Abstract
Schefflera oleifera honey (SH) is produced from the nectar of S. Oleifera by worker bees. Due to its unique properties and potential biological activities, this winter honey has attracted much attention. In this study, the physicochemical characteristics, antioxidant and antibacterial activities, antitumor effect against HepG2 cells, and its potential mechanisms of SH were systematically evaluated. The results showed that different SH samples differed significantly in their physicochemical characteristics. The 910 chemical components, including 52 kinds of phenols, phenolic acids, and flavonoids, were detected in the methanol extract of SH using UHPLC-MS/MS by non-targeted metabolomics. Based on our limited knowledge, solanine and soyasaponin I are the first determined components in honey, and they may be used as characteristic substances of SH for identification and adulteration. SH had a weaker inhibitory effect against Salmonella typhimurium and Staphylococcus aureus than MH (UMF 10+), analyzed by MBC and MIC assays. Network pharmacology analysis showed that 95 overlapping targets were found between the active ingredients of SH and liver cancer cells (HepG2), which were enriched in KEGG of the PI3K-Akt pathway, Lipid and atherosclerosis, Proteoglycans in cancer, etc. The IC50 of SH against HepG2 cells was 5.07% (dw/v), which is lower than the glucose, fructose, and sucrose contents in SH on HepG2 cells, of 16.24%, 9.60% dw/v, and 9.94% dw/v, respectively. SH significantly down-regulated the expression of EGFR, AKT1, and SRC in HepG2 cells (p < 0.05), determined by an enzyme-linked immunosorbent assay kit, and induced cell cycle arrest and apoptosis by multiple pathways. These results provide a theoretical basis for its potential application in developing functional foods and additives.
1. Introduction
Honey is produced from nectar or secretions of plants by worker bees, of which the process includes foraging, transformation, deposition, dehydration, and storage. It contains about 200 components, including sugars, water, proteins, amino acids, vitamins, minerals, enzymes, and phenolic compounds [1,2]. Honey is widely used as a food additive and as a drug to treat diseases and wounds [3,4,5]. Its biological activities include anti-cancer [6,7,8,9], anti-inflammatory [10,11,12], antibacterial [13,14,15], antioxidant [16,17,18], antiviral [4], and prebiotic activities [3]. The biological activities of honey are affected by many factors.
The antioxidant activity of different honey samples varies depending on their botanical origin [19], geographical region [20], processing and storage conditions [21], and the method used to measure the antioxidant activity [22]. Methods used to measure antioxidant activity include 2,2′-azino-bis-3-ethylbenzothiazolin-6-sulphonic acid (ABTS) [22], 2,2-diphenyl-1-picrylhydrazyl free radical (DPPH) [23], and Ferric Ion Reducing Antioxidant Power (FRAP) [24]. Phenolic compounds are the main source of the antioxidant activity of honey [19,25,26,27]. The total flavonoid content, total water-soluble vitamins and minerals, and protein content of honey also contribute to the antioxidant activity [19,20,24]. The botanical source of honey is closely related to its antioxidant properties, which is one of the mechanisms of antibacterial activity.
The antimicrobial effects of honey were attributed primarily to its high sugar concentration, antioxidant activity, acidic components, and production of hydrogen peroxide [13,15,28,29]. High osmotic pressure inhibits microbial growth because sugar molecules bind to water molecules, leaving bacteria without sufficient water to survive [30,31,32]. The acidic components of honey (partly due to its gluconic acid) neutralize the alkaline environment of chronic wounds, thereby reducing protease activity, increasing fibroblast activity, and increasing oxygen release [33,34], all of which are beneficial to the wound healing process. Hydrogen peroxide and non-hydrogen peroxide compounds are produced by glucose oxidase added by bees during the maturation process of honey [15,28,29]. The hydrogen peroxide gradient attracts macrophages to the wound and releases vascular endothelial growth factors and angiogenic factors, which are essential for wound healing [35,36,37,38]. Honey provides a steady supply of hydrogen peroxide to exhibit antimicrobial and physiologically nontoxic effects [39] when the honey is diluted. Also, the high sugar content in honey attracts liquid and forms a protective layer that prevents microbial invasion and keeps the wound dry [40,41,42,43,44]. Schefflera oleifera honey (SH), characterized by its light amber color and slightly bitter taste, is a typical winter honey produced primarily in the Fujian, Guangxi, and Guangdong provinces of southern China [45]. Although studies have investigated the antioxidant activity of SH [46], its antimicrobial and anti-hepatocarcinogenic properties have not been fully investigated.
Hepatocellular carcinoma (HCC) is one of the most common liver cancers. Numerous factors, including infection with hepatitis C or hepatitis B virus, obesity, diabetes, and genetic and social risk factors (such as excessive alcohol consumption) increase the incidence of HCC [47]. Despite significant advances in the treatment of HCC, the quality of life of patients remains poor. This suggests that more effective treatments should be achieved. Honey has strong and specific cytotoxicity to tumor cells and no cytotoxicity for normal cells. Egyptian clover honey significantly reduced the number of viable human hepatocellular carcinoma (HepG2) cells and nitric oxide (NO) levels [48]. Based on these findings, reactive oxygen species (ROS) may play a key role in the survival of HepG2 cell lines. Moderate levels of reactive oxygen species contribute to cell growth, division, and proliferation. The IC50 values of manuka honey were 6.92 ± 0.005% and 18.62 ± 0.07% for HepG2 and Hep3B cells, respectively [49]. A combination of selected drugs (Cisplatin, cyclophosphamide, and 5-Fluorouracil) and Nigella sativa honey may be an effective chemo-preventive and therapeutic strategy for treating diethylnitrosamine (DEN) and CCl4-induced HCC [50]. As an emerging research method, network pharmacology provides a powerful tool for understanding drug formulations and predicting potential new drugs or disease targets based on disciplines such as systems biology, genomics, and proteomics [51].
This study aims to explore the function of winter honey, SH, and its physicochemical properties, antioxidant activities, and antibacterial activities, as well as predict its targets and signaling pathways for the inhibition of HepG2 cells using network pharmacology methods and validation experiments. The results can provide a scientific basis and theoretical support for the development and utilization of SH in food additives and functional foods.
2. Materials and Methods
2.1. Honey Samples, Bacteria and Cells
SH samples were harvested from different bee farms in Zhangzhou, Fujian Province, China, in January 2024. Manuka honey (MH, UMF 10+) was purchased from Comvita New Zealand Ltd., (Paengaroa, New Zealand) in August 2019 and mailed to our laboratory. These honey samples were stored in a 4 °C refrigerator until the experiments.
Escherichia coli and Staphylococcus aureus were purchased from the Guangdong Microbiological Culture Collection Center. Listeria monocytogenes was isolated from the Microbiological Laboratory of Fujian Agriculture and Forestry University. Salmonella typhimurium ATCC 14028 was purchased from Guangdong Huankai Microbiology Co., Ltd., Zhaoqing, China. The HepG2 (CL-0103) cell line was purchased from Wuhan Procell Life Science and Technology (Wuhan, China).
Chemicals not otherwise specified were purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China.
2.2. Determination of Physicochemical Properties of SH Samples
The contents of water, pH, glucose, fructose, and sucrose in SH samples were determined according to the methods of our earlier report [52]. The total phenolic and total flavonoid contents were determined according to previous methods [53]. SH (1.0 g, dw) was diluted with ultrapure water to an amount of 10.0 mL. SH aqueous solution (1.0 mL) was mixed with 1.0 mL of Folin–Ciocalteu color reagent for 3–5 min. Sodium carbonate solution (20%, 3 mL) was diluted to 10 mL and mixed well. After being held at room temperature in the dark for 1 h, ultrapure water was selected as a blank control to measure the absorbance at 760 nm. Using gallic acid (10–100 mg/mL) as the standard, the results were expressed in gallic acid equivalents as mg GAE/100 g. SH aqueous solution (1.0 mL) was mixed with 0.5 mL of 5% sodium nitrite. The mixture was shaken well and stilled for 6 min. Aluminum nitrate (10%, 0.5 mL) was added and mixed for 6 min. Then, 4 mL of 4% sodium hydroxide was added and the mixture was shaken well and diluted to 10 mL with 80% methanol. It was mixed for 15 min. The absorbance at 510 nm was measured using enzyme-linked immunosorbent assay (ELISA) by a microplate reader (1510, Thermo Fisher Scientific, Waltham, MA, USA) using 80% methanol as a blank control. Rutin solution (10–100 mg/mL) was the standard solution; the results are expressed in rutin equivalents as mg RE/100 g. The data are shown based on the dry weight of each SH sample.
