Physicochemical and Perceived Olfactory Changes in Black Soldier Fly (Hermetia illucens) Larvae Oil Under Domestic Cooking Temperatures
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Reference Cooking Oils
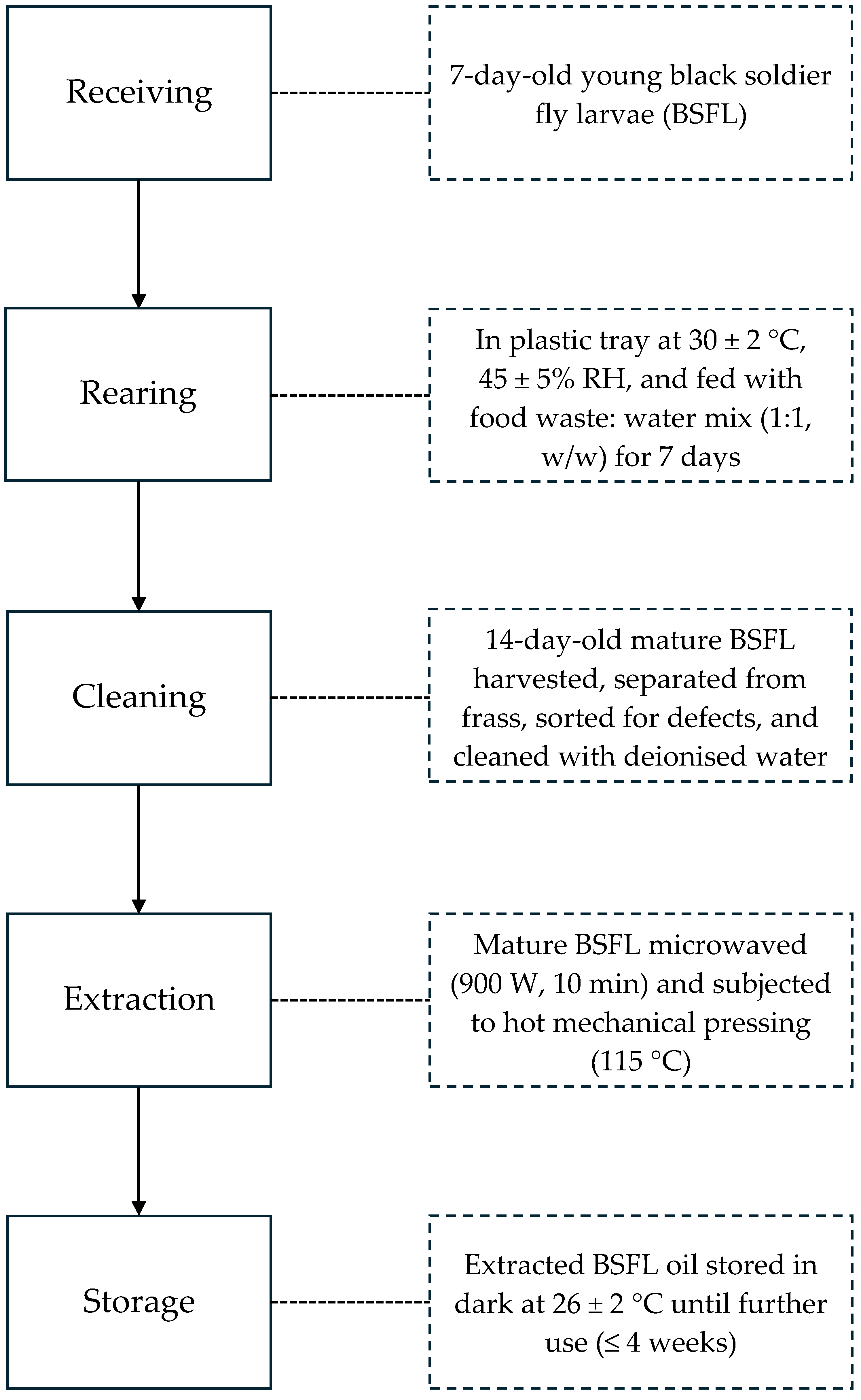
2.3. Black Soldier Fly Larvae Rearing and Oil Extraction
2.4. Exposure of BSFL Oil to Domestic Cooking Conditions
2.5. Physicochemical Analyses
2.6. Odour Characterisation
2.7. Statistical Analyses
3. Results and Discussion
3.1. Fatty Acid Composition of Oils Prior to Heating
3.2. Physicochemical Properties of Oils Prior to Heating
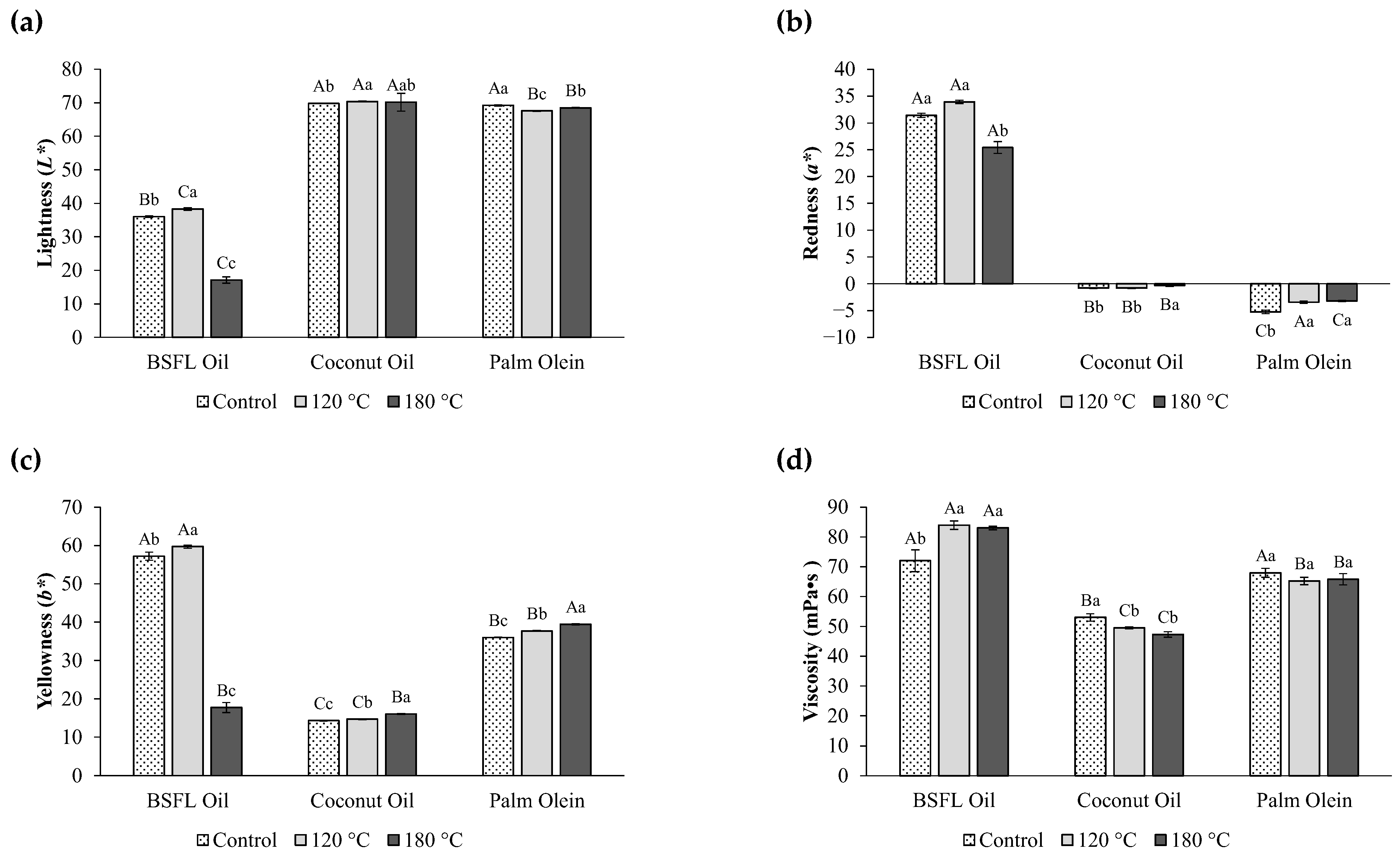
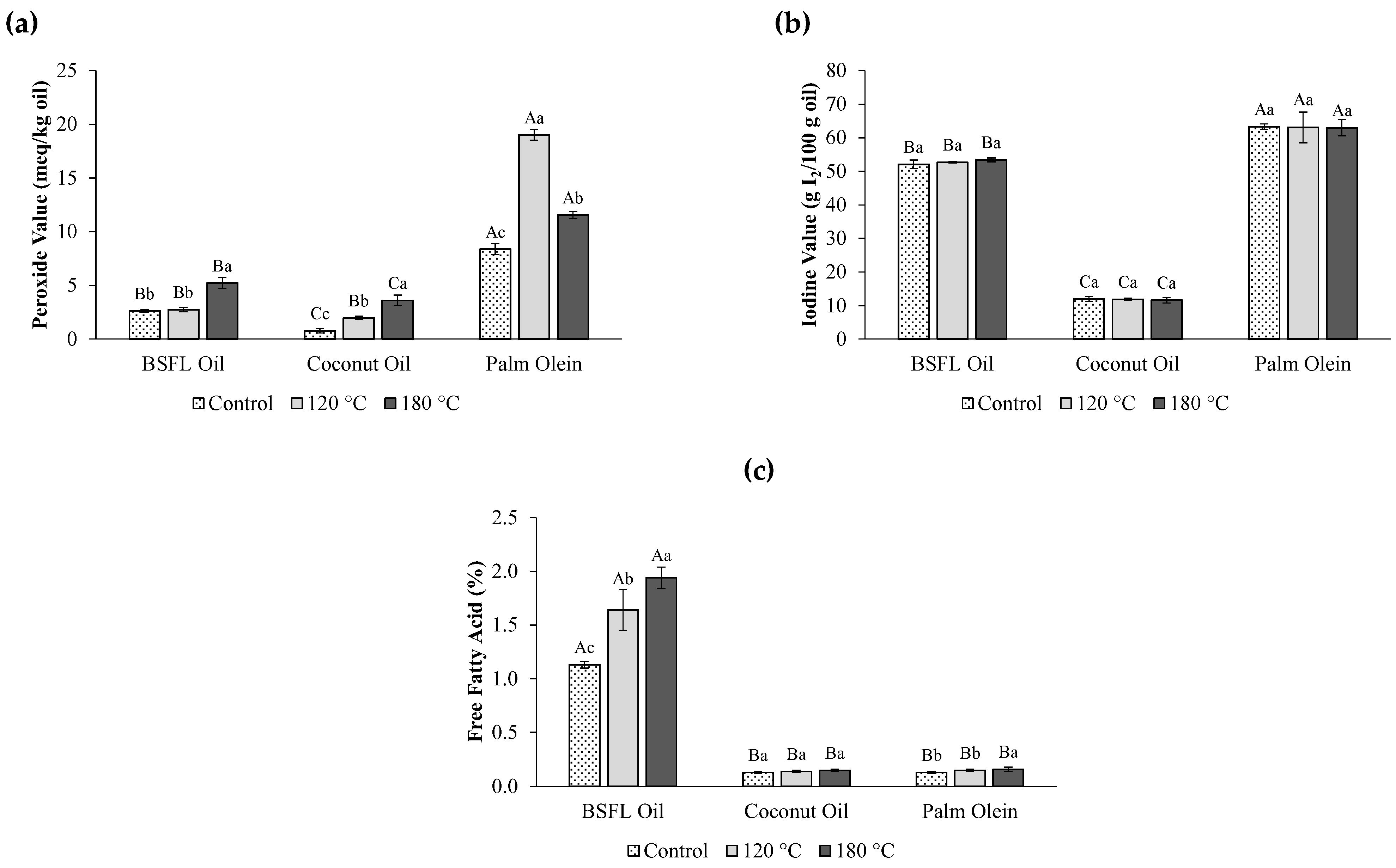
3.3. Physicochemical Properties of Oils After Heating
3.4. Changes in the Perceived Odour Intensity of BSFL Oils With and Without Heating
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tanga, C.M.; Xavier, C.; Kababu, M.; Subramanian, S.; Beesigamukama, D.; Shaphan, C.Y. Insect oils and food safety regulations. In Insect Oil as a Source of Nutraceuticals; Mariod, A.A., Ed.; Academic Press: Oxford, UK, 2025; pp. 227–248. ISBN 978-0-443-23934-2. [Google Scholar] [CrossRef]
- Huseynli, L.; Parviainen, T.; Kyllönen, T.; Aisala, H.; Vene, K. Exploring the protein content and odor-active compounds of black soldier fly larvae for future food applications. Future Foods 2023, 7, 100224. [Google Scholar] [CrossRef]
