Evaluation of the Biological Properties and Antibacterial Activities of the Natural Food Supplement “Epavin” for Liver Detoxification and Protection
Abstract
1. Introduction
2. Materials and Methods
2.1. Epavin
2.2. Chemicals
2.3. Chemical Characterization
2.4. Cell Culture
2.5. Evaluation of Cytotoxicity Using MTT Assay
2.6. Measurement of Intracellular ROS Production
2.7. Evaluation of Anti-Inflammatory Activity Using the Griess Assay
2.8. Antibacterial Studies
2.9. Statistical Analysis
3. Results
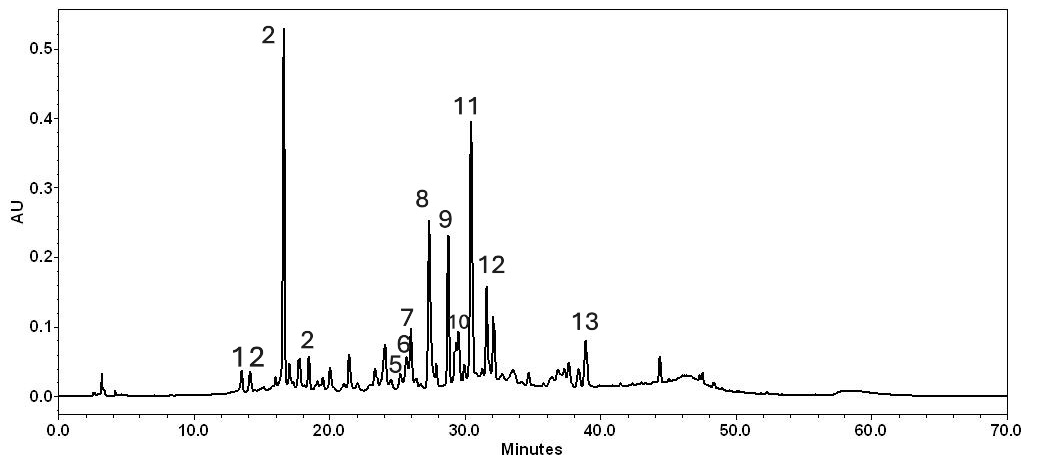
3.1. Epavin Chemical Composition
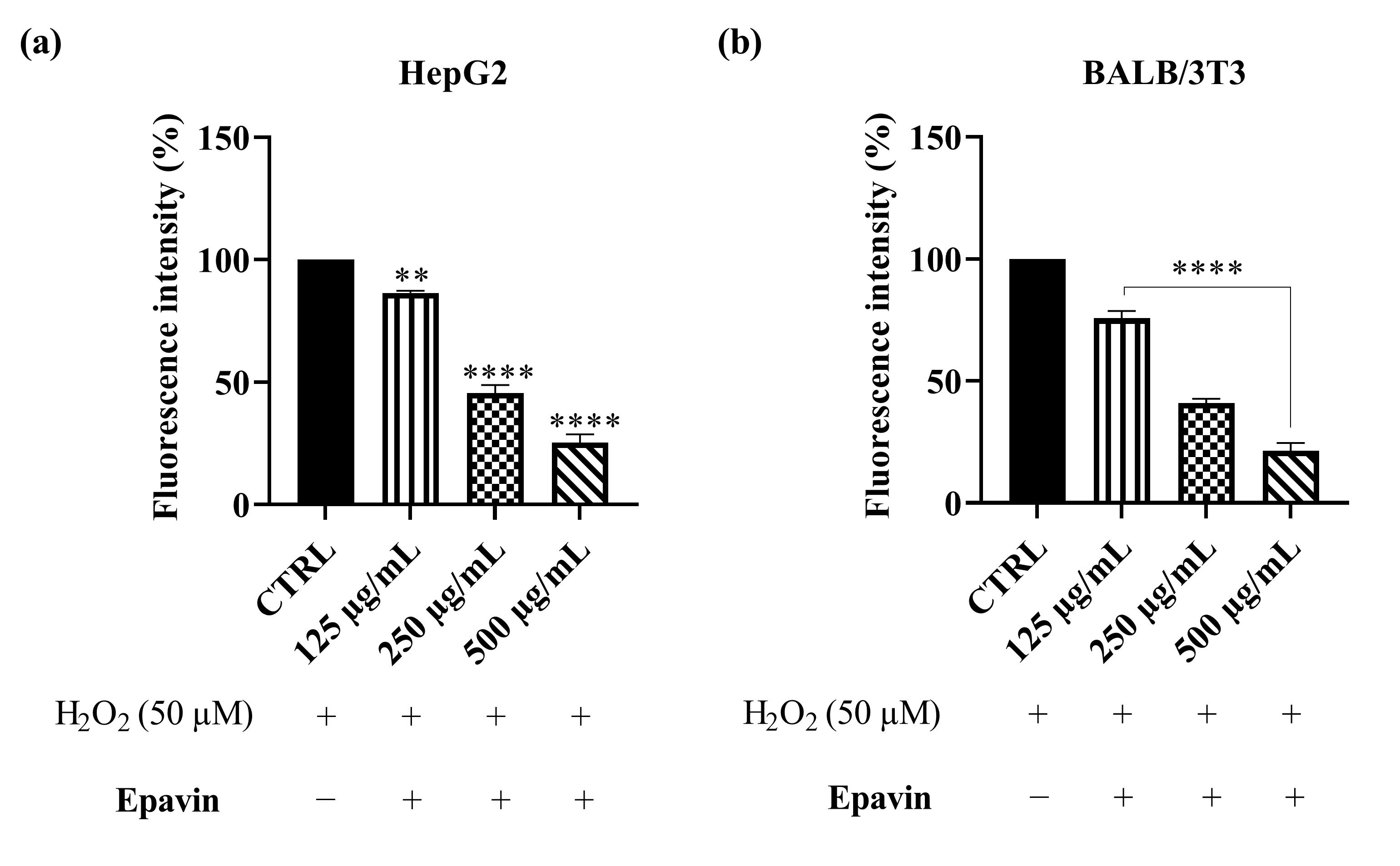
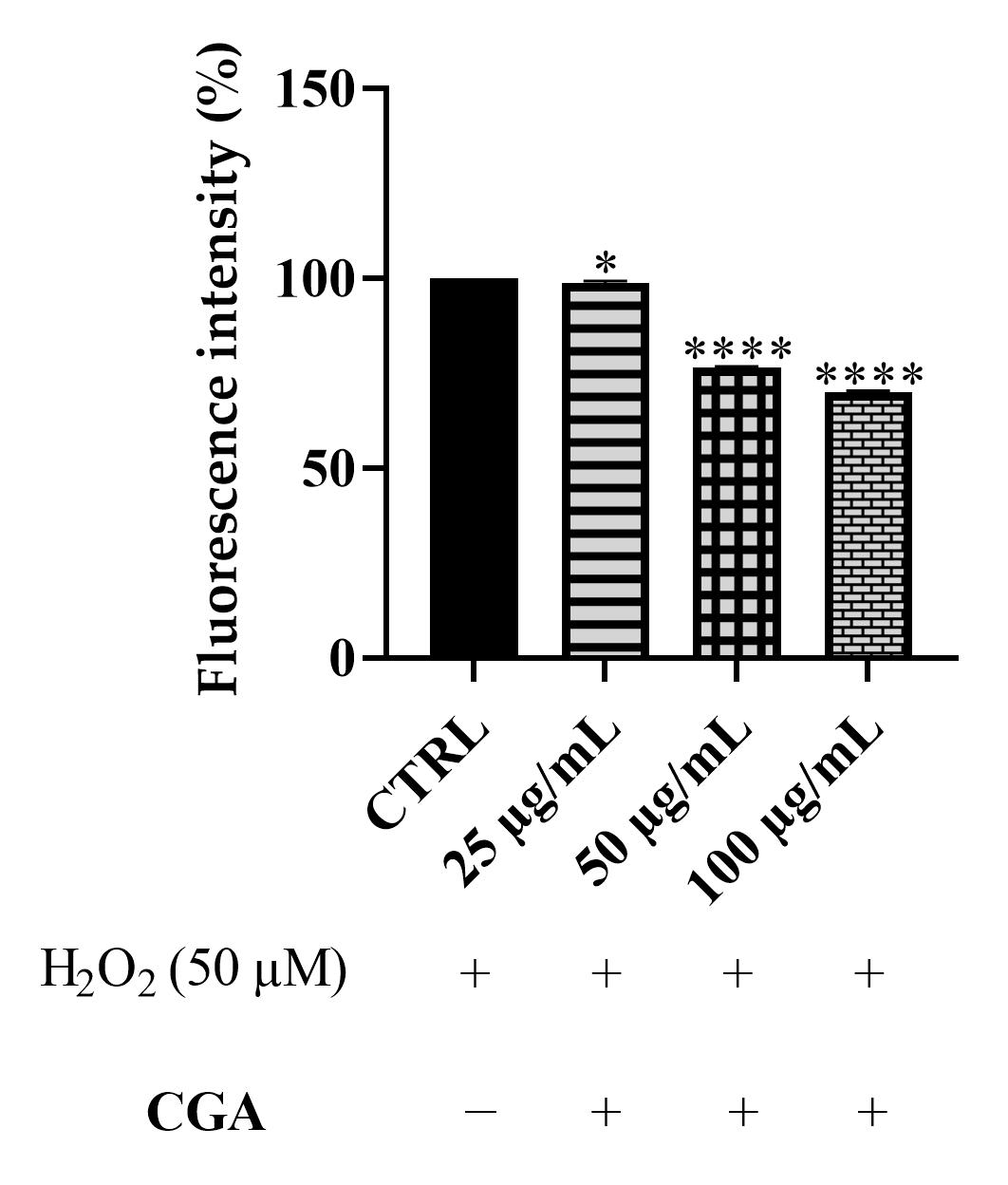
3.2. ROS Scavenging Effects of Epavin Against H2O2-Induced Oxidative Stress
3.3. Anti-Inflammatory Activity of Epavin Against LPS-Induced Nitric Oxide Production
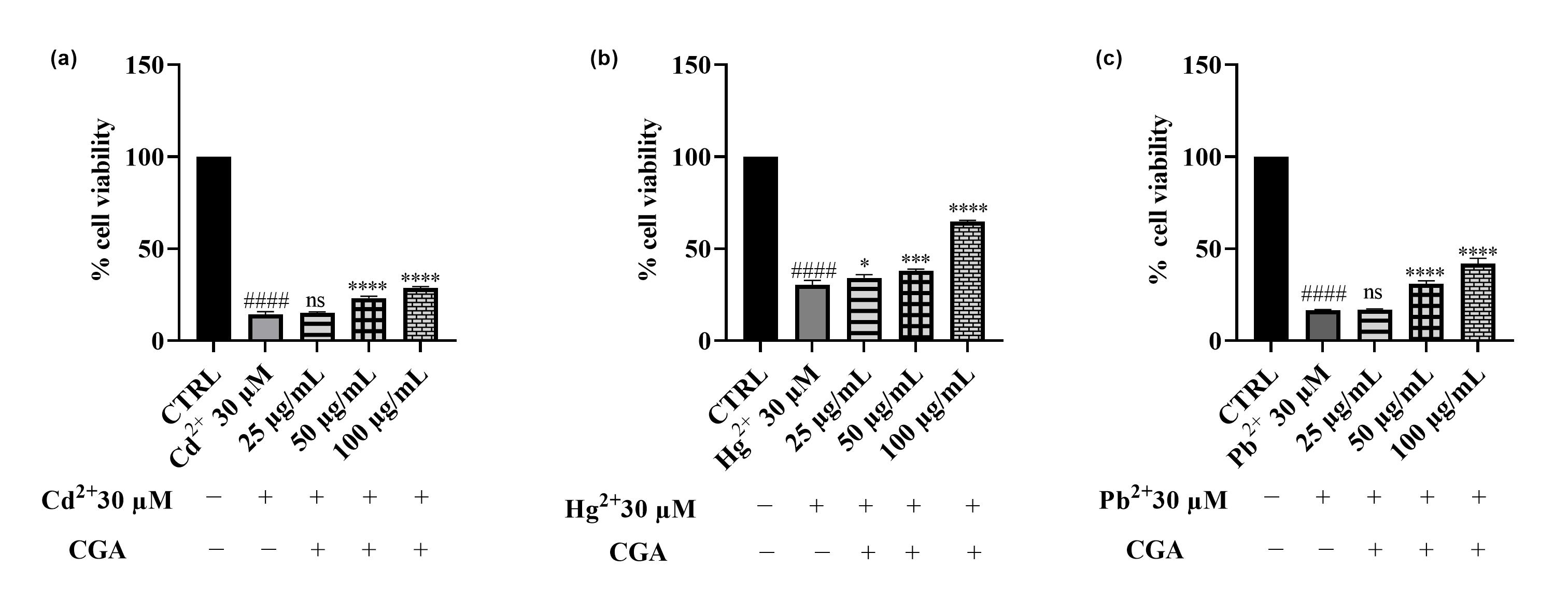
3.4. Effects of Cd2+, Hg2+, and Pb2+ on Cell Viability
3.5. Effect of Epavin on Cd2+-, Hg2+-, and Pb2+-Induced Cytotoxicity and ROS Production
3.6. Antibacterial Activity of Epavin
4. Discussion
4.1. ROS Scavenging Effects of Epavin Against H2O2-Induced Oxidative Stress
4.2. Anti-Inflammatory Activity of Epavin Against LPS-Induced Nitric Oxide Production
4.3. Effects of Cd2+, Hg2+, Pb2+, and Epavin on Cell Viability and the Cytoprotective Role of Epavin Against Heavy Metal-Induced Cytotoxicity and ROS Production
4.4. Antibacterial Activity of Epavin
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cione, E.; Abrego Guandique, D.M.; Caroleo, M.C.; Luciani, F.; Colosimo, M.; Cannataro, R. Liver Damage and microRNAs: An Update. Curr. Issues Mol. Biol. 2023, 45, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Uchida, D.; Takaki, A.; Oyama, A.; Adachi, T.; Wada, N.; Onishi, H.; Okada, H. Oxidative Stress Management in Chronic Liver Diseases and Hepatocellular Carcinoma. Nutrients 2020, 12, 1576. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, V.; Angeli, P.; Moreau, R.; Jalan, R.; Clària, J.; Trebicka, J.; Fernández, J.; Gustot, T.; Caraceni, P.; Bernardi, M.; et al. The systemic inflammation hypothesis: Towards a new paradigm of acute decompensation and multiorgan failure in cirrhosis. J. Hepatol. 2021, 74, 670–685. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-H.; Lee, C.-H.; Chang, C. Spontaneous Bacterial Peritonitis in Decompensated Liver Cirrhosis—A Literature Review. Livers 2022, 2, 214–232. [Google Scholar] [CrossRef]
