Elucidating Volatile Flavor Profiles and Metabolic Pathways in Northern Pike (Esox lucius) During Superchilled Storage: A Combined UPLC-Q-TOF/MS and GC-MS Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material and Sampling
2.2. Analysis of VOCs by GC-MS
2.3. Metabolomic Analysis
2.3.1. Metabolites Extraction
2.3.2. UHPLC-MS Analysis
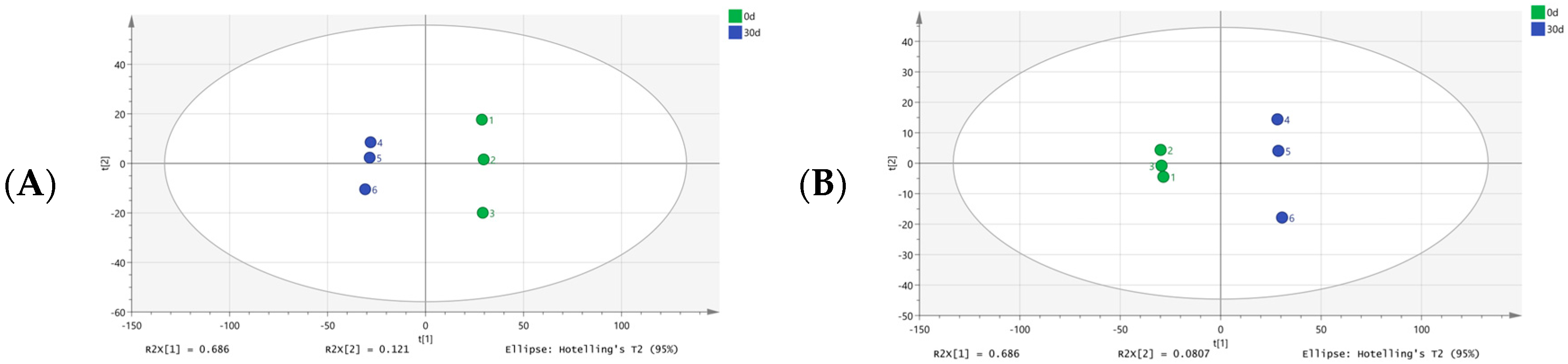
2.4. Data Processing and Statistical Analysis
3. Results
3.1. Analysis of VOCs
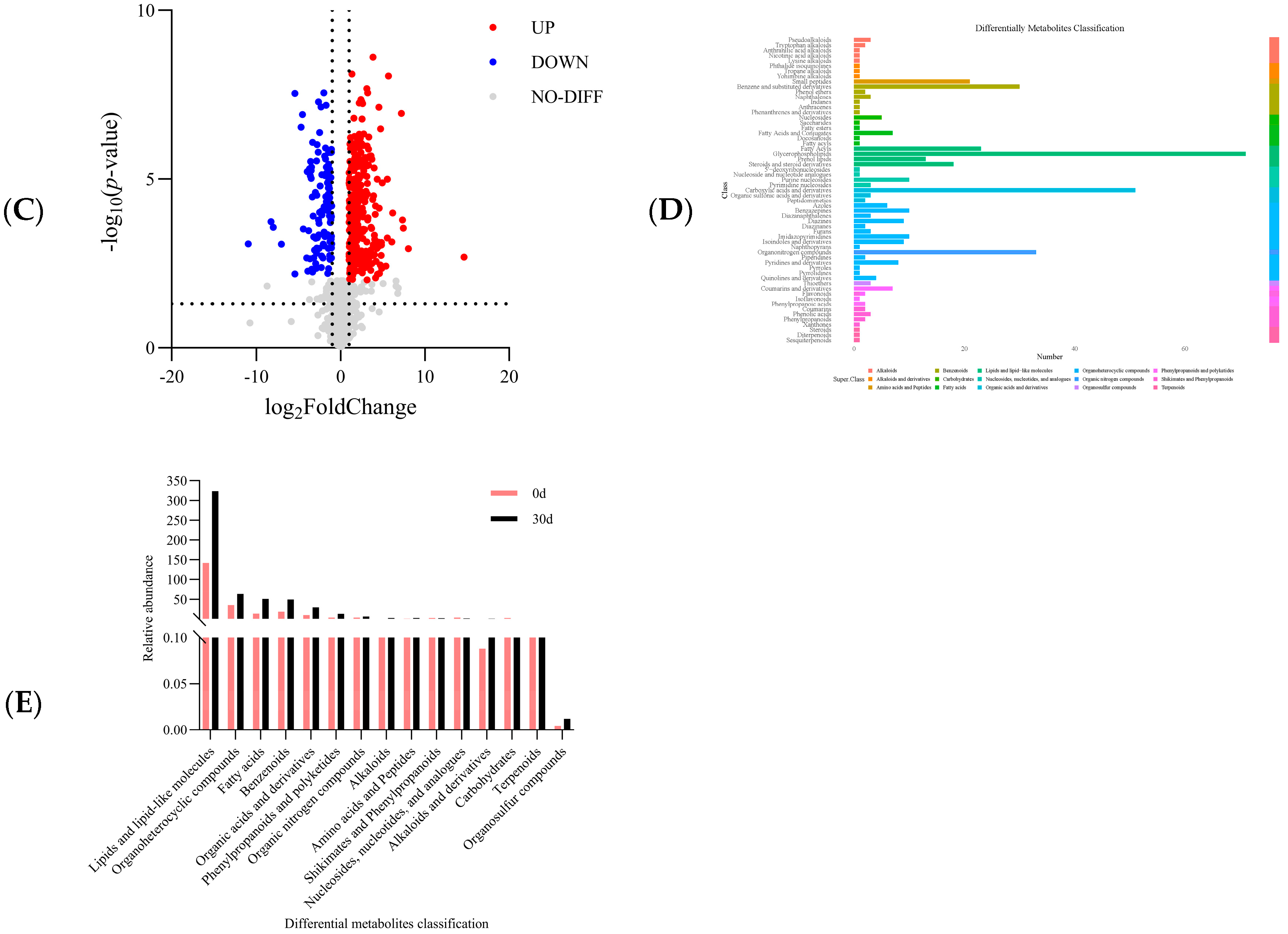
3.2. Identification and Classification of Metabolites
3.3. Analysis of Differential Metabolites
3.3.1. Differential Metabolites Related to Lipids and Lipid-like Substances
3.3.2. Differential Metabolites Related to Organoheterocyclic Compounds
3.3.3. Differential Metabolites Related to Fatty Acids
3.3.4. Differential Metabolites Related to Benzenoids
3.3.5. Differential Metabolites Related to Organic Acids and Derivatives
3.4. Screening for Key Metabolic Pathways
3.4.1. Glycerophospholipid Metabolism
3.4.2. Purine Metabolism
3.4.3. Pentose Phosphate Pathway
3.4.4. Arginine Biosynthesis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diaz-Suarez, A.; Noreikiene, K.; Kisand, V.; Burimski, O.; Svirgsden, R.; Rohtla, M.; Ozerov, M.; Gross, R.; Vetemaa, M.; Vasemagi, A. Temporally stable small-scale genetic structure of Northern pike (Esox lucius) in the coastal Baltic Sea. Fish. Res. 2022, 254, 106402. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.G.; Bichkaeva, F.A.A.; Vlasova, O.S.S.; Andronov, S.V.V.; Dvoretsky, V.G.G. Fatty Acid Composition of Northern Pike from an Arctic River (Northeastern Siberia, Russia). Foods 2023, 12, 764. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Bian, C.; Yu, H.; Mei, J.; Xie, J. Effect of ultrasound-assisted freezing combined with potassium alginate on the quality attributes and myofibril structure of large yellow croaker (Pseudosciaena crocea). LWT 2022, 167, 113869. [Google Scholar] [CrossRef]
- FAO. Fisheries and Aquaculture. Available online: https://www.fao.org/fishery/statistics/software/fishstatj (accessed on 24 March 2025).