- Gharby, S. Refining vegetable oils: Chemical and physical refining. Sci. World J. 2022, 2022, 627013. [Google Scholar] [CrossRef] [PubMed]
- Choe, E.; Min, D. Chemistry of Deep-Fat frying oils. J. Food Sci. 2007, 72, R77–R86. [Google Scholar] [CrossRef] [PubMed]
- Mai, H.C.; Dao, N.D.; Lam, T.D.; Nguyen, B.V.; Nguyen, D.C.; Bach, L.G. Purification Process, Physicochemical Properties, and Fatty Acid Composition of Black Soldier Fly (Hermetia illucens Linnaeus) Larvae Oil. J. Am. Oil Chem. Soc. 2019, 96, 1303–1311. [Google Scholar] [CrossRef]
- Mitrea, L.; Teleky, B.; Leopold, L.; Nemes, S.; Plamada, D.; Dulf, F.V.; Pop, I.; Vodnar, D.C. The physicochemical properties of five vegetable oils exposed at high temperature for a short-time-interval. J. Food Compos. Anal. 2022, 106, 104305. [Google Scholar] [CrossRef]
- Sobral, M.M.C.; Cunha, S.C.; Faria, M.A.; Ferreira, I.M. Domestic cooking of muscle foods: Impact on composition of nutrients and contaminants. Compr. Rev. Food Sci. Food Saf. 2018, 17, 309–333. [Google Scholar] [CrossRef]
- Akinoso, R.; Lawal, A.I.; Ayegbusi, F.D.; Obadeyi, F.O. Effect of repeated heating on palm oil and its fractions, oil stability and the cold storage quality of deep-fried “akara”. Food Humanit. 2025, 4, 100591. [Google Scholar] [CrossRef]
- Patil, R.S.; Waghmare, J.; Annapure, U. Comparative assessment of the frying performance of palm olein and sunflower oil during deep-fat frying of Indian battered food products. J. Agr. Food Res. 2023, 14, 100778. [Google Scholar] [CrossRef]
