Soy Sauce Fermentation with Cordyceps militaris: Process Optimization and Functional Profiling
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Koji Preparation and Optimization
2.3. Fermentation and Optimization
2.4. Determination of Quality and Functional Indicators
2.5. Statistical Analysis
3. Results and Discussion
3.1. Optimization of Koji Preparation
3.1.1. Effects of Single Factors on Koji Quality
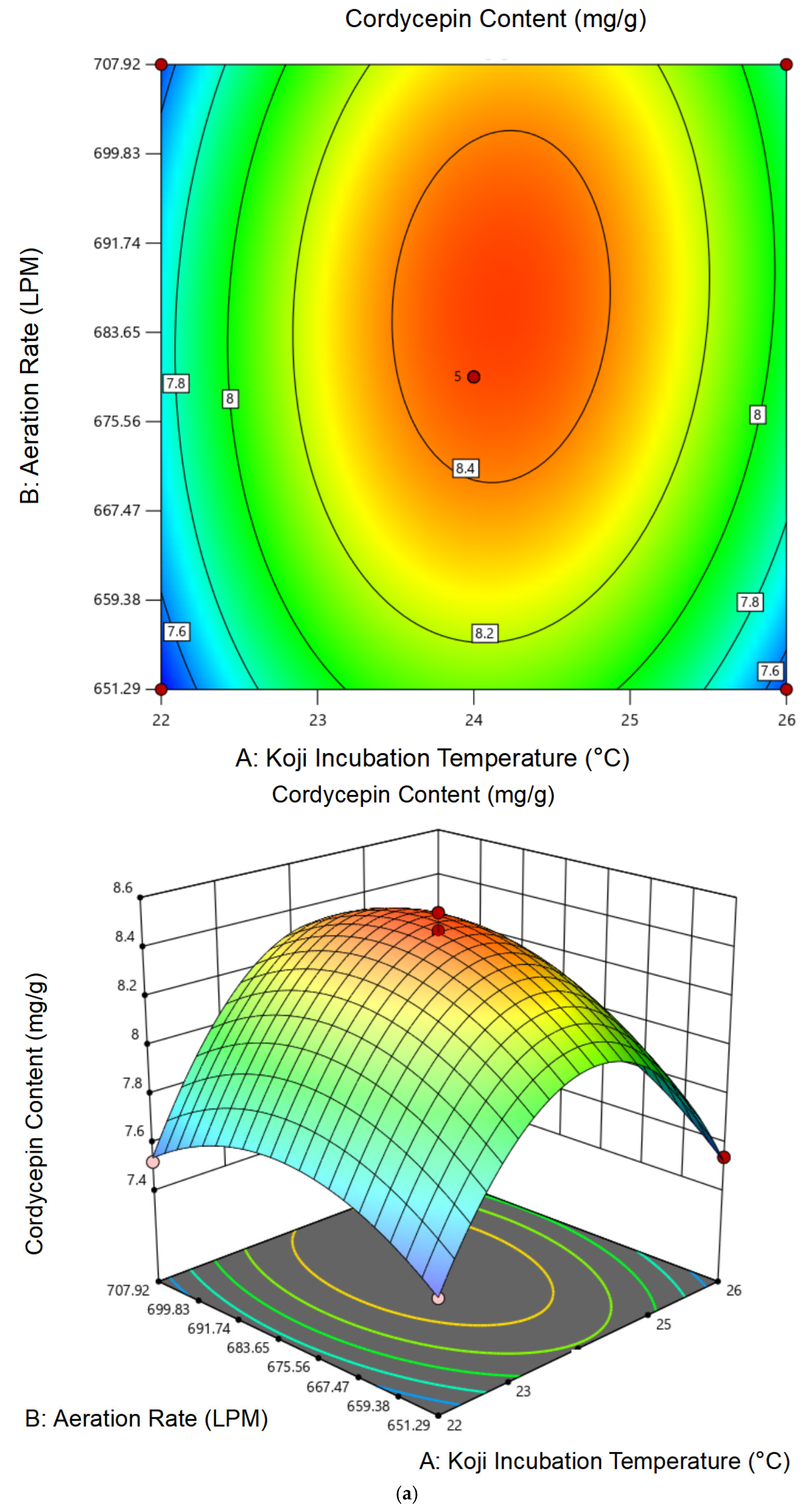
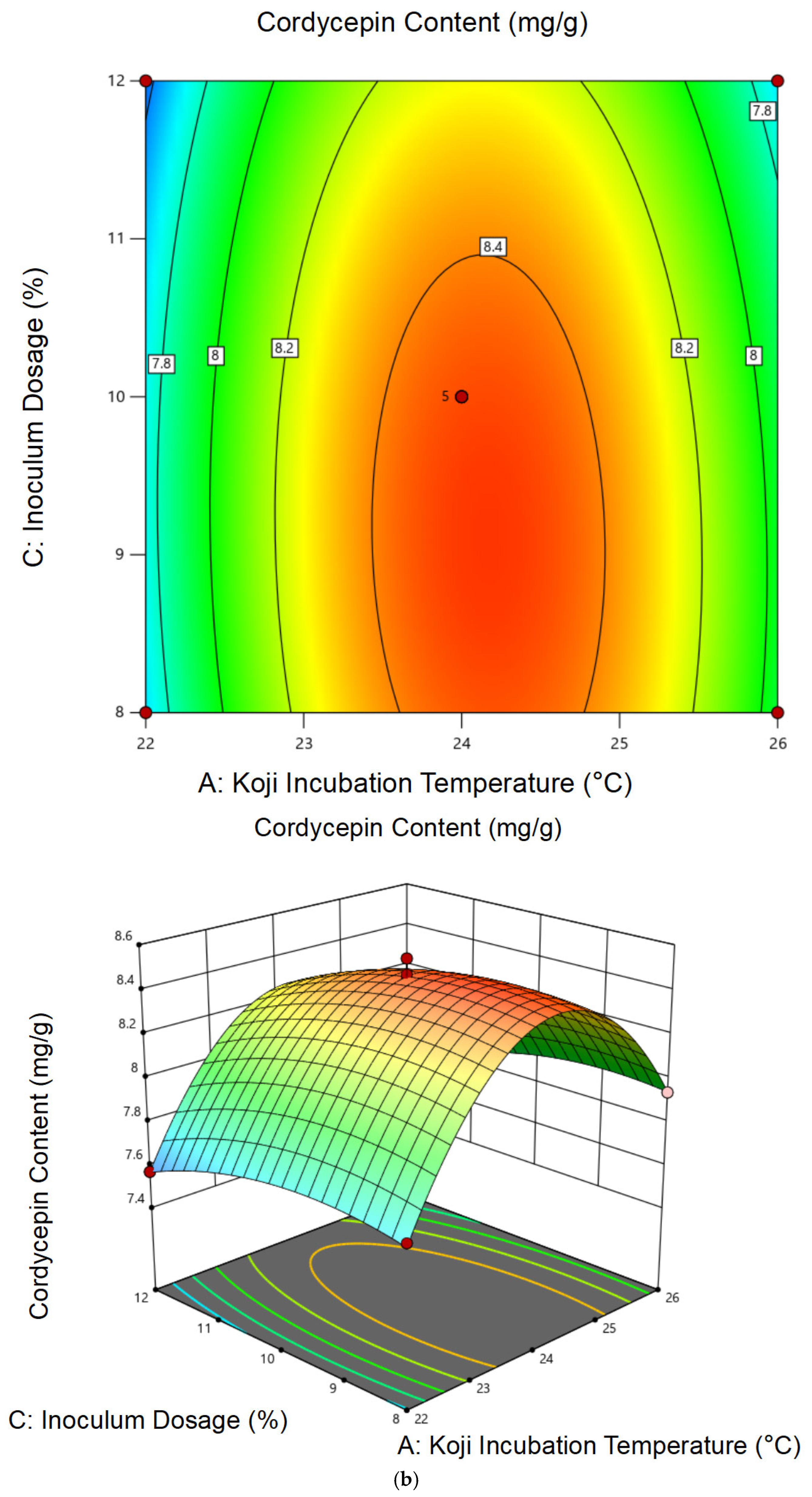
3.1.2. Response Surface Analysis and Model Fitting
3.1.3. Optimal Conditions and Experimental Validation of Koji Preparation
3.2. Optimization of Fermentation Conditions
3.2.1. Effects of Single Factors on Soy Sauce Quality
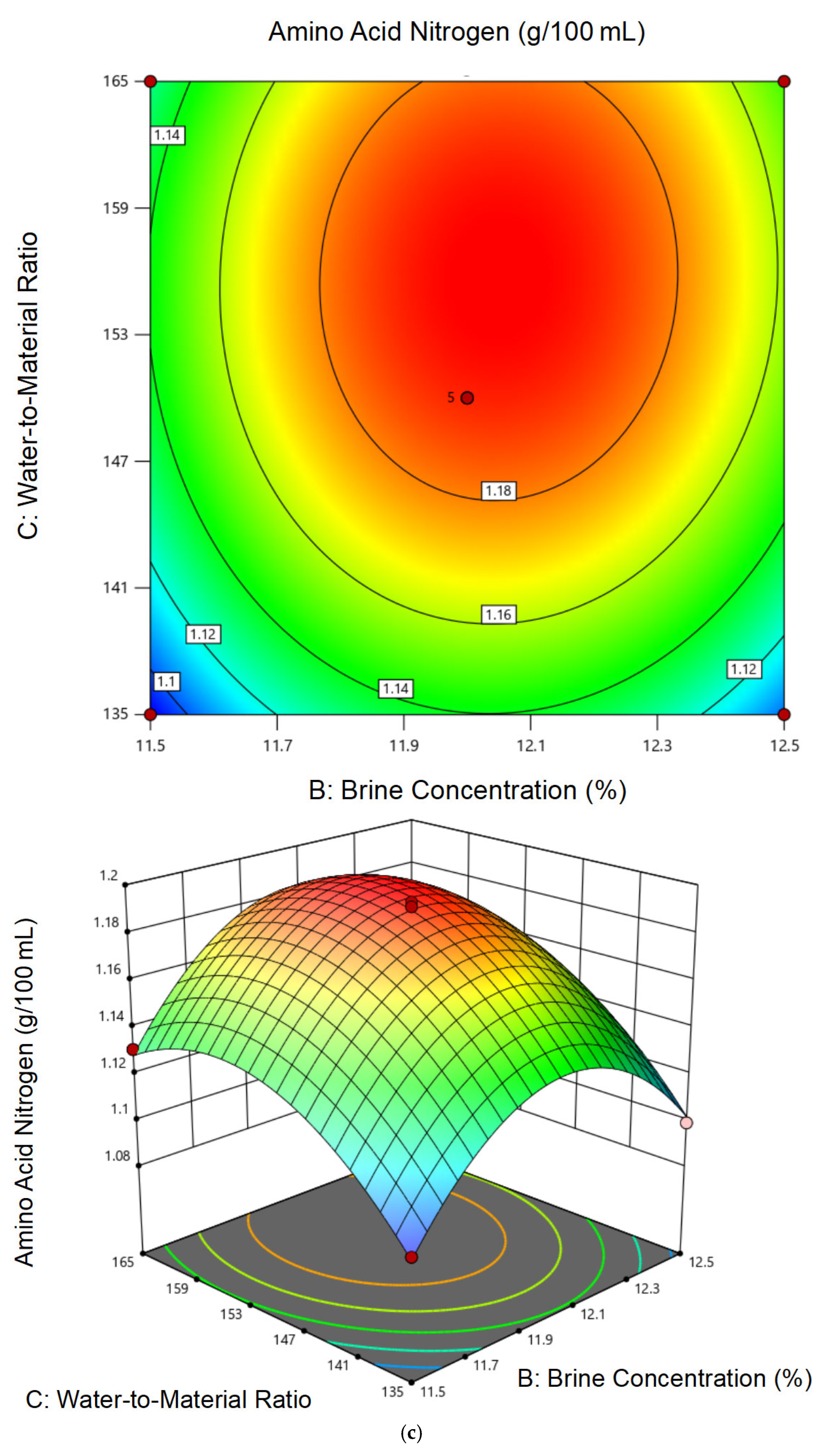
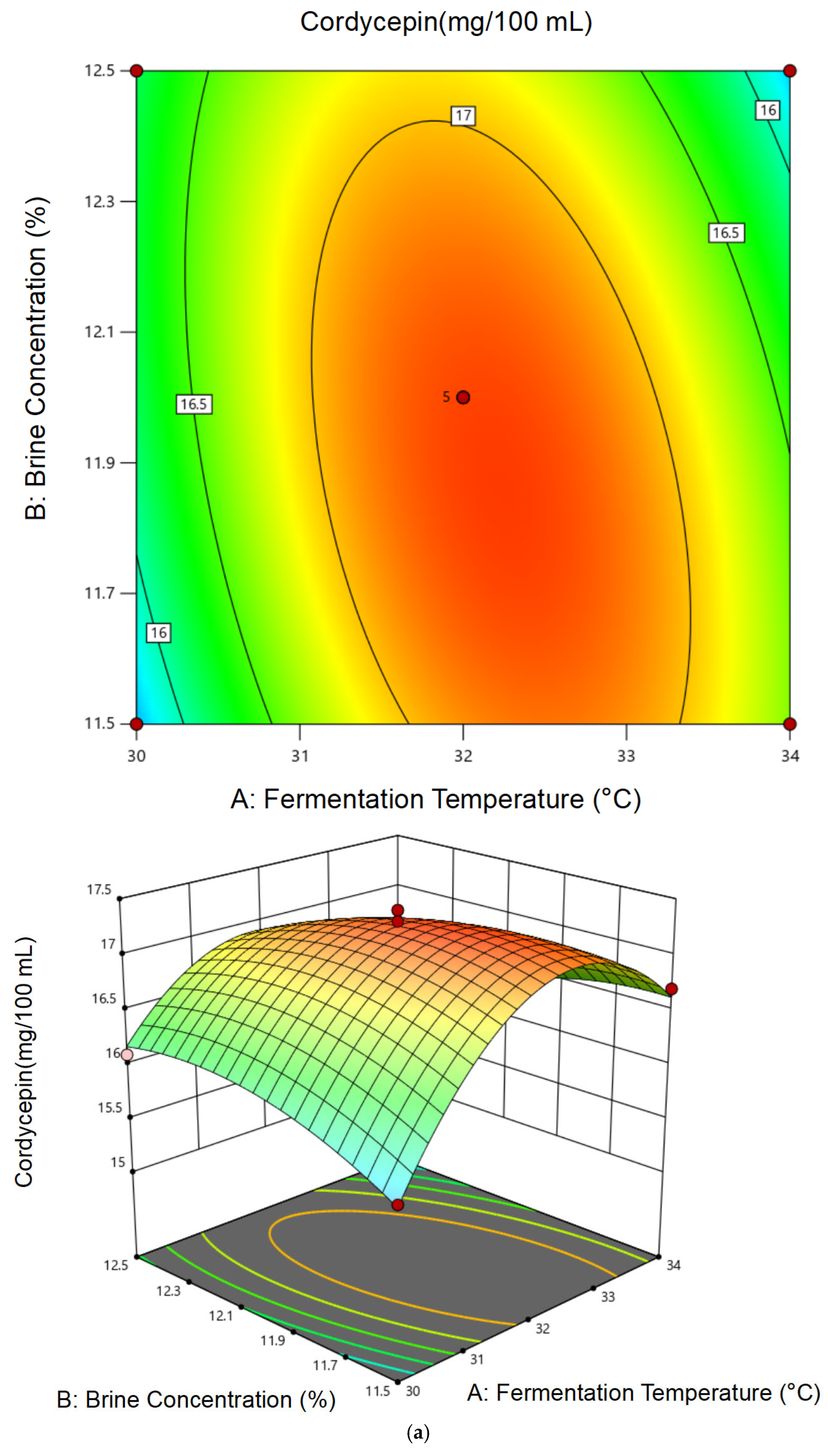
3.2.2. Response Surface Model Construction and Statistical Analysis
3.2.3. Optimal Conditions and Experimental Validation of Fermentation Conditions
3.3. Functional and Nutritional Characteristics of Soy Sauce
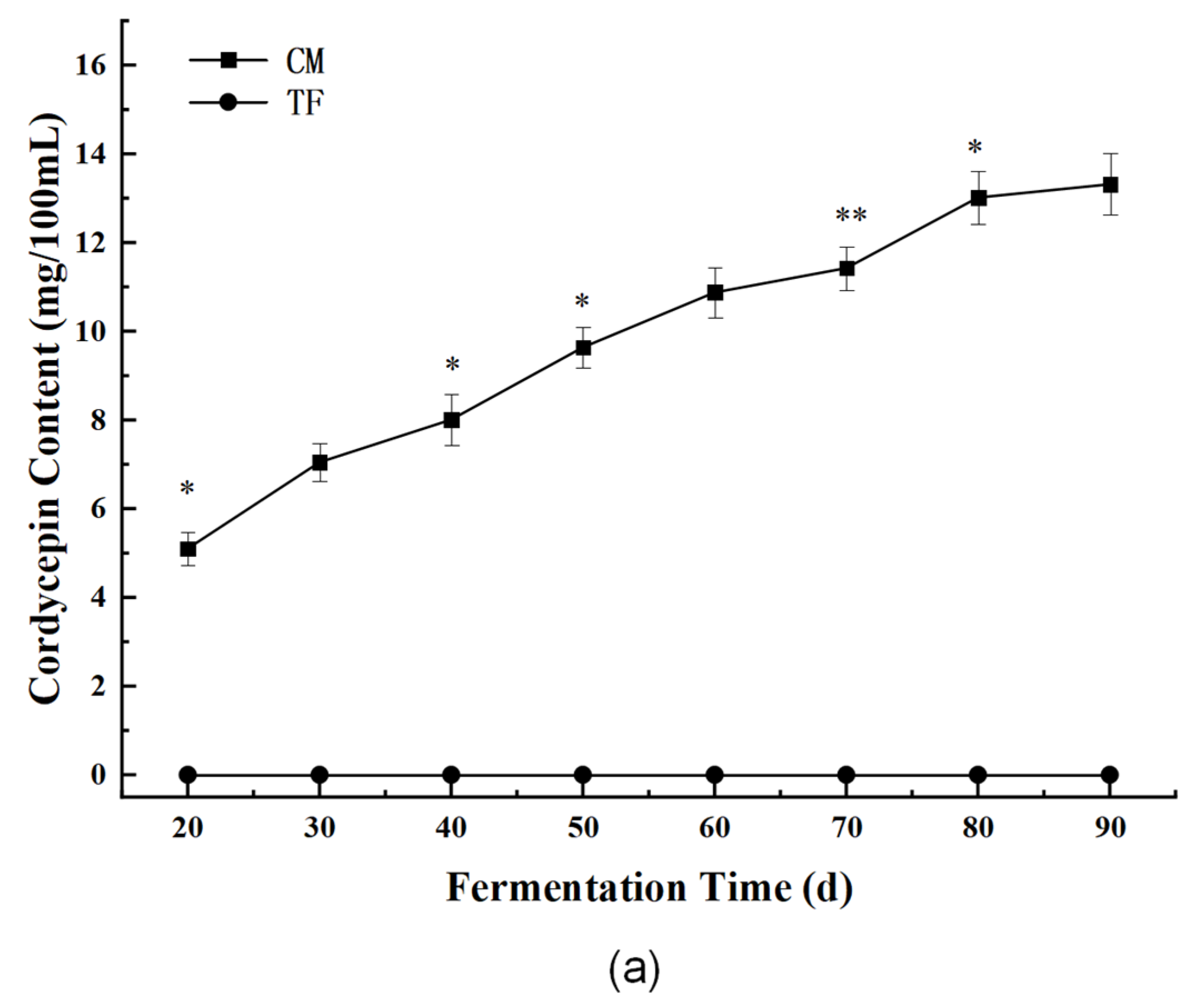
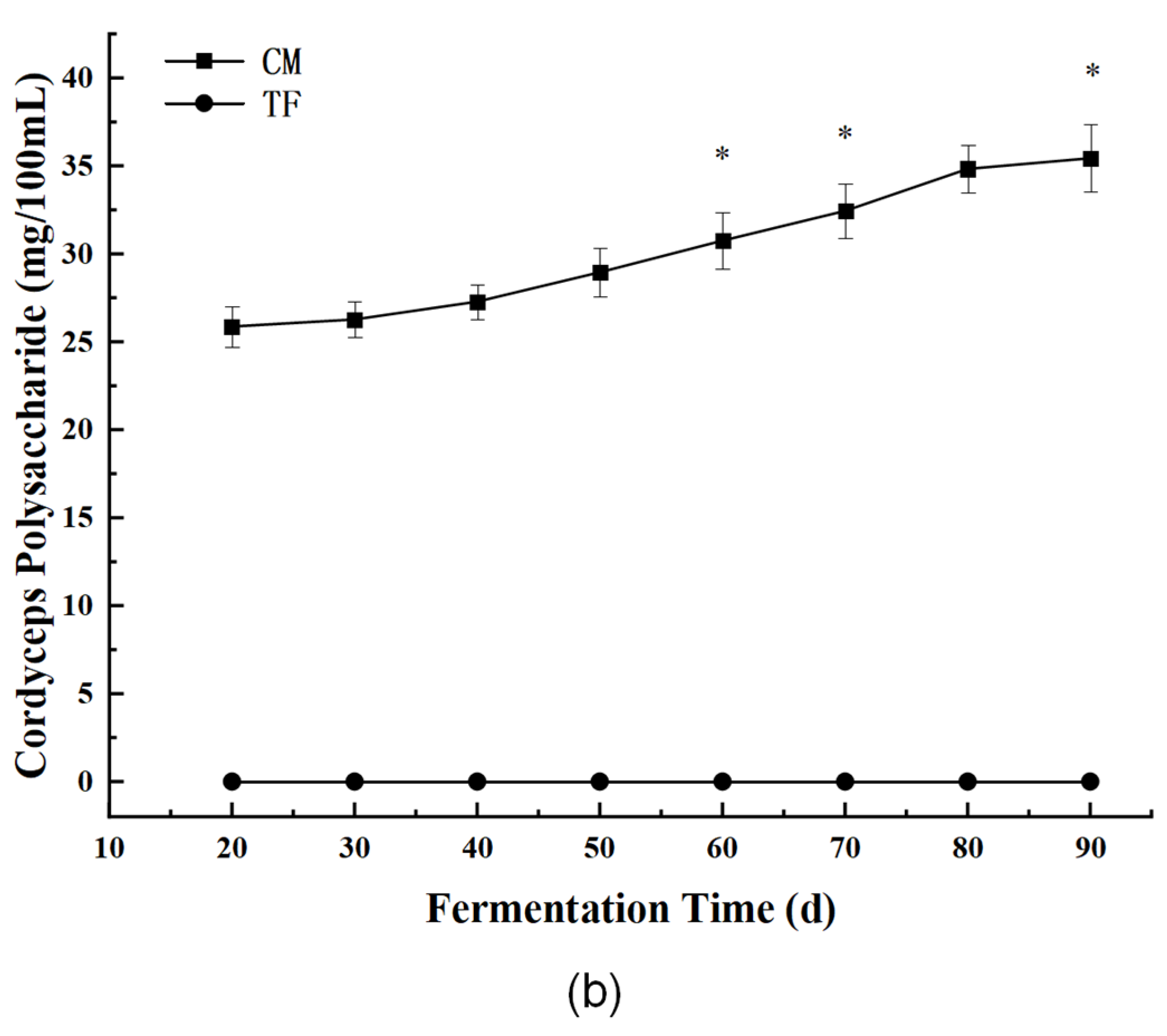
3.3.1. Bioactive Components: Cordycepin and Cordyceps Polysaccharides
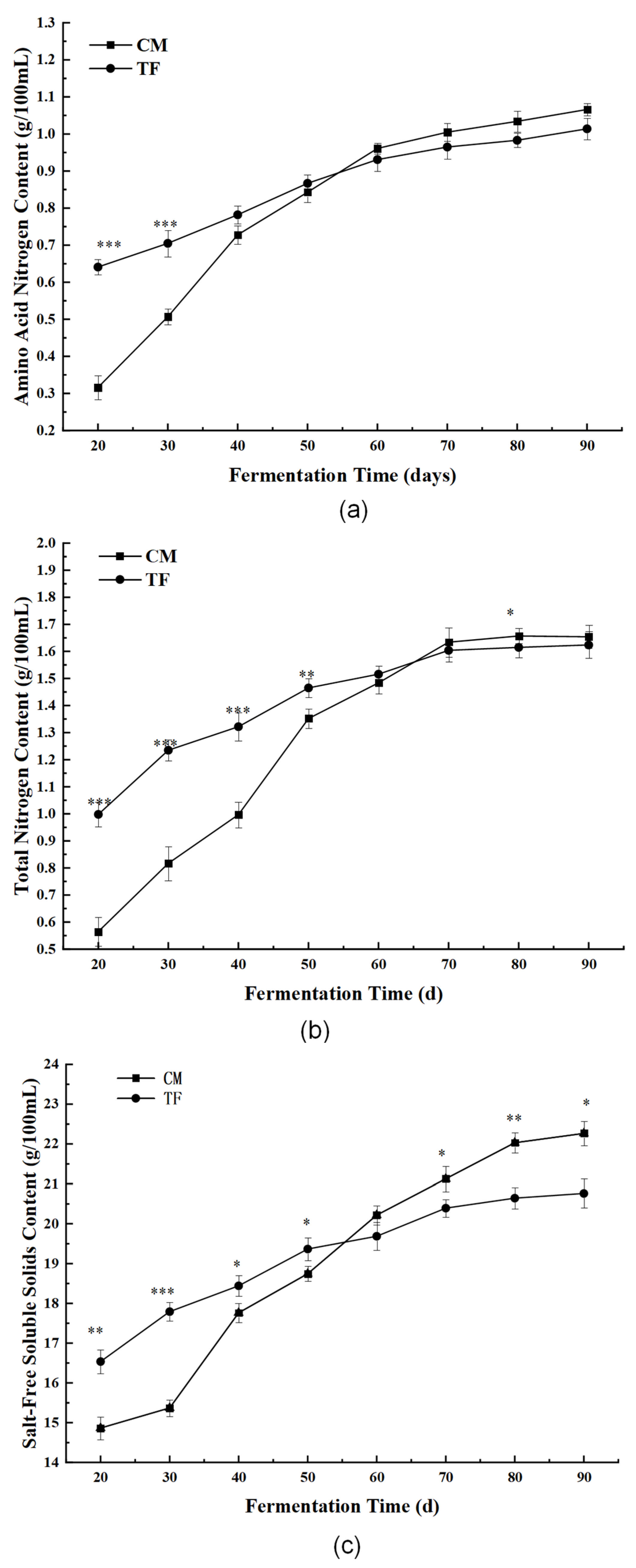
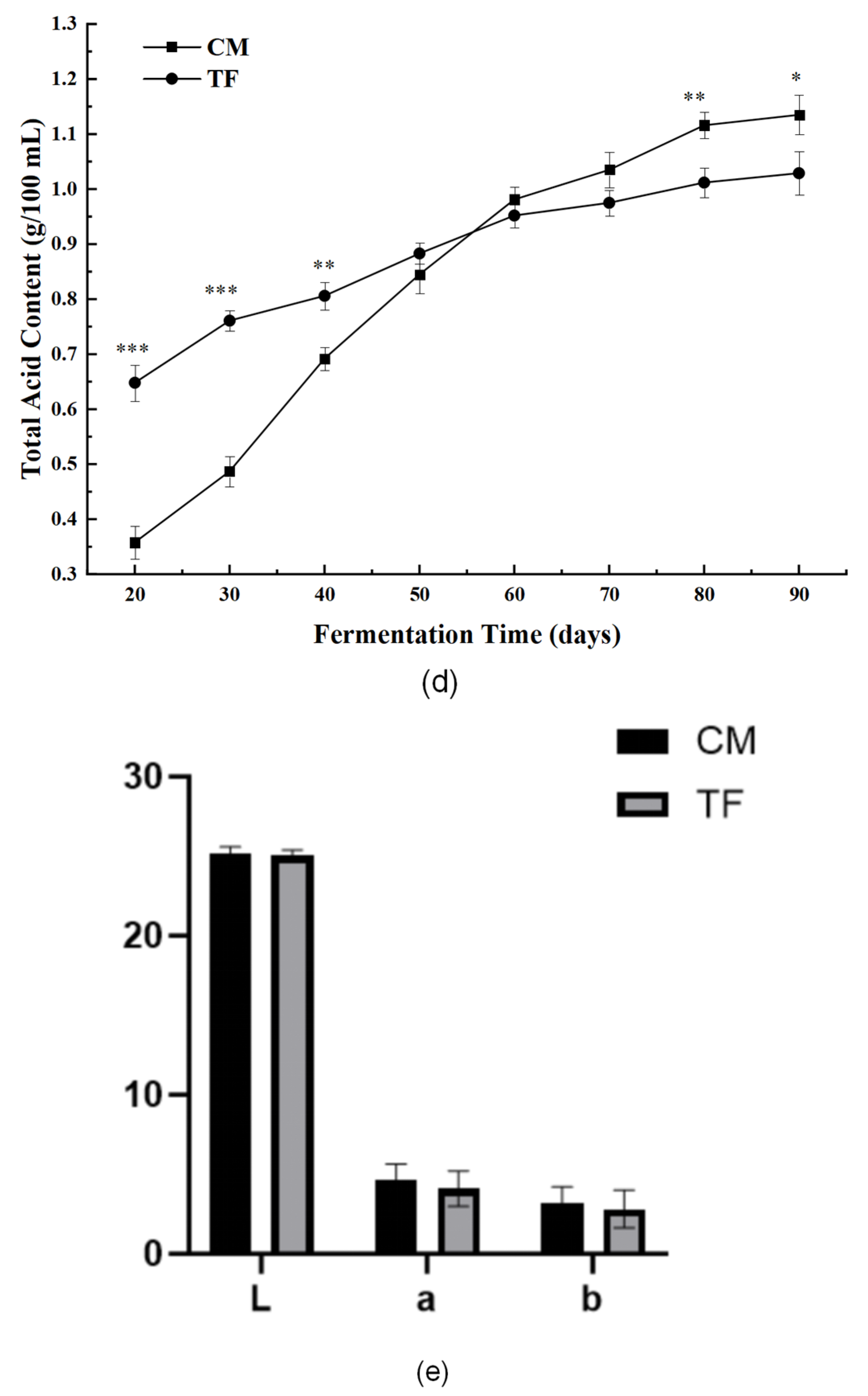
3.3.2. Nutritional and Physicochemical Indices
3.3.3. Volatile Flavor Compounds and Fatty Acid Profile
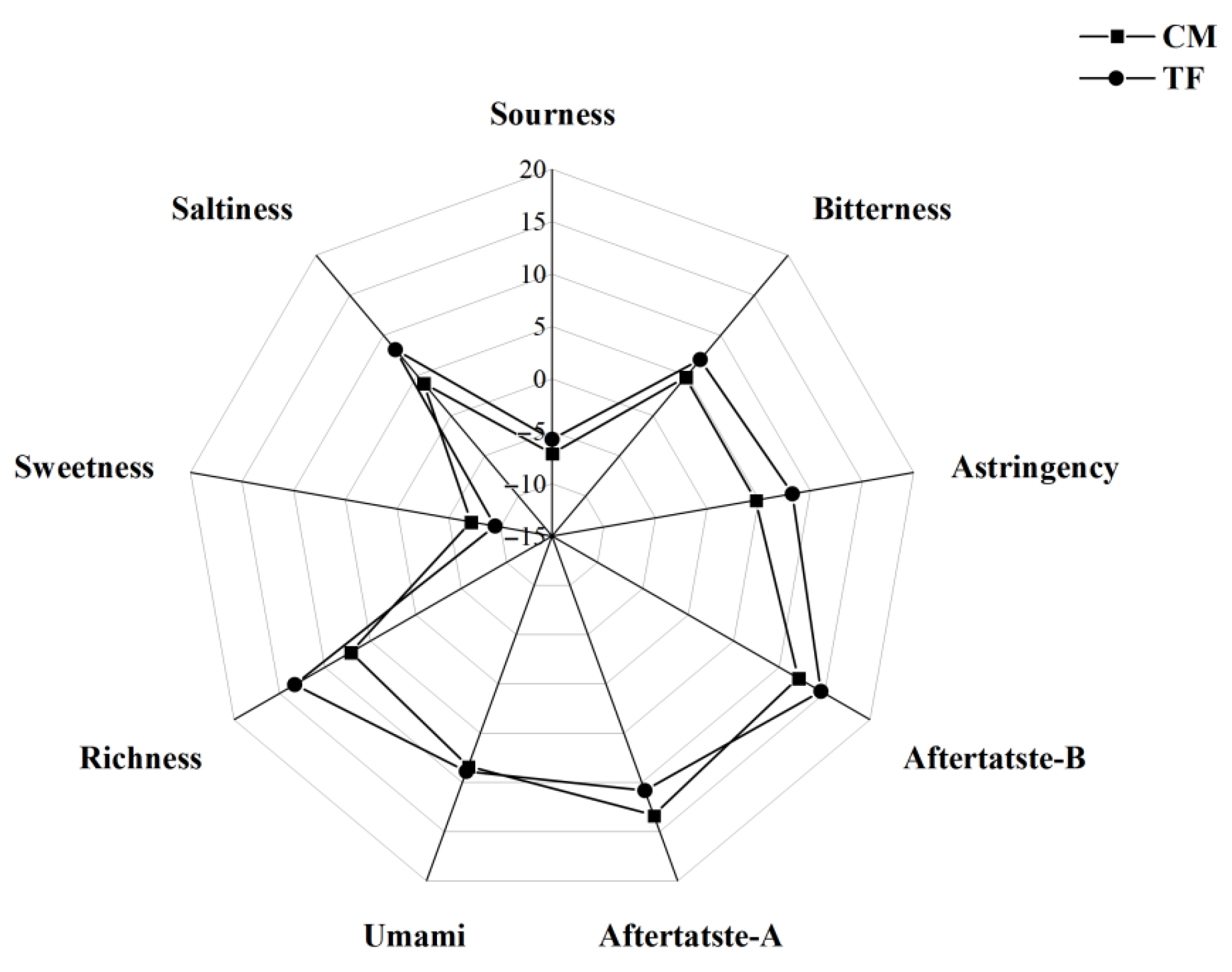
3.3.4. Taste Profile Analysis
3.3.5. Functional Value and Comparative Discussion