- Eliasson, S.; Arason, S.; Margeirsson, B.; Bergsson, A.B.; Palsson, O.P. The effects of superchilling on shelf-life and quality indicators of whole Atlantic cod and fillets. LWT 2019, 100, 426–434. [Google Scholar] [CrossRef]
- Kaale, L.D.; Eikevik, T.M. Changes of proteins during superchilled storage of Atlantic salmon muscle (Salmo salar). J. Food Sci. Technol. 2016, 53, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Hoang, H.M.; Brown, T.; Indergard, E.; Leducq, D.; Alvarez, G. Life cycle assessment of salmon cold chains: Comparison between chilling and superchilling technologies. J. Clean. Prod. 2016, 126, 363–372. [Google Scholar] [CrossRef]
- Cropotova, J.; Mozuraityte, R.; Standal, I.B.; Grovlen, M.S.; Rustad, T. Superchilled, chilled and frozen storage of Atlantic mackerel (Scomber scombrus) fillets-changes in texture, drip loss, protein solubility and oxidation. Int. J. Food Sci. Technol. 2019, 54, 2228–2235. [Google Scholar] [CrossRef]
- Kaale, L.D.; Eikevik, T.M.; Rustad, T.; Nordtvedt, T.S. Changes in water holding capacity and drip loss of Atlantic salmon (Salmo salar) muscle during superchilled storage. LWT 2014, 55, 528–535. [Google Scholar] [CrossRef]
- Mi, H.; Qian, C.; Zhao, Y.; Liu, C.; Mao, L. Comparisun of superchilling and freezing on the microstructure, muscle quality and protein denaturation of grass carp (Ctenopharyngodon idellus). J. Food Process. Preserv. 2013, 37, 546–554. [Google Scholar] [CrossRef]
- Lulijwa, R.; Alfaro, A.C.; Young, T. Metabolomics in salmonid aquaculture research: Applications and future perspectives. Rev. Aquac. 2022, 14, 547–577. [Google Scholar] [CrossRef]
- Shao, C.; Su, Y.; Li, Y.; Dong, Y.; Hao, H.; Ye, H. Comprehensive metabolomic profiling of nutrients in fish and shrimp. Food Chem. 2023, 407, 135037. [Google Scholar] [CrossRef] [PubMed]
- Sidira, M.; Agriopoulou, S.; Smaoui, S.; Varzakas, T. Omics-Integrated Approach (Metabolomics, Proteomics and Lipidomics) to Assess the Quality Control of Aquatic and Seafood Products. Appl. Sci. 2024, 14, 10755. [Google Scholar] [CrossRef]
- Deborde, C.; Hounoum, B.M.; Moing, A.; Maucourt, M.; Jacob, D.; Corraze, G.; Medale, F.; Fauconneau, B. Putative imbalanced amino acid metabolism in rainbow trout long term fed a plant-based diet as revealed by 1H-NMR metabolomics. J. Nutr. Sci. 2021, 10, e13. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Mei, J.; Xie, J. The odor deterioration of tilapia (Oreochromis mossambicus) fillets during cold storage revealed by LC-MS based metabolomics and HS-SPME-GC-MS analysis. Food Chem. 2023, 427, 136699. [Google Scholar] [CrossRef] [PubMed]
- Yingjie, L.; Mingzhu, Y.; Shanwei, L.; Zhongxiang, C.; Peng, W.; Yanchun, S. Research on Metabolomics Analysis Method for Fish Gills Target Organs Based on UPLC-QTOF MS. J. Instrum. Anal. 2022, 41, 1193–1199. [Google Scholar]
- Bi, S.J.; Gong, G.; Li, N.; Gao, P.; Hou, T.Y.; Zhu, J.F.; Abulikemu, B. Mechanism of flavor changes in Northern Pike during refrigeration revealed by UHPLC-MS metabolomics and GC-IMS. Food Biosci. 2025, 68, 106557. [Google Scholar] [CrossRef]
- Chu, Y.; Ding, Z.; Wang, J.; Xie, J. Exploration of the evolution and production of volatile compounds in grouper (Epinephelus coioides) during cold storage. Food Biosci. 2023, 52, 102496. [Google Scholar] [CrossRef]
