Apple Juice Fermented with Lactiplantibacillus plantarum Improves Its Flavor Profile and Probiotic Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Strains
2.2. Characterization of the Growth of L. plantarum
2.2.1. Growth Characteristics of L. plantarum
2.2.2. Growth Characteristics of L. plantarum in FAJ
2.3. Metabolic Activity
2.3.1. Analysis of Biogenic Amine Production Capability by L. plantarum
2.3.2. Hemolytic Activity Assay
2.3.3. Analysis of Biofilm Formation by L. plantarum
2.3.4. Preparation of Apple Juice for Fermentation
2.4. Fermentation of Apple Juice
2.5. Volatile Flavor Analysis Using Electronic Nose
2.6. In Vitro Colonic Fermentation
2.7. 16S rRNA Profiling
2.8. Statistical Analysis
3. Results and Discussion
3.1. Safety Assessment of L. plantarum
3.1.1. Qualitative Analysis of Biogenic Amine Production
3.1.2. Hemolytic Activity Assessment of L. plantarum
3.1.3. Analysis of Biofilm Formation Capacity in L. plantarum
3.2. Characterization of the Growth of L. plantarum
3.3. Response of the Electronic Nose to Signals of Flavor Substances in FAJ from Different Strains
3.4. Effect of FAJ on Gut Microbiota
3.4.1. FAJ-Induced Changes in Gut Microbial Composition at the Phylum Level
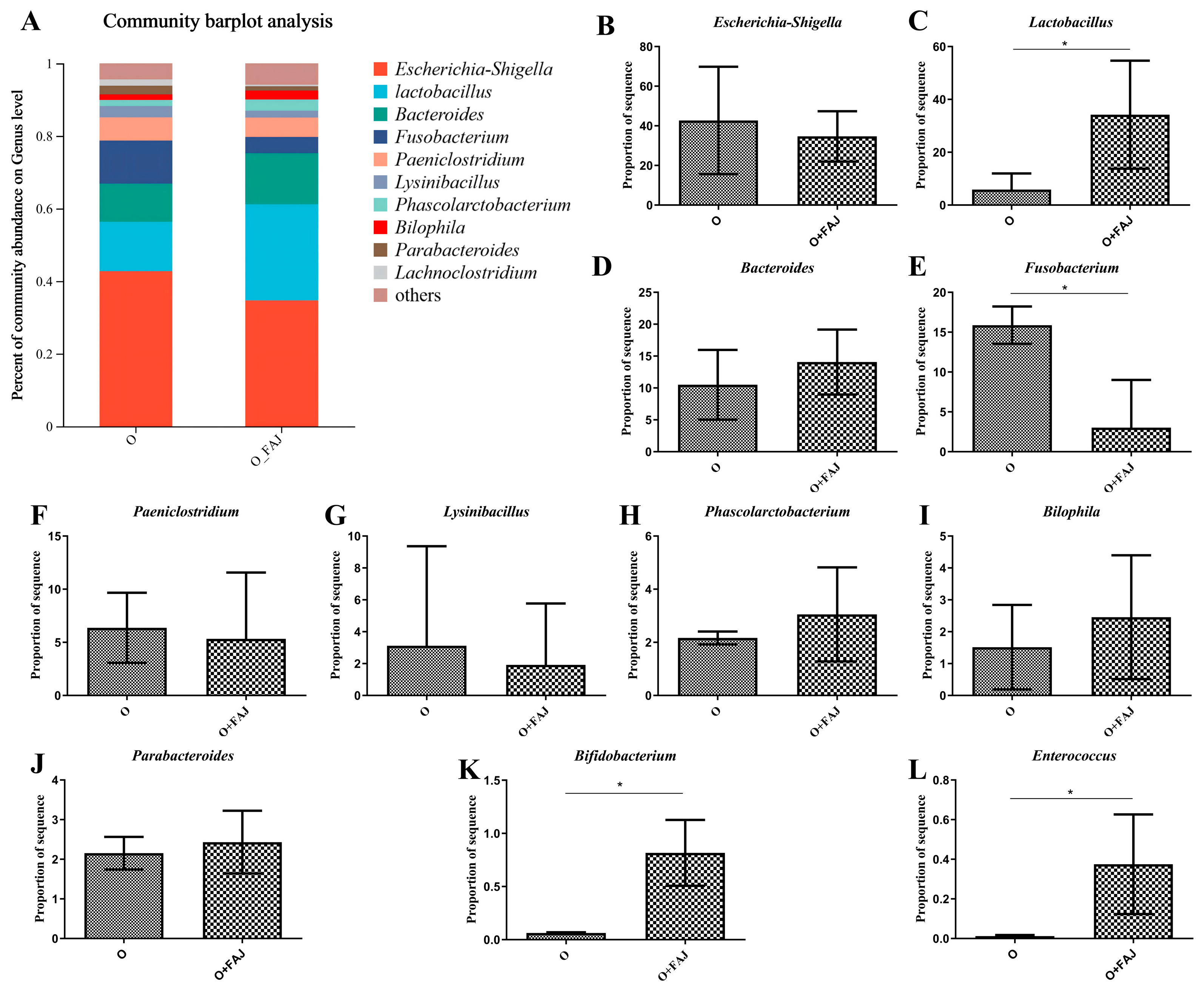
3.4.2. FAJ-Induced Changes in Gut Microbial Composition at the Genus Level
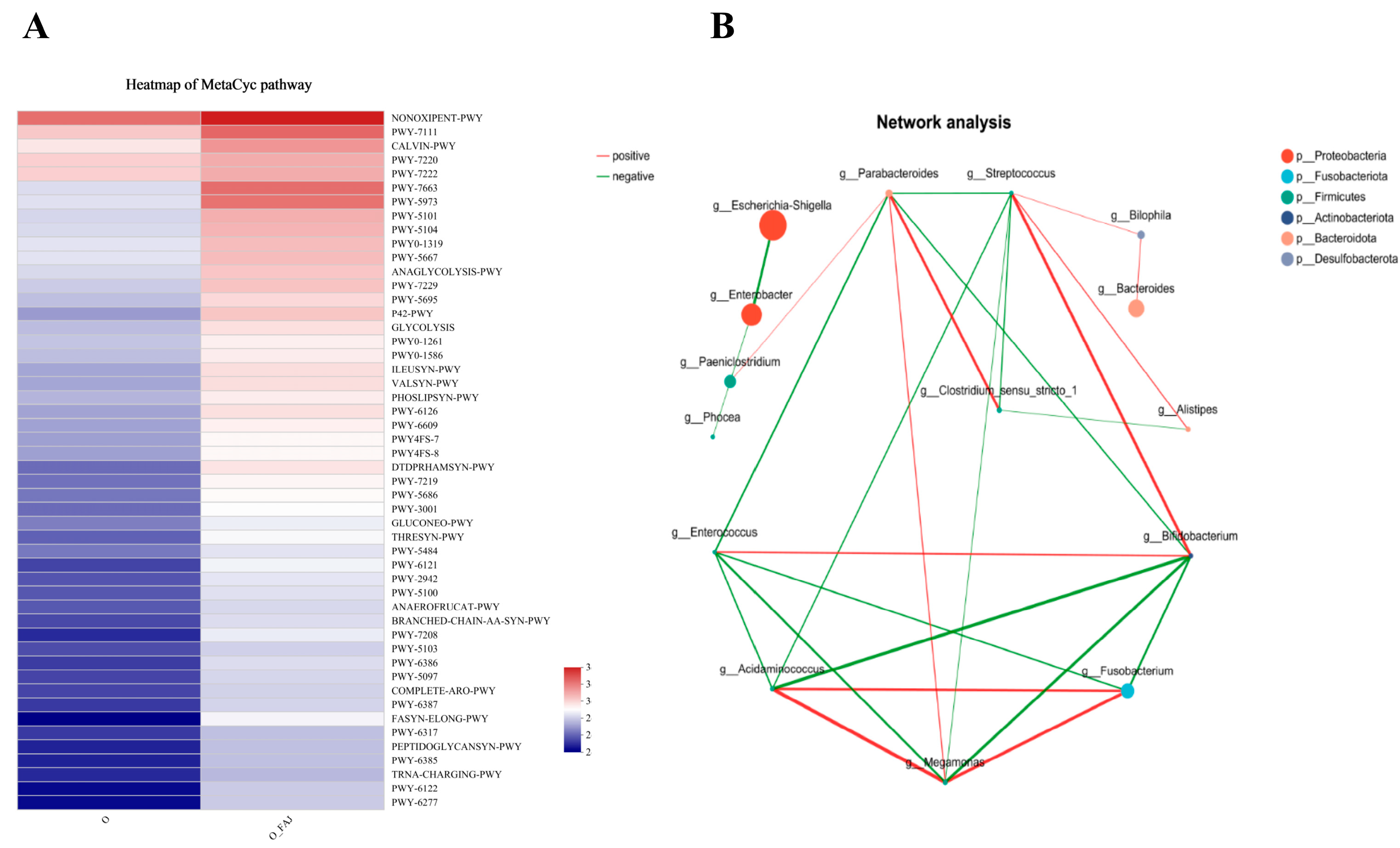
3.4.3. MetaCyc Pathway Analysis
3.4.4. Network Correlation Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiao, N.; Ding, Y.; Cui, B.; Li, R.; Qu, X.; Zhou, H.; Au, K.; Fan, X.; Xie, J.; Huang, Y. Navigating obesity: A comprehensive review of epidemiology, pathophysiology, complications and management strategies. Innov. Med. 2024, 2, 100090-1–100090-17. [Google Scholar] [CrossRef]
- Khan, S.; Luck, H.; Winer, S.; Winer, D.A. Emerging concepts in intestinal immune control of obesity-related metabolic disease. Nat. Commun. 2021, 12, 2598. [Google Scholar] [CrossRef]
- Bapat, S.P.; Whitty, C.; Mowery, C.T.; Liang, Y.; Yoo, A.; Jiang, Z.; Peters, M.C.; Zhang, L.-J.; Vogel, I.; Zhou, C. Obesity alters pathology and treatment response in inflammatory disease. Nature 2022, 604, 337–342. [Google Scholar] [CrossRef]
