Emerging Trends in Active Packaging for Food: A Six-Year Review
Abstract
1. Introduction
2. Biopolymers
3. Active Compounds
3.1. Essential Oils
3.1.1. Sources and Composition
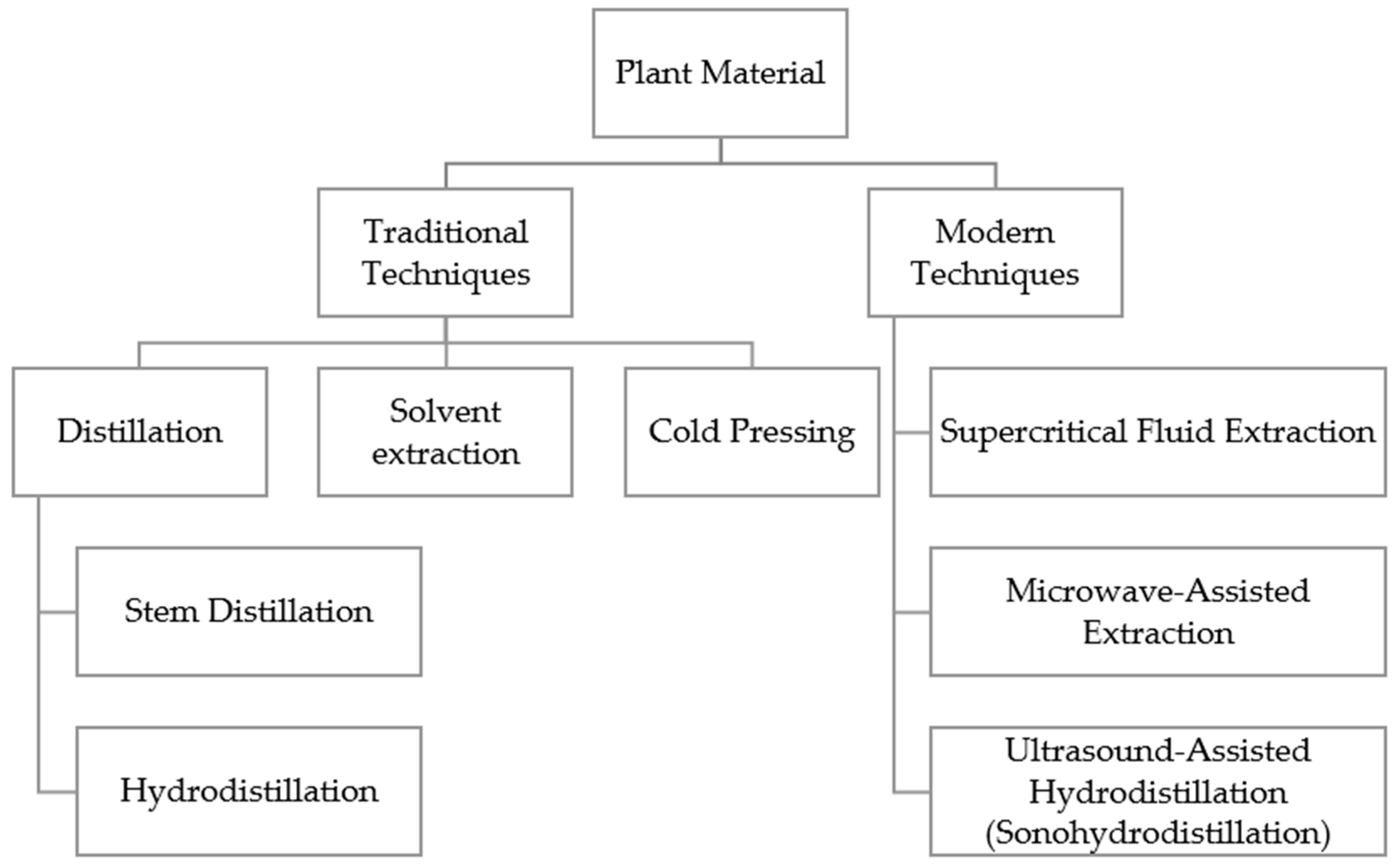
3.1.2. Extraction Methods
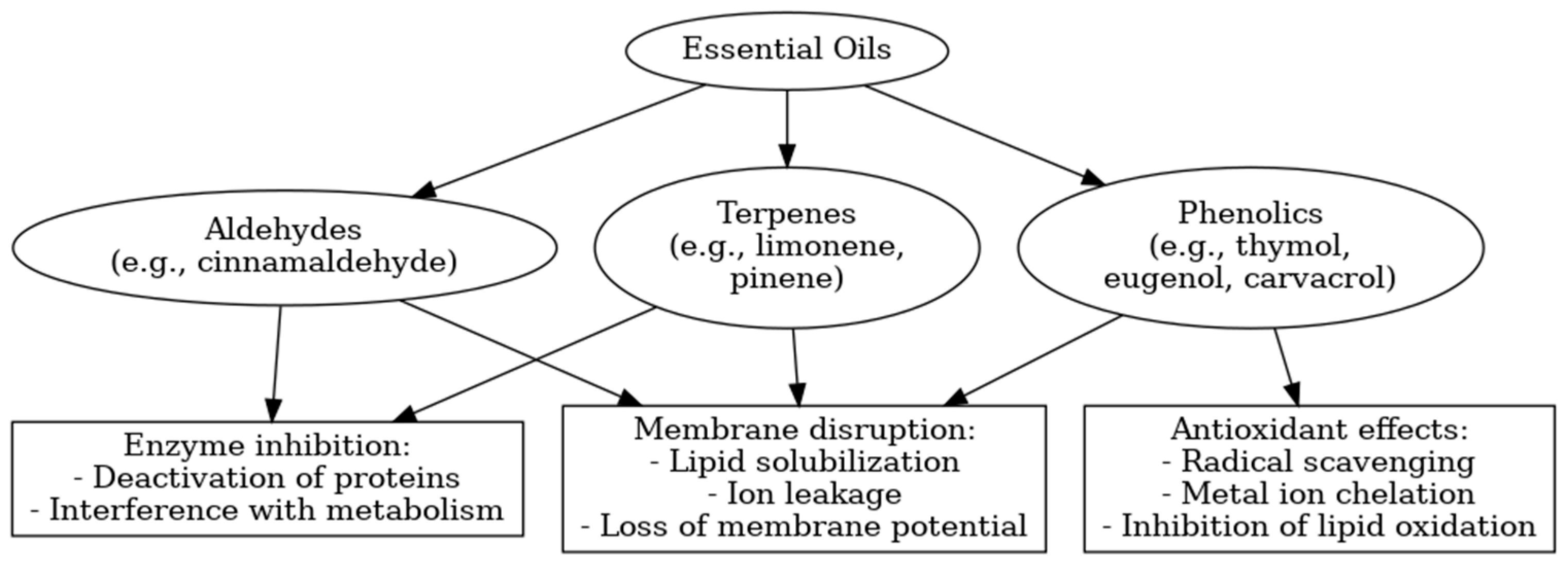
3.1.3. Bioactivity and Mechanisms of Action
3.1.4. Applications for Active Packaging
Innovations in Encapsulation and Controlled Release
Synergistic Combinations and Functional Blends
Polymer–EO Interactions and Performance
3.1.5. Limitations and Technological Solutions
3.1.6. Patent Developments
3.1.7. Regulatory, Economic, and Consumer Acceptance Challenges
3.2. Natural Extracts
3.2.1. Sources and Composition
3.2.2. Extraction Methods
3.2.3. Bioactivity and Mechanisms of Action
3.2.4. Applications for Active Packaging
3.3. Phenolic Compounds
3.3.1. Bioactivity and Mechanisms of Action
3.3.2. Applications for Active Packaging
3.3.3. Limitations and Technological Solutions
4. Examples of Application of New Active Food-Packaging Materials
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EO | Essential oil |
| UV | Ultraviolet radiation |
| IR | Infrared radiation |
| MAE | Microwave-assisted extraction |
| UAE | Ultrasound-assisted extraction |
| SFE | Supercritical fluid extraction |
| PLE | Pressurized liquid extraction |
| PLA | Poly (lactic acid) |
| MDA | Malonaldehyde |
| CS | Chitosan |
| CO | Clove oil |
| NI | Nisin |
| TEO | Turmeric essential oil |
| PVA | Poly(vinyl alcohol) |
| PEG | Poly(ethylene glycol) |
| PVP | Polyvinylpyrrolidone |
References
- European Parliament and Council. Regulation (EC) No 1935/2004 on Materials and Articles Intended to Come into Contact with Food. 2004. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32004R1935 (accessed on 8 June 2025).
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef]
- European Parliament and Council; European Commission; European Parliament and Council. European Commission Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on Food Additives. Off. J. Eur. Union. 2008, 354, 16–33. [Google Scholar]
- Barzegar, F.; Nabizadeh, S.; Kamankesh, M.; Ghasemi, J.B.; Mohammadi, A. Recent Advances in Natural Product-Based Nanoemulsions as Promising Substitutes for Hazardous Synthetic Food Additives: A New Revolution in Food Processing. Food Bioproc Tech. 2024, 17, 1087–1108. [Google Scholar] [CrossRef]
- Bautista-Hernández, I.; Gómez-García, R.; Martínez-Ávila, G.C.G.; Medina-Herrera, N.; González-Hernández, M.D. Unlocking Essential Oils’ Potential as Sustainable Food Additives: Current State and Future Perspectives for Industrial Applications. Sustainability 2025, 17, 2053. [Google Scholar] [CrossRef]
- Chen, X.; Shang, S.; Yan, F.; Jiang, H.; Zhao, G.; Tian, S.; Chen, R.; Chen, D.; Dang, Y. Antioxidant Activities of Essential Oils and Their Major Components in Scavenging Free Radicals, Inhibiting Lipid Oxidation and Reducing Cellular Oxidative Stress. Molecules 2023, 28, 4559. [Google Scholar] [CrossRef]
- Chen, X.; Lan, W.; Xie, J. Natural Phenolic Compounds: Antimicrobial Properties, Antimicrobial Mechanisms, and Potential Utilization in the Preservation of Aquatic Products. Food Chem. 2024, 440, 138198. [Google Scholar] [CrossRef]
- de la Rosa, L.A.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; Alvarez-Parrilla, E. Phenolic Compounds. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia, E.M., Ed.; Woodhead Publishing, Elsevier: Amsterdam, The Netherlands, 2019; pp. 253–271. ISBN 9780128132784. [Google Scholar]
- Ribeiro-Santos, R.; Andrade, M.; Sanches-Silva, A. Essential Oils. In Food Additives and Human Health; Bentham Science Publishers: Sharjah, United Arab Emirates, 2020; pp. 104–119. [Google Scholar]
- Ríos, J.-L. Essential Oils: What They Are and How the Terms Are Used and Defined. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 3–10. ISBN 9780124166417. [Google Scholar]
- Andrade, M.A.; Barbosa, C.H.; Shah, M.A.; Ahmad, N.; Vilarinho, F.; Khwaldia, K.; Silva, A.S.; Ramos, F. Citrus By-Products: Valuable Source of Bioactive Compounds for Food Applications. Antioxidants 2022, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Stobiecki, M.; Kachlicki, P. Isolation and Identification of Flavonoids. In The Science of Flavonoids; Springer: Berlin/Heidelberg, Germany, 2006; pp. 47–69. [Google Scholar]
- Cheynier, V. Phenolic Compounds: From Plants to Foods. Phytochem. Rev. 2012, 11, 153–177. [Google Scholar] [CrossRef]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Phenols, Polyphenols and Tannins: An Overview. In Plant Secondary Metabolites; Wiley: Hoboken, NJ, USA, 2006; pp. 1–24. [Google Scholar]
- Gómez-Contreras, P.; Figueroa-Lopez, K.J.; Hernández-Fernández, J.; Cortés Rodríguez, M.; Ortega-Toro, R. Effect of Different Essential Oils on the Properties of Edible Coatings Based on Yam (Dioscorea Rotundata, L.) Starch and Its Application in Strawberry (Fragaria Vesca L.) Preservation. Appl. Sci. 2021, 11, 11057. [Google Scholar] [CrossRef]
- Sabry, R.; Sayed, A.; El-Sayed, I.E.T.; Mahmoud, G.A. Optimizing Pectin-Based Biofilm Properties for Food Packaging via E-Beam Irradiation. Radiat. Phys. Chem. 2025, 229, 112474. [Google Scholar] [CrossRef]
- Liang, W.; Ge, X.; Lin, Q.; Niu, L.; Zhao, W.; Muratkhan, M.; Li, W. Ternary Composite Degradable Plastics Based on Alpinia Galanga Essential Oil Pickering Emulsion Templates: A Potential Multifunctional Active Packaging. Int. J. Biol. Macromol. 2024, 257, 128580. [Google Scholar] [CrossRef] [PubMed]
- Wardak, M.H.; Nkede, F.N.; Van, T.T.; Meng, F.; Xirui, Y.; Jothi, J.S.; Tanaka, F.; Tanaka, F. Development of a Coating Material Composed of Sodium Alginate and Kiwifruit Seed Essential Oil to Enhance Persimmon Fruit Quality Using a Novel Partial Coating Technique. Food Packag. Shelf Life 2024, 45, 101331. [Google Scholar] [CrossRef]
- Hong, S.J.; Riahi, Z.; Shin, G.H.; Kim, J.T. Development of Innovative Active Packaging Films Using Gelatin/Pullulan-Based Composites Incorporated with Cinnamon Essential Oil-Loaded Metal-Organic Frameworks for Meat Preservation. Int. J. Biol. Macromol. 2024, 267, 131606. [Google Scholar] [CrossRef] [PubMed]
- Sreekanth, K.; Sharath, K.P.; Midhun Dominic, C.D.; Divya, M.; Radhakrishnan, E.K. Microbial Load Reduction in Stored Raw Beef Meat Using Chitosan/Starch-Based Active Packaging Films Incorporated with Cellulose Nanofibers and Cinnamon Essential Oil. Meat Sci. 2024, 216, 109552. [Google Scholar] [CrossRef]
- Singh, A.; Ahuja, A.; Madan, M.; Singh, D.; Rastogi, V.K. Active Packaging Film of Poly(Lactic Acid) Incorporated with Plant-Based Essential Oils of Trachyspermum Ammi as an Antimicrobial Agent and Vanilla as an Aroma Corrector for Waffles. Int. J. Biol. Macromol. 2024, 278, 135086. [Google Scholar] [CrossRef]
- Gao, Y.; Li, R.; Wang, J.; Xu, H.; Wang, M.; Wang, H. Development of κ-Carrageenan/Tourmaline Composite for Active Food Packaging Applications: Improved Mechanical, Gas Barrier, and Antimicrobial. Carbohydr. Polym. 2025, 354, 123304. [Google Scholar] [CrossRef]
- Lima, D.A.S.; Grisi, C.V.B.; Florentino, G.I.B.; Santos, M.M.F.; Galvao, M.d.S.; Madruga, M.S.; Silva, F.A.P. da Development and Characterization of an Aromatic and Antioxidant Active Film Based on Myofibrillar Protein from Fish By-Products and Passion Fruit Essential Oil. Food Chem. 2025, 474, 143125. [Google Scholar] [CrossRef]
- Wu, C.; Wang, Y.; Gong, Y.; Yang, W.; Zhang, X.; Zhao, Y.; Wu, D. Preparation, Characterisation, and Application of Gelatin/Sodium Carboxymethyl Cellulose/Peach Gum Ternary Composite Microcapsules for Encapsulating Sweet Orange Essential Oil. Int. J. Biol. Macromol. 2025, 299, 140218. [Google Scholar] [CrossRef]
- Censi, V.; Saiano, F.; Bongiorno, D.; Indelicato, S.; Napoli, A.; Piazzese, D. Bioplastics: A New Analytical Challenge. Front. Chem. 2022, 10, 199–206. [Google Scholar] [CrossRef]
- Mastalygina, E.E.; Aleksanyan, K.V. Recent Approaches to the Plasticization of Poly(Lactic Acid) (PLA) (A Review). Polymers 2023, 16, 87. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, L.; Zhang, Y.; Huang, Y.; Cao, J.; Jiang, W. Antimicrobial and Controlled Release Properties of Nanocomposite Film Containing Thymol and Carvacrol Loaded UiO-66-NH2 for Active Food Packaging. Food Chem. 2023, 404, 134427. [Google Scholar] [CrossRef] [PubMed]
- Gumienna, M.; Górna, B. Antimicrobial Food Packaging with Biodegradable Polymers and Bacteriocins. Molecules 2021, 26, 3735. [Google Scholar] [CrossRef]
- Vianna, T.C.; Marinho, C.O.; Marangoni Júnior, L.; Ibrahim, S.A.; Vieira, R.P. Essential Oils as Additives in Active Starch-Based Food Packaging Films: A Review. Int. J. Biol. Macromol. 2021, 182, 1803–1819. [Google Scholar] [CrossRef]
- Falleh, H.; Ben Jemaa, M.; Saada, M.; Ksouri, R. Essential Oils: A Promising Eco-Friendly Food Preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef] [PubMed]
- Han Lyn, F.; Ismail-Fitry, M.R.; Noranizan, M.A.; Tan, T.B.; Nur Hanani, Z.A. Recent Advances in Extruded Polylactic Acid-Based Composites for Food Packaging: A Review. Int. J. Biol. Macromol. 2024, 266, 131340. [Google Scholar] [CrossRef]
- Jadhav, J.J.; Jadeja, G.C.; Desai, M.A. Ultrasound-Assisted Hydrodistillation for Extraction of Essential Oil from Clove Buds—A Step towards Process Improvement and Sustainable Outcome. Chem. Eng. Process. Process Intensif. 2023, 189, 109404. [Google Scholar] [CrossRef]
- Kalkan, S.; Ergínkaya, Z. Impact of Whey Protein Isolate Coatings Containing Different Antimicrobial Agents on Sliced Bologna-Type Sausage during Refrigerated Storage. Food Sci. Technol. 2020, 40, 136–145. [Google Scholar] [CrossRef]
- Surendhiran, D.; Roy, V.C.; Park, J.-S.; Chun, B.-S. Fabrication of Chitosan-Based Food Packaging Film Impregnated with Turmeric Essential Oil (TEO)-Loaded Magnetic-Silica Nanocomposites for Surimi Preservation. Int. J. Biol. Macromol. 2022, 203, 650–660. [Google Scholar] [CrossRef]
- Sethunga, M.; Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S.; Munaweera, I. Antimicrobial and Antioxidative Electrospun Cellulose Acetate-Essential Oils Nanofibrous Membranes for Active Food Packaging to Extend the Shelf Life of Perishable Fruits. Innov. Food Sci. Emerg. Technol. 2024, 97, 103802. [Google Scholar] [CrossRef]
- Khazani, B.; Almasi, H.; Mohtarami, F.; Amjadi, S. Incorporation of Artemisia Essential Oil Loaded Chitosomes in Salep Based Film for Use in Toast Bread Packaging: New Generation of Active Films. Food Packag. Shelf Life 2024, 43, 101305. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Z.; Jin, X.; Hu, J.; Tao, Y.; Lu, J.; Xia, X.; Tan, M.; Du, J.; Wang, H. Structurally Robust Chitosan-Based Active Packaging Film by Pickering Emulsion Containing Tree Essential Oil for Pork Preservation. Food Chem. 2025, 466, 142246. [Google Scholar] [CrossRef]
- Zhu, C.-Y.; Li, K.; Wang, Y.; Du, M.-T.; Chen, B.; Wang, Y.-T.; Zhou, Y.-F.; Bai, Y.-H. Antioxidant and Antimicrobial PSE-like Chicken Protein Isolate Films Loaded with Oregano Essential Oil Nanoemulsion for Pork Preservation. Food Chem. 2025, 475, 143355. [Google Scholar] [CrossRef]
