Quinoa Protein/Sodium Alginate Complex-Stabilized Pickering Emulsion for Sustained Release of Curcumin and Enhanced Anticancer Activity Against HeLa Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of Quinoa Protein/Sodium Alginate Complexes (QPI/SA)
2.2.1. Preparation of QPI/SA Complexes
2.2.2. Characterization of QPI/SA Interactions
2.2.3. Turbidity Measurement
2.2.4. Zeta Potential Analysis
2.2.5. Fourier-Transform Infrared (FTIR) Spectroscopic Analysis
2.2.6. Scanning Electron Microscopy (SEM)
2.2.7. Three-Phase Contact Angle Measurement
2.3. Preparation and Stability of Pickering Emulsions
2.3.1. Emulsion Preparation
2.3.2. Creaming Stability Assessment
2.3.3. Emulsion Droplet Size Analysis
2.4. Preparation and Characterization of Curcumin-Loaded Pickering Emulsions
2.4.1. Curcumin-Loaded Pickering Emulsions
2.4.2. In Vitro Release of Curcumin
2.4.3. Confocal Laser Scanning Microscopy Analysis
2.4.4. In Vitro Cytotoxicity
2.5. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Quinoa Protein/Sodium Alginate Complexes (QPI/SA)
3.1.1. Molecular Interactions in QPI/SA Complexes
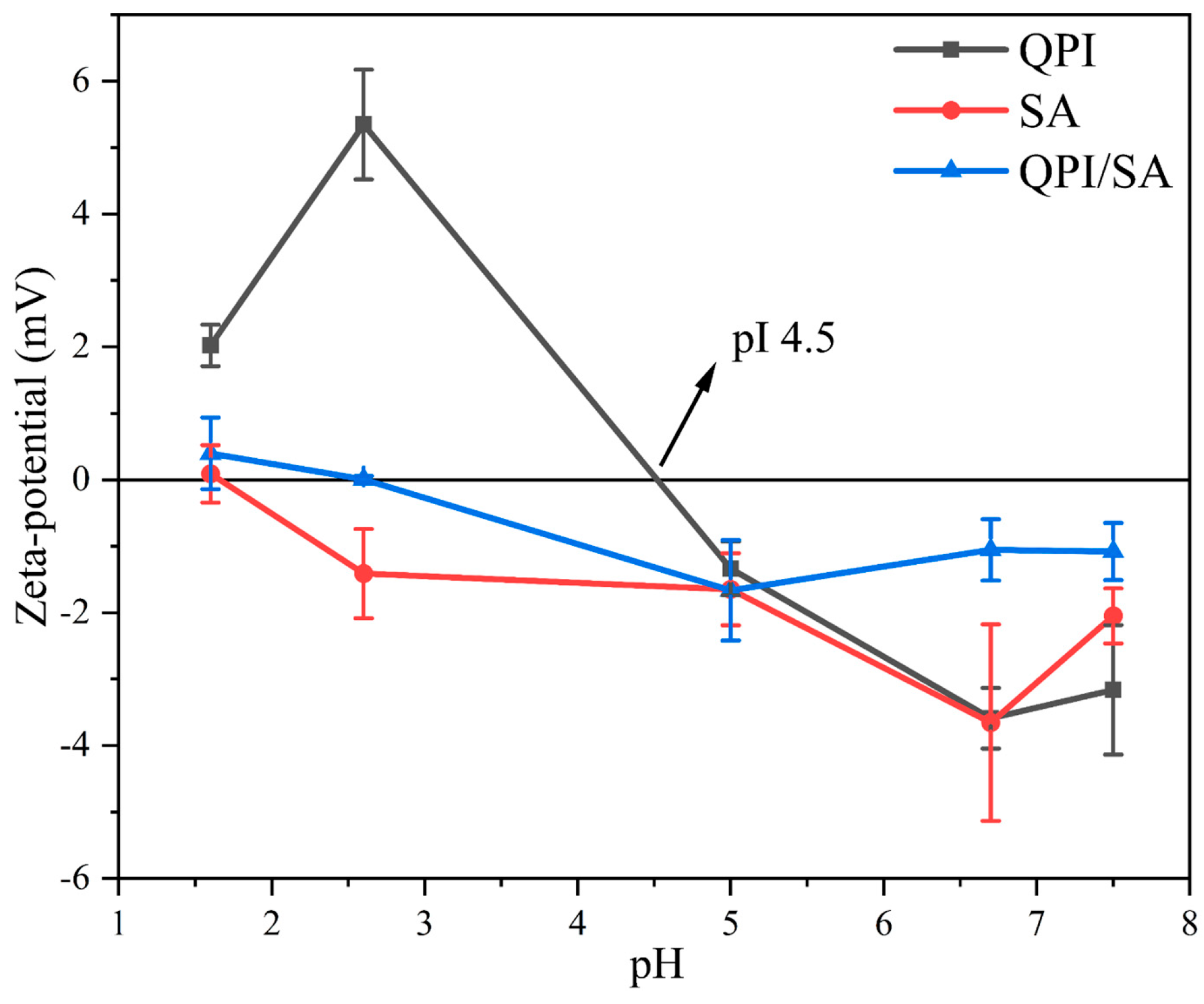
3.1.2. Zeta Potential
3.1.3. Fourier-Transform Infrared
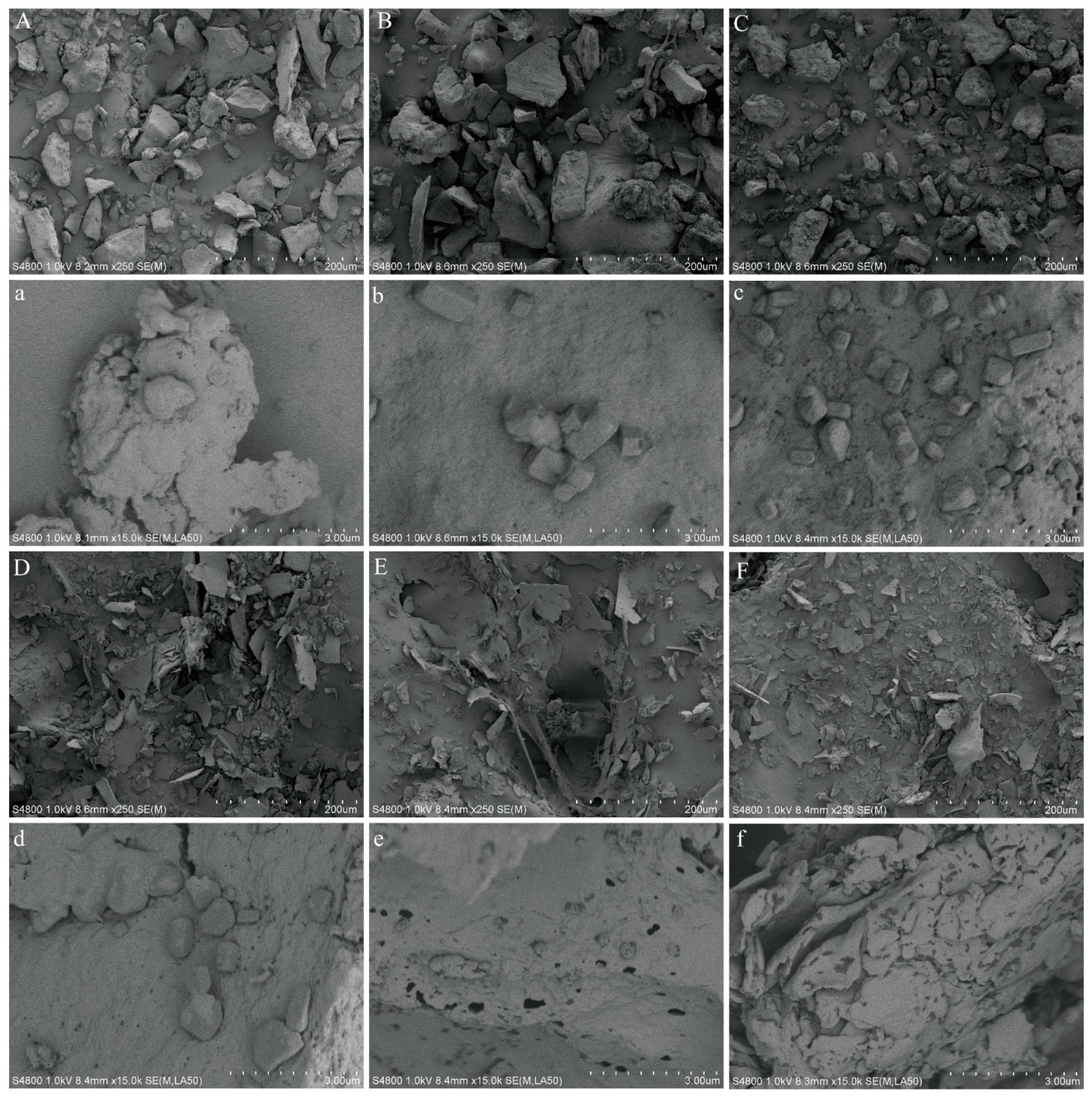
3.1.4. SEM
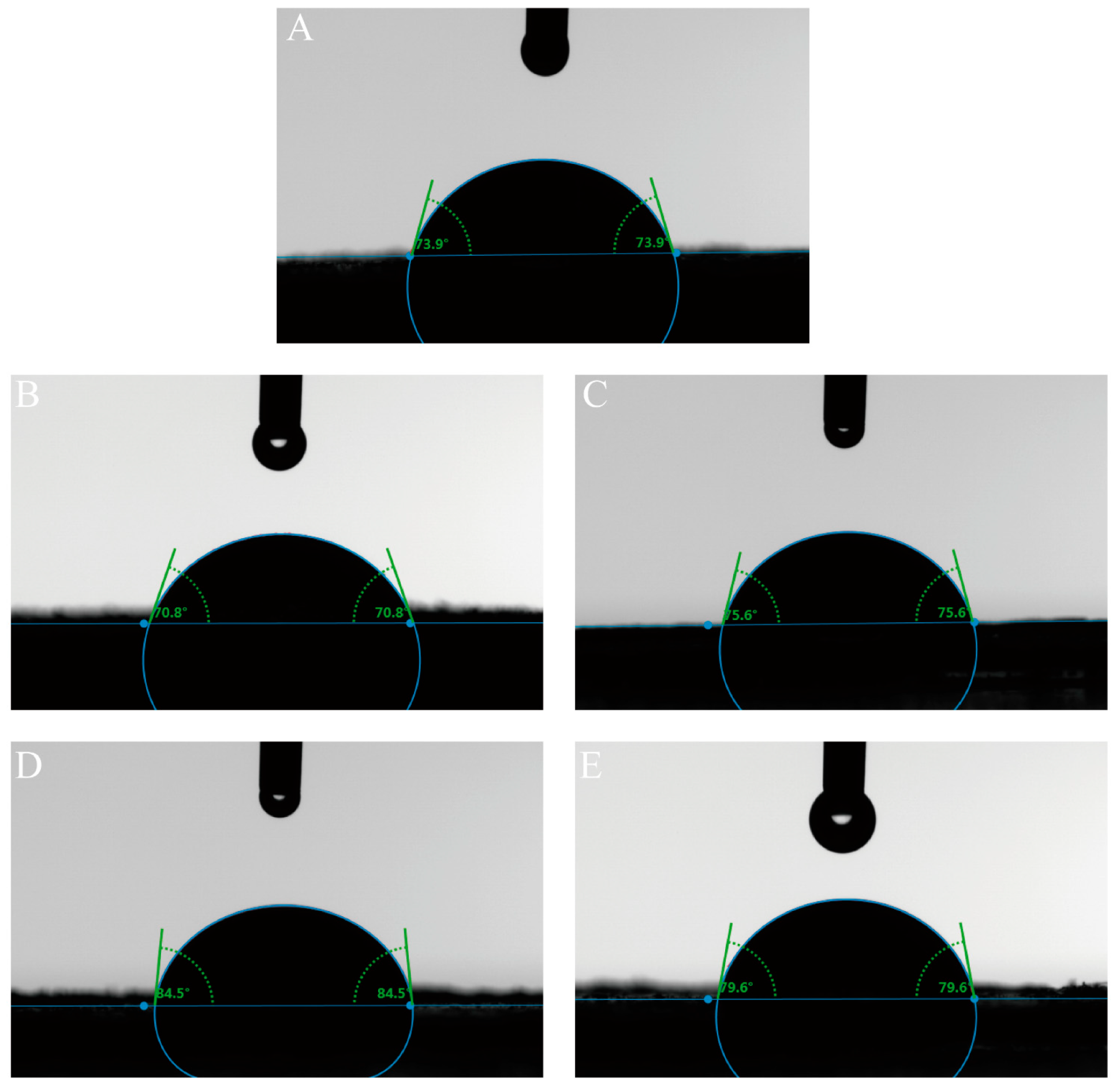
3.1.5. Three-Phase Contact Angle
3.2. Effects of Different Conditions on Pickering Emulsion Properties
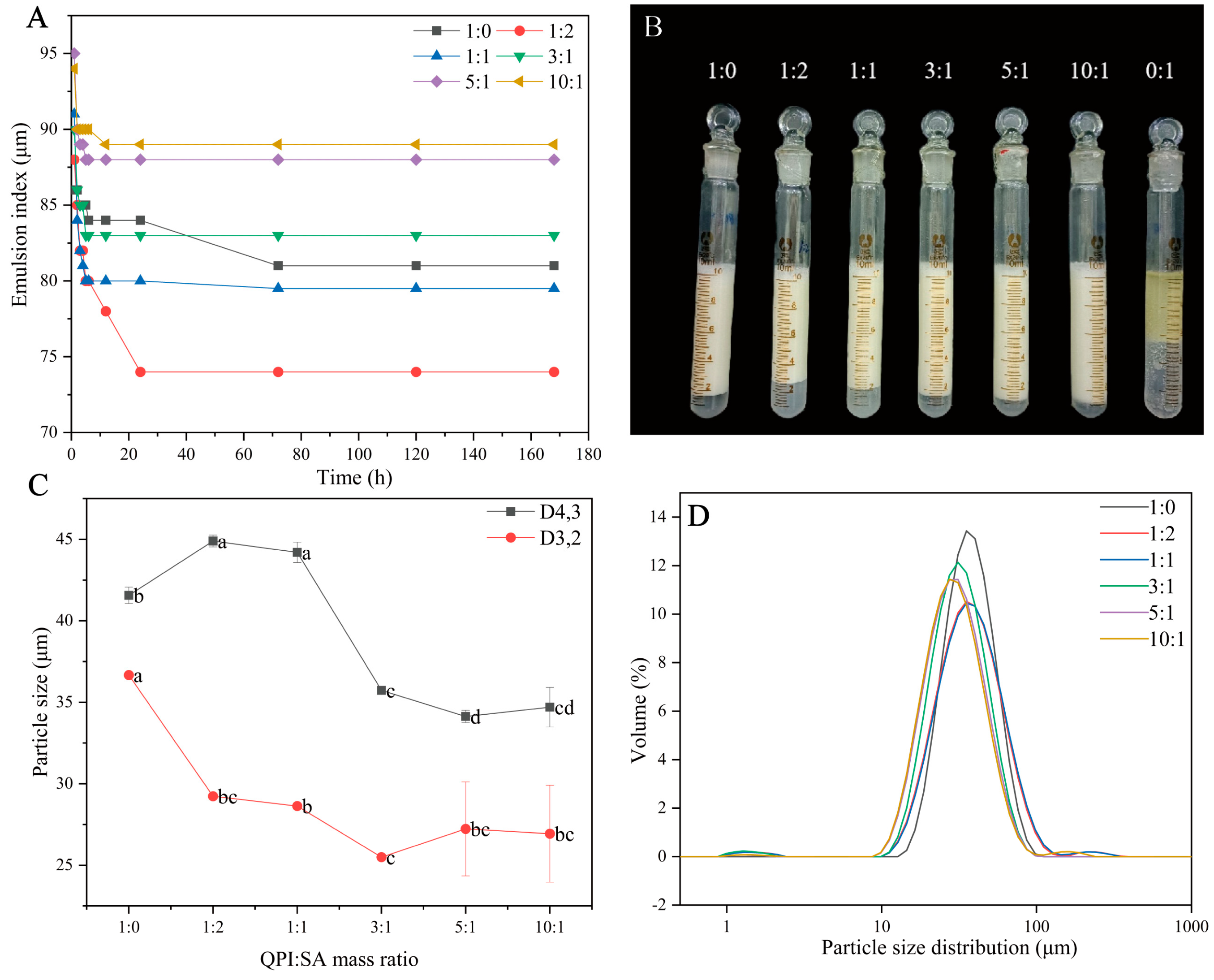
3.2.1. Effect of QPI/SA Mass Ratio on Physicochemical Properties of Pickering Emulsions
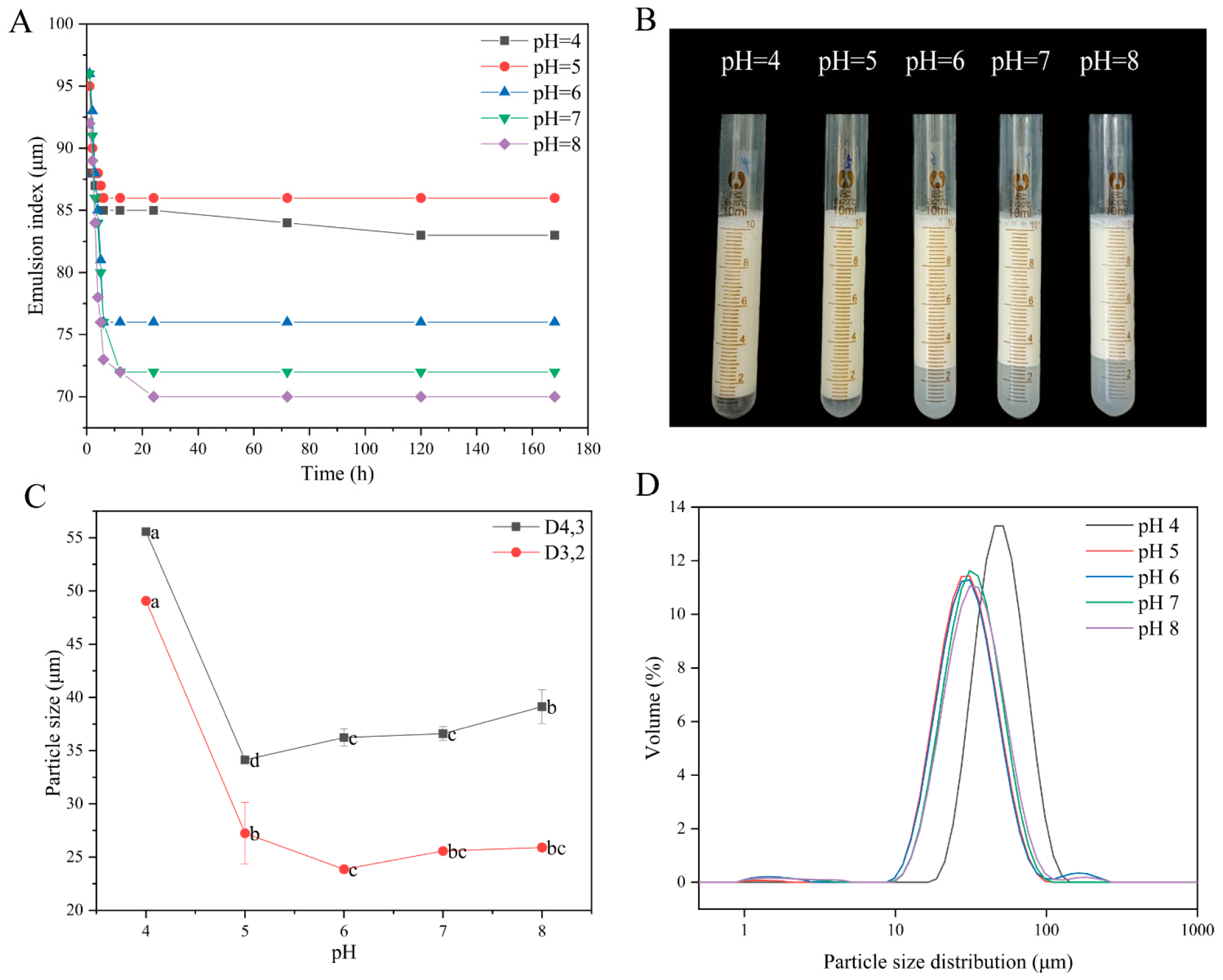
3.2.2. pH-Dependent Behavior of QPI/SA Complex-Stabilized Pickering Emulsions
3.2.3. Influence of Oil-Phase Volume Fraction on Pickering Emulsion Stability
3.3. Characterization of Curcumin-Loaded Pickering Emulsions
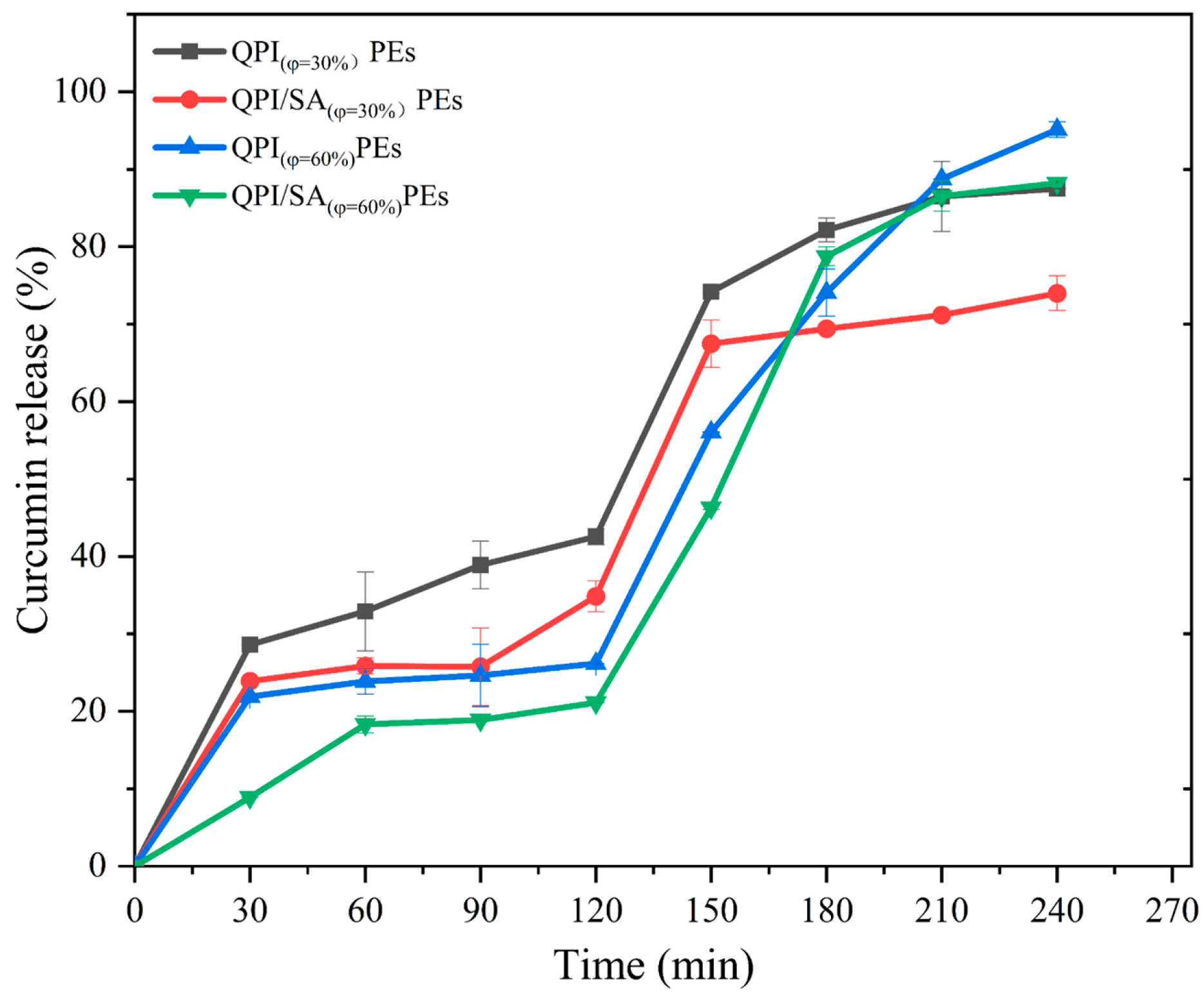
3.3.1. In Vitro Release Kinetics Analysis
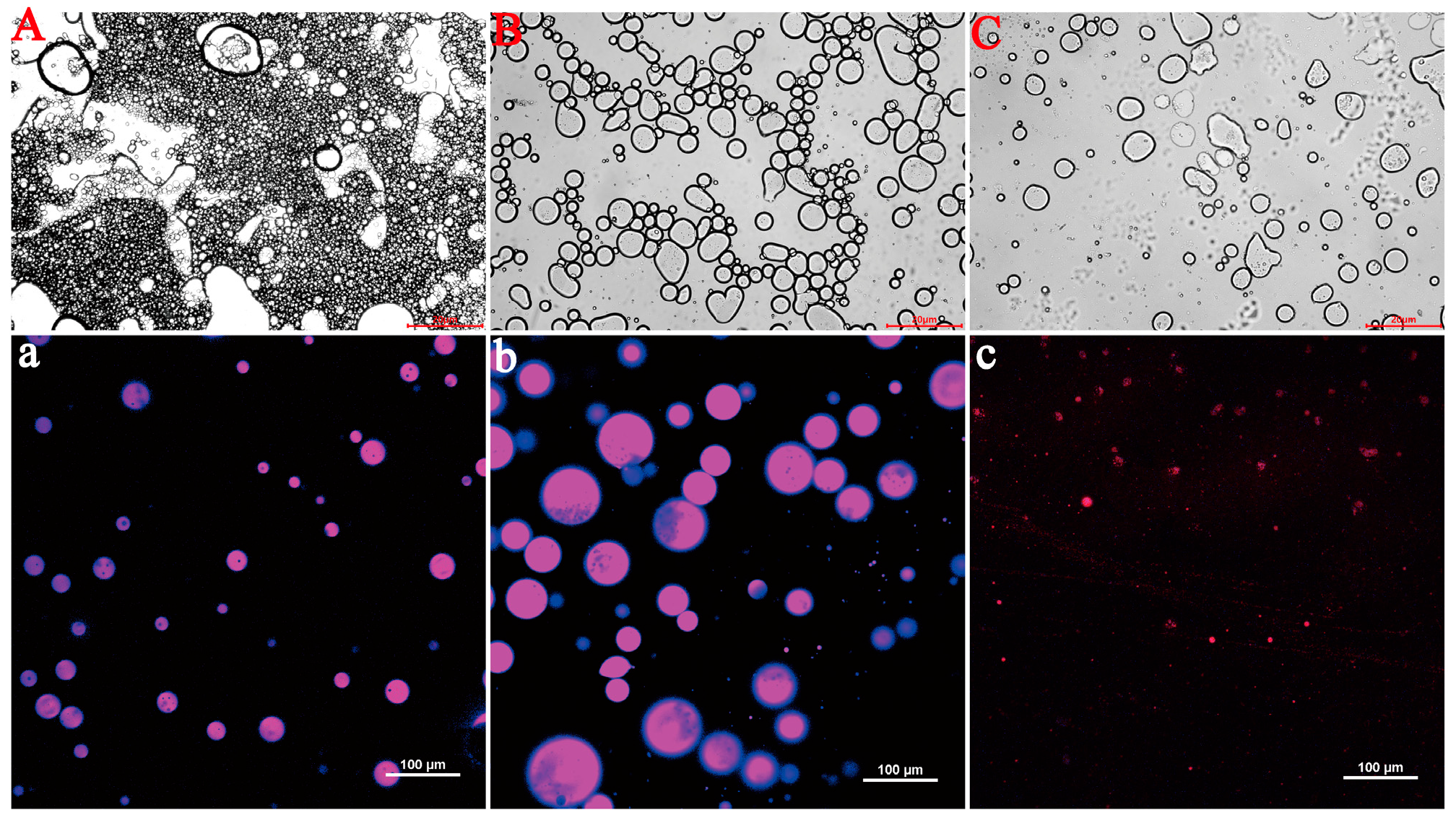
3.3.2. Microstructural Characterization by Confocal Laser Scanning Microscopy (CLSM)
3.3.3. Evaluation of Anticancer Efficacy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Linke, C.; Drusch, S. Pickering emulsions in foods-opportunities and limitations. Crit. Rev. Food Sci. Nutr. 2018, 58, 1971–1985. [Google Scholar] [CrossRef]
- Wang, C.; Wu, J.; Wang, C.; Mu, C.; Ngai, T.; Lin, W. Advances in Pickering emulsions stabilized by protein particles: Toward particle fabrication, interaction and arrangement. Food Res. Int. 2022, 157, 111380. [Google Scholar] [CrossRef]
