Efficient Hydrolysis of Earthworm Protein and the Lipid-Lowering Mechanism of Peptides in the Hydrolysate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Earthworm Protein Hydrolysate Production (EPH)
2.3. Degree of Hydrolysis (DH)
2.4. Soluble Peptide Yield (YSP)
2.5. Molecular Weight Distribution
2.6. Isolation of EPH
2.7. L02 Cell Culture, Modeling, and Treatment
2.8. Oil Red O Staining and Quantitative Analysis
2.9. Sequence Identification Analysis by LC-MS/MS
2.10. Western Blotting
2.11. Transcriptome Analysis
2.12. Real-Time Quantitative Polymerase Chain Reaction Validation (RT-qPCR)
2.13. Statistical Analysis
3. Results
3.1. Hydrolysis of Earthworm Proteins
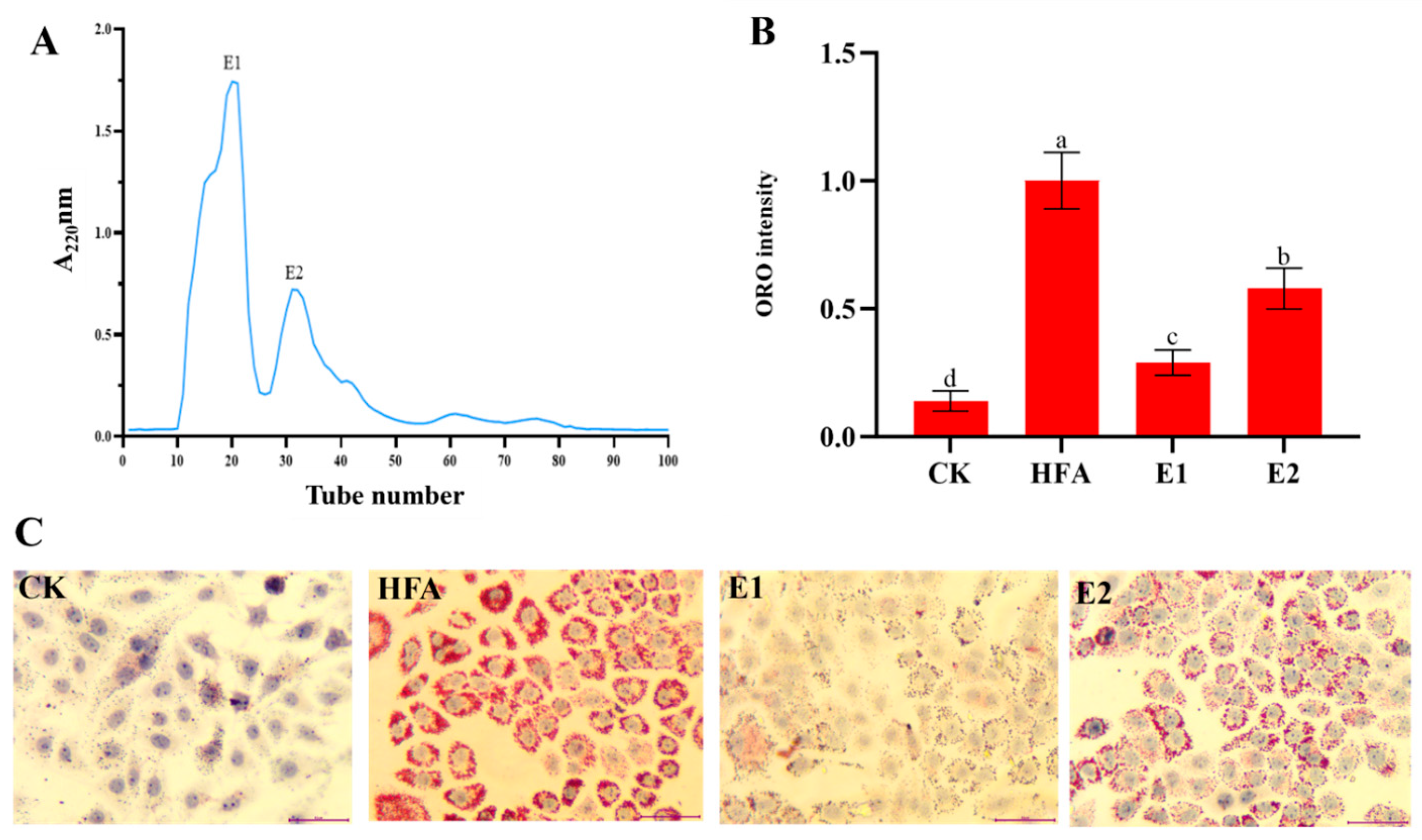
3.2. Lipid-Lowering Activity of EPH and Its Ultrafiltration Components
3.3. Isolation and Identification of Lipid-Lowering Peptides in <3 kDa Fractions
3.4. Effects of Earthworm Lipid-Lowering Peptides on the Levels of Crucial Proteins in Lipid Metabolism
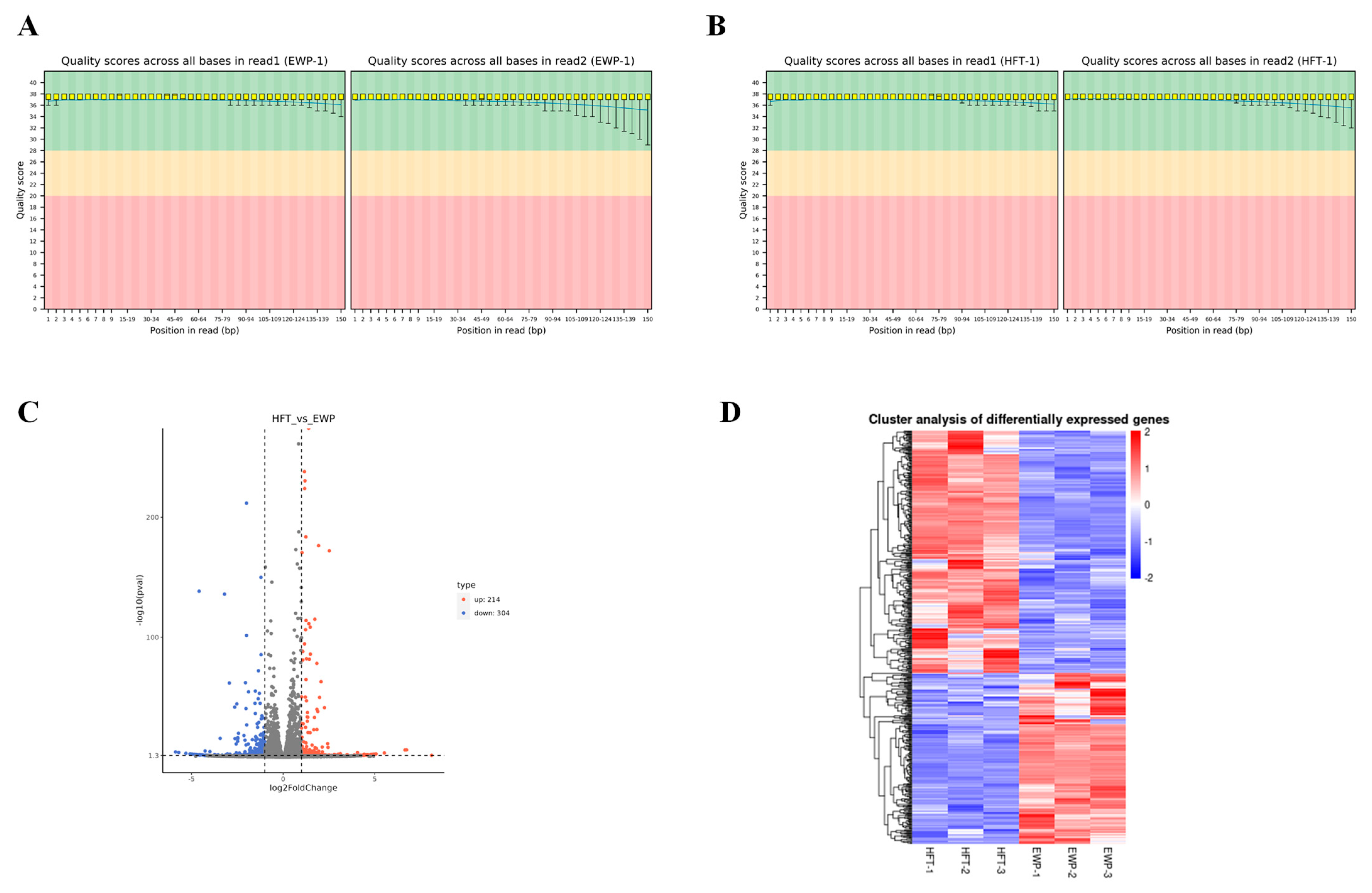
3.5. Transcriptome Analysis
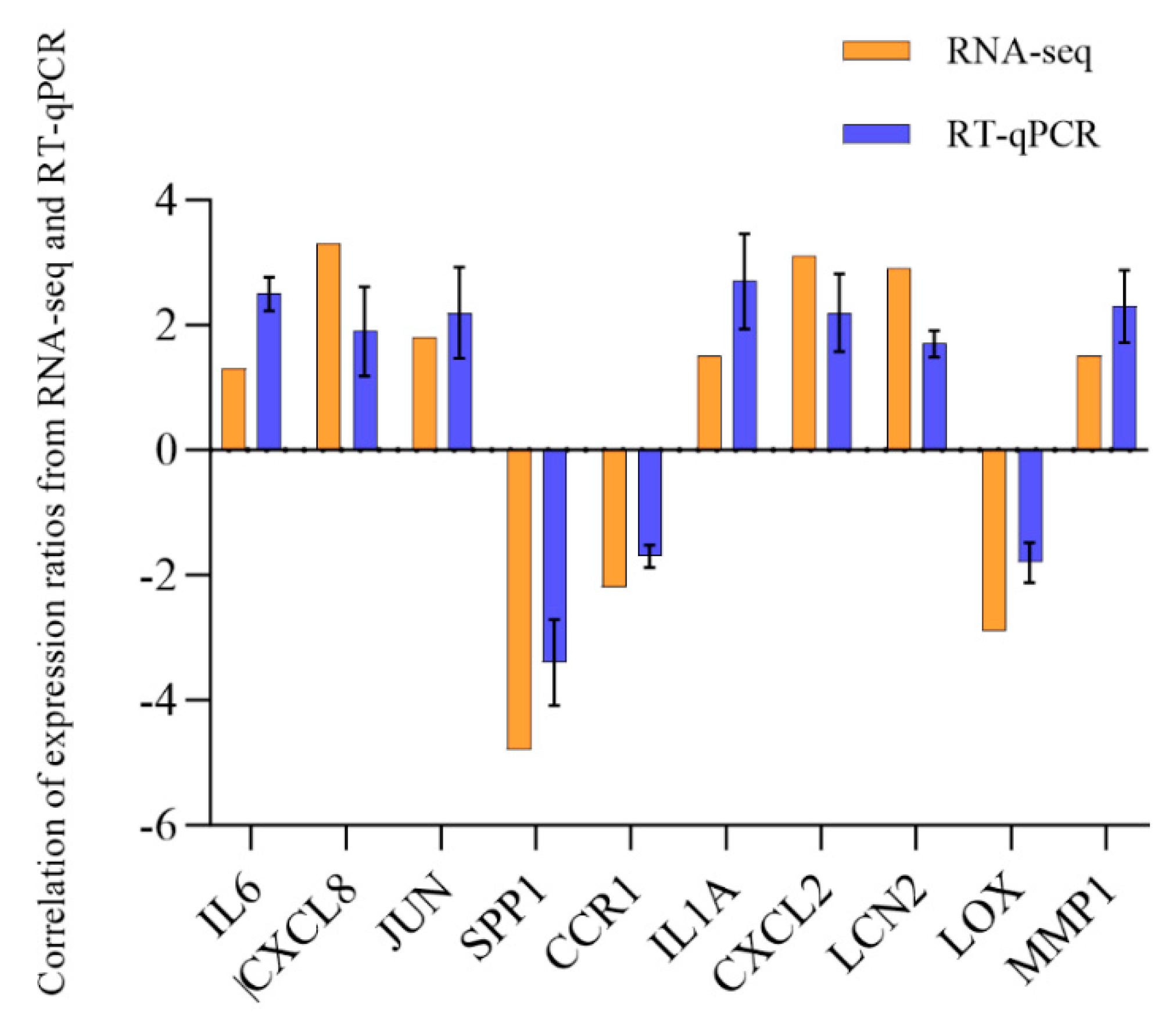
3.6. Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, P.; Zhang, Y.; Zhang, Y.; Zhang, L.; Lin, Z.; Sun, C.; Wu, H.; Zhang, M. Isolation, identification of antioxidant peptides from earthworm proteins and analysis of the structure–activity relationship of the peptides based on quantum chemical calculations. Food Chem. 2024, 431, 137137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, P.; Pan, L.; Lin, Z.; Yang, J.; Wu, H.; Zhang, M. Immunomodulatory effect of earthworm protein autolysates on Cyclophosphamide(CTX)-Induced immunosuppressed mice. Food Biosci. 2023, 56, 103297. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, H.; Zhang, M. Isolation, identification, and structure-activity relationship of novel ACE inhibitory peptides from earthworm protein in vitro gastrointestinal digestion product. Food Biosci. 2023, 55, 103010. [Google Scholar] [CrossRef]
