, 30, 40, and 50% | Whey protein dispersion | ↑ Protein content ↑ TPC ↓ Available amino groups | [180] |
| Sunflower pomace | Phenolic extract: 5, 10, and 20% | Strawberry puree Strawberry + yogurt beverage | ↑ Antioxidant potential (CUPRAC, ABTS, DPPH) before and after digestion | [16] |
| Sunflower seed cake | Flour: 5, 10, 15% flour substitution | Gluten-free bread | ↑ Protein, crude fiber, fat TPC: 89.30 to 222.33 mg GAE/100 g | [181] |
| Sunflower meal | Flour: 2, and 4% | Frankfurter | ↑ Protein and dietary fibre ↑ Mineral content: Mg, K, Fe, Zn, Cu, Mn ↑ TPC from 51 to 76 and 93 mg GAE/100 g, respectively. | [182] |
| Waste/By-Products | Form of the Ingredient | Food Product | The Final Quality of the Fortified Product | References |
|---|---|---|---|---|
| Flaxseed press cake | Flour: 4 and 8% flour substitution | Bread | ↑ Antioxidant potential (DPPH, ABTS and FRAP) ↓ Glucose release after in vitro digestion | [192] |
| Flaxseed marc | Flour: 5, 15, 25% flour substitution | Bread | ↑ Antioxidant potential (DPPH: from (control) 19.50% to 54.25%) | [187] |
| Flaxseed cake | Flour: 5, 7.5, and 10% flour substitution | Bread, sourdough bread | ↑ TPC and TFC ↑Antioxidant potential (DPPH, TEAC) | [198] |
| Flaxseed cake | Flour: 5, 7.5, and 10% flour substitution | Sourdough bread | ↑ Total phenolics and flavonoids ↑Antioxidant potential (DPPH, ABTS) | [199] |
| Flaxseed cake | Hot-distilled water extract: 25, 50, 75, and 100% | Gluten-free bread | ↑ Protein, ↓ carbohydrates ↑ Mineral content: P, K, Mg ↑ TPC: from (control) 0.096 to 0.234 mg GAE/g ↑ Antioxidant potential (DPPH, ABTS, FRAP) | [200] |
| Flaxseed meal | Flour: 5, 10, 15% flour substitution | Toast and cake | ↑ Mineral content: K, Mg, P, and Ca | [186] |
| Flaxseed cake | 5%, 10%, and 15% in water | Kefir-like fermented beverage with kefir grains, storage for 21 days at 6 °C | ↑ TPC, TFC, and antioxidant potential (DPPH, ABTS): linear with flaxseed ratio Stable phenolic and antioxidant potential during storage | [201] |
| Flaxseed cake | 35% in water | Camembert analog with Penicillium camemberti (PC) alone or with yeast Geotrichum candidum, | Storage for 28 days: ↑ TPC and TFC Antioxidant potential: ↑ 0–7/14 days ↓ 21–28 days | [202] |
| Flaxseed cake | 1.5 and 3% | Mortadella | ↓ TBARS level | [188] |
| Waste/By-Products | Form of the Additive | Food Product | The Final Quality of the Fortified Product | References |
|---|---|---|---|---|
| Sesame seed cake | Flour: 5, 5.15, 6, 8, and 10% flour substitution | Mayonnaise | ↓ PV, p-AV | [205] |
| Sesame seed meal | Soaked and filtered milk-like suspension: 6, 7, and 8% flour in water | Yogurt | ↑ Antioxidant potential (DPPH, FRAP, ABTS, ORAC) | [213] |
| Sesame seed cake | A filling mixture of date paste and sesame seed cake flour (80:20) | Biscuit | ↑ Protein, fiber, and ↓ reducing sugar, total sugar ↑ TPC and TFC, antioxidant activity (DPPH) ↑ Mineral content: Ca, Zn, Fe, Mg | [207] |
| Sesame seed cake | Flour: 20, 30, and 40% flour substitution | Biscuit | ↑ Protein, fiber, and ↓ carbohydrates ↑ Mineral content: P, Mg, Ca | [206] |
| Sesame seed cake | Flour: 5, 10, 20% flour substitution | Cookie | ↑ TPC, antioxidant potential (FRAP, TEAC) before and after digestion | [209] |
| Sesame seed meal | Flour | Cake | ↑ Protein, fiber, and ↓ carbohydrates ↑ Mineral content: Ca, Zn, Fe | [214] |
| Sesame seed cake | Flour: 6, 12, and 20% flour substitution | Bread | ↑ TPC, total lignans, and antioxidant potential (DPPH, ABTS) ↑ Caffeic acid, ferulic acid, sinapic acid, and p-coumaric acid, sesaminol diglucosides and sesaminol triglucosides | [215] |
| Sesame seed cake | Flour: 20, 40, and 60% soy protein isolate substitution | High-moisture extrusion-processed meat analogs | ↑ Free hydroxybenzoic acids ↓ Free and ↑ bound vitexin ↑ Free and bound quercetin, trans-cinnamic acid New flavonoids: naringenin, luteolin, and apigenin | [208] |
| Waste/By-Product | Extraction Method | Products | Advantages | Disadvantages |
|---|---|---|---|---|