- Seen, S. Chronic liver disease and oxidative stress—A narrative review. Expert. Rev. Gastroenterol. Hepatol. 2021, 15, 1021–1035. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lei, R.; Lei, H.; Xiong, Q.; Xie, F.; Yao, C.; Feng, P. Side Effect Profile of Pharmacologic Therapies for Liver Fibrosis in Nonalcoholic Fatty Liver Disease: A Systematic Review and Network Meta-Analysis. Eur. J. Gastroenterol. Hepatol. 2023, 35, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, N.K.; Mann, K.K. Mechanisms of Metal-Induced Hepatic Inflammation. Curr. Environ. Health Rep. 2024, 11, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Renu, K.; Chakraborty, R.; Myakala, H.; Koti, R.; Ademola, C.; Famurewa, A.C.; Madhyastha, H.; Vellingiri, B.; George, A.; Gopalakrishnan, A.V. Molecular mechanism of heavy metals (Lead, Chromium, Arsenic, Mercury, Nickel and Cadmium)—Induced hepatotoxicity—A review. Chemosphere 2021, 271, 129735. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Ma, X.; Jiang, Y.; Wen, J.; Yang, L.; Chen, D.; Cao, X.; Peng, C. A Comprehensive Review of Natural Products against Liver Fibrosis: Flavonoids, Quinones, Lignans, Phenols, and Acids. Evid.-Based Complement. Altern. Med. 2020, 2020, 7171498. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chen, S.; Wei, Y.; Sun, Y.; Yang, Y.; Lin, B.; Li, Y.; Wang, C. Glycyrrhizic acid restores the downregulated hepatic ACE2 signaling in the attenuation of mouse steatohepatitis. Eur. J. Pharmacol. 2024, 967, 176365. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.; Cheung, F.; Tan, H.Y.; Wang, N.; Yuen, M.F.; Feng, Y. Hepatoprotective Effects of Chinese Medicinal Herbs: A Focus on Anti-Inflammatory and Anti-Oxidative Activities. Int. J. Mol. Sci. 2016, 17, 465. [Google Scholar] [CrossRef] [PubMed]
- Barbarossa, A.; Rosato, A.; Tardugno, R.; Carrieri, A.; Corbo, F.; Limongelli, F.; Fumarola, L.; Fracchiolla, G.; Carocci, A. Antibiofilm Effects of Plant Extracts Against Staphylococcus aureus. Microorganisms 2025, 13, 454. [Google Scholar] [CrossRef] [PubMed]
- Ben Mrid, R.; Bouchmaa, N.; Ouedrhiri, W.; Ennoury, A.; Zouaou, I.Z.; Kabach, I.; Nhiri, M.; El Fatimy, R. Synergistic antioxidant effects of natural compounds on H2O2-induced cytotoxicity of human monocytes. Front. Pharm. 2022, 13, 830323. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Zhao, D.; Song, J.; Gao, R.; Wang, X.; Rao, H.; Gao, X.; Hao, J. Synergistic Antioxidant Activity of Lycium barbarum Polysaccharide and Chlorogenic Acid. and Its Effect on Inflammatory Response of NR8383 Cells. Foods 2024, 13, 3696. [Google Scholar] [CrossRef] [PubMed]