- Peng, C.; Lan, C.; Lin, P.; Kuo, Y. Effects of cooking method, cooking oil, and food type on aldehyde emissions in cooking oil fumes. J. Hazard. Mater. 2016, 324, 160–167. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, T.; Cha, J.Y.; Yong, H.I.; Kang, M.; Jang, H.W.; Choi, Y. Physicochemical characteristics and aroma patterns of oils prepared from edible insects. LWT 2022, 167, 113888. [Google Scholar] [CrossRef]
- Saviane, A.; Tassoni, L.; Naviglio, D.; Lupi, D.; Savoldelli, S.; Bianchi, G.; Cortellino, G.; Bondioli, P.; Folegatti, L.; Casartelli, M.; et al. Mechanical Processing of Hermetia illucens Larvae and Bombyx mori Pupae Produces Oils with Antimicrobial Activity. Animals 2021, 11, 783. [Google Scholar] [CrossRef] [PubMed]
- Aondoakaa, I.P.; Akoh, C.C. Microbial and insect oils: A sustainable approach to functional lipid. J. Am. Oil Chem. Soc. 2024, 102, 5–33. [Google Scholar] [CrossRef]
- Srisuksai, K.; Limudomporn, P.; Kovitvadhi, U.; Thongsuwan, K.; Imaram, W.; Lertchaiyongphanit, R.; Sareepoch, T.; Kovitvadhi, A.; Fungfuang, W. Physicochemical properties and fatty acid profile of oil extracted from black soldier fly larvae (Hermetia illucens). Vet. World 2024, 17, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Dong, J.; He, X.; Wang, J.; Li, C.; Dong, L.; Zhang, Y.; Zhou, X.; Wang, H.; Yi, Y.; et