The methanol compositions of SH were determined using UHPLC-MS/MS by non-targeted metabolomics at Novogene Co., Ltd. (Beijing, China) [53]. Honey samples (100 μL) were placed in EP tubes and suspended in pre-cooled 80% methanol (Shanghai MacLean Biochemical Technology Co., Ltd., Shanghai, China) by vortexing. Then, the samples were incubated on ice for 5 min and centrifuged at 15,000× g and 4 °C for 20 min. The supernatant was diluted with LC-MS grade water (Merck, Darmstadt, Germany) to a final concentration of 53% methanol and then centrifuged at 15,000× g and 4 °C for 20 min. The supernatant was injected into the LC-MS/MS system for analysis. UHPLC-MS/MS analysis was performed using a Vanquish UHPLC system (Thermo Fisher Scientific Inc., Dreieich, Germany) coupled with a Q Exactive™ HF/Q Exactive™ HF-X mass spectrometer (Thermo Fisher, Germany). The sample was injected into a Hypersil Gold column (100 mm 2.1 mm, 1.9 μm) at a flow rate of 0.2 mL/min with a 17 min linear gradient. The eluents in the positive polarity mode were eluent A (0.1% formic acid in water) and eluent B (methanol). The eluents in the negative polarity mode were eluent A (5 M ammonium acetate, pH 9.0) and eluent B (methanol). The solvent gradient was set as follows: 2% mobile phase for 1.5 min, mobile phase A 2–85% for 3 min, mobile phase B 85–100% for 10 min, mobile phase B 2% for 10.1 min, and mobile phase B 2% for 12 min. The Q Exactive™ HF/Q Exactive™ HF-X mass spectrometer (Thermo Fisher Scientific Inc., Dreieich, Germany) was operated in positive/negative polarity mode with a spray voltage of 3.5 kV, a capillary temperature of 320 °C, a sheath gas flow rate of 35 psi, an auxiliary gas flow rate of 10 L/min, an iontophoresis RF level of 60, and an auxiliary gas heater temperature of 350 °C. The raw data files were acquired for further analysis.
2.3. Determination of Antioxidant Activity of SH
The DPPH free radical scavenging activity and FRAP total antioxidant capacity of the SH and MH samples were measured at 517 nm and 593 nm, respectively, using an ultraviolet spectrophotometer (T6, Beijing Puxi General Instrument Co., Ltd., Beijing, China) according to previous methods [54]. The ABTS cation radical scavenging activities of SH and MH samples were determined at 734 nm [55]. The results were expressed as equivalents to Trolox (mg TE/100 g, dw), which was employed as the standard.
All antioxidant activities were expressed as the dry weight (dw) of SH.
2.4. Determination of Antibacterial Activity of SH
SH and MH were mixed with LB broth medium (Sangon Biotech (Shanghai) Co., Ltd., Shanghai, China) and prepared in different concentrations of 0, 5%, 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, and 50% (dry weight/volume of mixed liquid). These mediums were used to determine the antibacterial activity of SH and MH against E. coli, S. aureus, L. monocytogenes, and S. typhimurium.
2.4.1. Determination of Minimum Inhibitory Concentration
Determination of the minimum inhibitory concentration (MIC) was performed according to a previous method [56]. The bacterial suspension was adjusted to 1 × 108 CFU/mL with saline. The suspension (10 μL) was added into a 96-well plate. Then 190 μL of culture medium (broth medium containing SH or MH) was added and mixed thoroughly. It was incubated in a shaking incubator (HZQ-F100, Taicang Experimental Equipment Factory, Suzhou, China) at 37 °C and 80 rpm for 24 h. The mixed liquid was filtered with a 0.22 μm filter membrane (Biosharp Beijing Labgic Technology Co., Ltd., Beijing, China) for further determination of OD600 using an ELISA reader (1510, Thermo Fisher, Waltham, MA, USA).
2.4.2. Determination of Minimum Bactericidal Concentration
The culture medium (200 μL) in the 96-well plate was transferred to LB solid medium (Sangon Biotech (Shanghai) Co., Ltd., China) and cultured at 37 °C for 24 h. The growth of colonies in the solid medium was observed. The lowest honey concentration at which no colonies was recorded as the MBC [56].
2.5. The Mechanism of SH Against HepG2 Cells Based on Network Pharmacology
2.5.1. Retrieve the Targets of HepG2 and the Main Components of SH
The targets of the main components of SH were obtained from the EMBL-EBI (https://www.ebi.ac.uk/chembl/, accessed on 10 February 2025) and SEA Search Server (https://sea.bkslab.org/, accessed on 10 February 2025). The keyword-related targets were searched according to the keyword “Human hepatocellular carcinomas” in the “Genecard” database (https://www.genecards.org/, accessed on 10 February 2025). Effective targets with a score greater than 20 were screened. The target-related information was proofed in the UniProt database (https://www.uniprot.org/, accessed on 10 February 2025).
2.5.2. Retrieve the Interaction Targets and Bioinformatics Analysis
The overlapping targets of the main SH components and HepG2 cells were screened. The GO (p < 0.05) enrichment and KEGG (p < 0.05) pathway enrichment analyses were performed using the clusterProfiler package (http://bioconductor.org/packages/release/bioc/html/clusterProfiler.html, downloaded on 10 February 2025) in R (v4.4, https://cloud.r-project.org/, accessed on 10 February 2025). The protein–protein interaction (PPI) network diagram with a confidence level ≥ 0.7 among the overlapping targets was obtained through the STRING database (string-db.org, accessed on 10 February 2025) and Cytoscape software (version 3.10.2; JAVA: 17.0.5, https://cytoscape.org/download.html, accessed on 15 February 2025).
2.5.3. Experimental Verification
HepG2 cells were cultured with a complete culture medium (Wuhan Procell Life Science and Technology, China) in a constant temperature incubator (C150, Binder, Tuttlingen, Germany) at 37 °C and a CO2 concentration of 5%. The logarithmic growth phase HepG2 cells were processed with trypsin (Thermo Fisher Scientific Co., Ltd., Shanghai, China) and cultured in plates for subsequent experiments.
HepG2 cells (1 × 105 cells/well) were transfered into wells of a six-well plate. After the cells adhered to the wall, the culture medium was removed. The cells were rinsed 3 times with PBS (pH 7.2–7.4, Sinopharm Chemical Reagent Co., Ltd., Shanghai, China). Then, 2%, 4%, 8%, and 16% SH (final concentration, dw/v) in the culture medium and complete culture medium (blank control) were added to each group. The cells were cultured for 48 h. These experiments were repeated in triplicate with 6 replicate wells for each group.
Determination of SRC, EGFR, and AKT1 Contents of HepG2 Cells
The contents of SRC, EGFR, and AKT1 in cell culture supernatant, which was obtained after centrifuging at 1000× g for 20 min, were determined using the enzyme-linked immunosorbent assay kit (Shanghai ELISA Biotechnology Co., Ltd., Shanghai, China) [57]. The different concentrations of standard solution and samples (50 μL) were mixed with 100 μL of a horseradish peroxidase (HRP)-labeled detection antibody. The reaction wells were sealed with a sealing film and incubated at 37 °C in a water bath for 60 min. The cells were patted dry using absorbent paper after the medium was removed. A washing solution (350 uL) was added and stilled for 1 min. The washing solution was removed and the cells were patted dry with absorbent paper again. This washing and drying procedure was repeated 5 times. Substrates A and B (each for 50 μL) were added to each well and incubated at 37 °C in the dark for 15 min. A stop solution (50 μL) was added to stop the reaction. The OD values of the wells at a wavelength of 450 nm were determined within 15 min using a microplate reader. Nothing was added to the blank control well. The blank well was set as 0 to draw a linear regression curve of the standard, in which the horizontal and vertical axes were the standard concentrations and the corresponding OD value, respectively. The concentrations of each sample were obtained according to the regression equation.
Antiproliferation Effect of SH5 and Saccharides on HepG2 Cells
The honey concentration was diluted to 2%, 4%, 8%, and 16% (dry weight/volume of mixed solution). Glucose, fructose, and sucrose stock solutions were prepared according to their contents of SH5, which has relatively excellent antioxidant and antibacterial activity and total phenolic and flavonoid contents among all samples. The glucose stock solution is equal to the glucose content plus the sucrose content, while the fructose stock solution is equal to the fructose content plus the sucrose content. The sucrose stock solution is equal to the glucose content plus half of the fructose content plus the sucrose content. The saccharide concentrations were set to 2%, 4%, 8%, 16%, and 32% (dw/v).
HepG2 cells at a concentration of 5 × 104 cells/mL (100 μL) in a 96-well plate were incubated at 37 °C and 5% CO2 for 24 h. Cells were washed with PBS after the culture medium was removed. Complete culture mediums with different concentrations of SH or saccharide and complete culture medium (the control group) were added and incubated at 37 °C and 5% CO2 for 48 h. The wells with no cells were selected as the blank group. After 48 h, the cells were rinsed with PBS and 110 μL of complete culture medium containing 10 μL of CCK-8 solution was added (Beijing Solebao Technology Co., Ltd., Beijing, China), and the mixture was placed in the dark. The absorbance of each well at 450 nm was determined using the ELISA reader after 2 h of incubation [58]. There were 6 replicate wells in each group. The proliferation inhibition rate (%) was calculated as [(Ac − As)/(Ac − Ab)] × 100%, where As was the experimental group (cells, culture medium containing honey or saccharide), Ac was the control group (cells, culture medium containing CCK-8), and Ab was the blank group (no cells, culture medium containing CCK-8).