- Chang, C.; Li, X.; Zhai, J.; Su, Y.; Gu, L.; Li, J.; Yang, Y. Stability of protein particle based Pickering emulsions in various environments: Review on strategies to inhibit coalescence and oxidation. Food Chem. X 2023, 18, 100651. [Google Scholar] [CrossRef]
- de Carvalho-Guimarães, F.B.; Correa, K.L.; de Souza, T.P.; Rodriguez Amado, J.R.; Ribeiro-Costa, R.M.; Silva-Júnior, J.O.C. A review of Pickering emulsions: Perspectives and applications. Pharmaceuticals 2022, 15, 1413. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, Z.; Chen, X.; Zhang, W.; Xie, Y.; Chen, Y.; Liu, Z.; Yuan, W. An overview of Pickering emulsions: Solid-particle materials, classification, morphology, and applications. Front. Pharmacol. 2017, 8, 235054. [Google Scholar] [CrossRef]
- Trofimov, A.D.; Ivanova, A.A.; Zyuzin, M.V.; Timin, A.S. Porous inorganic carriers based on silica, calcium carbonate and calcium phosphate for controlled/modulated drug delivery: Fresh outlook and future perspectives. Pharmaceutics 2018, 10, 167. [Google Scholar] [CrossRef]
- Ribeiro, E.F.; Morell, P.; Nicoletti, V.R.; Quiles, A.; Hernando, I. Protein-and polysaccharide-based particles used for Pickering emulsion stabilisation. Food Hydrocoll. 2021, 119, 106839. [Google Scholar] [CrossRef]
- Jiang, Y.-H.; Cheng, J.-H.; Sun, D.-W. Effects of plasma chemistry on the interfacial performance of protein and polysaccharide in emulsion. Trends Food Sci. Technol. 2020, 98, 129–139. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Q.; Liu, Z.; Zhi, L.; Jiao, B.; Hu, H.; Ma, X.; Agyei, D.; Shi, A. Plant protein-based emulsifiers: Mechanisms, techniques for emulsification enhancement and applications. Food Hydrocoll. 2023, 144, 109008. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, W.; Jiang, J.; Khan, M.A.; Liang, L. A comprehensive review of protein-based carriers with simple structures for the co-encapsulation of bioactive agents. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2017–2042. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, R.; Li, L. The interaction between anionic polysaccharides and legume protein and their influence mechanism on emulsion stability. Food Hydrocoll. 2022, 131, 107814. [Google Scholar] [CrossRef]
- Xu, X.; Luo, L.; Liu, C.; McClements, D.J. Utilization of anionic polysaccharides to improve the stability of rice glutelin emulsions: Impact of polysaccharide type, pH, salt, and temperature. Food Hydrocoll. 2017, 64, 112–122. [Google Scholar] [CrossRef]
