Functional Foods in Clinical Trials and Future Research Directions
Abstract
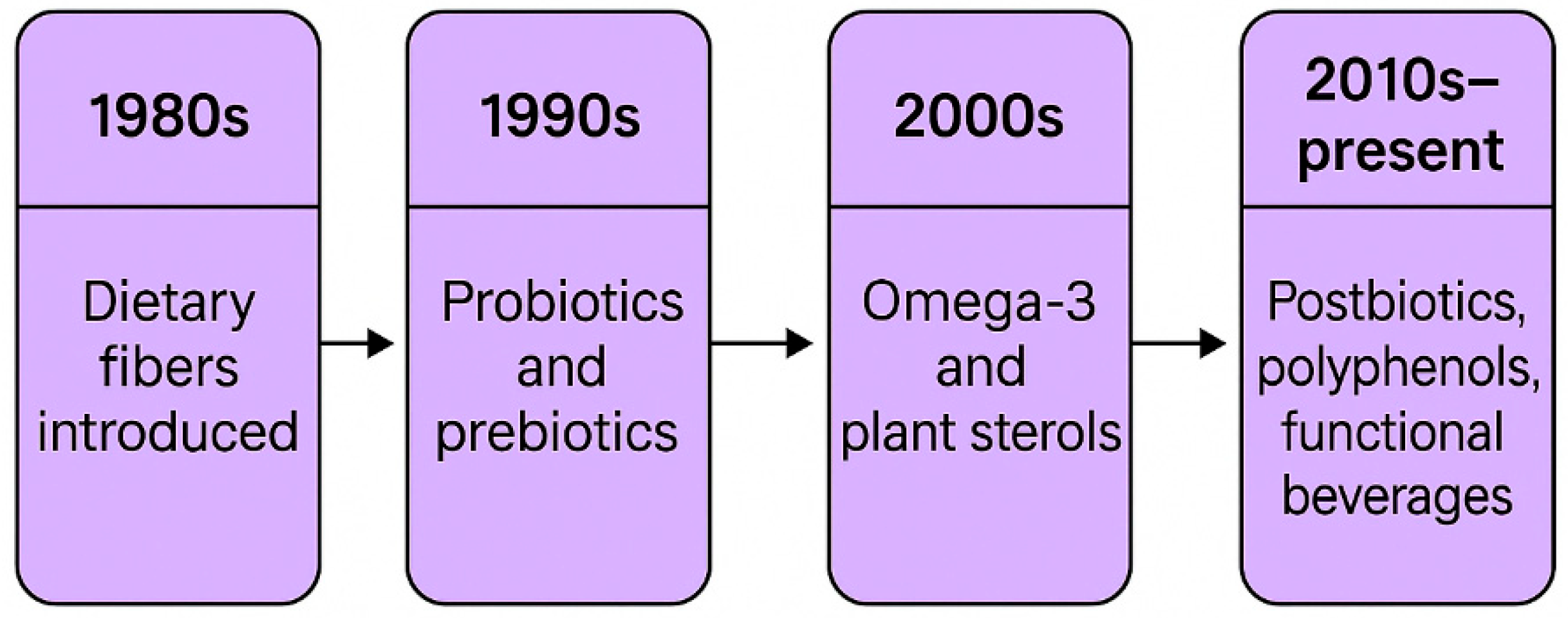
1. Introduction to Functional Foods
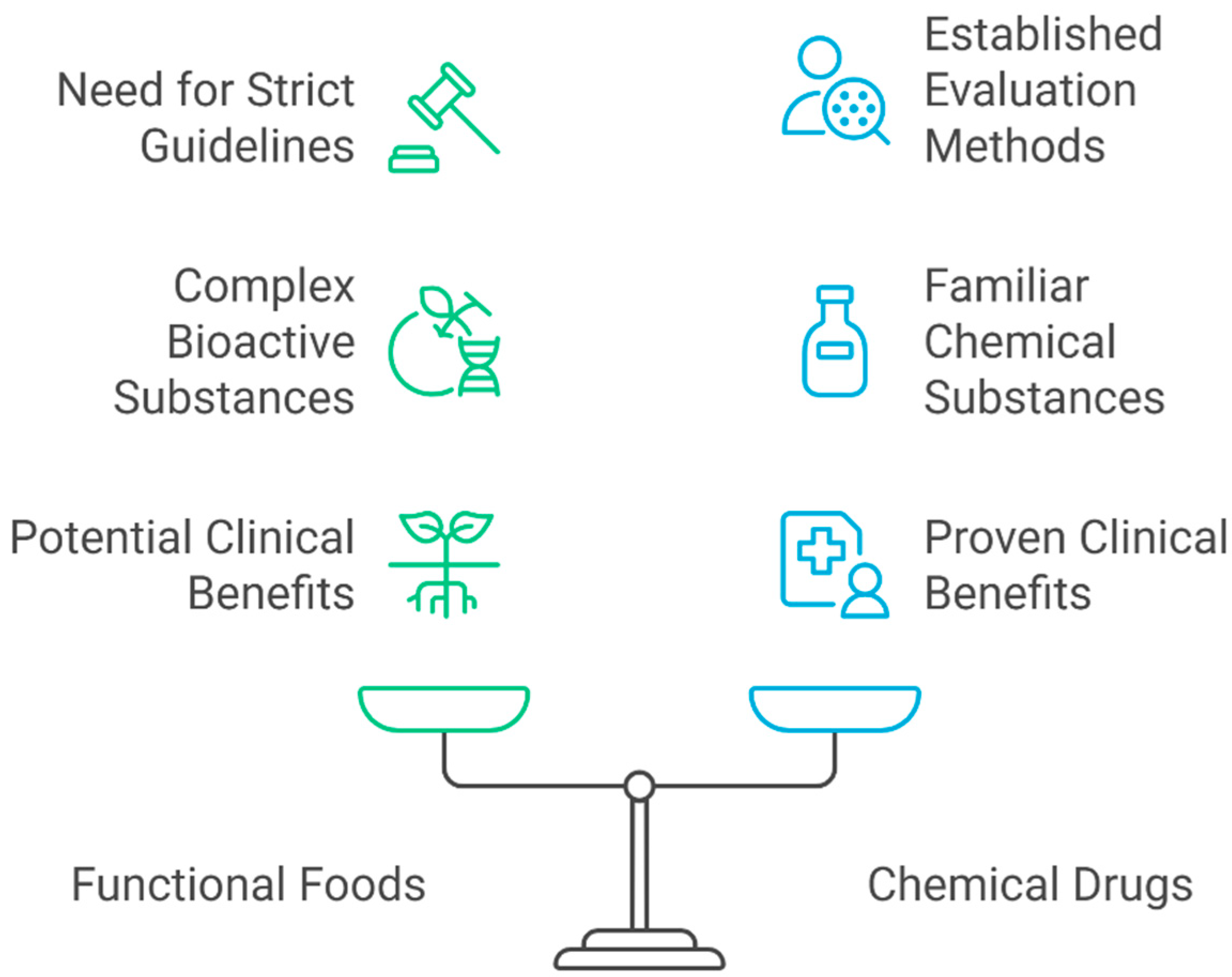
2. Importance of Clinical Trials
| Feature | Pharmaceutical Trials | Functional Food Trials | References |
|---|---|---|---|
| Primary goal | Efficacy and safety | Health promotion and prevention | [16] |
| Study design complexity | High (controlled, standardized) | High (dietary habits vary) | [15] |
| Regulatory oversight | Strict (FDA, EMA) | Emerging, diverse globally | [17] |
| Confounding variables | Minimally present | Highly present (diet, lifestyle) | [17] |
3. Bioactive Compounds in Functional Foods
3.1. Probiotics
3.2. Prebiotics
3.3. Postbiotics
3.4. Omega-3 Fatty Acids
3.5. Antioxidants
3.6. Peptides
3.7. Phenolic Compounds
3.8. Glucosinolates
3.9. Carotenoids
3.10. Phytosterols
3.11. Alkaloids
3.12. Saponins
4. Regulatory Framework
5. Functional Foods in Clinical Trials
| Trial Design | Primary Objective | Advantages | Limitations | References |
|---|---|---|---|---|
| Randomized controlled trial (RCT) | Assess efficacy and safety under controlled conditions | High internal validity, minimizes bias, provides high-quality evidence, gold standard in clinical evaluation | Time-consuming, resource-intensive, limited for pre/post-market evaluations, consumer behavior variability introduces bias | [100,101,102] |
| Crossover trial | Compare treatments within the same subjects | Requires fewer participants, reduces inter-subject variability | Risk of carry-over effects, longer study duration | [103,104] |
| Parallel-group trial | Compare outcomes between separate groups | Simple design, no carry-over effects | Requires larger sample sizes | [105,106] |
| Open-label trial | Evaluate treatment effect when blinding is not feasible | Easier to conduct, reflects real-world conditions | Increased risk of observer and participant bias | [107,108] |
| Blinded (single/double/triple) | Reduce bias in reporting and assessment | Enhances credibility of findings | Complex logistics, not always feasible in nutrition studies | [109] |
| Observational cohort study | Monitor outcomes in natural settings over time | Reflects real-life conditions, useful for long-term effects, explores prevention guidelines | Cannot establish causality, more confounding factors | [110,111] |
| Cross-sectional study | Examine correlations at a single point in time | Useful for identifying associations and informing hypotheses | Cannot establish causality, depends on timing of data collection | [112,113] |
| Authors (Year) | Design (N, Duration) | Key Findings | References |
|---|---|---|---|
| Timmers et al. (2016) | Double-blind, placebo-controlled crossover RCT; 17 T2D patients; 30 days (150 mg/d resveratrol vs. placebo) | Resveratrol did not improve hepatic or peripheral insulin sensitivity. It did enhance mitochondrial function but had no effect on insulin resistance. | [114] |
| Zare et al. (2019) | Triple-blind RCT (parallel groups); 140 T2D patients (BMI stratified); 3 months (500 mg × 2 daily cinnamon vs. placebo) | Cinnamon supplementation improved BMI and body fat, and significantly reduced fasting/postprandial glucose, HbA1c, fasting insulin and HOMA-IR. Total cholesterol, LDL and HDL cholesterol also improved (triglycerides unchanged). Benefits were greater in patients with BMI ≥ 27. | [115] |
| Dehghan et al. (2013) | RCT, placebo-controlled; 49 women with T2D; 8 weeks (10 g/d inulin vs. maltodextrin) | High-performance inulin significantly lowered fasting glucose, HbA1c, total cholesterol, triglycerides, LDL and LDL/HDL and TC/HDL ratios, while raising HDL. No significant changes in the placebo group. | [116] |
| Wolever et al. (2021) | Double-blind RCT; 207 adults (LDL 3.0–5.0 mmol/L); 4 weeks (3 × 1 g high-MW oat β-glucan drink/day vs. rice powder) | Oat β-glucan lowered LDL-C and total cholesterol. It also reduced non-HDL and TC:HDL ratio, translating to ~8% reduction in 10-year Framingham CVD risk. No changes in HDL, TG, glucose or insulin. | [117] |
| Ried et al. (2016) | Double-blind RCT; 88 patients with uncontrolled hypertension; 12 weeks (aged garlic extract 1.2 g/d vs. placebo) | Aged garlic extract significantly reduced blood pressure. Trends suggested improvements in central hemodynamics, arterial stiffness and inflammatory markers, though most were non-significant. | [118] |
| Atefi et al. (2018) | RCT; 77 women with T2D; 8 weeks (30 g/day: olive oil vs. canola oil vs. sunflower oil) | Replacing saturated-fat oil with canola or olive oil lowered inflammation. Both canola and olive oil groups showed significant reductions in CRP versus baseline and vs. sunflower oil (p < 0.05). No significant differences were seen in blood glucose or other lipids between groups. | [119] |
| Stote et al. (2020) | Double-blind RCT; 52 men with T2D; 8 weeks (22 g freeze-dried blueberry powder/day vs. placebo) | Blueberry intake improved glycemic and lipid markers. HbA1c was significantly lower in the blueberry group vs. placebo. Fructosamine, serum triglycerides, and liver enzymes (AST, ALT) also fell significantly with blueberries. Fasting glucose, insulin, cholesterol and CRP were unchanged. | [120] |
