Carcass Color in Broilers When Replacing Wheat with Corn in the Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
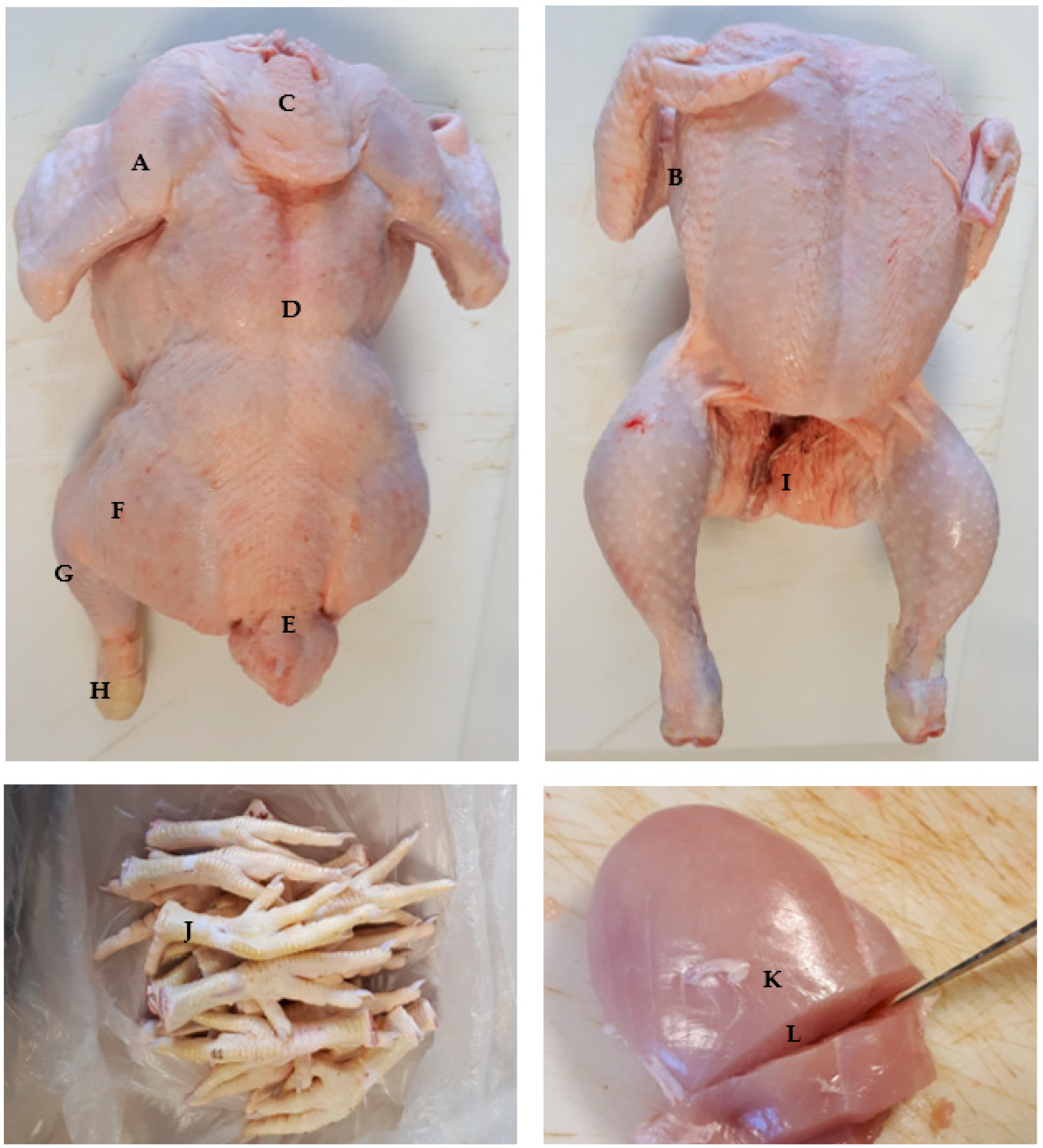
2.2. Carcass and Color Analyses
2.3. Statistical Analyses
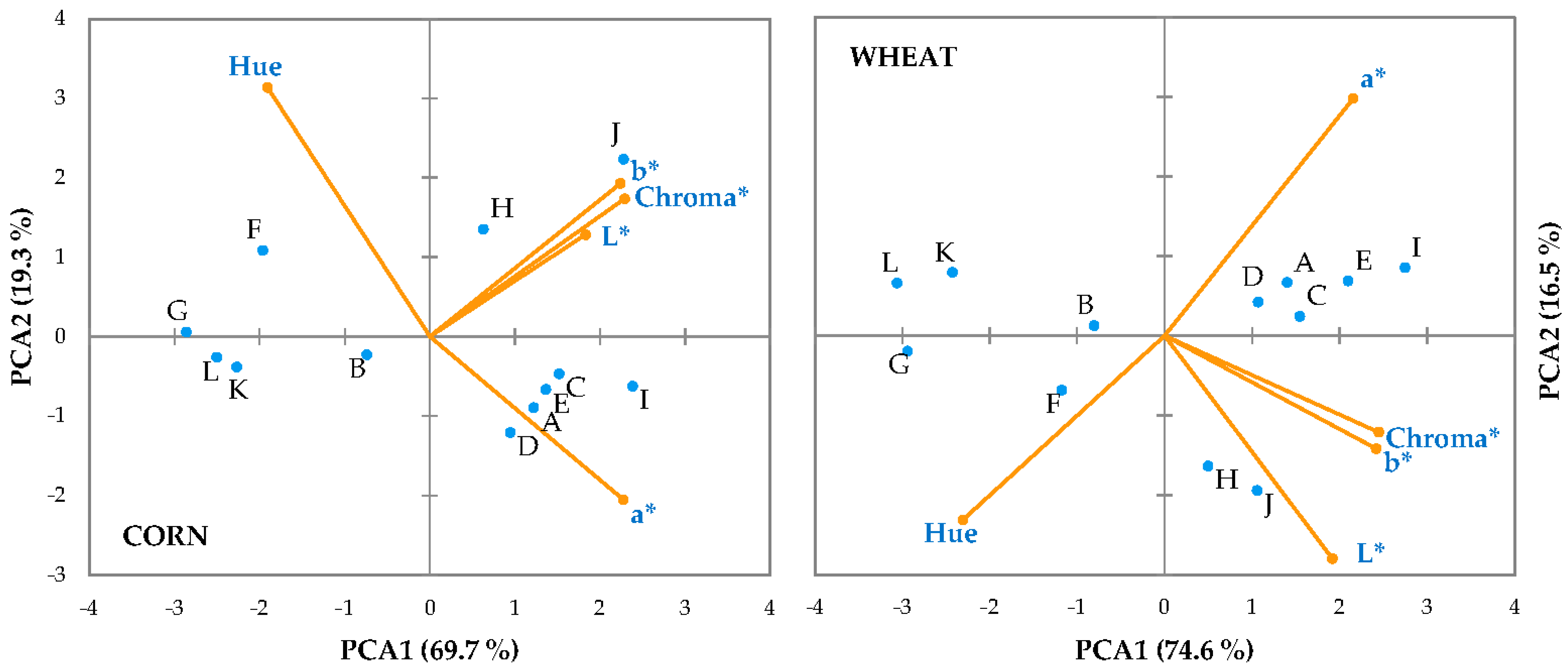
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kennedy, O.B.; Stewart-Knox, B.J.; Mitchell, P.C.; Thurnham, D.I. Consumer perceptions of poultry meat: A qualitative analysis. Nutr. Food Sci. 2004, 34, 122–129. [Google Scholar] [CrossRef]
- Castañeda, M.P.; Hirschler, E.M.; Sams, A.M. Skin pigmentation evaluation in broilers fed natural and synthetic pigments. Poult. Sci. 2005, 84, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Sirri, F.; Petracci, M.; Bianchi, M.; Meluzzi, A. Survey of skin pigmentation of yellow-skinned broiler chickens. Poult. Sci. 2010, 89, 1556–1561. [Google Scholar] [CrossRef] [PubMed]
- Torrey, S.; Mohammadigheisar, M.; Nascimento dos Santos, M.; Rothschild, D.; Dawson, L.C.; Liu, Z.; Kiarie, E.G.; Edwards, A.M.; Mandell, I.; Karrow, N.; et al. In pursuit of a better broiler: Growth, efficiency, and mortality of 16 strains of broiler chickens. Poult. Sci. 2021, 100, 100955. [Google Scholar] [CrossRef]
- Lusk, J.L. Consumer preferences for and beliefs about slow growth chicken. Poult. Sci. 2018, 97, 4159–4166. [Google Scholar] [CrossRef]
- Sunde, M.L. The scientific way to pigment poultry products. Poult. Sci. 1992, 71, 709–710. [Google Scholar] [CrossRef]
- Pérez-Vendrell, A.M.; Hernández, J.M.; Llauradó, L.; Schierle, J.; Brufau, J. Influence of source and ratio of xanthophyll pigments on broiler chicken pigmentation and performance. Poult. Sci. 2001, 80, 320–326. [Google Scholar] [CrossRef]
- Blanch, A. Getting the color of yolk and skin right. World Poult Sci. J. 1999, 15, 32–33. [Google Scholar]
- Alonso-Alvarez, C.; García-de-Blas, E.; Mateo, R. Dietary canthaxanthin reduces xanthophyll uptake and red coloration in adult red-legged partridges. J. Exp. Biol. 2018, 221, jeb185074. [Google Scholar] [CrossRef]