- Zhao, M.; Li, B.; He, H.; Hou, T. Preparation, identification, computational analysis of antioxidative peptides derived from Lumbricus protein and prevention of UV-B radiation-induced skin damaged. Food Biosci. 2023, 52, 102492. [Google Scholar] [CrossRef]
- Nikoo, M.; Xu, X.; Regenstein, J.M.; Noori, F. Autolysis of Pacific white shrimp (Litopenaeus vannamei) processing by-products: Enzymatic activities, lipid and protein oxidation, and antioxidant activity of hydrolysates. Food Biosci. 2021, 39, 100844. [Google Scholar] [CrossRef]
- Gaggini, M.; Vassalle, C. Lipids Metabolism and Cardiometabolic Diseases. Int. J. Mol. Sci. 2023, 24, 17460. [Google Scholar] [CrossRef]
- Xu, L.; Yang, Q.; Zhou, J. Mechanisms of Abnormal Lipid Metabolism in the Pathogenesis of Disease. Int. J. Mol. Sci. 2024, 25, 8465. [Google Scholar] [CrossRef]
- Kawakami, T.; Fujikawa, A.; Ishiyama, Y.; Hosojima, M.; Saito, A.; Kubota, M.; Fujimura, S.; Kadowaki, M. Protective effect of composite earthworm powder against diabetic complications via increased fibrinolytic function and improvement of lipid metabolism in ZDF rats. Biosci. Biotechnol. Biochem. 2016, 80, 1980–1989. [Google Scholar] [CrossRef][Green Version]
- Deng, Z.; Gao, S.; An, Y.; Huang, Y.; Liu, H.; Zhu, W.; Lu, W.; He, M.; Xie, W.; Yu, D.; et al. Effects of earthworm extract on the lipid profile and fatty liver induced by a high-fat diet in guinea pigs. Ann. Transl. Med. 2021, 9, 292. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, F.; Chen, S.; Zhao, Y.; Huang, X.; Huang, F.; Li, C. Improvement mechanism of umami peptides in oyster juice by cooperative enzymolysis of alcalase and trypsin based on peptidomics and molecular docking. J. Food Compos. Anal. 2024, 132, 106326. [Google Scholar] [CrossRef]
- Dhaval, A.; Yadav, N.; Purwar, S. Potential Applications of Food Derived Bioactive Peptides in Management of Health. Int. J. Pept. Res. Ther. 2016, 22, 377–398. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, M.; Zhou, Q.; Yang, Y.; Du, L.; Gao, X. Preparation, identification and in vivo study of antioxidant peptides from Haematococcus pluvialis residue. Food Biosci. 2025, 66, 106140. [Google Scholar] [CrossRef]
