Recent Advances in the Application Technologies of Surface Coatings for Fruits
Abstract
1. Introduction
1.1. Background
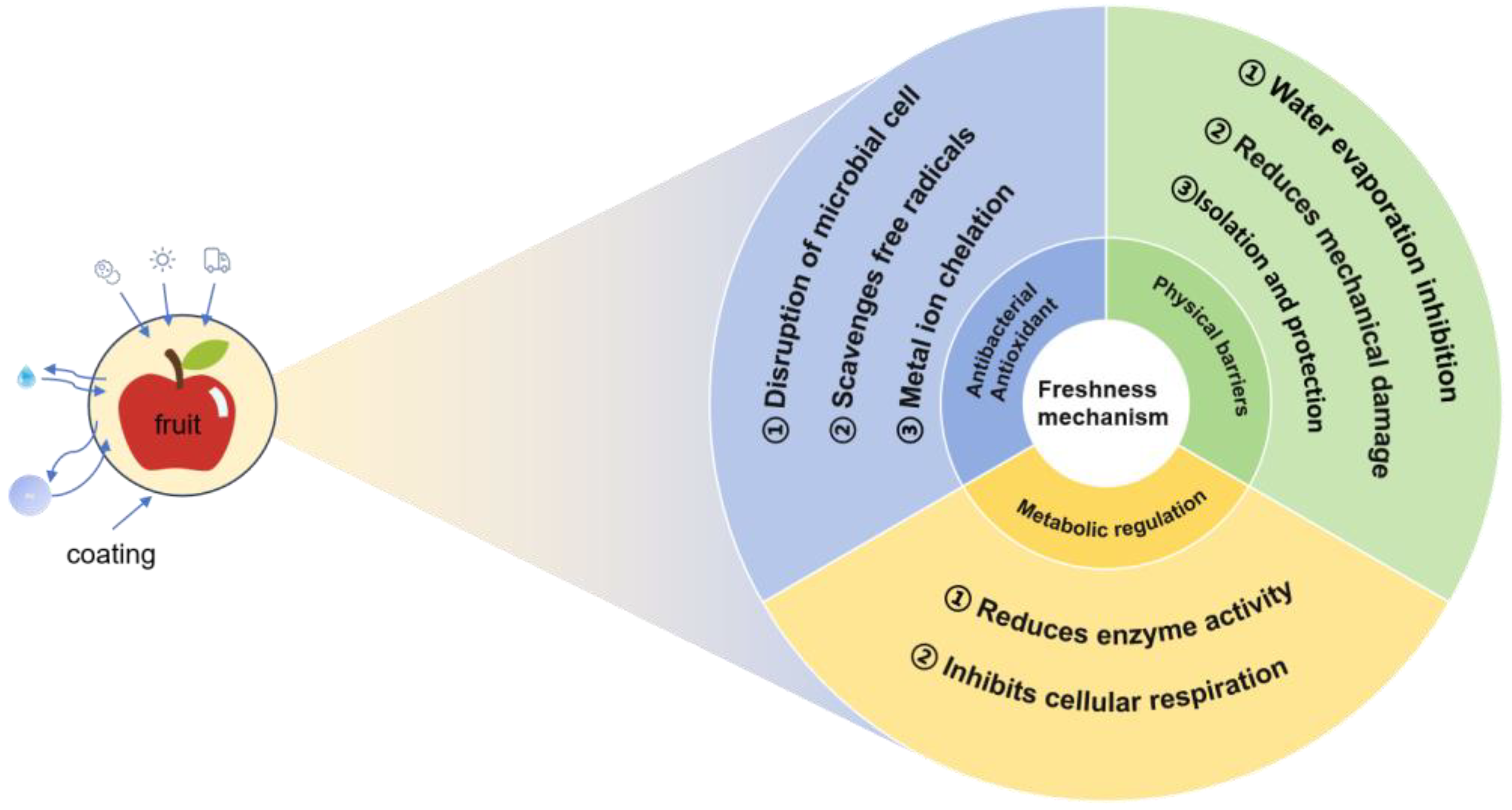
1.2. The Mechanism of Action of Coating Preservation
1.2.1. Physical Barrier Effect
1.2.2. Physiological Metabolism Regulatory Functions
1.2.3. Antibacterial and Antioxidant Effect
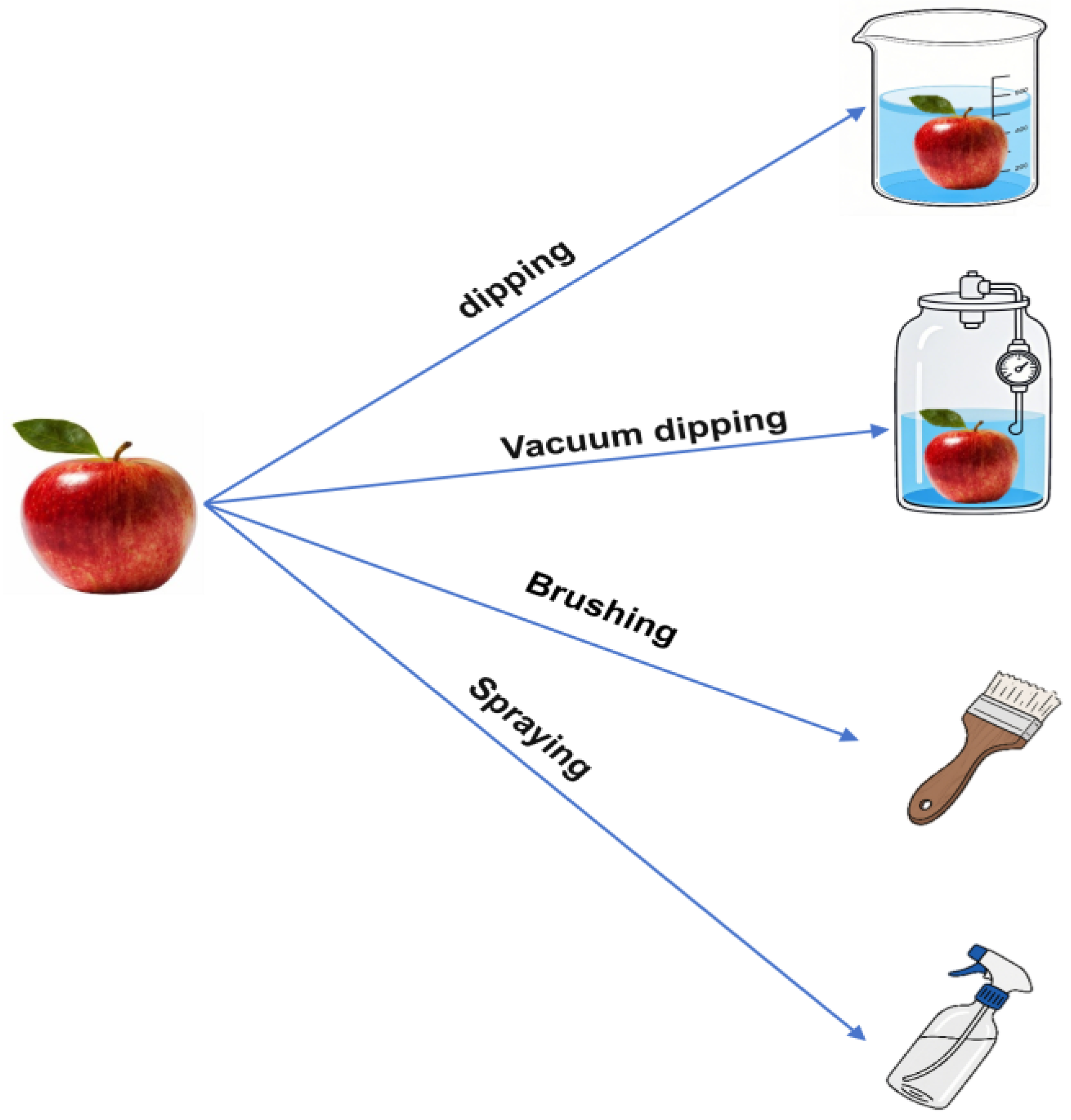
2. Application Techniques of Traditional Coatings
2.1. Dipping
2.2. Vacuum Dipping
2.3. Brushing
2.4. Spraying
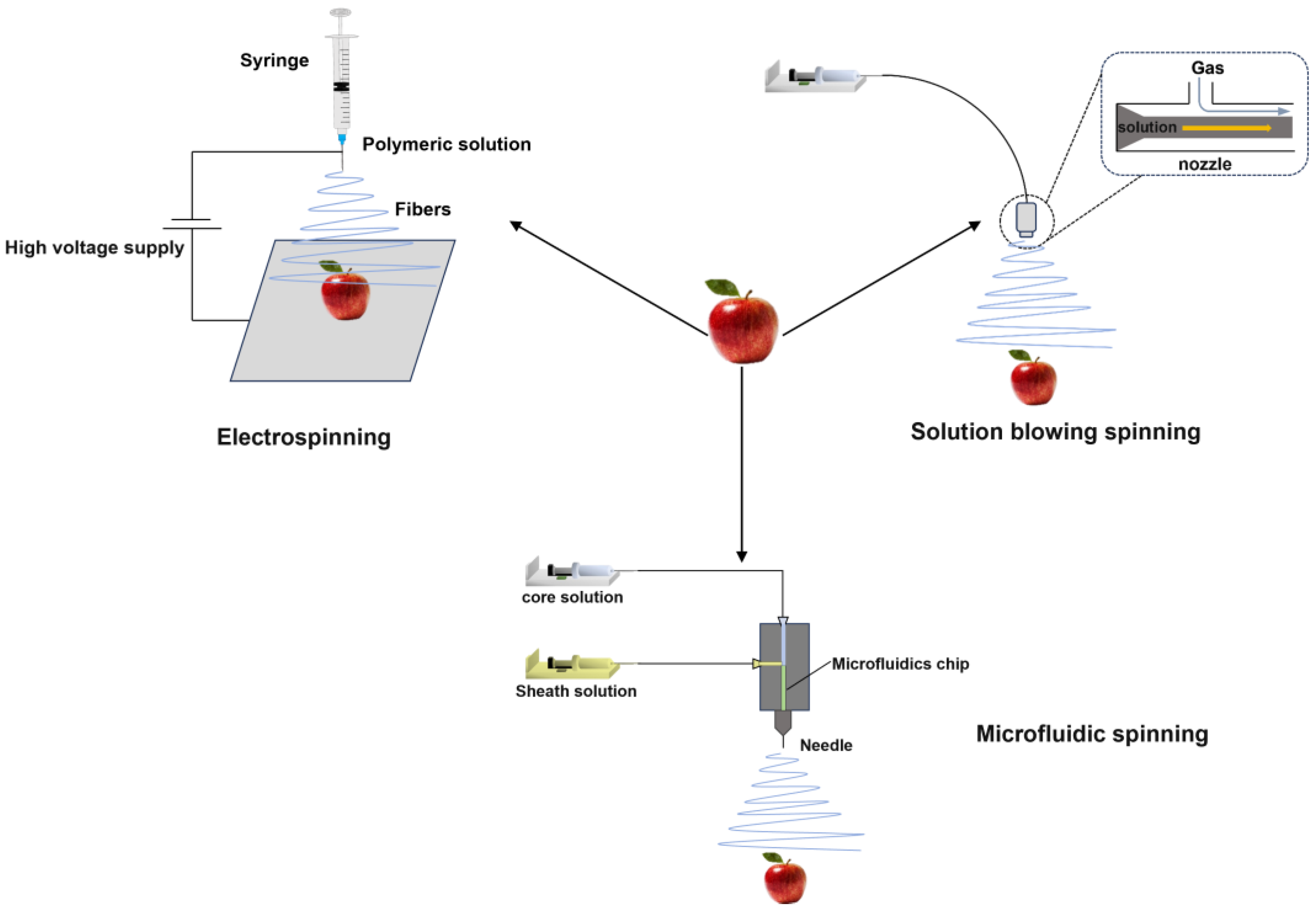
3. Fiber Coating-Forming Technology
3.1. Electrospinning Technology
3.1.1. Principle of Electrospinning
3.1.2. Classification and Application of Electrospinning
3.1.3. Factors Influencing Electrospinning
3.2. Solution-Blowing Spinning Technology
3.3. Microfluidic Spinning Technology
3.3.1. Solidification Methods for Microfluidic Spinning Fibers
3.3.2. Application of Microfluidic Spinning in Fruit Coatings
4. Conclusions and Future Trends
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| WHO | World Health Organization |
| FAO | Food and Agriculture Organization of the United Nations |
| CuNPs | Copper nanoparticles |
| TEO | Thyme essential oil |
| ATP | Adenosine triphosphat |
| AcXETs | Annona cherimola Xyloglucan Endotransglycosylases |
| AcEXPs | Annona cherimola Expansins |
| AcPE | Annona cherimola Pectinesterase |
| DNA | DeoxyriboNucleic Acid |
| CS | Chitosan |
| TA | Tannic acid |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| TPC | Total phenolic compound |
| PS | Polystyrene |
| PMMA | Polymethyl methacrylate |
| PC | Polycarbonate |
| CA | Cinnamaldehyde |
| GA | Glutaraldehyde |
| PCL | polycaprolactone |
| PVA | Polyvinyl alcohol |
| PEGDA | Poly (ethylene glycol) diacrylate |
| PVB | Polyvinyl butyral |
| PSF | Polysulfone |
| PES | Polyethersulfone |
| PAAS | Sodium Polyacrylate |
| PVP | Polyvinylpyrrolidone |
| KGM | Konjac glucomannan |
| EA | Elderberry anthocyanin |
| KEA | Konjac glucomannan and elderberry anthocyanin mixing |
| EC | Ethylcellulose |
| LAG | Low-acyl gellan gum |
| PLA | Polylactic acid |
| GCMC | Gelatin carboxymethyl cellulose membranes |
| AM | Aegle marmelos |
| CS-TA/MXene | Tannic acid/MXene assembly with chitosan |
| PEO | Corn soluble protein containing hexanal–poly(ethylene oxide) |
References
- Taguchi, M.; Beed, F.; Telemans, B.; Hassan, S. Fruit and Vegetables—Your Dietary Essentials; The International Year of Fruits and Vegetables, 2021 background paper; FAO: Rome, Italy, 2020. [Google Scholar]
- Jayawardena, R.; Jeyakumar, D.T.; Gamage, M.; Sooriyaarachchi, P.; Hills, A.P. Fruit and vegetable consumption among South Asians: A systematic review and meta-analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1791–1800. [Google Scholar] [CrossRef]
- FAO. Major Tropical Fruits Market Review. Preliminary Results. 2024. Available online: https://openknowledge.fao.org/handle/20.500.14283/cd3818en (accessed on 24 December 2024).
- Ammar, E.E.; Zou, X.B.; Ghosh, S.; Onyeaka, H.; Elmasry, S.A.; Alkeay, A.M.; Al-Farga, A.; Rady, H.A.; El-Shershaby, N.A.; Sallam, A.S. Fresh futures: Cutting-edge eco-friendly coating techniques for fruits. J. Food Process. Preserv. 2025, 2025, 5201632. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, Y.; Liang, L.; Dhanasekaran, S.; Zhang, X.; Yang, X.; Wu, M.; Song, Y.; Zhang, H. WSC1 regulates the growth, development, patulin production, and pathogenicity of penicillium expansum infecting pear fruits. J. Agric. Food Chem. 2024, 72, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, I.; Mandavgane, S.A. Valorization of fruit and vegetable waste for biofertilizer and biogas. J. Food Process Eng. 2021, 44, e13512. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.; Xu, B.; Mujumdar, A.S.; Guo, Z. Light-emitting diodes (below 700 nm): Improving the preservation of fresh foods during postharvest handling, storage, and transportation. Compr. Rev. Food Sci. Food Saf. 2022, 21, 106–126. [Google Scholar] [CrossRef]
- Pu, Y.; Zhou, Q.; Yu, L.; Li, C.; Dong, Y.; Yu, N.; Chen, X. Longitudinal analyses of lignin deposition in green asparagus by microscopy during high oxygen modified atmosphere packaging. Food Packag. Shelf Life 2020, 25, 100536. [Google Scholar] [CrossRef]
- Cui, H.Y.; Abdel-Samie, M.A.S.; Lin, L. Novel packaging systems in grape storage—A review. J. Food Process Eng. 2019, 42, e13162. [Google Scholar] [CrossRef]
- Umair, M.; Sultana, T.; Xun, S.; Jabbar, S.; Riaz Rajoka, M.S.; Albahi, A.; Abid, M.; Ranjha, M.; El-Seedi, H.R.; Xie, F.; et al. Advances in the application of functional nanomaterial and cold plasma for the fresh-keeping active packaging of meat. Food Sci. Nutr. 2023, 11, 5753–5772. [Google Scholar] [CrossRef]
- Hashim, S.B.H.; Tahir, H.E.; Mahdi, A.A.; Al-Maqtari, Q.A.; Shishir, M.R.I.; Mahunu, G.K.; Akpabli-Tsigbe, N.D.K.; Zhang, J.J.; Zou, X.B.; Shi, J.Y. Enhancing the functionality of the Origanum compactum essential oil capsules by combining sugarcane wax with various biopolymers. J. Food Meas. Charact. 2025, 19, 833–849. [Google Scholar] [CrossRef]
- Wang, H.; Ding, F.; Ma, L.; Zhang, Y. Recent advances in gelatine and chitosan complex material for practical food preservation application. Int. J. Food Sci. Technol. 2021, 56, 6279–6300. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Mahunu, G.K.; Castoria, R.; Yang, Q.Y.; Apaliya, M.T. Recent developments in the enhancement of some postharvest biocontrol agents with unconventional chemicals compounds. Trends Food Sci. Technol. 2018, 78, 180–187. [Google Scholar] [CrossRef]
- Ahima, J.; Zhang, H.; Apaliya, M.T.; Yang, Q.; Jiang, Z. The mechanism involved in enhancing the biological control efficacy of Rhodotorula mucilaginosa with salicylic acid to postharvest green mold decay of oranges. J. Food Meas. Charact. 2020, 14, 3146–3155. [Google Scholar] [CrossRef]
- Cui, H.Y.; Wu, J.; Li, C.Z.; Lin, L. Anti-listeria effects of chitosan-coated nisin-silica liposome on Cheddar cheese. J. Dairy Sci. 2016, 99, 8598–8606. [Google Scholar] [CrossRef]
- Zhao, Q.; Shi, Y.; Xu, C.; Jiang, Z.; Liu, J.; Sui, Y.; Zhang, H. Control of postharvest blue and gray mold in kiwifruit by Wickerhamomyces anomalus and its mechanism of antifungal activity. Postharvest Biol. Technol. 2023, 201, 112345. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Mahunu, G.K.; Castoria, R.; Apaliya, M.T.; Yang, Q.Y. Augmentation of biocontrol agents with physical methods against postharvest diseases of fruits and vegetables. Trends Food Sci. Technol. 2017, 69, 36–45. [Google Scholar] [CrossRef]
