Effect of Carboxymethyl Konjac Glucomannan on the Gel Properties of Silver Carp Surimi: A Study on the Regulatory Mechanism of Substitution Degree
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
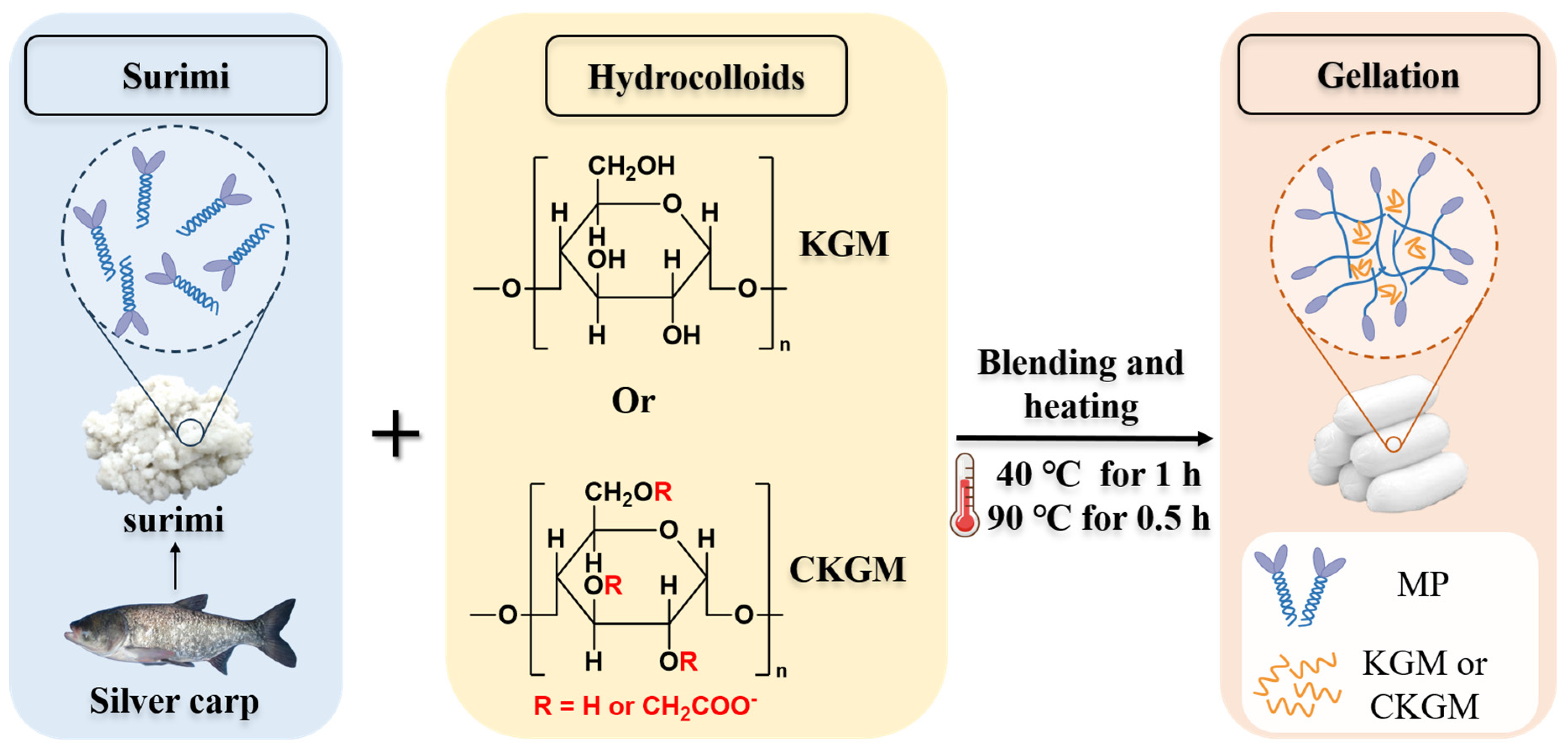
2.2. Preparation of CKGM
2.3. Preparation of Surimi Gels
2.4. Characteristics of Surimi Gels
2.4.1. Gel Strength
2.4.2. Rheological Properties
2.4.3. Scanning Electron Microscopy (SEM)
2.4.4. Low-Field Nuclear Magnetic Resonance (LF-NMR)
2.4.5. Magnetic Resonance Imaging (MRI)
2.4.6. Water-Holding Capacity (WHC)
2.4.7. Color Measurement
2.4.8. Chemical Interactions
2.4.9. Crosslinking Degree
2.4.10. Raman Spectra Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Gel Strength Properties
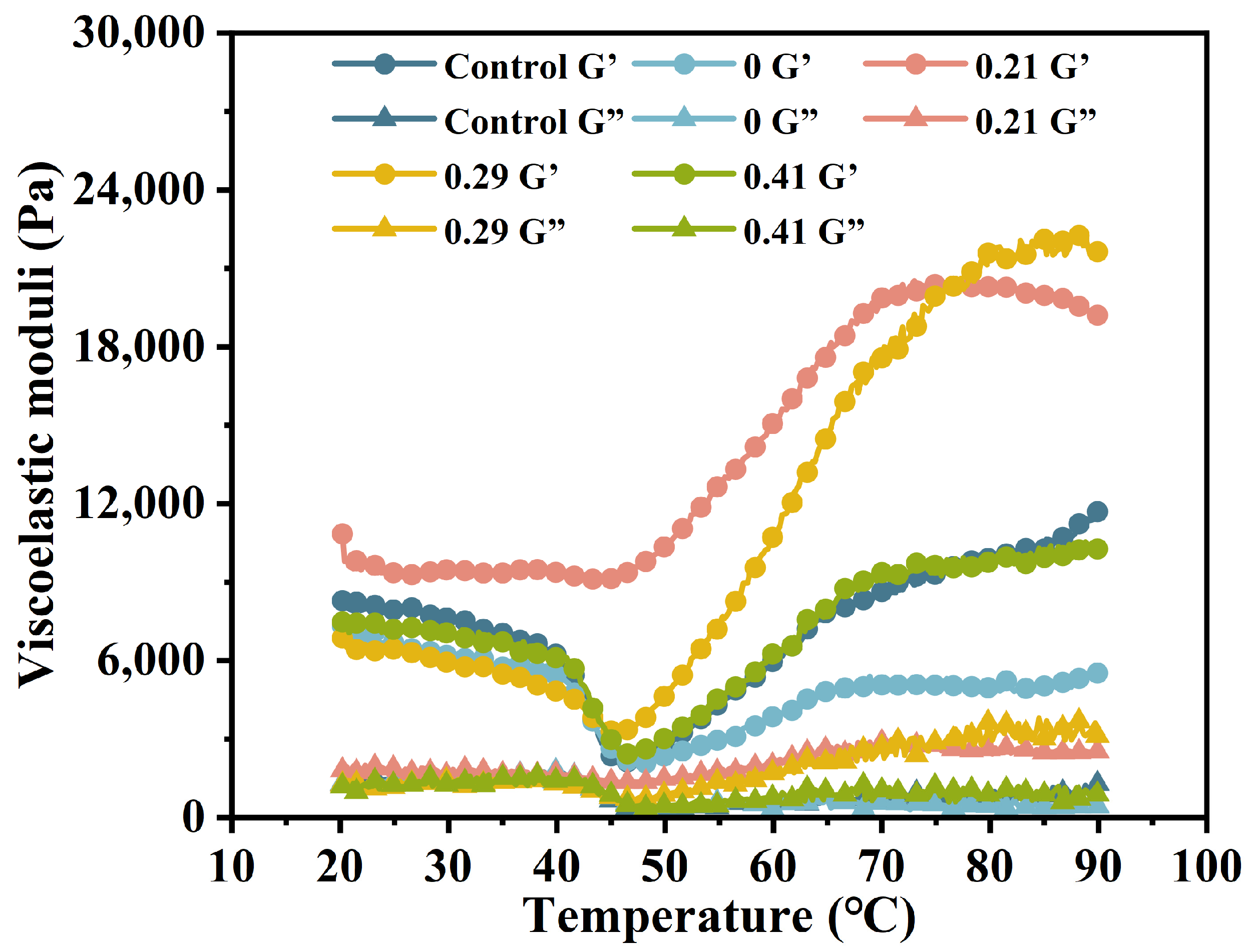
3.2. Dynamic Rheological Properties
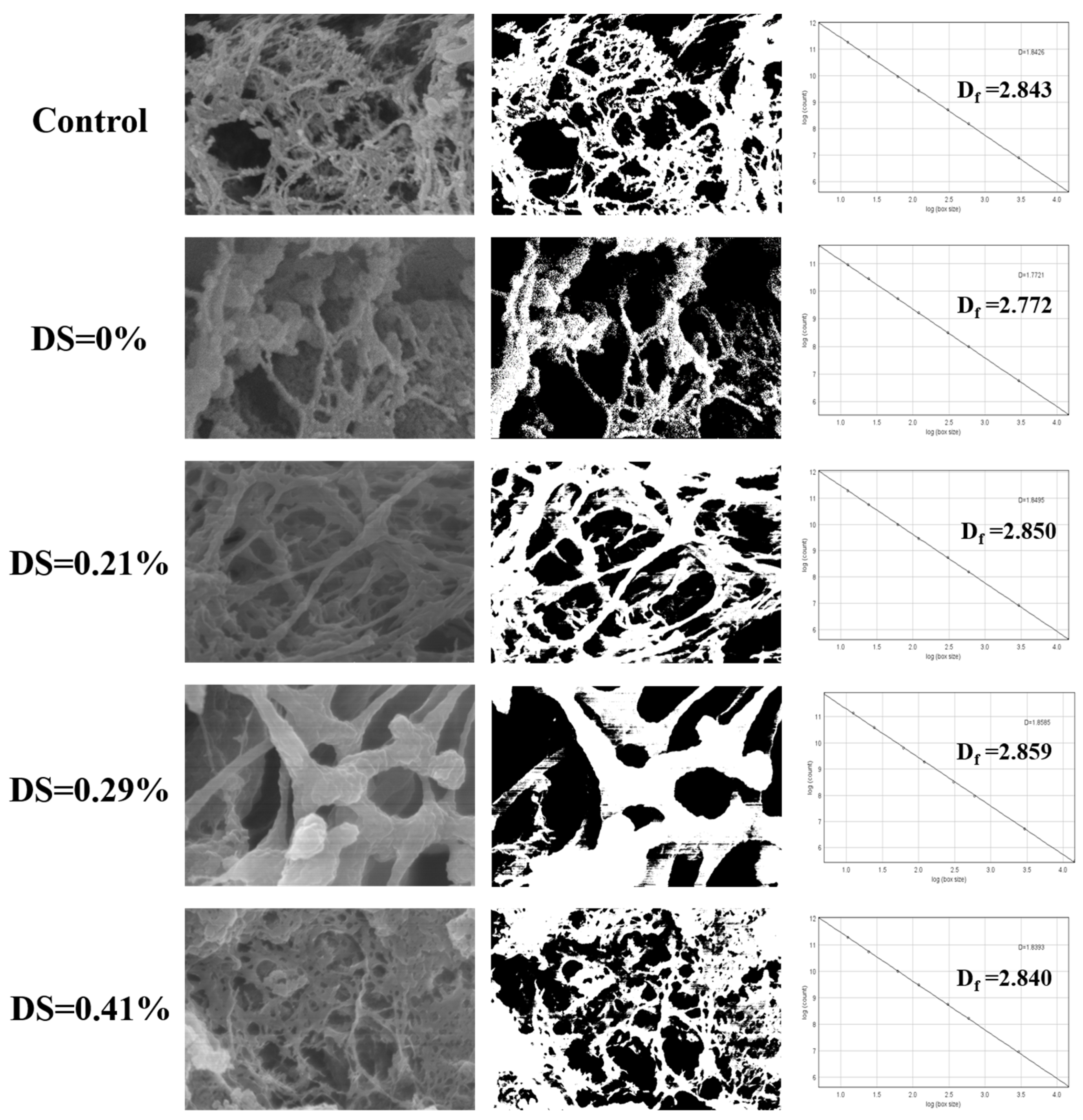
3.3. Microstructure Characteristics and Fractal Dimension Analysis
3.4. Water State and Distribution
3.4.1. Water State
3.4.2. Water Distribution
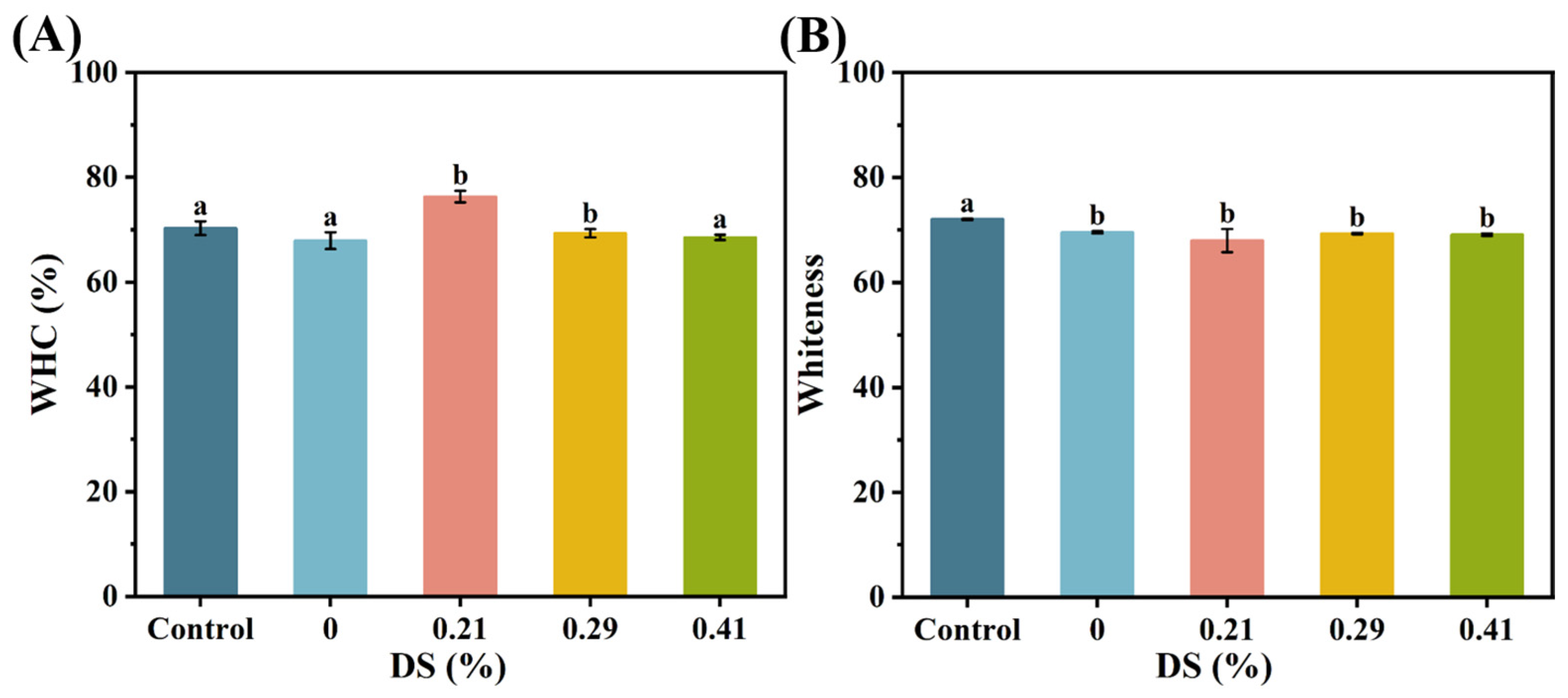
3.5. Water-Holding Capacity
3.6. Apparent Morphology
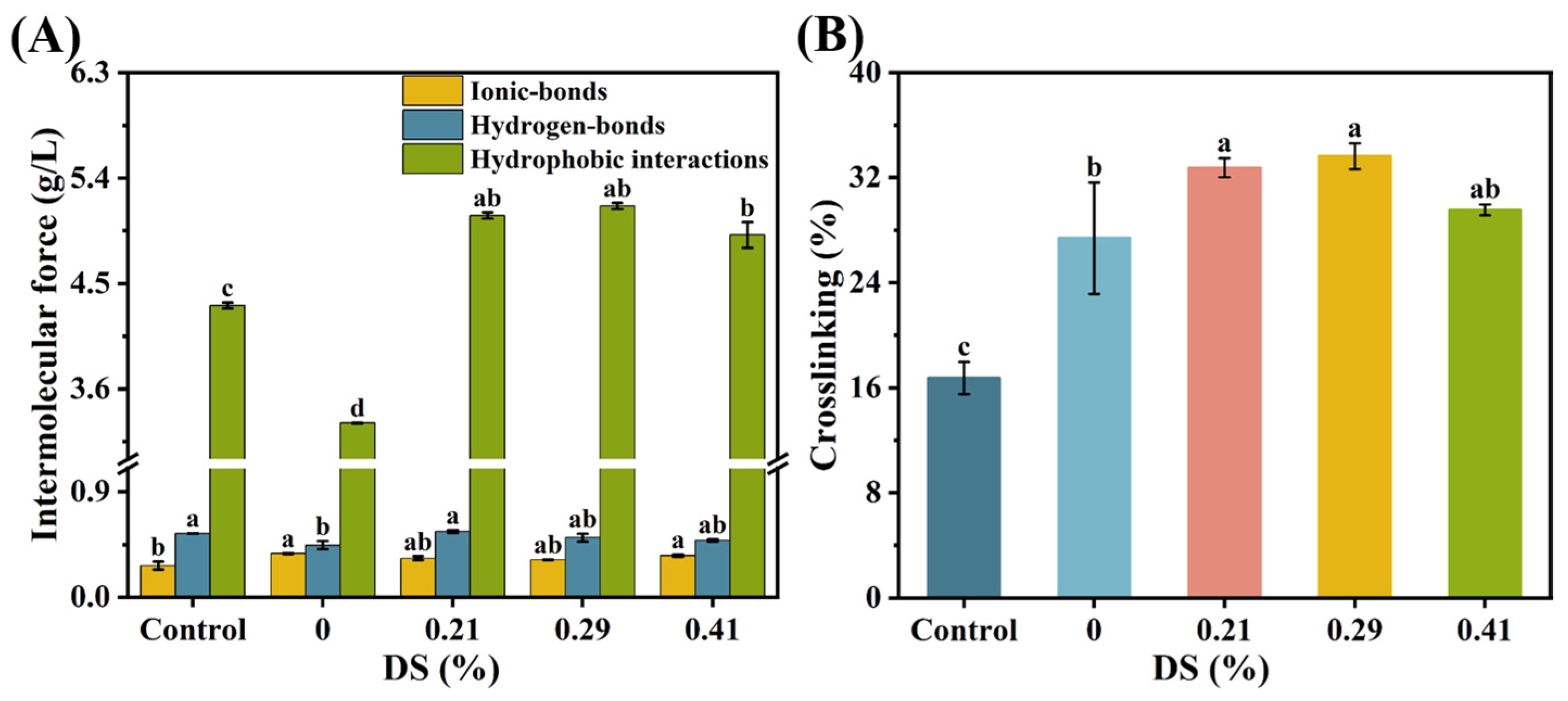
3.7. Chemical Forces
3.7.1. Ionic-Bonds
3.7.2. Hydrogen-Bonds
3.7.3. Hydrophobic Interactions
3.7.4. Degree of Crosslinking
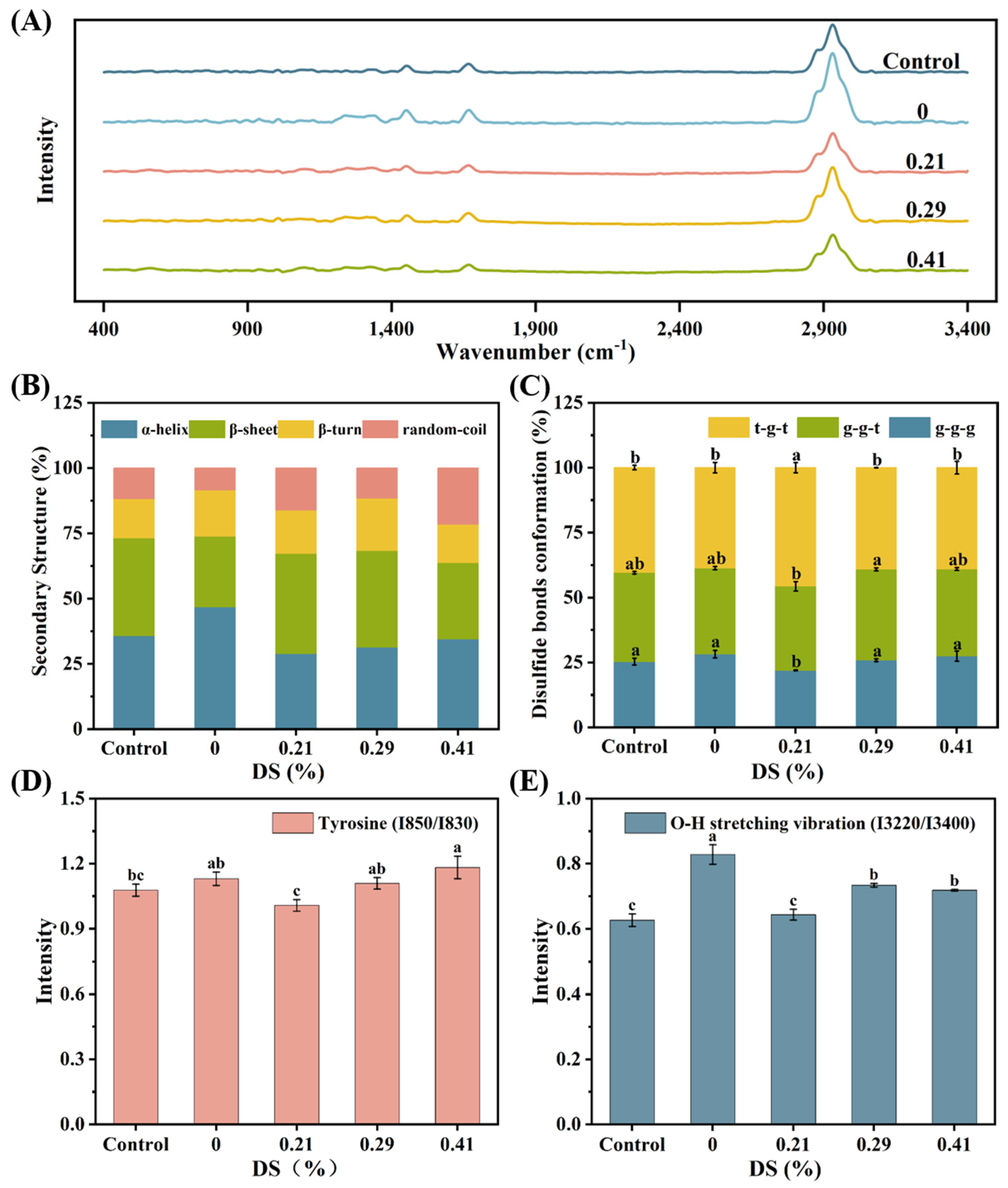
3.8. Raman Spectroscopy
3.8.1. Secondary Structure
3.8.2. S-S Stretching Vibrations and Disulfide Bond Conformations
3.8.3. Fermi Resonance of Tyrosine Residues
3.8.4. O-H Stretching Vibrations of Water Molecules
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, G.; Li, Y. A Review of Inhibition Mechanisms of Surimi Protein Hydrolysis by Different Exogenous Additives and Their Application in Improving Surimi Gel Quality. Food Chem. 2024, 456, 140002. [Google Scholar] [CrossRef]
- Yi, S.; Huo, Y.; Qiao, C.; Wang, W.; Li, X. Synergistic Gelation Effects in Surimi Mixtures Composed of Nemipterus virgatus and Hypophthalmichtys molitrix. J. Food Sci. 2019, 84, 3634–3641. [Google Scholar] [CrossRef]
- Fan, M.; Huang, Q.; Zhong, S.; Li, X.; Xiong, S.; Xie, J.; Yin, T.; Zhang, B.; Zhao, S. Gel Properties of Myofibrillar Protein as Affected by Gelatinization and Retrogradation Behaviors of Modified Starches with Different Crosslinking and Acetylation Degrees. Food Hydrocoll. 2019, 96, 604–616. [Google Scholar] [CrossRef]