3.3.6. Limitations and Future Perspectives
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| C. militaris | Cordyceps militaris |
| RSM | Response Surface Methodology |
| HPLC | High-Performance Liquid Chromatography |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| HS-SPME | Headspace Solid-Phase Microextraction |
| PCA | Principal Component Analysis |
References
- Zhou, X.; Guo, T.; Hadiatullah, H.; Lu, Y.; He, J.; Zhao, G. Metabolic behavior of Aspergillus oryzae in salt-reduced soy sauce and its regulation for the brewing process. Food Biosci. 2024, 59, 104206. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, Y.; Li, S.; Lin, J.; Lin, W.; Li, W.; Luo, L. Impacts of Aspergillus oryzae 3.042 on the flavor formation pathway in Cantonese soy sauce koji. Food Chem. 2024, 441, 138396. [Google Scholar] [CrossRef]
- Zhao, C.; Lin, J.; Zhang, Y.; Wu, H.; Li, W.; Lin, W.; Luo, L. Comprehensive analysis of flavor formation mechanisms in the mechanized preparation Cantonese soy sauce koji using absolute quantitative metabolomics and microbiomics approaches. Food Res. Int. 2024, 180, 114079. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xie, Z.; Huang, M.; Tong, X.; Hou, S.; Tin, H.; Zhao, M. Decoding temperature-driven microbial community changes and flavor regulation mechanism during winter fermentation of soy sauce. Food Res. Int. 2024, 177, 113756. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Zhao, S.; Wang, Q.; Wang, W.; He, J.; Dong, B.; Zhao, G. Influence of spatial and temporal diversity and succession of microbial communities on physicochemical properties and flavor substances of soy sauce. Food Chem. 2025, 463, 141041. [Google Scholar] [CrossRef]
- Ito, K.; Matsuyama, A. Koji molds for Japanese soy sauce brewing: Characteristics and key enzymes. J. Fungi 2021, 7, 658. [Google Scholar] [CrossRef]
- Liu, R.; Gao, G.; Bai, Y.; Hou, L. Fermentation of high-salt liquid–state soy sauce without any additives by inoculation of lactic acid bacteria and yeast. Food Sci. Technol. Int. 2020, 26, 642–654. [Google Scholar] [CrossRef]
- Gao, X.; Feng, T.; Sheng, M.; Wang, B.; Wang, Z.; Shan, P.; Zhang, Y.; Ma, H. Characterization of the aroma-active compounds in black soybean sauce, a distinctive soy sauce. Food Chem. 2021, 364, 130334. [Google Scholar] [CrossRef]
- Gu, C.; Zhang, D.; Zhai, W.; Zhang, H.; Wang, S.; Lv, S.; Bao, Y.; Zhu, D.; Feng, S.; Guo, S. Research progress on Cordyceps militaris polysaccharides. Food Biosci. 2022, 45, 101503. [Google Scholar] [CrossRef]
- Yang, L.; Li, G.; Chai, Z.; Gong, Q.; Guo, J. Synthesis of cordycepin: Current scenario and future perspectives. Fungal Genet. Biol. 2020, 143, 103431. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, M.; Liu, S.; Zhang, W.; Yu, H. Optimization of ultrasonic-microwave synergistic extraction of cordycepin and antioxidant activity from Cordyceps militaris mycelial symbiont. Chem. Pap. 2025, 79, 3107–3119. [Google Scholar] [CrossRef]