- Cui, L.; Chen, T.; Li, Z.; Yu, Z.; Liu, X.; Li, J.; Guo, Y.; Xu, D.; Wang, X. Association between dietary related factors and central obesity among married women: China Health and Nutrition Survey. Appetite 2022, 168, 105785. [Google Scholar] [CrossRef] [PubMed]
- Im, J.; Kim, M.; Park, K. Association between the phytochemical index and lower prevalence of obesity/abdominal obesity in Korean adults. Nutrients 2020, 12, 2312. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Zhu, P.; Diao, Z.; Tian, S.; Ding, Z.; Duan, Y.; Xu, T.; Zhou, X.; Wang, X.; et al. Raman flow cytometry based single-cell species classification, viable-cell counting and vitality test for probiotic products. iMetaOmics 2025, e70024. [Google Scholar] [CrossRef]
- Kullar, R.; Goldstein, E.J.; Johnson, S.; McFarland, L.V. Lactobacillus bacteremia and probiotics: A review. Microorganisms 2023, 11, 896. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, N.; Battista, N.; Prete, R.; Corsetti, A. Health-promoting role of Lactiplantibacillus plantarum isolated from fermented foods. Microorganisms 2021, 9, 349. [Google Scholar] [CrossRef]
- Panda, S.K.; Shetty, P.H. Innovations in Technologies for Fermented Food and Beverage Industries; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Wang, F.; Wang, M.; Xu, L.; Qian, J.; Xu, B.; Gao, X.; Ding, Z.; Cui, K. Application and Possible Mechanism of Microbial Fermentation and Enzyme Catalysis in Regulation of Food Flavour. Foods 2025, 14, 1909. [Google Scholar] [CrossRef]
- Liu, S.; He, Y.; He, W.; Song, X.; Peng, Y.; Hu, X.; Bian, S.; Li, Y.; Nie, S.; Yin, J. Exploring the biogenic transformation mechanism of polyphenols by Lactobacillus plantarum NCU137 fermentation and its enhancement of antioxidant properties in wolfberry juice. J. Agric. Food Chem. 2024, 72, 12752–12761. [Google Scholar] [CrossRef]
- Fan, J.; Lu, Y.; Li, X.; Huang, J.; Dong, L.; Luo, J.; Tian, F.; Ni, Y. Omics analysis of key pathway in flavour formation and B vitamins synthesis during chickpea milk fermentation by Lactiplantibacillus plantarum. Food Chem. 2025, 463, 141083. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Xiong, T.; Peng, Z.; Liu, C.; Huang, T.; Yu, H.; Xie, M. Correlation between microbiota and flavours in fermentation of Chinese Sichuan Paocai. Food Res. Int. 2018, 114, 123–132. [Google Scholar] [CrossRef]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—A systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef]