| Richter et al. (2021) | Double-blind crossover RCT; 40 overweight/obese adults with elevated BP; 8 weeks cranberry juice (500 mL/d) vs. placebo, 8-week washout | Cranberry supplementation had modest cardiovascular benefits. It did not change central BP but lowered 24 h ambulatory diastolic BP during daytime. It altered lipoproteins: large LDL particles and LDL size both increased with cranberry vs. placebo. No effect on central SBP or LDL-C concentration. | [121] |
| Chuengsamarn et al. (2012) | Double-blind RCT; 240 prediabetic adults; 9 months (750 mg curcumin/day vs. placebo) | Curcumin prevented progression to T2D p < 0. Curcumin also improved β-cell function (HOMA-β ↑, HOMA-IR ↓) and raised adiponectin. FPG and 2h-glucose remained stable in curcumin group but rose in controls. | [122] |
| Chatree et al. (2021) | RCT; 40 obese adults; 8 weeks (300 mg EGCG/day vs. placebo) | EGCG (green tea extract) significantly lowered metabolic risk factors. After 8 weeks, fasting triglycerides, systolic BP and diastolic BP. No significant changes in body weight, glucose or insulin were observed. | [123] |
| Berryman et al. (2015) | Controlled-feeding crossover RCT (6 wk each); 48 adults with high LDL-C; 1.5 oz/d almonds vs. isocaloric muffin | Almonds markedly improved lipids. Almonds also reduced abdominal fat, despite no change in body weight. | [124] |
6. Challenges in Conducting Trials
6.1. Participant Compliance
6.2. Funding Issues
7. Future Directions in Research
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martirosyan, D.; Ekblad, M. Functional Foods Classification System: Exemplifying through Analysis of Bioactive Compounds. Funct. Food Sci. Online 2022, 2, 94–123, ISSN 2767-3146. [Google Scholar] [CrossRef]
- Vlaicu, P.A.; Untea, A.E.; Varzaru, I.; Saracila, M.; Oancea, A.G. Designing Nutrition for Health—Incorporating Dietary By-Products into Poultry Feeds to Create Functional Foods with Insights into Health Benefits, Risks, Bioactive Compounds, Food Component Functionality and Safety Regulations. Foods 2023, 12, 4001. [Google Scholar] [CrossRef] [PubMed]
- Tur, J.A.; Bibiloni, M.M. Functional Foods. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 157–161. ISBN 978-0-12-384953-3. [Google Scholar]
- Iwatani, S.; Yamamoto, N. Functional Food Products in Japan: A Review. Food Sci. Hum. Wellness 2019, 8, 96–101. [Google Scholar] [CrossRef]
- Pravst, I. Functional Foods in Europe: A Focus on Health Claims. In Scientific, Health and Social Aspects of the Food Industry; IntechOpen: London, UK, 2012; ISBN 978-953-307-916-5. [Google Scholar]
- Alongi, M.; Anese, M. Re-Thinking Functional Food Development through a Holistic Approach. J. Funct. Foods 2021, 81, 104466. [Google Scholar] [CrossRef]
- Adefegha, S.A. Functional Foods and Nutraceuticals as Dietary Intervention in Chronic Diseases; Novel Perspectives for Health Promotion and Disease Prevention. J. Diet. Suppl. 2018, 15, 977–1009. [Google Scholar] [CrossRef]
- Essa, M.M.; Bishir, M.; Bhat, A.; Chidambaram, S.B.; Al-Balushi, B.; Hamdan, H.; Govindarajan, N.; Freidland, R.P.; Qoronfleh, M.W. Functional Foods and Their Impact on Health. J. Food Sci. Technol. 2023, 60, 820–834. [Google Scholar] [CrossRef]
- Cho, S. The Role of Functional Foods in Cutaneous Anti-Aging. J. Lifestyle Med. 2014, 4, 8–16. [Google Scholar] [CrossRef]
- Strouphauer, E.; Parke, M.; Perez-Sanchez, A.; Tantry, E.; Katta, R. Functional Foods in Dermatology. Dermatol. Pract. Concept. 2023, 13, e2023256. [Google Scholar] [CrossRef]
- Konstantinidi, M.; Koutelidakis, A.E. Functional Foods and Bioactive Compounds: A Review of Its Possible Role on Weight Management and Obesity’s Metabolic Consequences. Medecines 2019, 6, 94. [Google Scholar] [CrossRef]
- Mirmiran, P.; Bahadoran, Z.; Gaeini, Z. Common Limitations and Challenges of Dietary Clinical Trials for Translation into Clinical Practices. Int. J. Endocrinol. Metab. 2021, 19, e108170. [Google Scholar] [CrossRef]
- Marecek, S.; Martirosyan, D. An Assessment of Clinical Trials Used in Functional Food Science. Funct. Foods Health Dis. 2023, 13, 22–35. [Google Scholar] [CrossRef]
- Williams, K.; Oo, T.; Martirosyan, D. Exploring the Effectiveness of Lactobacillus Probiotics in Weight Management: A Literature Review. Funct. Food Sci. Online 2023, 3, 45–54, ISSN 2767-3146. [Google Scholar] [CrossRef]
- El Sheikha, A.F. Nutritional Profile and Health Benefits of Ganoderma Lucidum “Lingzhi, Reishi, or Mannentake” as Functional Foods: Current Scenario and Future Perspectives. Foods 2022, 11, 1030. [Google Scholar] [CrossRef]
- Reque, P.M.; Brandelli, A. Encapsulation of Probiotics and Nutraceuticals: Applications in Functional Food Industry. Trends Food Sci. Technol. 2021, 114, 1–10. [Google Scholar] [CrossRef]
- Altun, H.K.; Ermumcu, M.S.K.; Kurklu, N.S. Evaluation of Dietary Supplement, Functional Food and Herbal Medicine Use by Dietitians during the COVID-19 Pandemic. Public Health Nutr. 2021, 24, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Sanwal, N.; Bareen, M.A.; Barua, S.; Sharma, N.; Joshua Olatunji, O.; Prakash Nirmal, N.; Sahu, J.K. Trends in Functional Beverages: Functional Ingredients, Processing Technologies, Stability, Health Benefits, and Consumer Perspective. Food Res. Int. 2023, 170, 113046. [Google Scholar] [CrossRef] [PubMed]