- Ngolong Ngea, G.L.; Qian, X.; Yang, Q.; Dhanasekaran, S.; Ianiri, G.; Ballester, A.R.; Zhang, X.; Castoria, R.; Zhang, H. Securing fruit production: Opportunities from the elucidation of the molecular mechanisms of postharvest fungal infections. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2508–2533. [Google Scholar] [CrossRef]
- Rashid, A.; Qayum, A.; Bacha, S.A.S.; Liang, Q.; Liu, Y.; Kang, L.; Chi, Z.; Chi, R.; Han, X.; Ekumah, J.-N.; et al. Preparation and functional characterization of pullulan-sodium alginate composite film enhanced with ultrasound-assisted clove essential oil Nanoemulsions for effective preservation of cherries and mushrooms. Food Chem. 2024, 457, 140048. [Google Scholar] [CrossRef]
- Cui, H.; Surendhiran, D.; Li, C.; Lin, L. Biodegradable zein active film containing chitosan nanoparticle encapsulated with pomegranate peel extract for food packaging. Food Packag. Shelf Life 2020, 24, 100511. [Google Scholar] [CrossRef]
- Al-Maqtari, Q.A.; Al-Gheethi, A.A.S.; Ghaleb, A.D.S.; Mahdi, A.A.; Al-Ansi, W.; Noman, A.E.; Al-Adeeb, A.; Odjo, A.K.O.; Du, Y.; Wei, M.; et al. Fabrication and characterization of chitosan/gelatin films loaded with microcapsules of Pulicaria jaubertii extract. Food Hydrocoll. 2022, 129, 107624. [Google Scholar] [CrossRef]
- Atta, O.M.; Manan, S.; Shahzad, A.; Ul-Islam, M.; Ullah, M.W.; Yang, G. Biobased materials for active food packaging: A review. Food Hydrocoll. 2022, 125, 107419. [Google Scholar] [CrossRef]
- Jurić, M.; Maslov Bandić, L.; Carullo, D.; Jurić, S. Technological advancements in edible coatings: Emerging trends and applications in sustainable food preservation. Food Biosci. 2024, 58, 103835. [Google Scholar] [CrossRef]
- Lin, L.; Liu, X.; Shi, C.; Chen, X.C.; Aziz, T.; Al-Asmari, F.; Mohamed, A.A.; Cui, H.Y. Preparation and characterization of chitosan-Tremella fuciformis polysaccharide edible films for meat preservation. Packag. Technol. Sci. 2025, 38, 211–226. [Google Scholar] [CrossRef]
- Dai, L.M.; Wang, X.S.; Zhang, J.; Li, C.W. Application of chitosan and its derivatives in postharvest coating preservation of fruits. Foods 2025, 14, 1318. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ding, F.; Ma, L.; Zhang, Y. Edible films from chitosan-gelatin: Physical properties and food packaging application. Food Biosci. 2021, 40, 100871. [Google Scholar] [CrossRef]
- Jiao, X.; Xie, J.; Hao, M.; Li, Y.; Wang, C.; Zhu, Z.; Wen, Y. Chitosan Biguanidine/PVP antibacterial coatings for perishable fruits. Polymers 2022, 14, 2704. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.Y.; Yu, H.D.; Chen, K.J.; Cui, R.; Cao, J.X.; Wang, Z.X.; Zhang, Z.H.; Soteyome, T. Effects of chitosan/eugenol-loaded IRMOF-3 nanoparticles composite films on reactive oxygen species metabolism and microbial community dynamics in postharvest strawberries. Food Biosci. 2025, 63, 105652. [Google Scholar] [CrossRef]
- Dai, L.; Wang, X.; Mao, X.; He, L.; Li, C.; Zhang, J.; Chen, Y. Recent advances in starch-based coatings for the postharvest preservation of fruits and vegetables. Carbohydr. Polym. 2024, 328, 121736. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Liu, J.; Li, H.; Yuan, C.; Zhong, J.; Chen, Y. Influence of postharvest citric acid and chitosan coating treatment on ripening attributes and expression of cell wall related genes in cherimoya (Annona cherimola Mill.) fruit. Sci. Hortic. 2016, 198, 1–11. [Google Scholar] [CrossRef]
- Hosoya, N.; Motomura, K.; Tagawa, E.; Nagano, M.; Ogiwara, C.; Hosoya, H. Effects of the fungicide ortho-phenylphenol (OPP) on the early development of sea urchin eggs. Mar. Environ. Res. 2019, 143, 24–29. [Google Scholar] [CrossRef]
- Liang, F.; Liu, C.; Geng, J.; Chen, N.; Lai, W.; Mo, H.; Liu, K. Chitosan–fucoidan encapsulating cinnamaldehyde composite coating films: Preparation, pH-responsive release, antibacterial activity and preservation for litchi. Carbohydr. Polym. 2024, 333, 121968. [Google Scholar] [CrossRef]
- Li, M.; Chen, C.; Xia, X.; Betchem, G.; Shang, L.; Wang, Y. Proteomic analysis of the inhibitory effect of chitosan on Penicillium expansum. Food Sci. Technol. 2019, 40, 250–257. [Google Scholar] [CrossRef]
- Duan, C.; Meng, X.; Meng, J.; Khan, M.I.H.; Dai, L.; Khan, A.; An, X.; Zhang, J.; Huq, T.; Ni, Y. Chitosan as a Preservative for Fruits and Vegetables: A Review on Chemistry and Antimicrobial Properties. J. Bioresour. Bioprod. 2019, 4, 11–21. [Google Scholar] [CrossRef]
- Mehmood, A.; Aziz, T.; Al-Asmari, F.; Shami, A.; Haiying, C.; Xu, J.L.; Lin, L. Insight into the recent advances in the development of antimicrobial edible films for food packaging. Packag. Technol. Sci. 2025, 38, 487–509. [Google Scholar] [CrossRef]
- Umbayda, T.G.; Funga, A.D.; Mwakalesi, A.J. Novel edible coating based on Macadamia Nut oil and chitosan to maintain the antioxidant and physical properties of tomato fruits. Appl. Food Res. 2024, 4, 100434. [Google Scholar] [CrossRef]
- Pasquariello, M.S.; Di Patre, D.; Mastrobuoni, F.; Zampella, L.; Scortichini, M.; Petriccione, M. Influence of postharvest chitosan treatment on enzymatic browning and antioxidant enzyme activity in sweet cherry fruit. Postharvest Biol. Technol. 2015, 109, 45–56. [Google Scholar] [CrossRef]
- Liu, W.; Kang, S.; Zhang, Q.; Chen, S.; Yang, Q.; Yan, B. Self-assembly fabrication of chitosan-tannic acid/MXene composite film with excellent antibacterial and antioxidant properties for fruit preservation. Food Chem. 2023, 410, 135405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-H.; Peng, H.; Ma, H.; Zeng, X.-A. Effect of inlet air drying temperatures on the physicochemical properties and antioxidant activity of whey protein isolate-kale leaves chlorophyll (WPI-CH) microcapsules. J. Food Eng. 2019, 245, 149–156. [Google Scholar] [CrossRef]
- Florencia Cravero, C.; Stefani Juncos, N.; Rubén Grosso, N.; Horacio Olmedo, R. Autoxidation interference assay to evaluate the protection against lipid oxidation of antioxidant administration: Comparison of the efficiency of progressive release or total administration. Food Chem. 2024, 444, 138580. [Google Scholar] [CrossRef]
- Md Nor, S.; Ding, P. Trends and advances in edible biopolymer coating for tropical fruit: A review. Food Res. Int. 2020, 134, 109208. [Google Scholar] [CrossRef]