- Houghton, C.A. Sulforaphane: Its “Coming of Age” as a Clinically Relevant Nutraceutical in the Prevention and Treatment of Chronic Disease. Oxidative Med. Cell. Longev. 2019, 2019, 2716870. [Google Scholar] [CrossRef] [PubMed]
- Bresciani, L.; Martini, D.; Mena, P.; Tassotti, M.; Calani, L.; Brigati, G.; Brighenti, F.; Holasek, S.; Malliga, D.-E.; Lamprecht, M.; et al. Absorption Profile of (Poly)Phenolic Compounds after Consumption of Three Food Supplements Containing 36 Different Fruits, Vegetables, and Berries. Nutrients 2017, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.C.; Ring, M.; Dhruva, A.; Yeh, G.Y. A patient-centered approach to dietary supplements for patients with chronic liver disease. Hepatol. Commun. 2024, 8, e0552. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Reyes, S.; Velázquez-Villegas, L.A.; Vargas-Castillo, A.; Noriega, L.G.; Torres, N.; Tovar, A.R. Dietary bioactive compounds as modulators of mitochondrial function. J. Nutr. Biochem. 2021, 96, 108768. [Google Scholar] [CrossRef] [PubMed]
- Albillos, A.; Martin-Mateos, R.; Van der Merwe, S.; Wiest, R.; Jalan, R.; Álvarez-Mon, M. Cirrhosis-associated immune dysfunction. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 112–134. [Google Scholar] [CrossRef] [PubMed]
- Darbar, S.; Saha, S.; Pramanik, K.; Chattopadhyay, A. Antioxidant and immunomodulatory effect of AKSS16-LIV01–a multi herbal formulation against ethanol induced liver dysfunction in mice. Clin. Phytosci. 2021, 7, 80. [Google Scholar] [CrossRef]
- Li, H.Y.; Gan, R.Y.; Shang, A.; Mao, Q.Q.; Sun, Q.C.; Wu, D.T.; Geng, F.; He, X.Q.; Li, H.B. Plant-Based Foods and Their Bioactive Compounds on Fatty Liver Disease: Effects, Mechanisms, and Clinical Application. Oxidative Med. Cell Longev. 2021, 2021, 6621644. [Google Scholar] [CrossRef] [PubMed]
- Singla, R.K.; Singh, D.; Verma, R.; Kaushik, D.; Echeverría, J.; Garg, V.; Gupta, P.; Rahman, M.A.; Sharma, A.; Mittal, V.; et al. Fermented formulation of silybum marianum seeds: Optimization, heavy metal analysis, and hepatoprotective assessment. Phytomedicine 2024, 124, 155286. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Izzo, A.A.; Milić, N.; Cicala, C.; Santini, A.; Capasso, R. Milk thistle (Silybum marianum): A concise overview on its chemistry, pharmacological, and nutraceutical uses in liver diseases. Phytother. Res. 2018, 32, 2202–2213. [Google Scholar] [CrossRef] [PubMed]