- Lee, J.S.; Kwon, J.S.; Yun, J.S.; Pahk, J.W.; Shin, W.C.; Lee, S.Y.; Hong, E.K. Structural characterization of immunostimulating polysaccharide from cultured mycelia of Cordyceps militaris. Carbohydr. Polym. 2010, 80, 1011–1017. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, W.; Lu, J.; Wu, D.; Ge, F. Construction of a synthetic microbial community and its application in salt-reduced soy sauce fermentation. Food Microbiol. 2025, 128, 104738. [Google Scholar] [CrossRef]
- Hao, R.; Yang, Z.; Naseeb, J.; Sarwar, A.; Khan, A.A.; Aziz, T.; Zahra, N.; Alasmari, A.F.; Albekairi, T.H. Response surface analysis and kinetics modelling of the exopolysaccharide production by probiotic Lactiplantibacillus plantarum HMX2. Int. J. Biol. Macromol. 2025, 306, 141436. [Google Scholar] [CrossRef] [PubMed]
- Humberg, S.; Grund, S. Response surface analysis with missing data. Multivar. Behav. Res. 2022, 57, 581–602. [Google Scholar] [CrossRef]
- Abdul Manan, M.; Webb, C. Control strategies with variable air arrangements, forcefully aerated in single circular tray solid state bioreactors with modified Gompertz model and analysis of a distributed parameter gas balance. Biotechnol. Biotechnol. Equip. 2018, 32, 1455–1467. [Google Scholar] [CrossRef]
- GB/T 18186-2000; Fermented Soy Sauce; China Standards Press: Beijing, China, 2000.
