Molecular Fractionation Induced by Viscosity-Driven Segregative Phase Separation Behavior of Gum Arabic/Hydroxypropyl Methylcellulose
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Stock Solutions
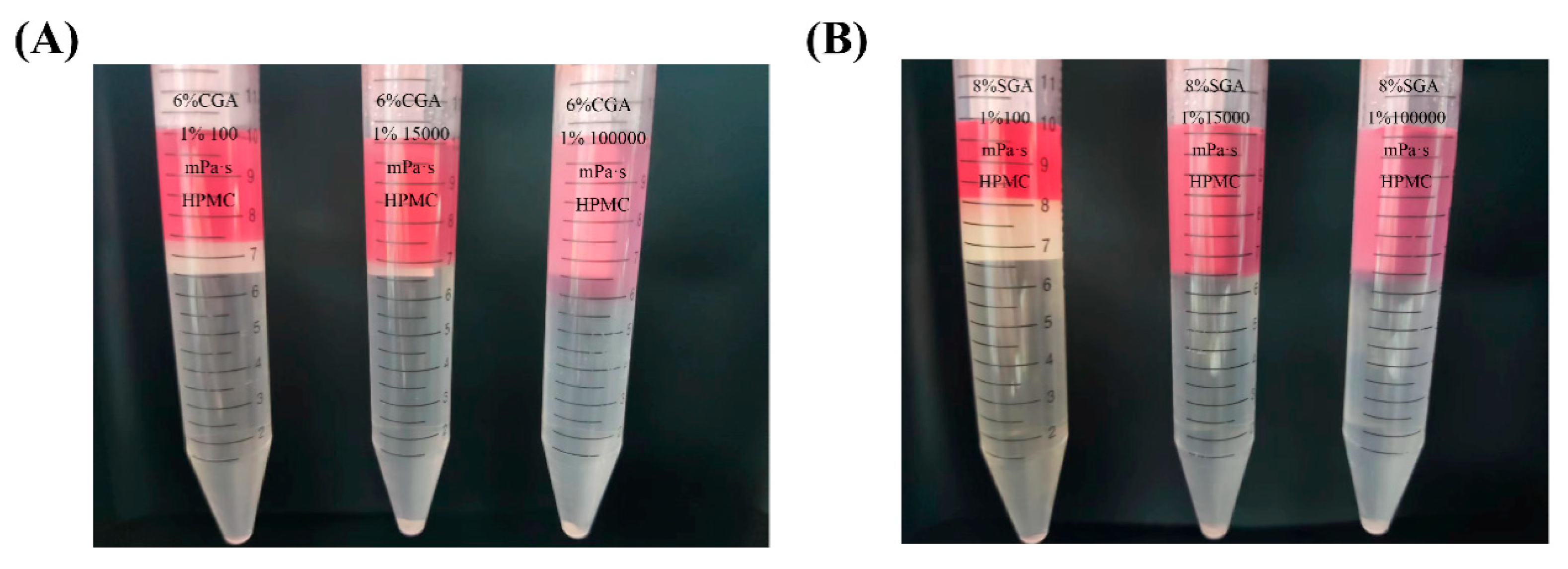
2.3. Preparation of GA/HPMC Mixed Aqueous Solutions
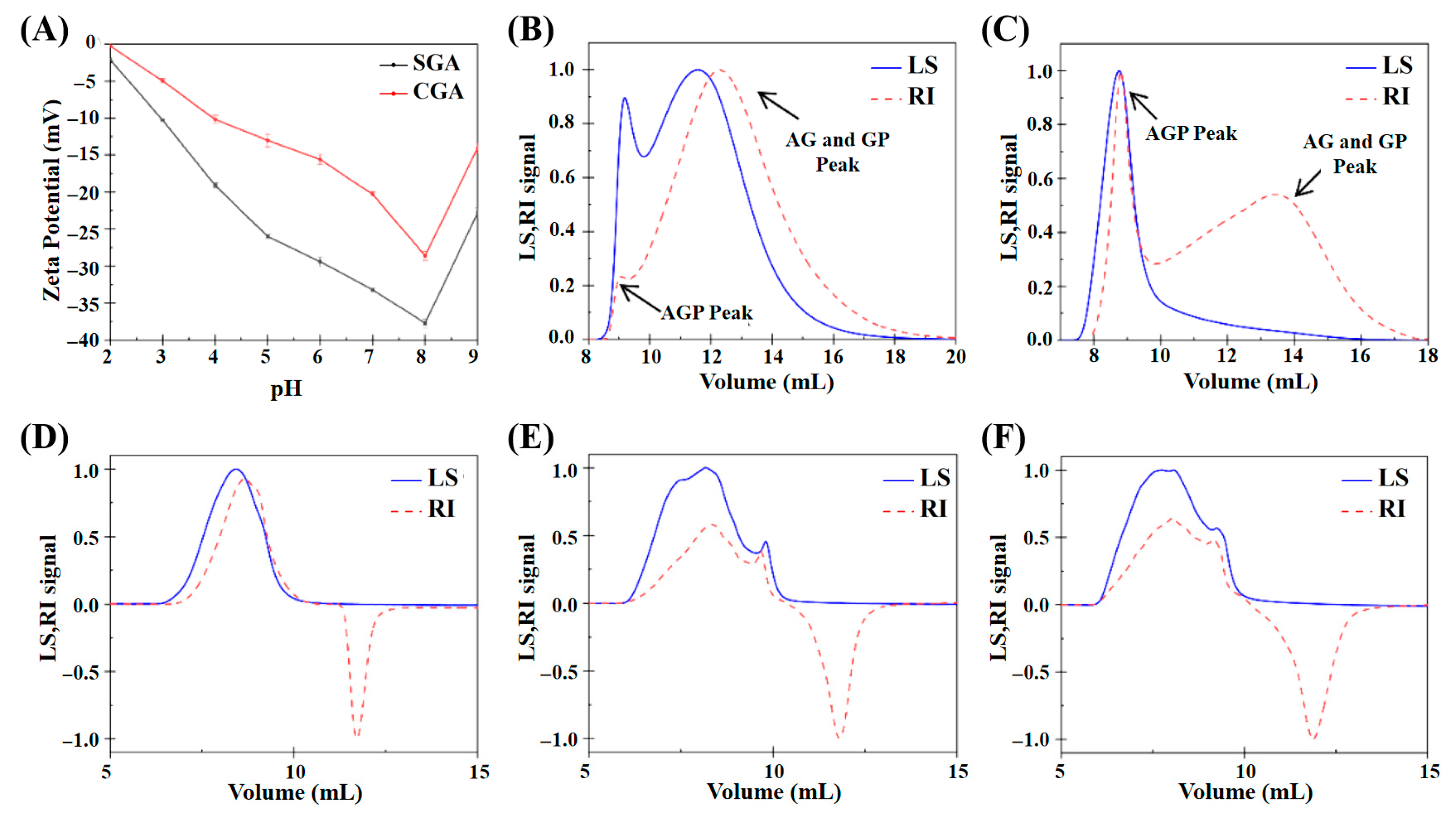
2.4. Zeta Potential Measurements
2.5. GPC-MALLS
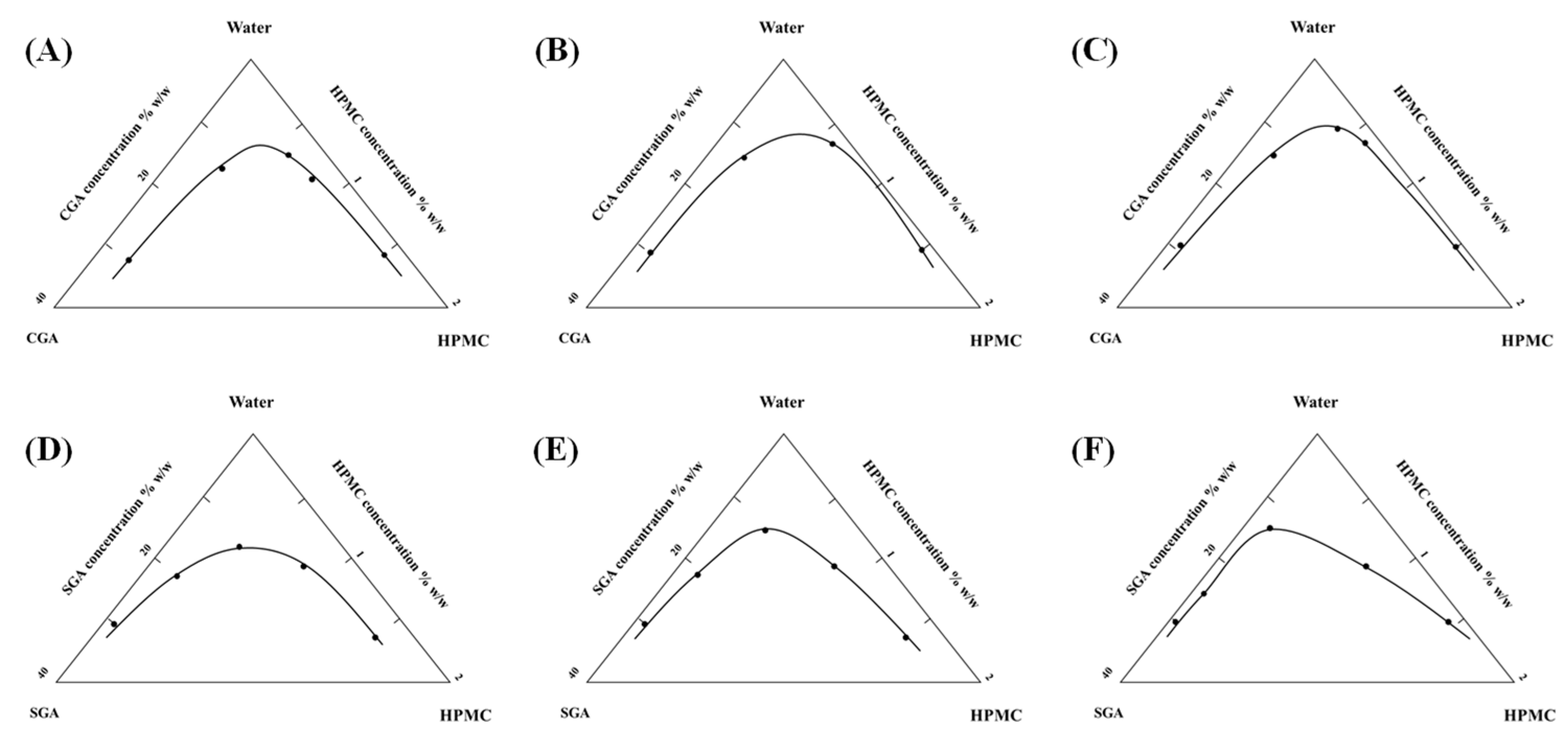
2.6. Determination of Separation Phase Diagram
2.7. Rheological Analyses
2.8. Statistical Analysis
3. Results
3.1. Characterisation of GA and HPMC
3.2. Viscosity-Dominated Phase Boundary Evolution
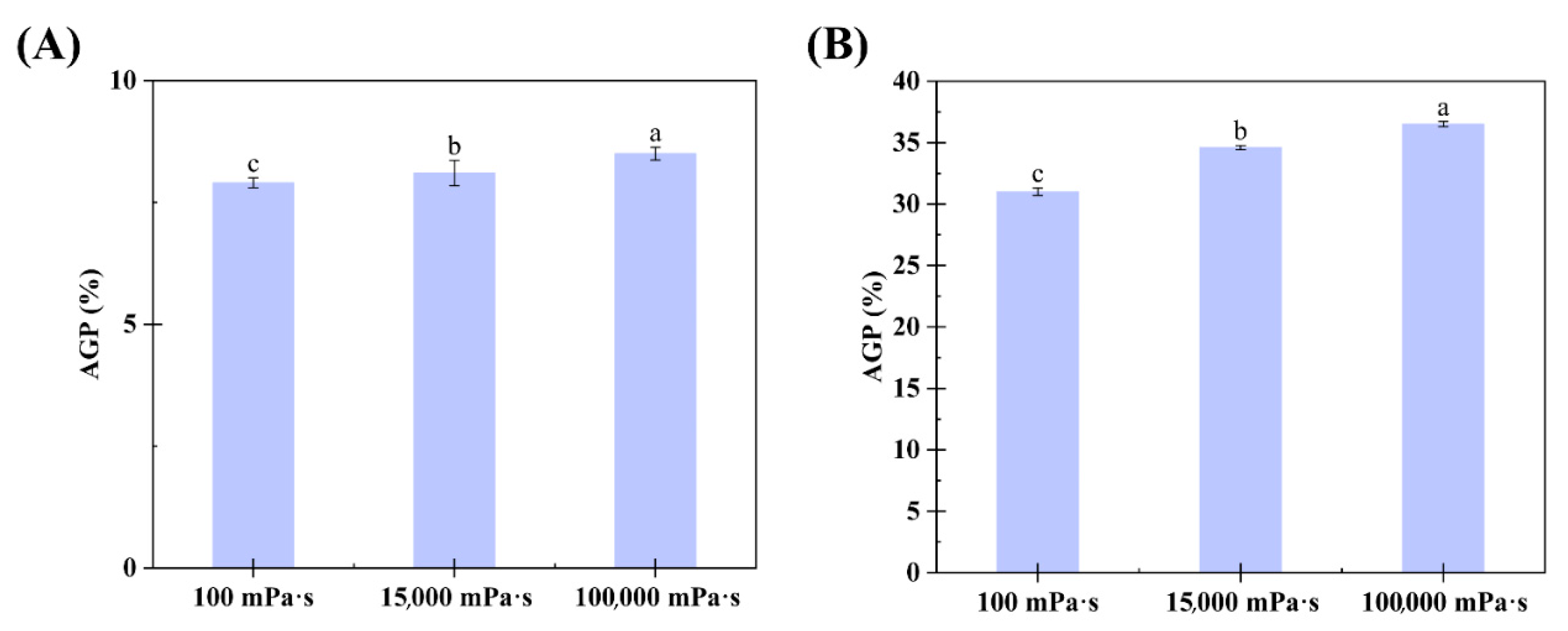
3.3. Viscosity-Driven AGP Enrichment Mechanics
3.4. Rheology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, B.; Zhang, C.; Zhu, J.; Yang, J.; Zheng, Q.; Zhang, X.; Cao, J.; Han, L. Liquid−liquid biopolymers aqueous solution segregative phase separation in food: From fundamentals to applications—A review. Int. J. Biol. Macromol. 2024, 265, 131044. [Google Scholar] [CrossRef]
- Hosseini, H.; Pasban Noghabi, V.; Saberian, H.; Jafari, S.M. The influence of different gums compared with surfactants as encapsulating stabilizers on the thermal, storage, and low-pH stability of chlorophyllin. Food Chem. X 2023, 20, 101020. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Wu, J.; Cai, L.; Li, M.; Dai, Z.; Li, D.; Liu, C.; Zhang, M. Effects of different hydrocolloids on the water migration, rheological and 3D printing characteristics of β-carotene loaded yam starch-based hydrogel. Food Chem. 2022, 393, 133422. [Google Scholar] [CrossRef]
