Valorisation of Beetroot Peel for the Development of Nutrient-Enriched Dehydrated Apple Snacks
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Processing Raw Ingredients
- ✓
- Selection and Sorting: We chose beetroots that were fresh and healthy, avoiding any that were overripe, mouldy, or damaged. Only healthy, well-grown roots were processed.
- ✓
- Cleaning: To remove any dust or debris, beetroots were thoroughly cleaned under the pressure of the water.
- ✓
- Peeling: The root peel was removed using a hygienic scraper, eliminating damaged or discoloured portions to ensure consistent quality.
- ✓
- Peel preparation: The clean peel was spread in a single layer on stainless steel trays, with each piece of peel being approximately 3 mm thick, in accordance with the recommendations in the specialist literature for optimal drying.
- ✓
- Drying: Trays were placed in a controlled hot-air chamber at 38 °C for six hours, until the peels were fully dehydrated and crisp.
- ✓
- Grinding: The beetroot peel was first dried thoroughly and then ground in a high-speed grinder until a fine, homogeneous powder was obtained. A subsequent stage of sieving through a fine sieve ensured the removal of oversized particles, resulting in particle sizes with values less than 40 µm, suitable for our experiment.
- ✓
- Packaging: The resulting fine powder was immediately transferred to hermetic moisture-resistant bags (zipper system) to prevent moisture absorption.
- ✓
- Storage: Packaged powder was stored in a cool, dark area (to prevent degradation from light exposure) with low relative humidity to maintain stability of colour and bioactive compounds.
2.3. Red Beetroot Peel Powder Characterisation
2.3.1. Physicochemical Analysis of Red Beetroot Peel Powder
2.3.2. Bioactive Compound Extraction from Red Beetroot Peel Powder
2.3.3. Total Betalain Content of Red Beetroot Peel Powder
2.3.4. Total Polyphenol Content of Red Beetroot Peel Powder
2.3.5. Antioxidant Activity of Red Beetroot Peel Powder
2.3.6. Colourimetric Analysis of the Red Beetroot Peel Powder
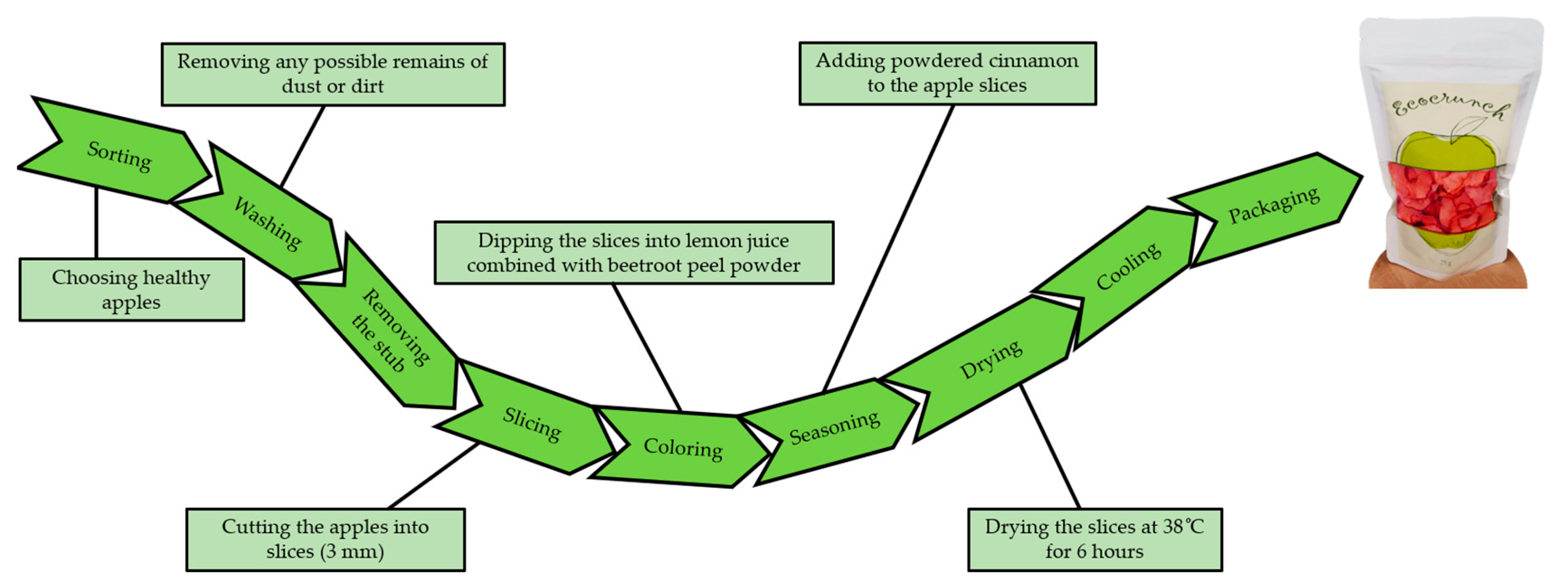
2.4. Preparation of Dried Apple Snack
2.5. Analysis of Dried Apple Snack
2.5.1. Physicochemical Characterisation
2.5.2. Extraction Procedure for Spectrophotometric and HPLC Determinations of Apple Snack
2.5.3. Total Betalain Content, Total Polyphenolic Content and Antioxidant Capacity
2.5.4. HPLC Analysis of Phenolic Compounds
2.6. Colourimetric Analysis of the Apple Snack
2.7. Sensory Characteristics
2.8. Statistical Test
3. Results and Discussion
3.1. Red Beetroot Peel Powder Characterisation
3.2. Apple Snack Characterisation
3.2.1. Physicochemical Characteristics of Dehydrated Apple Snacks
3.2.2. Bioactive Compound Content in Apple Snacks
3.3. HPLC Polyphenolic Profile of Apple Snacks
3.4. Colourimetric Analysis of Dehydrated Apple Snack
3.5. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Varghese, S.A.; Pulikkalparambil, H.; Promhuad, K.; Srisa, A.; Laorenza, Y.; Jarupan, L.; Nampitch, T.; Chonhenchob, V.; Harnkarnsujarit, N. Renovation of agro-waste for sustainable food packaging: A review. Polymers 2023, 15, 648. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.W.; Kumar, J.V.; Awad, M.; Saravanan, P.; Al-Sowayan, N.S.; Rosaiah, P.; Nivetha, M.S. Emerging trends in food process engineering: Integrating sensing technologies for health, sustainability, and consumer preferences. J. Food Process Eng. 2025, 48, e70035. [Google Scholar] [CrossRef]
- Zlati, C.; Grădinariu, G.; Istrate, M.; Draghia, L. Histological investigation on graft formation in pear/quince (Pyrus communis/Cydonia oblonga) combinations. Acta Hortic. 2011, 923, 291–298. [Google Scholar] [CrossRef]
- Milea, A.Ș.; Vasile, A.M.; Cîrciumaru, A.; Dumitrașcu, L.; Barbu, V.; Râpeanu, G.; Bahrim, G.E.; Stănciuc, N. Valorizations of sweet cherries skins phytochemicals by extraction, microencapsulation and development of value-added food products. Foods 2019, 8, 188. [Google Scholar] [CrossRef] [PubMed]