al. Impact of heating temperature and fatty acid type on the formation of lipid oxidation products during thermal processing. Front. Nutr. 2022, 9, 913297. [Google Scholar] [CrossRef]
- Phongpradist, R.; Semmarath, W.; Kiattisin, K.; Jiaranaikulwanitch, J.; Chaiyana, W.; Chaichit, S.; Phimolsiripol, Y.; Dejkriengkraikul, P.; Ampasavate, C. The in vitro effects of black soldier fly larvae (Hermitia illucens) oil as a high-functional active ingredient for inhibiting hyaluronidase, anti-oxidation benefits, whitening, and UVB protection. Front. Pharmacol. 2023, 14, 1243961. [Google Scholar] [CrossRef]
- Aydin, Ç.M. Characteristics of Hozat apricot seed oil as a new source of oilseed. Appl. Food Res. 2024, 4, 100396. [Google Scholar] [CrossRef]
- Tan, P.Y. Oxidative changes in repeatedly heated vegetable oils. J. Oil Palm Res. 2018, 30, 635–641. [Google Scholar] [CrossRef]
- Tarmizi, A.H.A.; Lin, S.W.; Kuntom, A. Palm-Based standard reference materials for iodine value and slip melting point. Anal. Chem. Insights 2008, 3, ACI.S1052. [Google Scholar] [CrossRef]
- Loganathan, R.; Tarmizi, A.H.A.; Vethakkan, S.R.; Teng, K.T. A review on lipid oxidation in edible oils. Malays. J. Anal. Sci. 2022, 26, 1378–1393. [Google Scholar]
- Pramitha, D.A.I.; Karta, I.W. Analysis of fatty acids in virgin coconut oil frying at various temperatures. J. Sains Dan Teknol. 2021, 10, 104–111. [Google Scholar] [CrossRef]