- Opara, U.L.; Fadiji, T.; Caleb, O.J.; Oluwole, A.O. Changes in Volatile Composition of Cape Hake Fillets under Modified Atmosphere Packaging Systems during Cold Storage. Foods 2022, 11, 1292. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yuan, L.; Weng, L.; Yu, C.; Hu, M.; Peng, B.; Tu, Z. Integration of lipidomics and flavoromics reveals the lipid-flavor transformation mechanism of fish oil from silver carp visceral with different enzymatic hydrolysis. Food Chem. 2025, 477, 143507. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, Y.; Xia, W.; Yu, D.; Wang, B.; Xu, J. Insight into the role of lipids in odor changes of frozen grass carp (Ctenopharyngodon idella) based on lipidomics and GC-MS-MS analysis: Impact of freeze-thaw cycles and heat treatment. Food Chem. 2024, 459, 140436. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Mei, J.; Xie, J. Integrated volatile compounds and non-targeted metabolomics analysis reveal the characteristic flavor formation of proteins in grouper (Epinephelus coioides) during cold storage. Food Res. Int. 2023, 172, 113145. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Cai, D.; Li, W.; Blank, I.; Liu, Y. Application of gas chromatography-ion mobility spectrometry (GC-IMS) and ultrafast gas chromatography electronic-nose (uf-GC E-nose) to distinguish four Chinese freshwater fishes at both raw and cooked status. J. Food Biochem. 2022, 46, e13840. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Wang, J.; Xie, J. Exploring the correlation of microbial community diversity and succession with protein degradation and impact on the production of volatile compounds during cold storage of grouper (Epinephelus coioides). Food Chem. 2024, 460, 140469. [Google Scholar] [CrossRef] [PubMed]
- Husein, Y.; Bruni, L.; Secci, G.; Taiti, C.; Belghit, I.; Lock, E.-J.; Parisi, G. Does sous-vide cooking preserve the chemical and volatile composition of Atlantic salmon (Salmo salar L.) fed Hermetia illucens larvae meal? J. Insects Food Feed. 2021, 7, 69–77. [Google Scholar] [CrossRef]
- Khodanazary, A.; Mohammadzadeh, B. Effect of alginate-gallic acid coating on freshness and flavor properties of Mackerel (Scomberomorus commerson) fillets under refrigerated storage. Int. J. Biol. Macromol. 2023, 249, 125999. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Feng, Q.; Zhang, Y.; Yu, Y.; Liao, W.; Li, G.; An, T. Odorous VOCs released from bio-decomposition and its interaction mechanism with bacteria: Compared inter-type with intra-type household garbage. J. Clean. Prod. 2024, 447, 141523. [Google Scholar] [CrossRef]
- Moser, B.; Steininger-Mairinger, T.; Jandric, Z.; Zitek, A.; Scharl, T.; Hann, S.; Troyer, C. Spoilage markers for freshwater fish: A comprehensive workflow for non-targeted analysis of VOCs using DHS-GC-HRMS. Food Res. Int. 2023, 172, 113123. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Mei, J.; Xie, J. Exploring the effects of lipid oxidation and free fatty acids on the development of volatile compounds in grouper during cold storage based on multivariate analysis. Food Chem.-X 2023, 20, 100968. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Bai, J.; Yuan, L.; Zhou, H.; Xu, L.; Yu, C.; Hu, M.; Tu, Z.; Peng, B. Comprehensive lipidomics and flavoromics reveals the accelerated oxidation mechanism of fish oil from silver carp (Hypophthalmichthys molitrix) viscera during heating. Food Chem. 2025, 478, 143651. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Wang, J.; Xie, J. Effects of interactions between microorganisms and lipids on inferior volatile compound production during cold storage of grouper (Epinephelus coioides). Food Chem.