- Nasef, M.A.; Yousef, M.I.; Ghareeb, D.A.; Augustyniak, M.; Aboul-Souds, M.A.M.; Wakil, A.E. Hepatoprotective effects of a chemically-characterized extract from artichoke (Cynara scolymus L.) against AFB1-induced toxicity in rats. Drug Chem. Toxicol. 2023, 46, 1070–1082. [Google Scholar] [CrossRef] [PubMed]
- Pfingstgraf, I.O.; Taulescu, M.; Pop, R.M.; Orăsan, R.; Vlase, L.; Uifalean, A.; Todea, D.; Alexescu, T.; Toma, C.; Pârvu, A.E. Protective Effects of Taraxacum officinale L. (Dandelion) Root Extract in Experimental Acute on Chronic Liver Failure. Antioxidants 2021, 10, 504. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Lupia, C.; Argentieri, M.P.; Bava, R.; Castagna, F.; Cozza, N.; Mollace, V.; Palma, E.; Statti, G. Foeniculum vulgare Mill. Aerial Parts (Italian ‘Finocchio di Isola Capo Rizzuto’ PGI): Valorization of Agri-Food Waste as a Potential Source of Lipase Inhibitors and Antioxidants. Resources 2024, 13, 89. [Google Scholar] [CrossRef]
- Barbarossa, A.; Ceramella, J.; Carocci, A.; Iacopetta, D.; Rosato, A.; Limongelli, F.; Carrieri, A.; Bonofiglio, D.; Sinicropi, M.S. Benzothiazole-Phthalimide Hybrids as Anti-Breast Cancer and Antimicrobial Agents. Antibiotics 2023, 12, 1651. [Google Scholar] [CrossRef] [PubMed]
- Barbarossa, A.; Mallamaci, R.; Spinozzi, E.; Maggi, F.; Sgobba, M.N.; Rosato, A.; Carocci, A.; Meleleo, D. Investigating Bergamot Essential Oil (BEO) Properties: Cytoprotection in Neuronal Cells Exposed to Heavy Metals and Antibacterial Activities. Antioxidants 2025, 14, 400. [Google Scholar] [CrossRef] [PubMed]
- Mallamaci, R.; Barbarossa, A.; Carrieri, A.; Meleleo, D.; Carocci, A. Evaluation of the Potential Cytoprotective Effect of Melatonin in Comparison with Vitamin E and Trolox against Cd2+-Induced Toxicity in SH-SY5Y, HCT 116, and HepG2 Cell Lines. Int. J. Mol. Sci. 2024, 25, 8055. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Hao, Z.; Li, M. Justino, G.C., Ed.; Isolation and Structure Identification of Flavonoids; IntechOpen: London, UK, 2017; pp. 17–43. [Google Scholar]