- Gazi, M.I. The finding of antiplaque features in Acacia Arabica type of chewing gum. J. Clin. Periodontol. 1991, 18, 75–77. [Google Scholar] [CrossRef]
- Campos Assumpção de Amarante, M.; MacCalman, T.; Harding, S.E.; Spyropoulos, F.; Gras, S.; Wolf, B. Atypical phase behaviour of quinoa protein isolate in mixture with maltodextrin. Food Res. Int. 2022, 162, 112064. [Google Scholar] [CrossRef]
- Jara, F.; Pilosof, A.M.R. Partitioning of α-lactalbumin and β-lactoglobulin in whey protein concentrate/hydroxypropylmethylcellulose aqueous two-phase systems. Food Hydrocoll. 2011, 25, 374–380. [Google Scholar] [CrossRef]
- Yan, S.; Jiang, P.; Zhang, X.; Guo, Y.; Fang, W. Cryogenic efficient phase separation of oil–water emulsions with amphiphilic hyperbranched poly(amido-amine). J. Mater. Chem. A 2023, 11, 14145–14158. [Google Scholar] [CrossRef]
- Matalanis, A.; Lesmes, U.; Decker, E.A.; McClements, D.J. Fabrication and characterization of filled hydrogel particles based on sequential segregative and aggregative biopolymer phase separation. Food Hydrocoll. 2010, 24, 689–701. [Google Scholar] [CrossRef]
- Drusch, S.; Eichhorn, M.; Heinert, S.; Weißbrodt, J.; Morales-Medina, R. Review on the impact of the molecular structure of pectin on the associative phase separation with proteins. Food Hydrocoll. 2024, 156, 110289. [Google Scholar] [CrossRef]
- Haug, I.; Williams, M.A.K.; Lundin, L.; Smidsrød, O.; Draget, K.I. Molecular interactions in, and rheological properties of, a mixed biopolymer system undergoing order/disorder transitions. Food Hydrocoll. 2003, 17, 439–444. [Google Scholar] [CrossRef]