- Ragavan, K.V.; Hernandez-Hernandez, O.; Martinez, M.M.; Gutiérrez, T.J. Organocatalytic esterification of polysaccharides for food applications: A review. Trends Food Sci. Technol. 2022, 119, 45–56. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, X.; Wang, Y.; Ye, W.; Lin, Y.; Zhang, Y.; Zhang, K.; Zhao, K.; Guo, H. The non-covalent and covalent interactions of whey proteins and saccharides: Influencing factor and utilization in food. Crit. Rev. Food Sci. Nutr. 2024, 65, 3896–3910. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, Z.; Lai, B.; Wang, C.; Wu, H. Recent advances in marine-derived protein/polysaccharide hydrogels: Classification, fabrication, characterization, mechanism and food applications. Trends Food Sci. Technol. 2024, 151, 104637. [Google Scholar] [CrossRef]
- Scanlin, L.; Lewis, K.; Dugger, P. Quinoa as a sustainable protein source: Production, nutrition, and processing. In Sustainable Protein Sources; Elsevier: Amsterdam, The Netherlands, 2024; pp. 381–398. [Google Scholar]
- de Carvalho Oliveira, L.; Martinez-Villaluenga, C.; Frias, J.; Cartea, M.E.; Francisco, M.; Cristianini, M.; Peñas, E. High pressure-assisted enzymatic hydrolysis potentiates the production of quinoa protein hydrolysates with antioxidant and ACE-inhibitory activities. Food Chem. 2024, 447, 138887. [Google Scholar] [CrossRef]
- Guo, H.; Hao, Y.; Yang, X.; Ren, G.; Richel, A. Exploration on bioactive properties of quinoa protein hydrolysate and peptides: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 2896–2909. [Google Scholar] [CrossRef]
- Xi, X.; Fan, G.; Xue, H.; Peng, S.; Huang, W.; Zhan, J. Harnessing the potential of quinoa: Nutritional profiling, bioactive components, and implications for health promotion. Antioxidants 2024, 13, 829. [Google Scholar] [CrossRef]
- Casalvara, R.F.A.; Ferreira, B.M.R.; Gonçalves, J.E.; Yamaguchi, N.U.; Bracht, A.; Bracht, L.; Comar, J.F.; de Sá-Nakanishi, A.B.; de Souza, C.G.M.; Castoldi, R. Biotechnological, nutritional, and therapeutic applications of quinoa (Chenopodium quinoa Willd.) and its by-products: A review of the past five-year findings. Nutrients 2024, 16, 840. [Google Scholar] [CrossRef]
- Zhong, L.; Lyu, W.; Lin, Z.; Lu, J.; Geng, Y.; Song, L.; Zhang, H. Quinoa ameliorates hepatic steatosis, oxidative stress, inflammation and regulates the gut microbiota in nonalcoholic fatty liver disease rats. Foods 2023, 12, 1780. [Google Scholar] [CrossRef]