- Zhong, C.; Bao, S.; Shen, K.; Shu, M.; Geng, J.; Wu, G. Characterization and Coating Application of Composite Gelatin Packaging Containing Eucalyptus Leaf Essential Oil Liposome and Phage Endolysin for Preservation of Pacific White Shrimp (Penaeus Vannamei). Food Control 2025, 169, 111017. [Google Scholar] [CrossRef]
- da Costa, R.C.; Ineichen, A.P.; Teixeira, C.D.S.; Bellettini, I.C.; Carli, L.N. Release of Oregano Essential Oil from PHBV Films in Simulated Food Conditionsa. Polímeros 2022, 32, e2022028. [Google Scholar] [CrossRef]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- De-Montijo-Prieto, S.; Razola-Díaz, M.d.C.; Gómez-Caravaca, A.M.; Guerra-Hernandez, E.J.; Jiménez-Valera, M.; Garcia-Villanova, B.; Ruiz-Bravo, A.; Verardo, V. Essential Oils from Fruit and Vegetables, Aromatic Herbs, and Spices: Composition, Antioxidant, and Antimicrobial Activities. Biology 2021, 10, 1091. [Google Scholar] [CrossRef] [PubMed]
- Abers, M.; Schroeder, S.; Goelz, L.; Sulser, A.; Rose, T.S.; Puchalski, K.; Langland, J. Antimicrobial Activity of the Volatile Substances from Essential Oils. BMC Complement. Med. Ther. 2021, 21, 124. [Google Scholar] [CrossRef]
- Kholiya, S.; Singh, M.; Chauhan, A.; Padalia, R.C.; Tiwari, A. Chemical Profiling and Bioactivity Analysis of Shoots and Roots Essential Oil of Indian Blumea Mollis (D. Don) Merr. Biochem. Syst. Ecol. 2024, 117, 104913. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front. Microbiol. 2012, 3, 37–60. [Google Scholar] [CrossRef]
- Yousuf, B.; Wu, S.; Siddiqui, M.W. Incorporating Essential Oils or Compounds Derived Thereof into Edible Coatings: Effect on Quality and Shelf Life of Fresh/Fresh-Cut Produce. Trends Food Sci. Technol. 2021, 108, 245–257. [Google Scholar] [CrossRef]
- de Souza Junior, E.T.; Siqueira, L.M.; Almeida, R.N.; Lucas, A.M.; Silva, C.G.F.d.; Cassel, E.; Vargas, R.M.F. Comparison of Different Extraction Techniques of Zingiber Officinale Essential Oil. Braz. Arch. Biol. Technol. 2020, 63, e20190213. [Google Scholar] [CrossRef]
- Xu, X.; Yang, S.; Lu, Q.; Zhu, M.; Chen, C. The Property Differences of Essential Oils Extracted by Two Methods from Dolichos Lablab Flowers: Chemical Composition, Anti-Microorganism and Cholinesterase Inhibition Activities. Sustain. Chem. Pharm. 2024, 42, 101843. [Google Scholar] [CrossRef]
- Miloudi, S.; Abbad, I.; Soulaimani, B.; Ferradous, A.; Abbad, A.; El Mouden, E.H. Optimization of Herbicidal Activity of Essential Oil Mixtures from Satureja Alpina, Thymus Satureioides and Myrtus Communis on Seed Germination and Post-Emergence Growth of Amaranthus Retroflexus L. Crop Prot. 2024, 180, 106642. [Google Scholar] [CrossRef]
- Manssouri, M.; Znini, M.; Majidi, L. Studies on the antioxidant activity of essential oil and various extracts of Ammodaucus leucotrichus Coss. & Dur. Fruits from Morocco. J. Taibah Univ. Sci. 2020, 14, 124–130. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Lee, D.-S.; Cho, S.-M.; Kim, J.-C.; Park, M.-J.; Choi, I.-G. Antioxidant Properties of 7 Domestic Essential Oils and Identification of Physiologically Active Components of Essential Oils against Candida Albicans. J. Korean Wood Sci. Technol. 2021, 49, 23–43. [Google Scholar] [CrossRef]
- Yasir, M.; Nawaz, A.; Ghazanfar, S.; Okla, M.K.; Chaudhary, A.; Al, W.H.; Ajmal, M.N.; AbdElgawad, H.; Ahmad, Z.; Abbas, F.; et al. Anti-Bacterial Activity of Essential Oils against Multidrug-Resistant Foodborne Pathogens Isolated from Raw Milk. Braz. J. Biol. 2024, 84, e259449. [Google Scholar] [CrossRef]
- Mangalagiri, N.P.; Panditi, S.K.; Jeevigunta, N.L.L. Antimicrobial Activity of Essential Plant Oils and Their Major Components. Heliyon 2021, 7, e06835. [Google Scholar] [CrossRef]
- Targino de Souza Pedrosa, G.; Pimentel, T.C.; Gavahian, M.; Lucena de Medeiros, L.; Pagán, R.; Magnani, M. The Combined Effect of Essential Oils and Emerging Technologies on Food Safety and Quality. LWT 2021, 147, 111593. [Google Scholar] [CrossRef]
- Alvarenga, J.P.; Braga, A.F.; Pacheco, F.V.; de Carvalho, A.A.; Pinto, J.E.B.P.; Bertolucci, S.K.V. Seasonal Variation in Essential Oil Content and Chemical Profile of Mint in Southeast of Brazil. Ciência Rural. 2021, 51, e20200979. [Google Scholar] [CrossRef]
- Cipriano, R.R.; Maia, B.H.L.N.S.; Deschamps, C. Chemical Variability of Essential Oils of Eugenia Uniflora L. Genotypes and Their Antioxidant Activity. Acad. Bras. Cienc. 2021, 93, e20181299. [Google Scholar] [CrossRef]
- Assaggaf, H.M.; Naceiri Mrabti, H.; Rajab, B.S.; Attar, A.A.; Alyamani, R.A.; Hamed, M.; El Omari, N.; El Menyiy, N.; Hazzoumi, Z.; Benali, T.; et al. Chemical Analysis and Investigation of Biological Effects of Salvia Officinalis Essential Oils at Three Phenological Stages. Molecules 2022, 27, 5157. [Google Scholar] [CrossRef]
- dos Santos, S.M.; Cardoso, C.A.L.; de Oliveira Junior, P.C.; da Silva, M.E.; Pereira, Z.V.; Silva, R.M.M.F.; Formagio, A.S.N. Seasonal and Geographical Variation in the Chemical Composition of Essential Oil from Allophylus Edulis Leaves. S. Afr. J. Bot. 2023, 154, 41–45. [Google Scholar] [CrossRef]
- Gandomi Hosnaroodi, V.; Ghavam, M. Evaluation of the Effect of Soil and Irrigation Water Characteristics on the Chemical Composition and Antimicrobial Activity of Mentha Spicata L. Essential Oil: A Plant Used in Traditional Medicine of Kashan People. Agric. Water Manag. 2025, 309, 109331. [Google Scholar] [CrossRef]
- Qian, Q.; Zhuo, Z.; Peng, Y.; Xu, D. Chemical Composition Variation in Essential Oil and Their Correlation with Climate Factors in Chinese Prickly Ash Peels (Zanthoxylum Armatum DC.) from Different Habitats. Molecules 2024, 29, 1343. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.F.; Hammer, K.A. Chemistry and Bioactivity of Essential Oils. In Lipids and Essential Oils as Antimicrobial Agents; Wiley: Hoboken, NJ, USA, 2011; pp. 203–238. [Google Scholar]
- Eslahi, H.; Fahimi, N.; Sardarian, A.R. Chemical Composition of Essential Oils. In Essential Oils in Food Processing; Wiley: Hoboken, NJ, USA, 2017; pp. 119–171. [Google Scholar]
- Zhang, J.; Zhang, M.; Bhandari, B.; Wang, M. Basic Sensory Properties of Essential Oils from Aromatic Plants and Their Applications: A Critical Review. Crit. Rev. Food Sci. Nutr. 2024, 64, 6990–7003. [Google Scholar] [CrossRef]
- Meng, F.; Lei, Y.; Zhang, Q.; Li, Y.; Chen, W.; Liu, D. Encapsulation of Zanthoxylum Bungeanum Essential Oil to Enhance Flavor Stability and Inhibit Lipid Oxidation of Chinese-style Sausage. J. Sci. Food Agric. 2022, 102, 4035–4045. [Google Scholar] [CrossRef]
- Soetjipto, H.; Aminu, N.R. Positive and Negative Impacts of the Use of Essential Oils in Food. In Essential Oils; Springer International Publishing: Cham, Switzerland, 2022; pp. 191–217. [Google Scholar]
- Cesca, R.S.; Fonseca, G.G.; da Paz, M.F.; Cortez-Vega, W.R. Advances and Perspectives on the Application of Essential Oils in Food Packaging Films, Coatings, and Nanoencapsulated Materials. Bragantia 2024, 83, e20230132. [Google Scholar] [CrossRef]
- De Farias, P.M.; De Sousa, R.V.; Maniglia, B.C.; Pascall, M.; Matthes, J.; Sadzik, A.; Schmid, M.; Fai, A.E.C. Biobased Food Packaging Systems Functionalized with Essential Oil via Pickering Emulsion: Advantages, Challenges, and Current Applications. ACS Omega 2025, 10, 4173–4186. [Google Scholar] [CrossRef]
- Vilela, C.; Kurek, M.; Hayouka, Z.; Röcker, B.; Yildirim, S.; Antunes, M.D.C.; Nilsen-Nygaard, J.; Pettersen, M.K.; Freire, C.S.R. A Concise Guide to Active Agents for Active Food Packaging. Trends Food Sci. Technol. 2018, 80, 212–222. [Google Scholar] [CrossRef]
- Tiekstra, S.; Dopico-Parada, A.; Koivula, H.; Lahti, J.; Buntinx, M. Holistic Approach to a Successful Market Implementation of Active and Intelligent Food Packaging. Foods 2021, 10, 465. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, C.; Rhein, S.; Sträter, K.F. Consumers’ Sustainability-Related Perception of and Willingness-to-Pay for Food Packaging Alternatives. Resour. Conserv. Recycl. 2022, 181, 106219. [Google Scholar] [CrossRef]
- Deshmukh, R.K.; Gaikwad, K.K. Natural Antimicrobial and Antioxidant Compounds for Active Food Packaging Applications. Biomass Convers. Biorefin. 2024, 14, 4419–4440. [Google Scholar] [CrossRef]
- Dutta, D.; Sit, N. Application of Natural Extracts as Active Ingredient in Biopolymer Based Packaging Systems. J. Food Sci. Technol. 2023, 60, 1888–1902. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, L.; Mao, C.; Jin, L.; Wu, S.; Zheng, Y.; Cui, Z.; Li, Z.; Zhang, Y.; Zhu, S.; et al. Natural Extracts for Antibacterial Applications. Small 2024, 20, e2306553. [Google Scholar] [CrossRef]
- Xie, Q.; Liu, G.; Zhang, Y.; Yu, J.; Wang, Y.; Ma, X. Active Edible Films with Plant Extracts: A Updated Review of Their Types, Preparations, Reinforcing Properties, and Applications in Muscle Foods Packaging and Preservation. Crit. Rev. Food Sci. Nutr. 2023, 63, 11425–11447. [Google Scholar] [CrossRef]
- Sheibani, S.; Jafarzadeh, S.; Qazanfarzadeh, Z.; Osadee Wijekoon, M.M.J.; Mohd Rozalli, N.H.; Mohammadi Nafchi, A. Sustainable Strategies for Using Natural Extracts in Smart Food Packaging. Int. J. Biol. Macromol. 2024, 267, 131537. [Google Scholar] [CrossRef]
- Pappas, V.M.; Samanidis, I.; Stavropoulos, G.; Athanasiadis, V.; Chatzimitakos, T.; Bozinou, E.; Makris, D.P.; Lalas, S.I. Analysis of Five-Extraction Technologies’ Environmental Impact on the Polyphenols Production from Moringa Oleifera Leaves Using the Life Cycle Assessment Tool Based on ISO 14040. Sustainability 2023, 15, 2328. [Google Scholar] [CrossRef]
- Peron, G.; Ferrarese, I.; Carmo Dos Santos, N.; Rizzo, F.; Gargari, G.; Bertoli, N.; Gobbi, E.; Perosa, A.; Selva, M.; Dall’Acqua, S. Sustainable Extraction of Bioactive Compounds and Nutrients from Agri-Food Wastes: Potential Reutilization of Berry, Honey, and Chicory Byproducts. Appl. Sci. 2024, 14, 10785. [Google Scholar] [CrossRef]
- Chaari, M.; Elhadef, K.; Akermi, S.; Ben Akacha, B.; Fourati, M.; Chakchouk Mtibaa, A.; Ennouri, M.; Sarkar, T.; Shariati, M.A.; Rebezov, M.; et al. Novel Active Food Packaging Films Based on Gelatin-Sodium Alginate Containing Beetroot Peel Extract. Antioxidants 2022, 11, 2095. [Google Scholar] [CrossRef]
- Rodrigues Arruda, T.; Campos Bernardes, P.; Robledo Fialho e Moraes, A.; de Fátima Ferreira Soares, N. Natural Bioactives in Perspective: The Future of Active Packaging Based on Essential Oils and Plant Extracts Themselves and Those Complexed by Cyclodextrins. Food Res. Int. 2022, 156, 111160. [Google Scholar] [CrossRef] [PubMed]
- Mellinas, A.C.; Jiménez, A.; Garrigós, M.C. Pectin-Based Films with Cocoa Bean Shell Waste Extract and ZnO/Zn-NPs with Enhanced Oxygen Barrier, Ultraviolet Screen and Photocatalytic Properties. Foods 2020, 9, 1572. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L.; Cocoletzi, H.H. Mango Leaf Extract Incorporated Chitosan Antioxidant Film for Active Food Packaging. Int. J. Biol. Macromol. 2019, 126, 1234–1243. [Google Scholar] [CrossRef]
- Todhanakasem, T.; Jaiprayat, C.; Sroysuwan, T.; Suksermsakul, S.; Suwapanich, R.; Maleenont, K.K.; Koombhongse, P.; Young, B.M. Active Thermoplastic Starch Film with Watermelon Rind Extract for Future Biodegradable Food Packaging. Polymers 2022, 14, 3232. [Google Scholar] [CrossRef]