2.6. Data Analysis
All experiments were performed in triplicate, and the results were expressed as mean ± standard error (SEM). Analysis of significant differences in the results was performed using the one-way ANOVA analysis function in GraphPad Prism 10.4.0 (p < 0.05 means the difference is significant). The correlation analyses among TPC, TFC, DPPH, ABTS, and FRAP were performed using the Pearson correlation function in GraphPad Prism 10.4.0. The structures of solanine and soyasaponin I were drawn on the MolView website (v2.4, https://molview.org/, accessed on 25 June 2025).
3. Results
3.1. Physicochemical Properties of SH Samples
3.1.1. Physicochemical Parameters of SH Samples
The physicochemical parameters of the SH samples are shown in Table 1. The water content, pH, glucose, fructose, sucrose, total phenols, and total flavonoid contents in the samples are significantly different.

Table 1.
Physicochemical parameters of SH samples.
3.1.2. Chemical Composition of Methanol Extract of SH
A total of 910 chemical components were detected in the methanol extract of SH by non-targeted metabolomics. The negative and positive ion spectra of the QC and methanol extract of the SH samples are presented in Figures S1–S8. There are two unique components of SH, solanine and soyasaponin I (Table 2 and Figure 1). There are 52 phenol, phenolic acid, and flavonoid components (Table 3). The other components are shown in Table S1.

Table 2.
The unique components determined in methanol extract of SH by non-targeted metabolomics.
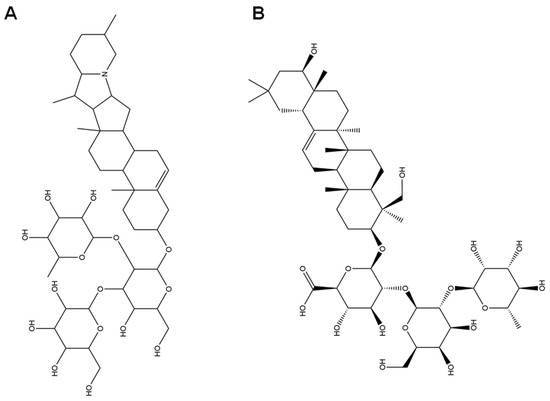
Figure 1.
Structures of solanine (A) and soyasaponin I (B).

Table 3.
Phenolics, phenolic acids and flavonoids determined in methanol extract of SH by non-targeted metabolomics (phenols: No 1–22, phenolic acids: No 23–27, flavonoids: No 28–52).
3.2. Antioxidant Activity of SH Samples
The antioxidant activity of the SH samples is shown in Figure 2. The correlations among TPC, TFC, DPPH, ABTS, and FRAP are presented in Table 4. The DPPH•, ABTS•+, and FRAP scavenging activities of these samples are significantly different.
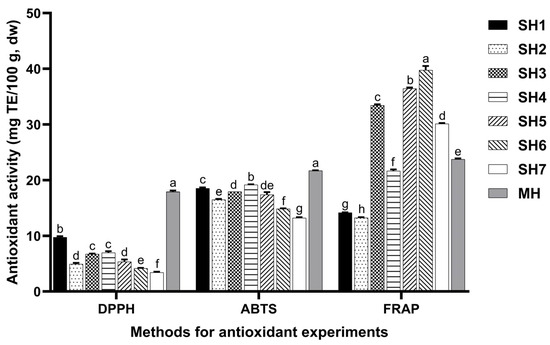
Figure 2.
Antioxidant activity of SH samples. Different lowercase letters mean significant differences among antioxidant activity of SH samples determined by the same method (p < 0.05). MH, SH, DPPH, ABTS, and FRAP mean manuka honey (UMF 10+), Schefflera oleifera honey, DPPH free radical scavenging activity, ABTS cation radical scavenging activity, and FRAP total antioxidant capacity, respectively.

Table 4.
The correlations among TPC, TFC, DPPH, ABTS, and FRAP.
3.3. Antibacterial Activity of SH Samples
As shown in Table 5, the MIC of SH samples against E. coli ranged from 30% to 40% and the MBC ranged from 30% to 40%; the MIC against S. aureus ranged from 30% to 40% and the MBC ranged from 35% to 45%; the MIC against L. monocytogenes ranged from 25% to 35% and the MBC ranged from 30% to 40%; and the MIC against S. typhimurium ranged from 15% to 20% and the MBC ranged from 20% to 30%. The antibacterial activity of SH is weaker than MH (UMF 10+).

Table 5.
Antibacterial activity of SH samples against four foodborne pathogens.
3.4. Overlapping Targets of Main Components of SH and HepG2 Cells
There were 545 targets of the main components of SH (phenols, flavonoids, saponins, triterpenes, and monoterpenes) collected through the SEA SearchEMBL-EBI. For HepG2, there were 895 targets. A Venn diagram of these targets was drawn (Figure 3). There were 95 overlapping targets between the main components of SH and HepG2: EGFR, AKT1, SRC, IL1B, ESR1, MMP9, TLR4, NFKB1, ALB, EP300, PIK3R1, RELA, PPARG, CXCL12, TLR2, PTGS2, PARP1, KDR, FGF2, PTPN11, MET, RHOA, PTPRC, IGF1R, CYP1A1, PTK2, MMP2, IL2, GSK3B, TNFRSF1A, SYK, IKBKG, CREB1, APP, PRKDC, CYP3A4, AR, RXRA, PPARA, LGALS3, IGFBP3, CYP1B1, CYP19A1, RARA, PLAU, PIK3CG, MMP3, ESR2, CYP1A2, INSR, IDO1, BRAF, NFE2L2, IGF2R, CYP17A1, COL18A1, ABL1, TOP2A, MPO, HSPD1, EPHX1, DPP4, CDK1, AHR, ABCG2, SLC2A1, PRNP, HNF4A, FASN, ERCC4, ERCC1, DNMT1, AXL, ARG1, ALK, TERT, RARB, NEK2, MMP13, CXCR1, COMT, ODC1, F2, CFTR, CA9, ABCC1, and ABCB1.
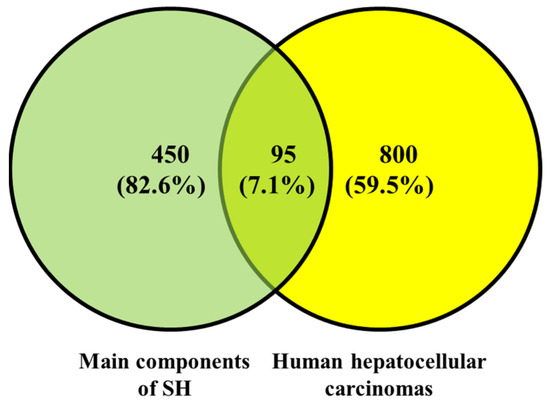
Figure 3.
Overlapping targets between the main components of SH and targets of human hepatocellular carcinoma.
3.5. GO Functional Enrichment, KEGG Pathway Enrichment and PPI Analysis Results
As shown in Figure 4, a total of 2304 relevant entries were screened out through GO enrichment analysis of the overlapping targets, of which 2035 entries were related to biological processes, mainly including regulation of inflammatory response, response to peptide hormone, positive regulation of kinase activity, gland development, positive regulation of transferase activity, response to amyloid-beta, cellular response to peptides, response to oxidative stress, negative regulation of cell adhesion and response to decreased oxygen levels. There were 71 entries related to cellular components, mainly including the membrane raft, membrane microdomain, external side of the plasma membrane, transferase complex, transferring phosphorus-containing groups, caveola, protein kinase complex, chromosomal region and le lumen. There were 198 items related to molecular functions, such as nuclear receptor activity, ligand-activated transcription factor activity, protein tyrosine kinase activity, transcription coregulator binding, RNA polymerase II-specific DNA-binding transcription factor binding, transcription coactivator binding, heme binding, tetrapyrrole binding, transmembrane receptor protein tyrosine kinase activity and DNA-binding transcription factor binding.
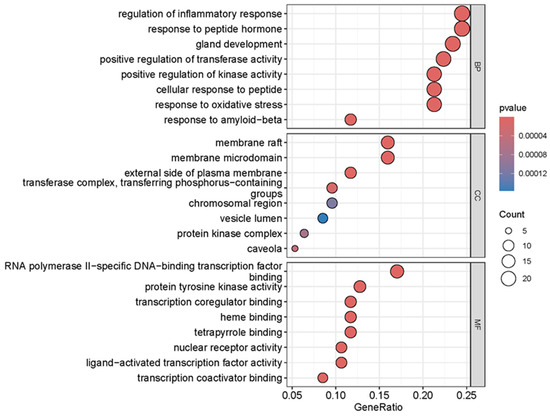
Figure 4.
GO enrichment analysis of overlapping targets of main components of SH and HepG2 cells.