- Hencken, H. Chemical and physiological behavior of feed carotenoids and their effects on pigmentation. Poult. Sci. 1992, 71, 711–717. [Google Scholar] [CrossRef]
- Odunitan-Wayas, F.A.; Kolanisi, U.; Chimonyo, M.; Siwela, M. Effect of provitamin A biofortified maize inclusion on quality of meat from indigenous chickens. J. Appl. Poult. Res. 2016, 25, 581–590. [Google Scholar] [CrossRef]
- Martínez Peña, M.; Cortés Cuevas, A.; Avila González, E. Evaluation of three pigment levels of marigold petals (Tagetes erecta) on skin pigmentation of broiler chicken. Tec. Pecu. Mex. 2004, 42, 105–111. [Google Scholar]
- Muñoz-Díaz, J.I.; Fuente-Martínez, B.; Hernández-Velasco, X.; Ávila-González, E. Skin pigmentation in broiler chickens fed various levels of metabolizable energy and xanthophylls from Tagetes erecta. J. Appl. Poult. Res. 2012, 21, 788–796. [Google Scholar] [CrossRef]
- Ponsano, E.H.G.; Pinto, M.F.; Garcia-Neto, M.; Lacava, P.M. Performance and color of broilers fed diets containing Rhodocyclus gelatinosus biomass. Braz. J. Poult. Sci. 2004, 6, 237–242. [Google Scholar] [CrossRef]
- Rajput, N.; Naeem, M.; Ali, S.; Rui, Y.; Tian, W. Effect of dietary supplementation of marigold pigment on immunity, skin and meat color, and growth performance of broiler chickens. Braz. J. Poult. Sci. 2012, 14, 233–304. [Google Scholar] [CrossRef]
- Abraham, M.E.; Weimer, S.L.; Scoles, K.; Vargas, J.I.; Johnson, T.A.; Robison, C.; Hoverman, L.; Rocheford, E.; Rocheford, T.; Ortiz, D.; et al. Orange corn diets associated with lower severity of footpad dermatitis in broilers. Poult. Sci. 2021, 100, 101054. [Google Scholar] [CrossRef]
- C.I.E. (Commission Internationale de L’Eclairage). Colorimetry; C.I.E.: Vienna, Austria, 1986. [Google Scholar]
- Kuttappan, V.A.; Lee, Y.; Erf, G.F.; Meullenet, J.F.; Owens, C.M. Consumer acceptance of visual appearance of broiler breast meat with varying degrees of white striping. Poult. Sci. 2012, 91, 1240–1247. [Google Scholar] [CrossRef]