- Dixit, V.; Joseph Kamal, S.W.; Bajrang Chole, P.; Dayal, D.; Chaubey, K.K.; Pal, A.K.; Xavier, J.; Manjunath, B.T.; Bachheti, R.K. Functional Foods: Exploring the Health Benefits of Bioactive Compounds from Plant and Animal Sources. J. Food Qual. 2023, 2023, 5546753. [Google Scholar] [CrossRef]
- Sharma, L.; Yadav, A. Role of Functional Foods in Human Health and Disease Prevention. In Bioactive Components: A Sustainable System for Good Health and Well-Being; Thakur, M., Belwal, T., Eds.; Springer Nature: Singapore, 2023; pp. 225–243. ISBN 978-981-19-2366-1. [Google Scholar]
- Marra, A.; Manousakis, V.; Zervas, G.P.; Koutis, N.; Finos, M.A.; Adamantidi, T.; Panoutsopoulou, E.; Ofrydopoulou, A.; Tsoupras, A. Avocado and Its By-Products as Natural Sources of Valuable Anti-Inflammatory and Antioxidant Bioactives for Functional Foods and Cosmetics with Health-Promoting Properties. Appl. Sci. 2024, 14, 5978. [Google Scholar] [CrossRef]
- Natali, P.G.; Piantelli, M.; Sottini, A.; Eufemi, M.; Banfi, C.; Imberti, L. A Step Forward in Enhancing the Health-Promoting Properties of Whole Tomato as a Functional Food to Lower the Impact of Non-Communicable Diseases. Front. Nutr. 2025, 12, 1519905. [Google Scholar] [CrossRef]
- AlAli, M.; Alqubaisy, M.; Aljaafari, M.N.; AlAli, A.O.; Baqais, L.; Molouki, A.; Abushelaibi, A.; Lai, K.-S.; Lim, S.-H.E. Nutraceuticals: Transformation of Conventional Foods into Health Promoters/Disease Preventers and Safety Considerations. Molecules 2021, 26, 2540. [Google Scholar] [CrossRef]
- Vandorou, M.; Plakidis, C.; Tsompanidou, I.M.; Adamantidi, T.; Panagopoulou, E.A.; Tsoupras, A. A Review on Apple Pomace Bioactives for Natural Functional Food and Cosmetic Products with Therapeutic Health-Promoting Properties. Int. J. Mol. Sci. 2024, 25, 10856. [Google Scholar] [CrossRef]
- Ampofo, J.; Abbey, L. Microalgae: Bioactive Composition, Health Benefits, Safety and Prospects as Potential High-Value Ingredients for the Functional Food Industry. Foods 2022, 11, 1744. [Google Scholar] [CrossRef]
- Ashraf, S.A.; Elkhalifa, A.E.O.; Ahmad, M.F.; Patel, M.; Adnan, M.; Sulieman, A.M.E. Probiotic Fermented Foods and Health Promotion. In African Fermented Food Products-New Trends; Elhadi Sulieman, A.M., Adam Mariod, A., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 59–88. ISBN 978-3-030-82902-5. [Google Scholar]
- Bock, P.M.; Martins, A.F.; Schaan, B.D. Understanding How Pre- and Probiotics Affect the Gut Microbiome and Metabolic Health. Am. J. Physiol. -Endocrinol. Metab. 2024, 327, E89–E102. [Google Scholar] [CrossRef] [PubMed]
- Mousanejadi, N.; Barzegar, H.; Alizadeh Behbahani, B.; Jooyandeh, H. Production and Evaluation of a Functional Fruit Beverage Consisting of Mango Juice and Probiotic Bacteria. Food Meas. 2023, 17, 3240–3253. [Google Scholar] [CrossRef]
- Allahdad, Z.; Manus, J.; Aguilar-Uscanga, B.R.; Salmieri, S.; Millette, M.; Lacroix, M. Physico-Chemical Properties and Sensorial Appreciation of a New Fermented Probiotic Beverage Enriched with Pea and Rice Proteins. Plant Foods Hum. Nutr. 2022, 77, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.B.; Huang, H.; Ning, Y.; Xiao, J. Probiotics in the New Era of Human Milk Oligosaccharides (HMOs): HMO Utilization and Beneficial Effects of Bifidobacterium Longum Subsp. Infantis M-63 on Infant Health. Microorganisms 2024, 12, 1014. [Google Scholar] [CrossRef]
- Capozza, M.; Laforgia, N.; Rizzo, V.; Salvatore, S.; Guandalini, S.; Baldassarre, M. Probiotics and Functional Gastrointestinal Disorders in Pediatric Age: A Narrative Review. Front. Pediatr. 2022, 10, 805466. [Google Scholar] [CrossRef]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef]
- Chaudhari, A.Z.; Teli, D.; Balar, P.C.; Gandhi, J.; Chavda, V.P. Dietary Supplements and Nutraceuticals Against the Variants of SARS-CoV-2. In SARS-CoV-2 Variants and Global Population Vulnerability; Apple Academic Press: Palm Bay, FL, USA, 2024; ISBN 978-1-003-46793-9. [Google Scholar]
- Ferreira-Lazarte, A.; Moreno, F.J.; Villamiel, M. Bringing the Digestibility of Prebiotics into Focus: Update of Carbohydrate Digestion Models. Crit. Rev. Food Sci. Nutr. 2021, 61, 3267–3278. [Google Scholar] [CrossRef]
- Fei, Y.; Chen, Z.; Han, S.; Zhang, S.; Zhang, T.; Lu, Y.; Berglund, B.; Xiao, H.; Li, L.; Yao, M. Role of Prebiotics in Enhancing the Function of Next-Generation Probiotics in Gut Microbiota. Crit. Rev. Food Sci. Nutr. 2023, 63, 1037–1054. [Google Scholar] [CrossRef]
- De Giani, A.; Sandionigi, A.; Zampolli, J.; Michelotti, A.; Tursi, F.; Labra, M.; Di Gennaro, P. Effects of Inulin-Based Prebiotics Alone or in Combination with Probiotics on Human Gut Microbiota and Markers of Immune System: A Randomized, Double-Blind, Placebo-Controlled Study in Healthy Subjects. Microorganisms 2022, 10, 1256. [Google Scholar] [CrossRef]
- Włodarczyk, M.; Śliżewska, K. Efficiency of Resistant Starch and Dextrins as Prebiotics: A Review of the Existing Evidence and Clinical Trials. Nutrients 2021, 13, 3808. [Google Scholar] [CrossRef]
- Piccioni, A.; Covino, M.; Candelli, M.; Ojetti, V.; Capacci, A.; Gasbarrini, A.; Franceschi, F.; Merra, G. How Do Diet Patterns, Single Foods, Prebiotics and Probiotics Impact Gut Microbiota? Microbiol. Res. 2023, 14, 390–408. [Google Scholar] [CrossRef]