- Ujong, A.; Emelike, N.; Owuno, F.; Okiyi, P. Effect of frying cycles on the physical, chemical and antioxidant properties of selected plant oils during deep-fat frying of potato chips. Food Chem. Adv. 2023, 3, 100338. [Google Scholar] [CrossRef]
- Gunstone, F.D. Vegetable Oils in Food Technology: Composition, Properties, and Uses; Blackwell Publishing Ltd.: Oxford, UK, 2011; Available online: https://onlinelibrary.wiley.com/doi/book/10.1002/9781444339925 (accessed on 30 May 2025).
- Standard for Named Vegetable Oils. CXS 210-1999; Codex Alimentarius, FAO: Rome, Italy, 1999. Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/list-standards/en/ (accessed on 30 May 2025).
- Hassim, N.A.M.; Ismail, N.H.; Kanagaratnam, S.; Isa, W.R.A.; Dian, N.L.H.M. Quality of commercial palm-based cooking oil packed in plastic pouch and polyethylene terephthalate (PET) bottle. J. Oil Palm Res. 2020, 33, 493–513. [Google Scholar] [CrossRef]
- MS 239:1987; Specification for Coconut Oil. Department of Standards Malaysia: Cyberjaya, Malaysia, 1987. Available online: https://mysol.jsm.gov.my/search-catalogue?keyword=MS+239%3A1987 (accessed on 30 May 2025).
- Palm Olein Specification. MS 816:2007; Department of Standards Malaysia: Cyberjaya, Malaysia, 2007. Available online: https://mysol.jsm.gov.my/search-catalogue?keyword=MS+816%3A2007 (accessed on 30 May 2025).