- King, A.H. Brown seaweed extracts (alginates). In Food Hydrocolloids; CRC Press: Boca Raton, FL, USA, 2019; pp. 115–188. [Google Scholar]
- Ye, S.; Xie, C.; Agar, O.T.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A. Alginates from brown seaweeds as a promising natural source: A review of its properties and health benefits. Food Rev. Int. 2024, 40, 2682–2710. [Google Scholar] [CrossRef]
- Sen, O.; Manna, S.; Nandi, G.; Jana, S.; Jana, S. Recent advances in alginate based gastroretentive technologies for drug delivery applications. Med. Nov. Technol. Devices 2023, 18, 100236. [Google Scholar] [CrossRef]
- Shaikh, M.A.J.; Alharbi, K.S.; Almalki, W.H.; Imam, S.S.; Albratty, M.; Meraya, A.M.; Alzarea, S.I.; Kazmi, I.; Al-Abbasi, F.A.; Afzal, O. Sodium alginate based drug delivery in management of breast cancer. Carbohydr. Polym. 2022, 292, 119689. [Google Scholar] [CrossRef]
- Shaikh, M.A.J.; Gupta, G.; Afzal, O.; Gupta, M.M.; Goyal, A.; Altamimi, A.S.A.; Alzarea, S.I.; Almalki, W.H.; Kazmi, I.; Negi, P. Sodium alginate-based drug delivery for diabetes management: A review. Int. J. Biol. Macromol. 2023, 236, 123986. [Google Scholar] [CrossRef]
- Szabó, L.; Gerber-Lemaire, S.; Wandrey, C. Strategies to functionalize the anionic biopolymer Na-alginate without restricting its polyelectrolyte properties. Polymers 2020, 12, 919. [Google Scholar] [CrossRef]
- Tao, L.; Shi, C.; Zi, Y.; Zhang, H.; Wang, X.; Zhong, J. A review on the chemical modification of alginates for food research: Chemical nature, modification methods, product types, and application. Food Hydrocoll. 2024, 147, 109338. [Google Scholar] [CrossRef]
- Zhao, N.; Zou, H.; Sun, S.; Yu, C. The interaction between sodium alginate and myofibrillar proteins: The rheological and emulsifying properties of their mixture. Int. J. Biol. Macromol. 2020, 161, 1545–1551. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, S.; Song, Y.; Jiang, Q.; Zhang, H. Preparation, characterization, and application of composite oleogels based on whey protein isolate and sodium alginate. Int. J. Biol. Macromol. 2025, 300, 140317. [Google Scholar] [CrossRef]
- Ye, H.; Chen, T.; Huang, M.; Ren, G.; Lei, Q.; Fang, W.; Xie, H. Exploration of the microstructure and rheological properties of sodium alginate-pectin-whey protein isolate stabilized Β-carotene emulsions: To improve stability and achieve gastrointestinal sustained release. Foods 2021, 10, 1991. [Google Scholar] [CrossRef]
