Incorporation of Pork Meat and Blood Plasma Proteins into a Cocoa Cream Matrix: Characterization, Comparison of Functional Properties, and In Vitro Simulated Digestion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The Chemical Composition of Commercial Proteins
2.3. The Determination of Amino Acids Using the HPLC-FLD Method
2.4. The Determination of Functional Properties of Proteins
2.4.1. Protein Solubility (WSI—Water Solubility Index)
2.4.2. Water-Holding Capacity (WHC)
2.4.3. Oil-Binding Capacity (OAC—Oil Absorption Capacity)
2.4.4. Foam Production (FC—Foam Capacity)
- A—the volume of the test sample measured after 1 min of vigorous mixing (mL),
- B—sample volume before mixing (mL).
2.4.5. Emulsifying Properties
- A0—absorbance of emulsion immediately after vortexing,
- N—dilution factor (100),
- L—the thickness of the cuvette used for the spectrophotometer, which was 1 cm,
- c—protein concentration in the sample (g/mL),
- φ—oil content in the emulsion,
- ΔA—change in absorbance during storage time in the refrigerator,
- t—time interval of storage in the refrigerator (24 h).
2.5. The Preparation of Cocoa Cream Products
2.6. In Vitro Digestion
2.7. The Degree of Hydrolysis (DH)
2.8. Total Phenol Content
2.9. Determination of ABTS Radical Scavenging Activity
2.10. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of Proteins
3.2. Amino Acid Profile
3.3. Functional Properties of Proteins
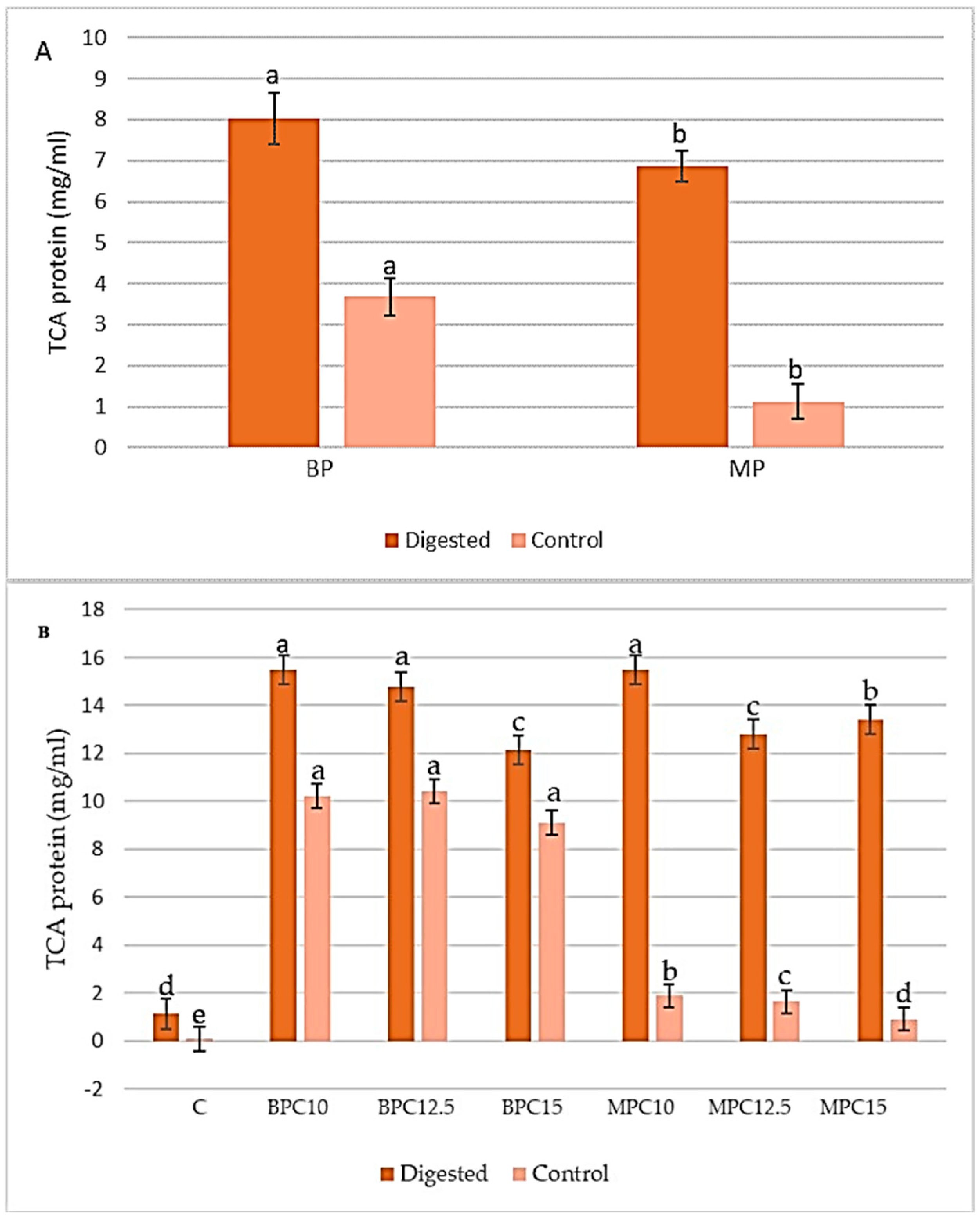
3.4. Determination of Degree Hydrolysis (DH)
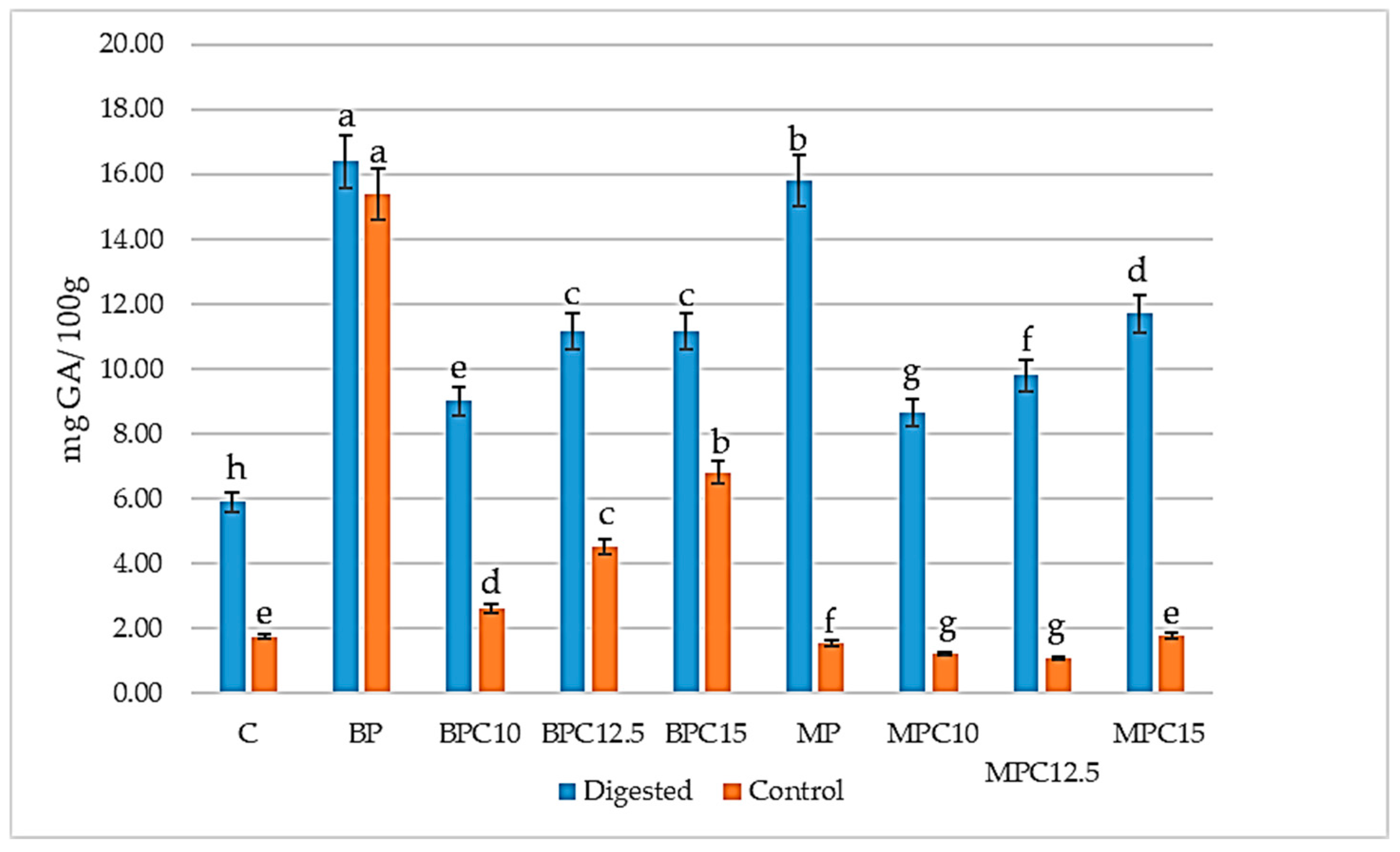
3.5. Total Phenol Content (TF)
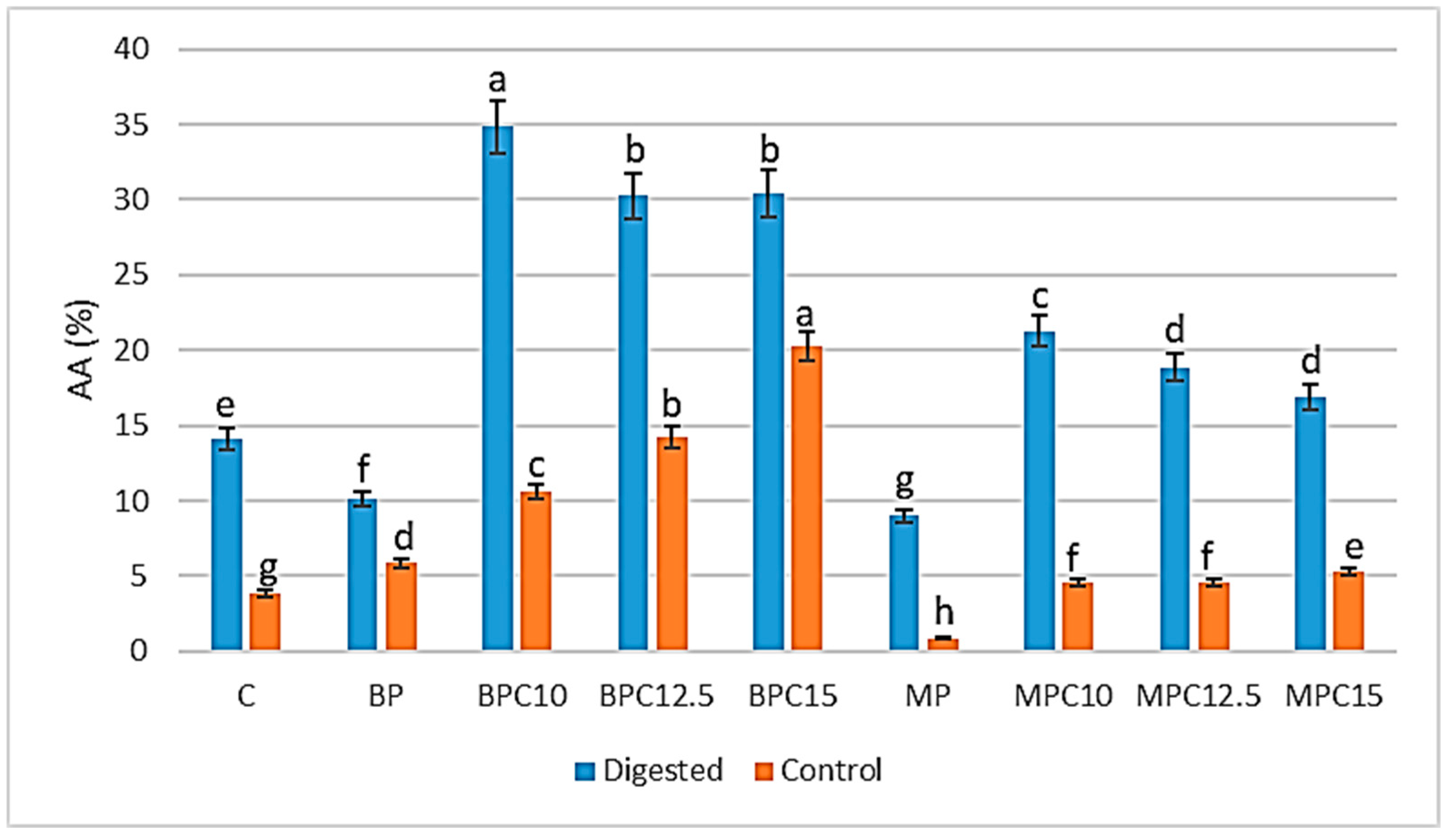
3.6. ABTS Radical Scavenging Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MP | Meat protein |
| BP | Blood plasma |
| WSI | Water solubility index |
| WHC | Water-holding capacity |
| OHC | Oil-holding capacity |
| FC | Foaming capacity |
| EC | Emulsion activity index |
| ES | Emulsion stability |
| DH | Degree of hydrolysis |
References
- Voigt, J. Chocolate and Cocoa Aroma. In Chocolate in Health and Nutrition; Humana Press: Totowa, NJ, USA, 2012; pp. 89–101. [Google Scholar] [CrossRef]
- Caballero, B.; Allen, L.; Prentice, A. Encyclopedia of Human Nutrition, 2nd ed.; Academic Press: Cambridge, MA, USA, 2005; pp. 150–300. [Google Scholar]
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Banovic, M.; Arvola, A.; Pennanen, K.; Duta, D.E.; Brückner-Gühmann, M.; Lähteenmäki, L.; Grunert, K.G. Foods with increased protein content: A qualitative study on European consumer preferences and perceptions. Appetite 2018, 125, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Adair, L.S.; Nq, S.W. Global Nutrition and the pandemic of obesity in developing countries. Nutr. Rev. 2012, 70, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Watford, M.; Wu, G. Protein. Adv. Nutr. 2018, 9, 651–653. [Google Scholar] [CrossRef] [PubMed]
- Vicente, F.; Pereira, P.C. Pork Meat Composition and Health: A Review of the Evidence. Foods 2024, 13, 1905. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. The Food and Agriculture Organization of the United Nations. 2019. Available online: https://www.fao.org/faostat/en/#home (accessed on 21 June 2025).