- Yan, Y.; Liu, N.; Hou, N.; Dong, L.; Li, J. Chlorogenic acid inhibits hepatocellular carcinoma In Vitro and In Vivo. J. Nutr. Biochem. 2017, 46, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Namvarjah, F.; Shokri-Afra, H.; Moradi-Sardareh, H.; Khorzoughi, R.B.; Pasalar, P.; Panahi, G.; Meshkani, R. Chlorogenic acid improves anti-lipogenic activity of metformin by positive regulating of AMPK signaling in HepG2 cells. Cell Biochem. Biophys. 2022, 80, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Rohilla, S.; Bhatt, D.C. Significance of hepatoprotective liver specific targeted drug delivery: A review on novel herbal and formulation approaches in the management of hepatotoxicity. Curr. Drug Targets 2018, 19, 1519–1549. [Google Scholar] [CrossRef] [PubMed]
- Yasgar, A.; Bougie, D.; Eastman, R.T.; Huang, R.; Itkin, M.; Kouznetsova, J.; Lynch, C.; McKnight, C.; Miller, M.; Ngan, D.K.; et al. Quantitative bioactivity signatures of dietary supplements and natural products. ACS Pharmacol. Transl. Sci. 2023, 6, 683–701. [Google Scholar] [CrossRef] [PubMed]
- Villiger, A.; Sala, F.; Suter, A.; Butterweck, V. In Vitro inhibitory potential of Cynara scolymus, Silybum marianum, Taraxacum officinale, and Peumus boldus on key enzymes relevant to metabolic syndrome. Phytomedicine 2015, 22, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, R.; Rezaee, R.; Shakeri, A.; Hayes, A.W.; Karimi, G. A review of the protective effects of chlorogenic acid against different chemicals. J. Food Biochem. 2022, 46, e14254. [Google Scholar] [CrossRef] [PubMed]
- Veeraswamy, S.D.; Raju, I.; Mohan, S. Phytochemical Evaluation and Antioxidant Potential of Polyherbal Extract Mixture—An In Vitro and In Silico Study. Appl. Biochem. Biotechnol. 2023, 195, 672–692. [Google Scholar] [CrossRef] [PubMed]
- Jurčacková, Z.; Hrčková, G.; Mudroňová, D.; Matiašová, A.A.; Biedermann, D. Flavonolignans silybin, silychristin and 2, 3-dehydrosilybin showed differential cytoprotective, antioxidant and anti-apoptotic effects on splenocytes from Balb/c mice. Sci. Rep. 2025, 15, 5631. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.B.; Unenkhuu, B.; Kim, G.J.; Kim, S.W.; Kim, H.S. Cynarin attenuates LPS-induced endothelial inflammation via upregulation of the negative regulator MKP-3. Animal Cells Syst. 2022, 26, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Mendoza, N.; Morales-González, Á.; Morales-Martínez, M.; Soriano-Ursúa, M.A.; Delgado-Olivares, L.; Sandoval-Gallegos, E.M.; Madrigal-Bujaidar, E.; Álvarez-González, I.; Madrigal-Santillán, E.; Morales-Gonzalez, J.A. Flavolignans from Silymarin as Nrf2 Bioactivators and Their Therapeutic Applications. Biomedicines 2020, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Inkanuwat, A.; Sukaboon, R.; Reamtong, O.; Asawanonda, P.; Pattaratanakun, A.; Saisavoey, T.; Sangtanoo, P.; Karnchanatat, A. Nitric Oxide Synthesis Inhibition and Anti-Inflammatory Effect of Polypeptide Isolated from Chicken Feather Meal in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. Food Technol. Biotechnol. 