- Bavisetty, S.C.B.; Venkatachalam, K. Physicochemical qualities and antioxidant properties of juice extracted from ripe and overripe wax apple as affected by pasteurization and sonication. J. Food Process. Preserv. 2021, 45, e15524. [Google Scholar] [CrossRef]
- Cai, D.; Li, X.; Liu, H.; Wen, L.; Qu, D. Machine learning and flavoromics-based research strategies for determining the characteristic flavor of food: A review. Trends Food Sci. Tech. 2024, 154, 104794. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.; Harris, H.M.; Mattarelli, P.; O’toole, P.W.; Pot, B.; Vandamme, P.; Walter, J. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Maimaitiyiming, R.; Wang, L. Effects of four individual lactic acid bacteria on the physical and chemical and antioxidant properties of Kuqa apple juice during fermentation. J. Food Process. Preserv. 2021, 45, e15385. [Google Scholar] [CrossRef]
- Kaprasob, R.; Kerdchoechuen, O.; Laohakunjit, N.; Sarkar, D.; Shetty, K. Fermentation-based biotransformation of bioactive phenolics and volatile compounds from cashew apple juice by select lactic acid bacteria. Process Biochem. 2017, 59, 141–149. [Google Scholar] [CrossRef]
- Wu, C.; Li, T.; Qi, J.; Jiang, T.; Xu, H.; Lei, H. Effects of lactic acid fermentation-based biotransformation on phenolic profiles, antioxidant capacity and flavor volatiles of apple juice. LWT-Food Sci. Technol. 2020, 122, 109064. [Google Scholar] [CrossRef]
- Han, M.; Zhang, M.; Wang, X.; Bai, X.; Yue, T.; Gao, Z. Cloudy apple juice fermented by Lactobacillus prevents obesity via modulating gut microbiota and protecting intestinal tract health. Nutrients 2021, 13, 971. [Google Scholar] [CrossRef]
- Park, S.; Son, H.K.; Chang, H.C.; Lee, J.J. Effects of cabbage-apple juice fermented by Lactobacillus plantarum EM on lipid profile improvement and obesity amelioration in rats. Nutrients 2020, 12, 1135. [Google Scholar] [CrossRef]
- Valles-Colomer, M.; Menni, C.; Berry, S.E.; Valdes, A.M.; Spector, T.D.; Segata, N. Cardiometabolic health, diet and the gut microbiome: A meta-omics perspective. Nat. Med. 2023, 29, 551–561. [Google Scholar] [CrossRef]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153.e4114. [Google Scholar] [CrossRef]
