Carcass and Meat Quality Characteristics and Changes of Lean and Fat Pigs After the Growth Turning Point
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
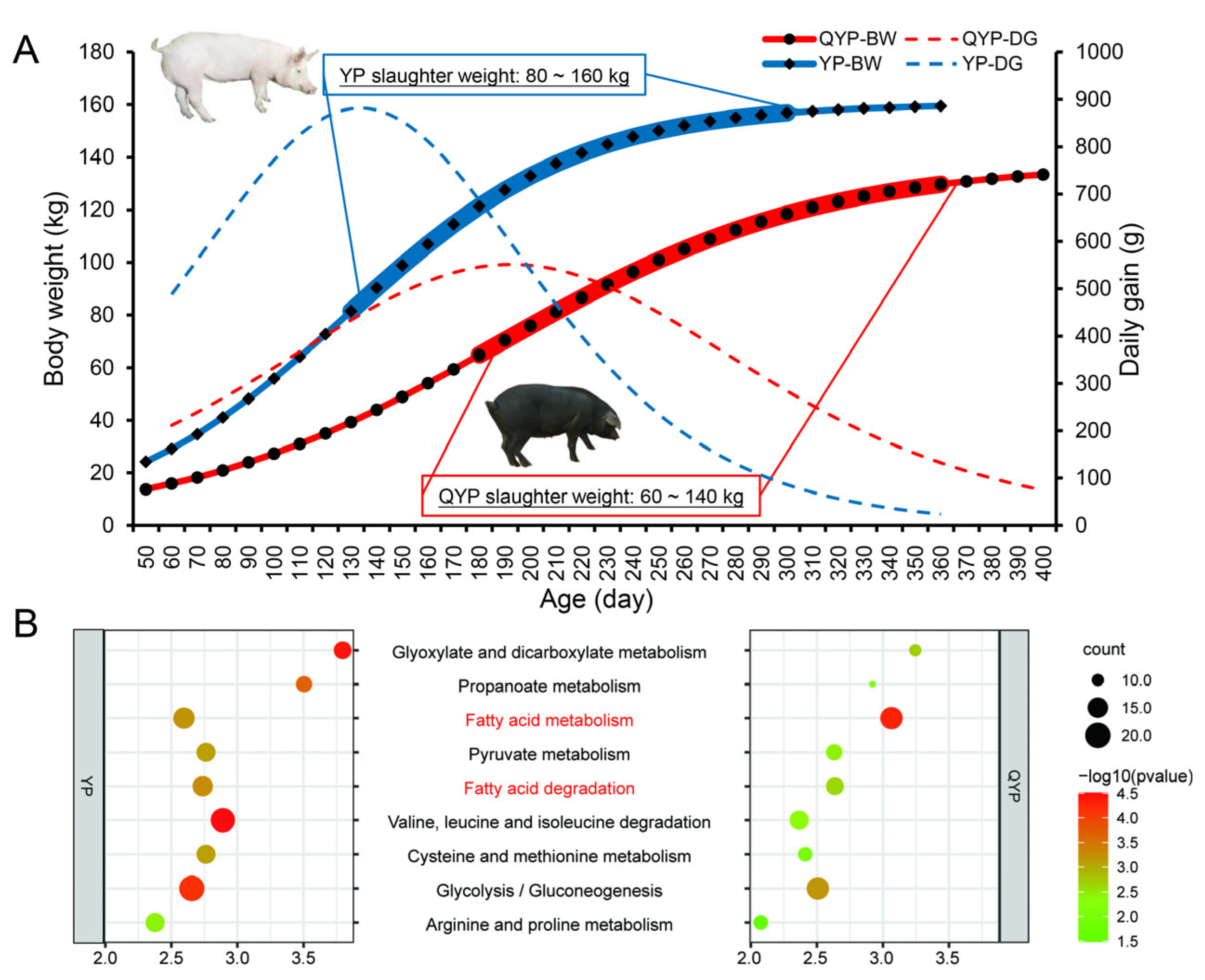
2.2. Growth Curve
2.3. Measurement of Carcass Traits
2.4. Measurement of Meat Quality
2.5. Chemical Composition
2.6. Total RNA Extraction and Real-Time Quantitative PCR
2.7. Bioinformatic Analysis
2.8. Statistical Analysis
3. Results
3.1. Differences in Growth and Development of Yorkshire Pigs and Qingyu Pigs
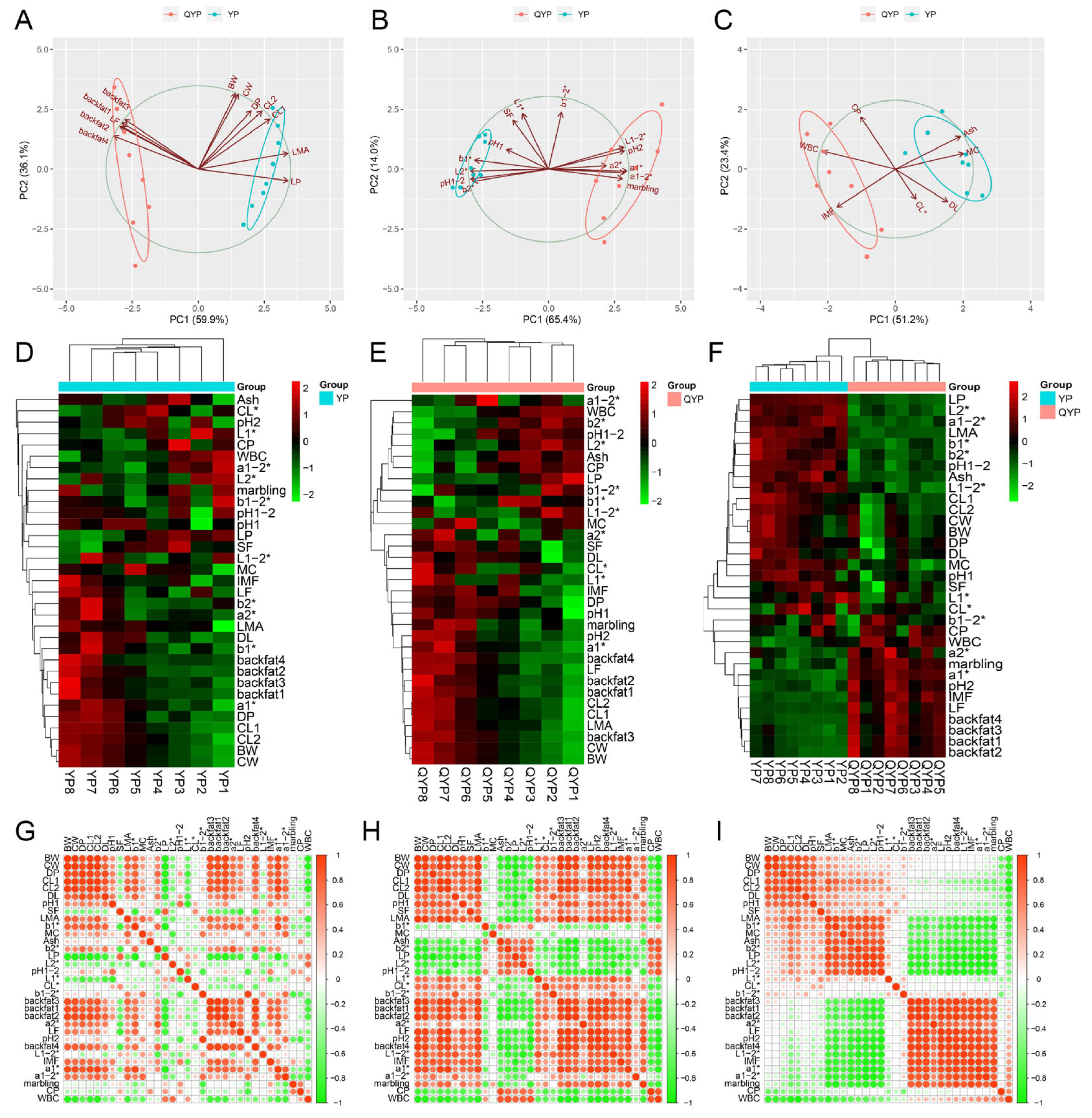
3.2. Overall Characteristics of Carcass and Meat Quality of Yorkshire Pigs and Qingyu Pigs
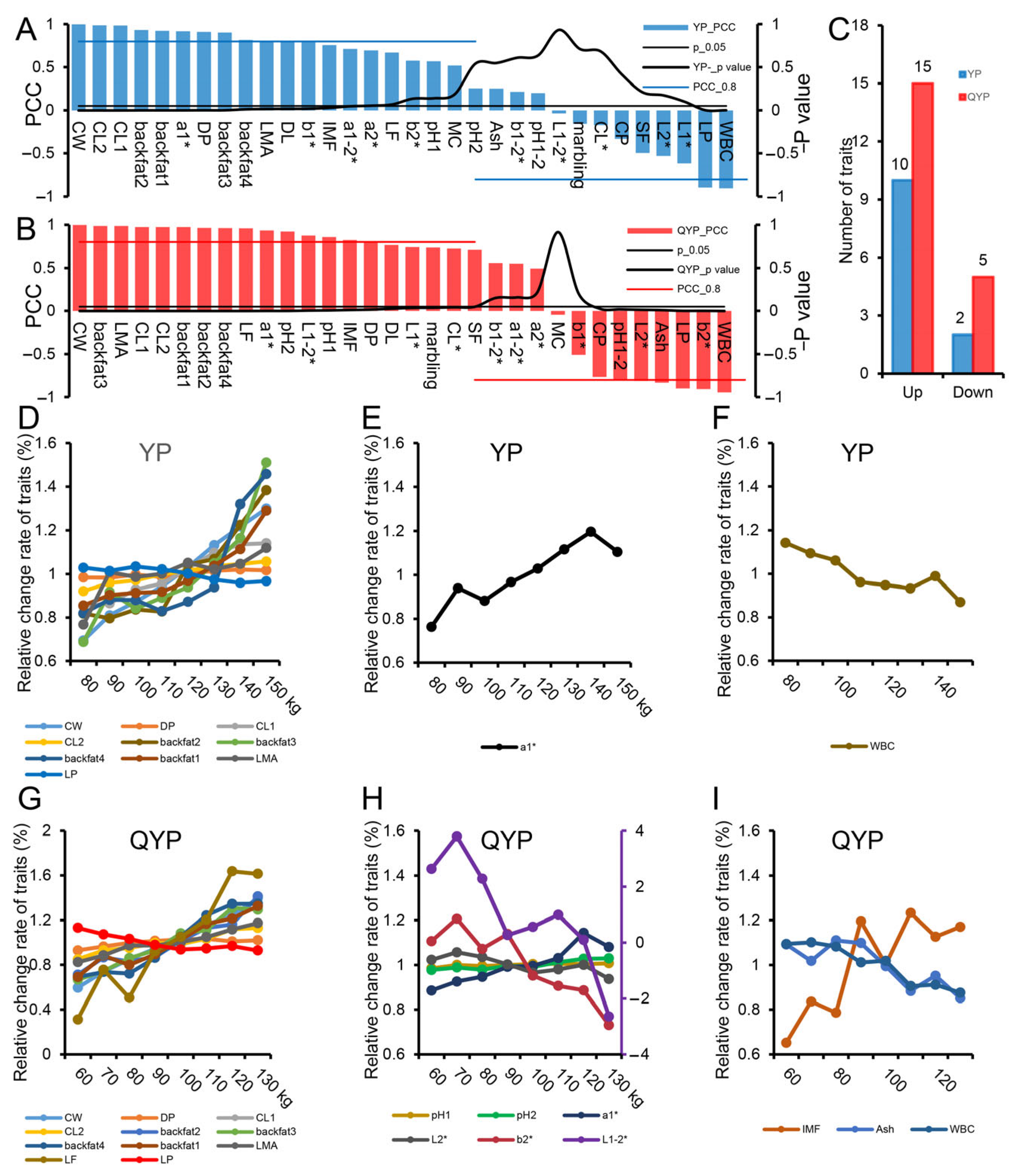
3.3. The Changes in Carcass and Meat Quality Indexes of Yorkshire Pigs and Qingyu Pigs
3.4. Differences in Muscle Development and Fat Deposition Between Yorkshire Pigs and Qingyu Pigs After Growth Inflection Point
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| YP | Yorkshire pig |
| QYP | Qingyu pig |
| LMA | Longissimus muscle area |
| IMF | Intramuscular fat |
| BW | Body weight |
| CW | Carcass weight |
| DP | Dressing percentage |
| LF | Leaf fat |
| LP | Lean percentage |
| DL | Drip loss |
| CL* | Cooking loss |
| SF | Shear force |
| WBC | Water-binding capacity |
| CP | Crude protein |
| MC | Moisture content |
| KEGG | Kyoto encyclopedia of genes and genomes |
References
- Lin-Schilstra, L.; Backus, G.; Snoek, H.; Mörlein, D. Consumers’ view on pork: Consumption motives and production preferences in ten European Union and four non-European Union countries. Meat Sci. 2022, 187, 108736. [Google Scholar] [CrossRef]
- Sasaki, K. Diversity of Japanese consumers’ requirements, sensory perceptions, and eating preferences for meat. Anim. Sci. J. 2022, 93, e13705. [Google Scholar] [CrossRef]