- Sipahi, R.E.; Castell-Perez, M.E.; Moreira, R.G.; Gomes, C.; Castillo, A. Improved multilayered antimicrobial alginate-based edible coating extends the shelf life of fresh-cut watermelon (Citrullus lanatus). LWT—Food Sci. Technol. 2013, 51, 9–15. [Google Scholar] [CrossRef]
- Saberi, B.; Golding, J.B.; Marques, J.R.; Pristijono, P.; Chockchaisawasdee, S.; Scarlett, C.J.; Stathopoulos, C.E. Application of biocomposite edible coatings based on pea starch and guar gum on quality, storability and shelf life of ‘Valencia’ oranges. Postharvest Biol. Technol. 2018, 137, 9–20. [Google Scholar] [CrossRef]
- Thakur, R.; Pristijono, P.; Bowyer, M.; Singh, S.P.; Scarlett, C.J.; Stathopoulos, C.E.; Vuong, Q.V. A starch edible surface coating delays banana fruit ripening. LWT 2019, 100, 341–347. [Google Scholar] [CrossRef]
- Ma, J.; Zhou, Z.; Li, K.; Li, K.; Liu, L.; Zhang, W.; Xu, J.; Tu, X.; Du, L.; Zhang, H. Novel edible coating based on shellac and tannic acid for prolonging postharvest shelf life and improving overall quality of mango. Food Chem. 2021, 354, 129510. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zou, X.; Li, Z.; Huang, X.; Zhai, X.; Zhang, W.; Shi, J.; Tahir, H.E. Improved postharvest quality of cold Stored blueberry by edible coating based on composite gum Arabic/roselle extract. Food Bioprocess Technol. 2019, 12, 1537–1547. [Google Scholar] [CrossRef]
- Nawab, A.; Alam, F.; Hasnain, A. Mango kernel starch as a novel edible coating for enhancing shelf- life of tomato (Solanum lycopersicum) fruit. Int. J. Biol. Macromol. 2017, 103, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, Y.J.F.C. Effects of chitosan coating on postharvest life and quality of longan fruit. Food Chem. 2001, 73, 139–143. [Google Scholar] [CrossRef]
- Mendy, T.K.; Misran, A.; Mahmud, T.M.M.; Ismail, S.I. Application of Aloe vera coating delays ripening and extend the shelf life of papaya fruit. Sci. Hortic. 2019, 246, 769–776. [Google Scholar] [CrossRef]
- Maftoonazad, N.; Ramaswamy, H.S. Application and evaluation of a pectin-based edible coating process for quality change kinetics and shelf-life extension of lime fruit (Citrus aurantifolium). Coatings 2019, 9, 285. [Google Scholar] [CrossRef]
- Iftikhar, A.; Rehman, A.; Usman, M.; Ali, A.; Ahmad, M.M.; Shehzad, Q.; Fatim, H.; Mehmood, A.; Moiz, A.; Shabbir, M.A.; et al. Influence of guar gum and chitosan enriched with lemon peel essential oil coatings on the quality of pears. Food Sci. Nutr. 2022, 10, 2443–2454. [Google Scholar] [CrossRef]
- Li, M.; Yang, Z.; Zhai, X.; Li, Z.; Huang, X.; Shi, J.; Zou, X.; Lv, G. Incorporation of Lactococcus lactis and chia mucilage for improving the physical and biological properties of gelatin-based coating: Application for strawberry preservation. Foods 2024, 13, 1102. [Google Scholar] [CrossRef]
- Hong, K.; Xie, J.; Zhang, L.; Sun, D.; Gong, D. Effects of chitosan coating on postharvest life and quality of guava (Psidium guajava L.) fruit during cold storage. Sci. Hortic. 2012, 144, 172–178. [Google Scholar] [CrossRef]
- Bleoanca, I.; Lanciu, A.; Patrașcu, L.; Ceoromila, A.; Borda, D. Efficacy of two stabilizers in nanoemulsions with whey proteins and thyme essential oil as edible coatings for Zucchini. Membranes 2022, 12, 326. [Google Scholar] [CrossRef] [PubMed]
- Suhag, R.; Kumar, N.; Petkoska, A.T.; Upadhyay, A. Film formation and deposition methods of edible coating on food products: A review. Food Res. Int. 2020, 136, 109582. [Google Scholar] [CrossRef]
- Tavassoli-Kafrani, E.; Shekarchizadeh, H.; Masoudpour-Behabadi, M. Development of edible films and coatings from alginates and carrageenans. Carbohydr. Polym. 2016, 137, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, B.J.; Bezerra, A.C.; Oliveira, L.L.; Arroyo, S.J.; Melo, E.A.d.; Santos, A.M.P. Antimicrobial active edible coating of alginate and chitosan add ZnO nanoparticles applied in guavas (Psidium guajava L.). Food Chem. 2020, 309, 125566. [Google Scholar] [CrossRef]
- Erceg, T.; Aćimović, M.; Šovljanski, O.; Lončar, B.; Tomić, A.; Pavlović, M.; Vukić, V.; Hadnađev, M. Preparation and characterization of carboxymethylated pullulan/butyric acid-modified chitosan active sustainable bi-layer coatings intended for packaging of cheese slices. Int. J. Biol. Macromol. 2024, 277, 134053. [Google Scholar] [CrossRef]
- Gupta, D.; Lall, A.; Kumar, S.; Patil, T.D.; Gaikwad, K.K. Plant-based edible films and coatings for food-packaging applications: Recent advances, applications, and trends. Sustain. Food Technol. 2024, 2, 1428–1455. [Google Scholar] [CrossRef]
- Aayush, K.; McClements, D.J.; Sharma, S.; Sharma, R.; Singh, G.P.; Sharma, K.; Oberoi, K. Innovations in the development and application of edible coatings for fresh and minimally processed Apple. Food Control 2022, 141, 109188. [Google Scholar] [CrossRef]
- Gautam, S.; Kathuria, D.; Hamid; Dobhal, A.; Singh, N. Vacuum impregnation: Effect on food quality, application and use of novel techniques for improving its efficiency. Food Chem. 2024, 460, 140729. [Google Scholar] [CrossRef]
- Vinod, B.R.; Asrey, R.; Sethi, S.; Menaka, M.; Meena, N.K.; Shivaswamy, G. Recent advances in vacuum impregnation of fruits and vegetables processing: A concise review. Heliyon 2024, 10, e28023. [Google Scholar] [CrossRef]
- Demir, N.; Alpaslan, M. Determination of impregnation parameters and volatile components in vacuum impregnated apricots. Heliyon 2024, 10, e28294. [Google Scholar] [CrossRef] [PubMed]
- Radziejewska-Kubzdela, E.; Biegańska-Marecik, R.; Kidoń, M. Applicability of vacuum impregnation to modify physico-chemical, sensory and nutritive characteristics of plant origin products—A review. Int. J. Mol. Sci. 2014, 15, 16577–16610. [Google Scholar] [CrossRef]
- Ruiz-Llacsahuanga, B.; Hamilton, A.M.; Anderson, K.; Critzer, F. Efficacy of cleaning and sanitation methods against Listeria innocua on apple packing equipment surfaces. Food Microbiol. 2022, 107, 104061. [Google Scholar] [CrossRef]
- Marmur, T.; Elkind, Y.; Nussinovitch, A. Increase in gloss of coated red peppers by different brushing procedures. LWT—Food Sci. Technol. 2013, 51, 531–536. [Google Scholar] [CrossRef]
- Njombolwana, N.S.; Erasmus, A.; van Zyl, J.G.; du Plooy, W.; Cronje, P.J.R.; Fourie, P.H. Effects of citrus wax coating and brush type on imazalil residue loading, green mould control and fruit quality retention of sweet oranges. Postharvest Biol. Technol. 2013, 86, 362–371. [Google Scholar] [CrossRef]