- Meng, J.; Dong, P.-P.; Zhu, M.-X.; Zhang, Z.; Chen, J.-H.; Meng, Y.; Ding, C.-H.; Du, H.; Zheng, D.-G.; Du, L.-G. Anti-obesity effects of Lactiplantibacillus plantarum BHP03 on high-fat diet mice and its regulatory function on intestinal microbiota. Food Biosci. 2024, 61, 104786. [Google Scholar] [CrossRef]
- Sang, X.; Li, K.; Zhu, Y.; Ma, X.; Hao, H.; Bi, J.; Zhang, G.; Hou, H. The impact of microbial diversity on biogenic amines formation in grasshopper sub shrimp paste during the fermentation. Front. Microbiol. 2020, 11, 782. [Google Scholar] [CrossRef]
- Sang, X.; Ma, X.; Zhang, Y.; Hao, H.; Bi, J.; Zhang, G.; Hou, H. Assessment of the distribution and safety of Tetragenococcus muriaticus for potential application in the preparation of Chinese grasshopper sub shrimp paste. Front. Microbiol. 2021, 12, 628838. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Hou, H.M.; Zhang, G.L.; Wang, Y.F.; Hao, H.S. AHLs regulate biofilm formation and swimming motility of Hafnia alvei H4. Front. Microbiol. 2019, 10, 1330. [Google Scholar] [CrossRef]
- Peng, W.; Meng, D.; Yue, T.; Wang, Z.; Gao, Z. Effect of the apple cultivar on cloudy apple juice fermented by a mixture of Lactobacillus acidophilus, Lactobacillus plantarum, and Lactobacillus fermentum. Food Chem. 2021, 340, 127922. [Google Scholar] [CrossRef]
- Sang, X. Microbial Diversity of Chinese Grasshopper Sub Shrimp Paste and the Correlation of Biogenic Amines Production. Ph.D. Thesis, Dalian Polytechnic University, Dalian, China, 2020. [Google Scholar]
- Wu, D.-T.; Nie, X.-R.; Gan, R.-Y.; Guo, H.; Fu, Y.; Yuan, Q.; Zhang, Q.; Qin, W. In vitro digestion and fecal fermentation behaviors of a pectic polysaccharide from okra (Abelmoschus esculentus) and its impacts on human gut microbiota. Food Hydrocolloid 2021, 114, 106577. [Google Scholar] [CrossRef]
- Zhou, W.; Yan, Y.; Mi, J.; Zhang, H.; Lu, L.; Luo, Q.; Li, X.; Zeng, X.; Cao, Y. Simulated digestion and fermentation in vitro by human gut microbiota of polysaccharides from bee collected pollen of Chinese wolfberry. J. Agric. Food Chem. 2018, 66, 898–907. [Google Scholar] [CrossRef]
- Sang, X.; Guan, X.; Tong, Y.; Wang, F.; Zhou, B.; Li, Y.; Zhao, Q. Sulfated polysaccharides from sea cucumber cooking liquid prevents obesity by modulating gut microbiome, transcriptome, and metabolite profiles in mice fed a high-fat diet. Foods 2024, 13, 2017. [Google Scholar] [CrossRef]