- Liang, Y.; Cheng, Y.; Xu, Y.; Hua, G.; Zheng, Z.; Li, H.; Han, L. Consumer Preferences for Animal Welfare in China: Optimization of Pork Production-Marketing Chains. Animals 2022, 12, 3021. [Google Scholar] [CrossRef]
- Verbeke, W.; Pérez-Cueto, F.J.A.; Barcellos, M.D.d.; Krystallis, A.; Grunert, K.G. European citizen and consumer attitudes and preferences regarding beef and pork. Meat Sci. 2010, 84, 284–292. [Google Scholar] [CrossRef]
- Razmaitė, V.; Šveistienė, R.; Jatkauskienė, V.; Šiukščius, A. Pork Consumption Frequencies, Attitudes and Sensory Acceptance of Traditional Products in Lithuania. Foods 2022, 11, 3292. [Google Scholar] [CrossRef]
- Van Loo, E.; Caputo, V.; Nayga, R.M.; Meullenet, J.-F.; Crandall, P.G.; Ricke, S.C. Effect of organic poultry purchase frequency on consumer attitudes toward organic poultry meat. J. Food Sci. 2010, 75, S384–S397. [Google Scholar] [CrossRef] [PubMed]
- Zomeño, C.; Gispert, M.; Brun, A.; Carabús, A.; Soler, J.; Font-I-Furnols, M. Productive performance and in vivo body composition across the growing and finishing period and carcass traits in pigs of four sex types. Meat Sci. 2022, 192, 108909. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jin, Y.; Zhou, S.; Wang, X.; Li, Y.; Luan, P.; Li, H.; Leng, L.; Wang, Y. A Study on the Growth and Development Characteristics of Lindian Chickens. Animals 2024, 14, 354. [Google Scholar] [CrossRef] [PubMed]
- Gurgeira, D.N.; Crisóstomo, C.; Sartori, L.V.C.; de Paz, C.C.P.; Delmilho, G.; Chay-Canul, A.J.; Bedoya, H.J.N.; Vega, W.H.O.; Bueno, M.S.; da Costa, R.L.D. Characteristics of growth, carcass and meat quality of sheep with different feed efficiency phenotypes. Meat Sci. 2022, 194, 108959. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Thakali, K.; Morse, P.; Shelby, S.; Chen, J.; Apple, J.; Huang, Y. Comparison of Growth Performance and Meat Quality Traits of Commercial Cross-Bred Pigs versus the Large Black Pig Breed. Animals 2021, 11, 200. [Google Scholar] [CrossRef]