- Andrade, M.A.; Ribeiro-Santos, R.; Guerra, M.; Sanches-Silva, A. Evaluation of the Oxidative Status of Salami Packaged with an Active Whey Protein Film. Foods 2019, 8, 387. [Google Scholar] [CrossRef]
- Istiqomah, A.; Prasetyo, W.E.; Firdaus, M.; Kusumaningsih, T. Antibacterial Evaluation of Garlic Extracts on Chitosan/Starch Packaging Film Using Response Surface Methodology and Its Application for Shelf-Life Extension of Bell Peppers (Capsicum Annuum). J. Food Sci. 2024, 14, 6523–6538. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ye, G.; Jia, S.; Ma, H.; Jia, D.; He, J.; Lv, J.; Chen, X.; Liu, F.; Gou, K.; et al. Preparation of Chitosan/Peony (Paeonia Suffruticosa Andr.) Leaf Extract Composite Film and Its Application in Sustainable Active Food Packaging. Int. J. Biol. Macromol. 2022, 222, 2200–2211. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zhang, B.; Zhang, X.; Ma, Z.; Feng, X. Incorporating Portulaca Oleracea Extract Endows the Chitosan-Starch Film with Antioxidant Capacity for Chilled Meat Preservation. Food Chem. X 2023, 18, 100662. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Concept, Mechanism, and Applications of Phenolic Antioxidants in Foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Andrade, M.; Madella, D.; Martinazzo, A.P.; de Aquino Garcia Moura, L.; de Melo, N.R.; Sanches-Silva, A. Revisiting an Ancient Spice with Medicinal Purposes: Cinnamon. Trends Food Sci. Technol. 2017, 62, 154–169. [Google Scholar] [CrossRef]
- Sun, J.; Leng, X.; Zang, J.; Zhao, G. Bio-Based Antibacterial Food Packaging Films and Coatings Containing Cinnamaldehyde: A Review. Crit. Rev. Food Sci. Nutr. 2024, 64, 140–152. [Google Scholar] [CrossRef]
- Doyle, A.A.; Stephens, J.C. A Review of Cinnamaldehyde and Its Derivatives as Antibacterial Agents. Fitoterapia 2019, 139, 104405. [Google Scholar] [CrossRef]
- Peng, J.; Song, X.; Yu, W.; Pan, Y.; Zhang, Y.; Jian, H.; He, B. The Role and Mechanism of Cinnamaldehyde in Cancer. J. Food Drug Anal. 2024, 32, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Aragón-Gutiérrez, A.; Heras-Mozos, R.; Gallur, M.; López, D.; Gavara, R.; Hernández-Muñoz, P. Hot-Melt-Extruded Active Films Prepared from EVOH/Trans-Cinnamaldehyde Blends Intended for Food Packaging Applications. Foods 2021, 10, 1591. [Google Scholar] [CrossRef]
- Yu, H.; Huang, X.; Zhou, L.; Wang, Y. Incorporation of Cinnamaldehyde, Carvacrol, and Eugenol into Zein Films for Active Food Packaging: Enhanced Mechanical Properties, Antimicrobial Activity, and Controlled Release. J. Food Sci. Technol. 2023, 60, 2846–2857. [Google Scholar] [CrossRef]
- Mohammadi, M.; Fasihi, M. Eco-Friendly Polylactic Acid/Modified Thermoplastic Starch Films Enhanced with Clove Essential Oil and Cochineal for Dual-Functional Active and Intelligent Food Packaging. Carbohydr. Polym. 2025, 354, 123320. [Google Scholar] [CrossRef] [PubMed]
- Doğan, C.; Akgul, Y.; Eticha, A.K.; Doğan, N.; Toptas, A. Innovative Electroblown Nanofibrous Mats Incorporating Sage Essential Oil for Extending Shelf Life of Spreadable Cheese. Food Bioprod. Process. 2025, 150, 338–349. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Lekjing, S. A Chitosan-Based Edible Film with Clove Essential Oil and Nisin for Improving the Quality and Shelf Life of Pork Patties in Cold Storage. RSC Adv. 2020, 10, 17777–17786. [Google Scholar] [CrossRef]
- Molina-Hernández, J.B.; Echeverri Castro, A.; Martinez-Correa, H.A.; Andrade-Mahecha, M.M. Edible Coating Based on Achira Starch Containing Garlic/Oregano Oils to Extend the Shelf Life of Double Cream Cheese. Rev. Fac. Nac. Agron. Medellin 2020, 73, 9099–9108. [Google Scholar] [CrossRef]
- Min, T.; Sun, X.; Yuan, Z.; Zhou, L.; Jiao, X.; Zha, J.; Zhu, Z.; Wen, Y. Novel Antimicrobial Packaging Film Based on Porous Poly(Lactic Acid) Nanofiber and Polymeric Coating for Humidity-Controlled Release of Thyme Essential Oil. LWT 2021, 135, 110034. [Google Scholar] [CrossRef]
- Balan, G.C.; Paulo, A.F.S.; Correa, L.G.; Alvim, I.D.; Ueno, C.T.; Coelho, A.R.; Ströher, G.R.; Yamashita, F.; Sakanaka, L.S.; Shirai, M.A. Production of Wheat Flour/PBAT Active Films Incorporated with Oregano Oil Microparticles and Its Application in Fresh Pastry Conservation. Food Bioproc. Technol. 2021, 14, 1587–1599. [Google Scholar] [CrossRef]
- Vasile, C.; Stoleru, E.; Darie-Niţa, R.N.; Dumitriu, R.P.; Pamfil, D.; Tarţau, L. Biocompatible Materials Based on Plasticized Poly(Lactic Acid), Chitosan and Rosemary Ethanolic Extract I. Effect of Chitosan on the Properties of Plasticized Poly(Lactic Acid) Materials. Polymers 2019, 11, 941. [Google Scholar] [CrossRef]
- Borzi, F.; Torrieri, E.; Wrona, M.; Nerín, C. Polyamide Modified with Green Tea Extract for Fresh Minced Meat Active Packaging Applications. Food Chem. 2019, 300, 125242. [Google Scholar] [CrossRef]
- Heras-Mozos, R.; Muriel-Galet, V.; López-Carballo, G.; Catalá, R.; Hernández-Muñoz, P.; Gavara, R. Development and Optimization of Antifungal Packaging for Sliced Pan Loaf Based on Garlic as Active Agent and Bread Aroma as Aroma Corrector. Int. J. Food Microbiol. 2019, 290, 42–48. [Google Scholar] [CrossRef] [PubMed]
- dos Passos Braga, S.; Magnani, M.; Madruga, M.S.; de Souza Galvão, M.; de Medeiros, L.L.; Batista, A.U.D.; Dias, R.T.A.; Fernandes, L.R.; de Medeiros, E.S.; de Souza, E.L. Characterization of Edible Coatings Formulated with Chitosan and Mentha Essential Oils and Their Use to Preserve Papaya (Carica Papaya L.). Innov. Food Sci. Emerg. Technol. 2020, 65, 102472. [Google Scholar] [CrossRef]
- Punia Bangar, S.; Whiteside, W.S.; Ozogul, F.; Dunno, K.D.; Cavender, G.A.; Dawson, P. Development of Starch-Based Films Reinforced with Cellulosic Nanocrystals and Essential Oil to Extend the Shelf Life of Red Grapes. Food Biosci. 2022, 47, 101621. [Google Scholar] [CrossRef]
- Ran, C.; Li, Q.; Zhao, M.; Cui, H.; Yang, Y.; Diao, K.; Liu, Y.; Lu, S.; Dong, J.; Wang, Q. Gelatin/Polyvinyl Alcohol Films Loaded with Doubly Stabilized Clove Essential Oil Chitosomes: Preparation, Characterization, and Application in Packing Marinated Steaks. Food Chem. 2024, 460, 140673. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, C.; Wang, J.; Xie, J. Effects of Orthogonal Dual-Frequency Ultrasound-Assisted Treatment Combined with Bioactive Coating Containing Melissa Officinalis L. Essential Oil on Changes in Quality, Lipid, and Protein of Large Yellow Croaker (Pseudosciaena Crocea) during Cold Storage. Food Chem. X 2024, 24, 101861. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, C.; Chen, Q.; Shi, Z.; Xu, K.; Niu, Y.; Rao, X. Preparation of Dehydroabietic Acid Modified Chitosan/Wintergreen Essential Oil Film and Mandarin Freshness Preservation Study. Food Chem. 2025, 464, 141836. [Google Scholar] [CrossRef]
- Kan, J.; Liu, J.; Yong, H.; Liu, Y.; Qin, Y.; Liu, J. Development of Active Packaging Based on Chitosan-Gelatin Blend Films Functionalized with Chinese Hawthorn (Crataegus Pinnatifida) Fruit Extract. Int. J. Biol. Macromol. 2019, 140, 384–392. [Google Scholar] [CrossRef]
- Hanif, J.; Khalid, N.; Khan, R.S.; Bhatti, M.F.; Hayat, M.Q.; Ismail, M.; Andleeb, S.; Mansoor, Q.; Khan, F.; Amin, F.; et al. Formulation of Active Packaging System Using Artemisia Scoparia for Enhancing Shelf Life of Fresh Fruits. Mater. Sci. Eng. C 2019, 100, 82–93. [Google Scholar] [CrossRef]
- Sun, M.; Liu, N.; Ni, S.; Bian, H.; Fu, Y.; Chen, X. Poplar Hot Water Extract Enhances Barrier and Antioxidant Properties of Chitosan/Bentonite Composite Film for Packaging Applications. Polymers 2019, 11, 1614. [Google Scholar] [CrossRef]
- Stoll, L.; Rech, R.; Flôres, S.H.; Nachtigall, S.M.B.; de Oliveira Rios, A. Poly(Acid Lactic) Films with Carotenoids Extracts: Release Study and Effect on Sunflower Oil Preservation. Food Chem. 2019, 281, 213–221. [Google Scholar] [CrossRef]
- Kadam, D.; Momin, B.; Palamthodi, S.; Lele, S.S. Physicochemical and Functional Properties of Chitosan-Based Nano-Composite Films Incorporated with Biogenic Silver Nanoparticles. Carbohydr. Polym. 2019, 211, 124–132. [Google Scholar] [CrossRef]
- Hu, X.; Yuan, L.; Han, L.; Li, S.; Song, L. Characterization of Antioxidant and Antibacterial Gelatin Films Incorporated with: Ginkgo Biloba Extract. RSC Adv. 2019, 9, 27449–27454. [Google Scholar] [CrossRef]
- Ahmadi, R.; Tanomand, A.; Kazeminava, F.; Kamounah, F.S.; Ayaseh, A.; Ganbarov, K.; Yousefi, M.; Katourani, A.; Yousefi, B.; Kafil, H.S. Fabrication and Characterization of a Titanium Dioxide (TiO2) Nanoparticles Reinforced Bio-Nanocomposite Containing Miswak (Salvadora Persica L.) Extract—The Antimicrobial, Thermo-Physical and Barrier Properties. Int. J. Nanomed. 2019, 14, 3439–3454. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Lopez, K.J.; Vicente, A.A.; Reis, M.A.M.; Torres-Giner, S.; Lagaron, J.M. Antimicrobial and Antioxidant Performance of Various Essential Oils and Natural Extracts and Their Incorporation into Biowaste Derived Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Layers Made from Electrospun Ultrathin Fibers. Nanomaterials 2019, 9, 144. [Google Scholar] [CrossRef]
- Tran, T.N.; Mai, B.T.; Setti, C.; Athanassiou, A. Transparent Bioplastic Derived from CO2 -Based Polymer Functionalized with Oregano Waste Extract toward Active Food Packaging. ACS Appl. Mater. Interfaces 2020, 12, 46667–46677. [Google Scholar] [CrossRef] [PubMed]
- Kalkan, S.; Otağ, M.R.; Engin, M.S. Physicochemical and Bioactive Properties of Edible Methylcellulose Films Containing Rheum Ribes L. Extract. Food Chem. 2020, 307, 125524. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, X.; Jiang, W. Development of Antioxidant Chitosan Film with Banana Peels Extract and Its Application as Coating in Maintaining the Storage Quality of Apple. Int. J. Biol. Macromol. 2020, 154, 1205–1214. [Google Scholar] [CrossRef]
- Safaei, M.; Roosta Azad, R. Preparation and Characterization of Poly-Lactic Acid Based Films Containing Propolis Ethanolic Extract to Be Used in Dry Meat Sausage Packaging. J. Food Sci. Technol. 2020, 57, 1242–1250. [Google Scholar] [CrossRef]
- Solaberrieta, I.; Jiménez, A.; Cacciotti, I.; Garrigós, M.C. Encapsulation of Bioactive Compounds from Aloe Vera Agrowastes in Electrospun Poly (Ethylene Oxide) Nanofibers. Polymers 2020, 12, 1323. [Google Scholar] [CrossRef]
- Menzel, C.; González-Martínez, C.; Vilaplana, F.; Diretto, G.; Chiralt, A. Incorporation of Natural Antioxidants from Rice Straw into Renewable Starch Films. Int. J. Biol. Macromol. 2020, 146, 976–986. [Google Scholar] [CrossRef]
- Martiny, T.R.; Raghavan, V.; de Moraes, C.C.; da Rosa, G.S.; Dotto, G.L. Bio-Based Active Packaging: Carrageenan Film with Olive Leaf Extract for Lamb Meat Preservation. Foods 2020, 9, 1759. [Google Scholar] [CrossRef]