- Peretto, G.; Du, W.-X.; Avena-Bustillos, R.J.; De, J.; Berrios, J.; Sambo, P.; McHugh, T.H. Electrostatic and conventional spraying of alginate-based edible coating with natural antimicrobials for preserving fresh strawberry quality. Food Bioprocess Technol. 2016, 10, 165–174. [Google Scholar] [CrossRef]
- Kumar, L.; Ramakanth, D.; Akhila, K.; Gaikwad, K.K. Edible films and coatings for food packaging applications: A review. Environ. Chem. Lett. 2021, 20, 875–900. [Google Scholar] [CrossRef]
- Andrade, R.D.; Skurtys, O.; Osorio, F.A. Atomizing spray systems for application of edible coatings. Compr. Rev. Food Sci. Food Saf. 2012, 11, 323–337. [Google Scholar] [CrossRef]
- Silva-Vera, W.; Zamorano-Riquelme, M.; Rocco-Orellana, C.; Vega-Viveros, R.; Gimenez-Castillo, B.; Silva-Weiss, A.; Osorio-Lira, F. Study of spray system applications of edible coating suspensions based on hydrocolloids containing cellulose nanofibers on grape surface (Vitis vinifera L.). Food Bioprocess Technol. 2018, 11, 1575–1585. [Google Scholar] [CrossRef]
- Dai, S.; Zhang, J.; Jia, W.; Ou, M.; Zhou, H.; Dong, X.; Chen, H.; Wang, M.; Chen, Y.; Yang, S. Experimental study on the droplet size and charge-to-mass ratio of an air-assisted electrostatic nozzle. Agriculture 2022, 12, 889. [Google Scholar] [CrossRef]
- Law, S.E.; Cooper, S.C. Air-assisted electrostatic sprays for postharvest control of fruit and vegetable spoilage microorganisms. IEEE Trans. Ind. Appl. 2001, 37, 1597–1602. [Google Scholar] [CrossRef]
- Zhang, F.; Zirwes, T.; Müller, T.; Wachter, S.; Jakobs, T.; Habisreuther, P.; Zarzalis, N.; Trimis, D.; Kolb, T. Effect of elevated pressure on air-assisted primary atomization of coaxial liquid jets: Basic research for entrained flow gasification. Renew. Sustain. Energy Rev. 2020, 134, 110411. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, H.; Chen, C.; Xiang, Q.J. Calculation and verification of formula for the range of sprinklers based on jet breakup length. Int. J. Agric. Biol. Eng. 2018, 11, 49–57. [Google Scholar] [CrossRef]
- Rahman, M.A.; Heidrick, T.; Fleck, B.A. Correlations between the two-phase gas/liquid spray atomization and the Stokes/aerodynamic Weber numbers. J. Phys. Conf. Ser. 2009, 147, 012057. [Google Scholar] [CrossRef]
- Yue, J.; Chao, C.; Hong, L.; Xiang, Q.J. Influences of nozzle parameters and low-pressure on jet breakup and droplet characteristics. Int. J. Agric. Biol. Eng. 2016, 9, 22–32. [Google Scholar] [CrossRef]
- Hussain, Z.; Liu, J.P.; Chauhdary, J.N.; Zhao, Y.Y. Evaluating the effect of operating pressure, nozzle size and mounting height on droplet characteristics of rotating spray plate sprinkler. Irrig. Sci. 2024, 1–16. [Google Scholar] [CrossRef]
- Liao, J.; Luo, X.W.; Wang, P.; Zhou, Z.Y.; O’Donnell, C.C.; Zang, Y.; Hewitt, A.J. Analysis of the influence of different parameters on droplet characteristics and droplet size classification categories for air induction nozzle. Agronomy 2020, 10, 256. [Google Scholar] [CrossRef]
- Lin, D.; Zhao, Y. Innovations in the development and application of edible coatings for fresh and minimally processed fruits and vegetables. Compr. Rev. Food Sci. Food Saf. 2007, 6, 60–75. [Google Scholar] [CrossRef]
- Bravin, B.; Peressini, D.; Sensidoni, A. Development and application of polysaccharide–lipid edible coating to extend shelf-life of dry bakery products. J. Food Eng. 2006, 76, 280–290. [Google Scholar] [CrossRef]
- Yakoubi, S.; Kobayashi, I.; Uemura, K.; Tounsi, M.S.; Nakajima, M.; Hiroko, I.; Neves, M.A. Enhancing plantain epicarp active edible coating performance through investigation of optimal spray coating conditions. Colloids Surf. A Physicochem. Eng. Asp. 2023, 678, 132474. [Google Scholar] [CrossRef]
- Gui, X.; Shang, B.; Yu, Y. Applications of electrostatic spray technology in food preservation. LWT 2023, 190, 115568. [Google Scholar] [CrossRef]
- Guo, J.; Dong, X.; Qiu, B. Analysis of the factors affecting the deposition coverage of air-assisted electrostatic spray on tomato leaves. Agronomy 2024, 14, 1108. [Google Scholar] [CrossRef]
- Xue, Y.; Liao, Y.; Wang, H.; Li, S.; Gu, Z.; Adu-Frimpong, M.; Yu, J.; Xu, X.; Smyth, H.D.C.; Zhu, Y. Preparation and evaluation of astaxanthin-loaded 2-hydroxypropyl-beta-cyclodextrin and Soluplus® nanoparticles based on electrospray technology. J. Sci. Food Agric. 2023, 103, 3628–3637. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, X.; Huang, X.; Li, Z.; Zhang, X.; Zou, X.; Shi, J. Effect of different coating methods on coating quality and mango preservation. Food Packag. Shelf Life 2023, 39, 101133. [Google Scholar] [CrossRef]
- Njie, A.; Dong, X.; Liu, Q.; Lu, C.; Pan, X.; Zhang, W. Melatonin treatment inhibits mango fruit (Cv. ‘Guiqi’) softening by maintaining cell wall and reactive oxygen metabolisms during cold storage. Postharvest Biol. Technol. 2023, 205, 112500. [Google Scholar] [CrossRef]
- Lin, L.; Zhu, Y.; Cui, H. Electrospun thyme essential oil/gelatin nanofibers for active packaging against Campylobacter jejuni in chicken. LWT 2018, 97, 711–718. [Google Scholar] [CrossRef]
- Nawaz, A.; Irshad, S.; Walayat, N.; Khan, M.R.; Iqbal, M.W.; Luo, X. Fabrication and characterization of apple-pectin–pva-based nanofibers for improved viability of probiotics. Foods 2023, 12, 3194. [Google Scholar] [CrossRef]
- Cui, H.; Bai, M.; Rashed, M.M.A.; Lin, L. The antibacterial activity of clove oil/chitosan nanoparticles embedded gelatin nanofibers against Escherichia coli O157:H7 biofilms on cucumber. Int. J. Food Microbiol. 2018, 266, 69–78. [Google Scholar] [CrossRef]
- Joshi, M.; Aayush, K.; Sharma, K.; Bose, I.; Khan, A.A.; Atanassova, M.; Yang, T.; Murariu, O.C.; Sharma, S.; Caruso, G. Fiber and nanofiber based edible packaging for enhancing the shelf life of food: A review. Food Biosci. 2024, 59, 103970. [Google Scholar] [CrossRef]
- Shen, C.; Yang, X.; Wang, D.; Li, J.; Zhu, C.; Wu, D.; Chen, K. Carboxymethyl chitosan and polycaprolactone-based rapid in-situ packaging for fruit preservation by solution blow spinning. Carbohydr. Polym. 2024, 326, 121636. [Google Scholar] [CrossRef]
- Ranjan, S.; Chandrasekaran, R.; Paliyath, G.; Lim, L.-T.; Subramanian, J. Effect of hexanal loaded electrospun fiber in fruit packaging to enhance the post harvest quality of peach. Food Packag. Shelf Life 2020, 23, 100447. [Google Scholar] [CrossRef]
- Yilmaz, A.; Bozkurt, F.; Cicek, P.K.; Dertli, E.; Durak, M.Z.; Yilmaz, M.T. A novel antifungal surface-coating application to limit postharvest decay on coated apples: Molecular, thermal and morphological properties of electrospun zein–nanofiber mats loaded with curcumin. Innov. Food Sci. Emerg. Technol. 2016, 37, 74–83. [Google Scholar] [CrossRef]