- Jian, W.; Wu, H.; Wu, L.; Wu, Y.; Jia, L.; Pang, J.; Sun, Y. Effect of Molecular Characteristics of Konjac Glucomannan on Gelling and Rheological Properties of Tilapia Myofibrillar Protein. Carbohydr. Polym. 2016, 150, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, J.A.; Uresti, R.M.; Velazquez, G.; Vázquez, M. Food Hydrocolloids as Additives to Improve the Mechanical and Functional Properties of Fish Products: A Review. Food Hydrocoll. 2011, 25, 1842–1852. [Google Scholar] [CrossRef]
- Mi, H.; Li, Y.; Wang, C.; Yi, S.; Li, X.; Li, J. The Interaction of Starch-Gums and Their Effect on Gel Properties and Protein Conformation of Silver Carp Surimi. Food Hydrocoll. 2021, 112, 106290. [Google Scholar] [CrossRef]
- Luo, H.; Guo, C.; Lin, L.; Si, Y.; Gao, X.; Xu, D.; Jia, R.; Yang, W. Combined Use of Rheology, LF-NMR, and MRI for Characterizing the Gel Properties of Hairtail Surimi with Potato Starch. Food Bioprocess Technol. 2020, 13, 637–647. [Google Scholar] [CrossRef]
- Hunt, A.; Park, J.W. Alaska Pollock Fish Protein Gels as Affected by Refined Carrageenan and Various Salts. J. Food Qual. 2013, 36, 51–58. [Google Scholar] [CrossRef]
- Yang, H.; Park, J.W. Effects of Starch Properties and Thermal-Processing Conditions on Surimi–Starch Gels. LWT-Food Sci. Technol. 1998, 31, 344–353. [Google Scholar] [CrossRef]
- Wu, C.; Yuan, C.; Chen, S.; Liu, D.; Ye, X.; Hu, Y. The Effect of Curdlan on the Rheological Properties of Restructured Ribbonfish (Trichiurus Spp.) Meat Gel. Food Chem. 2015, 179, 222–231. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, M.; He, D.; Li, X.; Shi, L.; Wang, L.; Xu, J.; Zheng, Y.; Yin, T. Cryoprotective Roles of Carboxymethyl Chitosan during the Frozen Storage of Surimi: Protein Structures, Gel Behaviors and Edible Qualities. Foods 2022, 11, 356. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Z.; Wang, Y.; Xue, Y.; Xue, C. Effects of Konjac Glucomannan on Heat-Induced Changes of Physicochemical and Structural Properties of Surimi Gels. Food Res. Int. 2016, 83, 152–161. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Ding, Y. Optimization of Adding Konjac Glucomannan to Improve Gel Properties of Low-Quality Surimi. Carbohydr. Polym. 2013, 92, 484–489. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, M.; Liu, X.-C.; Yao, X.; Wang, W.; Zheng, J.; Tomasevic, I.; Sun, W. Improved Qualities of Salt-Reduced Tilapia Surimi by Adding Konjac Glucomannan: Insight into the Edible Traits, Gel Properties and Anti-Freezing Ability. Food Hydrocoll. 2024, 153, 109971. [Google Scholar] [CrossRef]