- Campo, M.D.M.; Mur, L.; Guerrero, A.; Barahona, M.; Resconi, V.C.; Magalhaes, D.R.; Lisbinski, E.; Boito, B.; Oliveira, I.M.D.; Olleta, L. Differentiating breast myopathies through color and texture analyses in broiler. Foods 2020, 9, 824. [Google Scholar] [CrossRef]
- Soglia, F.; Mudalal, S.; Babini, E.; Di Nunzio, M.; Mazzoni, M.; Sirri, F.; Cavani, C.; Petracci, M. Histology, composition, and quality traits of chicken Pectoralis major muscle affected by wooden breast abnormality. Poult. Sci. 2016, 95, 651–659. [Google Scholar] [CrossRef]
- Peng, Y.; Tan, H.; Liu, S.; Li, H.; Chen, Y.; Lin, J. Effects of different grain sources in both maternal and offspring diets on pigmentation and growth performance in yellow-skinned chickens. J. Poult. Sci. 2017, 54, 228–235. [Google Scholar] [CrossRef]
- Lyon, B.G.; Smith, D.P.; Lyon, C.E.; Savage, E.M. Effects of diet and feed withdrawal on the sensory descriptive and instrumental profiles of broiler breast fillets. Poult. Sci. 2004, 83, 275–281. [Google Scholar] [CrossRef]
- Ciurescu, G.; Vasilachi, A.; Idriceanu, L.; Dumitru, M. Effects of corn replacement by sorghum in broiler chickens diets on performance, blood chemistry, and meat quality. Ital. J. Anim. Sci. 2023, 22, 537–547. [Google Scholar] [CrossRef]
- Moreno, J.A.; Diaz-Gomez, J.; Fuentes-Font, L.; Angulo, E.; Gosalvez, L.F.; Sandmann, G.; Portero-Otin, M.; Capell, T.; Zhu, C.; Christou, P.; et al. Poultry diets containing (keto)carotenoid-enriched maize improve egg yolk color and maintain quality. Anim. Feed Sci. Technol. 2020, 260, 114334. [Google Scholar] [CrossRef]
- Trono, D. Carotenoids in cereal food crops: Composition and retention throughout grain storage and food processing. Plants 2019, 8, 551. [Google Scholar] [CrossRef] [PubMed]
- Córdova-Noboa, H.A.; Oviedo-Rondón, E.O.; Sarsour, A.H.; Barnes, J.; Ferzola, P.; Rademacher-Heilshorn, M.; Braun, U. Performance, meat quality, and pectoral myopathies of broilers fed either corn or sorghum based diets supplemented with guanidinoacetic. Poult. Sci. 2018, 97, 2479–2493. [Google Scholar] [CrossRef]