The 95 overlapping targets between the main active ingredients of SH and HepG2 were enriched in the KEGG pathway (p < 0.05). The top 10 pathways were screened out (Figure 5), which were the PI3K-Akt pathway, Lipid and atherosclerosis, Proteoglycans in cancer, Chemical carcinogenesis—receptor activation, Chemical carcinogenesis—reactive oxygen species, Human cytomegalovirus infection, Kaposi sarcoma-associated herpesvirus infection, Prostate cancer, Fluid shear stress and atherosclerosis, and EGFR tyrosine kinase inhibitor resistance.
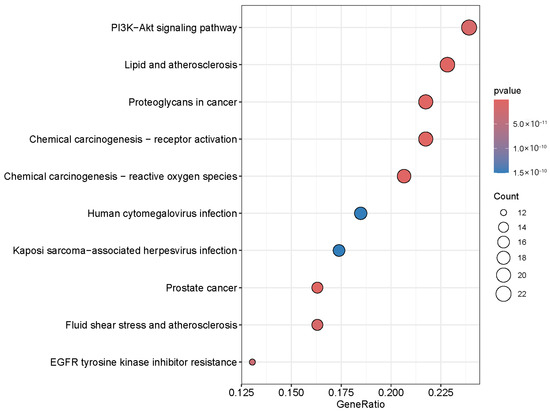
Figure 5.
KEGG enrichment analysis of overlapping targets of the main components of SH and HepG2 cells.
Among the overlapping targets between the main components of SH and HepG2, the protein–protein interactions with a confidence level of 0.7 are shown in Figure 6, among which the hub proteins are EGFR, AKT1, SRC, and IL1B. These traits represent the gene names of differentially expressed proteins, and the lines between genes represent the interactions of differentially expressed proteins. The darker the red and the larger the diameter mean the more related proteins among them.
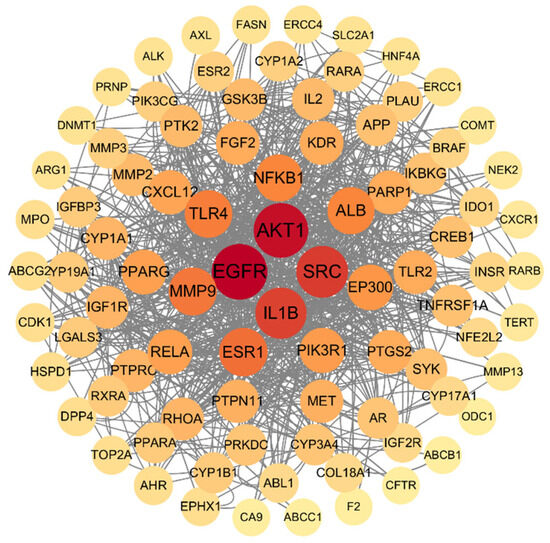
Figure 6.
PPI analysis of common targets between main components of honey and human hepatocellular carcinoma. The circles represent proteins, straight lines represent the interaction relationship between proteins. The darker color means more interaction among proteins.
3.6. Antiproliferative Effects of SH5 and Saccharides on HepG2 Cells
As shown in Figure 7, the inhibitory effects of SH5, glucose, fructose, and sucrose on the proliferation of HepG2 cells for 48 h were dose-dependent. The IC50 of SH, glucose, fructose, and sucrose on HepG2 cells was 5.07%, 16.24%, 9.60% dw/v, and 9.94% dw/v.
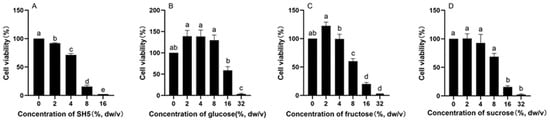
Figure 7.
Inhibitory effects of SH5 (A), glucose (B), fructose (C) and sucrose (D) on the proliferation of HepG2 cells. The concentrations of glucose, fructose, and sucrose mean percent of stock solution prepared according to their contents of SH5. Different characters in the figure indicate significant differences among different treatments.
3.7. Contents of EGFR, SRC and AKT1 in Culture Medium of HepG2 Cells
As shown in Figure 8, the contents of EGFR, SRC, and AKT1 in the medium of HepG2 cells treated with SH were significantly lower than those of untreated cells.
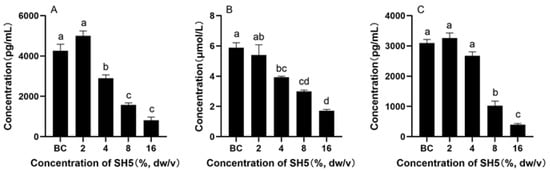
Figure 8.
Protein content in HepG2 cell medium: (A) EGFR, (B) AKT1, and (C) SRC. Different lowercase characters in the figure indicate significant differences between different treatments.
4. Discussion
The compounds of SH determine its function. The moisture content, pH, glucose content, fructose content, sucrose content, total phenolic content, total flavonoid content, antioxidant activity, and antibacterial activity of the SH samples were significantly different. SH6 has the highest sucrose content (4.073 g/100 g) and also has the highest moisture content, which may indicate that the sample is not fully mature or there is a high humidity microenvironment around the beehive [59]. There were 910 ingredients in the methanol extract of SH identified using the UHPLC-MS/MS system, which includes phenols (such as phloroglucinol and pyrogallol), flavonoids (such as quercetin and kaempferol), triterpenes (such as betulin and limonin), saponins (such as soyasaponin I and solanine) and monoterpenoids. These components in SH contributed to its bioactivity.
The total phenolic and flavonoid compounds of SH are closely related to the antioxidant capacity. The antioxidant activity of SH7 is low, which is mostly related to its low total phenolic and flavonoid content. These results are consistent with reference [60,61], which states that the antioxidant capacity of honey is positively correlated with its polyphenol composition. Quercetin has antioxidant properties and can directly scavenge free radicals. It can also interact with ascorbic acid (vitamin C) to form a cycle: quercetin is oxidized after scavenging free radicals, and ascorbic acid can reduce it to restore its antioxidant capacity. This cycle is repeated, effectively maintaining the redox balance of the system and reducing the damage of free radicals to cells [62]. Quercetin can activate the Nrf2/Keap1 pathway, increase the level of antioxidant factors, reduce oxidative stress markers, inhibit the NF-κB pathway, and reduce the level of inflammatory mediators, thereby protecting against pulmonary ischemia–reperfusion injury [63]. 7-O-methylchrysin was identified as a potential marker of Bauhinia championii honey and was strongly positively correlated with free radical scavenging ability. In addition, the interaction between 7-O-methylchrysin and mineral elements such as K and Na may be one of the potential mechanisms for promoting the enhanced antioxidant capacity of Bauhinia championii honey [64].
Another important antioxidant component is soyasaponin I, a triterpenoid saponin, which can not only enhance immune regulation by mediating the p105-Tpl2-ERK signaling pathway but also reduce serum endotoxin, D-lactic acid, and oxidative stress levels, as well as alleviate intestinal pathological damage and inflammation [65]. Solanine, a component detected in the methanol extract of SH, opens up the permeability transition (PT) channels in the membrane by lowering the membrane potential, leading to Ca2+ being transported down its concentration gradient, which in turn leads to a rise in the concentration of Ca2+ in the cell, initiating the mechanism for apoptosis [66]. Saponins may play an antioxidant role depending on the synergistic enhancement with other phenols [67]. Saponins can synergize with phenolic compounds (such as flavonoids and phenolic acids) to enhance their antioxidant and antibacterial effects [68]. Based on our limited knowledge, solanine and soyasaponin I are the first determined components in honey.
The different antioxidant activities of different SH samples may be related to the different test methods and the antioxidant components in different honey samples. The DPPH method mainly detects hydrogen atom donors, while the FRAP method is more sensitive to electron donors [69]. SH3 and SH5 have high FRAP values, but their DPPH and ABTS activities are moderate, suggesting that the FRAP test is more sensitive to specific reducing components (such as ascorbic acid or certain flavonoid glycosides) [70]. In in vivo experiments, Ohia Lehua honey was shown to significantly enhance antioxidant capacity and reduce oxidative stress markers, with a significant increase in total antioxidant capacity (TAC) and a significant decrease in total oxidative state (TOS) and oxidative stress index (OSI). In contrast, the antioxidant activity of Manuka honey was limited and dose-dependent, with a significant increase in TAC and sulfhydryl contents and a moderate decrease in TOS and OSI [71]. Phenolic acids in multifloral honey can inhibit NADPH oxidase and reduce the generation of superoxide anions [72]. Antioxidant activity is one of the causes of antibacterial activity.