- Sethunga, M.; Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S.; Munaweera, I. Antimicrobial and antioxidative electrospun cellulose acetate-essential oils nanofibrous membranes for active food packaging to extend the shelf life of perishable fruits. Innov. Food Sci. Emerg. Technol. 2024, 97, 103802. [Google Scholar] [CrossRef]
- de Barros, H.E.A.; Natarelli, C.V.L.; Santos, I.A.; Soares, L.S.; Nunes Carvalho, E.E.; de Oliveira, J.E.; Franco, M.; Vilas Boas, E.V.d.B. Development of poly(vinyl alcohol) nanofibers incorporated with aqueous plant extracts by solution blow spinning and their application as strawberry coatings. J. Food Eng. 2024, 363, 111761. [Google Scholar] [CrossRef]
- Lin, L.; Chen, X.; Hong, W.; Zhang, D.; Wen, X.; Bu, N.; Wen, C.; Mu, R.; Wang, L.; Pang, J. A rapid preparation strategy of konjac glucomannan-based fiber film incorporated with elderberry anthocyanins via microfluidic blow spinning for fresh-cut apple preservation. Int. J. Biol. Macromol. 2025, 299, 140122. [Google Scholar] [CrossRef]
- Guo, X.; Wu, M.; Zou, S.; Shi, X.; Chaiwong, S.; Wu, D.; Li, X.; Chen, K. In situ preparation of natamycin and trans-cinnamic acid loaded polycaprolactone/ethyl cellulose nanofibers on mangoes via handheld microfluidic-blow-spinning for freshness preservation. Food Packag. Shelf Life 2025, 48, 101448. [Google Scholar] [CrossRef]
- Salah, M.; Huang, J.; Zhu, C.; Sobhy, M.; Farag, M.A.; Fang, Y.; Sobhy, R.; Walayat, N.; Khalifa, I.; Maqsood, S.; et al. Chitosan dual gel-like functionalized with flavonoid extract and cinnamaldehyde oil using dual cross-linking agents: Characterization, antioxidant, and antimicrobial effects. Curr. Res. Food Sci. 2024, 9, 100826. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Wang, P.; Zhang, H. Electrospinning of nanofibers: Potentials and perspectives for active food packaging. Compr. Rev. Food Sci. Food Saf. 2020, 19, 479–502. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, X.; Zhang, M.; Ge, M.; Wang, J.; Tang, Y.; Zhang, Y.; Mi, J.; Cai, W.; Lai, Y.; et al. Rational design of electrospun nanofibers for gas purification: Principles, opportunities, and challenges. Chem. Eng. J. 2022, 446, 137099. [Google Scholar] [CrossRef]
- Zhang, Y.; Min, T.; Zhao, Y.; Cheng, C.; Yin, H.; Yue, J. The developments and trends of electrospinning active food packaging: A review and bibliometrics analysis. Food Control 2024, 160, 110291. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, M.; Xu, B.; Guo, Z. Fresh-cut orange preservation based on nano-zinc oxide combined with pressurized argon treatment. LWT 2021, 135, 110036. [Google Scholar] [CrossRef]
- Lin, L.; Mao, X.; Sun, Y.; Rajivgandhi, G.; Cui, H. Antibacterial properties of nanofibers containing chrysanthemum essential oil and their application as beef packaging. Int. J. Food Microbiol. 2019, 292, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Surendhiran, D.; Li, C.; Cui, H.; Lin, L. Fabrication of high stability active nanofibers encapsulated with pomegranate peel extract using chitosan/PEO for meat preservation. Food Packag. Shelf Life 2020, 23, 100439. [Google Scholar] [CrossRef]
- Lin, L.; Xue, L.; Duraiarasan, S.; Haiying, C. Preparation of ε-polylysine/chitosan nanofibers for food packaging against Salmonella on chicken. Food Packag. Shelf Life 2018, 17, 134–141. [Google Scholar] [CrossRef]
- Guan, Y.; Zhang, J.; Zhang, J.; Song, W.; Shi, J.; Huang, X.; Zhai, X.; Zhang, D.; Li, Z.; Zou, X. Preparation of active film based on cinnamon essential oil into β-cyclodextrin with high hydrophobic and its preservation for griskin. Food Control 2024, 160, 110344. [Google Scholar] [CrossRef]
- Dai, J.; Bai, M.; Li, C.; Cui, H.; Lin, L. The improvement of sodium dodecyl sulfate on the electrospinning of gelatin O/W emulsions for production of core-shell nanofibers. Food Hydrocoll. 2023, 145, 109092. [Google Scholar] [CrossRef]
- Zhang, J.; Qiu, C.; Wang, L.; Chen, R.; Ding, J.; Zhang, J.; Wan, H.; Guan, G. Inducing Cu2+ species to SrTiO3 nanofibers based on blend electrospinning for boosting CO2 photoreduction to CH3OH. Ceram. Int. 2024, 50, 39374–39381. [Google Scholar] [CrossRef]
- Isik, B.S.; Altay, F.; Capanoglu, E. The uniaxial and coaxial encapsulations of sour cherry (Prunus cerasus L.) concentrate by electrospinning and their in vitro bioaccessibility. Food Chem. 2018, 265, 260–273. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, J.; Yu, G.; Cardenas, R.; Wei, S.; Wujcik, E.K.; Guo, Z. Coaxial electrospun fibers: Applications in drug delivery and tissue engineering. WIREs Nanomed. Nanobiotechnol. 2016, 8, 654–677. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, H.; Li, S.; Xue, Y.; Chen, Y.; Adu-Frimpong, M.; Xu, Y.; Yu, J.; Xu, X.; Smyth, H.D.C.; et al. Preparation of astaxanthin-loaded composite micelles with coaxial electrospray technology for enhanced oral bioavailability and improved antioxidation capability. J. Sci. Food Agric. 2024, 104, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, K.; Kamilah, H.; Karim, A.A.; Ariffin, F. Enhancing the functional properties of fish gelatin mats by dual encapsulation of essential oils in β-cyclodextrins/fish gelatin matrix via coaxial electrospinning. Food Hydrocoll. 2023, 137, 108324. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Zhu, Z.; Jiao, X.; Shang, Y.; Wen, Y. Encapsulation of thymol in biodegradable nanofiber via coaxial eletrospinning and applications in fruit preservation. J. Agric. Food Chem. 2019, 67, 1736–1741. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Fang, H.; Li, C.; Dai, J.; Alharbi, M.; Cui, H. Advancing gelatin/cinnamaldehyde O/W emulsions electrospinability: Role of soybean lecithin in core-shell nanofiber fabrication. Food Chem. 2024, 449, 139305. [Google Scholar] [CrossRef]
- Al-Maqtari, Q.A.; Ghaleb, A.D.S.; Mahdi, A.A.; Al-Ansi, W.; Noman, A.E.; Wei, M.; Al-Adeeb, A.; Yao, W. Stabilization of water-in-oil emulsion of Pulicaria jaubertii extract by ultrasonication: Fabrication, characterization, and storage stability. Food Chem. 2021, 350, 129249. [Google Scholar] [CrossRef]
- Cui, Z.; Ren, G.; Li, D.; Lu, D.; Zheng, X.; Zhao, Y.; Zhang, Y.; Hu, S.; Sun, W.; Yu, H.; et al. Novel humidity-responsive Nanofiber film based on emulsion electrospinning for fruit preservation. J. Appl. Polym. Sci. 2025, 142, e56787. [Google Scholar] [CrossRef]