- Stoica, F.; Condurache, N.N.; Horincar, G.; Constantin, O.E.; Turturică, M.; Stănciuc, N.; Aprodu, I.; Croitoru, C.; Râpeanu, G. Value-added crackers enriched with red onion skin anthocyanins entrapped in different combinations of wall materials. Antioxidants 2022, 11, 1048. [Google Scholar] [CrossRef] [PubMed]
- Enache, I.-M.; Ciurlă, L.; Patraș, A.; Leonte, E.; Cârlescu, P.-M. Jelly candies with apple pomace—A circular economy solution for a food processing waste. Agriculture 2025, 15, 653. [Google Scholar] [CrossRef]
- Kujala, T.; Loponen, J.; Pihlaja, K. Betalains and phenolics in red beetroot (Beta vulgaris) peel extracts: Extraction and characterisation. Z. Naturforsch. 2001, 56, 343–348. [Google Scholar] [CrossRef] [PubMed]
- El-Beltagi, H.S.; El-Mogy, M.M.; Parmar, A.; Mansour, A.T.; Shalaby, T.A.; Ali, M.R. Phytochemical characterization and utilization of dried red beetroot (Beta vulgaris) peel extract in maintaining the quality of Nile Tilapia fish fillet. Antioxidants 2022, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Montenegro-Landívar, M.F.; Tapia-Quirós, P.; Vecino, X.; Reig, M.; Valderrama, C.; Granados, M.; Cortina, J.L.; Saurina, J. Fruit and vegetable processing wastes as natural sources of antioxidant-rich extracts: Evaluation of advanced extraction technologies by surface response methodology. J. Environ. Chem. Eng. 2021, 9, 105330. [Google Scholar] [CrossRef]
- Mello, F.R.; Bernardo, C.; Odebrecht Dias, C.; Gonzaga, L.; Amante, E.R.; Fett, R.; Candido, L.M.B. Antioxidant properties, quantification and stability of betalains from pitaya (Hylocereus undatus) peel. Cienc. Rural. 2015, 45, 323–328. [Google Scholar] [CrossRef]
- Minaxi, S.; Usmani, Z.; Kumar Gupta, V.; Bhat, R. Valorization of fruits and vegetable wastes and byproducts to produce natural pigments. Crit. Rev. Biotechnol. 2021, 41, 535–563. [Google Scholar] [CrossRef] [PubMed]
- Stoica, F.; Râpeanu, G.; Rațu, R.N.; Stănciuc, N.; Croitoru, C.; Țopa, D.; Jităreanu, G. Red beetroot and its by-products: A comprehensive review of phytochemicals, extraction methods, health benefits, and applications. Agriculture 2025, 15, 270. [Google Scholar] [CrossRef]
- Gandía-Herrero, F.; Simón-Carrillo, A.; Escribano, J.; García-Carmona, F. Determination of beet root betanin in dairy products by High-Performance Liquid Chromatography (HPLC). J. Chem. Educ. 2012, 89, 660–664. [Google Scholar] [CrossRef]
- Guldiken, B.; Toydemir, G.; Nur Memis, K.; Okur, S.; Boyacioglu, D.; Capanoglu, E. Home-processed red beetroot (Beta vulgaris L.) products: Changes in antioxidant properties and bioaccessibility. Int. J. Mol. Sci. 2016, 17, 858. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.L.; Teret, S.P.; Brownell, K.D. The food industry and self-regulation: Standards to promote success and to avoid public health failures framing health matters. Am. J. Public Health 2010, 100, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, V.G.; Weber, J.; Kneschke, E.M.; Denev, P.N.; Bley, T.; Pavlov, A.I. Antioxidant activity and phenolic content of betalain extracts from intact plants and hairy root cultures of the red beetroot (Beta vulgaris). Plant Foods Hum. Nutr. 2010, 65, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Pașcu, R.; Zlati, C.; Bernardis, R. Rehabilitation of the Dendrological Park in Buhusi, Bacau County. Scientific Papers. Series B, Horticulture. “Agric. Life, Life Agric.” Conf. Proceed. 2022, Volume LXVI, pp. 730–737, ISSN: 2285-5653, eISSN: 2286-1580. Available online: http://horticulturejournal.usamv.ro/pdf/2022/issue_1/Art106.pdf (accessed on 20 June 2025).
- Zlati, C.; Istrate, M.; Pascu, R.; Bernardis, R. An Assessment of Current Status, Future Trends and Opportunities for Improving Exotic and Underutilized Pome Fruit Species Production in Romania. Scientific Papers. Series B, Horticulture. 2024, Volume LXVIII, pp. 224–229, ISSN-L: 2285-5653, eISSN: 2286-1580. Available online: https://horticulturejournal.usamv.ro/index.php/scientific-papers/current-issue?id=1607 (accessed on 14 July 2025).