Antioxidant components in honey, such as polyphenols and flavonoids, also have an antibacterial effect by scavenging free radicals and reducing oxidative stress. Polyphenols inhibit the growth and reproduction of bacteria [73]. At the same time, they synergize with other antibacterial components in honey, such as hydroperoxides, to improve the overall antibacterial activity of honey [74]. In addition, the lowest pH value (2.907) of SH6 may be caused by the release of hydrogen peroxide, which inhibits bacterial enzyme activity [75]. Phloroglucinol and its derivatives exhibited anti-cancer effects in multiple pathways, including inducing cell apoptosis and inhibiting cell proliferation and migration [76,77]. In addition, phloroglucinol can also induce trained immunity by affecting metabolic and epigenetic pathways, thereby enhancing the body’s immune response to cancer cells [78]. In addition, saponins may regulate cell signaling pathways (such as PI3K-Akt and MAPK pathways) [79] to inhibit the proliferation and apoptosis of liver cancer cells.
SH, which has saponins, inhibits the proliferation of liver cancer cells. Cell proliferation of HepG2 cells was inhibited by SH in a dose-dependent manner (IC50 = 5.07% dw/v). Meanwhile, the IC50 value of SH’s saccharide components (glucose, fructose, sucrose) for HepG2 (9.60–16.24% dw/v) was significantly higher than that of SH, indicating that its anti-cancer effect mainly depends on the synergistic effect of sugars and non-sugar active ingredients (such as phenols and flavonoids) [80,81,82]. The mechanism of the antitumor effect against HepG2 cells depends on the complex components of SH.
The mechanism of the antitumor effect against HepG2 cells was analyzed by network pharmacology. There are 95 overlapping targets between the active ingredients of SH (total phenols, flavonoids, saponins, etc.) and HepG2 cells. The HepG2 cells were significantly inhibited after treatments of more than 4% (dw/v) SH. This result is in line with one study, which shows that honey can inhibit proliferation by scavenging free radicals and enhancing the total antioxidant state (TAS) [48]. SH has antioxidant activity and reduces the level of reactive oxygen in HepG2 cells; then, the proliferation is inhibited [83,84]. Long-term activation will disrupt the balance within the cell, leading to excessive cell stress response and inducing apoptosis [85,86]. The overlapping targets play an antitumor effect against HepG2 cells through pathways. KEGG enrichment analysis showed that the overlapping targets were mainly enriched in the following pathways: PI3K-Akt, Lipid and atherosclerosis, Proteoglycans in cancer, Chemical carcinogenesis—receptor activation, and Chemical carcinogenesis—reactive oxygen species. The PI3K/AKT/mTOR signaling pathway plays a key role in the occurrence and development of liver cancer. An increase in PI3K activity leads to phosphorylation of AKT, which in turn activates mTOR and promotes cell cycle progression and protein synthesis, thus promoting the development of liver cancer [87]. SH may induce HepG2 cell cycle arrest by inhibiting the phosphorylation of downstream effector molecules of the PI3K-Akt pathway (such as mTOR and BAD). TTFields treatment can activate the PI3K/AKT signaling pathway in cancer cells, and pharmacological inhibition of this pathway can enhance the therapeutic effect of TTFields. Combination therapy with PI3K inhibitors and TTFields can significantly reduce cell numbers, increase cell apoptosis, and reduce cloning ability. Inhibition of the PI3K-Akt pathway can significantly enhance the sensitivity of liver cancer cells to chemotherapy drugs [88]. SH provides a new strategy for combined therapy by targeting this pathway.
Another important pathway of the antitumor effect against HepG2 cells is Lipid and atherosclerosis. This pathway was the top pathway of the anti-atherosclerotic Tualang honey bioactive compound [89]. Proteoglycans in cancer is the top pathway when considering the different compounds and antiarrhythmic mechanisms of licorice before and after honey roasting [90]. It was also found in Jinfeng Pill, which is a Chinese medicine formula composed of nine kinds of herbs, which has been shown to alleviate damage to ovarian tissue induced by cyclophosphamide in a rat model of premature ovarian insufficiency [91]. Supplementation of honey for six months protects against DEN-induced inflammatory response and carcinogenesis in the liver of Sprague Dawley rats [92]. Honey and Nigella grains have a protective effect on methylnitrosourea-induced oxidative stress, inflammatory response, and carcinogenesis in Sprague Dawely rats [93]. SH inhibits the proliferation of HepG2 cells via multiple pathways, where DEPs play an inhibitory effect.
The PPI network analysis of DEPs in terms of SH’s antitumor effect against HepG2 cells confirmed that EGFR, AKT1, and SRC were the hub proteins. EGFR is one of the hub proteins that has an inhibition effect in terms of SH against HepG2 cells. EGF/EGFR can activate Akt/glycogen synthase kinase-3β (GSK-3 beta)/Snail pathway and mediate EMT to promote the proliferation and migration of HepG2 cells. Inhibiting the activation of the EGFR signaling pathway helps to partially reverse the EMT phenotype and inhibit the proliferation and migration of HepG2 cells [94]. Another hub protein is AKT1, of which down-regulation inhibited the proliferation of SMMC-7721 cells. In addition, the expression of AKT1 is closely related to cell apoptosis and cell cycle regulation. Knockdown of AKT1 significantly stimulated cell apoptosis and inhibited cell cycle progression [95]. Excessive phosphorylation of AKT1 leads to excessive activation of the mTOR pathway, thereby triggering autophagic cell death [66]. The third hub protein is SRC, which is highly expressed in a variety of cancers and activates the JAK/STAT pathway. It promotes the phosphorylation of STAT3, thereby enhancing the proliferation and survival of tumor cells [96]. The activation of SRC kinase may inhibit invasion and metastasis by destroying the stability of the cytoskeleton [97].
In conclusion, the down-regulation of key proteins such as EGFR, AKT1, and SRC was a possible mechanism of the antitumor effect of SH against HepG2. In the future, in-depth explorations of which bioactive components in SH have an anti-cancer effect and the specific effects of solanine and soybean saponin I can be conducted. In vivo experiments to verify the anti-cancer effect of SH in animals can be performed.
5. Conclusions
SH samples from different origins showed significant compositional diversity and weaker antibacterial activity than MH (UMF10+). The unique components solanine and soyasaponin I in SH may be used as characteristic substances for the identification of honey types and adulteration. The antitumor effect of SH on HepG2 cells was significantly stronger (IC50 = 5.07%) than that of carbohydrate components (IC50 = 9.60%~16.24%), of which the mechanism was seen in various pathways, PI3K-Akt, Lipid and atherosclerosis, Proteoglycans in cancer, Chemical carcinogenesis—receptor activation, and Chemical carcinogenesis—reactive oxygen species pathways, through network pharmacology analysis. The experimental results verified that the down-regulation of key proteins such as EGFR, AKT1, and SRC (p < 0.05) was a possible mechanism of the antitumor effect of SH against HepG2. This study expands the application boundaries of SH in the field of functional foods and food additives.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods14132376/s1, Figures S1–S8: The negative and positive ion spectra of QC and methanol extract of SH samples; Table S1: Components except phenolics, phenolic acids, flavonoids, solanine and soyasaponin I determined in methanol extract of SH by non-targeted metabolomics.