- Xu, Y.; Jia, Z.; Wang, J.; Sun, J.; Song, R. Property and stability of astaxanthin emulsion based on pickering emulsion templating with zein and sodium alginate as stabilizer. Int. J. Mol. Sci. 2022, 23, 9386. [Google Scholar] [CrossRef]
- Romo, I.; Abugoch, L.; Tapia, C. Soluble complexes between chenopodins and alginate/chitosan: Intermolecular interactions and structural-physicochemical properties. Carbohydr. Polym. 2020, 227, 115334. [Google Scholar] [CrossRef]
- Zhong, W.; Wang, Q.; Shen, X. Quinoa protein/polysaccharide electrostatic complex stabilized vegan high internal phase emulsions for 3D printing: Role of complex state and gelling-type polysaccharides. Food Chem. 2024, 434, 137447. [Google Scholar] [CrossRef]
- Hegde, M.; Kumar, A.; Girisa, S.; Aswani, B.S.; Vishwa, R.; Sethi, G.; Kunnumakkara, A.B. Nanoformulations of curcumin: An alliance for effective cancer therapeutics. Food Biosci. 2023, 56, 103095. [Google Scholar] [CrossRef]
- Yang, Z.; Yan, J.; Duan, Y.; Dai, L.; Wang, Y.; Sun, Q.; McClements, D.J.; Xu, X. Hydrolyzed rice glutelin nanoparticles as particulate emulsifier for Pickering emulsion: Structure, interfacial properties, and application for encapsulating curcumin. Food Hydrocoll. 2023, 134, 108105. [Google Scholar] [CrossRef]
- Zeng, Z.; Deng, S.; Liu, Y.; Li, C.; Fang, Z.; Hu, B.; Chen, H.; Wang, C.; Chen, S.; Wu, W. Targeting transportation of curcumin by soybean lipophilic protein nano emulsion: Improving its bioaccessibility and regulating intestinal microorganisms in mice. Food Hydrocoll. 2023, 142, 108781. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, H.; Feng, Z.; An, T.; Liu, F. A stable peony seed oil emulsion that enhances the stability, antioxidant activity, and bioaccessibility of curcumin. LWT 2023, 173, 114408. [Google Scholar] [CrossRef]
- Feng, J.; Tian, H.; Chen, X.; Cai, X.; Shi, X.; Wang, S. Interaction between fish gelatin and tremella polysaccharides from aqueous solutions to complex coacervates: Structure and rheological properties. Food Hydrocoll. 2023, 138, 108439. [Google Scholar] [CrossRef]
- Bai, Y.; Sun, Y.; Li, X.; Ren, J.; Sun, C.; Chen, X.; Dong, X.; Qi, H. Phycocyanin/lysozyme nanocomplexes to stabilize Pickering emulsions for fucoxanthin encapsulation. Food Res. Int. 2023, 173, 113386. [Google Scholar] [CrossRef]
- Ji, Y.; Han, C.; Liu, E.; Li, X.; Meng, X.; Liu, B. Pickering emulsions stabilized by pea protein isolate-chitosan nanoparticles: Fabrication, characterization and delivery EPA for digestion in vitro and in vivo. Food Chem. 2022, 378, 132090. [Google Scholar] [CrossRef]