- Roberts, M.M.; Perkins, S.D.; Anderson, B.L.; Sawyer, J.T.; Brandebourg, T.D. Characterization of Growth Performance, Pork Quality, and Body Composition in Mangalica Pigs. Foods 2023, 12, 554. [Google Scholar] [CrossRef] [PubMed]
- Helm, E.T.; Ross, J.W.; Patience, J.F.; Lonergan, S.M.; Huff-Lonergan, E.; Greiner, L.L.; Reever, L.M.; Hastad, C.W.; Arkfeld, E.K.; Gabler, N.K. Nutritional approaches to slow late finishing pig growth: Implications on carcass composition and pork quality. J. Anim. Sci. 2021, 99, skaa368. [Google Scholar] [CrossRef]
- Ferreira, Y.M.; Amaral, R.S.V.; Silva, B.G.V.; Moura, L.C.S.; Oliveira, D.A.; da Silva, T.E.; Hauschild, L.; Andretta, I.; Santos, L.S. A Simplified Daily Fit Model to Reduce Costs and Nutrient Intake in Growing-Finishing Pigs. Animals 2024, 14, 2922. [Google Scholar] [CrossRef]
- Ma, N.; Cai, K.; Liu, H.; He, L.; Liu, C.; Ma, X. Innovation in Swine Nutrition in China over the Past Decade. J. Nutr. 2025. [Google Scholar] [CrossRef]
- Sonea, C.; Gheorghe-Irimia, R.A.; Tapaloaga, D.; Gurau, M.R.; Udrea, L.; Tapaloaga, P.-R. Optimizing Animal Nutrition and Sustainability Through Precision Feeding: A Mini Review of Emerging Strategies and Technologies. Ann. Of‘valahia’Univ. Târgovişte. Agric. 2023, 15, 7–11. [Google Scholar] [CrossRef]
- Kellner, T.A.; Prusa, K.J.; Patience, J.F. Impact of dietary fat source and concentration and daily fatty acid intake on the composition of carcass fat and iodine value sampled in three regions of the pork carcass. J. Anim. Sci. 2014, 92, 5485–5495. [Google Scholar] [CrossRef]