- Spengler, R.N. Origins of the apple: The role of megafaunal mutualism in the domestication of Malus and Rosaceous trees. Front. Plant Sci. 2019, 10, 617. [Google Scholar] [CrossRef] [PubMed]
- Mureşan, E.A.; Muste, S.; Borşa, A.; Vlaic, R.A.; Mureşan, V. Evaluation of physical-chemical indexes, sugars, pigments and phenolic compounds of fruits from three apple varieties at the end of storage period. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca, Food Sci. Technol. 2014, 71, 45–50, ISSN-L 2344-2344; Print ISSN 2344-2344; Electronic ISSN 2344-5300. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koutsos, A.; Tuohy, K.; Lovegrove, J. Apples and cardiovascular health—Is the gut microbiota a core consideration? Nutrients 2015, 7, 3959–3998. [Google Scholar] [CrossRef] [PubMed]
- Patocka, J.; Bhardwaj, K.; Klimova, B.; Nepovimova, E.; Wu, Q.; Landi, M.; Kuka, K.; Valis, M.; Wu, W. Malus domestica: A review on nutritional features, chemical composition, traditional and medicinal value. Plants 2020, 9, 1408. [Google Scholar] [CrossRef] [PubMed]
- Irimia, L. Controlul și Expertiza Calității Legumelor, Fructelor și Produselor Derivate (Eng. Quality Control and Inspection of Vegetables, Fruit and Derived Products); Ion Ionescu de la Brad: Iași, Romania, 2013; pp. 1–276. ISBN 978-973-147-117-4. [Google Scholar]
- Cichowska-Bogusz, J.; Figiel, A.; Carbonell-Barrachina, A.A.; Pasławska, M.; Witrowa-Rajchert, D. Physicochemical properties of dried apple slices: Impact of osmo-dehydration, sonication, and drying methods. Molecules 2020, 25, 1078. [Google Scholar] [CrossRef] [PubMed]
- Sobukola, O.P.; Dairo, O.U.; Odunewu, A.V. Convective hot air drying of blanched yam slices. Int. J. Food Sci. Technol. 2008, 43, 1233–1238. [Google Scholar] [CrossRef]
- Çoklar, H.; Akbulut, M. Effect of sun, oven and freeze-drying on anthocyanins, phenolic compounds and antioxidant activity of black grape (Ekșikara) (Vitis vinifera L.). S. Afr. J. Enol. Vitic. 2017, 38, 264–272. [Google Scholar] [CrossRef]
- Mongi, R.J.; Ndabikunze, B.; Chove, B.; Wicklund, T. Descriptive sensory analysis, consumer liking and preference mapping for solar dried mango cv Dodo. Food Sci. Qualit. Manag. 2013, 16, 16–23. [Google Scholar]
- Kahraman, O.; Malvandi, A.; Vargas, L.; Feng, H. Drying characteristics and quality attributes of apple slices dried by a non-thermal ultrasonic contact drying method. Ultrason. Sonochem. 2021, 73, 105510. [Google Scholar] [CrossRef] [PubMed]
- Fardet, A.; Rock, E. Ultra-processed foods and food system sustainability: What are the links? Sustainability 2020, 12, 6280. [Google Scholar] [CrossRef]
- Nakra, S.; Tripathy, S.; Srivastav, P.P. Green and sustainable extraction of bioactive compounds from Centella asiatica leaves using microwave pretreatment and ultrasonication: Kinetics, process optimization, and biological activity. Food Biophys. 2025, 20, 56. [Google Scholar] [CrossRef]
- Gyurova, D.K.; Enikova, R.K. Dried fruits–brief characteristics of their nutritional values. Author’s own data for dietary fibers content, J. Food Nutr. Sci. 2014, 2, 105–109. [Google Scholar] [CrossRef]
- Ghinea, C.; Prisacaru, A.E.; Leahu, A. Physico-chemical and sensory quality of oven-dried and dehydrator-dried apples of the Starkrimson, Golden Delicious and Florina cultivars. Appl. Sci. 2022, 12, 2350. [Google Scholar] [CrossRef]
- Testa, R.; Rizzo, G.; Schifani, G.; Tinebra, I.; Farina, V.; Vella, F.; Migliore, G. Can dried fruits replace unhealthy snacking among millennials? An empirical study on dried fruit consumption in Italy. Sustainability 2023, 15, 7083. [Google Scholar] [CrossRef]
- Sabbe, S.; Verbeke, W.; Van Damme, P. Familiarity and purchasing intention of Belgian consumers for fresh and processed tropical fruit products. Br. Food J. 2008, 110, 805–818. [Google Scholar] [CrossRef]
- Jesionkowska, K.; Sijtsema, S.J.; Konopacka, D.; Symoneaux, R. Dried fruit and its functional properties from a consumer’s point of view. J. Hortic. Sci. Biotechnol. 2009, 84, 85–88. [Google Scholar] [CrossRef]
- ISO 11035; OECD Standards—ISO Sensory Analysis—Identification and Selection of Descriptors for Establishing a Sensory Profile by a Multidimensional Approach. ISO: Geneva, Switzerland, 1994.
- ISO 13299; OECD Standards—ISO Sensory Analysis—Methodology, General Guidance for Establishing a Sensory Profile. ISO: Geneva, Switzerland, 2003.
- ISO 8586:2012; OECD Standards—ISO Sensory Analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors. ISO: Geneva, Switzerland, 2012.
- Patras, A. Biochemistry (Ro: Biochimie); PIM: Iaşi, Romania, 2020; pp. 1–245. ISBN 978-606-13-5597-6. [Google Scholar]
- Murariu, O.C.; Irimia, L.M.; Robu, T. Analyzing and Controlling the Quality of Fruit and Vegetable Products, Practical Guide (Ro: Analiza și controlul calității produselor din fructe și legume, îndrumător de lucrări practice); Ion Ionescu de la Brad: Iași, Romania, 2017; pp. 1–310. ISBN 9789731472508. [Google Scholar]
- Wruss, J.; Waldenberger, G.; Huemer, S.; Uygun, P.; Lanzerstorfer, P.; Müller, U.; Höglinger, O.; Weghuber, J. Compositional characteristics of commercial beetroot products and beetroot juice prepared from seven beetroot varieties grown in Upper Austria. J. Food Compost. Anal. 2015, 42, 46–55. [Google Scholar] [CrossRef]
- Lazăr (Mistrianu), S.; Constantin, O.E.; Stănciuc, N.; Aprodu, I.; Croitoru, C.; Râpeanu, G. Optimization of betalain pigments extraction using beetroot by-products as a valuable source. Inventions 2021, 6, 50. [Google Scholar] [CrossRef]