- Liu, J.; Shim, Y.Y.; Reaney, M.J. Ionic strength and hydrogen bonding effects on whey protein isolate–flaxseed gum coacervate rheology. Food Sci. Nutr. 2020, 8, 2102–2111. [Google Scholar] [CrossRef]
- Huang, M.; Xu, Y.; Chen, X.; Xu, L.; Bai, Y.; Xu, X.; Zeng, X. Improved emulsifying properties of water-soluble myofibrillar proteins at acidic pH conditions: Emphasizing pH-regulated electrostatic interactions with chitosan. Int. J. Biol. Macromol. 2024, 257, 128557. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, R.; Decker, E.A.; McClements, D.J. Development of food-grade filled hydrogels for oral delivery of lipophilic active ingredients: pH-triggered release. Food Hydrocoll. 2015, 44, 345–352. [Google Scholar] [CrossRef]
- Liu, X.; Qin, X.; Wang, Y.; Zhong, J. Physicochemical properties and formation mechanism of whey protein isolate-sodium alginate complexes: Experimental and computational study. Food Hydrocoll. 2022, 131, 107786. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, Y.; Yuan, X.; Zhao, S.; Kang, Z.; Zhu, M.; He, H.; Ma, H. Physicochemical, conformational and functional changes of quinoa protein affected by high-pressure homogenization. LWT 2023, 173, 114343. [Google Scholar] [CrossRef]
- Wang, S.; Miao, S.; Sun, D.-W. Modifying structural and techno-functional properties of quinoa proteins through extraction techniques and modification methods. Trends Food Sci. Technol. 2024, 143, 104285. [Google Scholar] [CrossRef]
- Lade, B.D.; Dhowlaghar, N.; Pillai, S.S.; Patil, B.S. Physicochemical, mechanical, and antimicrobial properties of sodium alginate films as carriers of zein emulsion with pelargonic acid and eugenol for active food packing. Food Packag. Shelf Life 2023, 40, 101202. [Google Scholar] [CrossRef]
- Mir, N.A.; Riar, C.S.; Singh, S. Structural modification of quinoa seed protein isolates (QPIs) by variable time sonification for improving its physicochemical and functional characteristics. Ultrason. Sonochem. 2019, 58, 104700. [Google Scholar] [CrossRef]
- Li, M.; He, S. Utilization of zein-based particles in Pickering emulsions: A review. Food Rev. Int. 2023, 39, 4040–4060. [Google Scholar] [CrossRef]
- Derkach, S.R.; Voron’ko, N.G.; Sokolan, N.I.; Kolotova, D.S.; Kuchina, Y.A. Interactions between gelatin and sodium alginate: UV and FTIR studies. J. Dispers. Sci. Technol. 2020, 41, 690–698. [Google Scholar] [CrossRef]