- Han, L.; Hu, B.; Ma, R.; Gao, Z.; Nishinari, K.; Phillips, G.O.; Yang, J.; Fang, Y. Effect of arabinogalactan protein complex content on emulsification performance of gum arabic. Carbohydr. Polym. 2019, 224, 115170. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Han, L.; Gao, Z.; Zhang, K.; Al-Assaf, S.; Nishinari, K.; Phillips, G.O.; Yang, J.; Fang, Y. Effects of temperature and solvent condition on phase separation induced molecular fractionation of gum arabic/hyaluronan aqueous mixtures. Int. J. Biol. Macromol. 2018, 116, 683–690. [Google Scholar] [CrossRef]
- Lu, Z.; Lee, P.R.; Yang, H. Using HPMC to improve sensory properties of vegan omelet analogue: Effect of HPMC on water retention, oil adsorption, and thermal gelation. Food Hydrocoll. 2023, 144, 108938. [Google Scholar] [CrossRef]
- Wrona, M.; Cran, M.J.; Nerín, C.; Bigger, S.W. Development and characterisation of HPMC films containing PLA nanoparticles loaded with green tea extract for food packaging applications. Carbohydr. Polym. 2017, 156, 108–117. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, M.; Zhang, L.; Liu, Z.; Su, C.; Wu, M.; Wei, X.; Jiang, L.; Hou, J.; Jiang, Z. Hydroxypropyl methylcellulose (HPMC) reduces the hardening of fructose-containing and maltitol-containing high-protein nutrition bars during storage. LWT 2022, 163, 113607. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, C.; Hu, Z.; Shen, W.; Ji, Z.; Li, F.; Guo, C. Effect of zein-pectin composite particles on the stability and rheological properties of gelatin/hydroxypropyl methylcellulose water-water systems. Int. J. Biol. Macromol. 2024, 269, 131846. [Google Scholar] [CrossRef]