2019, 57, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Milic, N. Muriel, P., Ed.; Silymarin for liver disease. In Liver Pathophysiology; Academic Press: London, UK, 2017; pp. 621–631. [Google Scholar]
- Matsumoto, T.; Nakashima, S.; Nakamura, S.; Hattori, Y.; Ando, T.; Matsuda, H. Inhibitory Effects of Cynaropicrin and Related Sesquiterpene Lactones from Leaves of Artichoke (Cynara scolymus L.) on Induction of iNOS in RAW264.7 Cells and Its High-Affinity Proteins. J. Nat. Med. 2021, 75, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Zhang, X.; Tang, J.; Kong, Y.; Wang, X.; Wang, S. Chlorogenic acid protects against aluminum toxicity via MAPK/Akt signaling pathway in murine RAW264.7 macrophages. J. Inorg. Biochem. 2019, 190, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Arka, G.; Anindita, K.; Ankit, S.; Kumar, S.A.; Kumar, M.S. Preliminary evaluation of hepatoprotective potential of the polyherbal formulation. J. Intercult. Ethnopharmacol. 2014, 4, 118. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y.; Kashine, S.; Yoneyama, T.; Sakamoto, Y.; Matsui, Y.; Shibata, H. Iron chelation by chlorogenic acid as a natural antioxidant. Biosci. Biotechnol. Biochem. 1998, 62, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Huang, Y.; Zhu, Q.-F.; Song, M.; Xiong, S.; Manyande, A.; Du, H. The mechanism of chlorogenic acid inhibits lipid oxidation: An investigation using multi-spectroscopic methods and molecular docking. Food Chem. 2020, 333, 127528. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Li, X.; Liu, Y.; Wang, S.; Cheng, D. Protection Mechanisms Underlying Oral Administration of Chlorogenic Acid against Cadmium-Induced Hepatorenal Injury Related to Regulating Intestinal Flora Balance. J. Agric. Food Chem. 2021, 69, 1675–1683. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, Y.; Yang, X.; He, J.; Tang, W.; Chai, Y.; Duan, Z.; Li, W.; Zhao, D.; Wang, X.; et al. Chlorogenic Acid: A Promising Strategy for Milk Preservation by Inhibiting Staphylococcus aureus Growth and Biofilm Formation. Foods 2024, 13, 4104. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y.; Liu, Y.; Xu, M.; Yao, Z.; Zhang, X.; Sun, Y.; Zhou, T.; Shen, M. Effects of Chlorogenic Acid on Antimicrobial, Antivirulence, and Anti-Quorum Sensing of Carbapenem-Resistant Klebsiella Pneumoniae. Front. Microbiol. 2022, 13, 5051. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Qiao, M.; Guo, Y.; Wang, X.; Xu, Y.; Xia, X. Effect of subinhibitory concentrations of chlorogenic acid on reducing the virulence factor production by Staphylococcus aureus. Foodborne Pathog. Dis. 2014, 11, 677–683. [Google Scholar] [CrossRef] [PubMed]
- El-Sapagh, S.; Allam, N.G.; El-Sayed, M.N.E.-D.; El-Hefnawy, A.A.; Korbecka-Glinka, G.; Shala, A.Y. Effects of Silybum marianum L. Seed Extracts on Multi Drug Resistant (MDR) Bacteria. Molecules 2024, 29, 64. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.M.P.; Rathinasabapathy, T.; Esposito, D.; Komarnytsky, S. Structural Constraints and Importance of Caffeic Acid Moiety for Anti-Hyperglycemic Effects of Caffeoylquinic Acids from Chicory. Mol. Nutr. Food Res. 2017, 61, 1601118. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y. The biological activity mechanism of chlorogenic acid and its applications in food industry: A review. Front Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef] [PubMed]






| Peak n. | Compound | Rt (min) | µg/mL ± SD | UV (λ max, nm) | [M-H] (m/z) | ESI-MS/MS (%) |
|---|---|---|---|---|---|---|
| 1 | Silychristin | 13.48 | 0.48 ± 0.02 | 287.8; 325.8 | 481 | 451 (5); 301 (18); 285 (24); 283 (8); 273 (14); 257 (7); 169 (21) |
| 2 | Silydianin | 14.17 | 0.46 ± 0.02 | 272.5; 302.1 | 481 | 451 (5); 435 (9); 301 (18); 285 (24); 283 (8); 273 (14); 257 (7); 169 (21) |
| 3 | Chlorogenic acid | 16.62 | 3.30 ± 0.04 | 299; 327 | 353 | 191 (100) |
| 4 | Silybin A | 18.43 | 0.54 ± 0.03 | 287.8; 322.2 | 481 | 451 (5); 435 (9); 301 (18); 285 (24); 273 (14); 257 (7); 169 (21) |
| 5 | Silybin B | 25.28 | 0.47 ± 0.06 | 283.1; 328.2 | 481 | 451 (5); 435 (9); 301 (18); 285 (24); 283 (8); 273 (14); 169 (21) |
| 6 | Vitexin | 25.71 | 0.67 ± 0.04 | 268.9; 337.7 | 431 | 311 (25); 269 (100) |
| 7 | Luteolin-7-O-glucuronide | 26.04 | 0.93±0.09 | 266.6;298.3; 336.5 | 461 | 341 (5); 285 (16) |
| 8 | Cinaroside | 27.31 | 2.30 ± 0.10 | 256; 265.4; 347.2 | 447 | 327 (1) [M-120-H]−; 285 (100) [M-162-H]− |
| 9 | Cynarine | 28.77 | 2.25 ± 0.11 | 300; 325.8 | 515 | 353 (18); 191 (76); 179 (23); 173 (21); 135 (18) |
| 10 | Apigenin-7-O-glucoside | 29.48 | 0.79 ± 0.06 | 281.9; 334.1 | 431 | 269 (100) |