- Liu, H.; Huang, R.; Zhao, X.; Yang, S.; He, F.; Qin, W.; Huang, J.; Yu, G.; Feng, Y.; Li, J. Ca2+/pH-triggered gelation of Pickering emulsion in vitro digestion: Visualization and sustained-release performance. Food Hydrocoll. 2023, 140, 108583. [Google Scholar] [CrossRef]
- Meng, Y.; Nicolai, T. The effect of the contact angle on particle stabilization and bridging in water-in-water emulsions. J. Colloid Interface Sci. 2023, 638, 506–512. [Google Scholar] [CrossRef]
- Wang, W.; Sun, R.; Ji, S.; Xia, Q. Effects of κ-carrageenan on the emulsifying ability and encapsulation properties of pea protein isolate-grape seed oil emulsions. Food Chem. 2024, 435, 137561. [Google Scholar] [CrossRef] [PubMed]
- Gürbüz, G.; Kauntola, V.; Ramos Diaz, J.M.; Jouppila, K.; Heinonen, M. Oxidative and physical stability of oil-in-water emulsions prepared with quinoa and amaranth proteins. Eur. Food Res. Technol. 2018, 244, 469–479. [Google Scholar] [CrossRef]
- Kim, W.; Wang, Y.; Selomulya, C. Impact of sodium alginate on binary whey/pea protein-stabilised emulsions. J. Food Eng. 2022, 321, 110978. [Google Scholar] [CrossRef]
- Yi, J.; Gan, C.; Wen, Z.; Fan, Y.; Wu, X. Development of pea protein and high methoxyl pectin colloidal particles stabilized high internal phase pickering emulsions for β-carotene protection and delivery. Food Hydrocoll. 2021, 113, 106497. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhu, Y.; Li, F.; Du, J.; Huang, Q.; Sun-Waterhouse, D.; Li, D. Antioxidative pectin from hawthorn wine pomace stabilizes and protects Pickering emulsions via forming zein-pectin gel-like shell structure. Int. J. Biol. Macromol. 2020, 151, 193–203. [Google Scholar] [CrossRef]
- Song, X.; Pei, Y.; Qiao, M.; Ma, F.; Ren, H.; Zhao, Q. Preparation and characterizations of Pickering emulsions stabilized by hydrophobic starch particles. Food Hydrocoll. 2015, 45, 256–263. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, C.; Liu, X.; Mackie, A.; Zhang, M.; Dai, L.; Liu, J.; Mao, L.; Yuan, F.; Gao, Y. Co-encapsulation of curcumin and β-carotene in Pickering emulsions stabilized by complex nanoparticles: Effects of microfluidization and thermal treatment. Food Hydrocoll. 2022, 122, 107064. [Google Scholar] [CrossRef]
- Teixé-Roig, J.; Oms-Oliu, G.; Odriozola-Serrano, I.; Martín-Belloso, O. Enhancing the gastrointestinal stability of curcumin by using sodium alginate-based nanoemulsions containing natural emulsifiers. Int. J. Mol. Sci. 2022, 24, 498. [Google Scholar] [CrossRef]