- Pereira, C.G.; Locatelli, M.; Innosa, D.; Cacciagrano, F.; Polesná, L.; Santos, T.F.; Rodrigues, M.J.; Custódio, L. Unravelling the potential of the medicinal halophyte Eryngium maritimum L.: In vitro inhibition of diabetes-related enzymes, antioxidant potential, polyphenolic profile and mineral composition. S. Afr. J. Bot. 2019, SAJB-02064, 1–9. [Google Scholar] [CrossRef]
- Sielicka, M.; Mahecha, M.; Purłan, M. Comparison of the antioxidant capacity of lipid-soluble compounds in selected cold-pressed oils using photochemiluminescence assay (PCL) and DPPH method. Eur. J. Lipid Sci. Technol. 2014, 116, 388–394. [Google Scholar] [CrossRef]
- Ciurlă, L.; Enache, I.-M.; Buțerchi, I.; Mihalache, G.; Lipșa, F.D.; Patraș, A. A new approach to recover bioactive compounds from apple pomace: Healthy jelly candies. Foods 2024, 14, 39. [Google Scholar] [CrossRef] [PubMed]
- Irimia, L.M. (Coord.) Practical Manual, Horticulture Specialization, Vol. II (Ro: Manual de practică, specializarea horticultură, volumul II); Ion Ionescu de la Brad: Iași, Romania, 2021; pp. 1–338. ISBN 9789731474083. [Google Scholar]
- Kocabıyık, B.; Alkan, D. Effect of the production of dried fruit and fruit chips on chemical, sensory and bioactive properties. Explor. Foods Foodom. 2025, 3, 101077. [Google Scholar] [CrossRef]
- Filimon, R.V.; Bunea, C.I.; Bora, F.D.; Filimon, R.M.; Dunca, S.I.; Rózsa, S.; Ciurlă, L.; Patraș, A. Physico-chemical characterization, phenolic compound extraction and biological activity of grapevine (Vitis vinifera L.) canes. Horticulturae 2023, 9, 1164. [Google Scholar] [CrossRef]
- Lawless, H.; Heymann, H. Chapter 10: Descriptive analysis. In Sensory Evaluation of Food. Food Science Text Series; Springer: New York, NY, USA, 2010; pp. 227–257. [Google Scholar] [CrossRef]
- Nale, S.A.; Swami, S.B. Open sun drying of beetroot slices and its quality evaluation. Int. J. Food Ferment. Technol. 2023, 13, 27–39. [Google Scholar] [CrossRef]
- Sawicki, T.; Bączek, N.; Wiczkowski, W. Betalain profile, content and antioxidant capacity of red beetroot dependent on the genotype and root part. J. Funct. Foods 2016, 27, 249–261. [Google Scholar] [CrossRef]
- Salamatullah, A.M.; Hayat, K.; Alkaltham, M.S.; Ahmed, M.A.; Arzoo, S.; Husain, F.M.; Al-Dossari, A.M.; Shamlan, G.; Al-Harbi, L.N. Bioactive and antimicrobial properties of oven-dried beetroot (pulp and peel) using different solvents. Processes 2021, 9, 588. [Google Scholar] [CrossRef]
- Vulić, J.; Čanadanović-Brunet, J.; Ćetković, G.; Tumbas, V.; Djilas, S.; Četojević-Simin, D.; Čanadanović, V. Antioxidant and cell growth activities of beet root pomace extracts. J. Funct. Foods 2012, 4, 670–678. [Google Scholar] [CrossRef]
- Coy-Barrera, E. Chapter 17. Analysis of betalains (betacyanins and betaxanthins). In Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 593–619. [Google Scholar] [CrossRef]
- Tarasevičienė, Ž.; Paulauskienė, A.; Černiauskienė, J.; Degimienė, A. Chemical content and color of dried organic beetroot powder affected by different drying methods. Horticulturae 2024, 10, 733. [Google Scholar] [CrossRef]
- Bahriye, G.; Dadashi, S.; Dehghannya, J.; Ghaffari, H. Influence of processing temperature on production of red beetroot powder as a natural red colorant using foam-mat drying: Experimental and modeling study. Food Sci. Nutr. 2023, 11, 6955–6973. [Google Scholar] [CrossRef] [PubMed]
- González-Molina, E.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Natural bioactive compounds of Citrus limon for food and health. J. Pharm. Biomed. Anal. 2010, 51, 327–345. [Google Scholar] [CrossRef] [PubMed]
- Klimek-Szczykutowicz, M.; Szopa, A.; Ekiert, H. Citrus limon (lemon) phenomenon—A review of the chemistry, pharmacological properties, applications in the modern pharmaceutical, food, and cosmetics industries, and biotechnological studies. Plants 2020, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Qurban, F.; Hussain, S.; Waqas, M.; Shahzad, H.H.; Rukhsar, A.; Javed, A. Phytochemistry, nutritional, and pharmacological potential of Citrus limonum. Sci. Inq. Rev. 2024, 8, 1–23. [Google Scholar] [CrossRef]
- Huang, T.-C.; Fu, H.-Y.; Ho, C.-T.; Tan, D.; Huang, Y.-T.; Pan, M.-H. Induction of apoptosis by cinnamaldehyde from indigenous cinnamon Cinnamomum osmophloeum Kaneh through reactive oxygen species production, glutathione depletion, and caspase activation in human leukemia K562 cells. Food Chem. 2007, 103, 434–443. [Google Scholar] [CrossRef]
- Rao, P.V.; Gan, S.H. Review article: Cinnamon: A multifaceted medicinal plant. Evid. Based Complement. Alternat. Med. 2014, 2014, 642942. [Google Scholar] [CrossRef] [PubMed]
- Demiray, E.; Yazar, J.G.; Aktok, Ö.; Çulluk, B.; Çalışkan Koç, G.; Pandiselvam, R. The effect of drying temperature and thickness on the drying kinetic, antioxidant activity, phenolic compounds, and color values of apple slices. J. Food Qual. 2023, 2023, 426793. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A.; Sroka, P.; Satora, P.; Tuszyński, T. Production of flavored apple chips of high antioxidant activity. J. Food Proces. Preserv. 2010, 34, 728–742. [Google Scholar] [CrossRef]
- Suriati, L.; Utama, M.S.; Harsojuwono, B.A.; Gunam, I.B.W. Physicochemical characteristics of fresh-cut tropical fruit during storage. Int. J. Adv. Sci. Eng. Inform. Technol. 2020, 10, 1731–1736. [Google Scholar] [CrossRef]