- Drewnowski, A. Perspective: The place of pork meat in sustainable healthy diets. Adv. Nutr. 2024, 15, 100213. [Google Scholar] [CrossRef] [PubMed]
- Jayathilakan, K.; Sultana, K.; Radhakrishna, K.; Bawa, A.S. Utilization of byproducts and waste materials from meat, poultry and fish processing industries: A review. J. Food Sci. Technol. 2012, 49, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Sayeed, R.; Tiwari, P. Wealth from Meat Industry By-Products and Waste: A Review. In Sustainable Food Waste Management; Thakur, M., Modi, V.K., Khedkar, R., Singh, K., Eds.; Springer: Singapore, 2020; pp. 191–208. [Google Scholar] [CrossRef]
- Silva, V.D.; Silvestre, M.P. Functional properties of bovine blood plasma intended for use as a functional ingredient in human food. LWT-Food Sci. Technol. 2003, 36, 709–718. [Google Scholar] [CrossRef]
- Tseng, T.F.; Tsai, C.M.; Yang, J.H.; Chen, M.T. Porcine blood plasma transgluataminase combined with thrombin and fibrinogen as a binder in restructured meat. Asian-Australas. J. Anim. Sci. 2006, 19, 1054–1058. [Google Scholar] [CrossRef]
- Kim, S.; Jin, S.; Choi, J. Effects of the addition of blood plasma proteins on physico-chemical properties of emulsion-type pork sausage during cold storage. J. Sci. Food Agric. 2017, 97, 4501–4507. [Google Scholar] [CrossRef] [PubMed]
- Stader, C.; Judas, M.; Jira, W. A rapid UHPLC-MS/MS screening method for the detection of the addition of porcine blood plasma to emulsion-type pork sausages. Anal. Bioanal. Chem. 2019, 411, 6697–6709. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Ghosh, R.; Cui, Z. High-resolution plasma protein fractionation using ultrafiltration. Desalination 2002, 144, 301–306. [Google Scholar] [CrossRef]
- Mandal, P.K.; Rao, V.K.; Kowale, B.N.; Pal, U.K. Utilization of slaugther house blood in human food. J. Food Sci. Technol. 1999, 36, 91–105. [Google Scholar]
- AOAC (Association of Official Analytical Chemists). International, Official Methods of Analysis of the Association of Official Analytical Hemists International, 17th ed.; Association of Official Analytical Communities: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Wang, G.; Wang, T. Egg yolk protein modification by controlled enzymatic hydrolysis for improved functionalities. Int. J. Food Sci. Technol. 2009, 44, 763–769. [Google Scholar] [CrossRef]
- Diniz, F.M.; Martin, A.M. Effects of the extent of enzymatic hydrolysis on functional properties of shark protein hydrolysate. LWT-Food Sci. Technol. 1997, 30, 266–272. [Google Scholar] [CrossRef]
- Naczk, M.; Diosady, L.L.; Rubin, L.J. Functional properties of canola meals produced by a two-phase solvent extraction system. J. Food Sci. 1985, 50, 1685–1688. [Google Scholar] [CrossRef]
- Pearce, K.N.; Kinsella, J.E. Emulsifying properties of proteins: Evaluation of a turbidimetric technique. J. Agric. Food Chem. 1978, 26, 716–723. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Popović, L.; Peričin, D.; Vaštag, Ž.; Popović, S.; Krimer, V.; Torbica, A. Antioxidative and Functional Properties of Pumpkin Oil Cake Globulin Hydrolysates. J. Am. Oil Chem. Soc. 2013, 90, 1157–1165. [Google Scholar] [CrossRef]
- Lowry, O.; Rosenbrough, N.; Fair, A.; Randall, R. Protein measurement with the Folin–phenol reagent. J. Biolog. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Delaney, R.A. The nutritive value of porcine blood plasma concentrates prepared by ultrafiltration and spray drying. J. Sci. Food Agric. 1975, 26, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, E.B.; Delaney, R.A.M.; Kennedy, R. Studies on Slaughter Animal Blood Plasma: II. Heat Lability of Blood Plasma Proteins. Ir. J. Food Sci. Technol. 1978, 2, 39–44. Available online: https://www.jstor.org/stable/25557950 (accessed on 21 June 2025).