- Nguyen, P.-M.; Guiga, W.; Vitrac, O. Molecular thermodynamics for food science and engineering. Food Res. Int. 2016, 88, 91–104. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Zhang, N.; Li, Z.; Bao, J.; Zhang, X.; He, G.; Chen, C.; Song, Y. Tunable nano-wrinked channels of reduced graphene oxide membranes for molecular sieving gas separation. Carbon 2024, 216, 8. [Google Scholar] [CrossRef]
- Yeh, S.K.; Liao, Z.E.; Wang, K.C.; Ho, Y.T.; Kurniawan, V.; Tseng, P.C.; Tseng, T.W. Effect of molecular weight to the structure of nanocellular foams: Phase separation approach. Polymer 2020, 191, 122275. [Google Scholar] [CrossRef]
- Assaf, S.A.; Phillips, G.O.; Williams, P.A. Studies on acacia exudate gums. Part I: The molecular weight of Acacia senegal gum exudate. Food Hydrocoll. 2005, 19, 647–660. [Google Scholar] [CrossRef]
- Mao, P.; Zhao, M.; Zhang, F.; Fang, Y.; Phillips, G.O.; Nishinari, K.; Jiang, F. Phase separation induced molecular fractionation of gum arabic—Sugar beet pectin systems. Carbohydr. Polym. 2013, 98, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Sovilj, V.; Petrović, L. Interaction and phase separation in the system HPMC/NaCMC/SDS. Colloid Surf. A 2007, 298, 94–98. [Google Scholar] [CrossRef]
- Ji, Z.; Liu, H.; Yu, L.; Duan, Q.; Chen, Y.; Chen, L. pH controlled gelation behavior and morphology of gelatin/hydroxypropylmethylcellulose blend in aqueous solution. Food Hydrocoll. 2020, 104, 105733. [Google Scholar] [CrossRef]
- Molaahmadi Bahraseman, N.; Shekarchizadeh, H.; Goli, S.A.H. Segregative phase separation of gelatin and tragacanth gum solution and Mickering stabilization of their water-in-water emulsion with microgel particles prepared by complex coacervation. Int. J. Biol. Macromol. 2023, 237, 124250. [Google Scholar] [CrossRef]