- Zhang, T.; Xue, Y.; Li, Z.; Wang, Y.; Xue, C. Effects of Deacetylation of Konjac Glucomannan on Alaska Pollock Surimi Gels Subjected to High-Temperature (120 °C) Treatment. Food Hydrocoll. 2015, 43, 125–131. [Google Scholar] [CrossRef]
- Yuan, L.; Yu, J.; Mu, J.; Shi, T.; Sun, Q.; Jin, W.; Gao, R. Effects of Deacetylation of Konjac Glucomannan on the Physico-Chemical Properties of Surimi Gels from Silver Carp (Hypophthalmichthys molitrix). RSC Adv. 2019, 9, 19828–19836. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Shen, M.; Wang, Z.; Xie, J. Structure, Function and Food Applications of Carboxymethylated Polysaccharides: A Comprehensive Review. Trends Food Sci. Technol. 2021, 118, 539–557. [Google Scholar] [CrossRef]
- Zhang, C.; He, Y.; Zheng, Y.; Ai, C.; Cao, H.; Xiao, J.; El-Seedi, H.; Chen, L.; Teng, H. Effect of Carboxymethyl Cellulose (CMC) on Some Physico-Chemical and Mechanical Properties of Unrinsed Surimi Gels. LWT 2023, 180, 114653. [Google Scholar] [CrossRef]
- Zhang, K.; Tan, Z.; Zhang, Q.; Wu, Q.; Zhao, J.; Xu, W.; Liu, Y.; Liu, X.; Zhou, D.; Li, D. Construction and Characterisation of Mung Bean Protein Isolate/Carboxymethyl Konjac Glucomannan Sodium Hydrogels: Gel Properties, Structural Properties, Microstructure, Sodium Salt Release, and 3D Printing. Food Chem. 2025, 472, 142995. [Google Scholar] [CrossRef]
- Rahman, Z.; Zhang, S.; Khan, A.; You, J.; Liu, R.; Huang, Q.; Ma, H.; Benjakul, S.; Yin, T. Effects of Modified KGM on the Gelling Capability of Fish Protein: Novel Insights into Hooking Effect. Food Chem. 2025, 478, 143652. [Google Scholar] [CrossRef]
- Xiao, M.; Dai, S.; Wang, L.; Ni, X.; Yan, W.; Fang, Y.; Corke, H.; Jiang, F. Carboxymethyl Modification of Konjac Glucomannan Affects Water Binding Properties. Carbohydr. Polym. 2015, 130, 1–8. [Google Scholar] [CrossRef]
- Koroskenyi, B.; McCarthy, S.P. Synthesis of Acetylated Konjac Glucomannan and Effect of Degree of Acetylation on Water Absorbency. Biomacromolecules 2001, 2, 824–826. [Google Scholar] [CrossRef]
- Fang, M.; Xiong, S.; Hu, Y.; Yin, T.; You, J. In Vitro Pepsin Digestion of Silver Carp (Hypophthalmichthys Molitrix) Surimi Gels after Cross-Linking by Microbial Transglutaminase (MTGase). Food Hydrocoll. 2019, 95, 152–160. [Google Scholar] [CrossRef]