- Chollakup, R.; Kongtud, W.; Sukatta, U.; Premchookiat, M.; Piriyasatits, K.; Nimitkeatkai, H.; Jarerat, A. Eco-Friendly Rice Straw Paper Coated with Longan (Dimocarpus Longan) Peel Extract as Bio-Based and Antibacterial Packaging. Polymers 2021, 13, 3096. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, X.; Dai, Q.; Qin, Z. Antibacterial, Antioxidation, UV-Blocking, and Biodegradable Soy Protein Isolate Food Packaging Film with Mangosteen Peel Extract and ZnO Nanoparticles. Nanomaterials 2021, 11, 3337. [Google Scholar] [CrossRef]
- Wen, P.; Hu, T.G.; Wen, Y.; Li, K.E.; Qiu, W.P.; He, Z.L.; Wang, H.; Wu, H. Development of Nervilia Fordii Extract-Loaded Electrospun Pva/Pvp Nanocomposite for Antioxidant Packaging. Foods 2021, 10, 1728. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Tanwar, R.; Gupta, V.; Upadhyay, A.; Kumar, A.; Gaikwad, K.K. Pineapple Peel Extract Incorporated Poly(Vinyl Alcohol)-Corn Starch Film for Active Food Packaging: Preparation, Characterization and Antioxidant Activity. Int. J. Biol. Macromol. 2021, 187, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Mileriene, J.; Serniene, L.; Henriques, M.; Gomes, D.; Pereira, C.; Kondrotiene, K.; Kasetiene, N.; Lauciene, L.; Sekmokiene, D.; Malakauskas, M. Effect of Liquid Whey Protein Concentrate–Based Edible Coating Enriched with Cinnamon Carbon Dioxide Extract on the Quality and Shelf Life of Eastern European Curd Cheese. J. Dairy Sci. 2021, 104, 1504–1517. [Google Scholar] [CrossRef] [PubMed]
- Dordevic, S.; Dordevic, D.; Sedlacek, P.; Kalina, M.; Tesikova, K.; Antonic, B.; Tremlova, B.; Treml, J.; Nejezchlebova, M.; Vapenka, L.; et al. Incorporation of Natural Blueberry, Red Grapes and Parsley Extract By-Products into the Production of Chitosan Edible Films. Polymers 2021, 13, 3388. [Google Scholar] [CrossRef]
- Jancikova, S.; Dordevic, D.; Tesikova, K.; Antonic, B.; Tremlova, B. Active Edible Films Fortified with Natural Extracts: Case Study with Fresh-Cut Apple Pieces. Membranes 2021, 11, 684. [Google Scholar] [CrossRef] [PubMed]
- Luciano, C.G.; Tessaro, L.; Bonilla, J.; de Balieiro, J.C.C.; Trindade, M.A.; Sobral, P.J.d.A. Application of Bi-Layers Active Gelatin Films for Sliced Dried-Cured Coppa Conservation. Meat Sci. 2022, 189, 108821. [Google Scholar] [CrossRef] [PubMed]
- Avila, L.B.; Barreto, E.R.C.; Moraes, C.C.; Morais, M.M.; da Rosa, G.S. Promising New Material for Food Packaging: An Active and Intelligent Carrageenan Film with Natural Jaboticaba Additive. Foods 2022, 11, 792. [Google Scholar] [CrossRef]
- Khwaldia, K.; M’Rabet, Y.; Boulila, A. Active Food Packaging Films from Alginate and Date Palm Pit Extract: Physicochemical Properties, Antioxidant Capacity, and Stability. Food Sci. Nutr. 2023, 11, 555–568. [Google Scholar] [CrossRef]
- Rodrigues, P.V.; Vieira, D.M.; Martins, P.C.; Martins, V.G.; Castro, M.C.R.; Machado, A.V. Evaluation of Active LDPE Films for Packaging of Fresh Orange Juice. Polymers 2023, 15, 50. [Google Scholar] [CrossRef]
- Guo, Q.; Yuan, Y.; He, M.; Zhang, X.; Li, L.; Zhang, Y.; Li, B. Development of a Multifunctional Food Packaging for Meat Products by Incorporating Carboxylated Cellulose Nanocrystal and Beetroot Extract into Sodium Alginate Films. Food Chem. 2023, 415, 135799. [Google Scholar] [CrossRef]
- Yu, M.; Hou, Y.; Zheng, L.; Han, Y.; Wang, D. Soy Protein Isolate-Based Active Films Functionalized with Zanthoxylum Bungeanum by-Products: Effects on Barrier, Mechanical, Antioxidant and Cherry Tomato Preservation Performance. Int. J. Biol. Macromol. 2023, 253, 127539. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Al-Harrasi, A.; Shah, Y.A.; Jawad, M.; Al-Azri, M.S.; Ullah, S.; Anwer, M.K.; Aldawsari, M.F.; Koca, E.; Aydemir, L.Y. Physicochemical Characterization and Antioxidant Properties of Chitosan and Sodium Alginate Based Films Incorporated with Ficus Extract. Polymers 2023, 15, 1215. [Google Scholar] [CrossRef]
- Madureira, J.; Melgar, B.; Alves, V.D.; Moldão-Martins, M.; Margaça, F.M.A.; Santos-Buelga, C.; Barros, L.; Cabo Verde, S. Effect of Olive Pomace Extract Application and Packaging Material on the Preservation of Fresh-Cut Royal Gala Apples. Foods 2023, 12, 1926. [Google Scholar] [CrossRef]
- Nikmanesh, A.; Baghaei, H.; Mohammadi Nafchi, A. Development and Characterization of Antioxidant and Antibacterial Films Based on Potato Starch Incorporating Viola Odorata Extract to Improve the Oxidative and Microbiological Quality of Chicken Fillets during Refrigerated Storage. Foods 2023, 12, 2955. [Google Scholar] [CrossRef] [PubMed]
- Majdi, F.; Alizadeh Behbahani, B.; Barzegar, H.; Mehrnia, M.A.; Taki, M. Active Packaging Coating Based on Lepidium Sativum Seed Mucilage and Propolis Extract: Preparation, Characterization, Application and Modeling the Preservation of Buffalo Meat. PLoS ONE 2024, 19, e0311802. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Chen, H. Antioxidant Packaging Films Based upon Starch-Montmorillonite with Forsythia Flower Extract: Characterization and Application. Food Addit. Contam. Part A 2024, 41, 1679. [Google Scholar] [CrossRef]
- Barzan, G.; Sacco, A.; Giovannozzi, A.M.; Portesi, C.; Schiavone, C.; Salafranca, J.; Wrona, M.; Nerín, C.; Rossi, A.M. Development of Innovative Antioxidant Food Packaging Systems Based on Natural Extracts from Food Industry Waste and Moringa Oleifera Leaves. Food Chem. 2024, 432, 137088. [Google Scholar] [CrossRef]
- Saied, M.; Ward, A.; Hamieda, S.F. Effect of Apricot Kernel Seed Extract on Biophysical Properties of Chitosan Film for Packaging Applications. Sci. Rep. 2024, 14, 3430. [Google Scholar] [CrossRef]
- Dobrucka, R.; Pawlik, M.; Szymański, M. Green Packaging Films with Antioxidant Activity Based on Pectin and Camellia Sinensis Leaf Extract. Molecules 2024, 29, 4699. [Google Scholar] [CrossRef] [PubMed]
- Aldalbahi, A.; Thamer, B.M.; Abdulhameed, M.M.; El-Newehy, M.H. Fabrication of Biodegradable and Antibacterial Films of Chitosan/Polyvinylpyrrolidone Containing Eucalyptus Citriodora Extracts. Int. J. Biol. Macromol. 2024, 266, 131001. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Song, Y.Z.; Thakur, K.; Zhang, J.G.; Khan, M.R.; Ma, Y.L.; Wei, Z.J. Blueberry Anthocyanin Based Active Intelligent Wheat Gluten Protein Films: Preparation, Characterization, and Applications for Shrimp Freshness Monitoring. Food Chem. 2024, 453, 139676. [Google Scholar] [CrossRef]
- Hernández-Hernández, F.A.; Gómez-Aldapa, C.A.; Castro-Rosas, J.; Vargas-León, E.A.; Gutierrez, M.C.; Velazquez, G.; Jiménez-Regalado, E.J.; Aguirre-Loredo, R.Y. Hibiscus Sabdariffa L. Extract as a Natural Additive in Food Packaging Biodegradable Films to Improve Antioxidant, Antimicrobial, and Physicochemical Properties. Plant Foods Hum. Nutr. 2024, 79, 285–291. [Google Scholar] [CrossRef]
- Tonyali, B.; McDaniel, A.; Amamcharla, J.; Trinetta, V.; Yucel, U. Release Kinetics of Cinnamaldehyde, Eugenol, and Thymol from Sustainable and Biodegradable Active Packaging Films. Food Packag. Shelf Life 2020, 24, 100484. [Google Scholar] [CrossRef]
- Cui, R.; Yan, J.; Cao, J.; Qin, Y.; Yuan, M.; Li, L. Release Properties of Cinnamaldehyde Loaded by Montmorillonite in Chitosan-based Antibacterial Food Packaging. Int. J. Food Sci. Technol. 2021, 56, 3670–3681. [Google Scholar] [CrossRef]
- Bianchi, F.; Fornari, F.; Riboni, N.; Spadini, C.; Cabassi, C.S.; Iannarelli, M.; Carraro, C.; Mazzeo, P.P.; Bacchi, A.; Orlandini, S.; et al. Development of Novel Cocrystal-Based Active Food Packaging by a Quality by Design Approach. Food Chem. 2021, 347, 129051. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.-J.; Zhang, J.; Xu, W.-R.; Zhang, Y.-C. Development of Active Packaging Films Based on Liquefied Shrimp Shell Chitin and Polyvinyl Alcohol Containing β-Cyclodextrin/Cinnamaldehyde Inclusion. Int. J. Biol. Macromol. 2022, 214, 67–76. [Google Scholar] [CrossRef]
- Wang, K.; Li, W.; Wu, L.; Li, Y.; Li, H. Preparation and Characterization of Chitosan/Dialdehyde Carboxymethyl Cellulose Composite Film Loaded with Cinnamaldehyde@zein Nanoparticles for Active Food Packaging. Int. J. Biol. Macromol. 2024, 261, 129586. [Google Scholar] [CrossRef]
- Siddiqui, M.N.; Redhwi, H.H.; Tsagkalias, I.; Vouvoudi, E.C.; Achilias, D.S. Development of Bio-Composites with Enhanced Antioxidant Activity Based on Poly(Lactic Acid) with Thymol, Carvacrol, Limonene, or Cinnamaldehyde for Active Food Packaging. Polymers 2021, 13, 3652. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Ma, Y.; Wu, D.; Liu, P.; He, Y.; Chen, K. Preparation of Covalent Organic Framework-Based Nanofibrous Films with Temperature-Responsive Release of Thymol for Active Food Packaging. Food Chem. 2023, 410, 135460. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Salmas, C.E.; Moschovas, D.; Karabagias, V.K.; Karabagias, I.K.; Baikousi, M.; Georgopoulos, S.; Leontiou, A.; Katerinopoulou, K.; Zafeiropoulos, N.E.; et al. Development, Characterization, and Evaluation as Food Active Packaging of Low-Density-Polyethylene-Based Films Incorporated with Rich in Thymol Halloysite Nanohybrid for Fresh “Scaloppini” Type Pork Meat Fillets Preservation. Polymers 2023, 15, 282. [Google Scholar] [CrossRef]
- Lan, W.; Liang, X.; Lan, W.; Ahmed, S.; Liu, Y.; Qin, W. Electrospun Polyvinyl Alcohol/d-Limonene Fibers Prepared by Ultrasonic Processing for Antibacterial Active Packaging Material. Molecules 2019, 24, 767. [Google Scholar] [CrossRef]
- Hou, C.-Y.; Hazeena, S.H.; Hsieh, S.-L.; Li, B.-H.; Chen, M.-H.; Wang, P.-Y.; Zheng, B.-Q.; Liang, Y.-S. Effect of D-Limonene Nanoemulsion Edible Film on Banana (Musa Sapientum Linn.) Post-Harvest Preservation. Molecules 2022, 27, 6157. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.J.; Cabedo, L.; Lagaron, J.M.; Torres-Giner, S. Development of Electrospun Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Monolayers Containing Eugenol and Their Application in Multilayer Antimicrobial Food Packaging. Front. Nutr. 2020, 7, 140. [Google Scholar] [CrossRef]
- Jiang, L.; Han, Y.; Meng, X.; Xiao, Y.; Zhang, H. Cellulose Nanocrystals Reinforced Zein/Catechin/β-Cyclodextrin Inclusion Complex Nanoparticles Nanocomposite Film for Active Food Packaging. Polymers 2021, 13, 2759. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Xu, H.; Xu, E.; Jin, Z.; Zhao, H.; Yuan, C.; Zhao, M.; Wu, Z.; He, D.; Cui, B. Improving Structural and Functional Properties of Starch-Catechin-Based Green Nanofiber Mats for Active Food Packaging by Electrospinning and Crosslinking Techniques. Int. J. Biol. Macromol. 2024, 267, 131460. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Mohammadi Nafchi, A.; Baghaie, H. Development of an Active Packaging Based on Polyethylene Containing Linalool or Thymol for Mozzarella Cheese. Food Sci. Nutr. 2021, 9, 3732–3739. [Google Scholar] [CrossRef]
- Velázquez-Contreras, F.; García-Caldera, N.; Padilla de la Rosa, J.D.; Martínez-Romero, D.; Núñez-Delicado, E.; Gabaldón, J.A. Effect of PLA Active Packaging Containing Monoterpene-Cyclodextrin Complexes on Berries Preservation. Polymers 2021, 13, 1399. [Google Scholar] [CrossRef] [PubMed]
| Database | Search Keywords | Total Results | Results (2019–2024) | Results (2023–2024) | Top Journals | Article Types |
|---|---|---|---|---|---|---|
| PubMed | active food packaging | 6320 | 4428 | 2020 | Int. J. Biol. Macromol. (1045), Food Chem. (379), Polymers (347) | Not specified |