| Olive pomace | Solid–liquid extraction | Phenolics, fiber, proteins, fatty acids | Simple, widely used | Low yield, long time |
| Ultrasound-assisted | Phenolics, antioxidants | Faster, higher yield | Requires special equipment | |
| Pressurized liquid extraction | Phenolics, flavonoids | High phenolic content, fast | Thermal degradation risk | |
| NADES | Phenolics, flavonoids | Green, selective | Needs optimization | |
| Methanol maceration | Polyphenols, flavonoids | High efficiency | Toxic solvent | |
| Microwave-assisted | Phenolics | Fast, energy-efficient | Equipment cost | |
| Olive leaves | Ultrasound-alkaline-assisted extraction | Proteins, bioactive peptides | Mild conditions, preserves activity | Enzyme cost |
| Enzyme-assisted extraction | Proteins | Effective protein extraction | May denature some proteins | |
| Palm kernel cake | Alkaline solubilization | Proteins, bioactive peptides | Effective protein extraction | Possible protein denaturation |
| Enzyme hydrolysis | Antioxidant peptides | Functional peptides produced | Enzyme cost, control needed | |
| Microwave-assisted | Phenolics | High yield, fast extraction | Equipment cost | |
| Ultrasound-assisted | Phenolics, sterols, carotenoids | Enhanced recovery | Equipment needed | |
| NADES | Phenolics | Green, selective | Emerging method, optimization needed | |
| Avocado by-products | Mechanical, chemical, microwave, ultrasound, DES | Phenolics, carotenoids, proteins, lipids | Rich bioactives, green methods | Variability, limited protein data |
| Coconut by-products | Various (e.g., copra meal, press cake, fiber) | Proteins, fibers, phenolics | Nutritional value, functional properties | Some variability |
| Rapeseed meal | Alkaline extraction | Protein isolates | High yield, low cost | Protein denaturation, low solubility |
| NADES extraction | Enhanced protein | Green, selective, high solubility | High viscosity, limited scalability | |
| Aqueous ethanol extraction | Phenolic compounds | Food-grade, effective for free phenolics | Less effective for bound phenolics | |
| Sunflower meal | Aqueous extraction | Phenolics (e.g., chlorogenic acid) | Simple, cost-effective | Co-extraction of unwanted pigments |
| Dehulling + pressing | Protein-rich meal | Higher protein and phenolic content | Requires additional preprocessing | |
| Flaxseed cake | Steam explosion | Bound phenolics, lignans | High release efficiency | Energy-intensive |
| Ultrasound-assisted hydrolysis | Antioxidant peptides | Improved bioactivity and yield | Equipment cost | |
| Microwave treatment | Phenolics, minerals | Enhances extractability | Risk of thermal degradation | |
| Sesame seed cake | Enzymatic hydrolysis | Bioactive peptides | Strong antioxidant potential | Enzyme cost, optimization needed |
| NADES extraction | High-purity proteins | Eco-friendly, high solubility | Still limited at industrial scale | |
| Organic solvent extraction | Lignans, flavonoids | Effective and widely used | Solvent toxicity, environmental concerns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemli, E.; Günal-Köroğlu, D.; Apak, R.; Capanoglu, E. Potential of Plant-Based Oil Processing Wastes/By-Products as an Alternative Source of Bioactive Compounds in the Food Industry. Foods 2025, 14, 2718. https://doi.org/10.3390/foods14152718
Nemli E, Günal-Köroğlu D, Apak R, Capanoglu E. Potential of Plant-Based Oil Processing Wastes/By-Products as an Alternative Source of Bioactive Compounds in the Food Industry. Foods. 2025; 14(15):2718. https://doi.org/10.3390/foods14152718
Chicago/Turabian StyleNemli, Elifsu, Deniz Günal-Köroğlu, Resat Apak, and Esra Capanoglu. 2025. "Potential of Plant-Based Oil Processing Wastes/By-Products as an Alternative Source of Bioactive Compounds in the Food Industry" Foods 14, no. 15: 2718. https://doi.org/10.3390/foods14152718
APA StyleNemli, E., Günal-Köroğlu, D., Apak, R., & Capanoglu, E. (2025). Potential of Plant-Based Oil Processing Wastes/By-Products as an Alternative Source of Bioactive Compounds in the Food Industry. Foods, 14(15), 2718. https://doi.org/10.3390/foods14152718