- Walayat, N.; Xiong, Z.; Xiong, H.; Moreno, H.M.; Nawaz, A.; Niaz, N.; Hu, C.; Taj, M.I.; Mushtaq, B.S.; Khalifa, I. The Effect of Egg White Protein and β-Cyclodextrin Mixture on Structural and Functional Properties of Silver Carp Myofibrillar Proteins during Frozen Storage. LWT 2021, 135, 109975. [Google Scholar] [CrossRef]
- Feng, R.; Li, J.; Liu, C.; Xia, W.; Xu, Y. Effects of Actomyosin Dissociation on the Physicochemical and Gelling Properties of Silver Carp Myofibrillar Protein Sol during Freeze–Thaw Cycles. Food Res. Int. 2022, 162, 112075. [Google Scholar] [CrossRef] [PubMed]
- Xing, T.; Xu, Y.; Qi, J.; Xu, X.; Zhao, X. Effect of High Intensity Ultrasound on the Gelation Properties of Wooden Breast Meat with Different NaCl Contents. Food Chem. 2021, 347, 129031. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Xiao, S.; Ruan, Q.; Gao, Q.; An, Y.; Hu, Y.; Xiong, S. Differences in Flavor Characteristics of Frozen Surimi Products Reheated by Microwave, Water Boiling, Steaming, and Frying. Food Chem. 2022, 372, 131260. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wang, Y.; Ding, Y.; Xiang, X.; Yang, Q.; Wei, Z.; Song, H.; Liu, S.; Zhou, X. Improved Texture Properties and Toughening Mechanisms of Surimi Gels by Double Network Strategies. Food Hydrocoll. 2024, 152, 109900. [Google Scholar] [CrossRef]
- Ni, X.; Chen, W.; Xiao, M.; Wu, K.; Kuang, Y.; Corke, H.; Jiang, F. Physical Stability and Rheological Properties of Konjac Glucomannan-Ethyl Cellulose Mixed Emulsions. Int. J. Biol. Macromol. 2016, 92, 423–430. [Google Scholar] [CrossRef]
- Kampa, J.; Frazier, R.; Rodriguez-Garcia, J. Development of Saturated Fat Replacers: Conventional and Nano-Emulsions Stabilised by Lecithin and Hydroxylpropyl Methylcellulose. Foods 2022, 11, 2536. [Google Scholar] [CrossRef]
- Yu, S.; Pang, G.; Li, S.; Lv, S.; Wei, Z.; Wang, J.; Xiao, H.; Zhu, L. Impacts of Zein-Fucoidan Nanoparticles with and without Curcumin on Gel Properties of Golden Threadfin Bream (Nemipterus Virgatus) Surimi. Food Chem. 2025, 468, 142415. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, T.; Xue, Y.; Xue, C. Changes of Structural and Physical Properties of Semi-Gel from Alaska Pollock Surimi during 4 °C Storage. Food Hydrocoll. 2019, 87, 772–782. [Google Scholar] [CrossRef]