- Tan, F.; Wang, B.; Li, L.; Yu, Q.; Cai, J.; Zhang, M. The effect of non-Saccharomyces yeasts on biogenic amines in Sauvignon Blanc wine and Gewürztraminer wine. Food Biosci. 2024, 59, 104153. [Google Scholar] [CrossRef]
- Liang, Z.; Chu, H.; Gao, L.; Sun, X.; Guo, S.; Guo, W.; He, J.; Hou, Z.; Wang, C.; Li, C. Effects of probiotics on biogenic amines content in fermented milk during fermentation and storage. J. Food Compos. Anal. 2024, 127, 105985. [Google Scholar] [CrossRef]
- Lei, W.; Liu, C.; Pan, L.; Peng, C.; Wang, J.; Zhou, H. Screening of probiotic Lactobacilli with potential anti-allergic activity based on hyaluronidase inhibition and degranulation of RBL-2H3 cells in vitro. LWT 2021, 140, 110707. [Google Scholar] [CrossRef]
- Woo, I.-K.; Hyun, J.-H.; Jang, H.J.; Lee, N.-K.; Paik, H.-D. Probiotic Pediococcus acidilactici strains exert anti-inflammatory effects by regulating intracellular signaling pathways in LPS-induced RAW 264.7 cells. In Probiotics and Antimicrobial Proteins; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–12. [Google Scholar]
- Flemming, H.-C.; Wuertz, S. Bacteria and archaea on Earth and their abundance in biofilms. Nat. Rev. Microbiol. 2019, 17, 247–260. [Google Scholar] [CrossRef]
- Yahav, S.; Berkovich, Z.; Ostrov, I.; Reifen, R.; Shemesh, M. Encapsulation of beneficial probiotic bacteria in extracellular matrix from biofilm-forming Bacillus subtilis. Artif. Cell Nanomed. B. 2018, 46, 974–982. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, D.; Zhang, L.; Qu, X. Research progress on the regulatory mechanism of biofilm formation in probiotic lactic acid bacteria. Crit. Rev. Food Sci. 2024, 8, 1–15. [Google Scholar] [CrossRef]
- Ma, N.; Cai, K.; Zhao, J.; Liu, C.; Li, H.; Tan, P.; Li, Y.; Li, D.; Ma, X. Mannosylated MOF encapsulated in Lactobacillus biofilm for dual-targeting intervention against mammalian Escherichia coli infections. Adv. Mater. 2025, 2503056. [Google Scholar] [CrossRef] [PubMed]
- Mgomi, F.C.; Zhang, B.; Lu, C.; Yang, Z.; Yuan, L. Novel biofilm-inspired encapsulation technology enhances the viability of probiotics during processing, storage, and delivery. Trends Food Sci. Tech. 2025, 160, 105032. [Google Scholar] [CrossRef]