- Wei, K.; Wei, Y.; Xu, W.; Lu, F.; Ma, H. Corn peptides improved obesity-induced non-alcoholic fatty liver disease through relieving lipid metabolism, insulin resistance and oxidative stress. Food Funct. 2022, 13, 5782–5793. [Google Scholar] [CrossRef]
- Ye, H.; Xu, Y.; Sun, Y.; Liu, B.; Chen, B.; Liu, G.; Cao, Y.; Miao, J. Purification, identification and hypolipidemic activities of three novel hypolipidemic peptides from tea protein. Food Res. Int. 2023, 165, 112450. [Google Scholar] [CrossRef]
- Qin, S.; Wang, J.; Yuan, H.; He, J.; Luan, S.; Deng, Y. Liver function indicators and risk of hepatocellular carcinoma: A bidirectional mendelian randomization study. Front. Genet. 2024, 14, 1260352. [Google Scholar] [CrossRef]
- Yang, F.; Huang, J.; He, H.; Ju, X.; Ji, Y.; Deng, F.; Wang, Z.; He, R. Study on the hypolipidemic activity of rapeseed protein-derived peptides. Food Chem. 2023, 423, 136315. [Google Scholar] [CrossRef] [PubMed]
- Giammanco, A.; Spina, R.; Cefalù, A.B.; Averna, M. APOC-III: A Gatekeeper in Controlling Triglyceride Metabolism. Curr. Atheroscler. Rep. 2023, 25, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Sun, X. Cholesterol Metabolism: A Double-Edged Sword in Hepatocellular Carcinoma. Front. Cell. Dev. Biol. 2021, 9, 762828. [Google Scholar] [CrossRef]
- Libby, P.; Tokgözoğlu, L. Chasing LDL cholesterol to the bottom—PCSK9 in perspective. Nat. Cardiovasc. Res. 2022, 1, 554–561. [Google Scholar] [CrossRef]
- Li, N.; Li, X.; Ding, Y.; Liu, X.; Diggle, K.; Kisseleva, T.; Brenner, D.A. SREBP Regulation of Lipid Metabolism in Liver Disease, and Therapeutic Strategies. Biomedicines 2023, 11, 3280. [Google Scholar] [CrossRef]
- Sekar, M.; Thirumurugan, K. Autophagy: A molecular switch to regulate adipogenesis and lipolysis. Mol. Cell. Biochem. 2022, 477, 727–742. [Google Scholar] [CrossRef]
- Hu, M.; Yang, F.; Huang, Y.; You, X.; Liu, D.; Sun, S.; Sui, S. Structural insights into the mechanism of human NPC1L1-mediated cholesterol uptake. Sci. Adv. 2021, 7, eabg3188. [Google Scholar] [CrossRef] [PubMed]