- Li, P.; He, W.; Wu, G. Composition of amino acids in foodstuffs for humans and animals. In Amino Acids in Nutrition and Health: Amino Acids in Gene Expression, Metabolic Regulation, and Exercising Performance; Springer: Cham, Switzerland, 2021; pp. 189–210. [Google Scholar] [CrossRef]
- Sedlar, T. Agroindustry by-Product—Green Leaves: A New Source of Protein and Bioactive Compounds. Ph.D. Thesis, University of Novi Sad, Novi Sad, Serbia, 2022. [Google Scholar]
- Tang, Q.; Tan, P.; Ma, N.; Ma, X. Physiological functions of threonine in animals: Beyond nutrition metabolism. Nutrients 2021, 13, 2592. [Google Scholar] [CrossRef] [PubMed]
- Martínez, Y.; Li, X.; Liu, G.; Bin, P.; Yan, W.; Más, D.; Valdivié, M.; Hu, C.A.A.; Ren, W.; Yin, Y. The role of methionine on metabolism, oxidative stress, and diseases. Amino Acids 2017, 49, 2091–2098. [Google Scholar] [CrossRef] [PubMed]
- Almeida, F.N.; Htoo, J.K.; Thomson, J.; Stein, H.H. Comparative amino acid digestibility in US blood products fed to weanling pigs. Anim. Feed. Sci. Technol. 2013, 181, 80–86. [Google Scholar] [CrossRef]
- Li, Y.H.; Li, F.N.; Duan, Y.H.; Guo, Q.P.; Wen, C.Y.; Wang, W.L.; Huang, X.G.; Yin, Y.L. Low-protein diet improves meat quality of growing and finishing pigs through changing lipid metabolism, fiber characteristics, and free amino acid profile of the muscle. J. Anim. Sci. 2018, 96, 3221–3232. [Google Scholar] [CrossRef] [PubMed]
- Nursten, H.E. The Maillard Reaction. Chemistry, Biochemistry and Implications; Royal Society of Chemistry: Cambridge, UK, 2005. [Google Scholar]
- Meeker, D.L. North American Rendering: Processing high quality protein and fats for feed. Rev. Bras. Zootec. 2009, 38, 432–440. [Google Scholar] [CrossRef]
- WHO/FAO/UNU. Protein and Amino Acid Requirements in Human Nutrition. Report of a Joint WHO/FAO/UNU Expert Consultation; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- Kinsella, J.E.; Melachouris, N. Functional properties of proteins in foods: A survey. Crit. Rev. Food Sci. Nutr. 1976, 7, 219–280. [Google Scholar] [CrossRef]
- Álvarez, C.; Bances, M.; Rendueles, M.; Díaz, M. Functional properties of isolated porcine blood proteins. Int. J. Food Sci. Technol. 2009, 44, 807–814. [Google Scholar] [CrossRef]
- Li, X.; Wang, W.; Wang, S.; Shen, Y.; Pan, J.; Dong, X.; Li, S. The Solubility and Structures of Porcine Myofibrillar Proteins under Low-Salt Processing Conditions as Affected by the Presence of L-Lysine. Foods 2022, 11, 855. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.M.; Lee, S.H.; Choe, J.H.; Rhee, M.S.; Lee, S.K.; Joo, S.T.; Kim, B.C. Protein solubility is related to myosin isoforms, muscle fiber types, meat quality traits, and postmortem protein changes in porcine longissimus dorsi muscle. Livest. Sci. 2010, 127, 183–191. [Google Scholar] [CrossRef]
- Lam, A.C.Y.; Karaca, A.C.; Tyler, R.T.; Nickerson, M.T. Pea Protein Isolates: Structure, Extraction, and Functionality. Food Rev. Int. 2016, 34, 126–147. [Google Scholar] [CrossRef]
- Wu, J.; Xu, S.; Yan, X.; Zhang, X.; Xu, X.; Li, Q.; Ye, J.; Liu, C. Effect of Homogenization Modified Rice Protein on the Pasting Properties of Rice Starch. Foods 2022, 11, 1601. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Guo, Y.; Zheng, Y.; Liu, S.; Cheng, T.; Zhou, L.; Guo, Z. Effects of high-pressure homogenization on physicochemical and functional properties of enzymatic hydrolyzed soybean protein concentrate. Front. Nutr. 2022, 9, 1054326. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Liu, R.; Ni, K.; Wu, T.; Luo, X.; Liang, B.; Zhang, M. Reduction of particle size based on superfine grinding: Effects on structure, rheological and gelling properties of whey protein concentrate. J. Food Eng. 2016, 186, 69–76. [Google Scholar] [CrossRef]
- Kristensen, L.; Purslow, P. P The effect of ageing on the water-holding capacity of pork: Role of cytoskeletal proteins. Meat Sci. 2001, 58, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Szmańko, T.; Lesiów, T.; Górecka, J. The water-holding capacity of meat: A reference analytical method. Food Chem. 2021, 357, 129727. [Google Scholar] [CrossRef] [PubMed]