- Saleem, G.N.; Gu, R.; Qu, H.; Bahar Khaskheli, G.; Rashid Rajput, I.; Qasim, M.; Chen, X. Therapeutic Potential of Popular Fermented Dairy Products and Its Benefits on Human Health. Front. Nutr. 2024, 11, 1328620. [Google Scholar] [CrossRef]
- Liao, W.; Su, M.; Zhang, D. A Study on the Effect of Symbiotic Fermented Milk Products on Human Gastrointestinal Health: Double-Blind Randomized Controlled Clinical Trial. Food Sci. Nutr. 2022, 10, 2947–2955. [Google Scholar] [CrossRef]
- Maftei, N.-M.; Iancu, A.-V.; Goroftei Bogdan, R.E.; Gurau, T.V.; Ramos-Villarroel, A.; Pelin, A.-M. A Novel Symbiotic Beverage Based on Sea Buckthorn, Soy Milk and Inulin: Production, Characterization, Probiotic Viability, and Sensory Acceptance. Microorganisms 2023, 11, 736. [Google Scholar] [CrossRef]
- Kumar, A.; Green, K.M.; Rawat, M. A Comprehensive Overview of Postbiotics with a Special Focus on Discovery Techniques and Clinical Applications. Foods 2024, 13, 2937. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Ebrahimi, M.; Shahryari, S.; Kharazmi, M.S.; Jafari, S.M. Food Applications of Probiotic Yeasts; Focusing on Their Techno-Functional, Postbiotic and Protective Capabilities. Trends Food Sci. Technol. 2022, 128, 278–295. [Google Scholar] [CrossRef]
- Hijová, E. Postbiotics as Metabolites and Their Biotherapeutic Potential. Int. J. Mol. Sci. 2024, 25, 5441. [Google Scholar] [CrossRef] [PubMed]
- Dyall, S.C. Long-chain omega-3 fatty acids and the brain: A review of the independent and shared effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015, 7, 52. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tofiq, A.; Zetterberg, H.; Blennow, K.; Basun, H.; Cederholm, T.; Eriksdotter, M.; Faxén-Irving, G.; Hjorth, E.; Jernerén, F.; Schultzberg, M.; et al. Effects of Peroral Omega-3 Fatty Acid Supplementation on Cerebrospinal Fluid Biomarkers in Patients with Alzheimer’s Disease: A Randomized Controlled Trial—The OmegAD Study. J. Alzheimer’s Dis. 2021, 83, 1291–1301. [Google Scholar] [CrossRef]
- Pitkala, K.H.; Strandberg, T.E. Clinical Trials in Older People. Age Ageing 2022, 51, afab282. [Google Scholar] [CrossRef] [PubMed]
- Carta, M.G.; Cossu, G.; Pintus, E.; Zaccheddu, R.; Callia, O.; Conti, G.; Pintus, M.; Gonzalez, C.I.A.; Massidda, M.V.; Mura, G. Moderate Exercise Improves Cognitive Function in Healthy Elderly People: Results of a Randomized Controlled Trial. Clin. Pract. Epidemiol. Ment. Health 2021, 17, 75. [Google Scholar] [CrossRef] [PubMed]
- Barnes, L.L.; Dhana, K.; Liu, X.; Carey, V.J.; Ventrelle, J.; Johnson, K.; Hollings, C.S.; Bishop, L.; Laranjo, N.; Stubbs, B.J.; et al. Trial of the MIND Diet for Prevention of Cognitive Decline in Older Persons. N. Engl. J. Med. 2023, 389, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Dael, P. Role of N-3 Long-Chain Polyunsaturated Fatty Acids in Human Nutrition and Health: Review of Recent Studies and Recommendations. Nutr. Res. Pract. 2021, 15, 137. [Google Scholar] [CrossRef]
- Harris, W.S.; Tintle, N.L.; Imamura, F.; Qian, F.; Korat, A.V.A.; Marklund, M.; Djoussé, L.; Bassett, J.K.; Carmichael, P.-H.; Chen, Y.-Y. Blood N-3 Fatty Acid Levels and Total and Cause-Specific Mortality from 17 Prospective Studies. Nat. Commun. 2021, 12, 2329. [Google Scholar] [CrossRef]
- In Choi, J.; Lee, S.Y. Effects of Omega-3 Fatty Acids in Myocardial Infarction. In Omega-3 Fatty Acids: Keys to Nutritional Health and Disease; Zanwar, A.A., Adekar, S.P., Hegde, M.V., Eds.; Springer Nature: Cham, Switzerland, 2025; pp. 61–74. ISBN 978-3-031-84200-9. [Google Scholar]
- Golanski, J.; Szymanska, P.; Rozalski, M. Effects of Omega-3 Polyunsaturated Fatty Acids and Their Metabolites on Haemostasis—Current Perspectives in Cardiovascular Disease. Int. J. Mol. Sci. 2021, 22, 2394. [Google Scholar] [CrossRef]
- Parcheta, M.; Świsłocka, R.; Orzechowska, S.; Akimowicz, M.; Choińska, R.; Lewandowski, W. Recent Developments in Effective Antioxidants: The Structure and Antioxidant Properties. Materials 2021, 14, 1984. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several Lines of Antioxidant Defense against Oxidative Stress: Antioxidant Enzymes, Nanomaterials with Multiple Enzyme-Mimicking Activities, and Low-Molecular-Weight Antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Boutin, J.A.; Liberelle, M.; Yous, S.; Ferry, G.; Nepveu, F. Melatonin Facts: Lack of Evidence That Melatonin Is a Radical Scavenger in Living Systems. J. Pineal Res. 2024, 76, e12926. [Google Scholar] [CrossRef]
- Tumilaar, S.G.; Hardianto, A.; Dohi, H.; Kurnia, D. A Comprehensive Review of Free Radicals, Oxidative Stress, and Antioxidants: Overview, Clinical Applications, Global Perspectives, Future Directions, and Mechanisms of Antioxidant Activity of Flavonoid Compounds. J. Chem. 2024, 2024, 5594386. [Google Scholar] [CrossRef]
- Balboni, E.; Zagnoli, F.; Filippini, T.; Fairweather-Tait, S.J.; Vinceti, M. Zinc and Selenium Supplementation in COVID-19 Prevention and Treatment: A Systematic Review of the Experimental Studies. J. Trace Elem. Med. Biol. 2022, 71, 126956. [Google Scholar] [CrossRef] [PubMed]