- Llorens, B.; Pomar, C.; Goyette, B.; Rajagopal, R.; Andretta, I.; Latorre, M.A.; Remus, A. Precision feeding as a tool to reduce the environmental footprint of pig production systems: A life-cycle assessment. J. Anim. Sci. 2024, 102, skae225. [Google Scholar] [CrossRef]
- Blasco, A.; Nagy, I.; Hernández, P. Genetics of growth, carcass and meat quality in rabbits. Meat Sci. 2018, 145, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Hernández, P.; Aliaga, S.; Pla, M.; Blasco, A. The effect of selection for growth rate and slaughter age on carcass composition and meat quality traits in rabbits. J. Anim. Sci. 2004, 82, 3138–3143. [Google Scholar] [CrossRef] [PubMed]
- Lebret, B.; Lhuisset, S.; Labussière, E.; Louveau, I. Combining pig genetic and feeding strategies improves the sensory, nutritional and technological quality of pork in the context of relocation of feed resources. Meat Sci. 2023, 197, 109074. [Google Scholar] [CrossRef]
- Wang, L.; Gu, H.; Liao, T.; Lei, Y.; Qiu, Y.; Chen, Q.; Chen, L.; Zhang, S.; Wang, J.; Hao, X.; et al. tsRNA Landscape and Potential Function Network in Subcutaneous and Visceral Pig Adipose Tissue. Genes 2023, 14, 782. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.; Gan, M.; Lei, Y.; Wang, Y.; Chen, L.; Shen, L.; Zhu, L. Dynamic changes in the transcriptome of tRNA-derived small RNAs related with fat metabolism. Sci. Data 2023, 10, 703. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yang, Y.; Liao, K.; Liu, B.; Chen, Y.; Shen, L.; Chen, L.; Jiang, A.a.; Liu, Y.; Li, Q.; et al. Genetic parameter estimation for reproductive traits in QingYu pigs and comparison of carcass and meat quality traits to Berkshire×QingYu crossbred pigs. Asian-Australas. J. Anim. Sci. 2020, 33, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Aggrey, S.E. Logistic nonlinear mixed effects model for estimating growth parameters. Poult. Sci. 2009, 88, 276–280. [Google Scholar] [CrossRef]