- Montaigne, D.; Butruille, L.; Staels, B. PPAR control of metabolism and cardiovascular functions. Nat. Rev. Cardiol. 2021, 18, 809–823. [Google Scholar] [CrossRef]
- Kurano, M.; Hara, M.; Tsuneyama, K.; Okamoto, K.; Iso-O, N.; Matsushima, T.; Koike, K.; Tsukamoto, K. Modulation of lipid metabolism with the overexpression of NPC1L1 in mouse liver. J. Lipid. Res. 2012, 53, 2275–2285. [Google Scholar] [CrossRef]
- Yu Cai Lim, M.; Kiat Ho, H. Pharmacological modulation of cholesterol 7α-hydroxylase (CYP7A1) as a therapeutic strategy for hypercholesterolemia. Biochem. Pharmacol. 2024, 220, 115985. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Y.; Chen, B.; Xu, M.; Liu, S.; Su, Y.; Qiao, K.; Liu, Z. Advances in Research on Marine-Derived Lipid-Lowering Active Substances and Their Molecular Mechanisms. Nutrients 2023, 15, 5118. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.; Cui, Z.; Huang, X.; Zhang, D.; Guo, R.; Han, M. Inflammation and atherosclerosis: Signaling pathways and therapeutic intervention. Signal Transduct. Target. Ther. 2022, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Malekmohammad, K.; Bezsonov, E.E.; Rafieian-Kopaei, M. Role of Lipid Accumulation and Inflammation in Atherosclerosis: Focus on Molecular and Cellular Mechanisms. Front. Cardiovasc. Med. 2021, 8, 707529. [Google Scholar] [CrossRef]
- Cai, X.; Fang, C.; Hayashi, S.; Hao, S.; Zhao, M.; Tsutsui, H.; Nishiguchi, S.; Sheng, J. Pu-erh tea extract ameliorates high-fat diet-induced nonalcoholic steatohepatitis and insulin resistance by modulating hepatic IL-6/STAT3 signaling in mice. J. Gastroenterol. 2016, 51, 819–829. [Google Scholar] [CrossRef]
- Serrano-Marco, L.; Rodríguez-Calvo, R.; El Kochairi, I.; Palomer, X.; Michalik, L.; Wahli, W.; Vázquez-Carrera, M. Activation of Peroxisome Proliferator–Activated Receptor-β/-δ (PPAR-β/-δ) Ameliorates Insulin Signaling and Reduces SOCS3 Levels by Inhibiting STAT3 in Interleukin-6–Stimulated Adipocytes. Diabetes 2011, 60, 1990–1999. [Google Scholar] [CrossRef]