- Brown, R.A. An appreciation of the Folin-Lowry protein assay. J. Pharm. Pharmacol. 1992, 44, 369. [Google Scholar] [CrossRef]
- Xu, L.; Wang, F.; Zhang, Z.; Terry, N. Optimization of polysaccharide production from Cordyceps militaris by solid-state fermentation on rice and its antioxidant activities. Foods 2019, 8, 590. [Google Scholar] [CrossRef] [PubMed]
- Kan, Q.; Cao, L.; He, L.; Wang, P.; Deng, G.; Li, J.; Fu, J.; Huang, Q.; Ho, C.-T.; Li, Y. Tracing the change of the volatile compounds of soy sauce at different fermentation times by PTR-TOF-MS, E-nose and GC–MS. Food Chem. X 2025, 25, 102002. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Ashraf, S.A.; Khan, S.; Alshammari, E.; Awadelkareem, A.M. Effect of pH, temperature and incubation time on cordycepin production from Cordyceps militaris using solid-state fermentation on various substrates. CyTA-J. Food 2017, 15, 617–621. [Google Scholar] [CrossRef]
- Wen, T.; Long, F.; Kang, C.; Wang, F.; Zeng, W. Effects of additives and bioreactors on cordycepin production from Cordyceps militaris in liquid static culture. Mycosphere 2017, 8, 886–898. [Google Scholar] [CrossRef]
- Park, J.P.; Kim, Y.M.; Kim, S.W.; Hwang, H.J.; Cho, Y.J.; Lee, Y.S.; Song, C.H.; Yun, J.W. Effect of aeration rate on the mycelial morphology and exo-biopolymer production in Cordyceps militaris. Process Biochem. 2002, 37, 1257–1262. [Google Scholar] [CrossRef]
- Mao, X.B.; Zhong, J.J. Hyperproduction of cordycepin by two-stage dissolved oxygen control in submerged cultivation of medicinal mushroom Cordyceps militaris in bioreactors. Biotechnol. Prog. 2004, 20, 1408–1413. [Google Scholar] [CrossRef]
- Shi, M.; Yang, Y.; Guan, D.; Wang, Y.; Zhang, Z. Evaluation of solid-state fermentation by Ganoderma lucidum using soybean curd residue. Food Bioprocess Technol. 2013, 6, 1856–1867. [Google Scholar] [CrossRef]
- Rashid, J.; Samat, N.; Mohtar, W.; Yusoff, W. Optimization of temperature, moisture content and inoculum size in solid state fermentation to enhance mannanase production by Aspergillus terreus SUK-1 using RSM. Pak. J. Biol. Sci. PJBS 2011, 14, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Ding, L.-L.; Yao, Y.; Cao, Y.; Pan, Z.-H.; Kong, D.-H. Extracellular proteome analysis and flavor formation during soy sauce fermentation. Front. Microbiol. 2018, 9, 1872. [Google Scholar] [CrossRef]
- Ng, T.; Wang, H. Pharmacological actions of Cordyceps, a prized folk medicine. J. Pharm. Pharmacol. 2005, 57, 1509–1519. [Google Scholar] [CrossRef]
- Yang, D.; Peng, N.; Zhang, H.; Qiu, Z.; Xu, L.; Pan, M. Cordycepin ameliorates autoimmunity by promoting STING degradation via autophagy pathway. Br. J. Pharmacol. 2025, 182, 1546–1560. [Google Scholar] [CrossRef]
- Khan, M.A.; Tania, M. Cordycepin and kinase inhibition in cancer. Drug Discov. Today 2023, 28, 103481. [Google Scholar] [CrossRef]
- Borde, M.; Singh, S.K. Enhanced production of cordycepin under solid-state fermentation of Cordyceps militaris by using combinations of grains/substrates. Braz. J. Microbiol. 2023, 54, 2765–2772. [Google Scholar] [CrossRef]
- Suparmin, A.; Kato, T.; Dohra, H.; Park, E.Y. Insight into cordycepin biosynthesis of Cordyceps militaris: Comparison between a liquid surface culture and a submerged culture through transcriptomic analysis. PLoS ONE 2017, 12, e0187052. [Google Scholar] [CrossRef]
- Jiao, Y.; Xu, F.; Chang, Y.J.S.T. Improvement of Cordyceps militaris Polysaccharides Production by a Sole Corn Steep Liquor Medium, and its Antioxidant Activity; Springer: Berlin/Heidelberg, Germany, 2025; pp. 1–11. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, J.; Guo, Z.; Li, Q.; Zhang, L.; Zhao, L.; Zhou, X. Immunomodulatory effect of cordyceps militaris polysaccharide on RAW 264.7 macrophages by regulating MAPK signaling pathways. Molecules 2024, 29, 3408. [Google Scholar] [CrossRef]
- Lee, C.-T.; Huang, K.-S.; Shaw, J.-F.; Chen, J.-R.; Kuo, W.-S.; Shen, G.; Grumezescu, A.M.; Holban, A.M.; Wang, Y.-T.; Wang, J.-S. Trends in the immunomodulatory effects of Cordyceps militaris: Total extracts, polysaccharides and cordycepin. Front. Pharmacol. 2020, 11, 575704. [Google Scholar] [CrossRef]
- Miao, M.; Yu, W.-Q.; Li, Y.; Sun, Y.-L.; Guo, S.-D. Structural elucidation and activities of Cordyceps militaris-derived polysaccharides: A review. Front. Nutr. 2022, 9, 898674. [Google Scholar] [CrossRef]
- Cai, T.; Hai, N.; Guo, P.; Feng, Z.; Zhang, Y.; Wang, J.; Yu, Z.; Liu, H.; Ding, L. Characteristics of Umami Taste of Soy Sauce Using Electronic Tongue, Amino Acid Analyzer, and MALDI− TOF MS. Foods 2024, 13, 2242. [Google Scholar] [CrossRef]
- Kuang, X.; Su, H.; Li, W.; Lin, L.; Lin, W.; Luo, L. Effects of microbial community structure and its co-occurrence on the dynamic changes of physicochemical properties and free amino acids in the Cantonese soy sauce fermentation process. Food Res. Int. 2022, 156, 111347. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Hou, S.; Wu, C.; Cao, Y.; Tong, X.; Chen, Y. Improving protein utilization and fermentation quality of soy sauce by adding protease. J. Food Compos. Anal. 2023, 121, 105399. [Google Scholar] [CrossRef]
- Kaneko, S.; Kumazawa, K. Comparative aroma extract dilution analysis of changes in aroma components of seasoned soy sauce prepared from soy sauce and mirin during heating. Food Sci. Technol. Res. 2020, 26, 725–733. [Google Scholar] [CrossRef]
- Masuda, S.; Yamaguchi, H.; Kurokawa, T.; Shirakami, T.; Tsuji, R.F.; Nishimura, I. Immunomodulatory effect of halophilic lactic acid bacterium Tetragenococcus halophilus Th221 from soy sauce moromi grown in high-salt medium. Int. J. Food Microbiol. 2008, 121, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Zhang, K.; Zhang, Z.; Zhang, C.; Sun, Y.; Feng, Z. Fermented soybean foods: A review of their functional components, mechanism of action and factors influencing their health benefits. Food Res. Int. 2022, 158, 111575. [Google Scholar] [CrossRef]
- Hu, G.; Chen, J.; Du, G.; Fang, F. Moromi mash dysbiosis trigged by salt reduction is relevant to quality and aroma changes of soy sauce. Food Chem. 2023, 406, 135064. [Google Scholar] [CrossRef]
- Zou, M.; Zhu, X.; Li, X.; Zeng, X. Changes in lipids distribution and fatty acid composition during soy sauce production. Food Sci. Nutr. 2019, 7, 764–772. [Google Scholar] [CrossRef]
- Chin, X.H.; Soh, R.; Chan, G.; Ng, P.; Thong, A.; Elhalis, H.; Yoganathan, K.; Chow, Y.; Liu, S.Q. Modulating the aroma and taste profile of soybean using novel strains for fermentation. Curr. Res. Food Sci. 2025, 10, 100933. [Google Scholar] [CrossRef] [PubMed]
- Diez-Simon, C.; Eichelsheim, C.; Mumm, R.; Hall, R.D. Chemical and sensory characteristics of soy sauce: A review. J. Agric. Food Chem. 2020, 68, 11612–11630. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Feng, Y.; Hadiatullah, H.; Zheng, F.; Yao, Y. Chemical characteristics of three kinds of Japanese soy sauce based on electronic senses and GC-MS analyses. Front. Microbiol. 2021, 11, 579808. [Google Scholar] [CrossRef]
- Long, L.; Liu, Z.; Wang, Y.; Lin, Q.; Ding, S.; Li, C.; Deng, C. High-level production of cordycepin by the xylose-utilising Cordyceps militaris strain 147 in an optimised medium. Bioresour. Technol. 2023, 388, 129742. [Google Scholar] [CrossRef]
- Zhou, K.; Zhang, X.; Li, B.; Shen, C.; Sun, Y.-M.; Yang, J.; Xu, Z.-L. Citrulline accumulation mechanism of Pediococcus acidilactici and Weissella confusa in soy sauce and the effects of phenolic compound on citrulline accumulation. Front. Microbiol. 2021, 12, 757542. [Google Scholar] [CrossRef]
- Hong, K.-Q.; Fu, X.-M.; Lei, F.-F.; Chen, D.; He, D.-P. Selection of Salt-Tolerance and Ester-Producing Mutant Saccharomyces cerevisiae to Improve Flavour Formation of Soy Sauce during Co-Fermentation with Torulopsis globosa. Foods 2023, 12, 3449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Y.; Hu, Z. The effects of catabolism relationships of leucine and isoleucine with BAT2 gene of Saccharomyces cerevisiae on high alcohols and esters. Genes 2022, 13, 1178. [Google Scholar] [CrossRef]
- Anisha, C.; Radhakrishnan, E. Metabolite analysis of endophytic fungi from cultivars of Zingiber officinale Rosc. identifies myriad of bioactive compounds including tyrosol. 3 Biotech 2017, 7, 146. [Google Scholar] [CrossRef]
- Tan, G.; Wang, Y.; Hu, M.; Li, X.; Li, X.; Pan, Z.; Li, M.; Li, L.; Zheng, Z. Comparative evaluation of the microbial diversity and metabolite profiles of Japanese-style and Cantonese-style soy sauce fermentation. Front. Microbiol. 2022, 13, 976206. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.; Bhandari, A.; Shrestha, J. Effect of different doses of triacontanol on growth and yield of kohlrabi (Brassica oleracea L. var. gongylodes). Heliyon 2021, 7, e08242. [Google Scholar] [CrossRef]
- Zhang, L.; Xiong, S.; Du, T.; Xu, Y.; Zhao, X.; Huang, G.; Guan, Q.; Xiong, T. Unraveling the core functional microbiota involved in metabolic network of characteristic flavor development during soy sauce fermentation. Food Biosci. 2024, 58, 103697. [Google Scholar] [CrossRef]
- Zhang, L.; Xiong, S.; Du, T.; Xu, Y.; Madjirebaye, P.; Huang, G.; Guan, Q.; Xiong, T. Effect of microbiota succession on the dynamics of characteristic flavors and physicochemical properties during the soy sauce fermentation. Food Biosci. 2023, 54, 102883. [Google Scholar] [CrossRef]
- El Jaddaoui, I.; Rangel, D.E.; Bennett, J.W. Fungal volatiles have physiological properties. Fungal Biol. 2023, 127, 1231–1240. [Google Scholar] [CrossRef]
- Delompré, T.; Guichard, E.; Briand, L.; Salles, C. Taste perception of nutrients found in nutritional supplements: A review. Nutrients 2019, 11, 2050. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-W.; Jin, C.-Y.; Kim, G.-Y.; Lee, J.-D.; Park, C.; Kim, G.-D.; Kim, W.-J.; Jung, W.-K.; Seo, S.K.; Choi, I.-W. Anti-inflammatory effects of cordycepin via suppression of inflammatory mediators in BV2 microglial cells. Int. Immunopharmacol. 2010, 10, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tian, S.; Ruan, S.; Wei, J.; Wei, S.; Chen, W.; Hu, H.; Qin, W.; Li, Y.; Yuan, H. Neuroprotective effects of cordycepin on MPTP-induced Parkinson’s disease mice via suppressing PI3K/AKT/mTOR and MAPK-mediated neuroinflammation. Free Radic. Biol. Med. 2024, 216, 60–77. [Google Scholar] [CrossRef]
- Sun, H.; Yu, X.; Li, T.; Zhu, Z. Structure and hypoglycemic activity of a novel exopolysaccharide of Cordyceps militaris. Int. J. Biol. Macromol. 2021, 166, 496–508. [Google Scholar] [CrossRef]
- Gu, L.; Yu, T.; Liu, J.; Lu, Y. Evaluation of the mechanism of cordyceps polysaccharide action on rat acute liver failure. Arch. Med. Sci. 2020, 16, 94236. [Google Scholar] [CrossRef] [PubMed]
- Azemi, N.A.; Azemi, A.K.; Abu-Bakar, L.; Sevakumaran, V.; Muhammad, T.S.T.; Ismail, N. Effect of linoleic acid on cholesterol levels in a high-fat diet-induced hypercholesterolemia rat model. Metabolites 2022, 13, 53. [Google Scholar] [CrossRef] [PubMed]
- GB 16740—2014; Food Safety National Health Food Standards. China Standards Press: Beijing, China, 2014.