- Xu, K.; Wang, A.; Brown, S. Genetic characterization of the Ma locus with pH and titratable acidity in apple. Mol. Breed. 2011, 30, 899–912. [Google Scholar] [CrossRef]
- Owusu, J.; Ma, H.; Wang, Z.; Amissah, A. Effect of drying methods on physicochemical properties of pretreated tomato (Lycopersion esculentum mill.) slices. Croat. J. Food Technol. Biotechnol. Nutr. 2012, 7, 106–111. [Google Scholar]
- Ullah, F.; Hasrat, K.; Iqbal, S.; Hussain, I.; Hussain, A.; Mumtaz, Y. An approach to evaluate dehydration of apples (Malus domestica L) with the effect of temperature and time interval under the Response Surface Method. Int. J. Fruit Sci. 2021, 21, 657–669. [Google Scholar] [CrossRef]
- Mongi, R.J.; Ndabikunze, B.K.; Wicklund, T.; Chove, L.M.; Chove, B.E. Effect of solar drying methods on total phenolic contents and antioxidant activity of commonly consumed fruits and vegetable (mango, banana, pineapple and tomato) in Tanzania. Afr. J. Food Sci. 2015, 9, 291–300. [Google Scholar] [CrossRef]
- Pomeranz, I.; Meloan, E.C. Chapter 35: Ash and Minerals. In Food Analysis; Chapman & Hall, Inc.: New York, NY, USA; London, UK, 1994; pp. 602–603. [Google Scholar]
- Orak, H.H.; Aktas, T.; Yagar, H.; Selen Isbilir, S.; Ekinci, N.; Hasturk Sahin, F. Effects of hot air and freeze drying methods on antioxidant activity, colour and some nutritional characteristics of strawberry tree (Arbutus unedo L.) fruit. Food Sci. Technol. Int. 2012, 18, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Masamba, K.G.; Mkandawire, M.; Chiputula, J.; Nyirenda, K.S. Evaluation of sensory quality attributes and extent of vitamin C degradation in dried pineapple, mango and banana fruit pieces pre–treated with sodium metabisulphite and lemon juice. Int. Res. J. Agric. Sci. Soil Sci. 2013, 3, 75–80. [Google Scholar]
- Azeredo, H.M.C. Betalains: Properties, sources, applications, and stability—A review. Int. J. Food Sci. Technol. 2009, 44, 2365–2376. [Google Scholar] [CrossRef]
- Quitral, V.; Sepúlveda, M.; Schwartz, M. Antioxidant capacity and total polyphenol content in different apple varieties cultivated in Chile. Rev. Iberoameric. Tecnol. Postcosec. 2013, 14, 31–39. [Google Scholar]
- Vujčić Bok, V.; Šola, I.; Rusak, G. Lemon juice formulations modulate in vitro digestive recovery of spinach phytochemicals. Food Technol. Biotechnol. 2022, 60, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Burgos, E.; Gomez-Serranillos Cuadrado, P. Effect of phenolic compounds on human health. Nutrients 2021, 13, 3922. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, D.V.; Shvachko, N.A.; Mikhailova, A.S.; Popov, V.S.; Solovyeva, A.E.; Khlestkina, E.K. Characterization of betalain content and antioxidant activity variation dynamics in table beets (Beta vulgaris L.) with differently colored roots. Agronomy 2024, 14, 999. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Székely, D.; Furulyás, D.; Stéger-Máté, M. Investigation of mineral and vitamin C contents in different parts of beetroots (Beta vulgaris L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 615–620. [Google Scholar] [CrossRef]
- Bendich, A.; Machlin, L.J.; Scandurra, O.; Burton, G.W.; Wayner, D.D.M. The antioxidant role of vitamin C. Adv. Free Radic. Biol. Med. 1986, 2, 419–444. [Google Scholar] [CrossRef]
- Oikeh, E.I.; Omoregie, E.S.; Oviasogie, F.E.; Oriakhi, K. Phytochemical, antimicrobial, and antioxidant activities of different citrus juice concentrates. Food Sci. Nutr. 2016, 4, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Tarko, T.; Krankowski, F.; Duda-Chodak, A. The Impact of compounds extracted from wood on the quality of alcoholic beverages. Molecules 2023, 28, 620. [Google Scholar] [CrossRef] [PubMed]
- Constantin, O.E.; Stoica, F.; Lazăr (Mistrianu), S.; Andronoiu, D.G.; Turturică, M.; Stănciuc, N.; Rațu, R.N.; Croitoru, C.; Râpeanu, G. A sustainable approach: Repurposing red beetroot peels for innovative meringue products. Foods 2025, 14, 317. [Google Scholar] [CrossRef] [PubMed]
- Lazăr (Mistrianu), S.; Constantin, O.E.; Horincar, G.; Andronoiu, D.G.; Stănciuc, N.; Muresan, C.; Râpeanu, G. Beetroot by-product as a functional ingredient for obtaining value-added mayonnaise. Processes 2022, 10, 227. [Google Scholar] [CrossRef]
- Feng, S.; Yi, J.; Li, X.; Wu, X.; Zhao, Y.; Ma, Y.; Bi, J. Systematic review of phenolic compounds in apple fruits: Compositions, distribution, absorption, metabolism, and processing stability. J. Agric. Food Chem. 2021, 69, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Uğurlu, S.; Bakkalbaşı, E. A comparison of phenolic compounds, antioxidant activity, and α-glucosidase inhibitory activities of apple chips dried and fried by vacuum combined infrared radiation. J. Food Meas. Charact. 2024, 18, 3783–3792. [Google Scholar] [CrossRef]
- Cvetković, B.; Bajić, A.; Belović, M.; Pezo, L.; Dragojlović, D.; Šimurina, O.; Djordjević, M.; Korntheuer, K.; Philipp, C.; Eder, R. Assessing antioxidant properties, phenolic compound profiles, organic acids, and sugars in conventional apple cultivars (Malus domestica): A chemometric approach. Foods 2024, 13, 2291. [Google Scholar] [CrossRef] [PubMed]
- Liaudanskas, M.; Viškelis, P.; Kviklys, D.; Raudonis, R.; Janulis, V. A comparative study of phenolic content in apple fruits. Int. J. Food Prop. 2015, 18, 945–953. [Google Scholar] [CrossRef]