- Ding, J.; Zhao, X.; Li, X.; Huang, Q. Effects of Different Recovered Sarcoplasmic Proteins on the Gel Performance, Water Distribution and Network Structure of Silver Carp Surimi. Food Hydrocoll. 2022, 131, 107835. [Google Scholar] [CrossRef]
- Chen, M.; Sun, Z.; Ma, A.; Shi, G.; Xiong, G.; Qiao, Y.; Chen, S.; Wu, W.; Liu, J.; Tu, Z.; et al. Enhancing Surimi Gel Properties in Silver Carp with Innovative Ultra-Micro Crayfish Shell Powder. Food Chem. 2025, 478, 143605. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Munir, S.; Yu, X.; Yin, T.; You, J.; Liu, R.; Xiong, S.; Hu, Y. Double-Crosslinked Effect of TGase and EGCG on Myofibrillar Proteins Gel Based on Physicochemical Properties and Molecular Docking. Food Chem. 2021, 345, 128655. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Guo, X.; Xiong, Z.; Wang, X.; Monto, A.R.; Jin, W.; Li, J.; Gao, R. Effects of Sturgeon Oil and Its Pickering Emulsion on the Quality of Sturgeon Surimi Gel. Food Chemistry: X 2024, 22, 101451. [Google Scholar] [CrossRef]
- Montero, P.; Gómez-Guillén, C. Thermal Aggregation of Sardine Muscle Proteins during Processing. J. Agric. Food Chem. 1996, 44, 3625–3630. [Google Scholar] [CrossRef]
- An, Y.; You, J.; Xiong, S.; Yin, T. Short-Term Frozen Storage Enhances Cross-Linking That Was Induced by Transglutaminase in Surimi Gels from Silver Carp (Hypophthalmichthys molitrix). Food Chem. 2018, 257, 216–222. [Google Scholar] [CrossRef]
- Kong, W.; Zhang, T.; Feng, D.; Xue, Y.; Wang, Y.; Li, Z.; Yang, W.; Xue, C. Effects of Modified Starches on the Gel Properties of Alaska Pollock Surimi Subjected to Different Temperature Treatments. Food Hydrocoll. 2016, 56, 20–28. [Google Scholar] [CrossRef]
- Xu, H.; Tian, H.; Deng, J.; Zhuo, Q.; Cui, J.; Wang, J.; Yin, Y.; Yu, P. Review of Influence of Steric Effect on Aggregation Behavior of Fine Particles. Minerals Engineering 2023, 203, 108304. [Google Scholar] [CrossRef]
- Jiang, X.; Liang, Q.; Shi, W. Influence of Starch on Freeze-Thaw Stability of Hypophthalmichthys Molitrix Surimi Gel Observed via Ice Crystal Distribution and Gel Properties. Food Chem. X 2024, 24, 101995. [Google Scholar] [CrossRef]