- Bai, S.; Wang, G.; Zhang, W.; Zhang, S.; Rice, B.B.; Cline, M.A.; Gilbert, E.R. Broiler chicken adipose tissue dynamics during the first two weeks post-hatch. Comp. Biochem. Physiol. A 2015, 189, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Muzhingi, T.; Yeum, K.J.; Russell, R.M.; Johnson, E.L.; Qin, J.; Tang, G. Determination of carotenoids in yellow maize, the effects of saponification and food preparations. Int. J. Vitam. Nutr. Res. 2008, 78, 112–120. [Google Scholar] [CrossRef]
- Kean, E.G.; Bordenave, N.; Ejeta, G.; Hamaker, B.R.; Ferruzzi, M.G. Carotenoid bioaccessibility from whole grain and decorticated yellow endosperm sorghum porridge. J. Cereal Sci. 2011, 54, 450–459. [Google Scholar] [CrossRef]
- Branellec, J.C. La pigmentation du poulet de chair. Aliscope 1985, 85, 1–13. [Google Scholar]
- Riveros Lizana, R.; Zea Mendoza, O.; Vilchez Perales, C. Evaluation of tarsus pigmentation in chickens fed with different levels of xanthophyll pigment: A practical application of the CIELab system. Int. J. Poult. Sci. 2020, 19, 265–269. [Google Scholar] [CrossRef]
- Deming, D.M.; Erdman, J.W., Jr. Mammalian carotenoid absorption and metabolism. Pure Appl. Chem. 1999, 71, 2213–2223. [Google Scholar] [CrossRef]
- Gao, Y.Y.; Lin, Z.X.; Zhang, M.; Yang, R.; Guo, P.T.; Zhang, J.; Wang, C.K.; Jin, L. Health effects of astaxanthin in the intestinal tract of yellow-feathered broilers. Poult. Sci. 2025, 104, 104768. [Google Scholar] [CrossRef]
- Phelan, D.; Prado-Cabrero, A.; Nolan, J.M. Analysis of lutein, zeaxanthin, and meso-zeaxanthin in the organs of carotenoid-supplemented chickens. Foods 2018, 7, 20. [Google Scholar] [CrossRef]
- Wang, Y.; Illingworth, D.R.; Connor, S.L.; Duell, P.B.; Connor, W.E. Competitive inhibition of carotenoid transport and tissue concentrations by high dose supplements of lutein, zeaxanthin and beta-carotene. Eur. J. Nutr. 2010, 49, 327–336. [Google Scholar] [CrossRef]
- Abril, M.; Campo, M.M.; Önenç, A.; Sañudo, C.; Albertí, P.; Negueruela, A.I. Beef color evolution as a function of ultimate pH. Meat Sci. 2001, 58, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Brennan, M.A.; Brennan, C.S. The effect of semolina replacement with protein powder from fish (Pseudophycis bachus) on the physicochemical characteristics of pasta. Food Sci. Technol. 2018, 89, 52–57. [Google Scholar] [CrossRef]