- Qian, C.; Decker, E.A.; Xiao, H.; McClements, D.J. Nanoemulsion delivery systems: Influence of carrier oil on β-carotene bioaccessibility. Food Chem. 2012, 135, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Gallier, S.; Ye, A.; Singh, H. Structural changes of bovine milk fat globules during in vitro digestion. J. Dairy Sci. 2012, 95, 3579–3592. [Google Scholar] [CrossRef]
- Bellesi, F.A.; Martinez, M.J.; Ruiz-Henestrosa, V.M.P.; Pilosof, A.M. Comparative behavior of protein or polysaccharide stabilized emulsion under in vitro gastrointestinal conditions. Food Hydrocoll. 2016, 52, 47–56. [Google Scholar] [CrossRef]
- Han, J.; Chen, F.; Gao, C.; Zhang, Y.; Tang, X. Environmental stability and curcumin release properties of Pickering emulsion stabilized by chitosan/gum arabic nanoparticles. Int. J. Biol. Macromol. 2020, 157, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Loyola, A.C.; Dao, K.; Shang, R.; Zhang, L.; Dutta, P.; Fowler, C.; Li, J.; Li, W.X. Streptonigrin at low concentration promotes heterochromatin formation. Sci. Rep. 2020, 10, 3478. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Shen, Q.; Wang, Y.; Wen, Y.; Dong, S.; Shi, X.; Shao, S. Rapidly and Directly Formed O/W Pickering Emulsion Gels Stabilized by Zein/Pectin Complex Nanoparticles: Encapsulation, Delivery, and In Vitro Gastrointestinal Digestion Behavior of Curcumin. Food Biophys. 2025, 20, 63. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Li, J.; Liu, S.; Yang, H.; Lu, F.; Zhu, M. Quinoa Protein/Sodium Alginate Complex-Stabilized Pickering Emulsion for Sustained Release of Curcumin and Enhanced Anticancer Activity Against HeLa Cells. Foods 2025, 14, 2705. https://doi.org/10.3390/foods14152705
Zhu Y, Li J, Liu S, Yang H, Lu F, Zhu M. Quinoa Protein/Sodium Alginate Complex-Stabilized Pickering Emulsion for Sustained Release of Curcumin and Enhanced Anticancer Activity Against HeLa Cells. Foods. 2025; 14(15):2705. https://doi.org/10.3390/foods14152705
Chicago/Turabian StyleZhu, Yiqun, Jianan Li, Shuhong Liu, Hongli Yang, Fei Lu, and Minpeng Zhu. 2025. "Quinoa Protein/Sodium Alginate Complex-Stabilized Pickering Emulsion for Sustained Release of Curcumin and Enhanced Anticancer Activity Against HeLa Cells" Foods 14, no. 15: 2705. https://doi.org/10.3390/foods14152705
APA StyleZhu, Y., Li, J., Liu, S., Yang, H., Lu, F., & Zhu, M. (2025). Quinoa Protein/Sodium Alginate Complex-Stabilized Pickering Emulsion for Sustained Release of Curcumin and Enhanced Anticancer Activity Against HeLa Cells. Foods, 14(15), 2705. https://doi.org/10.3390/foods14152705