- Liao, L.; Zhang, W.; Zhang, B.; Cai, Y.; Gao, L.; Ogutu, C.; Sun, J.; Zheng, B.; Wang, L.; Li, L.; et al. Evaluation of chlorogenic acid accumulation in cultivated and wild apples. J. Food Compos. Anal. 2021, 104, 104156. [Google Scholar] [CrossRef]
- Liu, H.; Cao, J.; Jiang, W. Anti-inflammatory procyanidins and triterpenes in 109 apple varieties. J. Agric. Food Chem. 2012, 60, 10603–10613. [Google Scholar] [CrossRef] [PubMed]
- Amzad Hossain, M.; Salehuddin, S.M.; Kabir, M.J.; Rahman, S.M.M.; Rupasinghe, H.P.V. Sinensetin, rutin, 3′-hydroxy-5,6,7,4′-tetramethoxyflavone and rosmarinic acid contents and antioxidative effect of the skin of apple fruit. Food Chem. 2009, 113, 185–190. [Google Scholar] [CrossRef]
- Lee, J.; Chan, B.L.S.; Mitchell, A.E. Identification/quantification of free and bound phenolic acids in peel and pulp of apples (Malus domestica) using high resolution mass spectrometry (HRMS). Food Chem. 2017, 215, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.C.; Taciana Ribeiro, É.; Kuskoski, E.M.; Valdemiro Gonzaga, L.; Lima, A.; Mancini Filho, J.; Fett, R. Composition of phenolic acids content in apple (Malus sp.) pomace. Semin. Ciênc. Agrár. 2008, 29, 339–347. [Google Scholar] [CrossRef]
- Hammad, K.S.M.; Elsayed, N.; Elkashef, H. Development of a whey protein concentrate/apple pomace extract edible coating for shelf life extension of fresh-cut apple. Int. Food Res. J. 2021, 28, 377–385. [Google Scholar] [CrossRef]
- Liaudanskas, M.; Viškelis, P.; Jakštas, V.; Raudonis, R.; Kviklys, D.; Milašius, A.; Janulis, V. Application of an optimized HPLC method for the detection of various phenolic compounds in apples from Lithuanian cultivars. J. Chem. 2014, 2014, 542121. [Google Scholar] [CrossRef]
- Borjan, D.; Šeregelj, V.; Andrejč, D.C.; Pezo, L.; Šaponjac, V.T.; Knez, Ž.; Vulić, J.; Marevci, M.K. Green techniques for preparation of red beetroot extracts with enhanced biological potential. Antioxidants 2022, 11, 805. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Lee, S.H.; Nam, J.-S. Comparison of the antioxidant properties and phenolic compositions of different varieties of beets (Beta vulgaris L.) cultivated in Korea. J. Korean Soc. Food Sci. Nutr. 2021, 50, 1058–1064. [Google Scholar] [CrossRef]
- Vulić, J.J.; Ćebović, T.N.; Čanadanović-Brunet, J.M.; Ćetković, G.S.; Čanadanović, V.M.; Djilas, S.M.; Tumbas Šaponjac, V.T. In vivo and in vitro antioxidant effects of beetroot pomace extracts. J. Funct. Foods 2014, 6, 168–175. [Google Scholar] [CrossRef]
- Ertekin Filiz, B.; Seydim, A.C. Kinetic changes of antioxidant parameters, ascorbic acid loss, and hydroxymethyl furfural formation during apple chips production. J. Food Biochem. 2018, 42, e12676. [Google Scholar] [CrossRef]
- Abdo, E.; El-Sohaimy, S.; Shaltout, O.; Abdalla, A.; Zeitoun, A. Nutritional evaluation of beetroots (Beta vulgaris L.) and its potential application in a functional beverage. Plants 2020, 9, 1752. [Google Scholar] [CrossRef] [PubMed]
- Wach, A.; Pyrzyńska, K.; Biesaga, M. Quercetin content in some food and herbal samples. Food Chem. 2007, 100, 699–704. [Google Scholar] [CrossRef]
- Celik, F.; Gundogdu, M.; Ercisli, S.; Kaki, B.; Berk, S.; Ilhan, G.; Sagbas, H.I. Variation in organic acid, sugar and phenolic compounds in fruits of historical apple cultivars. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 622–629. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, P.; Cheng, L. Developmental changes of carbohydrates, organic acids, amino acids, and phenolic compounds in ‘Honeycrisp’ apple flesh. Food Chem. 2010, 123, 1013–1018. [Google Scholar] [CrossRef]
- Özcan, M.M.; Uslu, N. Changes in bioactive compounds, antioxidant activity, and polyphenols of red beetroots dehydrated in oven, microwave, and infrared systems. JSFA Rep. 2023, 3, 582–587. [Google Scholar] [CrossRef]
- Geană, E.-I.; Ciucure, C.T.; Ionete, R.E.; Ciocârlan, A.; Aricu, A.; Ficai, A.; Andronescu, E. Profiling of phenolic compounds and triterpene acids of twelve apple (Malus domestica Borkh.) cultivars. Foods 2021, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, C.; Wilches-Pérez, D.; Hallmann, E.; Kazimierczak, R.; Rembiałkowska, E. Organic versus conventional beetroot. Bioactive compounds and antioxidant properties. LWT 2019, 116, 108552. [Google Scholar] [CrossRef]
- Płatosz, N.; Sawicki, T.; Wiczkowski, W. Profile of phenolic acids and flavonoids of red beet and its fermentation products. does long-term consumption of fermented beetroot juice affect phenolics profile in human blood plasma and urine? Polish J. Food Nutr. Sci. 2020, 70, 55–65. [Google Scholar] [CrossRef]
- Spence, C. On the psychological impact of food colour. Flavour 2015, 4, 1–16. [Google Scholar] [CrossRef]
- Zlati, C.; Istrate, M.; Dascălu, M.; Pașcu, R.; Bernardis, R.; Strugariu-Eisenhauer, E. Scientific Papers. Series B, Horticulture. “Agriculture for Life, Life for Agriculture” Conference Proceedings; ISSN: 2285-5653, eISSN: 2286-1580; 2023; Volume LXVII, pp. 215–218. [Google Scholar]
- Pașcu, R.; Zlati, C.; Calance, A.; Bernardis, R.; Dodu, D. Methods of rehabilitation of a degraded area in Orăştie. Scientific Papers. Series B, Horticulture. ”Agriculture for Life, Life for Agriculture” Conf. Proceed. 2021, Volume LXV, pp. 664–693, ISSN: 2285-5653, eISSN: 2286-1580. Available online: http://horticulturejournal.usamv.ro/pdf/2021/issue_1/vol2021_1.pdf (accessed on 20 June 2025).