| Starter (0–14 d) Crumble | Grower (15–41 d) Pellet | |||
|---|---|---|---|---|
| WHEAT | CORN | WHEAT | CORN | |
| Ingredients % | ||||
| Corn | 5.00 | 30.0 | 0 | 45.0 |
| Wheat | 30.0 | 15.0 | 45.1 | 0 |
| Barley | 20.0 | 6.70 | 25.0 | 22.9 |
| Soybean meal, 47% | 30.0 | 30.0 | 20.8 | 25.4 |
| Full-fat soybean meal | 5.00 | 6.00 | 0 | 0 |
| Soybean oil | 0 | 0 | 2.51 | 2.25 |
| Sunflower meal, 34% | 3.00 | 4.00 | 3.00 | 1.00 |
| Tallow | 1.60 | 1.60 | 0 | 0 |
| Mucose, hydrolyzed | 2.00 | 2.65 | 0 | 0 |
| Dicalcium phosphate | 0.920 | 0.968 | 0.965 | 1.10 |
| Limestone | 0.786 | 0.712 | 0.981 | 0.871 |
| Vitamin–mineral premix 1 | 0.500 | 0.500 | 0.500 | 0.500 |
| MHA-Ca 2 | 0.447 | 0.447 | 0.327 | 0.346 |
| L-Lysine HCl | 0.278 | 0.260 | 0.293 | 0.233 |
| Salt (NaCl) | 0.168 | 0.153 | 0.167 | 0.217 |
| Sodium bicarbonate | 0.119 | 0.100 | 0.189 | 0.137 |
| L-Threonine | 0.130 | 0.115 | 0.106 | 0.071 |
| Calculated nutrients % | ||||
| Dry matter | 89.5 | 88.4 | 90.0 | 87.9 |
| ME poultry, kcal/kg | 2980 | 2980 | 3064 | 3050 |
| Crude protein | 29.0 | 23.8 | 18.7 | 18.3 |
| Lysine, total | 1.47 | 1.47 | 1.09 | 1.10 |
| Lysine, digestible | 1.31 | 1.31 | 0.970 | 0.970 |
| Crude fiber | 3.91 | 3.81 | 3.72 | 3.15 |
| Crude fat | 4.09 | 4.82 | 4.00 | 4.77 |
| Ash | 5.54 | 5.51 | 5.10 | 5.00 |
| Calcium | 0.965 | 0.950 | 1.00 | 0.995 |
| Phosphorus, total | 0.597 | 0.609 | 0.551 | 0.553 |
| Phosphorus, digestible | 0.440 | 0.440 | 0.420 | 0.420 |
| FEED | SEM | p | ||
|---|---|---|---|---|
| WHEAT | CORN | |||
| n | 15 | 15 | ||
| Carcass weight (kg) | 2.15 | 2.27 | 0.306 | 0.279 |
| Carcass breast yield % | 33.2 | 32.8 | 2.05 | 0.573 |
| pH | 5.89 | 5.90 | 0.107 | 0.842 |
| Wooden breast | ||||
| Normal | 10 | 12 | 0.378 | |
| Moderate | 1 | 2 | ||
| Severe | 4 | 1 | ||
| White striping | ||||
| Normal | 14 | 12 | 0.291 | |