Author Contributions
Conceptualization, W.Y.; methodology, J.L.; formal analysis, J.L., Y.W. and J.W.; writing—original draft preparation, J.L.; writing—review and editing, W.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded for the Undergraduate Innovation Program by Fujian Agriculture and Forestry University, grant number FAFUXMPC20250429001.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ranneh, Y.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A.; Zakaria, Z.A.; Albujja, M.; Bakar, M.F.A. Honey and its nutritional and anti-inflammatory value. BMC Complement. Med. Ther. 2021, 21, 30. [Google Scholar] [CrossRef]
- Seraglio, S.K.T.; Schulz, M.; Brugnerotto, P.; Silva, B.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. Quality, composition and health-protective properties of citrus honey: A review. Food Res. Int. 2021, 143, 110268. [Google Scholar] [CrossRef]
- Ali, A.; Paramanya, A.; Poojari, P.; Arslan-Acaroz, D.; Acaroz, U.; Kostić, A.Ž. The utilization of bee products as a holistic approach to managing polycystic ovarian syndrome-related infertility. Nutrients 2023, 15, 1165. [Google Scholar] [CrossRef]
- Asma, S.T.; Bobiş, O.; Bonta, V.; Acaroz, U.; Shah, S.R.A.; Istanbullugil, F.R.; Arslan-Acaroz, D. General nutritional profile of bee products and their potential antiviral properties against mammalian viruses. Nutrients 2022, 14, 3579. [Google Scholar] [CrossRef]
- Tricou, L.P.; Guirguis, N.; Djebbar, S.; Freedman, B.R.; Matoori, S. Bee better: The role of honey in modern wound care. Adv. Ther. 2025, 8, 2400502. [Google Scholar] [CrossRef]
- Waheed, M.; Hussain, M.B.; Javed, A.; Mushtaq, Z.; Hassan, S.; Shariati, M.A.; Khan, M.U.; Majeed, M.; Nigam, M.; Mishra, A.P.; et al. Honey and cancer: A mechanistic review. Clin. Nutr. 2019, 38, 2499–2503. [Google Scholar] [CrossRef]
- Nurhidayah, I.; Rustina, Y.; Hastono, S.P.; Mediani, H.S. The effect of honey in oral care intervention against chemotherapy-induced mucositis in pediatric cancer patients: A pilot study. BMC Complement. Med. Ther. 2024, 24, 415. [Google Scholar] [CrossRef]
- Martinotti, S.; Bonsignore, G.; Ranzato, E. Understanding the anticancer properties of honey. Int. J. Mol. Sci. 2024, 25, 11724. [Google Scholar] [CrossRef]
- Bose, D.; Famurewa, A.C.; Akash, A.; Othman, E.M. The therapeutic mechanisms of honey in mitigating toxicity from anticancer chemotherapy toxicity: A review. J. Xenobiot. 2024, 14, 1109–1129. [Google Scholar] [CrossRef]
- Tomblin, V.; Ferguson, L.R.; Han, D.Y.; Murray, P.; Schlothauer, R. Potential pathway of anti-inflammatory effect by New Zealand honeys. Int. J. Gen. Med. 2014, 7, 149–158. [Google Scholar] [CrossRef]
- El-Hakam, F.E.Z.A.; Laban, G.A.; El-Din, S.B.; El-Hamid, H.A.; Farouk, M.H. Apitherapy combination improvement of blood pressure, cardiovascular protection, and antioxidant and anti-inflammatory responses in dexamethasone model hypertensive rats. Sci. Rep. 2022, 12, 20765. [Google Scholar] [CrossRef]
- Navaei-Alipour, N.; Mastali, M.; Ferns, G.A.; Saberi-Karimian, M.; Ghayour-Mobarhan, M. The effects of honey on pro- and anti-inflammatory cytokines: A narrative review. Phytother. Res. 2021, 35, 3690–3701. [Google Scholar] [CrossRef]
- Matharu, R.K.; Ahmed, J.; Seo, J.; Karu, K.; Golshan, M.A.; Edirisinghe, M.; Ciric, L. Antibacterial properties of honey nanocomposite fibrous meshes. Polymers 2022, 14, 5155. [Google Scholar] [CrossRef]
- Majtan, J.; Bucekova, M.; Kafantaris, I.; Szweda, P.; Hammer, K.; Mossialos, D. Honey antibacterial activity: A neglected aspect of honey quality assurance as functional food. Trends Food Sci. Technol. 2021, 118, 870–886. [Google Scholar] [CrossRef]
- Bucekova, M.; Buriova, M.; Pekarik, L.; Majtan, V.; Majtan, J. Phytochemicals-mediated production of hydrogen peroxide is crucial for high antibacterial activity of honeydew honey. Sci. Rep. 2018, 8, 9061. [Google Scholar] [CrossRef]
- Ahmed, S.; Sulaiman, S.A.; Baig, A.A.; Ibrahim, M.; Liaqat, S.; Fatima, S.; Jabeen, S.; Shamim, N.; Othman, N.H. Honey as a potential natural antioxidant medicine: An insight into its molecular mechanisms of action. Oxidative Med. Cell. Longev. 2018, 2018, 8367846. [Google Scholar] [CrossRef]
- Pereira, C.; Barreira, J.C.; Calhelha, R.C.; Lopes, M.; Queiroz, M.J.; Vilas-Boas, M.; Barros, L.; Ferreira, I.C. Is honey able to potentiate the antioxidant and cytotoxic properties of medicinal plants consumed as infusions for hepatoprotective effects? Food Funct. 2015, 6, 1435–1442. [Google Scholar] [CrossRef]
- Biluca, F.C.; da Silva, B.; Caon, T.; Mohr, E.T.B.; Vieira, G.N.; Gonzaga, L.V.; Vitali, L.; Micke, G.; Fett, R.; Dalmarco, E.M.; et al. Investigation of phenolic compounds, antioxidant and anti-inflammatory activities in stingless bee honey (Meliponinae). Food Res. Int. 2020, 129, 108756. [Google Scholar] [CrossRef]
- Escuredo, O.; Míguez, M.; Fernández-González, M.; Seijo, M.C. Nutritional value and antioxidant activity of honeys produced in a European Atlantic area. Food Chem. 2013, 138, 851–866. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Avila, F.J.; Escudero-Gilete, M.L.; Pajuelo, A.G.; Heredia, F.J.; Hernanz, D.; Terrab, A. Physicochemical properties, colour, chemical composition, and antioxidant activity of Spanish Quercus honeydew honeys. Eur. Food Res. Technol. 2019, 245, 2017–2026. [Google Scholar] [CrossRef]
- Scepankova, H.; Majtan, J.; Estevinho, L.M.; Saraiva, J.A. The high pressure preservation of honey: A comparative study on quality changes during storage. Foods 2024, 13, 989. [Google Scholar] [CrossRef]
- Martinello, M.; Mutinelli, F. Antioxidant activity in bee products: A review. Antioxidants 2021, 10, 71. [Google Scholar] [CrossRef]
- Lawag, I.L.; Islam, M.K.; Sostaric, T.; Lim, L.Y.; Hammer, K.; Locher, C. Antioxidant activity and phenolic compound identification and quantification in Western Australian honeys. Antioxidants 2023, 12, 189. [Google Scholar] [CrossRef]
- Chua, L.S.; Rahaman, N.L.A.; Adnan, N.A.; Tan, T.T.E. Antioxidant activity of three honey samples in relation with their biochemical components. J. Anal. Methods Chem. 2013, 2013, 313798. [Google Scholar] [CrossRef]
- Becerril-Sánchez, A.L.; Quintero-Salazar, B.; Dublán-García, O.; Escalona-Buendía, H.B. Phenolic compounds in honey and their relationship with antioxidant activity, botanical origin, and color. Antioxidants 2021, 10, 1700. [Google Scholar] [CrossRef]
- Zawawi, N.; Chong, P.J.; Tom, N.N.M.; Anuar, N.S.S.; Mohammad, S.M.; Ismail, N.; Jusoh, A.Z. Establishing relationship between vitamins, total phenolic and total flavonoid content and antioxidant activities in various honey types. Molecules 2021, 26, 4399. [Google Scholar] [CrossRef]
- Kędzierska-Matysek, M.; Stryjecka, M.; Teter, A.; Skałecki, P.; Domaradzki, P.; Florek, M. Relationships between the content of phenolic compounds and the antioxidant activity of Polish honey varieties as a tool for botanical discrimination. Molecules 2021, 26, 1810. [Google Scholar] [CrossRef]
- Almasaudi, S. The antibacterial activities of honey. Saudi J. Biol. Sci. 2021, 28, 2188–2196. [Google Scholar] [CrossRef]
- Khataybeh, B.; Jaradat, Z.; Ababneh, Q. Anti-bacterial, anti-biofilm and anti-quorum sensing activities of honey: A review. J. Ethnopharmacol. 2023, 317, 116830. [Google Scholar] [CrossRef]
- Cui, T.T.; Wu, X.K.; Mou, T.; Fan, F.H. Water usability as a descriptive parameter of thermodynamic properties and water mobility in food solids. NPJ Sci. Food. 2023, 7, 30. [Google Scholar] [CrossRef]
- Getzke, F.; Wang, L.; Chesneau, G.; Böhringer, N.; Mesny, F.; Denissen, N.; Wesseler, H.; Adisa, P.T.; Marner, M.; Schulze-Lefert, P.; et al. Physiochemical interaction between osmotic stress and a bacterial exometabolite promotes plant disease. Nat. Commun. 2024, 15, 4438. [Google Scholar] [CrossRef]