| Web of Science | active food packaging | 5740 | 4191 | 1968 | Int. J. Biol. Macromol. (500), Food Packag. Sheld Life (315), Food Hydrocolloids (245) | Reviews: 800 Research papers: 3312 |
| Patents | Title | Year of Publication |
|---|---|---|
| CN110396224A | A preparation method of anti-oxidative and antibacterial film equipped with cinnamon essential oil Pickering emulsion | 2019 |
| CN108752610B | A kind of edible antibacterial film of slow-release essential oil and preparation method thereof | 2020 |
| AU2020103921A4 | Antibacterial compound fiber film, method for preparing the same and application thereof | 2020 |
| CN113354853B | A kind of biodegradable high barrier antibacterial composite film and preparation method thereof | 2022 |
| CN114411331A | Nanometer film added with oregano essential oil cyclodextrin inclusion compound and preparation method and application thereof | 2022 |
| CN113846490B | Preservative film with intelligent microbial self-inhibition effect and preparation method thereof | 2022 |
| CN111440344B | A kind of composite seaweed polysaccharide-based double-layer packaging film and its preparation method and application | 2022 |
| CN113956633B | A degradable active packaging material based on ginger essential oil and polylactic acid and its preparation method thereof | 2022 |
| CN113712072B | A clove essential oil Pickering emulsion film preservative and its preparation method and application | 2023 |
| CN114249926B | Edible film and preparation method thereof | 2023 |
| JP2024513989A | Edible spray or coating compositions and methods of making and uses thereof for extending the shelf life of perishable items | 2024 |
| CN115516046B | Coating for food packaging comprising antimicrobial active ingredient | 2024 |
| KR102735729B1 | Paper wrapper for packing food with excellent fragrance activity and antimicrobial activity | 2024 |
| CN113907128B | Edible pepper essential oil film for fruit and vegetable fresh-keeping and preparation method thereof | 2024 |
| Compound | PubMed | Web of Science | Scopus |
|---|---|---|---|
| Thymol | 87 | 125 | 466 |
| Cinnamaldehyde | 68 | 112 | 420 |
| Limonene | 38 | 37 | 257 |
| Carvacrol | 80 | 161 | 483 |
| Eugenol | 46 | 83 | 376 |
| Vanillin | 19 | 15 | 145 |
| Linalool | 29 | 29 | 180 |
| Citral | 18 | 31 | 138 |
| α-Pinene | 7 | 8 | 117 |
| Citric acid | 65 | 84 | 567 |
| Gallic acid | 91 | 100 | 582 |
| Quercetin | 50 | 56 | 397 |
| Resveratrol | 16 | 14 | 172 |
| Catechin | 39 | 37 | 305 |
| Epicatechin | 42 | 12 | 122 |
| Essential Oil | Polymer | Packaging Preparation Method | Food Matrix (Applied) | Main Results | Ref. |
|---|---|---|---|---|---|
| Mentha piperita; Mentha x villosa Huds | Chitosan (CS) | Coatings | Papaya (Carica papaya L.) | EOs were incorporated into chitosan-based coatings to inhibit fungi growth during papaya storage in refrigerators. Formulated coatings did not affect papaya sensory acceptability. | [105] |
| Basil, coriander, pimento, rosemary, thyme | Whey protein isolate | Coating | Sliced bologna-type sausage | Coatings incorporating pimento EO provided the most effective inactivation against Listeria innocua followed by thyme, basil, coriander, and rosemary EO. Coatings containing thyme essential oil were the best sensory coating types. | [35] |
| Clove (C) | CS | Casting | Pork patties in cold storage | Based on sensory and microbiological evaluations, the shelf life of pork patties was 6 days for control, 9 days for CS and CS-Nisin, and 12 days for CS-C and CS-C-Nisin (CS-C-NI). C showed high antioxidant activity, and the combination of C and CS may enhance oxidative stability of pork patties during storage. CS-C-NI combination treatment has excellent microbial inhibition due to synergistic bactericidal effects. | [98] |
| Thyme (encapsulated into porous poly (lactic acid) nanofibers—PLA) | Poly(vinyl alcohol)/poly(ethylene glycol)—(PVA/PEG) | Electrospinning | Strawberries | Thyme essential oil significantly inhibited bacterial survival in vitro. The slower release of TEO from the PLA/TEO/PVA/PEG composite films, compared to the PLA/TEO nanofibers, contributed to the extended shelf life of the strawberries. PLA/TEO/PVA/PEG film shows higher microbial activity against Escherichia coli and Staphylococcus aureus. | [100] |
| Oregano (free and microencapsulate) | Wheat flour and poly (butylene co-terephthalate adipate) | Blown extrusion | Brazilian fresh pastry (known as pastel) | Fresh pastries packaged with film incorporating oregano essential oil microparticles exhibited lower mold and yeast counts during 28 days of refrigerated storage compared to those packaged with control film or film containing free oregano EO. This may have occurred due to the slow and gradual migration of the OEO from the film to the food surface. | [101] |
| Turmeric (TEO); turmeric (encapsulated into magnetic/silica porous core–shell nanocomposites-MNPs/Si) | CS | Casting | Surimi | The CS/TEO film effectively inhibited Bacillus cereus growth, significantly reducing the bacterial population for up to 6 days of storage. However, due to the rapid and uncontrolled release of TEO when directly incorporated into the film, bacterial growth resumed. In contrast, the CS/MNPs/Si/TEO film maintained reduced bacterial proliferation, until the end of storage (14 days), likely due to the slow release of TEO. Both films prevented surimi protein oxidation, suggesting TEO’s antioxidant potential. | [36] |
| Clove | Cellulosic nanocrystals obtained from the Kudzu plant (Pueraria montana), and corn starch | Casting | Red grapes | The films loaded with essential oil exhibited remarkable antimicrobial properties against S. aureus and E. coli. The antimicrobial effect was stronger on S. aureus. In addition, films with essential oils were found to be more efficient in maintaining the fruit’s physical and chemical stability for 15 days at 5 °C. | [106] |
| Clove (doubly stabilized oil chitosome nanoparticles (CNPs)) | Gelatin/PVA (GEL/PVA) | Casting | Marinated steaks | The presence of CNPs in film suppressed microbial proliferation, decelerated meat product degradation, and preserved the color and freshness of the meat products during storage. This was attributed to the antimicrobial effect of the CNPs. | [107] |
| Trachyspermum ammi | PLA | Tape casting | Waffles | Waffles packed in PLA films containing 50 wt% blend of both oils had their shelf life extended up to 30 days compared to 2 days for the neat PLA film. Vanilla was found to be effective in masking the unpleasant odor of Tammi. | [23] |
| Cinnamon bark and clove bud | Cellulose acetate nanofibers | Electrospinning | Fresh grapes and tomatoes | The use of 50% w/w cinnamon oil (55.56% w/w cinnamaldehyde) and CBO (with 75.82% w/w eugenol)-loaded CANFs as an active food packaging membrane for a shelf-life study of fresh grapes and tomatoes at 4 °C confirmed the microbiological safety of consumption for 40 days and enhanced sensory and physicochemical properties for up to 30 days, compared to just 15 days for the controls. | [37] |
| Artemisia absinthium | Salep gum containing chitosomes (chitosan-coated essential oil-loaded nanoliposomes)—Salep-NLPs-CH | The film-forming solutions were desiccated at 35 °C for 48 h after being cast onto polystyrene plates | Toast bread | Salep–NLPs–CH film proved most effective in preserving bread color over time due to its antioxidant and antifungal properties. Mold growth was not detected until day 44, attributed to the chitosan and AEO slow release. In contrast, mold appeared earlier on samples packaged with Salep–NLPs and Salep-free AEO films. The Salep–NLPs and Salep–NLPs–CH films exhibited higher overall acceptance, likely due to the preservation of color, aroma, and texture during storage. | [38] |
| Cinnamon | CS/ Starch | Casting | Raw beef meat | Film packaging incorporated into EO with or without cellulose nanofibers had the ability to effectively reduce the bacterial load of raw beef meat samples and thereby enhance the shelf life. This property could be due to the combined effect of the chitosan in starch/chitosan/cellulose along with the CEO present in the active packaging material. | [22] |
| Cinnamon | Gelatin/pullulan | The film-forming solution was poured onto a Teflon-coated glass plate and dried at room temperature for 60 h | Meat | The low pH change indicated that meat packaged with active gelatin/pullulan-based composite films incorporated with cinnamon essential oil-loaded metal–organic frameworks can inhibit food quality deterioration after 16 days. The active film maintained the microbial load low. The results show that the incorporation of cinnamon essential oil-loaded metal–organic frameworks helped to prolong the shelf life of beef. | [21] |
| Alpinia galanga | PVA-acetylated pullulan polysaccharides | Casting | Chicken meat | Alpinia galanga essential oil components in the composite plastic provided favorable inhibiting the oxidation of proteins and lipids during shelf-life and inhibitory effects against E. coli and S. aureus. | [19] |
| Melissa officinalis L | Carboxymethyl chitosan, and locust bean gum | Coating | Large yellow croakers (Pseudosciaena crocea) | Ultrasound treatment (US) and a bioactive coating (CMCS), alone and combined, significantly inhibited microbial growth and lipid oxidation in yellow croakers during cold storage. The US+CMCS treatment was the most effective, extending shelf life considerably, compared to control and individual treatments. | [108] |
| Kiwifruit seed | Sodium alginate | Film by casting and coating material | Persimmon fruit | Applying the coating material to persimmon fruit resulted in reduced weight loss and helped maintain both firmness and respiration rate. The antifungal properties of the coating were further enhanced by the addition of EO. | [20] |
| Lavender flowers | Polyvinylpyrrolidone (PVP) | Centrifugal spinning | Minced lamb meat | Over the storage period, 1%, 3.5%, and 7% LEO nanofiber mats effectively suppressed meat oxidation. Microbial counts remained below acceptable limits for all samples (except for the 1% LEO PVP sample). After 5 days, yeast and mold count in the 3.5% and 7% LEO-containing samples were lower than their initial levels, likely from the intense and controlled release of LEO. The positive antimicrobial effect of the PVP film is attributable to the LEO’s activity against aerobic bacteria. | [97] |
| Clove | PLA; polylactic acid/modified thermoplastic starch (TPS) | Hot pressing | Shrimp | Shrimp packaged in polylactic acid incorporated with clove essential oil film (PC) maintained better quality than control (without EO). PC-packaged shrimp stayed below spoilage pH and microbial limits until day 10, compared to the control. The PC film, with its dense, crystalline structure, facilitated the controlled release of clove EO, extending shrimp shelf life. | [96] |
| Tea tree | CS | Solution was poured into a film-forming container and dried | Fresh cut pork | The soybean separation protein (SPI)–carboxymethyl cellulose (CMC) emulsion (SCCE) containing tea tree essential oil (TTO) incorporated into CS matrix controlled the slow release of antibacterial and antioxidant TTO into the packaging microenvironments, prolonging the pork shelf life by 6 days. | [39] |