| Moderate | 1 | 3 | ||
| Severe | 0 | 0 | ||
| Spaghetti meat | ||||
| Normal | 13 | 15 | 0.150 | |
| Moderate | 1 | 0 | ||
| Severe | 1 | 0 | ||
| Lightness | SEM | p-Value | Redness | SEM | p-Value | Yellowness | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WHEAT | CORN | WHEAT | CORN | WHEAT | CORN | |||||||
| n | 15 | 15 | 15 | 15 | 15 | 15 | ||||||
| Apterial latero-pectoral area | 74.43 cdeX | 72.28 cY | 0.47 | 0.020 | 0.78 dY | 2.05 deX | 0.30 | 0.030 | 7.78 deY | 11.64 efX | 0.58 | <0.001 |
| Back | 74.89 bcdX | 72.58 cY | 0.33 | <0.001 | 2.84 cY | 4.76 bcX | 0.32 | 0.001 | 13.17 cY | 15.21 deX | 0.40 | 0.009 |
| Breast interior | 51.95 g | 51.59 f | 0.67 | 0.791 | −0.94 eY | 0.80 efX | 0.20 | <0.001 | 6.53 efY | 10.93 fX | 0.46 | <0.001 |
| Breast surface | 54.55 g | 54.50 e | 0.46 | 0.959 | −0.32 deY | 1.02 efX | 0.17 | <0.001 | 7.39 deY | 10.58 fX | 0.45 | <0.001 |
| Drumette | 72.71 deX | 71.31 cY | 0.31 | 0.021 | 3.46 bcY | 5.12 bX | 0.31 | 0.005 | 14.35 bcY | 17.75 cdX | 0.45 | <0.001 |
| Ham | 66.05 f | 65.81 d | 0.48 | 0.805 | −0.43 eY | 0.25 fX | 0.12 | 0.002 | 4.62 f | 5.47 g | 0.42 | 0.320 |
| Hock | 77.49 ab | 76.19 a | 0.35 | 0.057 | −0.36 deY | 2.14 deX | 0.30 | <0.001 | 16.39 abY | 21.52 bcX | 1.23 | 0.035 |
| Neck | 75.97 bcX | 73.49 bcY | 0.37 | <0.001 | 3.19 bcY | 5.17 bX | 0.28 | <0.001 | 14.98 bcY | 19.70 bcX | 0.52 | <0.001 |
| Saddle | 73.02 de | 73.00 c | 0.34 | 0.975 | 4.31 ab | 5.08 b | 0.26 | 0.137 | 16.58 abY | 18.57 cdX | 0.44 | 0.020 |
| Shank | 78.99 aX | 75.84 abY | 0.32 | <0.001 | −0.27 deY | 3.32 cdX | 0.33 | <0.001 | 18.70 aY | 31.59 aX | 1.23 | <0.001 |
| Thigh | 72.93 de | 71.57 c | 0.37 | 0.064 | −0.39 de | 0.15 f | 0.14 | 0.062 | 9.50 d | 10.86 f | 0.57 | 0.237 |
| Vent | 72.09 e | 71.93 c | 0.37 | 0.830 | 5.30 aY | 6.78 aX | 0.31 | 0.015 | 18.65 aY | 23.03 bX | 0.58 | <0.001 |
| SEM | 0.63 | 0.59 | 0.17 | 0.19 | 0.39 | 0.56 | ||||||
| P | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||||
| Chroma* | SEM | p-Value | Hue° | SEM | p-Value | ΔE | |||
|---|---|---|---|---|---|---|---|---|---|