- Wu, Y.; Li, S.; Tao, Y.; Li, D.; Han, Y.; Show, P.L.; Wen, G.; Zhou, J. Fermentation of blueberry and blackberry juices using Lactobacillus plantarum, Streptococcus thermophilus and Bifidobacterium bifidum: Growth of probiotics, metabolism of phenolics, antioxidant capacity in vitro and sensory evaluation. Food Chem. 2021, 348, 129083. [Google Scholar] [CrossRef]
- Zhu, D.; Ren, X.; Wei, L.; Cao, X.; Ge, Y.; Liu, H.; Li, J. Collaborative analysis on difference of apple fruits flavour using electronic nose and electronic tongue. Sci. Hortic. 2020, 260, 108879. [Google Scholar] [CrossRef]
- Jiao, W.; Sang, Y.; Wang, X.; Wang, S. Metabonomics and the gut microbiome analysis of the effect of 6-shogaol on improving obesity. Food Chem. 2023, 404, 134734. [Google Scholar] [CrossRef]
- Lee, N.R.; Kwon, T.J.; Chung, E.C.; Bae, J.; Soung, S.H.; Tak, H.J.; Choi, J.Y.; Lee, Y.E.; Hwang, N.W.; Lee, J.S. Combination of Lacticaseibacillus paracasei BEPC22 and Lactiplantibacillus plantarum BELP53 attenuates fat accumulation and alters the metabolome and gut microbiota in mice with high-fat diet-induced obesity. Food Funct. 2024, 15, 647–662. [Google Scholar] [CrossRef]
- Min, B.H.; Devi, S.; Kwon, G.H.; Gupta, H.; Jeong, J.-J.; Sharma, S.P.; Won, S.-M.; Oh, K.-K.; Yoon, S.J.; Park, H.J. Gut microbiota-derived indole compounds attenuate metabolic dysfunction-associated steatotic liver disease by improving fat metabolism and inflammation. Gut Microbes 2024, 16, 2307568. [Google Scholar] [CrossRef]
- Zhao, R.; Coker, O.O.; Wu, J.; Zhou, Y.; Zhao, L.; Nakatsu, G.; Bian, X.; Wei, H.; Chan, A.W.; Sung, J.J. Aspirin reduces colorectal tumor development in mice and gut microbes reduce its bioavailability and chemopreventive effects. Gastroenterology 2020, 159, 969–983. [Google Scholar] [CrossRef]
- Leite, G.; Pimentel, M.; Barlow, G.M.; Chang, C.; Hosseini, A.; Wang, J.; Parodi, G.; Sedighi, R.; Rezaie, A.; Mathur, R. Age and the aging process significantly alter the small bowel microbiome. Cell Rep. 2021, 36, 109765. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, L.; Wang, X.; Yi, Y.; Shan, Y.; Liu, B.; Zhou, Y.; Lü, X. Roles of intestinal Parabacteroides in human health and diseases. FEMS Microbiol. Lett. 2022, 369, fnac072. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, X.; Ho, C.L. Recent development of probiotic bifidobacteria for treating human diseases. Front. Bioeng. Biotech. 