- Villois, A.; Capasso Palmiero, U.; Mathur, P.; Perone, G.; Schneider, T.; Li, L.; Salvalaglio, M.; deMello, A.; Stavrakis, S.; Arosio, P. Droplet Microfluidics for the Label-Free Extraction of Complete Phase Diagrams and Kinetics of Liquid–Liquid Phase Separation in Finite Volumes. Small 2022, 18, 2202606. [Google Scholar] [CrossRef] [PubMed]
- Tipvarakarnkoon, T.; Einhorn-Stoll, U.; Senge, B. Effect of modified Acacia gum (SUPER GUM™) on the stabilization of coconut o/w emulsions. Food Hydrocoll. 2010, 24, 595–601. [Google Scholar] [CrossRef]
- Siddig, N.E.; Osman, M.E.; Al-Assaf, S.; Phillips, G.O.; Williams, P.A. Studies on acacia exudate gums, part IV. Distribution of molecular components in Acacia seyal in relation to Acacia senegal. Food Hydrocoll. 2005, 19, 679–686. [Google Scholar] [CrossRef]
- Al-Assaf, S.; Phillips, G.O.; Sasaki, Y.; Katayama, T. Modified Gum Arabic. PCT/JP2004/005050, 16 April 2004. [Google Scholar]
- Hayashi, H. Enhancement Method of Gum Arabic Under the Atmosphere of 30–100% of Relative Humidity at over 401C. US Patent 2005/0158440, 21 July 2005. [Google Scholar]
- Al-Assaf, S.; Phillips, G.O.; Williams, P.A. Studies on Acacia exudate gums: Part II. Molecular weight comparison of the Vulgares and Gummiferae series of Acacia gums. Food Hydrocoll. 2005, 19, 661–667. [Google Scholar] [CrossRef]
- Renard, D.; Garnier, C.; Lapp, A.; Schmitt, C.; Sanchez, C. Structure of arabinogalactan-protein from Acacia gum: From porous ellipsoids to supramolecular architectures. Carbohydr. Polym. 2012, 90, 322–332. [Google Scholar] [CrossRef]
- Jin, W.; Ge, H.; Wang, Y.; Du, X.; Li, B. Molecular migration of konjac glucomannan and gum Arabic phase separation and its application in oil-water interfaces. Food Hydrocoll. 2016, 61, 868–876. [Google Scholar] [CrossRef]