| WHEAT | CORN | WHEAT | CORN | ||||||
| n | 15 | 15 | 15 | 15 | |||||
| Apterial latero-pectoral area | 7.89 efY | 11.90 eX | 0.60 | <0.001 | 84.91 cd | 80.81 cd | 1.48 | 0.169 | 4.60 |
| Back | 13.50 dY | 16.04 dX | 0.43 | 0.002 | 77.93 deX | 72.55 eY | 1.12 | 0.014 | 3.63 |
| Breast interior | 6.62 fgY | 10.98 eX | 0.46 | <0.001 | 97.58 abX | 85.91 abcY | 1.33 | <0.001 | 4.75 |
| Breast surface | 7.41 efY | 10.65 eX | 0.46 | <0.001 | 93.05 bcX | 84.56 bcY | 1.06 | <0.001 | 3.46 |
| Drumette | 14.80 cdY | 18.53 cdX | 0.49 | <0.001 | 76.70 de | 73.92 e | 0.86 | 0.106 | 4.03 |
| Ham | 4.76 g | 5.50 f | 0.40 | 0.365 | 102.70 aX | 88.90 abY | 3.36 | 0.037 | 1.12 |
| Hock | 16.40 bcY | 21.67 bcX | 1.24 | 0.030 | 91.29 bcX | 84.14 bcY | 0.94 | <0.001 | 5.92 |
| Neck | 15.34 cdY | 20.40 bcX | 0.55 | <0.001 | 78.06 deX | 75.25 deY | 0.71 | 0.047 | 5.69 |
| Saddle | 17.16 abcY | 19.29 cdX | 0.46 | 0.018 | 75.64 de | 74.60 e | 0.65 | 0.429 | 2.13 |
| Shank | 18.71 abY | 31.77 aX | 1.24 | <0.001 | 90.98 bcX | 84.06 bcY | 0.64 | <0.001 | 13.75 |
| Thigh | 9.53 e | 10.90 e | 0.57 | 0.232 | 93.04 bc | 90.74 a | 0.89 | 0.200 | 2.00 |
| Vent | 19.45 aY | 24.02 bX | 0.61 | <0.001 | 74.35 e | 73.64 e | 0.62 | 0.573 | 4.62 |
| SEM | 0.40 | 0.58 | 0.89 | 0.58 | |||||
| P | <0.001 | <0.001 | <0.001 | <0.001 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campo, M.d.M.; Mur, L. Carcass Color in Broilers When Replacing Wheat with Corn in the Diet. Foods 2025, 14, 2558. https://doi.org/10.3390/foods14152558
Campo MdM, Mur L. Carcass Color in Broilers When Replacing Wheat with Corn in the Diet. Foods. 2025; 14(15):2558. https://doi.org/10.3390/foods14152558
Chicago/Turabian StyleCampo, Maria del Mar, and Leticia Mur. 2025. "Carcass Color in Broilers When Replacing Wheat with Corn in the Diet" Foods 14, no. 15: 2558. https://doi.org/10.3390/foods14152558
APA StyleCampo, M. d. M., & Mur, L. (2025). Carcass Color in Broilers When Replacing Wheat with Corn in the Diet. Foods, 14(15), 2558. https://doi.org/10.3390/foods14152558