- Kabwanga, I.T.; Ozturkoglu-Budak, S.; Kesari, K.K. Chapter 10—Bioactive Peptides Derived from Milk: Formation and Functional Benefits. In Bioactive Microbial Metabolites; Mishra, V., Mishra, J., Arora, N.K., Eds.; Developments in Applied Microbiology and Biotechnology; Academic Press: Cambridge, MA, USA, 2024; pp. 201–217. ISBN 978-0-443-18568-7. [Google Scholar]
- Saubenova, M.; Oleinikova, Y.; Rapoport, A.; Maksimovich, S.; Yermekbay, Z.; Khamedova, E. Bioactive Peptides Derived from Whey Proteins for Health and Functional Beverages. Fermentation 2024, 10, 359. [Google Scholar] [CrossRef]
- Adams, C.; Sawh, F.; Green-Johnson, J.M.; Jones Taggart, H.; Strap, J.L. Characterization of Casein-Derived Peptide Bioactivity: Differential Effects on Angiotensin-Converting Enzyme Inhibition and Cytokine and Nitric Oxide Production. J. Dairy Sci. 2020, 103, 5805–5815. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Jin, Z.; Qiu, C. Polyphenols as Plant-Based Nutraceuticals: Health Effects, Encapsulation, Nano-Delivery, and Application. Foods 2022, 11, 2189. [Google Scholar] [CrossRef] [PubMed]
- Aatif, M. Current Understanding of Polyphenols to Enhance Bioavailability for Better Therapies. Biomedicines 2023, 11, 2078. [Google Scholar] [CrossRef]
- Nanoencapsulation of Flavonoid Bioactives Using the Nanoprecipitation Technique—Review. In Connecting Expertise Multidisciplinary Development for the Future; Seven Editora: São José dos Pinhais, Brazil, 2024.
- Bischoff, K.L. Chapter 53—Glucosinolates. In Nutraceuticals, 2nd ed.; Gupta, R.C., Lall, R., Srivastava, A., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 903–909. ISBN 978-0-12-821038-3. [Google Scholar]
- Keap1–Nrf2 Signaling: A Target for Cancer Prevention by Sulforaphane. In Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2012; pp. 163–177. ISBN 978-3-642-34574-6.
- Alibrahem, W.; Nguyen, D.H.H.; Kharrat Helu, N.; Tóth, F.; Nagy, P.T.; Posta, J.; Prokisch, J.; Oláh, C. Health Benefits, Applications, and Analytical Methods of Freshly Produced Allyl Isothiocyanate. Foods 2025, 14, 579. [Google Scholar] [CrossRef]
- Li, L.; Ma, P.; Nirasawa, S.; Liu, H. Formation, Immunomodulatory Activities, and Enhancement of Glucosinolates and Sulforaphane in Broccoli Sprouts: A Review for Maximizing the Health Benefits to Human. Crit. Rev. Food Sci. Nutr. 2024, 64, 7118–7148. [Google Scholar] [CrossRef]
- Paiva, S.A.R.; Russell, R.M. β-Carotene and Other Carotenoids as Antioxidants. J. Am. Coll. Nutr. 1999, 18, 426–433. [Google Scholar] [CrossRef]
- Song, J.; Zhang, Y.; Wang, H.; Li, Y. The Influence of Food Matrix and Processing Methods on the Bioaccessibility of Lutein: A Review. J. Food Bioact. 2024, 26. [Google Scholar] [CrossRef]
- Ferreira, A.L.A.; Corrêa, C.R. Lycopene Bioavailability and Its Effects on Health. In Food Quality, Safety and Technology; Lima, G.P.P., Vianello, F., Eds.; Springer: Vienna, Austria, 2013; pp. 63–76. [Google Scholar]
- Rodríguez-Roque, M.J.; de Ancos, B.; Sánchez-Vega, R.; Sánchez-Moreno, C.; Cano, M.P.; Elez-Martínez, P.; Martín-Belloso, O. Food Matrix and Processing Influence on Carotenoid Bioaccessibility and Lipophilic Antioxidant Activity of Fruit Juice-Based Beverages. Food Funct. 2016, 7, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Poli, A.; Marangoni, F.; Corsini, A.; Manzato, E.; Marrocco, W.; Martini, D.; Medea, G.; Visioli, F. Phytosterols, Cholesterol Control, and Cardiovascular Disease. Nutrients 2021, 13, 2810. [Google Scholar] [CrossRef] [PubMed]
- The Bioavailability and Biological Activities of Phytosterols as Modulators of Cholesterol Metabolism. Available online: https://www.mdpi.com/1420-3049/27/2/523 (accessed on 8 July 2025).
- Bacchetti, T.; Masciangelo, S.; Bicchiega, V.; Bertoli, E.; Ferretti, G. Phytosterols, Phytostanols and Their Esters: From Natural to Functional Foods. Mediterr. J. Nutr. Metab. 2011, 4, 165–172. [Google Scholar] [CrossRef]
- Pardeshi, V.N.; Lokhande, T.; Mahajan, S.K.; Surana, K.R. Novel Applications of Functional Foods in Brain Health. In Applications of Functional Foods in Disease Prevention; Apple Academic Press: Palm Bay, FL, USA, 2024; pp. 71–88. [Google Scholar]
- Vignesh, A.; Amal, T.C.; Sarvalingam, A.; Vasanth, K. A Review on the Influence of Nutraceuticals and Functional Foods on Health. Food Chem. Adv. 2024, 5, 100749. [Google Scholar] [CrossRef]
- Chen, M.L.; Takeda, K.; Sundrud, M.S. Emerging Roles of Bile Acids in Mucosal Immunity and Inflammation. Mucosal Immunol. 2019, 12, 851–861. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Wadhwa, S.S. Industry-Relevant Approaches for Minimising the Bitterness of Bioactive Compounds in Functional Foods: A Review. Food Bioprocess. Technol. 2013, 6, 607–627. [Google Scholar] [CrossRef]
- Che, Y.; Xu, W.; Ding, C.; He, T.; Xu, X.; Shuai, Y.; Huang, H.; Wu, J.; Wang, Y.; Wang, C.; et al. Bile Acids Target Mitofusin 2 to Differentially Regulate Innate Immunity in Physiological versus Cholestatic Conditions. Cell Rep. 2023, 42, 112011. [Google Scholar] [CrossRef]
- Kamioka, H. Current Status and Issues on the Foods with Function Claims System in Japan: Evidence of Functionality of the Foods. Yakugaku Zasshi 2023, 143, 931–940. [Google Scholar] [CrossRef]
- Essential Guide to China’s Updated Health Food Regulations 2024. Available online: https://www.freyrsolutions.com/blog/essential-guide-to-chinas-new-health-food-and-supplements-regulations (accessed on 9 July 2025).