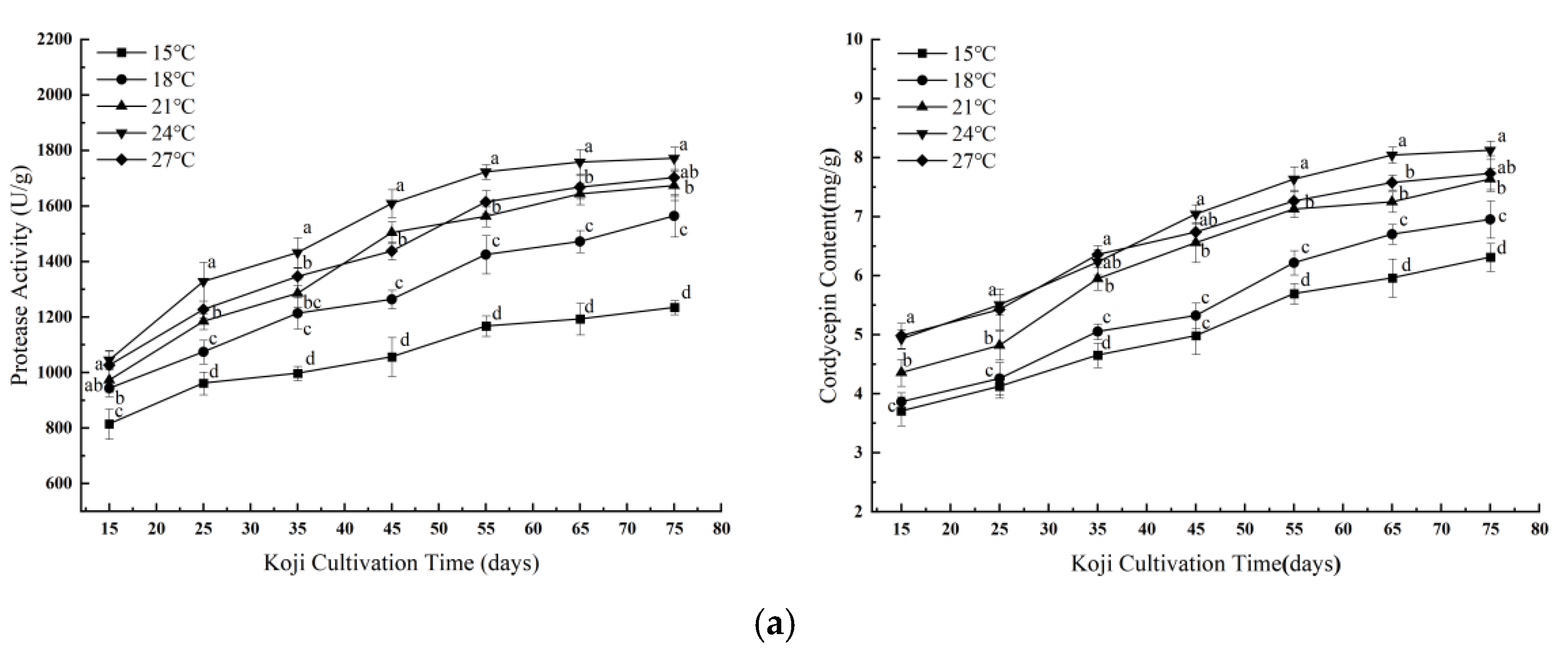
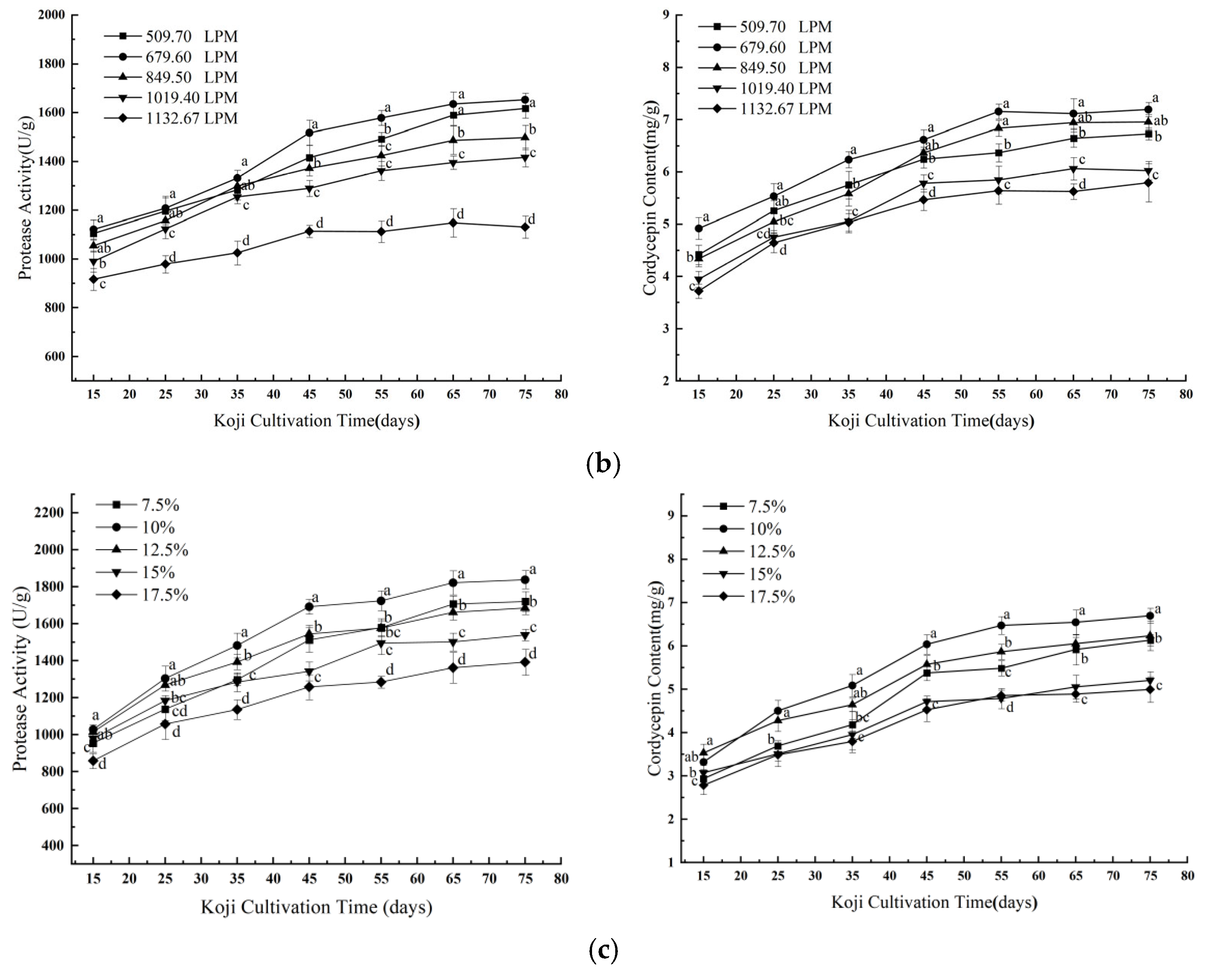
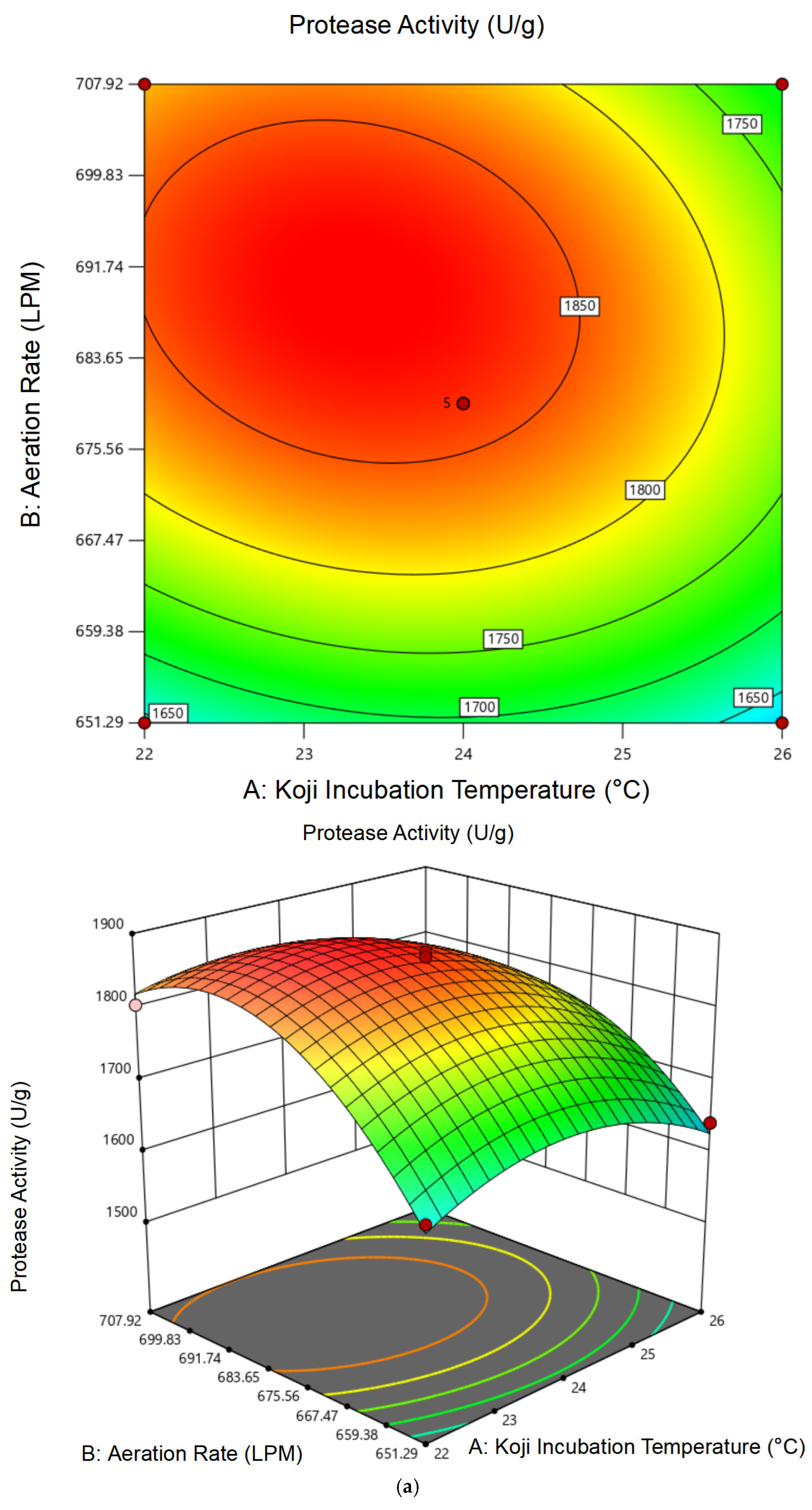
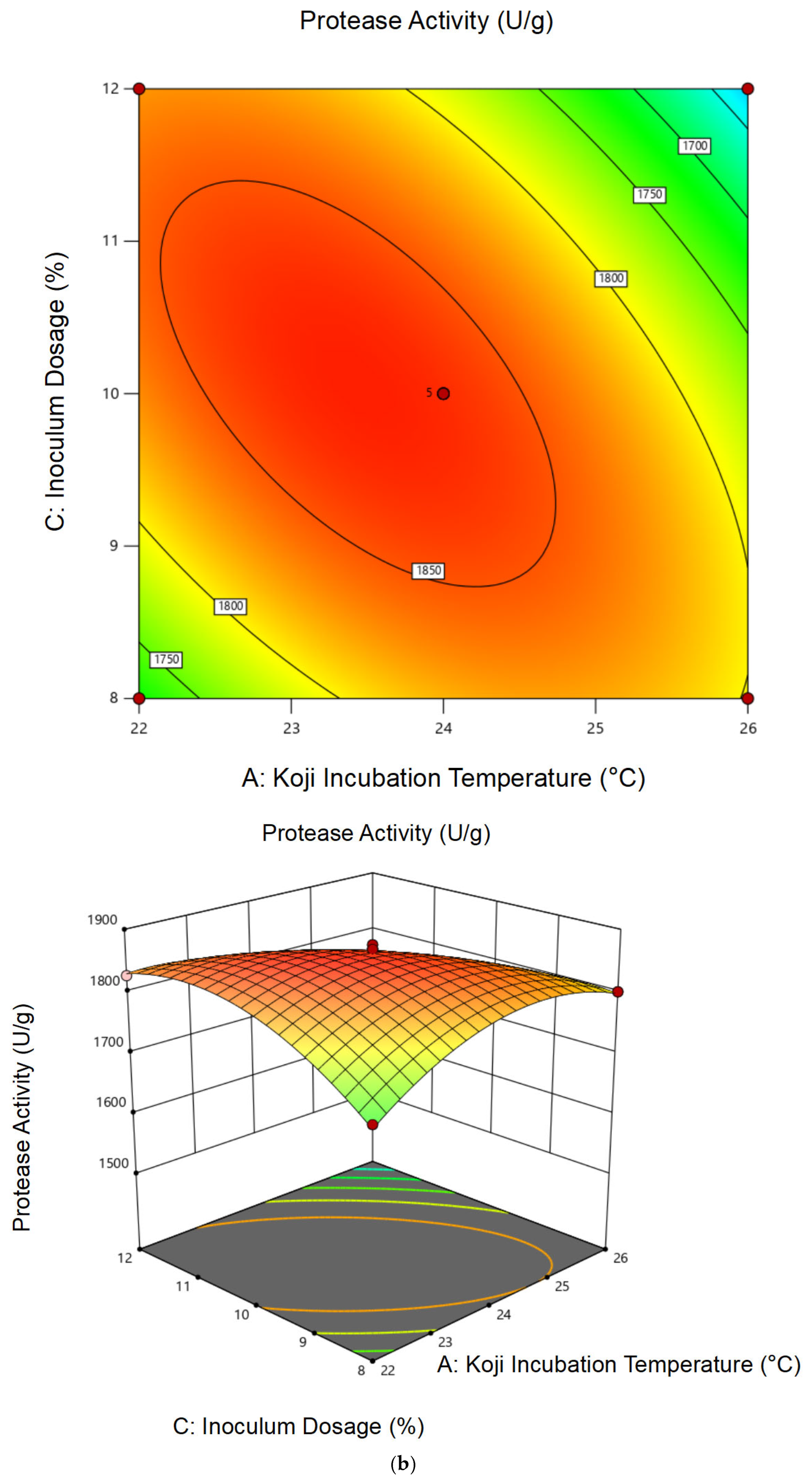







| Experiment No. | A | B | C | Protease Activity (U/g) | Cordycepin Content (mg/g) |
|---|---|---|---|---|---|
| 1 | 24 | 23 | 8 | 1685.32 | 8.06 |
| 2 | 24 | 25 | 12 | 1817.24 | 8.11 |
| 3 | 22 | 23 | 10 | 1652.37 | 7.46 |
| 4 | 24 | 23 | 12 | 1562.8 | 7.95 |
| 5 | 26 | 25 | 10 | 1694.72 | 7.86 |
| 6 | 26 | 24 | 12 | 1617.58 | 7.69 |
| 7 | 24 | 25 | 8 | 1739.27 | 8.34 |
| 8 | 22 | 25 | 10 | 1804.23 | 7.52 |
| 9 | 24 | 24 | 10 | 1875.77 | 8.44 |
| 10 | 26 | 24 | 8 | 1801.13 | 7.94 |
| 11 | 24 | 24 | 10 | 1851.05 | 8.47 |
| 12 | 24 | 24 | 10 | 1847.51 | 8.39 |
| 13 | 24 | 24 | 10 | 1869.51 | 8.46 |
| 14 | 22 | 24 | 12 | 1826.84 | 7.57 |
| 15 | 22 | 24 | 8 | 1727.06 | 7.72 |
| 16 | 24 | 24 | 10 | 1868.61 | 8.54 |
| 17 | 26 | 23 | 10 | 1641.42 | 7.54 |
| Source of Variation | Sum of Squares | Degrees of Freedom | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 1.584 × 105 | 9 | 17,605.23 | 56.80 | <0.0001 | *** |
| A | 8169.62 | 1 | 8169.62 | 26.36 | 0.0013 | ** |
| B | 32,966.19 | 1 | 32,966.19 | 106.35 | <0.0001 | *** |
| C | 2058.12 | 1 | 2058.12 | 6.64 | 0.0366 | * |
| AB | 2428.52 | 1 | 2428.52 | 7.83 | 0.0266 | * |
| AC | 20,068.97 | 1 | 20,068.97 | 64.75 | <0.0001 | *** |
| BC | 10,048.66 | 1 | 10,048.66 | 32.42 | 0.0007 | *** |
| A2 | 15,747.35 | 1 | 15,747.35 | 50.80 | 0.0002 | *** |
| B2 | 44,799.24 | 1 | 44,799.24 | 144.53 | <0.0001 | *** |
| C2 | 14,253.24 | 1 | 14,253.24 | 45.98 | 0.0003 | *** |
| Residual | 2169.75 | 7 | 309.96 | |||
| Lack of Fit | 1551.39 | 9 | 517.13 | 3.35 | 0.1369 | ns |
| Pure Error | 618.37 | 1 | 154.59 | |||
| Total | 1.606 × 105 | 1 | ||||
| R2 | 0.9865 | 1 | ||||
| R2Adj | 0.9691 | 1 |
| Source of Variation | Sum of Squares | Degrees of Freedom | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 2.31 | 9 | 0.2562 | 132.86 | <0.0001 | *** |
| A | 0.0722 | 1 | 0.0722 | 37.44 | 0.0005 | *** |
| B | 0.0841 | 1 | 0.0841 | 43.58 | 0.0003 | *** |
| C | 0.0685 | 1 | 0.0685 | 35.49 | 0.0006 | *** |
| AB | 0.0169 | 1 | 0.0169 | 8.76 | 0.0211 | * |
| AC | 0.0025 | 1 | 0.0025 | 1.30 | 0.2924 | ns |
| BC | 0.0036 | 1 | 0.0036 | 1.87 | 0.2141 | ns |
| A2 | 1.64 | 1 | 1.64 | 852.83 | <0.0001 | *** |
| B2 | 0.2425 | 1 | 0.2425 | 125.75 | <0.0001 | *** |
| C2 | 0.0464 | 1 | 0.0464 | 24.07 | 0.0017 | ** |
| Residual | 0.0135 | 7 | 0.0019 | |||
| Lack of Fit | 0.0017 | 3 | 0.0006 | 0.1921 | 0.8967 | ns |
| Pure Error | 0.0118 | 4 | 0.0029 | |||
| Total | 2.32 | 16 | ||||
| R2 | 0.9942 | |||||
| R2Adj | 0.9867 |
| Run | A | B | C | Amino Acid Nitrogen (g/100 mL) | Cordycepin (mg/100 mL) |
|---|---|---|---|---|---|
| 1 | 32 | 12.5 | 1.35:1 | 1.099 | 15.9 |
| 2 | 30 | 11.5 | 1.5:1 | 1.131 | 15.7 |
| 3 | 30 | 12 | 1.65:1 | 1.165 | 15.8 |
| 4 | 32 | 12 | 1.5:1 | 1.193 | 17.4 |
| 5 | 34 | 12 | 1.65:1 | 1.167 | 15.8 |
| 6 | 34 | 12 | 1.35:1 | 1.142 | 15.7 |
| 7 | 32 | 12.5 | 1.65:1 | 1.147 | 16.7 |
| 8 | 30 | 12.5 | 1.5:1 | 1.121 | 16.1 |
| 9 | 32 | 12 | 1.5:1 | 1.186 | 17.2 |
| 10 | 30 | 12 | 1.35:1 | 1.107 | 15.3 |
| 11 | 32 | 11.5 | 1.35:1 | 1.091 | 16.2 |
| 12 | 32 | 12 | 1.5:1 | 1.191 | 17.2 |
| 13 | 34 | 12.5 | 1.5:1 | 1.159 | 15.7 |
| 14 | 32 | 11.5 | 1.65:1 | 1.131 | 16.6 |
| 15 | 34 | 11.5 | 1.5:1 | 1.109 | 16.7 |
| 16 | 32 | 12 | 1.5:1 | 1.191 | 17.3 |
| 17 | 32 | 12 | 1.5:1 | 1.187 | 17.2 |
| Source of Variation | Sum of Squares | Degrees of Freedom | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 0.0195 | 9 | 0.0022 | 121.08 | <0.0001 | *** |