| Oregano | PSE-like chicken protein isolate (PPI) | The film-forming solution was poured into polyethylene and dried in the oven | Fresh pork | Oregano EO-loaded nanoemulsion PPI films were tested for antibacterial activity against E. coli and S. aureus using the disk diffusion method. However, no significant effect against E. coli was revealed. The film with 2.5% oregano EO proved the highest effective in inhibiting bacterial growth and quality deterioration in refrigerated pork, thus extending its shelf life. | [40] |
| Wintergreen | Dehydroabietic acid (DHA) modified chitosan | The film-forming solution was poured into the mold to form a film | Mandarin oranges | Films incorporated into EO were able to delay the loss of antioxidant activity, improve the antifungal property against penicillium and prolonging the shelf life of mandarins up to 18 days. | [109] |
| Natural Extract | Polymer | Incorporation Method | Food Matrix | Main Results | Ref. |
|---|---|---|---|---|---|
| Green tea extract | Food contact polyamide (Nylon 6) | Adsorption technique | Fresh minced beef | The active film incorporated with green tea extract presented excellent antioxidant capacity. The polyamide exhibited good film-forming properties with green tea extract incorporated. The active film protected the beef’s color, as well as its lipid oxidation and variation in metmyoglobin values up to 23 days at 4 °C. | [103] |
| Chinese hawthorn fruit extract | Chitosan–gelatin blend film | Casting method | - | Chinese hawthorn fruit extract was successfully incorporated in a chitosan–gelatin blend film. The active film presented significantly improved mechanical and water vapor barrier properties. The addition of the extract also improved the light barrier and antioxidant properties of chitosan–gelatin films. The main polyphenols identified were epicatechin, chlorogenic acid, and procyanidin B2. | [110] |
| Mango leaf extract | Chitosan | Casting method | Cashew nuts | The addition of mango leaf extract increased the film’s antioxidant activity, thickness, opacity, tensile strength, and surface hydrophobicity. On the contrary, it reduced the water vapor permeability, water solubility, and elongation at break. The active film was able to protect cashew nuts from oxidation for 28 days, compared to commercial films. | [83] |
| Silver nanoparticle extract of Artemisia scoparia | Calcium alginate | Incorporation/casting method | Strawberries and loquats | A significant enhancement was observed in the quality parameters of strawberries and loquats, including reduced acidity loss, minimized soluble solid content and weight loss, and overall quality preservation. The active coating also demonstrated high antimicrobial activities. | [111] |
| Poplar hot water extract | Bentonite and chitosan | Casting method | - | The active film with poplar hot water extract incorporated presented greater antioxidant properties, enhanced UV blocking properties, and improved water vapor and oxygen barrier properties. The authors concluded that the new active film is a potential sustainable food packaging material. | [112] |
| Garlic extract | Polyethylene, Ethylene-vinyl alcohol copolymer and zein | - | Sliced pan loaf | The new active film with garlic extract developed presented antifungal activity against Penicillium expansum. The active film successfully delayed fungal growth in the bread, compared to the control, during the 30 days of storage. | [104] |
| Chitosan and rosemary extract | Poly (lactic acid) | Melt mixing | - | The incorporation of rosemary extract and chitosan on the PLA matrix resulted in a film packaging with improved elongation at break, mechanical strength, and thermal stability, as well as antibacterial and antioxidant properties. The authors concluded that this new film can be a potential active packaging with the controlled release of antimicrobial/antioxidant compounds. | [102] |
|
Carotenoids extracts β-carotene and lycopene extracted from carrots and tomatoes and bixin extracted from annatto seeds | Poly (lactic acid) | Casting method | Sunflower oil | The incorporation of carotenoid extracts into PLA films successfully improved the shelf life of sunflower oil as it delayed its oxidation. Films with lycopene and β-carotene extracts exhibited better protection against UV light and oxygen barrier properties. Nonetheless, films with bixin extract demonstrated superior capacities in protecting sunflower oil with the best antioxidant properties. | [113] |
| Silver nanoparticles from Nigella sativa seedcake extract | Chitosan | Casting method | - | The incorporation of silver nanoparticles improved the film’s mechanical properties; specifically, it improved the film’s tensile strength and elongation and reduced the water vapor permeability. The silver nanoparticles also enhanced the film with antibacterial properties. | [114] |
| Rosemary extract | Whey Protein | Casting method | Salami | The whey protein film incorporated with rosemary extract was effective against the lipid oxidation of salami during 90 days at 5 °C. The active film was able to delay lipid oxidation of salami, as the samples presented lower values of MDA and hexanal in comparison with the control. | [85] |
| Ginkgo biloba extract | Gelatin | Casting method | - | The authors concluded that the incorporation of ginkgo biloba increased the films’ tensile strength and decreased their elongation at break, moisture content, solubility, and water vapor permeability. The extract also added to the film antioxidant properties and antimicrobial activity against S. aureus and Candida albicans. | [115] |
| Salvadora persica L. extract and titanium dioxide nanoparticles | Carboxymethyl cellulose | Casting method | - | The study concluded that the incorporation of Miswak (Salvadora persica L.) extract and titanium dioxide nanoparticles into a nanocomposite carboxymethyl cellulose film improved its properties, specifically the thermal stability, oxygen and water vapor permeability, and antimicrobial activity, namely against E. coli and S. aureus. | [116] |
| Oregano essential oil, rosemary extract, and green tea extract | Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) | Electrospinning | - | The addition of the active compounds to the film increased its opacity and decreased its hydrophobicity. The active compounds also conferred antioxidant and antimicrobial properties to the film. | [117] |
| Extract from oregano waste | Carbon dioxide-derived poly (propylene carbonate) and cellulose acetate | Casting method | - | The new food packaging developed presented good mechanical, thermal, and water vapor barrier properties. The addition of oregano waste extract also improved the film’s antioxidant and antimicrobial properties. The authors also concluded that the film is biodegradable, posing as a candidate for a new sustainable active food packaging material. | [118] |
| Rheum ribes L. Extract | Methylcellulose polymer | Casting method | - | The authors found that the addition of the extract increased the thickness and opacity of the film while decreasing solubility and water vapor permeability. In addition, it enhanced the tensile strength of the films but reduced their elongation at break. The active film presented increased antioxidant capacity and antimicrobial activity. | [119] |
| Banana peel extract | Chitosan | Casting method | Apple fruit | The chitosan film incorporated with banana peel extract presented increased thickness, opacity, and tensile strength but reduced elongation at break, solubility, and water vapor permeability. The addition of the extract also improved the film’s antioxidant properties. The active coating was applied to apples and demonstrated to increase their shelf life, as those apples presented lower respiration rates, weight loss, and soluble solid content, as well as higher firmness, titratable acidity, and ascorbic acid content, compared to the control. | [120] |
| Propolis extract | Poly lactic acid | Casting method | Meat sausage | Incorporating propolis ethanolic extract into PLA films can increase the films’ thickness and opacity but decrease the tensile strength and elongation at break. When applied to sausage slices, the active film presented enhanced antioxidant activity and antimicrobial activity. | [121] |
| Aloe Vera skin extract | Poly(ethylene oxide) | Electrospinning technique | - | The new active film was evaluated by different assays that concluded that the aloe vera skin extract was successfully incorporated into the poly(ethylene oxide), obtaining a smooth, defect-free, non-woven, and self-standing film. The active film presented a slight reduction in the thermal stability but increased antioxidant activity. | [122] |
| Aqueous rice straw extract | Native potato starch | Melt blending and compression molding techniques | - | The incorporation of rice straw extract significantly enhanced the films’ antioxidant activity, slightly modified the mechanical strength, and also improved the films’ barrier properties by reducing water vapor permeability. The authors concluded that the films could help protect food from moisture loss and oxidation, thus extending the shelf life of food products. | [123] |
| Cocoa bean shell extract and zinc oxide nanoparticles | Pectin-based films | Casting method | - | Zinc oxide nanoparticles and cocoa bean shell extract addition to the pectin-based film improved the oxygen, thermal, and UV barrier properties of the films, with the oxygen barrier improved by 50% and the screen to UV radiation reaching 98%. | [82] |
| Olive leaf extract | Carrageenan | Casting method | Lamb meat | The addition of the extract to the film led to increased opacity and altered mechanical strength but maintained adequate flexibility and barrier properties suitable for packaging applications. The film also presented antimicrobial activity against E. coli. The active film was able to preserve lamb meat under refrigeration, as it significantly inhibited microbial growth, helped stabilize the pH of the meat, prevented the oxidation of lipids and proteins, and improved the sensory attributes, given that it helped maintain the red color of the meat, reduce discoloration, and retain moisture, thereby preventing excessive drying and texture deterioration. | [124] |
| Longan ( Dimocarpus longan ) peels | Paper from rice straw fibers | Casting method | - | The authors developed paper with adequate strength and flexibility to be used as packaging material with good moisture resistance and strong antimicrobial activity against both S. aureus and E. coli bacteria. | [125] |
| Mangosteen peel extract and zinc oxide nanoparticles | Soy protein isolate | Casting method | - | The addition of the active compounds significantly improved the film’s mechanical properties, as an increased tensile strength and elongation at break was observed. In addition, the active film presented excellent UV-blocking properties. The active films exhibited notable antioxidant activity and effective antibacterial activity against common foodborne pathogens, including E. coli and S. aureus. | [126] |