- Wang, X.; McClements, D.J.; Xu, Z.; Meng, M.; Qiu, C.; Long, J.; Jin, Z.; Chen, L. Recent advances in the optimization of the sensory attributes of fried foods: Appearance, flavor, and texture. Trends Food Sci. Technol. 2023, 138, 297–309. [Google Scholar] [CrossRef]
- Singh, N.M.K.; Kumar, N.A.; Kumar, N.R.; Kumar, N.P.S.; Selvakumar, N.P.; Chourasia, N.A. Effects of repeated deep frying on refractive index and peroxide value of selected vegetable oils. Int. Res. J. Biotechnol. 2022, 9, 28–31. [Google Scholar] [CrossRef]
- Jurid, L.S.; Zubairi, S.I.; Kasim, Z.M.; Kadir, I.A.A. The effect of repetitive frying on physicochemical properties of refined, bleached and deodorized Malaysian tenera palm olein during deep-fat frying. Arab. J. Chem. 2020, 13, 6149–6160. [Google Scholar] [CrossRef]
- Park, J.; Kim, J. Monitoring of used frying oils and frying times for frying chicken nuggets using peroxide value and acid value. Korean J. Food Sci. Anim. Resour. 2016, 36, 612–616. [Google Scholar] [CrossRef]
- Liu, L.; Liu, C.; Dou, S.; Dong, L. Volatile metabolite profiling of linolenic acid oxidation in the heating process. Food Sci. Technol. 2023, 43, e121622. [Google Scholar] [CrossRef]
- Nyangena, D.N.; Mutungi, C.; Imathiu, S.; Kinyuru, J.; Affognon, H.; Ekesi, S.; Nakimbugwe, D.; Fiaboe, K.K.M. Effects of traditional processing techniques on the nutritional and microbiological quality of four edible insect species used for food and feed in East Africa. Foods 2020, 9, 574. [Google Scholar] [CrossRef]
- Li, L.; Perea-Sanz, L.; López-Díez, J.J.; Salvador, A.; Belloch, C.; Flores, M. Aroma enhancement in dry cured loins by the addition of nitrogen and sulfur precursors. Meat Sci. 2021, 184, 108698. [Google Scholar] [CrossRef]
- Sajib, M.; Langeland, M.; Undeland, I. Effect of antioxidants on lipid oxidation in herring (Clupea harengus) co-product silage during its production, heat-treatment and storage. Sci. Rep. 2022, 12, 3362. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossain, A. Role of lipids in food flavor generation. Molecules 2022, 27, 5014. [Google Scholar] [CrossRef]
| Carbon No. | Fatty Acid | BSFL Oil | Coconut Oil | Palm Olein | One-Way ANOVA Result |
|---|---|---|---|---|---|
| Saturated Fatty Acids (SAFA) | |||||
| C6:0 | Caproic | ND | 0.92 ± 0.01 | ND | - |
| C8:0 | Caprylic | ND | 9.05 ± 0.01 | ND | - |
| C10:0 | Capric | 0.51 ± 0.01 | 8.09 ± 0.01 | ND | - |
| C12:0 | Lauric | 9.66 ± 0.04 b | 24.88 ± 0.21 a | 0.61 ± 0.01 c | F(2,6) = 29,058, p = 0.001 |
| C14:0 | Myristic | 4.90 ± 0.02 b | 18.77 ± 0.11 a | 1.80 ± 0.03 c | F(2,6) = 56,103, p = 0.001 |
| C16:0 | Palmitic | 23.69 ± 0.07 b | 15.09 ± 0.04 c | 30.24 ± 0.06 a | F(2,6) = 53,014, p = 0.001 |
| C18:0 | Stearic | 4.46 ± 0.01 c | 5.90 ± 0.01 b | 6.51 ± 0.04 a | F(2,6) = 6276, p = 0.001 |
| C20:0 | Arachidic | ND | ND | 0.59 ± 0.01 | - |
| ΣSAFA | NA | 43.22 | 82.70 | 39.75 | - |
| Monounsaturated Fatty Acids (MUFA) | |||||
| C14:1 | Myristoleic | 0.60 ± 0.01 | ND | ND | - |
| C16:1 | Palmitoleic | 3.46 ± 0.02 | ND | ND | - |
| C18:1n9c | Oleic | 30.90 ± 0.06 b | 13.97 ± 0.04 c | 45.02 ± 0.01 a | F(2,6) = 381,447, p = 0.001 |
| Polyunsaturated Fatty Acids (PUFA) | |||||
| C18:2n6c | Linoleic | 21.81 ± 0.11 a | 3.32 ± 0.04 c | 15.22 ± 0.03 b | F(2,6) = 59,282, p = 0.001 |
| Total Unsaturated Fatty Acids (TUFA) | |||||
| ΣMUFA | NA | 34.96 | 13.97 | 45.02 | - |