- Duttaroy, A.K. Chapter 19—Regulation of Functional Foods in European Union: Assessment of Health Claim by the European Food Safety Authority. In Nutraceutical and Functional Food Regulations in the United States and around the World, 3rd ed.; Bagchi, D., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 267–276. ISBN 978-0-12-816467-9. [Google Scholar]
- Bröring, S.; Khedkar, S. Regulatory Compliance and Company Strategies: The Case of the Nutrition and Health Claims RegulatioKalogerakoun (EC) No. 1924/2006. In Regulating and Managing Food Safety in the EU: A Legal-Economic Perspective; Bremmers, H., Purnhagen, K., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 105–128. ISBN 978-3-319-77045-1. [Google Scholar]
- Sato, K.; Kodama, K.; Sengoku, S. Optimizing the Relationship between Regulation and Innovation in Dietary Supplements: A Case Study of Food with Function Claims in Japan. Nutrients 2023, 15, 476. [Google Scholar] [CrossRef]
- Komala, M.G.; Ong, S.G.; Qadri, M.U.; Elshafie, L.M.; Pollock, C.A.; Saad, S. Investigating the Regulatory Process, Safety, Efficacy and Product Transparency for Nutraceuticals in the USA, Europe and Australia. Foods 2023, 12, 427. [Google Scholar] [CrossRef]
- Barroga, E.; Matanguihan, G. A Practical Guide to Writing Quantitative and Qualitative Research Questions and Hypotheses in Scholarly Articles. J. Korean Med. Sci. 2022, 37, e121. [Google Scholar] [CrossRef]
- Karunarathna, I.; De Alvis, K.; Gunasena, P.; Jayawardana, A. Designing and Conducting Clinical Research: Methodological Approaches; Uva Clinical Anaesthesia: Charlottesville, VA, USA, 2024. [Google Scholar]
- Kalogerakou, T.; Antoniadou, M. The Role of Dietary Antioxidants, Food Supplements and Functional Foods for Energy Enhancement in Healthcare Professionals. Antioxidants 2024, 13, 1508. [Google Scholar] [CrossRef]
- Vargas-Alvarez, M.A.; Navas-Carretero, S.; Palla, L.; Martínez, J.A.; Almiron-Roig, E. Impact of Portion Control Tools on Portion Size Awareness, Choice and Intake: Systematic Review and Meta-Analysis. Nutrients 2021, 13, 1978. [Google Scholar] [CrossRef]
- Zahren, C.; Harvey, S.; Weekes, L.; Bradshaw, C.; Butala, R.; Andrews, J.; O’Callaghan, S. Clinical Trials Site Recruitment Optimisation: Guidance from Clinical Trials: Impact and Quality. Clin. Trials 2021, 18, 174077452110159. [Google Scholar] [CrossRef]
- Shariq, S.; Cardoso Pinto, A.M.; Budhathoki, S.S.; Miller, M.; Cro, S. Barriers and Facilitators to the Recruitment of Disabled People to Clinical Trials: A Scoping Review. Trials 2023, 24, 171. [Google Scholar] [CrossRef] [PubMed]
- Agazie, G.; Anumarlapudi, A.; Archibald, A.M.; Baker, P.T.; Bécsy, B.; Blecha, L.; Bonilla, A.; Brazier, A.; Brook, P.R.; Burke-Spolaor, S. The NANOGrav 15 Yr Data Set: Constraints on Supermassive Black Hole Binaries from the Gravitational-Wave Background. Astrophys. J. Lett. 2023, 952, L37. [Google Scholar] [CrossRef]
- Shamloo, M.; Granger, M.J.; Trautwein, E.A.; House, J.D.; MacKay, D. Genetic Basis for Prediction of Non-Responders to Dietary Plant Sterol Intervention (GenePredict-PS): A Study Protocol for a Double-Blind, Placebo-Controlled, Randomized Two-Period Crossover Study. Trials 2020, 21, 452. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.-W.; Boutron, I.; Hopewell, S.; Moher, D.; Schulz, K.; Collins, G.; Tunn, R.; Aggarwal, R.; Berkwits, M.; Berlin, J.; et al. SPIRIT 2025 Statement: Updated Guideline for Protocols of Randomised Trials. BMJ 2025, 389, e081477. [Google Scholar] [CrossRef]
- Medicinal Mushrooms: Bioactive Compounds, Use, and Clinical Trials. Available online: https://www.mdpi.com/1422-0067/22/2/634 (accessed on 12 June 2025).
- Božić, D.; Živičnjak, M.; Stanković, R.; Ignjatić, A. Impact of the Product Master Data Quality on the Logistics Process Performance. Logistics 2024, 8, 43. [Google Scholar] [CrossRef]
- Badawy, S.; Liu, Y.; Guo, M.; Liu, Z.; Xie, C.; Marawan, M.A.; Ares, I.; Lopez-Torres, B.; Martínez, M.; Maximiliano, J.-E. Conjugated Linoleic Acid (CLA) as a Functional Food: Is It Beneficial or Not? Food Res. Int. 2023, 172, 113158. [Google Scholar] [CrossRef]
- Hadjimbei, E.; Botsaris, G.; Chrysostomou, S. Beneficial Effects of Yoghurts and Probiotic Fermented Milks and Their Functional Food Potential. Foods 2022, 11, 2691. [Google Scholar] [CrossRef]
- Di Francesco, F.; Lanza, A.; Di Blasio, M.; Vaienti, B.; Cafferata, E.A.; Cervino, G.; Cicciù, M.; Minervini, G. Application of Botulinum Toxin in Temporomandibular Disorders: A Systematic Review of Randomized Controlled Trials (RCTs). Appl. Sci. 2022, 12, 12409. [Google Scholar] [CrossRef]
- Lam, T.Y.; Cheung, M.F.; Munro, Y.L.; Lim, K.M.; Shung, D.; Sung, J.J. Randomized Controlled Trials of Artificial Intelligence in Clinical Practice: Systematic Review. J. Med. Internet Res. 2022, 24, e37188. [Google Scholar] [CrossRef] [PubMed]
- Abdelazeem, B.; Abbas, K.S.; Amin, M.A.; El-Shahat, N.A.; Malik, B.; Kalantary, A.; Eltobgy, M. The Effectiveness of Incentives for Research Participation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2022, 17, e0267534. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.K.; Lewis, C.E.; Varady, K.A.; Su, Y.R.; Madhur, M.S.; Lackland, D.T.; Reis, J.P.; Wang, T.J.; Lloyd-Jones, D.M.; Allen, N.B. Effect of Dietary Sodium on Blood Pressure: A Crossover Trial. JAMA 2023, 330, 2258–2266. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.P.; Wood, F.A.; Finegold, J.A.; Nowbar, A.N.; Thompson, D.M.; Arnold, A.D.; Rajkumar, C.A.; Connolly, S.; Cegla, J.; Stride, C.; et al. Side Effect Patterns in a Crossover Trial of Statin, Placebo, and No Treatment. J. Am. Coll. Cardiol. 2021, 78, 1210–1222. [Google Scholar] [CrossRef]
- Reignier, J.; Plantefève, G.; Mira, J.-P.; Argaud, L.; Asfar, P.; Aissaoui, N.; Badie, J.; Botoc, N.-V.; Brisard, L.; Bui, H.-N. Low versus Standard Calorie and Protein Feeding in Ventilated Adults with Shock: A Randomised, Controlled, Multicentre, Open-Label, Parallel-Group Trial (NUTRIREA-3). Lancet Respir. Med. 2023, 11, 602–612. [Google Scholar] [CrossRef]