| Nervilia fordii extracts | Poly (vinyl alcohol) and polyvinyl (pyrrolidone) | Electrospinning technique | Encapsulated fish oil | The researchers were able to develop a film with uniform diameters and smooth surfaces. The addition of the extract enhanced the tensile strength and flexibility of the films and attributed a significant antioxidant capacity. | [127] |
| Pineapple peel extract | Poly (vinyl alcohol)–corn starch | Casting method | - | The incorporation of the extract decreased the transparency and the tensile strength of the film but increased the elongation at break and the water vapor permeability. The active film presented improved thermal stability and significantly enhanced antioxidant activity. | [128] |
| Chinese cinnamon (Cinnamomum cassia) extract | Whey protein concentrate | - | Eastern European curd cheese | The study demonstrated that the edible coating could efficiently prolong the shelf life of perishable curd cheese as it successfully inhibited microbial growth. Sensory evaluations (odor, taste, texture, appearance, and overall acceptability) indicated that the active coating did not negatively affect the cheese’s overall acceptability. | [129] |
| Blueberry, red grape, and parsley by-product extracts | Chitosan | Casting method | - | The addition of the extracts increased the water vapor transmission rate but decreased oxygen permeability. The swelling degree decreased with higher concentrations of extracts, indicating improved structural integrity. Both the antioxidant and antimicrobial activity of the films was enhanced by the incorporation of plant extracts. | [130] |
| Red cabbage (Brassica oleracea), sweet potato (Ipomoea batatas), and blue tea (Clitoria ternatea) extracts | Carrageenan and chitosan | Casting method | Freshly cut apple pieces | The incorporation of different extracts into carrageenan-based films resulted in films with higher mechanical strength, total polyphenol content, and antioxidant activity. In addition, when applied to freshly cut apples, the films presented reduced browning intensity and improved antioxidant activity compared to the control. | [131] |
| Beetroot peel extract | Gelatin–sodium alginate | Casting method | Beef meat | The study concluded that the inclusion of beetroot peel extract significantly improved the total phenolic content and consequently the antioxidant capacity. The active film was robust and flexible, demonstrating good tensile strength and elongation at break. In addition, the active film presented a reduction in water vapor permeability. The active film successfully increased the minced beef meat shelf life as it led to a reduction in thiobarbituric acid reactive substances values and inhibited microbial growth. | [80] |
| Pitanga leaf hydroethanolic extract and/or nisin | Gelatin | Mechanical spreading technique | Sliced dried-cured coppa | The authors concluded that the bi-layer active film effectively maintained the quality and sensory properties of the meat during storage. The active film reduced moisture loss, which maintained the texture and prevented excessive drying of the meat. The active film inhibited lipid oxidation and microbial growth during storage, extending the shelf life of the coppa slices. In addition, the active film helped retain the characteristic flavor and aroma of the coppa and maintain a more stable color profile during storage. | [132] |
| Jaboticaba peel extract | Carrageenan | Casting method | - | The jaboticaba peel extract presented excellent antioxidant and antimicrobial properties. The incorporation of the extract into the carrageenan matrix increased the film’s thickness and Young’s modulus and decreased the elongation capacity, tensile strength, water vapor permeability, and swelling. Nonetheless, the extract improved the opacity of the film, giving it UV–vis light barrier properties. | [133] |
| Watermelon rind extract | Polyvinyl alcohol, corn starch, glycerol | Casting method | Freshly cut purple cabbage | The addition of the watermelon rind extract to the composite film improved the barrier, antioxidant, and antimicrobial properties of the film. The active film was able to significantly reduce the microbial count of freshly cut purple cabbage, and it did not affect its sensory attributes. | [84] |
| Peony leaf extract | Chitosan | Casting method | Apples | The incorporation of peony leaf extract into chitosan film improved the film’s water vapor permeability, thermal stability, and opacity, but it negatively influenced the packaging appearance. Nonetheless, it presented as a good UV and light protector of the packed food. The active packaging was effective in retarding the natural browning process of fresh apples during storage. | [87] |
| Date palm pit extract | Alginate | Casting method | - | The active film demonstrated significant antioxidant activity. The active film presented good oxygen and grease barrier properties and a glossy appearance, and it was water-soluble and tasteless. The incorporation of date palm pit extracts improved water vapor barrier properties, tensile strength, and elongation at break. | [134] |
| Green tea extract | Low-density polyethylene | Extrusion process | Fresh orange juice | The study concluded that the active films with green tea extract were effective in extending the shelf life of fresh orange juice. The active packaging inhibited microbial, yeast, and mold growth for up to 14 days. The films decreased oxidation processes, with low levels of ascorbic acid degradation and the development of brown pigments, preventing the degradation of the juice’s quality over time. | [135] |
| Carboxylated cellulose nanocrystal and beetroot extract | Sodium alginate | External gelation method | Fresh pork external fat | The active film presented improved mechanical and antioxidant properties. The active compound enhanced the ability to block UV light and functioned as a real-time freshness indicator by changing color when spoilage thresholds were exceeded during storage. | [136] |
| Portulaca oleracea extract | Chitosan–starch | Casting method | Chilled pork meat | The developed active film presented excellent antioxidant capacity, good water barrier properties, and mechanical strength. The film was applied to chilled pork meat and was able to delay the lipid oxidation and meat spoilage; in addition, it protected the meat’s color during storage. | [88] |
| Zanthoxylum bungeanum leaf extract | Soy protein isolate | Casting method | Cherry tomatoes | The incorporation of the extract improved the films’ tensile strength, water barrier properties, UV-light blocking properties, and antioxidant activities. When applied to cherry tomatoes, the active film effectively maintained the quality of the tomatoes during storage, reducing weight loss and delaying spoilage compared to control. | [137] |
| Ficus racemosa fruit extract | Chitosan and sodium alginate | Casting method | - | The extract was successfully incorporated into the chitosan–sodium alginate matrix, originating a uniform and smooth surface and an improvement of the thermal stability of the films. The active films exhibited enhanced antioxidant activity with the incorporation of the F. racemosa extract. | [138] |
| Olive pomace extract | Poly lactic acid and polypropylene | - | Freshly cut Royal Gala apples | The natural extract reduced the growth of mesophilic bacteria and filamentous fungi for at least five days and inhibited the growth of coliforms for up to 12 days. The extract increased the antioxidant activity of the fruits without significant changes in their firmness and preserved their color after the initial browning of the samples. | [139] |
| Viola odorata flower extract | Potato starch | Casting method | Chicken filets | The incorporation of the extract into the film improved its phenolic content, antioxidant capacity, and antibacterial efficacy against common foodborne pathogens, including E. coli, S. aureus, and Salmonella typhimurium. The active film presented good light-blocking activity, especially against UV waves and improved permeability to water vapor. The active films effectively inhibited lipid oxidation and microbial growth in the chicken filets, thereby extending their shelf life compared to control samples. | [140] |
| Garlic extract | Chitosan–starch | - | Green and yellow bell peppers | The chitosan–starch garlic extract film demonstrated its potential as food packaging as it protected the bell peppers from bacterial growth and weight loss, protecting their general appearance during storage. | [86] |
| Propolis extract | Lepidium sativum seed mucilage | - | Buffalo meat | The active coating developed exhibited significant antioxidant and antimicrobial properties. The active coating was able to reduce lipid oxidation and microbial growth in buffalo meat during storage. The active coating was also able to minimize weight and texture losses during display and enhance the overall acceptability of the meat. | [141] |
| Forsythia flower extract | Starch–montmorillonite | Solution flow delay method | Cherry tomatoes | The authors concluded that the incorporation of the extract improved the film’s antioxidant and UV protection properties, as well as its thermal stability. When applied to fresh tomatoes, the active film preserved firmness, minimized nutrient loss, boosted vitamin C content, reduced decay rates, and consequently prolonged the tomatoes’ shelf life. | [142] |
| Olive pomace, grape marc, and moringa leaves extracts | Cellulose | - | Ground beef | The packaging material’s antioxidant qualities were significantly enhanced by the use of natural extracts, in addition to successfully decreased lipid peroxidation in food products. Additionally, over a 16-day period, the active packaging reduced lipid oxidation by at least 50% when applied to ground beef. | [143] |
| Apricot kernel seed extract | Chitosan | Casting method | - | The authors concluded that the incorporation of apricot kernel seed extract into the chitosan matrix significantly improves its mechanical strength, thermal stability, and barrier properties. Additionally, the active films exhibit enhanced antioxidant and antimicrobial activities, which are crucial for extending the shelf life and ensuring the safety of packaged food products. | [144] |
| Camellia sinensis leaf extract | Pectin | Casting method | - | The addition of the extract to the pectin-based film significantly improved its antioxidant activity due to the high polyphenol content in green tea. The active films demonstrated improved water resistance, reducing the permeability of moisture and oxygen. However, a slight reduction in film strength was observed with higher extract concentrations, but the overall flexibility and integrity remained within an acceptable range. | [145] |