- Zhuang, Z.; Li, S.; Ding, R.; Yang, M.; Zheng, E.; Yang, H.; Gu, T.; Xu, Z.; Cai, G.; Wu, Z.; et al. Meta-analysis of genome-wide association studies for loin muscle area and loin muscle depth in two Duroc pig populations. PLoS ONE 2019, 14, e0218263. [Google Scholar] [CrossRef]
- Suzuki, K.; Kadowaki, H.; Shibata, T.; Uchida, H.; Nishida, A. Selection for daily gain, loin-eye area, backfat thickness and intramuscular fat based on desired gains over seven generations of Duroc pigs. Livest. Prod. Sci. 2005, 97, 193–202. [Google Scholar] [CrossRef]
- Shen, L.; Lei, H.; Zhang, S.; Li, X.; Li, M.; Jiang, X.; Zhu, K.; Zhu, L. Comparison of energy metabolism and meat quality among three pig breeds. Anim. Sci. J. 2014, 85, 770–779. [Google Scholar] [CrossRef]
- Honikel, K.O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef]
- Chromý, V.; Vinklárková, B.; Šprongl, L.; Bittová, M. The Kjeldahl method as a primary reference procedure for total protein in certified reference materials used in clinical chemistry. I. A review of Kjeldahl methods adopted by laboratory medicine. Crit. Rev. Anal. Chem. 2015, 45, 106–111. [Google Scholar] [CrossRef]
- Sander, L.C. Soxhlet Extractions. J. Res. Natl. Inst. Stand. Technol. 2017, 122, 1. [Google Scholar] [CrossRef]
- Zia, Q.; Alawami, M.; Mokhtar, N.F.K.; Nhari, R.M.H.R.; Hanish, I. Current analytical methods for porcine identification in meat and meat products. Food Chem. 2020, 324, 126664. [Google Scholar] [CrossRef]