- Fiorentino, G.; Coppola, A.; Izzo, R.; Annunziata, A.; Bernardo, M.; Lombardi, A.; Trimarco, V.; Santulli, G.; Trimarco, B. Effects of Adding L-Arginine Orally to Standard Therapy in Patients with COVID-19: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Trial. Results of the First Interim Analysis. EClinicalMedicine 2021, 40, 101125. [Google Scholar] [CrossRef]
- Raffaelli, B.; De Icco, R.; Corrado, M.; Terhart, M.; Ailani, J. Open-Label Trials for CGRP-Targeted Drugs in Migraine Prevention: A Narrative Review. Cephalalgia 2023, 43, 03331024221137091. [Google Scholar] [CrossRef]
- Kliewer, K.L.; Gonsalves, N.; Dellon, E.S.; Katzka, D.A.; Abonia, J.P.; Aceves, S.S.; Arva, N.C.; Besse, J.A.; Bonis, P.A.; Caldwell, J.M. One-Food versus Six-Food Elimination Diet Therapy for the Treatment of Eosinophilic Oesophagitis: A Multicentre, Randomised, Open-Label Trial. Lancet Gastroenterol. Hepatol. 2023, 8, 408–421. [Google Scholar] [CrossRef]
- Flanary, J.; Rengel, Z.; Sathianathen, N.; Lane, R.; Jarosek, S.; Barkve, N.; Weight, C. Rates of editor-authored Manuscripts among Urology Journals Using Blinded or non-blinded Review. Learn. Publ. 2023, 36, 171–177. [Google Scholar] [CrossRef]
- Staplin, N.; de la Sierra, A.; Ruilope, L.M.; Emberson, J.R.; Vinyoles, E.; Gorostidi, M.; Ruiz-Hurtado, G.; Segura, J.; Baigent, C.; Williams, B. Relationship between Clinic and Ambulatory Blood Pressure and Mortality: An Observational Cohort Study in 59 124 Patients. Lancet 2023, 401, 2041–2050. [Google Scholar] [CrossRef] [PubMed]
- Bass, G.A.; Kaplan, L.J.; Ryan, É.J.; Cao, Y.; Lane-Fall, M.; Duffy, C.C.; Vail, E.A.; Mohseni, S. The Snapshot Audit Methodology: Design, Implementation and Analysis of Prospective Observational Cohort Studies in Surgery. Eur. J. Trauma Emerg. Surg. 2023, 49, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Cvetkovic-Vega, A.; Maguiña, J.L.; Soto, A.; Lama-Valdivia, J.; Correa López, L.E. Cross-Sectional Studies. Rev. Fac. Med. Humana 2021, 21, 164–170. [Google Scholar]
- Hunziker, S.; Blankenagel, M. Cross-Sectional Research Design. In Research Design in Business and Management; Springer Fachmedien Wiesbaden: Wiesbaden, Germany, 2024; pp. 187–199. ISBN 978-3-658-42738-2. [Google Scholar]
- Timmers, S.; de Ligt, M.; Phielix, E.; van de Weijer, T.; Hansen, J.; Moonen-Kornips, E.; Schaart, G.; Kunz, I.; Hesselink, M.K.C.; Schrauwen-Hinderling, V.B.; et al. Resveratrol as Add-on Therapy in Subjects with Well-Controlled Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Care 2016, 39, 2211–2217. [Google Scholar] [CrossRef]
- Zare, R.; Nadjarzadeh, A.; Zarshenas, M.M.; Shams, M.; Heydari, M. Efficacy of Cinnamon in Patients with Type II Diabetes Mellitus: A Randomized Controlled Clinical Trial. Clin. Nutr. 2019, 38, 549–556. [Google Scholar] [CrossRef]
- Dehghan, P.; Gargari, B.P.; Asgharijafarabadi, M. Effects of High Performance Inulin Supplementation on Glycemic Status and Lipid Profile in Women with Type 2 Diabetes: A Randomized, Placebo-Controlled Clinical Trial. Health Promot. Perspect. 2013, 3, 55. [Google Scholar][Green Version]
- Wolever, T.; Rahn, M.; Dioum, E.; Spruill, S.E.; Ezatagha, A.; Campbell, J.E.; Jenkins, A.L.; Chu, Y. An Oat β-Glucan Beverage Reduces LDL Cholesterol and Cardiovascular Disease Risk in Men and Women with Borderline High Cholesterol: A Double-Blind, Randomized, Controlled Clinical Trial. J. Nutr. 2021, 151, 2655–2666. [Google Scholar] [CrossRef]
- Ried, K.; Travica, N.; Sali, A. The Effect of Aged Garlic Extract on Blood Pressure and Other Cardiovascular Risk Factors in Uncontrolled Hypertensives: The AGE at Heart Trial. Integr. Blood Press. Control 2016, 9, 9–21. [Google Scholar] [CrossRef]
- Atefi, M.; Pishdad, G.R.; Faghih, S. The Effects of Canola and Olive Oils on Insulin Resistance, Inflammation and Oxidative Stress in Women with Type 2 Diabetes: A Randomized and Controlled Trial. J. Diabetes Metab. Disord. 2018, 17, 85–91. [Google Scholar] [CrossRef]
- Stote, K.S.; Wilson, M.M.; Hallenbeck, D.; Thomas, K.; Rourke, J.M.; Sweeney, M.I.; Gottschall-Pass, K.T.; Gosmanov, A.R. Effect of Blueberry Consumption on Cardiometabolic Health Parameters in Men with Type 2 Diabetes: An 8-Week, Double-Blind, Randomized, Placebo-Controlled Trial. Curr. Dev. Nutr. 2020, 4, nzaa030. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.K.; Skulas-Ray, A.C.; Gaugler, T.L.; Meily, S.; Petersen, K.S.; Kris-Etherton, P.M. Effects of Cranberry Juice Supplementation on Cardiovascular Disease Risk Factors in Adults with Elevated Blood Pressure: A Randomized Controlled Trial. Nutrients 2021, 13, 2618. [Google Scholar] [CrossRef] [PubMed]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Luechapudiporn, R.; Phisalaphong, C.; Jirawatnotai, S. Curcumin Extract for Prevention of Type 2 Diabetes. Diabetes Care 2012, 35, 2121–2127. [Google Scholar] [CrossRef] [PubMed]
- Chatree, S.; Sitticharoon, C.; Maikaew, P.; Pongwattanapakin, K.; Keadkraichaiwat, I.; Churintaraphan, M.; Sripong, C.; Sririwichitchai, R.; Tapechum, S. Corrigendum: Epigallocatechin Gallate Decreases Plasma Triglyceride, Blood Pressure, and Serum Kisspeptin in Obese Human Subjects. Exp. Biol. Med. 2024, 249, 10179. [Google Scholar] [CrossRef]
- Berryman, C.E.; West, S.G.; Fleming, J.A.; Bordi, P.L.; Kris-Etherton, P.M. Effects of Daily Almond Consumption on Cardiometabolic Risk and Abdominal Adiposity in Healthy Adults with Elevated LDL-Cholesterol: A Randomized Controlled Trial. J. Am. Heart Assoc. 2015, 4, e000993. [Google Scholar] [CrossRef]
- Laville, M.; Segrestin, B.; Alligier, M.; Ruano-Rodríguez, C.; Serra-Majem, L.; Hiesmayr, M.; Schols, A.; La Vecchia, C.; Boirie, Y.; Rath, A.; et al. Evidence-Based Practice within Nutrition: What Are the Barriers for Improving the Evidence and How Can They Be Dealt With? Trials 2017, 18, 425. [Google Scholar] [CrossRef]
- Gómez-Llorente, H.; Fernández-Segovia, I.; Pérez-Esteve, É.; Ribes, S.; Rivas, A.; Ruiz-Rico, M.; Barat, J.M. Immobilization of Natural Antimicrobial Compounds on Food-Grade Supports as a New Strategy to Preserve Fruit-Derived Foods. Foods 2023, 12, 2060. [Google Scholar] [CrossRef]
- Rivero-Pino, F. Bioactive Food-Derived Peptides for Functional Nutrition: Effect of Fortification, Processing and Storage on Peptide Stability and Bioactivity within Food Matrices. Food Chem. 2023, 406, 135046. [Google Scholar] [CrossRef]