- Wang, S.; Zhan, Y.; Wu, X.; Ye, T.; Li, Y.; Wang, L.; Chen, Y.; Li, B. Dissolution and Rheological Behavior of Deacetylated Konjac Glucomannan in Urea Aqueous Solution. Carbohydr. Polym. 2014, 101, 499–504. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Zheng, B.; Guo, Z. Effects of High Pressure Processing on Gelation Properties and Molecular Forces of Myosin Containing Deacetylated Konjac Glucomannan. Food Chem. 2019, 291, 117–125. [Google Scholar] [CrossRef]
- Zhuang, X.; Han, M.; Jiang, X.; Bai, Y.; Zhou, H.; Li, C.; Xu, X.; Zhou, G. The Effects of Insoluble Dietary Fiber on Myofibrillar Protein Gelation: Microstructure and Molecular Conformations. Food Chem. 2019, 275, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Walayat, N.; Wang, X.; Liu, J.; Nawaz, A.; Zhang, Z.; Khalifa, I.; Rincón Cervera, M.Á.; Pateiro, M.; Lorenzo, J.M.; Nikoo, M.; et al. Kappa-Carrageenan as an Effective Cryoprotectant on Water Mobility and Functional Properties of Grass Carp Myofibrillar Protein Gel during Frozen Storage. LWT 2022, 154, 112675. [Google Scholar] [CrossRef]
- Zhao, Y.; Piao, X.; Zheng, B.; Gao, P.; Miao, W.; Wen, Z.; Zhang, X.; Mei, G.; Zhou, R.; Deng, S. Enhancement of Surimi Gel Properties through the Synergetic Effect of Fucoidan and Oligochitosan. Food Hydrocoll. 2023, 140, 108626. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, L.; Zaky, A.A.; Tie, S.; Cui, G.; Liu, R.; Abd El-Aty, A.M.; Tan, M. High Internal Phase Pickering Emulsion by Spanish Mackerel Proteins-Procyanidins: Application for Stabilizing Astaxanthin and Surimi. Food Hydrocoll. 2022, 133, 107999. [Google Scholar] [CrossRef]
- Yoon, W.B.; An, S.; Oyinloye, T.M.; Kim, J. Developing a Quality Control System in a Continuous Hot Air Heating Process in Surimi Seafood Processing Using Image Analysis and Artificial Intelligence. Processes 2023, 11, 3187. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, X.; Chuai, P.; Jiang, Z.; Zhu, Y.; Zhang, B.; Ni, H.; Li, Q. Effects of Crude Fucoidan on Physicochemical Properties, Antioxidation and Bacteriostasis of Surimi Products. Food Control 2021, 122, 107806. [Google Scholar] [CrossRef]
- Yi, S.; Li, Q.; Qiao, C.; Zhang, C.; Wang, W.; Xu, Y.; Mi, H.; Li, X.; Li, J. Myofibrillar Protein Conformation Enhance Gel Properties of Mixed Surimi Gels with Nemipterus virgatus and Hypophthalmichthys molitrix. Food Hydrocoll. 2020, 106, 105924. [Google Scholar] [CrossRef]