- Alshammari, J.; Dhowlaghar, N.; Xie, Y.C.; Xu, J.; Tang, J.M.; Sablani, S.; Zhu, M.J. Survival of Salmonella and Enterococcus faecium in high fructose corn syrup and honey at room temperature (22 °C). Food Control 2021, 123, 107765. [Google Scholar] [CrossRef]
- Tsuruda, J.M.; Chakrabarti, P.; Sagili, R.R. Honey bee nutrition. Vet. Clin. N. Am. Food Anim. Pract. 2021, 37, 505–519. [Google Scholar] [CrossRef]
- Kapoor, N.; Yadav, R. Manuka honey: A promising wound dressing material for the chronic nonhealing discharging wounds: A retrospective study. Natl. J. Maxillofac. Surg. 2021, 12, 233–237. [Google Scholar] [CrossRef]
- Zuchelkowski, B.E.; Peñaloza, H.F.; Xiong, Z.; Wang, L.; Cifuentes-Pagano, E.; Rochon, E.; Yang, M.; Gingras, S.; Gladwin, M.T.; Lee, J.S. Increased Neutrophil H2O2 Production and Enhanced Pulmonary Clearance of Klebsiella pneumoniae in G6PD A-Mice. Res. Sq. 2024, rs.3.rs-3931558. [Google Scholar] [CrossRef]
- Dai, X.M.; Li, Y.; Zhang, Y.J.; Zou, Y.Q.; Yuan, S.Y.; Gao, F. pH/H2O2 dual-responsive macrophage-targeted chitosaccharides nanoparticles to combat intracellular bacterial infection. Colloids Surf. B Biointerfaces 2025, 248, 114465. [Google Scholar] [CrossRef]
- Imlay, J.A. The barrier properties of biological membranes dictate how cells experience oxidative stress. Mol. Microbiol. 2025, 123, 454–463. [Google Scholar] [CrossRef]
- Zhu, G.Y.; Wang, Q.; Lu, S.L.; Niu, Y.W. Hydrogen peroxide: A potential wound therapeutic target? Med. Princ. Pract. 2017, 26, 301–308. [Google Scholar] [CrossRef]
- Brudzynski, K. A current perspective on hydrogen peroxide production in honey. A review. Food Chem. 2020, 332, 127229. [Google Scholar] [CrossRef]
- Mieles, J.Y.; Vyas, C.; Aslan, E.; Humphreys, G.; Diver, C.; Bartolo, P. Honey: An advanced antimicrobial and wound healing biomaterial for tissue engineering applications. Pharmaceutics 2022, 14, 1663. [Google Scholar] [CrossRef]
- Ogwu, M.C.; Izah, S.C. Honey as a natural antimicrobial. Antibiotics 2025, 14, 255. [Google Scholar] [CrossRef]
- Khan, S.U.; Anjum, S.I.; Rahman, K.; Ansari, M.J.; Khan, W.U.; Kamal, S.; Khattak, B.; Muhammad, A.; Khan, H.U. Honey: Single food stuff comprises many drugs. Saudi J. Biol. Sci. 2018, 25, 320–325. [Google Scholar] [CrossRef]
- Nolan, V.C.; Harrison, J.; Cox, J.A.G. Dissecting the antimicrobial composition of honey. Antibiotics 2019, 8, 251. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Biological properties and therapeutic activities of honey in wound healing: A narrative review and meta-analysis. J. Tissue. Viability 2016, 25, 98–118. [Google Scholar] [CrossRef]
- Zhu, H.Q.; Du, Y.N.; Lin, X.; Huang, Z.L.; Dong, J.; Qiao, J.T.; Zhang, H.C. Terpenoids identification and authenticity evaluation of longan, litchi and schefflera honey. Food Sci. Hum. Wellness 2025, 14, 9250073. [Google Scholar] [CrossRef]
- Hailu, D.; Belay, A. Melissopalynology and antioxidant properties used to differentiate Schefflera abyssinica and polyfloral honey. PLoS ONE 2020, 15, e0240868. [Google Scholar] [CrossRef]
- Venook, A.P.; Papandreou, C.; Furuse, J.; de Guevara, L.L. The incidence and epidemiology of hepatocellular carcinoma: A global and regional perspective. Oncologist 2010, 15, 5–13. [Google Scholar] [CrossRef]
- Hassan, M.I.; Mabrouk, G.M.; Shehata, H.H.; Aboelhussein, M.M. Antineoplastic effects of bee honey and Nigella sativa on hepatocellular carcinoma cells. Integr. Cancer Ther. 2012, 11, 354–363. [Google Scholar] [CrossRef]
- Al Refaey, H.R.; Newairy, A.S.A.; Wahby, M.M.; Albanese, C.; Elkewedi, M.; Choudhry, M.U.; Sultan, A.S. Manuka honey enhanced sensitivity of HepG2, hepatocellular carcinoma cells, for Doxorubicin and induced apoptosis through inhibition of Wnt/β-catenin and ERK1/2. Biol. Res. 2021, 54, 16. [Google Scholar] [CrossRef]
- Mohamed, N.Z.; Aly, H.F.; El-Mezayen, H.A.M.; El-Salamony, H.E. Effect of co-administration of bee honey and some chemotherapeutic drugs on dissemination of hepatocellular carcinoma in rats. Toxicol. Rep. 2019, 6, 875–888. [Google Scholar] [CrossRef]
- Hopkins, A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef]
- Yang, W.; Shen, M.; Kuang, H.; Liu, X.; Zhang, C.; Tian, Y.; Xu, X. The botanical sources, entomological proteome and antibiotic properties of wild honey. Innov. Food Sci. Emerg. Technol. 2020, 67, 102589. [Google Scholar] [CrossRef]
- Tan, W.; Tian, Y.; Zhang, Q.; Miao, S.; Wu, W.; Miao, X.; Kuang, H.; Yang, W. Antioxidant and antibacterial activity of Apis laboriosa honey against Salmonella enterica serovar Typhimurium. Front. Nutr. 2023, 10, 1181492. [Google Scholar] [CrossRef]
- Anand, S.; Pang, E.; Livanos, G.; Mantri, N. Characterization of physico-chemical properties and antioxidant capacities of bioactive honey produced from Australian Grown Agastache rugosa and its correlation with colour and poly-phenol content. Molecules 2018, 23, 108. [Google Scholar] [CrossRef]
- Bellik, Y.; Selles, S.M.A. In vitro synergistic antioxidant activity of honey-Mentha spicata combination. J. Food Meas. Charact. 2017, 11, 111–118. [Google Scholar] [CrossRef]
- Girma, A.; Seo, W.; She, R.C. Antibacterial activity of varying UMF-graded Manuka honeys. PLoS ONE 2019, 14, e0224495. [Google Scholar] [CrossRef]
- Tabatabaei, M.S.; Ahmed, M. Enzyme-linked immunosorbent assay (ELISA). Methods Mol. Biol. 2022, 2508, 115–134. [Google Scholar] [CrossRef]
- Tian, Y.Y.; Liu, X.Q.; Wang, J.; Zhang, C.; Yang, W.C. Antitumor effects and the potential mechanism of 10-HDA against SU-DHL-2 cells. Pharmaceuticals 2024, 17, 1088. [Google Scholar] [CrossRef]
- da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Di Marco, G.; Gismondi, A.; Panzanella, L.; Canuti, L.; Impei, S.; Leonardi, D.; Canini, A. Botanical influence on phenolic profile and antioxidant level of Italian honeys. J. Food Sci. Technol. 2018, 55, 4042–4050. [Google Scholar] [CrossRef]
- Jaśkiewicz, K.; Szczęsna, T.; Jachuła, J. How phenolic compounds profile and antioxidant activity depend on botanical origin of honey-A case of polish varietal honeys. Molecules 2025, 30, 360. [Google Scholar] [CrossRef]
- Veiko, A.G.; Lapshina, E.A.; Zavodnik, I.B. Comparative analysis of molecular properties and reactions with oxidants for quercetin, catechin, and naringenin. Mol. Cell. Biochem. 2021, 476, 4287–4299. [Google Scholar] [CrossRef]
- Zardak, M.Y.; Keshavarz, F.; Mahyaei, A.; Gholami, M.; Moosavi, F.S.; Abbasloo, E.; Abdollahi, F.; Rezaei, M.H.; Madadizadeh, E.; Soltani, N.; et al. Quercetin as a therapeutic agent activate the Nrf2/Keap1 pathway to alleviate lung ischemia-reperfusion injury. Sci. Rep. 2024, 14, 23074. [Google Scholar] [CrossRef]
- Liu, R.J.; Zhang, X.H.; Liu, H.H.; Huang, Y.Y.; Zhang, Y.; Wu, Y.X.; Nie, J.F. Revealing the key antioxidant compounds and potential action mechanisms of Bauhinina championii honey based on non-targeted metabolomics, mineralogical analysis and physicochemical characterization. Food Chem. 2025, 477, 143456. [Google Scholar] [CrossRef]
- Li, M.H.; Zhao, D.Y.; Meng, J.X.; Pan, T.X.; Li, J.Y.; Guo, J.L.; Huang, H.B.; Wang, N.; Zhang, D.; Wang, C.F.; et al. Bacillus halotolerans attenuates inflammation induced by enterotoxigenic Escherichia coli infection in vivo and in vitro based on its metabolite soyasaponin I regulating the p105-Tpl2-ERK pathway. Food Funct. 2024, 15, 6743–6758. [Google Scholar] [CrossRef]
- Gao, S.Y.; Wang, Q.J.; Ji, Y.B. Effect of solanine on the membrane potential of mitochondria in HepG2 cells and [Ca2+]i in the cells. World J. Gastroenterol. 2006, 12, 3359–3367. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Zhang, B.; Deng, Z. The synergistic and antagonistic antioxidant interactions of dietary phytochemical combinations. Crit. Rev. Food Sci. Nutr. 2022, 62, 5658–5677. [Google Scholar] [CrossRef]