- Huang, S.; Nahm, A.Y.; Song, Z. Government Subsidies of New Energy Vehicle Industry and Enterprise Innovation: Moderating Role of Chief Executive Officers’ Technical Background. Manag. Decis. Econ. 2023, 44, 2137–2147. [Google Scholar] [CrossRef]
- Abdul Hakim, B.N.; Xuan, N.J.; Oslan, S.N.H. A Comprehensive Review of Bioactive Compounds from Lactic Acid Bacteria: Potential Functions as Functional Food in Dietetics and the Food Industry. Foods 2023, 12, 2850. [Google Scholar] [CrossRef]
- Dai, X.; Qin, K.; Wu, L. Study on Effect of Collaborative Governance Participation Willingness of Online Food Delivery Platform Restaurants and Consumers from Perspective of Control Theory: Based on Moderating Effects of Perceived Risks. Front. Psychol. 2023, 14, 1149538. [Google Scholar] [CrossRef]
- Puri, V.; Nagpal, M.; Singh, I.; Singh, M.; Dhingra, G.A.; Huanbutta, K.; Dheer, D.; Sharma, A.; Sangnim, T. A Comprehensive Review on Nutraceuticals: Therapy Support and Formulation Challenges. Nutrients 2022, 14, 4637. [Google Scholar] [CrossRef]
- Evemy, J.; Berry, C.; Yates, E. Low Interest Rates, Low Productivity, Low Growth? A Multi-Sector Case Study of UK-Based Firms’ Funding and Investment Strategies in the Context of Loose Monetary Policy. New Political Econ. 2024, 29, 240–259. [Google Scholar] [CrossRef]
- Varacca, A.; Soregaroli, C.; Kardung, M.; Espa, I.; Colombo, I.; Cortesi, B.; Wesseler, J. The Effect of the EU’s Novel Food Regulations on Firm Investment Decisions. J. Agric. Econ. 2025, 76, 211–229. [Google Scholar] [CrossRef]
- Mishra, R.; Tripathi, A.D.; Singh, R.B.; Tomar, R.S.; Wilson, D.W.; Smail, M.M. Estimates of Functional Food and Nutraceutical Availability in the World, with Reference to Food Peroxidation and Food Safety. In Functional Foods and Nutraceuticals in Metabolic and Non-Communicable Diseases; Academic Press: Cambridge, MA, USA, 2022; pp. 23–42. [Google Scholar]
- Topolska, K.; Florkiewicz, A.; Filipiak-Florkiewicz, A. Functional Food—Consumer Motivations and Expectations. Int. J. Environ. Res. Public Health 2021, 18, 5327. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M. Functionality of Food Components and Emerging Technologies. Foods 2021, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Martirosyan, D.; Lampert, T.; Lee, M. A Comprehensive Review on the Role of Food Bioactive Compounds in Functional Food Science. Funct. Food Sci.-Online 2022, 2, 64–78. [Google Scholar] [CrossRef]
- John, R.; Singla, A. Functional Foods: Components, Health Benefits, Challenges, and Major Projects. DRC Sustain. Future 2021, 2, 61–72. [Google Scholar]
- Stankevic, E.; Kern, T.; Borisevich, D.; Poulsen, C.S.; Madsen, A.L.; Hansen, T.H.; Jonsson, A.; Schubert, M.; Nygaard, N.; Nielsen, T. Genome-Wide Association Study Identifies Host Genetic Variants Influencing Oral Microbiota Diversity and Metabolic Health. Sci. Rep. 2024, 14, 14738. [Google Scholar] [CrossRef]
| Bioactive Compound | Examples | Food Sources | Health Benefit | References |
|---|---|---|---|---|
| Probiotics | Lactobacillus, Bifidobacterium, Saccharomyces, Enterococcus | Yogurt, kefir, fermented dairy products, freeze-dried fruit juice gummy supplements, probiotic powder. | Improves gut health, supports immunity, delivers bioactive peptides, strain-specific protection against enteric pathogens. | [26,27,28,30] |
| Prebiotics | Inulin, Fructooligosaccharides (FOS), Galacto-oligosaccharides (GOS), Xylo-oligosaccharides | Bananas, garlic, chicory, soy milk, fermented dairy products. | Stimulates growth of beneficial gut bacteria, increases Short-chain fatty acids (SCFAs), reduces harmful bacteria, lowers inflammation. | [36,37,38] |
| Postbiotics | Short-chain fatty acids (e.g., butyrate), bacterial metabolites | Fermented foods. | Modulates immunity, maintains gut barrier integrity, promotes digestive health, balances microbiome. | [42,43,44] |
| Omega-3 Fatty Acids | Eicosapentaenoic acid (EPA), Docosahexaenoic acid (DHA), DPA | Fish oil, flaxseed, seaweed oil, marine concentrate. | Supports cardiovascular and cognitive health, improves memory, lowers agitation. | [45,46,48] |
| Antioxidants | Vitamin C, Vitamin E, Polyphenols, Flavonoids, Melatonin | Berries, citrus, tea, green tea, lycopene. | Reduces oxidative stress, lowers inflammation, prevents oxidative damage to DNA, proteins and lipids. | [55,56,57] |
| Peptides | Bioactive milk peptides | Fermented dairy products. | Blood pressure regulation (ACE inhibitors). | [59,60,61] |
| Phenolic Compounds | Flavonoids, phenolic acids | Tea, berries, olive oil, cocoa. | Antioxidant, anti-inflammatory, vascular health. | [62,63,64] |
| Glucosinolates | Sulforaphane | Cruciferous vegetables. | Detoxification, anti-cancer properties. | [66,67,68] |
| Carotenoids | Beta-carotene, lutein, lycopene | Carrots, tomatoes, spinach. | Eye health, antioxidant, cancer prevention. | [69,70,71] |
| Phytosterols | β-sitosterol | Fortified margarines. | Lower cholesterol, clinically significant reductions in serum LDL-C. | [73,74,75] |
| Alkaloids | Caffeine, theobromine | Coffee, cocoa. | Mental vigilance and focus, thermogenesis, lipid catabolism. | [76,77] |
| Saponins | Ginsenosides | Ginseng, legumes. | Immune modulation, anti-inflammatory activities, cholesterol-lowering. | [78,79,80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajzer, Z.E.; Alibrahem, W.; Kharrat Helu, N.; Oláh, C.; Prokisch, J. Functional Foods in Clinical Trials and Future Research Directions. Foods 2025, 14, 2675. https://doi.org/10.3390/foods14152675
Hajzer ZE, Alibrahem W, Kharrat Helu N, Oláh C, Prokisch J. Functional Foods in Clinical Trials and Future Research Directions. Foods. 2025; 14(15):2675. https://doi.org/10.3390/foods14152675
Chicago/Turabian StyleHajzer, Zsuzsa Emma, Walaa Alibrahem, Nihad Kharrat Helu, Csaba Oláh, and József Prokisch. 2025. "Functional Foods in Clinical Trials and Future Research Directions" Foods 14, no. 15: 2675. https://doi.org/10.3390/foods14152675
APA StyleHajzer, Z. E., Alibrahem, W., Kharrat Helu, N., Oláh, C., & Prokisch, J. (2025). Functional Foods in Clinical Trials and Future Research Directions. Foods, 14(15), 2675. https://doi.org/10.3390/foods14152675