-X 2025, 25, 102183. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, N.; Guclu, G.; Kelebek, H.; Mazi, H.; Selli, S. Characterization of volatile compounds in the water samples from rainbow trout aquaculture ponds eliciting off-odors: Understanding locational and seasonal effects. Environ. Sci. Pollut. Res. Int. 2024, 31, 61819–61834. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Wang, W.; Gu, S.; Xu, M.; Yao, H.; Zhou, X.; Ding, Y. Effect of chitosan-epigallocatechin gallate coating on volatile flavor compounds retention in bighead carp (Aristichthys nobilis) fillets during chilled storage. LWT 2022, 169, 114027. [Google Scholar] [CrossRef]
- Xue, C.; You, J.; Zhang, H.; Zhao, L.; Xiong, S.; Yin, T.; Huang, Q. Hydrophobic bonds-dominated key off-odors/silver carp myofibrillar protein interactions, and their binding characteristics at cold storage and oral temperatures. Food Chem.-X 2022, 15, 100396. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yin, T.; Xiong, S.; Huang, Q. Effect of refrigeration and reheating on the lipid oxidation and volatile compounds in silver carp surimi gels. J. Sci. Food Agric. 2025, 105, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Phetsang, H.; Panpipat, W.; Panya, A.; Phonsatta, N.; Chaijan, M. Occurrence and Development of Off-Odor Compounds in Farmed Hybrid Catfish (Clarias macrocephalus × Clarias gariepinus) Muscle during Refrigerated Storage: Chemical and Volatilomic Analysis. Foods 2021, 10, 1841. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, C.; Sun, Y.; Cui, N.; Zhong, B.; Peng, B.; Hu, M.; Li, J.; Tu, Z. Characterization of key off-odor compounds in grass carp cube formed during room temperature storage by molecular sensory science approach. Food Chem.-X 2024, 24, 102011. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.-Q.; Xue, C.-H.; Zhang, H.-W.; Xu, L.-L.; Wang, X.-H.; Bi, S.-J.; Xue, Q.-Q.; Xue, Y.; Li, Z.-J.; Velasco, J.; et al. Concomitant oxidation of fatty acids other than DHA and EPA plays a role in the characteristic off-odor of fish oil. Food Chem. 2023, 404, 134724. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Ding, Z.; Xie, J. Molecular Mechanism of the Impact of Lipid Oxidation Products (2,4-Decadienal) on the Conformation of Grouper (Epinephelus coioides) Myofibrillar Proteins During Refrigeration and its Implications on the Overall Protein Flavor. Food Bioprocess Technol. 2024, 17, 5299–5311. [Google Scholar] [CrossRef]
- Hou, M.; Sun, W.; Ma, Y.; Ye, H.; Zhai, X.; Xue, Y.; Tang, R.; Teng, S.; Wu, R.; Luo, H. Comparative analysis for nutrients, flavor compounds, and lipidome revealed the edible value of pond-cultured male Pelodiscus sinensis with different ages. Food Chem. 2024, 454, 139795. [Google Scholar] [CrossRef] [PubMed]
- Amoah, K.; Dong, X.-H.; Tan, B.-P.; Zhang, S.; Chi, S.-Y.; Yang, Q.-H.; Liu, H.-Y.; Yan, X.-B.; Yang, Y.-Z.; Zhang, H. Ultra-Performance Liquid Chromatography-Mass Spectrometry-Based Untargeted Metabolomics Reveals the Key Potential Biomarkers for Castor Meal-Induced Enteritis in Juvenile Hybrid Grouper (Epinephelus fuscoguttatus♀ × E. lanceolatus♂). Front. Nutr. 2022, 9, 847425. [Google Scholar] [CrossRef] [PubMed]