- Zhu, J.; Liu, Y.; Zhu, C.; Wei, M. Effects of different drying methods on the physical properties and sensory characteristics of apple chip snacks. LWT Food Sci. Technol. 2022, 154, 112829. [Google Scholar] [CrossRef]
- Patras, A. Stability and colour evaluation of red cabbage waste hydroethanolic extract in presence of different food additives or ingredients. Food Chem. 2019, 275, 539–548. [Google Scholar] [CrossRef] [PubMed]
- González-Montelongo, R.; Lobo, M.G.; González, M. Antioxidant activity in banana peel extracts: Testing extraction conditions and related bioactive compounds. Food Chem. 2010, 119, 1030–1039. [Google Scholar] [CrossRef]
- Sogi, D.S.; Siddiq, M.; Greiby, I.; Dolan, K.D. Total Phenolics, Antioxidant activity, and functional properties of ‘tommy atkins’ mango peel and kernel as affected by drying methods. Food Chem. 2013, 141, 2649–2655. [Google Scholar] [CrossRef] [PubMed]
- Calín-Sánchez, Á.; Figiel, A.; Lech, K.; Szumny, A.; Carbonell-Barrachina, Á.A. Effects of drying methods on the composition of thyme (Thymus vulgaris L.) essential oil. Drying Technol. 2013, 31, 224–235. [Google Scholar] [CrossRef]
- Harker, F.R.; Marsh, K.B.; Young, H.; Murray, S.H.; Gunson, F.A.; Walker, S.B. Sensory interpretation of instrumental measurements 2: Sweet and acid taste of apple fruit. Postharvest Biol. Technol. 2002, 24, 241–250. [Google Scholar] [CrossRef]
- Skendrović Babojelić, M.; Ivančić, K.; Družić, J.; Kovač, A.; Voća, S. Chemical and sensory characteristics of three apple cultivars (Malus x domestica Borkh.). Agric. Conspec. Sci. 2007, 72, 317–322. [Google Scholar]
- Antal, T.; Kerekes, B.; Sikolya, L.; Tarek, M. Quality and drying characteristics of apple cubes subjected to combined drying (Fd pre-drying and Had finish-drying). J. Food Proces. Preserv. 2015, 39, 994–1005. [Google Scholar] [CrossRef]
- Abdo, E.M.; Allam, M.G.; Gomaa, M.A.E.; Shaltout, O.E.; Mansour, H.M.M. Valorization of whey proteins and beetroot peels to develop a functional beverage high in proteins and antioxidants. Front. Nutr. 2022, 9, 984891. [Google Scholar] [CrossRef] [PubMed]




| Sample | Treatment Details |
|---|---|
| Control | Apple slices |
| S1 | Apple slices dipped in 5% RBPP in water, seasoned with cinnamon powder |
| S2 | Apple slices dipped in 10% RBPP in water, seasoned with cinnamon powder |
| S3 | Apple slices dipped in 5% RBPP in 50% lemon juice, seasoned with cinnamon powder |
| S4 | Apple slices dipped in 10% RBPP in 50% lemon juice, seasoned with cinnamon powder |
| Attribute | 0—Minimum | 10—Maximum |
|---|---|---|
| External appearance | Non-attractive | Very attractive |
| Colour | Bright | Dark |
| Overall aroma | Unpleasant | Very pleasant |
| Consistency | Very hard | Soft |
| Sweet taste | Imperceptible | Very intense |
| Sour taste | Imperceptible | Very intense |
| Flavour | Uncharacteristic | Aromatic, palatable |
| Acceptability | Poor quality | Very good quality |
| Parameter | Results |
|---|---|
| pH | 5.40 ± 0.23 |
| Soluble dry matter (°Bx) | 85.50 ± 0.41 |
| Total dry matter (%) | 98.41 ± 0.11 |
| Total acidity (% malic acid) | 1.32 ± 0.07 |
| Total betalain content (mg betalains/100 g DM) | 1361.30 ± 2.45 |
| Total polyphenol content (mg GAE/100 g DM) | 2780.01 ± 68.38 |
| Antioxidant activity (µmol TE/g DM) | 503.96 ± 1.83 |
| L* | 28.09 ± 0.27 |
| a* | 34.74 ± 0.42 |
| b* | 6.36 ± 0.03 |
| c* | 35.32 ± 0.42 |
| h* | 10.38 ± 0.02 |
| Sample | Control | S1 | S2 | S3 | S4 |
|---|---|---|---|---|---|
| Total soluble solids (%) | 27.40 ± 0.01 e | 35.03 ± 0.03 d | 42.08 ± 0.02 b | 38.11 ± 0.04 c | 49.50 ± 0.03 a |
| pH | 3.80 ± 0.01 b | 4.07 ± 0.02 a | 4.13 ± 0.03 a | 3.48 ± 0.01 d | 3.60 ± 0.01 c |
| Total acidity (% malic acid) | 0.75 ± 0.09 b | 0.56 ± 0.10 b | 0.63 ± 0.08 b | 2.13 ± 0.06 a | 2.47 ± 0.08 a |
| Moisture (%) | 12.27 ± 0.01 d | 12.36 ± 0.01 c | 12.56 ± 0.01 ab | 12.49 ± 0.03 b | 12.63 ± 0.01 a |
| Total dry matter (%) | 87.73 ± 0.18 ns | 87.64 ± 0.02 ns | 87.44 ± 0.03 ns | 87.51 ± 0.00 ns | 87.37 ± 0.03 ns |
| Ash (%) | 0.46 ± 0.01 d | 0.65 ± 0.00 c | 0.72 ± 0.01 b | 0.71 ± 0.00 b | 0.78 ± 0.01 a |
| Reducing sugars (% Glucose) | 21.30 ± 0.03 e | 25.20 ± 0.01 d | 27.60 ± 0.00 b | 26.10 ± 0.02 c | 28.40 ± 0.02 a |
| Ascorbic acid (mg/100 g product) | 3.87 ± 0.01 e | 6.45 ± 0.01 d | 8.26 ± 0.03 c | 35.08 ± 0.02 b | 39.20 ± 0.01 a |
| Parameter | Sample | ||||
|---|---|---|---|---|---|
| Control | S1 | S2 | S3 | S4 | |