- Shi, L.; Zhou, B.; Li, P.; Schinckel, A.P.; Liang, T.; Wang, H.; Li, H.; Fu, L.; Chu, Q.; Huang, R. MicroRNA-128 targets myostatin at coding domain sequence to regulate myoblasts in skeletal muscle development. Cell Signal. 2015, 27, 1895–1904. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Xu, J.; Wang, C.; Jin, E.; Gu, Y.; Li, S.; Li, Q. Identification of differentially expressed genes in longissimus dorsi muscle between Wei and Yorkshire pigs using RNA sequencing. Genes Genom. 2018, 40, 413–421. [Google Scholar] [CrossRef]
- Luo, J.; Shen, L.; Gan, M.; Jiang, A.; Chen, L.; Ma, J.; Jin, L.; Liu, Y.; Tang, G.; Jiang, Y.; et al. Profiling of skeletal muscle tissue for long non-coding RNAs related to muscle metabolism in the QingYu pig at the growth inflection point. Anim. Biosci. 2021, 34, 1309–1320. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.; Vazquez, J.A.; Lorenzo, J.M. Growth performance, carcass and meat quality of the Celta pig crossbred with Duroc and Landrance genotypes. Meat Sci. 2014, 96, 195–202. [Google Scholar] [CrossRef]
- Lan, Y.H.; McKeith, F.K.; Novakofski, J.; Carr, T.R. Carcass and muscle characteristics of Yorkshire, Meishan, Yorkshire x Meishan, Meishan x Yorkshire, Fengjing x Yorkshire, and Minzhu x Yorkshire pigs. J. Anim. Sci. 1993, 71, 3344–3349. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fu, X.; Yang, G.; Du, M. Review: Enhancing intramuscular fat development via targeting fibro-adipogenic progenitor cells in meat animals. Animal 2020, 14, 312–321. [Google Scholar] [CrossRef]

| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|
| YP | BW (kg) | 84.93 ± 2.80 | 95.46 ± 3.05 | 104.80 ± 2.72 | 114.72 ± 3.10 | 123.37 ± 2.83 | 134.25 ± 2.76 | 142.88 ± 3.10 | 153.79 ± 2.81 |
| ADG (g) | 399.6 | 414.2 | 426.4 | 436 | 442.8 | 447.6 | 450.6 | 452.4 | |