- Liang, Y.; Guo, B.; Zhou, A.; Xiao, S.; Liu, X. Effect of High Pressure Treatment on Gel Characteristics and Gel Formation Mechanism of Bighead Carp (Aristichthys nobilis) Surimi Gels. J. Food Process. Preserv. 2017, 41, e13155. [Google Scholar] [CrossRef]
- Mankova, A.A.; Nagaeva, A.I.; Brandt, N.N.; Chikishev, A.Y. Comparison of Vibrational Spectra of Proteins with Similar Secondary and Different Tertiary Structures. Vib. Spectrosc. 2022, 120, 103375. [Google Scholar] [CrossRef]
- Zhuang, X.; Han, M.; Bai, Y.; Liu, Y.; Xing, L.; Xu, X.; Zhou, G. Insight into the Mechanism of Myofibrillar Protein Gel Improved by Insoluble Dietary Fiber. Food Hydrocoll. 2018, 74, 219–226. [Google Scholar] [CrossRef]
- Sun, J.; Li, X.; Xu, X.; Zhou, G. Influence of Various Levels of Flaxseed Gum Addition on the Water-Holding Capacities of Heat-Induced Porcine Myofibrillar Protein. J. Food Sci. 2011, 76, C472–C478. [Google Scholar] [CrossRef]
- Xu, Y.; Yin, Y.; Wang, R.; Zhao, H.; Li, X.; Yi, S.; Li, J.; Xie, J. Effect of Deacetylated Konjac Glucomannan on Heat-Induced Structural Changes and Flavor Binding Ability of Fish Myosin. Food Chem. 2021, 365, 130540. [Google Scholar] [CrossRef]
- Chen, H.; Han, M. Raman Spectroscopic Study of the Effects of Microbial Transglutaminase on Heat-Induced Gelation of Pork Myofibrillar Proteins and Its Relationship with Textural Characteristics. Food Res. Int. 2011, 44, 1514–1520. [Google Scholar] [CrossRef]
- Feng, X.; Yu, X.; Yang, Y.; Tang, X. Improving the Freeze-Thaw Stability of Fish Myofibrils and Myofibrillar Protein Gels: Current Methods and Future Perspectives. Food Hydrocoll. 2023, 144, 109041. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, X.; Zou, W.; Tang, H.; Yang, F.; Wang, L.; Elfalleh, W. Raman Spectroscopy Analysis of the Effect of Electrolysis Treatment on the Structure of Soy Protein Isolate. J. Food Meas. Charact. 2021, 15, 1294–1300. [Google Scholar] [CrossRef]
- Chen, X.; Wu, J.; Li, X.; Yang, F.; Yu, L.; Li, X.; Huang, J.; Wang, S. Investigation of the Cryoprotective Mechanism and Effect on Quality Characteristics of Surimi during Freezing Storage by Antifreeze Peptides. Food Chem. 2022, 371, 131054. [Google Scholar] [CrossRef] [PubMed]
- Hernández, B.; Coïc, Y.; Pflüger, F.; Kruglik, S.G.; Ghomi, M. All Characteristic Raman Markers of Tyrosine and Tyrosinate Originate from Phenol Ring Fundamental Vibrations. J. Raman Spectrosc. 2016, 47, 210–220. [Google Scholar] [CrossRef]