| ΣPUFA | NA | 21.81 | 3.32 | 15.22 | - |
| ΣTUFA | NA | 56.77 | 17.29 | 60.24 | - |
| Properties | BSFL Oil | Coconut Oil | Palm Olein | One-Way ANOVA Result |
|---|---|---|---|---|
| Physical | ||||
| L* | 36.00 ± 0.25 b | 69.80 ± 0.01 a | 69.20 ± 0.20 a | F(2,6) = 11,225, p = 0.001 |
| a* | 31.40 ± 0.40 a | −0.80 ± 0.10 b | −5.23 ± 0.32 c | F(2,6) = 13,162, p = 0.001 |
| b* | 57.23 ± 1.05 a | 14.30 ± 0.10 c | 36.00 ± 0.10 b | F(2,6) = 3692, p = 0.001 |
| Viscosity (mPa•s) | 72.00 ± 3.65 a | 53.00 ± 1.25 b | 67.93 ± 1.53 a | F(2,6) = 52, p = 0.001 |
| Chemical | ||||
| Peroxide Value (meq/kg oil) | 2.62 ± 0.14 b | 0.78 ± 0.20 c | 8.38 ± 0.51 a | F(2,6) = 448, p = 0.001 |
| Iodine Value (g I2/100 g oil) | 52.08 ± 1.25 b | 12.02 ± 0.71 c | 63.31 ± 0.82 a | F(2,6) = 2403, p = 0.001 |
| Free Fatty Acid Value (%) | 1.13 ± 0.03 a | 0.13 ± 0.01 b | 0.13 ± 0.01 b | F(2,6) = 3231, p = 0.001 |
| Odour Attribute | Definition |
|---|---|
| Fishy | Perception of odour associated with the fishiness of any seafood product. |
| Nutty | Perception of odour associated with a mix of cashew nut and almond. |
| Oily | Perception of odour associated with rancid vegetable oil. |
| Meaty/savoury | Perception of odour associated with roasted or deep-fried meat with its crispy cracklings. |
| Roasted | Perception of odour associated with any roasted food product. |
| Pungent | Perception of odour associated with a noticeable odour that can be somewhat irritating. |
| Odour Attribute | Control | 120 °C | 180 °C | One-Way ANOVA Result |
|---|---|---|---|---|
| Fishy | 75.47 ± 17.22 b | 98.98 ± 29.47 a | 96.09 ± 26.57 ab | F(2,18) = 3.79, p = 0.028 |
| Nutty | 85.18 ± 27.26 a | 73.40 ± 25.66 a | 93.55 ± 27.21 a | F(2,18) = 2.01, p = 0.143 |
| Oily | 71.81 ± 23.82 b | 85.80 ± 27.79 ab | 97.85 ± 20.14 a | F(2,18) = 4.27, p = 0.019 |
| Meaty/Savoury | 71.05 ± 19.43 a | 78.23 ± 17.66 a | 88.90 ± 18.18 a | F(2,18) = 2.16, p = 0.124 |
| Roasted | 77.49 ± 25.58 a | 71.11 ± 26.13 a | 92.81 ± 23.52 a | F(2,18) = 2.06, p = 0.137 |
| Pungent | 44.56 ± 22.68 a | 52.38 ± 36.89 a | 59.46 ± 32.52 a | F(2,18) = 0.81, p = 0.451 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, K.A.; Leong, S.Y.; Chew, L.Y.; Lim, C.Q.; Lim, M.J.; Ong, Z.; Chan, S.W. Physicochemical and Perceived Olfactory Changes in Black Soldier Fly (Hermetia illucens) Larvae Oil Under Domestic Cooking Temperatures. Foods 2025, 14, 2333. https://doi.org/10.3390/foods14132333
Chang KA, Leong SY, Chew LY, Lim CQ, Lim MJ, Ong Z, Chan SW. Physicochemical and Perceived Olfactory Changes in Black Soldier Fly (Hermetia illucens) Larvae Oil Under Domestic Cooking Temperatures. Foods. 2025; 14(13):2333. https://doi.org/10.3390/foods14132333
Chicago/Turabian StyleChang, Kian Aun, Sze Ying Leong, Lye Yee Chew, Ching Qi Lim, Meng Jack Lim, Zongwei Ong, and Sook Wah Chan. 2025. "Physicochemical and Perceived Olfactory Changes in Black Soldier Fly (Hermetia illucens) Larvae Oil Under Domestic Cooking Temperatures" Foods 14, no. 13: 2333. https://doi.org/10.3390/foods14132333
APA StyleChang, K. A., Leong, S. Y., Chew, L. Y., Lim, C. Q., Lim, M. J., Ong, Z., & Chan, S. W. (2025). Physicochemical and Perceived Olfactory Changes in Black Soldier Fly (Hermetia illucens) Larvae Oil Under Domestic Cooking Temperatures. Foods, 14(13), 2333. https://doi.org/10.3390/foods14132333