- Valizadeh, A.; Mussa Farkhani, S. Electrospinning and electrospun nanofibres. IET Nanobiotechnol. 2014, 8, 83–92. [Google Scholar] [CrossRef]
- Yao, Z.-C.; Chang, M.-W.; Ahmad, Z.; Li, J.-S. Encapsulation of rose hip seed oil into fibrous zein films for ambient and on demand food preservation via coaxial electrospinning. J. Food Eng. 2016, 191, 115–123. [Google Scholar] [CrossRef]
- Benavides, R.E.; Jana, S.C.; Reneker, D.H. Nanofibers from scalable gas jet process. ACS Macro Lett. 2012, 1, 1032–1036. [Google Scholar] [CrossRef]
- Rodrigues, M.Á.V.; Bertolo, M.R.V.; Horn, M.M.; Lugão, A.B.; Mattoso, L.H.C.; de Guzzi Plepis, A.M. Comparing solution blow spinning and electrospinning methods to produce collagen and gelatin ultrathin fibers: A review. Int. J. Biol. Macromol. 2024, 283, 137806. [Google Scholar] [CrossRef]
- Alvarenga, A.D.; Correa, D.S. Composite nanofibers membranes produced by solution blow spinning modified with CO2-activated sugarcane bagasse fly ash for efficient removal of water pollutants. J. Clean. Prod. 2021, 285, 125376. [Google Scholar] [CrossRef]
- Shen, C.; Wu, M.; Sun, C.; Li, J.; Wu, D.; Sun, C.; He, Y.; Chen, K. Chitosan/PCL nanofibrous films developed by SBS to encapsulate thymol/HPβCD inclusion complexes for fruit packaging. Carbohydr. Polym. 2022, 286, 119267. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.J.; Soares Filho, J.E.; Simões, T.A.; Oliveira, J.E.; Medeiros, E.S. Experimental investigation of solution blow spinning nozzle geometry and processing parameters on fiber morphology. ACS Appl. Polym. Mater. 2024, 6, 9735–9743. [Google Scholar] [CrossRef]
- Bonan, R.F.; Bonan, P.R.F.; Batista, A.U.D.; Perez, D.E.C.; Castellano, L.R.C.; Oliveira, J.E.; Medeiros, E.S. Poly(lactic acid)/poly(vinyl pyrrolidone) membranes produced by solution blow spinning: Structure, thermal, spectroscopic, and microbial barrier properties. J. Appl. Polym. Sci. 2017, 134, 44802. [Google Scholar] [CrossRef]
- da Silva Parize, D.D.; de Oliveira, J.E.; Foschini, M.M.; Marconcini, J.M.; Mattoso, L.H.C. Poly(lactic acid) fibers obtained by solution blow spinning: Effect of a greener solvent on the fiber diameter. J. Appl. Polym. Sci. 2016, 133, 43379. [Google Scholar] [CrossRef]
- Abdal-Hay, A.; Abdelrazek Khalil, K.; Al-Jassir, F.F.; Gamal-Eldeen, A.M. Biocompatibility properties of polyamide 6/PCL blends composite textile scaffold using EA.hy926 human endothelial cells. Biomed. Mater. 2017, 12, 035002. [Google Scholar] [CrossRef]
- Liu, J.P.; Liu, X.F.; Zhu, X.Y.; Yuan, S.Q. Droplet characterisation of a complete fluidic sprinkler with different nozzle dimensions. Biosyst. Eng. 2016, 148, 90–100. [Google Scholar] [CrossRef]
- Dias, F.T.G.; Rempel, S.P.; Agnol, L.D.; Bianchi, O. The main blow spun polymer systems: Processing conditions and applications. J. Polym. Res. 2020, 27, 205. [Google Scholar] [CrossRef]
- Jun, Y.; Kang, E.; Chae, S.; Lee, S.-H. Microfluidic spinning of micro- and nano-scale fibers for tissue engineering. Lab Chip 2014, 14, 2145–2160. [Google Scholar] [CrossRef]
- Li, R.; Feng, Y.; Zhang, H.; Liu, J.; Wang, J. Recent advances in fabricating, characterizing, and applying food-derived fibers using microfluidic spinning technology. Food Hydrocoll. 2023, 144, 108947. [Google Scholar] [CrossRef]
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.B.; Hanson, R.L.; Almughamsi, H.M.; Pang, C.; Fish, T.R.; Woolley, A.T. Microfluidics: Innovations in materials and their fabrication and functionalization. Anal. Chem. 2020, 92, 150–168. [Google Scholar] [CrossRef]
- He, T.; Ma, M.; Li, H.; Zhang, F.; Liu, F.; Liu, Z.; Li, X. Integrated wireless microfluidic liquid sensors based on low temperature co-fired ceramic (LTCC) technology. Sens. Actuators A Phys. 2022, 346, 113840. [Google Scholar] [CrossRef]
- Mu, R.; Bu, N.; Pang, J.; Wang, L.; Zhang, Y. Recent trends of microfluidics in food science and technology: Fabrications and applications. Foods 2022, 11, 3727. [Google Scholar] [CrossRef]
- Campbell, S.B.; Wu, Q.; Yazbeck, J.; Liu, C.; Okhovatian, S.; Radisic, M. Beyond polydimethylsiloxane: Alternative materials for fabrication of organ-on-a-chip devices and microphysiological systems. ACS Biomater. Sci. Eng. 2021, 7, 2880–2899. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Ciprioti, S.V. Characterization of hybrid materials prepared by sol-gel method for biomedical implementations. A critical review. Materials 2021, 14, 1788. [Google Scholar] [CrossRef]
- Tian, L.; Ma, J.; Li, W.; Zhang, X.; Gao, X.J.M.B. Microfiber fabricated via microfluidic spinning toward tissue engineering applications. Macromol. Biosci. 2023, 23, 2200429. [Google Scholar] [CrossRef] [PubMed]
- Tahir, H.E.; Zhihua, L.; Mahunu, G.K.; Xiaobo, Z.; Arslan, M.; Xiaowei, H.; Yang, Z.; Mariod, A.A. Effect of gum arabic edible coating incorporated with African baobab pulp extract on postharvest quality of cold stored blueberries. Food Sci. Biotechnol. 2020, 29, 217–226. [Google Scholar] [CrossRef]
- Li, C.Z.; Chen, W.Q.; Siva, S.; Cui, H.Y.; Lin, L. Electrospun phospholipid nanofibers encapsulated with cinnamaldehyde/HP-β-CD inclusion complex as a novel food packaging material. Food Packag. Shelf Life 2021, 28, 100647. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, Z.; Shi, J.; Zou, X.; Zhai, X.; Huang, X.; Li, Z.; Holmes, M.; Daglia, M.; Xiao, J. Physical properties and bioactivities of chitosan/gelatin-based films loaded with tannic acid and its application on the preservation of fresh-cut apples. LWT 2021, 144, 111223. [Google Scholar] [CrossRef]
- He, X.-H.; Wang, W.; Deng, K.; Xie, R.; Ju, X.-J.; Liu, Z.; Chu, L.-Y. Microfluidic fabrication of chitosan microfibers with controllable internals from tubular to peapod-like structures. RSC Adv. 2015, 5, 928–936. [Google Scholar] [CrossRef]
- Zhao, J.; Xiong, W.; Yu, N.; Yang, X. Continuous jetting of alginate microfiber in atmosphere based on a microfluidic chip. Micromachines 2017, 8, 8. [Google Scholar] [CrossRef]
- Yang, H.; Guo, M. Bioinspired polymeric helical and superhelical microfibers via microfluidic spinning. Macromol. Rapid Commun. 2019, 40, 1900111. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Tian, M.; Sun, B.; Qu, L.; Zhu, S.; Zhang, X. Hydrodynamic alignment and microfluidic spinning of strength-reinforced calcium alginate microfibers. Mater. Lett. 2018, 230, 148–151. [Google Scholar] [CrossRef]