| Total betalain content (mg betalains/100 g DM) | n.a. d | 22.83 ± 0.74 c | 34.56 ± 1.93 b | 41.20 ± 1.65 b | 65.01 ± 4.26 a |
| Total polyphenol content (mg GAE/100 g DM) | 373.11 ± 18.53 d | 497.68 ± 17.08 c | 601.33 ± 17.98 b | 544.51 ± 15.58 bc | 903.22 ± 16.71 a |
| Antioxidant activity (µmol TE/g DM) | 28.07 ± 0.22 c | 33.30 ± 0.29 b | 35.51 ± 1.25 ab | 33.90 ± 0.16 b | 37.11 ± 0.31 a |
| Phenolic Compound (µg/g DM) | Sample | ||||
|---|---|---|---|---|---|
| Control | S1 | S2 | S3 | S4 | |
| Gallic acid | n.d. c | 3.44 ± 0.16 c | 3.17 ± 0.11 c | 19.60 ± 0.97 b | 32.79 ± 1.41 a |
| p -Hydroxybenzoic acid | n.d. c | 2.08 ± 0.18 b | 2.71 ± 0.10 b | 2.12 ± 0.15 b | 4.70 ± 0.32 a |
| Caffeic acid | n.d. c | n.d. c | n.d. c | 0.90 ± 0.01 a | 0.24 ± 0.01 b |
| Catechin | 1.24 ± 0.01 c | 4.86 ± 0.07 b | 5.84 ± 0.19 a | 4.76 ± 0.12 b | 6.06 ± 0.02 a |
| Chlorogenic acid | 690.53 ± 13.86 ns | 692.78 ± 13.11 ns | 705.34 ± 15.50 ns | 691.29 ± 18.15 ns | 697.63 ± 24.53 ns |
| Syringic acid | 12.57 ± 0.57 b | 17.67 ± 0.57 a | 18.14 ± 0.70 a | 17.15 ± 1.15 a | 18.54 ± 1.18 a |
| Coumaric acid | 6.39 ± 0.21 ns | 5.26 ± 0.38 ns | 5.35 ± 0.28 ns | 6.31 ± 0.31 ns | 5.97 ± 0.17 ns |
| Epicatechin | 10.13 ± 0.88 d | 24.41 ± 0.83 c | 28.97 ± 0.98 bc | 31.15 ± 1.09 b | 41.61 ± 1.37 a |
| Ferulicacid | 0.81 ± 0.03 c | 0.80 ± 0.03 c | 0.81 ± 0.03 c | 1.33 ± 0.07 a | 1.08 ± 0.05 b |
| Sinapic acid | n.d. b | 2.54 ± 0.39 b | 3.48 ± 0.21 b | 16.31 ± 1.89 a | 21.17 ± 2.40 a |
| Salicylic acid | 72.14 ± 5.67 b | 76.36 ± 4.89 b | 75.15 ± 4.50 b | 118.02 ± 5.52 a | 117.09 ± 5.96 a |
| Resveratrol | 3.61 ± 0.25 ns | 4.34 ± 0.36 ns | 5.07 ± 0.41 ns | 3.93 ± 0.31 ns | 4.98 ± 0.50 ns |
| Rosmarinic acid | 92.13 ± 4.39 ns | 107.91 ± 4.84 ns | 108.56 ± 5.36 ns | 108.69 ± 5.95 ns | 112.52 ± 4.60 ns |
| Quercetin | 1.74 ± 0.17 b | 1.78 ± 0.16 b | 1.93 ± 0.18 b | 3.55 ± 0.36 a | 3.78 ± 0.29 a |
| Colour Parameter | Sample | ||||
|---|---|---|---|---|---|
| Control | S1 | S2 | S3 | S4 | |
| L* | 76.62 ± 0.37 a | 58.37 ± 0.43 b | 50.10 ± 0.28 c | 40.82 ± 0.35 d | 35.02 ± 0.16 e |
| a* | 12.35 ± 0.07 c | 20.50 ± 0.18 b | 22.70 ± 0.10 ab | 25.53 ± 1.52 a | 25.53 ± 1.52 a |
| b* | 2.67 ± 0.05 d | 3.94 ± 0.04 c | 2.51 ± 0.08 d | 5.26 ± 0.12 b | 5.84 ± 0.10 a |
| c* | 12.63 ± 0.06 e | 20.87 ± 0.18 d | 23.15 ± 0.12 c | 27.70 ± 0.19 b | 29.68 ± 0.51 a |
| h* | 12.19 ± 0.24 b | 10.89 ± 0.09 c | 23.15 ± 0.12 a | 10.95 ± 0.16 c | 11.41 ± 0.12 c |
| ΔE* | - e | 20.28 ± 0.37 d | 28.79 ± 0.16 c | 38.95 ± 0.34 b | 45.06 ± 0.04 a |
| Sensory Parameter | Sample | ||||
|---|---|---|---|---|---|
| Control | S1 | S2 | S3 | S4 | |
| External appearance | 8.50 ± 0.02 e | 9.20 ± 0.02 d | 9.35 ± 0.01 c | 9.60 ± 0.02 b | 9.90 ± 0.02 a |
| Colour | 8.90 ± 0.03 e | 9.20 ± 0.01 d | 9.40 ± 0.02 c | 9.70 ± 0.02 b | 9.90 ± 0.01 a |
| Overall aroma | 7.00 ± 0.10 d | 8.70 ± 0.05 c | 8.95 ± 0.02 b | 9.70 ± 0.01 a | 9.90 ± 0.02 a |
| Consistency | 9.00 ± 0.03 d | 9.20 ± 0.02 c | 9.25 ± 0.01 c | 9.50 ± 0.03 b | 9.70 ± 0.02 a |
| Sweet taste | 8.50 ± 0.01 c | 9.60 ± 0.02 a | 9.60 ± 0.05 a | 8.75 ± 0.01 b | 8.50 ± 0.01 c |
| Sour taste | 4.80 ± 0.05 e | 6.30 ± 0.02 d | 6.60 ± 0.02 c | 9. 00 ± 0.03 b | 9.50 ± 0.05 a |
| Flavour | 6.80 ± 0.05 e | 8.43 ± 0.04 d | 8.65 ± 0.02 c | 9.90 ± 0.05 a | 9.20 ± 0.03 b |
| Acceptability | 7.80 ± 0.05 e | 8.25 ± 0.03 d | 8.75 ± 0. 00 c | 9.80 ± 0.02 a | 9.20 ± 0.02 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buțerchi, I.; Ciurlă, L.; Enache, I.-M.; Patraș, A.; Teliban, G.-C.; Irimia, L.-M. Valorisation of Beetroot Peel for the Development of Nutrient-Enriched Dehydrated Apple Snacks. Foods 2025, 14, 2560. https://doi.org/10.3390/foods14152560
Buțerchi I, Ciurlă L, Enache I-M, Patraș A, Teliban G-C, Irimia L-M. Valorisation of Beetroot Peel for the Development of Nutrient-Enriched Dehydrated Apple Snacks. Foods. 2025; 14(15):2560. https://doi.org/10.3390/foods14152560
Chicago/Turabian StyleBuțerchi, Ioana, Liliana Ciurlă, Iuliana-Maria Enache, Antoanela Patraș, Gabriel-Ciprian Teliban, and Liviu-Mihai Irimia. 2025. "Valorisation of Beetroot Peel for the Development of Nutrient-Enriched Dehydrated Apple Snacks" Foods 14, no. 15: 2560. https://doi.org/10.3390/foods14152560
APA StyleBuțerchi, I., Ciurlă, L., Enache, I.-M., Patraș, A., Teliban, G.-C., & Irimia, L.-M. (2025). Valorisation of Beetroot Peel for the Development of Nutrient-Enriched Dehydrated Apple Snacks. Foods, 14(15), 2560. https://doi.org/10.3390/foods14152560