- Tagousop, C.N.; Tamokou, J.D.; Kengne, I.C.; Ngnokam, D.; Voutquenne-Nazabadioko, L. Antimicrobial activities of saponins from Melanthera elliptica and their synergistic effects with antibiotics against pathogenic phenotypes. Chem. Cent. J. 2018, 12, 97. [Google Scholar] [CrossRef]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu assays to assess the antioxidant capacity of lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef]
- Benzie, I.F.; Devaki, M. The ferric reducing/antioxidant power (FRAP) assay for non-enzymatic antioxidant capacity: Concepts, procedures, limitations and applications. In Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications; Wiley: Hoboken, NJ, USA, 2018; pp. 77–106. [Google Scholar] [CrossRef]
- Morar, I.I.; Pop, R.M.; Peitzner, E.; Ranga, F.; Orasan, M.S.; Cecan, A.D.; Chera, E.I.; Bonci, T.I.; Usatiuc, L.O.; Ticolea, M.; et al. Phytochemical composition and antioxidant activity of Manuka honey and Ohia Lehua honey. Nutrients 2025, 17, 276. [Google Scholar] [CrossRef]
- Faúndez, X.; Báez, M.E.; Martínez, J.; Zúñiga-López, M.C.; Espinoza, J.; Fuentes, E. Evaluation of the generation of reactive oxygen species and antibacterial activity of honey as a function of its phenolic and mineral composition. Food Chem. 2023, 426, 136561. [Google Scholar] [CrossRef]
- Chanu, N.R.; Gogoi, P.; Barbhuiya, P.A.; Dutta, P.P.; Pathak, M.P.; Sen, S. Natural flavonoids as potential therapeutics in the management of diabetic wound: A review. Curr. Top. Med. Chem. 2023, 23, 690–710. [Google Scholar] [CrossRef] [PubMed]
- Balázs, V.L.; Nagy-Radványi, L.; Filep, R.; Kerekes, E.; Kocsis, B.; Kocsis, M.; Farkas, A. In vitro antibacterial and antibiofilm activity of Hungarian honeys against respiratory tract bacteria. Foods 2021, 10, 1632. [Google Scholar] [CrossRef]
- Farkasovska, J.; Bugarova, V.; Godocikova, J.; Majtan, V.; Majtan, J. The role of hydrogen peroxide in the antibacterial activity of different floral honeys. Eur. Food Res. Technol. 2019, 245, 2739–2744. [Google Scholar] [CrossRef]
- Huang, S.M.; Cheung, C.W.; Chang, C.S.; Tang, C.H.; Liu, J.F.; Lin, Y.H.; Chen, J.H.; Ko, S.H.; Wong, K.L.; Lu, D.Y. Phloroglucinol derivative MCPP induces cell apoptosis in human colon cancer. J. Cell. Biochem. 2011, 112, 643–652. [Google Scholar] [CrossRef]
- Kim, R.K.; Suh, Y.; Yoo, K.C.; Cui, Y.H.; Hwang, E.; Kim, H.J.; Kang, J.S.; Kim, M.J.; Lee, Y.Y.; Lee, S.J. Phloroglucinol suppresses metastatic ability of breast cancer cells by inhibition of epithelial-mesenchymal cell transition. Cancer Sci. 2015, 106, 94–101. [Google Scholar] [CrossRef]
- Castelo, J.; Araujo-Aris, S.; Barriales, D.; Pasco, S.T.; Seoane, I.; Peña-Cearra, A.; Palacios, A.; Simó, C.; Garcia-Cañas, V.; Khamwong, M.; et al. The microbiota metabolite, phloroglucinol, confers long-term protection against inflammation. Gut Microbes 2024, 16, 2438829. [Google Scholar] [CrossRef]
- Zhan, Y.; Liu, R.; Wang, W.; Li, J.; Yang, X.O.; Zhang, Y. Total saponins isolated from Radix et Rhizoma Leonticis suppresses tumor cells growth by regulation of PI3K/Akt/mTOR and p38 MAPK pathways. Environ. Toxicol. Pharmacol. 2016, 41, 39–44. [Google Scholar] [CrossRef]
- Ahmed, S.; Othman, N.H. Honey as a potential natural anticancer agent: A review of its mechanisms. Evid. Based Complement. Altern. Med. 2013, 2013, 829070. [Google Scholar] [CrossRef]
- Eteraf-Oskouei, T.; Najafi, M. Uses of natural honey in cancer: An updated review. Adv. Pharm. Bull. 2022, 12, 248–261. [Google Scholar] [CrossRef]
- Porcza, L.M.; Simms, C.; Chopra, M. Honey and cancer: Current status and future directions. Diseases 2016, 4, 30. [Google Scholar] [CrossRef]
- Ren, J.L.; Yan, G.L.; Yang, L.; Kong, L.; Guan, Y.; Sun, H.; Liu, C.; Liu, L.; Han, Y.; Wang, X.J. Cancer chemoprevention: Signaling pathways and strategic approaches. Signal Transduct. Target. Ther. 2025, 10, 113. [Google Scholar] [CrossRef]
- Erejuwa, O.O.; Sulaiman, S.A.; Ab Wahab, M.S. Effects of honey and its mechanisms of action on the development and progression of cancer. Molecules 2014, 19, 2497–2522. [Google Scholar] [CrossRef]
- Ma, S.Y.; Liu, Y.M.; Wang, J. Potential bidirectional regulatory effects of botanical drug metabolites on tumors and cardiovascular diseases based on the PI3K/Akt/mTOR pathway. Front. Pharmacol. 2025, 16, 1467894. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, D.; Zheng, X.; Huang, B.; Xia, X.; Pan, X. Quercetin exerts bidirectional regulation effects on the efficacy of tamoxifen in estrogen receptor-positive breast cancer therapy: An in vitro study. Environ. Toxicol. 2020, 35, 1179–1193. [Google Scholar] [CrossRef]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef]
- Klein-Goldberg, A.; Voloshin, T.; Tov, E.Z.; Paz, R.; Somri-Gannam, L.; Volodin, A.; Koren, L.; Lifshitz, L.; Meir, A.; Shabtay-Orbach, A.; et al. Role of the PI3K/AKT signaling pathway in the cellular response to Tumor Treating Fields (TTFields). Cell Death Dis. 2025, 16, 210. [Google Scholar] [CrossRef]
- Azman, A.N.S.S.; Tan, J.J.; Abdullah, M.N.H.; Bahari, H.; Lim, V.; Yong, Y.K. Network pharmacology and molecular docking analysis of active compounds in tualang honey against atherosclerosis. Foods 2023, 12, 1779. [Google Scholar] [CrossRef]
- Lu, L.J.; Wang, W.X.; Sun, P.J.; Yan, S.W.; Chen, H.X.; Liu, X.; Dong, J.J.; Chen, L.H.; Lu, T.L. Differential compounds of licorice before and after honey roasted and anti-arrhythmia mechanism via LC-MS/MS and network pharmacology analysis. J. Liq. Chromatogr. Relat. Technol. 2023, 46, 1–11. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Zhong, R.H.; Guo, X.J.; Li, G.T.; Zhou, J.Y.; Yang, W.J.; Ren, B.T.; Zhu, Y. Jinfeng pills ameliorate premature ovarian insufficiency induced by cyclophosphamide in rats and correlate to modulating IL-17A/IL-6 axis and MEK/ERK signals. J. Ethnopharmacol. 2023, 307, 116242. [Google Scholar] [CrossRef]
- El-kott, A.F.; Kandeel, A.A.; El-Aziz, S.F.A.; Ribea, H.M. Anti-tumor effects of bee honey on PCNA and P53 expression in the rat hepatocarcinogenesis. Int. J. Cancer Res. 2012, 8, 130–139. [Google Scholar] [CrossRef]
- Mabrouk, G.M.; Moselhy, S.S.; Zohny, S.F.; Ali, E.M.; Helal, T.E.; Amin, A.A.; Khalifa, A.A. Inhibition of methylnitrosourea (MNU) induced oxidative stress and carcinogen-esis by orally administered bee honey and Nigella grains in Sprague Dawely rats. J. Exp. Clin. Cancer Res. 2002, 21, 341–346. [Google Scholar] [PubMed]
- Gao, J.F.; Huo, Z.; Song, X.Y.; Shao, Q.Q.; Ren, W.W.; Huang, X.L.; Zhou, S.P.; Tang, X.L. EGFR mediates epithelial-mesenchymal transition through the Akt/GSK-3β/Snail signaling pathway to promote liver cancer proliferation and migration. Oncol. Lett. 2023, 27, 59. [Google Scholar] [CrossRef]
- Chen, J.; Liang, J.; Liu, S.H.; Song, S.N.; Guo, W.X.; Shen, F.Z. Differential regulation of AKT1 contributes to survival and proliferation in hepatocellular carcinoma cells by mediating Notch1 expression. Oncol. Lett. 2018, 15, 6857–6864. [Google Scholar] [CrossRef]
- Shi, D.D.; Tao, J.J.; Man, S.L.; Zhang, N.; Ma, L.; Guo, L.P.; Huang, L.Q.; Gao, W.Y. Structure, function, signaling pathways and clinical therapeutics: The translational potential of STAT3 as a target for cancer therapy. Biochim. Biophys. Acta (BBA) Rev. Cancer 2024, 1879, 189207. [Google Scholar] [CrossRef]
- Hao, H.F.; Bian, Y.; Yang, N.; Ji, X.Z.; Bao, J.; Zhu, K.K. Discovery of anti-tumor small molecule lead compounds targeting the SH3 domain of c-Src protein through virtual screening and biological evaluation. Arch. Biochem. Biophys. 2025, 764, 110286. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).