2021, 9, 770248. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, E.; Corr, S.C. Lactobacillus spp. for gastrointestinal health: Current and future perspectives. Front. Immunol. 2022, 13, 840245. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Yamashita, T.; Osone, T.; Hosooka, T.; Shinohara, M.; Kitahama, S.; Sasaki, K.; Sasaki, D.; Yoneshiro, T.; Suzuki, T. Bacteroides spp. promotes branched-chain amino acid catabolism in brown fat and inhibits obesity. Iscience 2021, 24, 103342. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, X.; Stamatiou, M.; Hambly, C.; Huang, Y.; Ma, J.; Li, Y.; Speakman, J.R. Higher than predicted resting energy expenditure and lower physical activity in healthy underweight Chinese adults. Cell Metab. 2022, 34, 1413–1415. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.; Zhang, C.; Dong, M.; Jiang, J.; Xu, H.; Yan, C.; Liu, X.; Zhou, H.; Zhang, H.; Chen, L. Myristoleic acid produced by Enterococci reduces obesity through brown adipose tissue activation. Gut. 2020, 69, 1239–1247. [Google Scholar] [CrossRef]
- Dong, T.; Hu, G.; Fan, Z.; Wang, H.; Gao, Y.; Wang, S.; Xu, H.; Yaffe, M.B.; Vander Heiden, M.G.; Lv, G. Activation of GPR3-β-arrestin2-PKM2 pathway in Kupffer cells stimulates glycolysis and inhibits obesity and liver pathogenesis. Nat. Commun. 2024, 15, 807. [Google Scholar] [CrossRef]
- Martin, A.J.; Serebrinsky-Duek, K.; Riquelme, E.; Saa, P.A.; Garrido, D. Microbial interactions and the homeostasis of the gut microbiome: The role of Bifidobacterium. Microbiome Res. Rep. 2023, 2, 17. [Google Scholar] [CrossRef]
- Song, X.; Qiao, L.; Dou, X.; Chang, J.; Zeng, X.; Deng, T.; Yang, G.; Liu, P.; Wang, C.; Xu, Q. Hypertriglyceridemia-modulated gut microbiota promotes lysophosphatidylcholine generation to aggravate acute pancreatitis in a TLR4-dependent manner. iMeta 2025, 4, e70003. [Google Scholar] [CrossRef]
- Griffin, M.E.; Espinosa, J.; Becker, J.L.; Luo, J.-D.; Carroll, T.S.; Jha, J.K.; Fanger, G.R.; Hang, H.C. Enterococcus peptidoglycan remodeling promotes checkpoint inhibitor cancer immunotherapy. Science 2021, 373, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Miyamoto, J.; Hisa, K.; Ohue-Kitano, R.; Takada, H.; Yamano, M.; Nishida, A.; Sasahara, D.; Masujima, Y.; Watanabe, K. Sucrose-preferring gut microbes prevent host obesity by producing exopolysaccharides. Nat. Commun. 2025, 16, 1145. [Google Scholar] [CrossRef]