- Yuan, Q.; Tang, B.; Zhang, C. Signaling pathways of chronic kidney diseases, implications for therapeutics. Signal Transduct. Target. Ther. 2022, 7, 182. [Google Scholar] [CrossRef] [PubMed]
- Gierens, H.; Nauck, M.; Roth, M.; Schinker, R.; Schürmann, C.; Scharnagl, H.; Neuhaus, G.; Wieland, H.; März, W. Interleukin-6 Stimulates LDL Receptor Gene Expression via Activation of Sterol-Responsive and Sp1 Binding Elements. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1777–1783. [Google Scholar] [CrossRef]
- Momtazi-Borojeni, A.A.; Sabouri-Rad, S.; Gotto, A.M.J.; Pirro, M.; Banach, M.; Awan, Z.; Barreto, G.E.; Sahebkar, A. PCSK9 and inflammation: A review of experimental and clinical evidence. Eur. Heart J.-Cardiovasc. Pharmacother. 2019, 5, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Pan, X.; Li, Y.; Li, Y.; Zhao, X.; Lai, K.; Wei, J. Total Flavonoids of Engelhardia roxburghiana Wall. Attenuated Nonalcoholic Fatty Liver Disease by Regulating Lipid Metabolism and Inflammation Through Targeting SREBP1 and PPARα. Chem. Biodivers. 2025, e00190. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Shin, D.; Kim, K.; Lee, J.; Paik, T.; Jo, E. Roles of Reactive Oxygen Species in CXCL8 and CCL2 Expression in Response to the 30-kDa Antigen of Mycobacterium tuberculosis. J. Clin. Immunol. 2009, 29, 46–56. [Google Scholar] [CrossRef]
- Wang, H.; Huang, X.; Xu, P.; Liu, X.; Zhou, Z.; Wang, F.; Li, J.; Wang, Y.; Xian, X.; Liu, G.; et al. Apolipoprotein C3 aggravates diabetic nephropathy in type 1 diabetes by activating the renal TLR2/NF-κB pathway. Metabolism 2021, 119, 154740. [Google Scholar] [CrossRef]
- Tao, D.; Li, Y.; Tian, X.; Liao, X.; Yu, Z.; Xiang, Z. Effect of FoxO1 on Cardiomyocyte Apoptosis and Inflammation in Viral Myocarditis via Modultion of the TLR4/NF-κB Signaling Pathway. Int. Heart J. 2023, 64, 732–740. [Google Scholar] [CrossRef]