- Du, X.Y.; Li, Q.; Wu, G.; Chen, S. Multifunctional micro/nanoscale fibers based on microfluidic spinning technology. Adv. Mater. 2019, 31, 1903733. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Avaro, J.; Hammer, T.; Hämmerle, L.; Silva, B.F.; Boesel, L.F.; Rossi, R.M.; Wei, K. Hydrogel-assisted microfluidic wet spinning of poly(lactic acid) fibers from a green and pro-crystallization spinning dope. Chem. Eng. J. 2024, 481, 148417. [Google Scholar] [CrossRef]
- Zhao, G.; Wu, T.; Wang, R.; Li, Z.; Yang, Q.; Wang, L.; Zhou, H.; Jin, B.; Liu, H.; Fang, Y.; et al. Hydrogel-assisted microfluidic spinning of stretchable fibers via fluidic and interfacial self-adaptations. Sci. Adv. 2023, 9, eadj5407. [Google Scholar] [CrossRef]
- Liu, J.D.; Du, X.Y.; Chen, S.J.A.C.I.E. A phase inversion-based microfluidic fabrication of helical microfibers towards versatile artificial abdominal skin. Angew. Chem. Int. Ed. 2021, 60, 25089–25096. [Google Scholar] [CrossRef]
- Ni, Y.; Liu, Y.; Zhang, W.; Shi, S.; Zhu, W.; Wang, R.; Zhang, L.; Chen, L.; Sun, J.; Pang, J.; et al. Advanced konjac glucomannan-based films in food packaging: Classification, preparation, formation mechanism and function. LWT 2021, 152, 112338. [Google Scholar] [CrossRef]
- Ma, K.; Du, X.-Y.; Zhang, Y.-W.; Chen, S. In situ fabrication of halide perovskite nanocrystals embedded in polymer composites via microfluidic spinning microreactors. J. Mater. Chem. C 2017, 5, 9398–9404. [Google Scholar] [CrossRef]
- Rashid, M.T.; Ma, H.; Safdar, B.; Jatoi, M.A.; Wali, A.; Sarpong, F.; Zhou, C.S. Synergy of ultrasound and osmotic dehydration in improving drying kinetics and quality of dried sweet potato (Ipomea batatas L.). J. Food Saf. Food Qual. -Arch. Fur Leb. 2019, 70, 72–81. [Google Scholar] [CrossRef]
- Song, Y.; Yu, X.Q.; Chen, S.J. Recent advances in microfluidic fiber-spinning chemistry. J. Polym. Sci. 2024, 62, 447–462. [Google Scholar] [CrossRef]
- Golecki, H.M.; Yuan, H.; Glavin, C.; Potter, B.; Badrossamay, M.R.; Goss, J.A.; Phillips, M.D.; Parker, K.K. Effect of solvent evaporation on fiber morphology in rotary jet spinning. Langmuir 2014, 30, 13369–13374. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.-A.; Wu, M.; Shen, C.; Yang, X.; Wang, D.; Li, J.; Wu, D.; Chen, K. Microfluidic-blow-spinning of carvacrol-loaded porphyrin metal—Organic framework nanofiber films with synergistic antibacterial capabilities for food packaging. Food Chem. 2024, 460, 140707. [Google Scholar] [CrossRef] [PubMed]
- Barhoum, A.; Pal, K.; Rahier, H.; Uludag, H.; Kim, I.S.; Bechelany, M. Nanofibers as new-generation materials: From spinning and nano-spinning fabrication techniques to emerging applications. Appl. Mater. Today 2019, 17, 1–35. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, J.; Su, Y.; Wang, H.; Wang, X.-X.; Huang, L.-P.; Yu, M.; Ramakrishna, S.; Long, Y.-Z. Recent progress and challenges in solution blow spinning. Mater. Horiz. 2021, 8, 426–446. [Google Scholar] [CrossRef]
- Wu, M.; Deng, Z.-A.; Shen, C.; Yang, Z.; Cai, Z.; Wu, D.; Chen, K. Fabrication of antimicrobial PCL/EC nanofibrous films containing natamycin and trans-cinnamic acid by microfluidic blow spinning for fruit preservation. Food Chem. 2024, 442, 138436. [Google Scholar] [CrossRef]
- Zhao, Y.-T.; Zhang, J.; Gao, Y.; Liu, X.-F.; Liu, J.-J.; Wang, X.-X.; Xiang, H.-F.; Long, Y.-Z. Self-powered portable melt electrospinning for in situ wound dressing. J. Nanobiotechnol. 2020, 18, 111. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, H.; Shen, Y.; Dai, X.; Wang, X.; Deng, K.; Long, X.; Liu, L.; Zhang, X.; Li, Y.J.; et al. Instant in-situ tissue repair by biodegradable PLA/Gelatin nanofibrous membrane using a 3D printed handheld electrospinning device. Front. Bioeng. Biotechnol. 2021, 9, 684105. [Google Scholar] [CrossRef]
- Haik, J.; Kornhaber, R.; Blal, B.; Harats, M. The Feasibility of a handheld electrospinning device for the application of nanofibrous wound dressings. Adv. Wound Care 2017, 6, 166–174. [Google Scholar] [CrossRef]
- Du, L.; Huang, X.; Li, Z.; Qin, Z.; Zhang, N.; Zhai, X.; Shi, J.; Zhang, J.; Shen, T.; Zhang, R.; et al. Application of smart packaging in fruit and vegetable preservation: A review. Foods 2025, 14, 447. [Google Scholar] [CrossRef] [PubMed]
| Object | Coating Method | Formulation | Coating Effects | References |
|---|---|---|---|---|
| Watermelon | Spraying | Sodium-alginate, pectin and calcium lactate | The shelf life of watermelon has been extended from 7 days to 12–15 days. | [42] |
| Orange | Spraying | Pea starch and guar gum | It is better than commercial wax in terms of extending shelf life (4 weeks refrigerated and 1 week on the shelf), maintaining organoleptic quality and inhibiting decay. | [43] |
| Banana | Spraying | Sucrose esters and rice starch | Effectively delayed ethylene biosynthesis and reduced respiration rate, extending the shelf life by 12 days compared to untreated controls. | [44] |
| Mango | Dipping | Bleached shellac, tannic acid, glycerol | TA-shellac extends shelf life by about 10 days compared to the control group, with significant improvement in browning inhibition, weight loss and flavor retention | [45] |
| Blueberry | Dipping | Gum Arabic, roselle flower extract, calcium chloride and glycerin | Gum Arabic coatings with roselle extract are more effective than plain ones in inhibiting microorganisms, reducing enzyme activity and anthocyanin degradation, increasing total phenolic content, and lowering the decay rate. | [46] |
| Tomato | Dipping | Mango kernel starch, Glycerin, sorbitol | The mango kernel starch coating delayed the ripening process of tomatoes up to 20 days during storage at 20 °C without negatively affecting post-harvest quality. | [47] |
| Longan | Dipping | 0.5%, 1.0%, and 2.0% chitosan | Chitosan coating treatment reduced the respiration rate and oxidase activity, and the increased chitosan concentration effectively prolonged the storage time and quality of longan. | [48] |
| Papaya | Dipping | 15%, 25%, and 50% aloe vera gel | Aloe vera coating effectively delays papaya ripening and extends shelf life, and can still be sold after 15 days of storage, and with better results than higher concentrations of aloe vera. | [49] |
| Lime | Dipping | Pectin, sorbitol, beeswax and monoglycerides | Compared with the control sample, the respiration rate of coated limes was inhibited, and fruit weight loss and firmness were reduced to a lower level. | [50] |