- Brøndum, J.; Munck, L.; Henckel, P.; Karlsson, A.; Tornberg, E.; Engelsen, S.B. Prediction of water-holding capacity and composition of porcine meat by comparative spectroscopy. Meat Sci. 2000, 55, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Đermanovć, B.; Vujetić, J.; Sedlar, T.; Dragojlović, D.; Popović, L.; Kojić, P.; Jovanov, P.; Šarić, B. Optimization of Protein Extraction from Rapeseed Oil Cake by Dephenolization Process for Scale-Up Application Using Artificial Neural Networks. Foods 2025, 14, 1762. [Google Scholar] [CrossRef] [PubMed]
- Marseglia, A.; Dellafiora, L.; Prandi, B.; Lolli, V.; Sforza, S.; Cozzini, P.; Tedeschi, T.; Galaverna, G.; Caligiani, A. Simulated Gastrointestinal Digestion of Cocoa: Detection of Resistant Peptides and In Silico/In Vitro Prediction of Their Ace Inhibitory Activity. Nutrients 2019, 11, 985. [Google Scholar] [CrossRef] [PubMed]
- Haliza, W.; Purwani, E.Y.; Fardiaz, D.; Suhartono, M.T. In vitro protein digestibility of enzymatically pre-treated cocoa bean powder using commercial protease. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 828, p. 012024. [Google Scholar]
- Liu, R.; Xing, L.; Fu, Q.; Zhou, G.-H.; Zhang, W.-G. A Review of Antioxidant Peptides Derived from Meat Muscle and By-Products. Antioxidants 2016, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Borrajo, P.; Pateiro, M.; Barba, F.J.; Mora, L.; Franco, D.; Toldrá, F.; Lorenzo, J.M. Antioxidant and antimicrobial activity of peptides extracted from meat by-products: A review. Food Anal. Methods 2019, 12, 2401–2415. [Google Scholar] [CrossRef]
- López-Pedrouso, M.; Lorenzo, J.M.; Bou, R.; Vazquez, J.A.; Valcarcel, J.; Toldrà, M.; Franco, D. Valorisation of pork by-products to obtain antioxidant and antihypertensive peptides. Food Chem. 2023, 423, 136351. [Google Scholar] [CrossRef] [PubMed]
- D’ Archivio, M.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources, and bioavailability. Ann. Ist. Super. 2007, 43, 348. [Google Scholar]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [CrossRef] [PubMed]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein–phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- You, J.; Luo, Y.; Wu, J. Conjugation of ovotransferrin with catechin shows improved antioxidant activity. J. Agric. Food Chem. 2014, 62, 2581–2587. [Google Scholar] [CrossRef] [PubMed]
- Labuckas, D.O.; Maestri, D.M.; Perelló, M.; Martínez, M.L.; Lamarque, A.L. Phenolics from walnut (Juglans regia L.) kernels: Antioxidant activity and interactions with proteins. Food Chem. 2008, 107, 607–612. [Google Scholar] [CrossRef]
- Bešlo, D.; Golubić, N.; Rastija, V.; Agić, D.; Karnaš, M.; Šubarić, D.; Lučić, B. Antioxidant Activity, Metabolism, and Bioavailability of Polyphenols in the Diet of Animals. Antioxidants 2023, 12, 1141. [Google Scholar] [CrossRef] [PubMed]
- Chedea, V.S.; Palade, L.M.; Pelmus, R.S.; Dragomir, C.; Taranu, I. Red Grape Pomace Rich in Polyphenols Diet Increases the Antioxidant Status in Key Organs—Kidneys, Liver, and Spleen of Piglets. Animals 2019, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- da Silveira Almeida, B.C.; Ludke, M.d.C.M.M.; Bertol, T.M.; Ludke, J.V.; Bernardi, D.M.; Cunha, A., Jr.; Coldebella, A. Growth Performance, Meat Quality, and Lipid Oxidation in Pigs’ Fed Diets Containing Grape Pomace. Appl. Biosci. 2024, 3, 378–391. [Google Scholar] [CrossRef]
- Pastorelli, G.; Benamri, R.; Faustini, M.; De Bellis, R.; Serra, V.; Turin, L.; Haumont, M.; Durand, P.; Bianchessi, L.; Prost-Camus, E.; et al. Partial Replacement of Synthetic Vitamin E by Polyphenols in Post-Weaning Piglets. Antioxidants 2023, 12, 1752. [Google Scholar] [CrossRef] [PubMed]