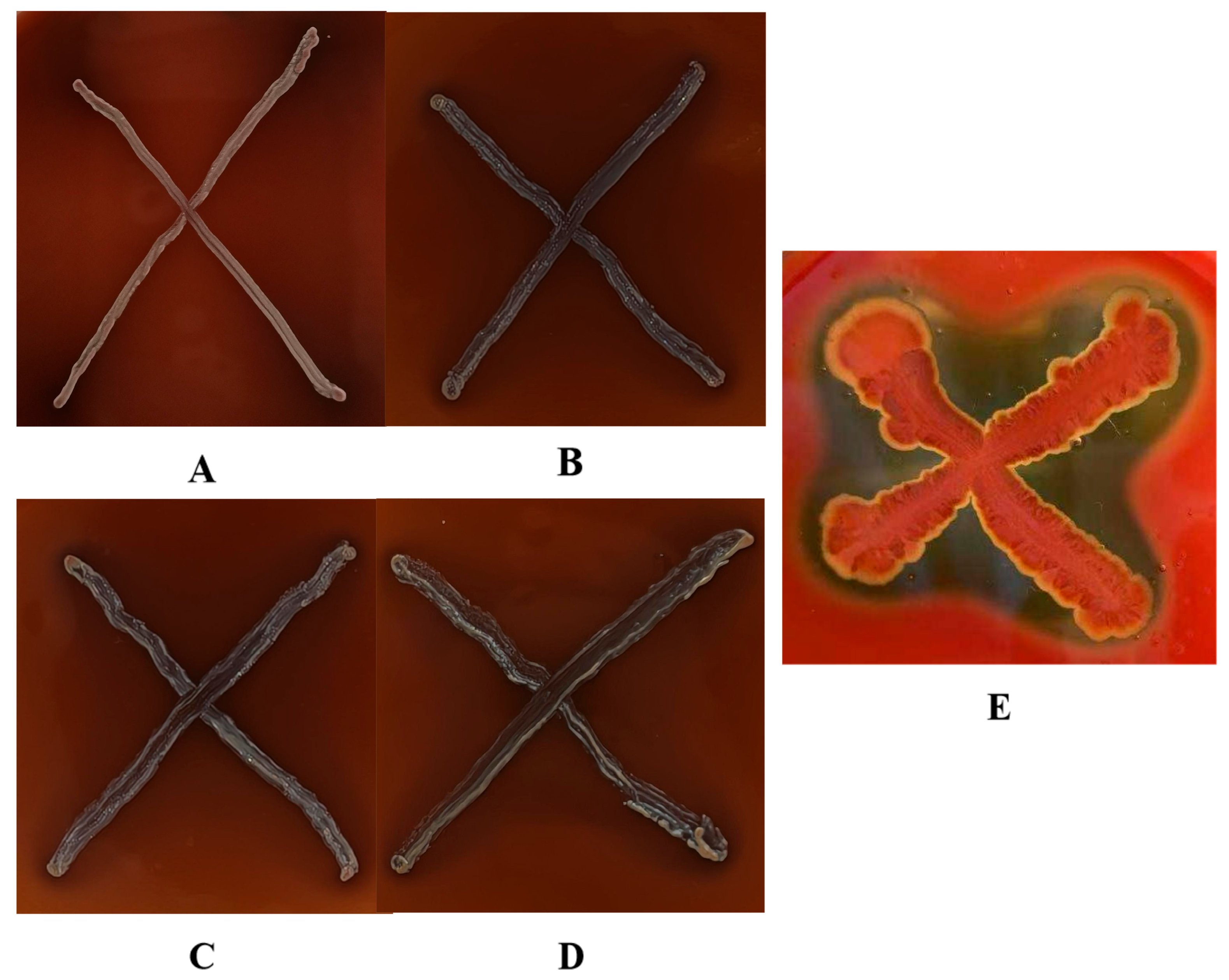
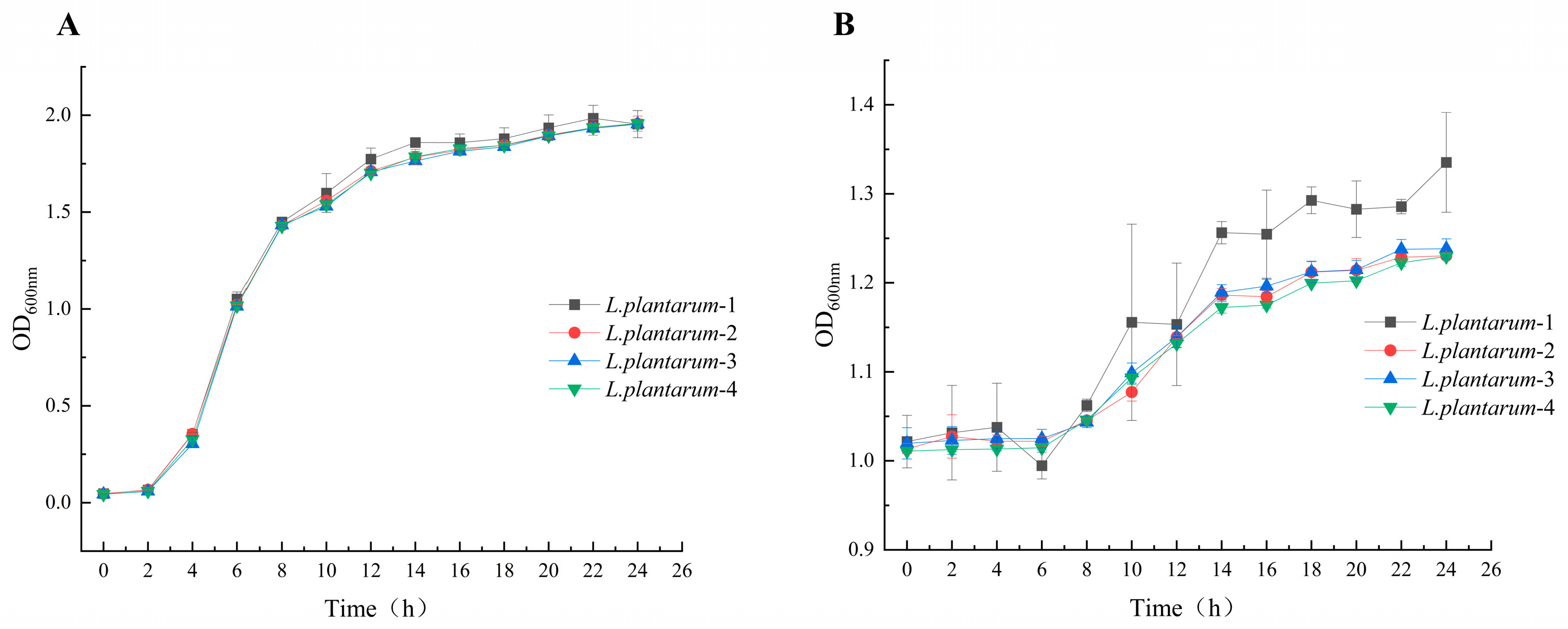
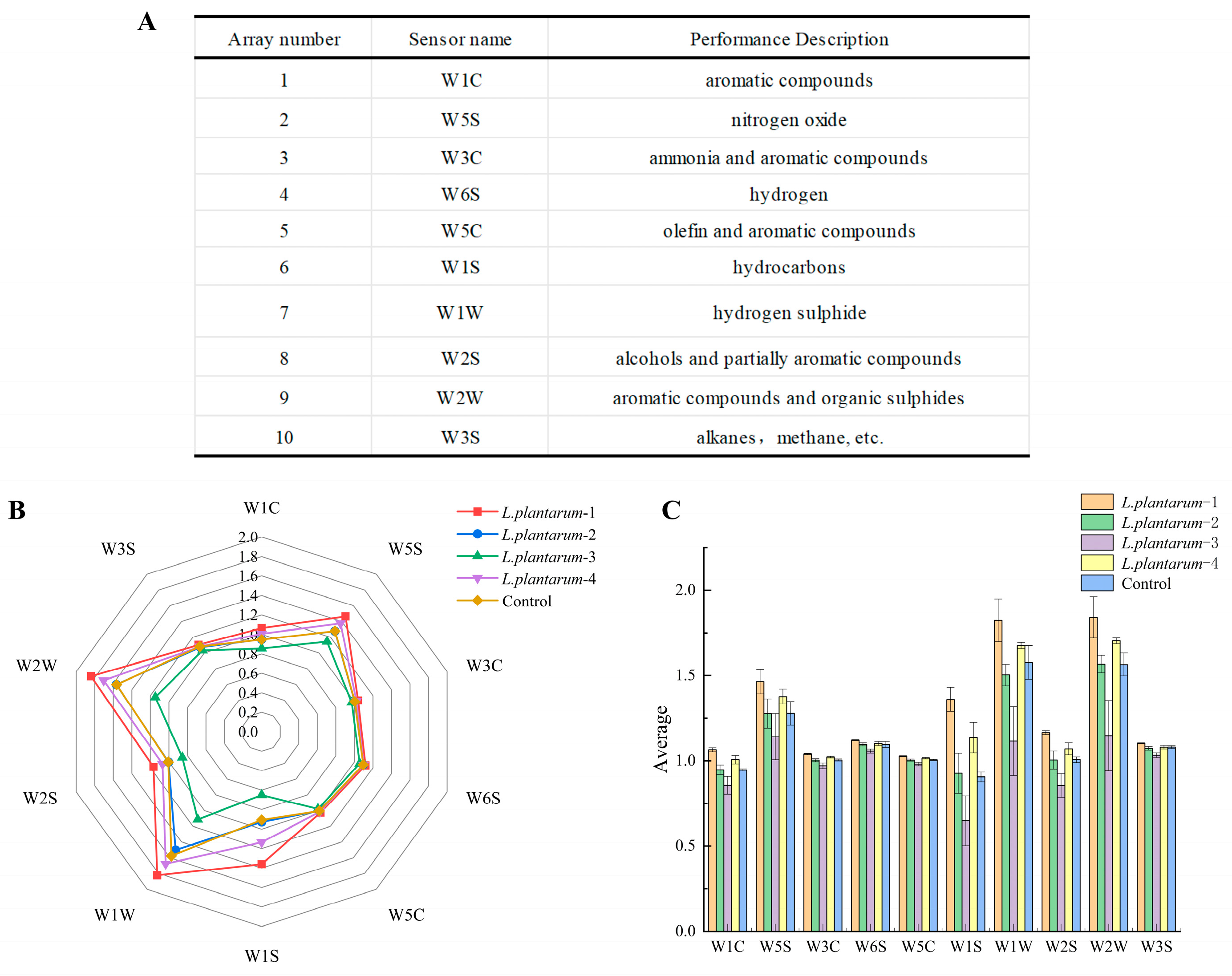
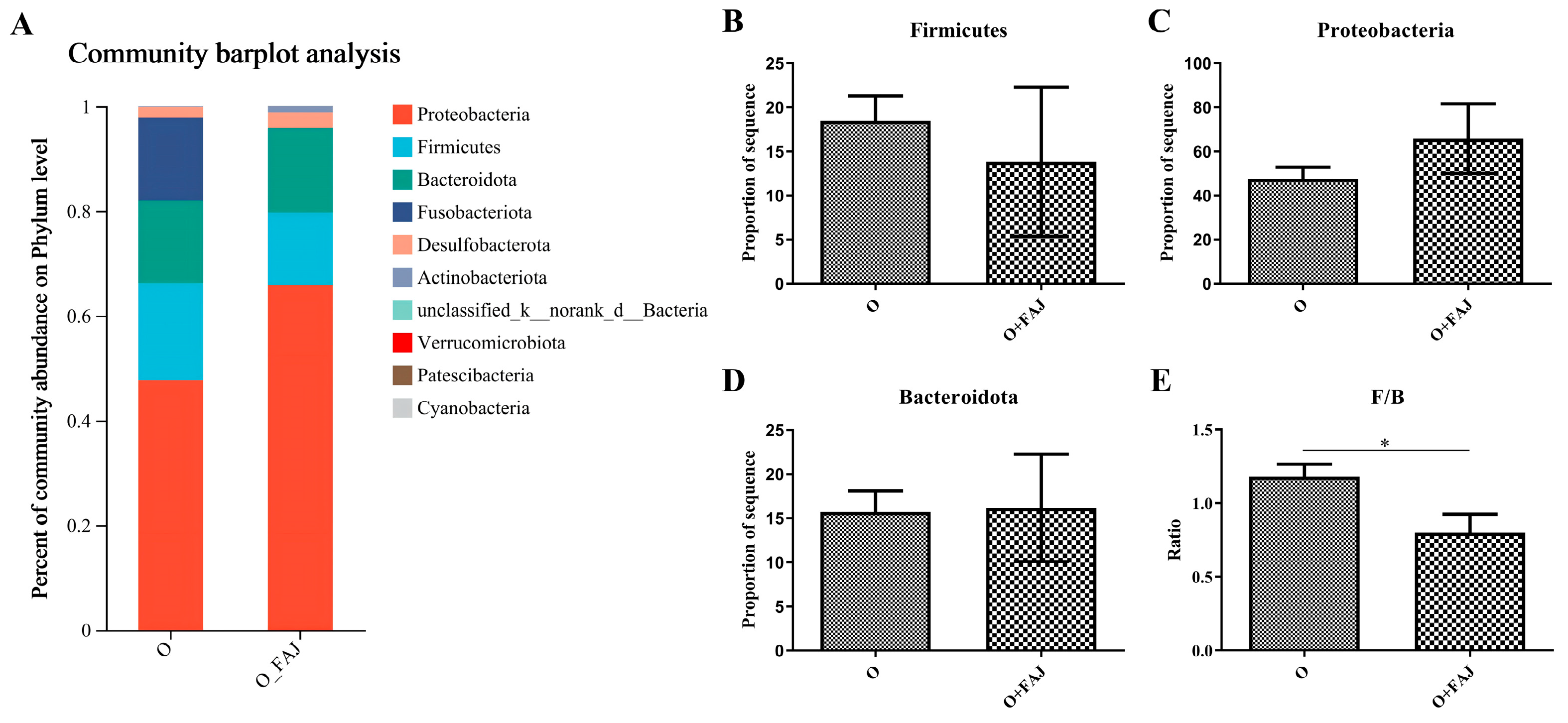
| Strains | mg/L | ||||
|---|---|---|---|---|---|
| Putrescine | Cadaverine | Histamine | Tyramine | Total BAs | |
| L. plantarum-1 | 30.55 ± 1.20 d | 0.59 ± 0.25 a | n.d. | n.d. | 31.14 ± 1.04 d |
| L. plantarum-2 | 33.52 ± 1.30 c | 0.30 ± 0.02 b | n.d. | n.d. | 33.82 ± 1.28 c |
| L. plantarum-3 | 47.28 ± 1.04 b | 0.15 ± 0.12 c | n.d. | n.d. | 47.43 ± 1.16 b |
| L. plantarum-4 | 50.74 ± 1.00 a | 0.61 ± 0.27 a | n.d. | n.d. | 51.35 ± 0.73 a |
| Strain Number | OD600nm | OD590nm |
|---|---|---|
| Control | −0.545 ± 0.229 | −0.029 ± 0.005 |
| Staphylococcus aureus ATCC6538 | 0.431 ± 0.101 a | 0.300 ± 0.038 a |
| L. plantarum-1 | 0.360 ± 0.108 a | 0.230 ± 0.004 b |
| L. plantarum-2 | 0.420 ± 0.142 a | 0.199 ± 0.017 b |
| L. plantarum-3 | 0.356 ± 0.090 a | 0.206 ± 0.022 b |
| L. plantarum-4 | 0.372 ± 0.059 a | 0.219 ± 0.009 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, B.; Xing, Z.; Wang, Y.; Guan, X.; Wang, F.; Yin, J.; Li, Z.; Zhao, Q.; Hou, H.; Sang, X. Apple Juice Fermented with Lactiplantibacillus plantarum Improves Its Flavor Profile and Probiotic Potential. Foods 2025, 14, 2373. https://doi.org/10.3390/foods14132373
Zhou B, Xing Z, Wang Y, Guan X, Wang F, Yin J, Li Z, Zhao Q, Hou H, Sang X. Apple Juice Fermented with Lactiplantibacillus plantarum Improves Its Flavor Profile and Probiotic Potential. Foods. 2025; 14(13):2373. https://doi.org/10.3390/foods14132373
Chicago/Turabian StyleZhou, Boqian, Zhuobin Xing, Yiting Wang, Xin Guan, Fuyi Wang, Jiaqi Yin, Zhibo Li, Qiancheng Zhao, Hongman Hou, and Xue Sang. 2025. "Apple Juice Fermented with Lactiplantibacillus plantarum Improves Its Flavor Profile and Probiotic Potential" Foods 14, no. 13: 2373. https://doi.org/10.3390/foods14132373
APA StyleZhou, B., Xing, Z., Wang, Y., Guan, X., Wang, F., Yin, J., Li, Z., Zhao, Q., Hou, H., & Sang, X. (2025). Apple Juice Fermented with Lactiplantibacillus plantarum Improves Its Flavor Profile and Probiotic Potential. Foods, 14(13), 2373. https://doi.org/10.3390/foods14132373