- Bhosale, P.B.; Kim, H.H.; Abusaliya, A.; Vetrivel, P.; Ha, S.E.; Park, M.Y.; Lee, H.J.; Kim, G.S. Structural and Functional Properties of Activator Protein-1 in Cancer and Inflammation. Evid.-Based Complement Altern. Med. 2022, 2022, 9797929. [Google Scholar] [CrossRef]
- Fan, F.; Podar, K. The Role of AP-1 Transcription Factors in Plasma Cell Biology and Multiple Myeloma Pathophysiology. Cancers 2021, 13, 2326. [Google Scholar] [CrossRef]
- Du, S.; Li, Z.; Xie, X.; Xu, C.; Shen, X.; Wang, N.; Shen, Y. IL-17 stimulates the expression of CCL2 in cardiac myocytes via Act1/TRAF6/p38MAPK-dependent AP-1 activation. Scand. J. Immunol. 2020, 91, e12840. [Google Scholar] [CrossRef]
- Du, M.; Wang, Y.; Liu, Z.; Wang, L.; Cao, Z.; Zhang, C.; Hao, Y.; He, H. Effects of IL-1β on MMP-9 Expression in Cementoblast-Derived Cell Line and MMP-Mediated Degradation of Type I Collagen. Inflammation 2019, 42, 413–425. [Google Scholar] [CrossRef]
- Ding, C.; Xiao, T.; Deng, Y.; Yang, H.; Xu, B.; Li, J.; Lv, Z. The Teleost CXCL13–CXCR5 Axis Induces Inflammatory Cytokine Expression through the Akt–NF-κB, p38–AP-1, and p38–NF-κB Pathways. J. Immunol. 2023, 212, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Cai, Q.; Wang, S.; Li, L.; Wang, Y.; Zou, S.; Gao, X.; Wei, Y. The Ameliorative Effect and Mechanisms of Ruditapes philippinarum Bioactive Peptides on Obesity and Hyperlipidemia Induced by a High-Fat Diet in Mice. Nutrients 2022, 14, 5066. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Shen, W.; Weng, P.; Wu, Z.; Liu, Y. Novel Chlorella pyrenoidosa peptides attenuate weight gain, insulin resistance, and lipid levels in mice fed a high-fat diet. J. Funct. Foods. 2024, 112, 105908. [Google Scholar] [CrossRef]
- Dong, X.; Ma, Y.; Xie, Y.; Cui, W.; Zhou, H.; Zhou, K.; Xu, F.; Xu, B. Identification and Mechanism Elucidation of Anti-Inflammatory Peptides in Jinhua Ham: An Integrative In Silico and In Vitro Study. J. Agric. Food. Chem. 2023, 71, 17097–17111. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, F.; Cao, Y.; Li, B.; Zhang, S.; Zhang, Y. Peptide GTSFTTTAER From Rapana venosa Alleviates TNBS-Induced Inflammatory Bowel Disease in a Zebrafish Model via Multi-Pathway Regulation. Food Sci. Nutr. 2025, 13, e70427. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, H.; Chen, F.; Guo, X.; Liu, Q.; Hou, J. The double-edged effects of IL-6 in liver regeneration, aging, inflammation, and diseases. Exp. Hematol. Oncol. 2024, 13, 62. [Google Scholar] [CrossRef]
- Huang, F.; Zhao, N.; Cai, P.; Hou, M.; Yang, S.; Zheng, B.; Ma, Q.; Jiang, J.; Gai, X.; Mao, Y.; et al. Active AKT2 stimulation of SREBP1/SCD1-mediated lipid metabo-lism boosts hepatosteatosis and cancer. Transl. Res. 2024, 268, 51–62. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, C.Y. G protein-coupled receptors as potential targets for nonalco-holic fatty liver disease treatment. World J. Gastroenterol. 2021, 27, 677–691. [Google Scholar] [CrossRef]
- Chaudhary, P.K.; Kim, S. An Insight into GPCR and G-Proteins as Cancer Drivers. Cells 2021, 10, 3288. [Google Scholar] [CrossRef]
- Li, S.; Yu, Y.; Wang, B.; Qiao, S.; Hu, M.; Wang, H.; Fu, C.; Dong, B. Overexpression of G Protein-Coupled Receptor 40 Protects Obesity-Induced Cardiomyopathy Through the SIRT1/LKB1/AMPK Pathway. Hum. Gene Ther. 2022, 33, 598–613. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yu, W.; Kou, X.; Niu, Y.; Ji, J.; Shao, Y.; Wu, S.; Liu, M.; Xue, Z. Recent advances in the effect of simulated gastrointestinal digestion and encapsulation on peptide bioactivity and stability. Food Funct. 2025, 16, 1634–1655. [Google Scholar] [CrossRef] [PubMed]
- Wen, K.; Luo, D.; Xie, J.; Xue, B.; Bian, X.; Sun, T. Encapsulation of Antarctic krill peptide by polysaccharide-based nanoparticles: Preparation, characterization, evaluation of stability and hypoglycemic activity. Food Biosci. 2024, 62, 105458. [Google Scholar] [CrossRef]









| Molecular Weight Range (Da) | Percentage of Peak Area (%) |
|---|---|
| >10,000 | 0.91 |
| 10,000~5000 | 3.00 |
| 5000~3000 | 1.89 |
| 3000~2000 | 2.66 |
| 2000~1000 | 8.66 |
| 1000~500 | 20.48 |
| 500~180 | 36.51 |
| <180 | 25.88 |