| N | 8 | 22 | 37 | 40 | 20 | 26 | 17 | 14 | |
| QYP | BW (kg) | 64.85 ± 3.70 | 77.48 ± 2.02 | 85.87 ± 2.60 | 94.07 ± 3.11 | 105.89 ± 3.33 | 113.52 ± 2.11 | 123.88 ± 3.12 | 135.53 ± 4.67 |
| ADG (g) | 318.83 | 331.98 | 342.21 | 350.43 | 356.99 | 361.59 | 364.96 | 367.49 | |
| N | 7 | 6 | 6 | 17 | 11 | 6 | 12 | 8 | |
| Breed | Number | PCC | A | B | K | P-BW | P-Age | MDG |
|---|---|---|---|---|---|---|---|---|
| YP | 400 | 0.991 | 160.493 | 16.901 | 0.022 | 80.25 | 128.52 | 882.71 |
| QYP | 126 | 0.993 | 137.948 | 20.059 | 0.016 | 68.97 | 187.42 | 551.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, T.; Gan, M.; Zhu, Y.; Lei, Y.; Yang, Y.; Zheng, Q.; Niu, L.; Zhao, Y.; Chen, L.; Wu, Y.; et al. Carcass and Meat Quality Characteristics and Changes of Lean and Fat Pigs After the Growth Turning Point. Foods 2025, 14, 2719. https://doi.org/10.3390/foods14152719
Liao T, Gan M, Zhu Y, Lei Y, Yang Y, Zheng Q, Niu L, Zhao Y, Chen L, Wu Y, et al. Carcass and Meat Quality Characteristics and Changes of Lean and Fat Pigs After the Growth Turning Point. Foods. 2025; 14(15):2719. https://doi.org/10.3390/foods14152719
Chicago/Turabian StyleLiao, Tianci, Mailin Gan, Yan Zhu, Yuhang Lei, Yiting Yang, Qianli Zheng, Lili Niu, Ye Zhao, Lei Chen, Yuanyuan Wu, and et al. 2025. "Carcass and Meat Quality Characteristics and Changes of Lean and Fat Pigs After the Growth Turning Point" Foods 14, no. 15: 2719. https://doi.org/10.3390/foods14152719
APA StyleLiao, T., Gan, M., Zhu, Y., Lei, Y., Yang, Y., Zheng, Q., Niu, L., Zhao, Y., Chen, L., Wu, Y., Zhou, L., Xue, J., Zhou, X., Wang, Y., Shen, L., & Zhu, L. (2025). Carcass and Meat Quality Characteristics and Changes of Lean and Fat Pigs After the Growth Turning Point. Foods, 14(15), 2719. https://doi.org/10.3390/foods14152719