| A | 0.0004 | 1 | 0.0004 | 19.59 | 0.0031 | ** |
| B | 0.0005 | 1 | 0.0005 | 28.57 | 0.0011 | ** |
| C | 0.0037 | 1 | 0.0037 | 203.95 | <0.0001 | *** |
| AB | 0.0009 | 1 | 0.0009 | 50.22 | 0.0002 | *** |
| AC | 0.0003 | 1 | 0.0003 | 15.19 | 0.0059 | ** |
| BC | 0.0000 | 1 | 0.0000 | 0.8928 | 0.3762 | ns |
| A2 | 0.0010 | 1 | 0.0010 | 57.73 | 0.0001 | *** |
| B2 | 0.0081 | 1 | 0.0081 | 453.30 | <0.0001 | *** |
| C2 | 0.0035 | 1 | 0.0035 | 193.18 | <0.0001 | *** |
| Residual | 0.0001 | 7 | 0.0000 | |||
| Lack of Fit | 0.0001 | 3 | 0.0000 | 3.42 | 0.1330 | ns |
| Pure Error | 0.0000 | 4 | 8.800 × 10−6 | |||
| Total | 0.0197 | 16 | ||||
| R2 | 0.9936 | |||||
| R2Adj | 0.9854 |
| Source of Variation | Sum of Squares | Degrees of Freedom | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 7.62 | 9 | 0.8470 | 58.13 | <0.0001 | *** |
| A | 0.1250 | 1 | 0.1250 | 8.58 | 0.0221 | * |
| B | 0.0800 | 1 | 0.0800 | 5.49 | 0.0516 | ns |
| C | 0.4050 | 1 | 0.4050 | 27.79 | 0.0012 | ** |
| AB | 0.4900 | 1 | 0.4900 | 33.63 | 0.0007 | *** |
| AC | 0.0400 | 1 | 0.0400 | 2.75 | 0.1415 | ns |
| BC | 0.0400 | 1 | 0.0400 | 2.75 | 0.1415 | ns |
| A2 | 3.84 | 1 | 3.84 | 263.54 | <0.0001 | *** |
| B2 | 0.2738 | 1 | 0.2738 | 18.79 | 0.0034 | ** |
| C2 | 1.81 | 1 | 1.81 | 123.97 | <0.0001 | *** |
| Residual | 0.1020 | 7 | 0.0146 | |||
| Lack of Fit | 0.0700 | 3 | 0.0233 | 2.92 | 0.1639 | ns |
| Pure Error | 0.0320 | 4 | 0.0080 | |||
| Total | 7.72 | 16 | ||||
| R2 | 0.9868 | |||||
| R2Adj | 0.9698 |
| No. | Compound | Molecular Formula | Molecular Weight | TF Relative Content (%) | CM Relative Content (%) | Significance |
|---|---|---|---|---|---|---|
| 1 | Furfural Alcohol | C5H6O2 | 98.037 | 0.31 ± 0.061 | ND | *** |
| 2 | Phenol | C6H6O | 94.042 | 0.24 ± 0.014 | ND | *** |
| 3 | 2-n-Pentylfuran | C9H14O | 138.104 | 0.08 ± 0.006 | ND | *** |
| 4 | n-Hexanoic Acid | C6H12O2 | 116.084 | 0.16 ± 0.009 | 0.04 ± 0.002 | ** |
| 5 | 2,3-Dimethyl-5-hydroxy-2-cyclopenten-1-one | C7H10O2 | 126.068 | 0.11 ± 0.006 | ND | *** |
| 6 | 2-Acetylpyrrole | C6H7NO | 109.053 | 0.86 ± 0.053 | 1.04 ± 0.103 | * |
| 7 | 4-Methyl-5,6-dihydro-2H-pyran-2-one | C6H8O2 | 112.052 | 0.32 ± 0.019 | ND | *** |
| 8 | Benzoic Acid | C7H6O2 | 122.037 | 1.12 ± 0.093 | 1.18 ± 0.071 | ns |
| 9 | Azelaic Acid | C9H16O4 | 188.105 | 17.55 ± 1.013 | ND | *** |
| 10 | Phenylacetic Acid | C8H8O2 | 136.052 | 1.68 ± 0.095 | 1.39 ± 0.067 | ** |
| 11 | 2,6-Dimethoxyphenol (Syringol) | C8H10O3 | 154.063 | 0.67 ± 0.047 | 0.64 ± 0.043 | ns |
| 12 | 2,6-Di-tert-butyl-p-cresol (BHT) | C15H24O | 220.183 | 2.7 ± 0.170 | 7.71 ± 0.458 | *** |
| 13 | Diethyl Phthalate | C12H14O4 | 222.089 | 2.49 ± 0.217 | 0.46 ± 0.027 | *** |
| 14 | Cycloundecanone | C11H20O | 168.151 | 0.57 ± 0.033 | ND | *** |
| 15 | 2,4-Di-tert-butylphenol | C14H22O | 206.167 | 0.27 ± 0.016 | ND | *** |
| 16 | Methyl Linoleate | C19H34O2 | 294.256 | 1.09 ± 0.063 | 2.28 ± 0.004 | *** |
| 17 | 3-Hydroxy-4-methoxybenzoic Acid | C8H8O4 | 168.042 | 0.5 ± 0.029 | 1.25 ± 0.109 | *** |
| 18 | Vanillic Acid | C8H8O4 | 168.042 | 0.96 ± 0.055 | 0.76 ± 0.044 | ** |
| 19 | Myristic Acid | C14H28O2 | 228.209 | 4.2 ± 0.243 | ND | *** |
| 20 | 2-Octylcyclopropaneoctanal | C19H36O | 280.277 | 0.6 ± 0.042 | ND | *** |
| 21 | Dehydroacetic Acid | C8H8O4 | 168.042 | 0.2 ± 0.011 | ND | *** |
| 22 | Oleamide | C18H35NO | 281.272 | 0.48 ± 0.028 | 1.03 ± 0.009 | *** |
| 23 | Palmitic Acid | C16H32O2 | 256.24 | 5.59 ± 0.436 | ND | *** |