| Signaling Pathway | Genes |
|---|---|
| IL-17 signaling pathway | IL6/CXCL8/CXCL3/CXCL2/FOSL1/CCL20/JUN/LCN2/ FOSB/MMP1/MMP3/S100A8/MAPK15/MMP13 |
| Cytokine–cytokine receptor interaction | IL6/CXCL8/CXCL3/CXCL2/CCL20/IL11/TNFRSF9/GDF15/INHBA/IL13RA2/ IL24/IL1A/NGFR/IL20/CCR3/TNFSF10/CCR1/INHBC/TSLP/TNFRSF8 |
| Rheumatoid arthritis | IL6/CXCL8/CXCL3/CXCL2/CCL20/JUN/IL11/IL1A/HLA-DQB1/MMP1/MMP3/ANGPT1/HLA-DQA1 |
| Staphylococcus aureus infection | KRT17/KRT16/HLA-DQB1/ FGG/KRT14/CAMP/KRT34/LOC100653049/CFI/HLA-DQA1 |
| Hematopoietic cell lineage | IL6/IL11/IL1A/HLA-DQB1/CD36/CR2/CD3E/CD5/ITGA4/HLA-DQA1 |
| Viral protein interaction with cytokine and cytokine receptor | IL6/CXCL8/CXCL3/CXCL2/CCL20/IL24/IL20/CCR3/TNFSF10/CCR1 |
| Amoebiasis | IL6/CXCL8/CXCL3/CXCL2/SERPINB4/SERPINB3/GNA14/PRKCG/COL3A1 |
| Leishmaniasis | JUN/IL1A/HLA-DQB1/NCF2/NCF1/ITGA4/HLA-DQA1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Mai, X.; Yang, S.; Huang, Y.; Zhang, L.; Ren, W.; Bai, W.; Xin, X.; Zhao, W.; Hao, L. Efficient Hydrolysis of Earthworm Protein and the Lipid-Lowering Mechanism of Peptides in the Hydrolysate. Foods 2025, 14, 2338. https://doi.org/10.3390/foods14132338
Zhang M, Mai X, Yang S, Huang Y, Zhang L, Ren W, Bai W, Xin X, Zhao W, Hao L. Efficient Hydrolysis of Earthworm Protein and the Lipid-Lowering Mechanism of Peptides in the Hydrolysate. Foods. 2025; 14(13):2338. https://doi.org/10.3390/foods14132338
Chicago/Turabian StyleZhang, Mengmeng, Xiang Mai, Shanghua Yang, Yuhua Huang, Lina Zhang, Wenbin Ren, Weidong Bai, Xuan Xin, Wenhong Zhao, and Lisha Hao. 2025. "Efficient Hydrolysis of Earthworm Protein and the Lipid-Lowering Mechanism of Peptides in the Hydrolysate" Foods 14, no. 13: 2338. https://doi.org/10.3390/foods14132338
APA StyleZhang, M., Mai, X., Yang, S., Huang, Y., Zhang, L., Ren, W., Bai, W., Xin, X., Zhao, W., & Hao, L. (2025). Efficient Hydrolysis of Earthworm Protein and the Lipid-Lowering Mechanism of Peptides in the Hydrolysate. Foods, 14(13), 2338. https://doi.org/10.3390/foods14132338