- Anagnostopoulos, D.A.; Parlapani, F.F.; Mallouchos, A.; Angelidou, A.; Syropoulou, F.; Minos, G.; Boziaris, I.S. Volatile Organic Compounds and 16S Metabarcoding in Ice-Stored Red Seabream Pagrus major. Foods 2022, 11, 666. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Cao, Q.; Jiang, S.; Huangfu, J.; Wang, W.; Niu, Z. Evaluation of Dynamic Changes of Volatile Organic Components for Fishmeal during Storage by HS-SPME-GC-MS with PLS-DA. Foods 2024, 13, 1290. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Wang, B.; Jiang, H.; Li, W.; Zhang, Y.; Ji, C.; Lin, X.; Zhang, S. Quality assessment and microbial community succession of finely chopped common carp (Cyprinus carpio) under low-temperature storage: Implications for nutritional composition and spoilage indicators. Food Control 2025, 171, 111103. [Google Scholar] [CrossRef]
- Chen, J.-N.; Zhang, Y.-Y.; Huang, X.; Wang, H.-P.; Dong, X.; Zhu, B.; Qin, L. Analysis of Lipid Molecule Profiling and Conversion Pathway in Mandarin Fish (Siniperca chuatsi) during Fermentation via Untargeted Lipidomics. J. Agric. Food Chem. 2023, 71, 8673–8684. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Zhou, Z.; Zhao, J.; Xu, H.; Limbu, S.M.; Xu, Q. Dietary β-conglycinin induces intestinal enteritis and affects glycerophospholipid and arginine metabolism in mirror carp (Cyprinus carpio). Aquaculture 2023, 567, 739257. [Google Scholar] [CrossRef]
- Wang, Z.-R.; Li, S.-Y.; Zhang, Y.-Z.; Li, Y.-A.; Huo, H.-H.; Yu, C.-Q.; Zhou, Q.-B. Metabolomic and transcriptomic profiling reveals the effect of dietary protein and lipid levels on growth performance in loach (Paramisgurnus dabryanus). Front. Immunol. 2023, 14, 1236812. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, J.; Hu, M.; Shen, X.; Gao, R.; Yan, H.; Liu, Q.; Liu, Y.; Tian, Y.; Wang, H.; et al. Physiological responses to different temperature in the liver of Takifugu rubripes larvae revealed by integrated transcriptomic and metabolomic analyses. Comp. Biochem. Physiol. D-Genom. Proteom. 2025, 54, 101371. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhang, Y.; Wu, D.; Lu, Z.; Xiao, J.; Huang, H.; Fu, Q.; Guo, Z. Multi-omics analysis revealed the differences in lipid metabolism of the gut between adult and juvenile yellowfin tuna (Thunnus albacares). Front. Microbiol. 2024, 14, 1326247. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, Y.; Xiang, H.; Sun-Waterhouse, D.; Zhao, Y.; Chen, S.; Li, L.; Wang, Y. UHPLC-Q-Exactive Orbitrap MS/MS-based untargeted lipidomics reveals molecular mechanisms and metabolic pathways of lipid changes during golden pomfret (Trachinotus ovatus) fermentation. Food Chem. 2022, 396, 133676. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Liu, H.; Fu, L.; Han, D.; Zhu, X.; Jin, J.; Yang, Y.; Xie, S. Dietary inosine monophosphate improved liver health and flesh quality of gibel carp (Carassius auratus gibelio) via activating AMPK signalling pathway and enhancing the contents of muscle fat and flavour substance. Front. Mar. Sci. 2022, 9, 940732. [Google Scholar] [CrossRef]
- Cao, Y.; Gao, Q.; Li, X.; Zhou, Y.; Dong, S.; Wang, Y.; Dai, Z. Integrated Analysis of Metabolomics and Transcriptomics for Assessing Effects of Fish Meal and Fish Oil Replacement on the Metabolism of Rainbow Trout (Oncorhynchus mykiss). Front. Mar. Sci. 2022, 9, 843637. [Google Scholar] [CrossRef]