| Eucalyptus citriodora leaf extract | Chitosan/ polyvinylpyrrolidone | Casting method | - | The authors concluded that the incorporation of the extract into the films effectively inhibited microbial growth and improved the mechanical properties by making them more robust and durable. The extract improved the films’ tensile modulus, yield strength, and tension at the break. | [146] |
| Blueberry anthocyanin extract | Wheat gluten protein and apple pectin | Casting method | Shrimp | The active film exhibited a uniform and compact structure after incorporation of the extract, effective water vapor permeability, and improved mechanical strength. The addition of the extract improved the antioxidant activity, which can help in delaying lipid oxidation in food products. When used to monitor shrimp spoilage, films changed color with volatile amine release, visually indicating freshness over 18 days. | [147] |
| Hibiscus sabdariffa l. extract | Potato starch and polyvinyl alcohol | - | - | The active film presented significant antioxidant capacity and antibacterial activity against common foodborne pathogens, including E. coli and S. aureus. The active film presented improved the tensile strength and flexibility and reduced water vapor permeability. | [148] |
| Compound | Polymer | Packaging Preparation Method | Food Matrix (If Applied) | Main Results | Ref. |
|---|---|---|---|---|---|
| Cinnamaldehyde (CIN) | Ethylene vinyl alcohol (EVOH) | Solvent casting and melt extrusion | NA | The study successfully developed bioactive EVOH films containing 1, 3, and 5% of cinnamaldehyde using a hybrid solvent-casting and melt-extrusion method. The films exhibited antioxidant activity, UV-blocking properties, and antifungal properties against Penicillium expansum. The incorporation of cinnamaldehyde also improved the films’ flexibility and transparency while maintaining their mechanical integrity, making them suitable for industrial-scale food packaging applications. | [94] |
| Cinnamaldehyde, carvacrol, and eugenol | Zein, polyethylene glycol (as a hydrophilic plasticizer), oleic acid (as a hydrophobic plasticizer) | Solvent casting | NA | The study successfully developed biodegradable zein-based films incorporated with cinnamaldehyde, carvacrol, and eugenol with antimicrobial properties, with cinnamaldehyde showing the strongest activity, particularly against S. aureus for a period up to 96 h. However, while higher cinnamaldehyde concentrations improved film flexibility, they reduced tensile strength, with PEG proving more effective than OA as a plasticizer for enhancing mechanical properties. The results highlight the potential of these zein–PEG films containing 5% cinnamaldehyde as sustainable, antimicrobial packaging material. | [95] |
| Thymol, eugenol, and cinnamaldehyde | Pullulan | Lipid nanoparticle encapsulation | NA | This study investigated the release kinetics of thymol, eugenol, and cinnamaldehyde from pullulan-based biodegradable films, comparing liquid-lipid nanoparticles and solid-lipid nanoparticles as carriers. The results showed that solid-lipid nanoparticles films provide a faster release of antimicrobials due to the expulsion of active compounds during lipid crystallization. Among the tested compounds, thymol exhibits the highest release rate. | [149] |
| Cinnamaldehyde | Chitosan and acidified montmorillonite (MMT) | Solvent casting | NA | In this study, chitosan-based films with acidified MMT loaded with cinnamaldehyde were developed and evaluated. The films exhibited improved mechanical strength, UV resistance, and prolonged cinnamaldehyde release (using isooctane as a fatty food simulant), along with significant inhibition of S. aureus and E. coli. | [150] |
| Carvacrol, thymol, and cinnamaldehyde | Low-density polyethylene (LDPE) and chitosan | Cocrystallization and Solvent casting | White grapes | This study developed a cocrystal-based active packaging using carvacrol, thymol, and cinnamaldehyde anchored to a chitosan-coated LDPE. The active films showed antimicrobial activity against E. coli, Salmonella Typhimurium, S. aureus, and MR S. aureus. The films extended the shelf-life of white grapes by 7 days, maintaining their sensory quality. | [151] |
| Cinnamaldehyde | Chitin from shrimp and polyvinyl alcohol (PVA) | Solution casting method | Cherry tomatoes | Active packaging films by incorporating β-cyclodextrin/CIN inclusion complexes into chitin/PVA blends using a solution casting method. The films demonstrated a sustained release of CIN, enhanced antimicrobial activity against S. aureus, Bacillus subtilis, S. typhimurium, Aspergillus niger, Aspergillus flavus, and Penicillium citrinum. The film with 3% β-cyclodextrin/CIN effectively preserved the cherry tomatoes by reducing weight loss, maintaining hardness, and inhibiting microbial growth over 10 days. | [152] |
| Cinnamaldehyde | Chitosan and dialdehyde carboxymethyl cellulose (DCMC) | Solution casting method | Strawberries | Active packaging chitosan/DCMC-based films with zein nanoparticles loaded with CIN were successfully produced. The films exhibited improvements in mechanical strength, water vapor and oxygen barrier properties and UV-blocking ability. The film with 35% zein nanoparticles and CIN effectively preserved the strawberries by reducing microbial growth, weight loss, and maintaining quality over 7 days of storage. | [153] |
| Thymol, carvacrol, limonene and cinnamaldehyde | Polylactic acid (PLA) | Solvent-casting technique | NA | This study developed a PLA-based active film incorporating thymol, carvacrol, limonene, or CIN using solvent casting. The films exhibited significant antioxidant activity, with carvacrol demonstrating superior performance compared to the other individual compounds and their triple blends. Regarding the films’ characteristics, the active films exhibited plasticization effects and maintained PLA’s crystallinity, with CIN slightly reducing thermal stability. | [154] |
| Thymol | Polycaprolactone (PCL) | Solution blow spinning | NA | Thymol was encapsulated in covalent organic frameworks and incorporated in PCL through solution blow spinning. The films exhibited controlled thymol release and antimicrobial activity against S. aureus and E. coli. | [155] |
| Thymol | LDPE | Melt extrusion process | Fresh “scaloppini”-type pork meat filets | Halloysite nanotubes were impregnated with thymol to form a hybrid nanostructure. Then, this nanostructure was incorporated in LDPE at 5, 10, and 15%. The active films exhibit significant antioxidant activity and improved barrier properties. The LDPE with 10% of the thymol nanostructure optimally preserved pork meat by significantly reducing lipid oxidation. The research also established a linear correlation between TBARS and heme iron measurements, offering a faster method to assess meat spoilage. | [156] |
| D-Limonene | PVA | Electrospinning, with additional ultrasonic processing | NA | PVA/D-limonene composite fibers, with the optimized ratio of 7:3 and an ultrasonic processing time of 15 min, were developed. The active composite fibers presented antimicrobial activity against E. coli and S. aureus and enhanced degradability and homogeneity due to ultrasonic treatment. | [157] |
| D-Limonene | Sodium alginate | Phase inversion and brush-coated application | Bananas (Musa sapientum Linn.) | The study developed an edible coating using a sodium alginate D-limonene nanoemulsion, which demonstrated antimicrobial activity against S. aureus, L. monocytogenes, Salmonella enterica, and E. coli, and effectively extended the shelf life of bananas by reducing weight loss and delaying ripening. The 1.0% D-limonene concentration was optimal, while higher concentrations caused undesirable visual effects. | [158] |
| Eugenol | Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) | Electrospinning | NA | The authors developed multilayer food packaging using electrospun PHBV fibers loaded with eugenol, which demonstrated antimicrobial activity against S. aureus and E. coli. The optimized 15 wt.% eugenol–PHBV monolayer, exhibited enhanced barrier properties, hydrophobicity, and thermal stability. | [159] |
| Catechin | Zein reinforced with cellulose nanocrystals | Solution casting | Soybean oil | An antioxidant-active nanocomposite film using zein, catechin/β-cyclodextrin nanoparticles, and cellulose nanocrystals was created in this study. The active film exhibited enhanced mechanical strength, barrier properties, and oxidative stability. Also, the film effectively inhibited lipid oxidation in soybean oil, demonstrating potential for active food packaging applications. | [160] |
| Catechin | Octenyl succinic anhydride starch and Pullulan | Electrospinning and glutaraldehyde vapor phase crosslinking | Strawberries | In this study, a starch-based nanofiber film was developed using electrospinning and glutaraldehyde crosslinking, incorporated with catechin. The active film presented antimicrobial activity against S. aureus and E. coli along with in vitro antioxidant activity. The active film successfully extended the strawberries’ shelf life, maintaining their freshness by 6 days. | [161] |
| Linalool and thymol | Polyethylene | Direct mixing and compression molding | Mozzarella cheese | The study developed antimicrobial polyethylene films infused with linalool or thymol, which significantly inhibited S. aureus and E. coli growth in mozzarella cheese, extending its shelf life. Thymol (2%) was most effective, preventing bacterial contamination and reducing mold/yeast proliferation. | [162] |
| Thymol and carvacrol | PLA | Melt-processed by injection molding | Blackberries and raspberries | This study evaluated a PLA packaging containing thymol or carvacrol complexed with β-cyclodextrins for preserving blackberries and raspberries during cold storage. The active packaging showed significant antioxidant and antimicrobial properties, improving fruit quality and extending shelf life by one week over commercial packaging. Sensory evaluation confirmed no negative impact on flavor or aroma, supporting the potential of these natural compounds for food preservation. | [163] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, M.A.; Barbosa, C.H.; Ribeiro-Santos, R.; Tomé, S.; Fernando, A.L.; Silva, A.S.; Vilarinho, F. Emerging Trends in Active Packaging for Food: A Six-Year Review. Foods 2025, 14, 2713. https://doi.org/10.3390/foods14152713
Andrade MA, Barbosa CH, Ribeiro-Santos R, Tomé S, Fernando AL, Silva AS, Vilarinho F. Emerging Trends in Active Packaging for Food: A Six-Year Review. Foods. 2025; 14(15):2713. https://doi.org/10.3390/foods14152713
Chicago/Turabian StyleAndrade, Mariana A., Cássia H. Barbosa, Regiane Ribeiro-Santos, Sidney Tomé, Ana Luísa Fernando, Ana Sanches Silva, and Fernanda Vilarinho. 2025. "Emerging Trends in Active Packaging for Food: A Six-Year Review" Foods 14, no. 15: 2713. https://doi.org/10.3390/foods14152713
APA StyleAndrade, M. A., Barbosa, C. H., Ribeiro-Santos, R., Tomé, S., Fernando, A. L., Silva, A. S., & Vilarinho, F. (2025). Emerging Trends in Active Packaging for Food: A Six-Year Review. Foods, 14(15), 2713. https://doi.org/10.3390/foods14152713