- Viveros, A.; Chamorro, S.; Pizarro, M.; Arija, I.; Centeno, C.; Brenes, A. Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks. Poult. Sci. 2011, 90, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F. Polyphenol compounds in the chicken/animal diet: From the past to the future. J. Anim. Physiol. Anim. Nutr. 2014, 98, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Fiesel, A.; Gessner, D.K.; Erika Most, E.; Eder, K. Effects of dietary polyphenol-rich plant products from grape or hop on pro-inflammatory gene expression in the intestine, nutrient digestibility and faecal microbiota of weaned pigs. BMC Vet. Res. 2014, 10, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Jin, S.; Li, X.; Shen, J.; Zeng, X.; Wang, Y.; Zhou, G.; Tang, C. Biotinylated caffeic acid covalent binding with myofibrillar proteins in alkaline conditions: Identification of protein-phenol adducts and alterations in protein properties. Food Chem. 2023, 416, 135818. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhu, Y.; Li, X.; Xiang, F.; Deng, M.; Zhang, W.; Song, W.; Sun, H.; Tang, C. Identification of Protein–Phenol Adducts in Meat Proteins: A Molecular Probe Technology Study. Foods 2023, 12, 4225. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Kai, G. A review of dietary polyphenol-plasma protein interactions: Characterization, influence on the bioactivity, and structure-affinity relationship. Crit. Rev. Food Sci. Nutr. 2012, 52, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Maleyki, M.A.; Ismail, A. Antioxidant properties of cocoa powder. J. Food Biochem. 2010, 34, 111–128. [Google Scholar] [CrossRef]
- Moure, A.; Domínguez, H.; Parajó, J.C. Antioxidant properties of ultrafiltration-recovered soy protein fractions from industrial effluents and their hydrolysates. Process Biochem. 2006, 41, 447–456. [Google Scholar] [CrossRef]
- Klompong, V.; Benjakul, S.; Kantachote, D.; Shahidi, F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2007, 102, 1317–1327. [Google Scholar] [CrossRef]
- You, L.; Zhao, M.; Cui, C.; Zhao, H.; Yang, B. Effect of degree of hydrolysis on the antioxidant activity of loach (Misgurnus anguillicaudatus) protein hydrolysates. Innov. Food Sci. Emerg. Technol. 2009, 10, 235–240. [Google Scholar] [CrossRef]
- Vaštag, Ž.; Popović, L.; Popović, S.; Peričin-Starčević, I.; Krimer-Malešević, V. In vitro study on digestion of pumpkin oil cake protein hydrolysate: Evaluation of impact on bioactive properties. Int. J. Food Sci. Nutr. 2013, 64, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Su, G.; Ren, J.; Gu, L.; You, L.; Zhao, M. Isolation and characterization of an oxygen radical absorbance activity peptide from defatted peanut meal hydrolysate and its antioxidant properties. J. Agric. Food Chem. 2012, 60, 5431–5437. [Google Scholar] [CrossRef] [PubMed]
- Aluko, R.E. Antihypertensive Properties of Plant-Derived Inhibitors of Angiotensin I-Converting Enzyme Activity: A Review; Studium Press LLC.: Houston, TX, USA, 2008. [Google Scholar]
- Hartmann, R.; Meisel, H. Food-derived peptides with biological activity: From research to food applications. Curr. Opin. Biotechnol. 2007, 18, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Meisel, H. Multifunctional peptides encrypted in milk proteins. Biofactors 2004, 21, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; De Mejia, E.G. A new frontier in soy bioactive peptides that may prevent age-related chronic diseases. Compr. Rev. Food Sci. Food Saf. 2005, 4, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Pripp, A.H.; Isaksson, T.; Stepaniak, L.; Sørhaug, T.; Ardö, Y. Quantitative structure activity relationship modelling of peptides and proteins as a tool in food science. Trends Food Sci. Technol. 2005, 16, 484–494. [Google Scholar] [CrossRef]
- Chen, H.M.; Muramoto, K.; Yamauchi, F.; Fujimoto, K.; Nokihara, K. Antioxidative properties of histidine-containing peptides designed from peptide fragments found in the digests of a soybean protein. J. Agric. Food Chem. 1998, 46, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, N.; Mendis, E.; Jung, W.K.; Je, J.Y.; Kim, S.K. Purification of a radical scavenging peptide from fermented mussel sauce and its antioxidant properties. Food Res. Int. 2005, 38, 175–182. [Google Scholar] [CrossRef]
- Qian, Z.J.; Jung, W.K.; Kim, S.K. Free radical scavenging activity of a novel antioxidative peptide purified from hydrolysate of bullfrog skin, Rana catesbeiana Shaw. Bioresour. Technol. 2008, 99, 1690–1698. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.M.; Decker, E.A.; Feustman, C. Endogenous skeletal muscle antioxidants. Crit. Rev. Food Sci. Nutr. 1994, 34, 403–426. [Google Scholar] [CrossRef] [PubMed]