- Du, H.; Xiong, S.; Lv, H.; Zhao, S.; Manyande, A. Comprehensive analysis of transcriptomics and metabolomics to understand the flesh quality regulation of crucian carp (Carassius auratus) treated with short term micro-flowing water system. Food Res. Int. 2021, 147, 110519. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wang, J.; Li, B.; Song, Z.; Li, P.; Huang, B.; Wang, C.; Sun, Y.; Wang, X.; Hao, T. Analyses of regulatory network and discovery of potential biomarkers for Korean rockfish (Sebastes schlegelii) in responses to starvation stress through transcriptome and metabolome. Comp. Biochem. Physiol. D-Genom. Proteom. 2023, 46, 101061. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; You, J.; Wang, L.; Shi, L.; Liao, T.; Huang, Q.; Xiong, S.; Yin, T. Insight into the mechanism on texture change of Wuchang bream muscle during live transportation using a UPLC-QTOF-MS based metabolomics method. Food Chem. 2023, 398, 133796. [Google Scholar] [CrossRef] [PubMed]
- Britt, E.C.; Lika, J.; Giese, M.A.; Schoen, T.J.; Seim, G.L.; Huang, Z.; Lee, P.Y.; Huttenlocher, A.; Fan, J. Switching to the cyclic pentose phosphate pathway powers the oxidative burst in activated neutrophils. Nat. Metab. 2022, 4, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Drechsel, V.; Schneebauer, G.; Sandbichler, A.M.; Fiechtner, B.; Pelster, B. Oxygen consumption and acid secretion in isolated gas gland cells of the European eel Anguilla anguilla. J. Comp. Physiol. B-Biochem. Syst. Environ. Physiol. 2022, 192, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Facchinello, N.; Astone, M.; Audano, M.; Oberkersch, R.E.; Spizzotin, M.; Calura, E.; Marques, M.; Crisan, M.; Mitro, N.; Santoro, M.M. Oxidative pentose phosphate pathway controls vascular mural cell coverage by regulating extracellular matrix composition. Nat. Metab. 2022, 4, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zheng, Z.; Guo, D.; Wang, Y.; Wu, Q.; Gao, Y. Creatine in giant grouper (Epinephelus lanceolatus): Insights into biosynthesis and transporter genes molecular characterization, regulation and impact on muscle metabolism. Aquaculture 2025, 595, 741468. [Google Scholar] [CrossRef]

| Volatile Compounds | RT (min) | Relative Abundance (%) | VIP | p-Value | Log2Foldchange | |
|---|---|---|---|---|---|---|
| 0 d | 30 d | |||||
| 1-Hexanol | 14.59 | 1.83 | ND | 1.25 | 0.001 | −12.513 |
| (Z)-3-Dodecen-1-ol | 14.86 | 1.14 | ND | 1.251 | 0.006 | −11.831 |
| 1-Pentanol | 11.73 | 0.78 | ND | 1.25 | 0.003 | −11.284 |
| 2-Methyl-butanal | 2.94 | 0.52 | ND | 1.255 | 0.010 | −10.682 |
| Phenylethyl Alcohol | 27.53 | 29.12 | 0.16 | 1.22 | 0.001 | −4.0467 |
| 6-Methyl-5-hepten-2-ol | 17.46 | 0.16 | 0.13 | 1.22 | 0.000 | 3.2401 |
| 2-Heptanone | 9.44 | 41.30 | 38.08 | 1.16 | 0.0011 | 3.366 |
| 2-Nonanone | 15.39 | 18.32 | 21.58 | 1.21 | 0.001 | 3.719 |
| 3-Octanone | 11.55 | 0.38 | 0.52 | 1.25 | 0.001 | 3.946 |
| 2,2-Dimethyl-3-octanone | 29.61 | 0.05 | 0.11 | 1.05 | 0.001 | 4.633 |
| (E)-6-Nonen-1-ol | 19.76 | 0.09 | 0.23 | 1.00 | 0.000 | 4.810 |
| 2-Octanone | 12.46 | 4.75 | 13.69 | 1.23 | 0.000 | 5.011 |
| 3-Heptanone | 8.52 | 0.01 | 0.03 | 1.11 | 0.000 | 5.436 |
| 2-Nonanol | 18.96 | 1.33 | 5.98 | 1.22 | 0.000 | 5.649 |
| 6-Methyl-2-heptanol | 15.22 | 0.17 | 0.77 | 1.003 | 0.0030 | 5.7040 |
| 2-Decanol | 20.31 | ND | 0.04 | 1.25 | 0.002 | 9.676 |
| 2-Pentadecanol | 25.07 | ND | 0.07 | 1.25 | 0.009 | 10.726 |
| 2-Heptyl acetate | 11.90 | ND | 0.10 | 1.25 | 0.001 | 11.236 |
| 3-Nonanol | 18.29 | ND | 0.11 | 1.25 | 0.004 | 11.299 |
| Indole | 37.13 | 0.05 | 15.86 | 1.24 | 0.003 | 11.920 |
| 3-Octanol | 15.73 | ND | 0.29 | 1.25 | 0.000 | 12.723 |
| Methyl butyrate | 4.15 | ND | 0.41 | 1.251 | 0.009 | 13.222 |