- Cai, Z.; Badr, R.G.M.; Hauer, L.; Chaudhuri, K.; Skabeev, A.; Schmid, F.; Pham, J.T. Phase separation dynamics in wetting ridges of polymer surfaces swollen with oils of different viscosities. Soft Matter. 2024, 20, 7300–7312. [Google Scholar] [CrossRef]
- Panda, S.K.; Alawani, N.A.; Lajami, A.R.; Al-Qunaysi, T.A.; Muller, H. Characterization of aromatic hydrocarbons and sulfur heterocycles in Saudi Arabian heavy crude oil by gel permeation chromatography and ultrahigh resolution mass spectrometry. Fuel 2019, 235, 1420–1426. [Google Scholar] [CrossRef]
- Liu, M.; Huang, Y.; Chen, S.; Pan, D.; Chen, M.; Chu, Q.; Liu, Y.; Yin, Q.; Zhang, Z. Progress and challenges in the fabrication of DPS shells for ICF. MRE 2019, 4, 018401. [Google Scholar] [CrossRef]
- Grasselli, M.; Poiatti, A. A phase separation model for binary fluids with hereditary viscosity. Math. Methods Appl. Sci. 2022, 45, 11031–11066. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Daneshfar, R.; Dehaghani, A.H.S.; Su, C.-H. The effect of shear rate on aggregation and breakage of asphaltenes flocs: Experimental study and model-based analysis. J. Mol. Liq. 2021, 325, 114861. [Google Scholar] [CrossRef]
- Aho, J.; Halme, A.; Boetker, J.; Water, J.J.; Bohr, A.; Sandler, N.; Rantanen, J.; Baldursdottir, S. The effect of HPMC and MC as pore formers on the rheology of the implant microenvironment and the drug release in vitro. Carbohydr. Polym. 2017, 177, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Qiu, J.; Jiang, H.; Kong, X.; Guo, Z.; Xiang, J.; Sun, X. Effect of hydroxypropyl methyl cellulose on coarse tailings cemented backfill: Rheology, stability, strength and microstructure. Constr. Build. Mater. 2024, 425, 136042. [Google Scholar] [CrossRef]