- Montero Castillo, P.M.; Martelo Gómez, R.J.; Paternina Sierra, K. Effect of lyophilized porcine plasma and Zaragoza bean (Phaseolus vulgaris) flour on the technological and sensory quality parameters of frankfurter-type sausages. Cienc. Tecnol. Agropecu. 2022, 23, e2293. [Google Scholar] [CrossRef]
- Jin, S.K.; Choi, J.S.; Kim, G.D. Effect of porcine plasma hydrolysate on physicochemical, antioxidant, and antimicrobial properties of emulsion-type pork sausage during cold storage. Meat Sci. 2021, 171, 108293. [Google Scholar] [CrossRef] [PubMed]
- Csurka, T.; Varga-Tóth, A.; Kühn, D.; Hitka, G.; Badak-Kerti, K.; Alpár, B.; Surányi, J.; Friedrich, L.F.; Pásztor-Huszár, K. Comparison of techno-functional and sensory properties of sponge cakes made with egg powder and different quality of powdered blood products for substituting egg allergen and developing functional food. Front. Nutr. 2022, 9, 979594. [Google Scholar] [CrossRef] [PubMed]

| MP | BP | |
|---|---|---|
| Proteins | 90.67 ± 0.6223 a | 86.85 ± 0.5869 b |
| Carbohydrate | 0 a | 1.59 ± 0.1 b |
| Fiber | 2.23 ± 0.071 a | 0.9 ± 0.106 b |
| Fat | 0.9 ± 0.02 a | 0 b |
| Moisture | 3.87 ± 0.034 a | 4.12 ± 0.0216 b |
| Ash | 2.33 ± 0.0141 a | 6.54 ± 0.0071 b |
| Energy (kJ/100 g) | 1541.39 | 1510.68 |
| Amino Acid (mg/100 g) | MP | BP |
|---|---|---|
| Aspartic acid | 2.19 ± 0.38 a | 6.91 ± 0.14 b |
| Glutamic acid | 9.09 ± 1.11 a | 11.10 ± 0.01 b |
| Serine | 2.95 ± 0.77 a | 10.12 ± 0.53 a |
| Histidine | 2.28 ± 0.58 a | 69.73 ± 3.65 b |
| Glycine | 118.42 ± 14.19 a | 66.93 ± 3.98 a |
| Threonine | 34.21 ± 1.79 a | 70.60 ± 8.17 b |
| Arginine | 27.63 ± 3.86 a | 18.59 ± 3.09 b |
| Alanine | 13.67 ± 1.63 a | 27.56 ± 2.24 b |
| Tyrosine | 3.34 ± 1.08 a | 7.73 ± 1.34 b |
| Cystine | 328.15 ± 36.63 a | 718.22 ± 54.67 b |
| Valine | 4.86 ± 0.78 a | 22.45 ± 0.96 b |
| Methionine | 25.95 ± 2.25 a | 94.35 ± 1.04 b |
| Tryptophane | 0.38 ± 0.11 a | 0.97 ± 0.01 b |
| Phenylalanine | 5.37 ± 0.64 a | 15.70 ± 1.23 b |
| Isoleucine | 6.27 ± 0.77 a | 2.34 ± 0.35 b |
| Leucine | 11.22 ± 1.35 a | 35.53 ± 1.66 b |
| Lysine | n.d. 1,a | 0.26 ± 0.00 b |
| Proline | n.d. a | n.d. a |
| MP | BP | |
|---|---|---|
| WSI (%) | 13.4 ± 0.3464 a | 95.7 ± 0.4582 b |
| WHC (%) | 83.4 ± 1.0583 a | 100 ± 1.2135 b |
| OHC (%) | 93.6 ± 1.1358 a | 85.8 ± 1.3868 b |
| FC (%) | - | 72.3 ± 0.8185 b |
| EAI (m2/g) | 183 ± 0.8185 a | 243 ± 1.6093 b |
| ES (min) | 28 ± 0.5 a | 88 ± 1.5620 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stožinić, M.; Ačkar, Đ.; Šojić, B.; Sedlar, T.; Popović, L.; Pajin, B.; Flanjak, I.; Bulatović, M.; Petrović, J.; Nikolić, I.; et al. Incorporation of Pork Meat and Blood Plasma Proteins into a Cocoa Cream Matrix: Characterization, Comparison of Functional Properties, and In Vitro Simulated Digestion. Foods 2025, 14, 2547. https://doi.org/10.3390/foods14142547
Stožinić M, Ačkar Đ, Šojić B, Sedlar T, Popović L, Pajin B, Flanjak I, Bulatović M, Petrović J, Nikolić I, et al. Incorporation of Pork Meat and Blood Plasma Proteins into a Cocoa Cream Matrix: Characterization, Comparison of Functional Properties, and In Vitro Simulated Digestion. Foods. 2025; 14(14):2547. https://doi.org/10.3390/foods14142547
Chicago/Turabian StyleStožinić, Milica, Đurđica Ačkar, Branislav Šojić, Tea Sedlar, Ljiljana Popović, Biljana Pajin, Ivana Flanjak, Maja Bulatović, Jovana Petrović, Ivana Nikolić, and et al. 2025. "Incorporation of Pork Meat and Blood Plasma Proteins into a Cocoa Cream Matrix: Characterization, Comparison of Functional Properties, and In Vitro Simulated Digestion" Foods 14, no. 14: 2547. https://doi.org/10.3390/foods14142547
APA StyleStožinić, M., Ačkar, Đ., Šojić, B., Sedlar, T., Popović, L., Pajin, B., Flanjak, I., Bulatović, M., Petrović, J., Nikolić, I., & Lončarević, I. (2025). Incorporation of Pork Meat and Blood Plasma Proteins into a Cocoa Cream Matrix: Characterization, Comparison of Functional Properties, and In Vitro Simulated Digestion. Foods, 14(14), 2547. https://doi.org/10.3390/foods14142547