- Sánchez-González, I.; Carmona, P.; Moreno, P.; Borderías, J.; Sánchez-Alonso, I.; Rodríguez-Casado, A.; Careche, M. Protein and Water Structural Changes in Fish Surimi during Gelation as Revealed by Isotopic H/D Exchange and Raman Spectroscopy. Food Chem. 2008, 106, 56–64. [Google Scholar] [CrossRef]

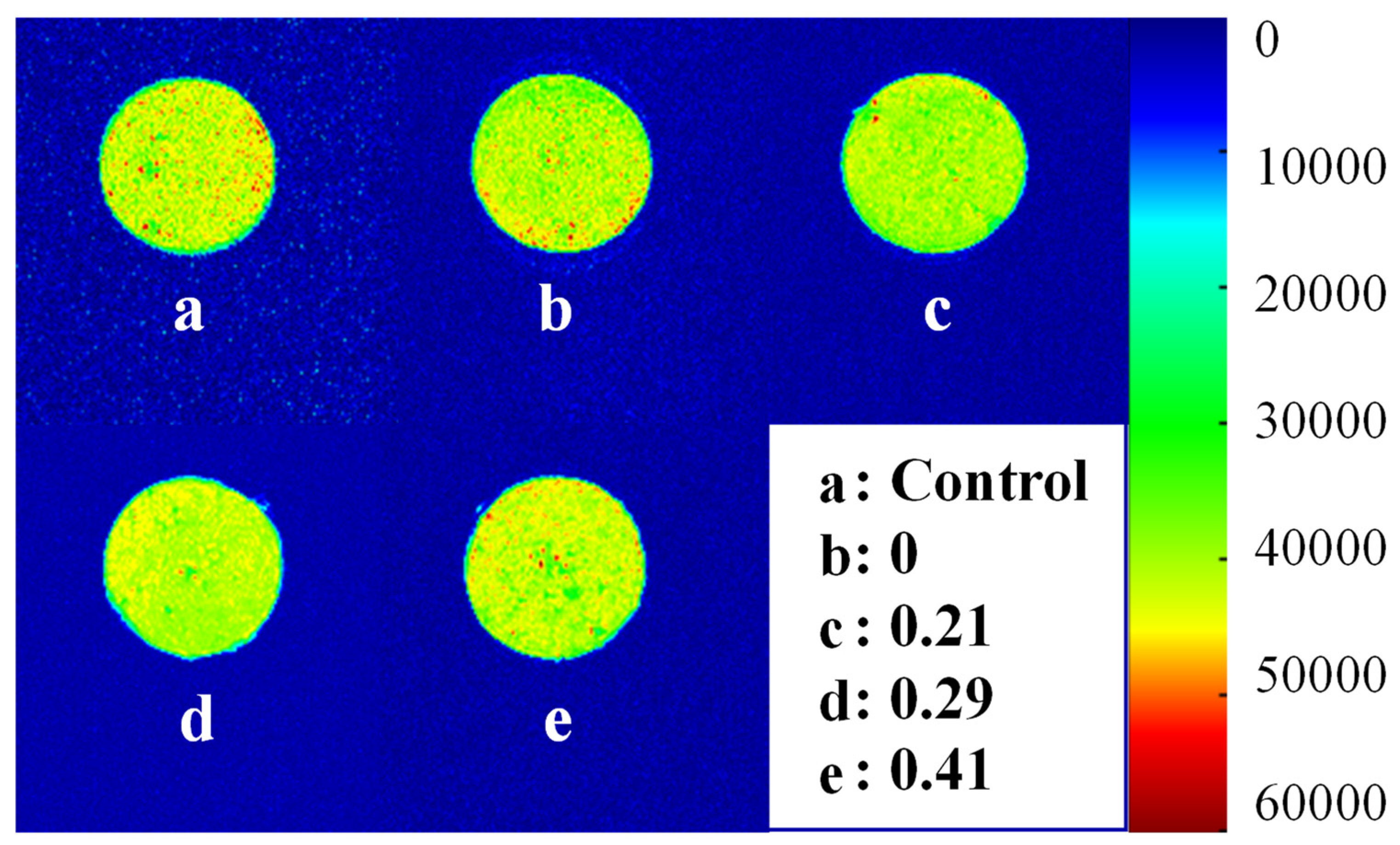
| KGM Substitution Degrees (%) | T21 (ms) | T22 (ms) | T23 (ms) | P21 (%) | P22 (%) | P23 (%) |
|---|---|---|---|---|---|---|
| Control | 0.83 ± 0.30 b | 110.83 ± 0.87 a | 1067.72 ± 85.41 a | 1.17 ± 0.22 bc | 97.38 ± 0.21 a | 1.45 ± 0.15 e |
| 0 | 0.74 ± 0.14 b | 108.02 ± 0.30 b | 709.46 ± 1.47 b | 1.81 ± 0.55 ab | 92.73 ± 0.58 b | 5.46 ± 0.05 d |
| 0.21 | 4.01 ± 0.29 a | 106.91 ± 0.43 b | 587.77 ± 6.94 c | 0.17 ± 0.21 c | 87.90 ± 0.21 c | 11.93 ± 0.04 c |
| 0.29 | 0.78 ± 0.16 b | 98.25 ± 1.24 d | 545.29 ± 13.96 a | 2.65 ± 0.63 a | 78.57 ± 0.28 e | 18.78 ± 0.43 a |
| 0.41 | 0.86 ± 0.15 b | 102.16 ± 0.56 c | 600.9 ± 12.06 ab | 2.17 ± 0.56 ab | 84.91 ± 1.17 d | 12.91 ± 0.61 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, W.; Ouyang, Z.; Luo, X.; Xiao, R.; Liao, S.; Jiang, F.; Li, Y.; Xiong, S.; Yin, T.; Zhu, X. Effect of Carboxymethyl Konjac Glucomannan on the Gel Properties of Silver Carp Surimi: A Study on the Regulatory Mechanism of Substitution Degree. Foods 2025, 14, 2715. https://doi.org/10.3390/foods14152715
Yan W, Ouyang Z, Luo X, Xiao R, Liao S, Jiang F, Li Y, Xiong S, Yin T, Zhu X. Effect of Carboxymethyl Konjac Glucomannan on the Gel Properties of Silver Carp Surimi: A Study on the Regulatory Mechanism of Substitution Degree. Foods. 2025; 14(15):2715. https://doi.org/10.3390/foods14152715
Chicago/Turabian StyleYan, Wenli, Zhihan Ouyang, Xiaoying Luo, Rankun Xiao, Siqiao Liao, Fatang Jiang, Yonghui Li, Shanbai Xiong, Tao Yin, and Xiangwei Zhu. 2025. "Effect of Carboxymethyl Konjac Glucomannan on the Gel Properties of Silver Carp Surimi: A Study on the Regulatory Mechanism of Substitution Degree" Foods 14, no. 15: 2715. https://doi.org/10.3390/foods14152715
APA StyleYan, W., Ouyang, Z., Luo, X., Xiao, R., Liao, S., Jiang, F., Li, Y., Xiong, S., Yin, T., & Zhu, X. (2025). Effect of Carboxymethyl Konjac Glucomannan on the Gel Properties of Silver Carp Surimi: A Study on the Regulatory Mechanism of Substitution Degree. Foods, 14(15), 2715. https://doi.org/10.3390/foods14152715