| 2-Pentanol | 8.14 | ND | 0.48 | 1.25 | 0.007 | 13.446 |
| Pyrazine | 10.30 | ND | 0.55 | 1.25 | 0.002 | 13.645 |
| 2-Hexanone | 6.48 | ND | 0.80 | 1.25 | 0.005 | 14.178 |
| ID | Name | RT | Relative Abundance | VIP | p-Value | Log_Foldchange | Up/Down | |
|---|---|---|---|---|---|---|---|---|
| 0 d | 30 d | |||||||
| 1 | IMP | 256.5 | 2.075410253 | 0.172349174 | 1.305 | 0.000 | −3.589 | down |
| 2 | GMP | 263.7 | 0.157221115 | 0.014306665 | 1.308 | 0.000 | −3.458 | down |
| 3 | Imidazoleacetic acid | 191.3 | 0.402646941 | 0.054648313 | 1.34 | 0.004 | −2.881 | down |
| 4 | Adenosine | 85.4 | 0.505515164 | 0.081046236 | 1.34 | 0.000 | −2.640 | down |
| 5 | Deoxyadenosine | 58.9 | 0.019532804 | 0.003480467 | 1.33 | 0.000 | −2.488 | down |
| 6 | Carnosine | 246.5 | 1.248932617 | 0.34588395 | 1.34 | 0.000 | −1.852 | down |
| 7 | Guanine | 139.8 | 4.12418659 | 1.466873231 | 1.34 | 0.000 | −1.491 | down |
| 8 | Fumaric acid | 215.5 | 0.043522088 | 0.016290857 | 1.35 | 0.000 | −1.417 | down |
| 9 | Dimethylglycine | 181.3 | 0.005597956 | 0.011406206 | 1.34 | 0.000 | 1.026 | up |
| 10 | Hypoxanthine | 82.5 | 15.10863255 | 31.39561676 | 1.34 | 0.000 | 1.055 | up |
| 11 | ADP | 264.5 | 0.014330009 | 0.030932311 | 1.34 | 0.000 | 1.110 | up |
| 12 | dGDP | 264.5 | 0.014330009 | 0.030932311 | 1.34 | 0.000 | 1.110 | up |
| 13 | Deoxyinosine | 95.9 | 0.182593159 | 0.410807127 | 1.355 | 0.000 | 1.169 | up |
| 14 | CDP-Ethanolamine | 83.8 | 0.207212681 | 0.492886833 | 1.34 | 0.003 | 1.250 | up |
| 15 | Xanthosine | 184.2 | 0.007277305 | 0.017887743 | 1.24 | 0.002 | 1.297 | up |
| 16 | Xanthine | 123.5 | 0.631407431 | 1.773516428 | 1.34 | 0.000 | 1.489 | up |
| 17 | 1-Acyl-sn-glycero-3-phosphocholine | 19.5 | 2.108224746 | 6.131611592 | 1.35 | 0.000 | 1.540 | up |
| 18 | Glutamate | 229.1 | 0.086630744 | 0.294221196 | 1.32 | 0.000 | 1.763 | up |
| 19 | Uric acid | 191.2 | 0.006596723 | 0.023092878 | 1.33 | 0.000 | 1.807 | up |
| 20 | Pyruvate | 47.5 | 3.782425277 | 13.2657556 | 1.34 | 0.000 | 1.810 | up |
| 21 | Gluconic acid | 227.4 | 0.084084384 | 0.545685796 | 1.31 | 0.000 | 2.698 | up |
| 22 | Glyceric acid | 179 | 0.01225653 | 0.082420234 | 1.32 | 0.000 | 2.749 | up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, S.; Li, N.; Gong, G.; Gao, P.; Zhu, J.; Abulikemu, B. Elucidating Volatile Flavor Profiles and Metabolic Pathways in Northern Pike (Esox lucius) During Superchilled Storage: A Combined UPLC-Q-TOF/MS and GC-MS Approach. Foods 2025, 14, 2556. https://doi.org/10.3390/foods14152556
Bi S, Li N, Gong G, Gao P, Zhu J, Abulikemu B. Elucidating Volatile Flavor Profiles and Metabolic Pathways in Northern Pike (Esox lucius) During Superchilled Storage: A Combined UPLC-Q-TOF/MS and GC-MS Approach. Foods. 2025; 14(15):2556. https://doi.org/10.3390/foods14152556
Chicago/Turabian StyleBi, Shijie, Na Li, Gao Gong, Peng Gao, Jinfang Zhu, and Batuer Abulikemu. 2025. "Elucidating Volatile Flavor Profiles and Metabolic Pathways in Northern Pike (Esox lucius) During Superchilled Storage: A Combined UPLC-Q-TOF/MS and GC-MS Approach" Foods 14, no. 15: 2556. https://doi.org/10.3390/foods14152556
APA StyleBi, S., Li, N., Gong, G., Gao, P., Zhu, J., & Abulikemu, B. (2025). Elucidating Volatile Flavor Profiles and Metabolic Pathways in Northern Pike (Esox lucius) During Superchilled Storage: A Combined UPLC-Q-TOF/MS and GC-MS Approach. Foods, 14(15), 2556. https://doi.org/10.3390/foods14152556