- Hundschell, C.S.; Bäther, S.; Drusch, S.; Wagemans, A.M. Osmometric and viscometric study of levan, β-lactoglobulin and their mixtures. Food Hydrocoll. 2020, 101, 105580. [Google Scholar] [CrossRef]

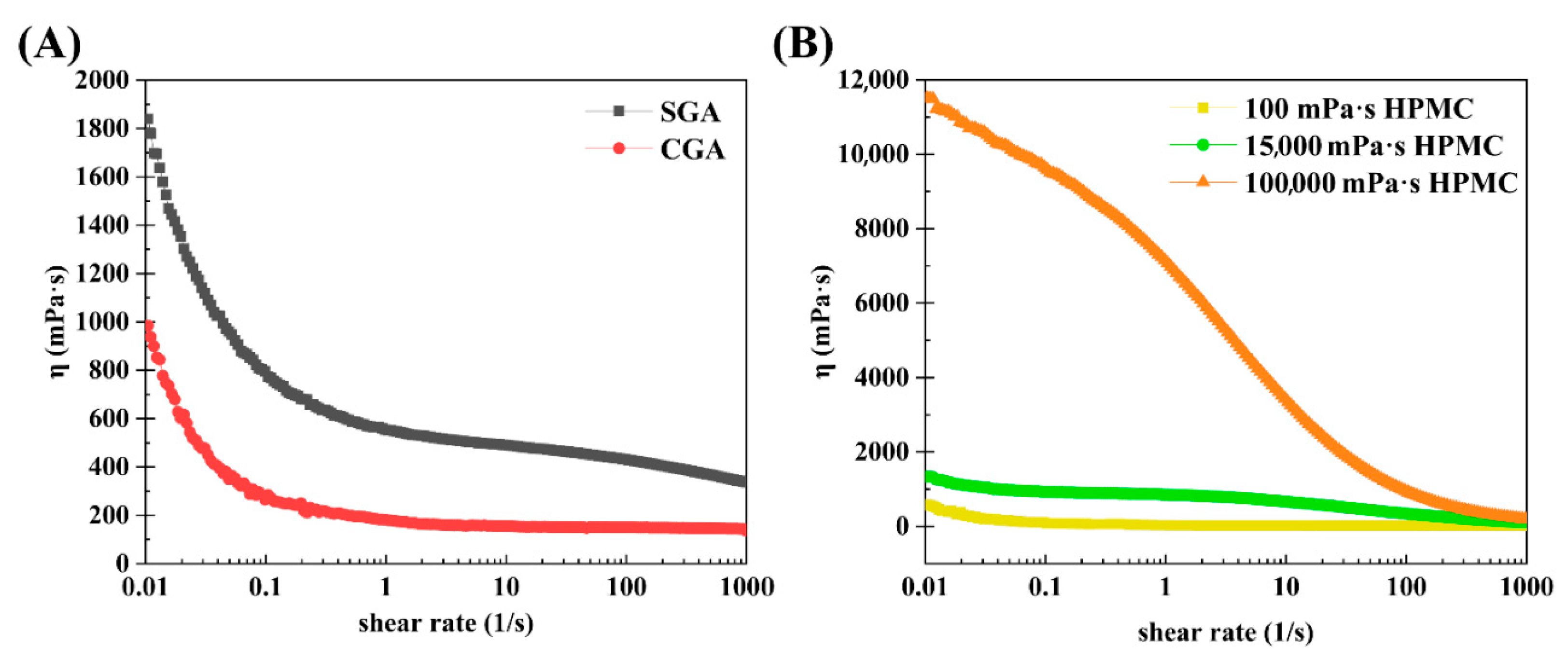
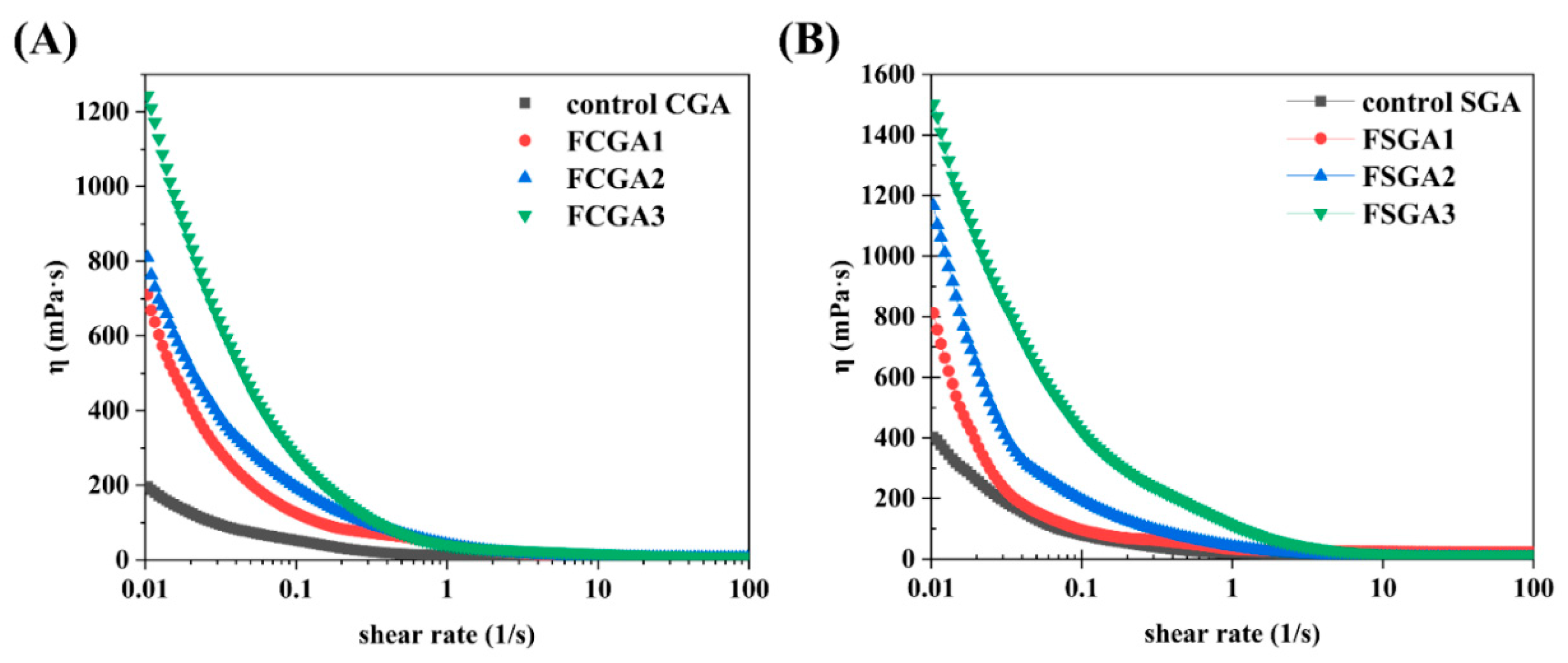
| Sample | Signals | Mw (105 Da) | Polydispersity (Mw/Mn) | Rg (nm) | Mass Recovery (%) |
|---|---|---|---|---|---|
| CGA | Peak 1 (AGP) | 15.15 ± 0.10 c | 1.06 ± 0.05 e | 24.20 ± 0.46 f | 6.30 ± 0.07 |
| Peak 2 (AG + GP) | 3.76 ± 0.09 ef | 1.47 ± 0.04 cde | 9.13 ± 0.10 i | 93.70 ± 0.23 | |
| Peak 3 (whole gum) | 5.23 ± 0.31 d | 1.66 ± 0.09 bcd | 12.68 ± 0.08 h | 100.00 | |
| SGA | Peak 1 (AGP) | 66.01 ± 0.27 a | 1.79 ± 0.10 bc | 57.11 ± 0.15 c | 27.30 ± 0.17 |
| Peak 2 (AG + GP) | 3.87 ± 0.11 e | 2.10 ± 0.60 b | 22.40 ± 0.10 g | 72.70 ± 0.12 | |
| Peak 3 (whole gum) | 28.23 ± 0.47 b | 8.41 ± 0.18 a | 31.43 ± 0.13 e | 100.00 | |
| 100 mPa·s HPMC | - | 0.73 ± 0.06 g | 1.46 ± 0.13 cde | 34.71 ± 0.17 d | - |
| 15,000 mPa·s HPMC | - | 3.33 ± 0.21 f | 1.35 ± 0.08 cde | 66.61 ± 0.14 b | - |
| 100,000 mPa·s HPMC | - | 5.88 ± 0.17 d | 1.23 ± 0.06 de | 87.65 ± 0.13 a | - |
| Mixture | VU/VL | Mw of FCGA/FSGA (×106 Da) | AGP in FCGA/FSGA (%) | Mw of AGP in FCGA/FSGA (×106 Da) |
|---|---|---|---|---|
| Control CGA | - | 0.52 ± 0.03 f | 6.30 ± 0.13 f | 1.52 ± 0.03 f |
| 6% CGA + 1.0% 100 mPa·s HPMC | 0.35 ± 0.02 | 0.64 ± 0.04 e | 7.90 ± 0.05 e | 1.64 ± 0.05 e |
| 6% CGA + 1.0% 15,000 mPa·s HPMC | 0.42 ± 0.06 | 0.65 ± 0.03 e | 8.10 ± 0.06 e | 1.65 ± 0.01 e |