| 24 | Methyl Oleate | C19H36O2 | 296.272 | 0.79 ± 0.000017 | ND | *** |
| 25 | Petroselinic Acid | C18H34O2 | 282.256 | 0.04 ± 0.002 | ND | *** |
| 26 | Linoleic Acid | C18H32O2 | 280.24 | 7.83 ± 0.28 | 15.68 ± 0.132 | *** |
| 27 | 2-Methylheptadecane | C15H28 | 208.219 | 0.11 ± 0.006 | ND | *** |
| 28 | Bis(2-ethylhexyl) Adipate | C22H42O4 | 370.308 | 0.72 ± 0.043 | ND | *** |
| 29 | (9Z)-Octadeca-9,17-dienal | C18H32O | 264.245 | 0.54 ± 0.031 | 1.27 ± 0.122 | *** |
| 30 | Di(2-ethylhexyl) Phthalate | C24H38O4 | 390.277 | 0.75 ± 0.044 | ND | *** |
| 31 | Hexahydro-3-(phenylmethyl)-1H-pyrrolo [1,2-a]pyrazine-1,4-dione | C14H16N2O2 | 244.121 | 3.06 ± 0.107 | 1.9 ± 0.034 | *** |
| 32 | trans-13-Octadecenoic Acid | C18H34O2 | 282.256 | 0.28 ± 0.016 | 0.08 ± 0.146 | *** |
| 33 | 2-Chloroethyl Linoleate | C20H35ClO2 | 342.233 | 0.05 ± 0.003 | ND | *** |
| 34 | trans-Squalene | C30H50 | 410.391 | 0.36 ± 0.014 | 0.69 ± 0.172 | * |
| 35 | 2-Hydroxycyclopentadecanone | C15H28O2 | 240.209 | 0.3 ± 0.001 | 0.4 ± 0.0492 | * |
| 36 | Glyceryl Monooleate | C21H40O4 | 356.293 | 0.01 ± 0.082 | ND | ns |
| 37 | Acetylpropionic Acid | C8H16O2 | 144.115 | ND | 0.42 ± 0.046 | *** |
| 38 | Nicotinamide | C6H6N2O | 122.048 | ND | 0.22 ± 0.014 | *** |
| 39 | Phenylmalonic Acid | C9H8O4 | 180.042 | ND | 0.03 ± 0.002 | ns |
| 40 | 3,4-Dimethoxyphenylacetone | C10H12O3 | 180.079 | ND | 0.24 ± 0.014 | *** |
| 41 | Dehydroacetic Acid | C8H8O4 | 168.042 | ND | 0.51 ± 0.029 | *** |
| 42 | Triacontanol | C30H62O | 438.48 | ND | 1.37 ± 0.079 | *** |
| 43 | Tetrahydro-α-ionone | C13H24O | 196.183 | ND | 0.47 ± 0.0001 | *** |
| 44 | Oleamide | C18H35NO | 281.272 | ND | 1.03 ± 0.009 | *** |
| 45 | Methyl Palmitate | C17H34O2 | 270.256 | ND | 1.04 ± 0.132 | *** |
| 46 | n-Nonacosane | C29H60 | 408.47 | ND | 0.34 ± 0.030 | *** |
| 47 | Cyclotriacontane | C30H52O4 | 420.47 | ND | 0.15 ± 0.023 | *** |
| 48 | Cyclo(L-Leu-L-Leu) | C12H22N2O2 | 226.168 | ND | 0.52 ± 0.074 | *** |
| 49 | (Z)-13-Octadecenal | C18H34O | 266.261 | ND | 2.52 ± 0.013 | *** |
| 50 | n-Hexacosane | C26H54 | 366.423 | ND | 2.29 ± 0.018 | *** |
| 51 | Eicosane | C20H42 | 282.329 | ND | 0.22 ± 0.007 | *** |
| 52 | 13-Tetradecen-1-ol Acetate | C16H30O2 | 254.225 | ND | 0.59 ± 0.035 | *** |
| 53 | n-Tricosane | C23H48 | 324.376 | ND | 0.31 ± 0.024 | *** |
| 54 | Tetracosane | C24H50 | 338.391 | ND | 0.12 ± 0.013 | *** |
| 55 | 1-Eicosene | C20H40 | 280.313 | ND | 0.32 ± 0.015 | *** |
| 56 | Erucamide | C22H43NO | 337.334 | ND | 0.23 ± 0.012 | *** |
| 57 | Dodecenyl Succinic Anhydride | C16H26O3 | 266.188 | ND | 1.91 ± 0.032 | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, W.; Zhang, X.; Yang, H.; Liu, H.; Wei, B. Soy Sauce Fermentation with Cordyceps militaris: Process Optimization and Functional Profiling. Foods 2025, 14, 2711. https://doi.org/10.3390/foods14152711
Song W, Zhang X, Yang H, Liu H, Wei B. Soy Sauce Fermentation with Cordyceps militaris: Process Optimization and Functional Profiling. Foods. 2025; 14(15):2711. https://doi.org/10.3390/foods14152711
Chicago/Turabian StyleSong, Wanying, Xinyue Zhang, Huiyi Yang, Hanyu Liu, and Baodong Wei. 2025. "Soy Sauce Fermentation with Cordyceps militaris: Process Optimization and Functional Profiling" Foods 14, no. 15: 2711. https://doi.org/10.3390/foods14152711
APA StyleSong, W., Zhang, X., Yang, H., Liu, H., & Wei, B. (2025). Soy Sauce Fermentation with Cordyceps militaris: Process Optimization and Functional Profiling. Foods, 14(15), 2711. https://doi.org/10.3390/foods14152711