| Pear | Dipping | Chitosan, guar gum and lemon peel essential oil (1, 1.5, 2, 2.5, and 3.0%) | The guar gum and chitosan coating with lemon peel essential oil significantly reduces weight loss and improves firmness of pears when stored at 4 ± 2 °C for up to 45 days. In addition, the coating with 3% lemon peel essential oil had a higher overall acceptability. | [51] |
| Strawberry | Dipping | Lactobacillus lactis, Bacillus cinerea, chia seed mucus and gelatin | Lactobacillus lactis and kiwifruit mucilage improved the quality of strawberries after harvest, and the addition of 2–4% lactobacilli effectively improved the storage quality of strawberries. | [52] |
| Guava | Dipping | 0.5%, 1.0%, and 2.0% chitosan | The chitosan coating helped to retard the ripening process of guava fruits during cold storage with better quality retention at a 2% concentration compared to 0.5% and 1%. | [53] |
| Zucchini | Brushing | Whey protein concentrate, Arabic gum, guar gum, glycerol, thyme essential oil | Coatings containing guar and gum Arabic (S) are rheologically superior to Tween 20 (T) coatings; T coatings are superior in reducing weight loss, retaining hardness, and maintaining sensory characteristics, and are more effective in extending shelf life. | [54] |
| Coating Method | Advantages | Disadvantages |
|---|---|---|
| Dipping | 1. Can process large quantities of small fruits (such as blueberries and cherries) at a time, with high batch efficiency. 2. Can penetrate into the recesses of fruit stems, providing good coverage. 3. Only requires an open immersion tank, with simple operation and low cost. | 1. Gravity causes more coating liquid to accumulate at the bottom of the fruit, resulting in uneven coating thickness. 2. Open slot dip coating easily leads to the accumulation of impurities, requiring frequent replacement of the coating. 3. Not suitable for large fruits such as watermelons and cantaloupes, which have long dripping times and low drying efficiency. |
| Vacuum dipping | 1. In a vacuum environment, the coating can penetrate into micropores (such as the gaps between strawberry seeds), increasing the penetration area and enhancing the fresh-keeping effect. 2. Vacuum adsorption reduces dripping loss and minimizes coating waste. | 1. Vacuum systems are expensive. 2. Vacuum environments can easily cause soft fruit cells to rupture, posing a risk of damage. 3. Precision control of parameters such as vacuum level and pressure is required, making operation difficult. |
| Brushing | 1. No complicated equipment is required, and the cost is extremely low. 2. Local repairs (such as apple stem marks) can be made. | 1. Prone to brush marks, bubbles, uneven coating, and poor decorative properties. 2. Repeated use of brush bristles may cause cross-contamination. |
| Spraying | 1. The atomized spray provides comprehensive coverage and forms an even coating, suitable for smooth fruit surfaces. 2. Automated assembly line operation, fast speed, and adjustable spray heads for different fruits. 3. Good adaptability to curved and irregular surfaces. | 1. High atomization loss and high paint loss. 2. Requires equipment such as spray guns and air compressors, resulting in high equipment costs. 3. Not suitable for porous fruits such as strawberries and bayberries, as excessive coating thickness can cause anaerobic respiration and produce an ethanol odor. |
| Object | Coating Method | Formulation | Coating Effects | References |
|---|---|---|---|---|
| Peach | Electrospinning | Zein, ethanol, and polyethylene oxide | The shelf life of peaches was extended by 4 days, and the fiber prepared from glutaraldehyde, corn protein, and PEO in a 1:5:5 ratio had a better preservation effect. | [93] |
| Apple | Electrospinning | Zein, ethanol, and curcumin | At 23 °C and 75% humidity, after 6 days, the diameter of the green mold lesions on the coated apples was reduced by nearly 50% compared to the uncoated apples. | [94] |
| Grapes and tomatoes | Electrospinning | Cinnamon bark oil, clove bud oil, cellulose acetate, dimethyl formaldehyde, and acetone | Using cellulose acetate nanofiber membranes loaded with 50% cinnamon bark oil and clove bud oil, the shelf life of fresh grapes and tomatoes was extended to 30 days at 4 °C, with minimal deterioration in physical and chemical properties. | [95] |
| Strawberry | microfluidic blow spinning | Polyvinyl alcohol, aqueous extract of acai pulp, cocoa shell, jabuticaba peel, and carrot pomace | Compared with the control fruit, jabuticaba peel and PVA as strawberry coatings resulted in less color change, reduced degradation of antioxidant activity and TPCs, and a 50% reduction in the incidence of rotten fruit during storage. | [96] |
| Apple | microfluidic blow spinning | Konjac glucomannan polyvinylpyrrolidone, ethanol, and Elderberry anthocyanin | KEA/PVP membranes exhibit excellent antioxidant properties, with DPPH and ABTS radical scavenging rates of 74.69% and 96.18%, respectively. Compared to the control group, fresh-cut apples showed the best preservation effect. | [97] |
| Mango | handheld microfluidic-blow-spinning | Polycaprolactone, ethyl cellulose, 2,2,2-trifluoroethanol, natamycin, and trans-cinnamic acid | PCL/EC/Nt-p nanofiber membrane treatment resulted in the smallest diameter of mango lesions and a 20% lower decay index compared to the untreated group. After 9 days of storage, the decline in antioxidant enzyme activity was delayed. | [98] |
| Cherry tomatoes | solution blow spinning | 2,2,2-Trifluoroethanol, polycaprolactone, carboxymethyl chitosan, curcumin, thymol, Nisin, and natamycin | The film forms a barrier on the surface of cherry tomatoes, limiting water penetration, reducing fruit respiration, thereby reducing weight and hardness, and delaying the ripening of cherry tomatoes after harvest. | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, L.; Luo, D.; Li, C.; Chen, Y. Recent Advances in the Application Technologies of Surface Coatings for Fruits. Foods 2025, 14, 2471. https://doi.org/10.3390/foods14142471
Dai L, Luo D, Li C, Chen Y. Recent Advances in the Application Technologies of Surface Coatings for Fruits. Foods. 2025; 14(14):2471. https://doi.org/10.3390/foods14142471
Chicago/Turabian StyleDai, Limin, Dong Luo, Changwei Li, and Yuan Chen. 2025. "Recent Advances in the Application Technologies of Surface Coatings for Fruits" Foods 14, no. 14: 2471. https://doi.org/10.3390/foods14142471
APA StyleDai, L., Luo, D., Li, C., & Chen, Y. (2025). Recent Advances in the Application Technologies of Surface Coatings for Fruits. Foods, 14(14), 2471. https://doi.org/10.3390/foods14142471