| 6%CGA + 1.0% 100,000 mPa·s HPMC | 0.51 ± 0.02 | 0.64 ± 0.06 e | 8.50 ± 0.06 e | 1.60 ± 0.03 ef |
| Control SGA | - | 2.82 ± 0.06 d | 27.30 ± 0.63 d | 6.60 ± 0.09 c |
| 8% SGA + 1.0% 100 mPa·s HPMC | 0.21 ± 0.04 | 4.03 ± 0.09 c | 31.00 ± 0.53 c | 6.88 ± 0.07 b |
| 8% SGA + 1.0% 15,000 mPa·s HPMC | 0.51 ± 0.10 | 5.19 ± 0.16 b | 34.60 ± 0.55 b | 7.20 ± 0.12 a |
| 8% SGA + 1.0% 100,000 mPa·s HPMC | 0.60 ± 0.03 | 5.81 ± 0.13 a | 36.50 ± 0.31 a | 6.32 ± 0.13 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, L.; Zhang, C.; Dong, N.; Yang, J.; Zheng, Q.; Zhang, X.; Liu, R.; Cao, J.; Hu, B. Molecular Fractionation Induced by Viscosity-Driven Segregative Phase Separation Behavior of Gum Arabic/Hydroxypropyl Methylcellulose. Foods 2025, 14, 2642. https://doi.org/10.3390/foods14152642
Han L, Zhang C, Dong N, Yang J, Zheng Q, Zhang X, Liu R, Cao J, Hu B. Molecular Fractionation Induced by Viscosity-Driven Segregative Phase Separation Behavior of Gum Arabic/Hydroxypropyl Methylcellulose. Foods. 2025; 14(15):2642. https://doi.org/10.3390/foods14152642
Chicago/Turabian StyleHan, Lingyu, Cunzhi Zhang, Nuo Dong, Jixin Yang, Qiuyue Zheng, Xiaobo Zhang, Ronggang Liu, Jijuan Cao, and Bing Hu. 2025. "Molecular Fractionation Induced by Viscosity-Driven Segregative Phase Separation Behavior of Gum Arabic/Hydroxypropyl Methylcellulose" Foods 14, no. 15: 2642. https://doi.org/10.3390/foods14152642
APA StyleHan, L., Zhang, C., Dong, N., Yang, J., Zheng, Q., Zhang, X., Liu, R., Cao, J., & Hu, B. (2025). Molecular Fractionation Induced by Viscosity-Driven Segregative Phase Separation Behavior of Gum Arabic/Hydroxypropyl Methylcellulose. Foods, 14(15), 2642. https://doi.org/10.3390/foods14152642