| 11 | 3,4-dicaffeoylquinic acid | 30.43 | 2.68 ± 0.06 | 300.2; 329.4 | 515 | 353 (18); 191 (76); 179 (23); 173 (21); 135 (18) |
| 12 | 4,5-dicaffeoylquinic acid | 31.62 | 1.03 ± 0.07 | 300.9; 327 | 515 | 353 (18); 191 (76); 179 (23); 173 (21); 135 (18) |
| 13 | Luteolin | 38.93 | 0.95 ± 0.13 | 254; 268; 349.5 | 285 | 133 (100) |
| Epavin | CGA | Levofloxacin | |||
|---|---|---|---|---|---|
| Gram-Positive Strains | MIC | MBC | MIC | MBC | MIC |
| E. faecalis ATCC 29212 | 1.5 | 1.5 | 3.0 | 6.0 | 2 |
| E. faecalis BS | 1.5 | 1.5 | 3.0 | 6.0 | 8 |
| S. aureus ATCC 25923 | 0.7 | 0.7 | 1.5 | 3.0 | 0.5 |
| S. aureus ATCC 29213 | 1.5 | 1.5 | 3.0 | 6.0 | 0.5 |
| S. aureus ATCC 43300 | 3.0 | 6.0 | 6.0 | 12.0 | 1 |
| S. aureus BS | 0.4 | 0.8 | 1.5 | 3.0 | 1 |
| Gram-negative strains | |||||
| Escherichia coli ATCC 25922 | 3.0 | 6.0 | 6.0 | 12.0 | 0.02 |
| Escherichia coli BS (ESBL) | 3.0 | 6.0 | 6.0 | 12.0 | 1 |
| Klebsiella pneumoniae ATCC 13883 | 6.0 | 12.0 | 6.0 | 12.0 | 8 |
| Klebsiella pneumoniae ATCC 700603 | 6.0 | 12.0 | 6.0 | 12.0 | 8 |
| Klebsiella pneumoniae BS | 6.0 | 12.0 | 12.0 | 24.0 | 32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbarossa, A.; Argentieri, M.P.; Diella, M.V.; Caforio, A.; Carrieri, A.; Corbo, F.; Rosato, A.; Carocci, A. Evaluation of the Biological Properties and Antibacterial Activities of the Natural Food Supplement “Epavin” for Liver Detoxification and Protection. Foods 2025, 14, 2600. https://doi.org/10.3390/foods14152600
Barbarossa A, Argentieri MP, Diella MV, Caforio A, Carrieri A, Corbo F, Rosato A, Carocci A. Evaluation of the Biological Properties and Antibacterial Activities of the Natural Food Supplement “Epavin” for Liver Detoxification and Protection. Foods. 2025; 14(15):2600. https://doi.org/10.3390/foods14152600
Chicago/Turabian StyleBarbarossa, Alexia, Maria Pia Argentieri, Maria Valeria Diella, Anita Caforio, Antonio Carrieri, Filomena Corbo, Antonio Rosato, and Alessia Carocci. 2025. "Evaluation of the Biological Properties and Antibacterial Activities of the Natural Food Supplement “Epavin” for Liver Detoxification and Protection" Foods 14, no. 15: 2600. https://doi.org/10.3390/foods14152600
APA StyleBarbarossa, A., Argentieri, M. P., Diella, M. V., Caforio, A., Carrieri, A., Corbo, F., Rosato, A., & Carocci, A. (2025). Evaluation of the Biological Properties and Antibacterial Activities of the Natural Food Supplement “Epavin” for Liver Detoxification and Protection. Foods, 14(15), 2600. https://doi.org/10.3390/foods14152600








