Loop-Mediated Isothermal Amplification for Detecting Four Major Foodborne Pathogens in Meat and Meat Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Bacterial Recovery Culture and Genomic DNA Extraction
2.3. LAMP and PCR Primer Design
2.4. Optimization of LAMP Reaction Systems
2.5. Specificity Assessment
2.6. Sensitivity Assessment
2.7. PCR Reaction System of Four Foodborne Pathogenic Bacteria
2.8. Detection of Four Foodborne Pathogenic Bacteria in Meat and Meat Products
2.9. Culture-Based Methods for Salmonella and E. coli O157:H7
3. Results and Discussion
3.1. Optimization of LAMP Reactions
3.2. Specificity of the Established LAMP Assays
3.3. Sensitivity of the Established LAMP Methods
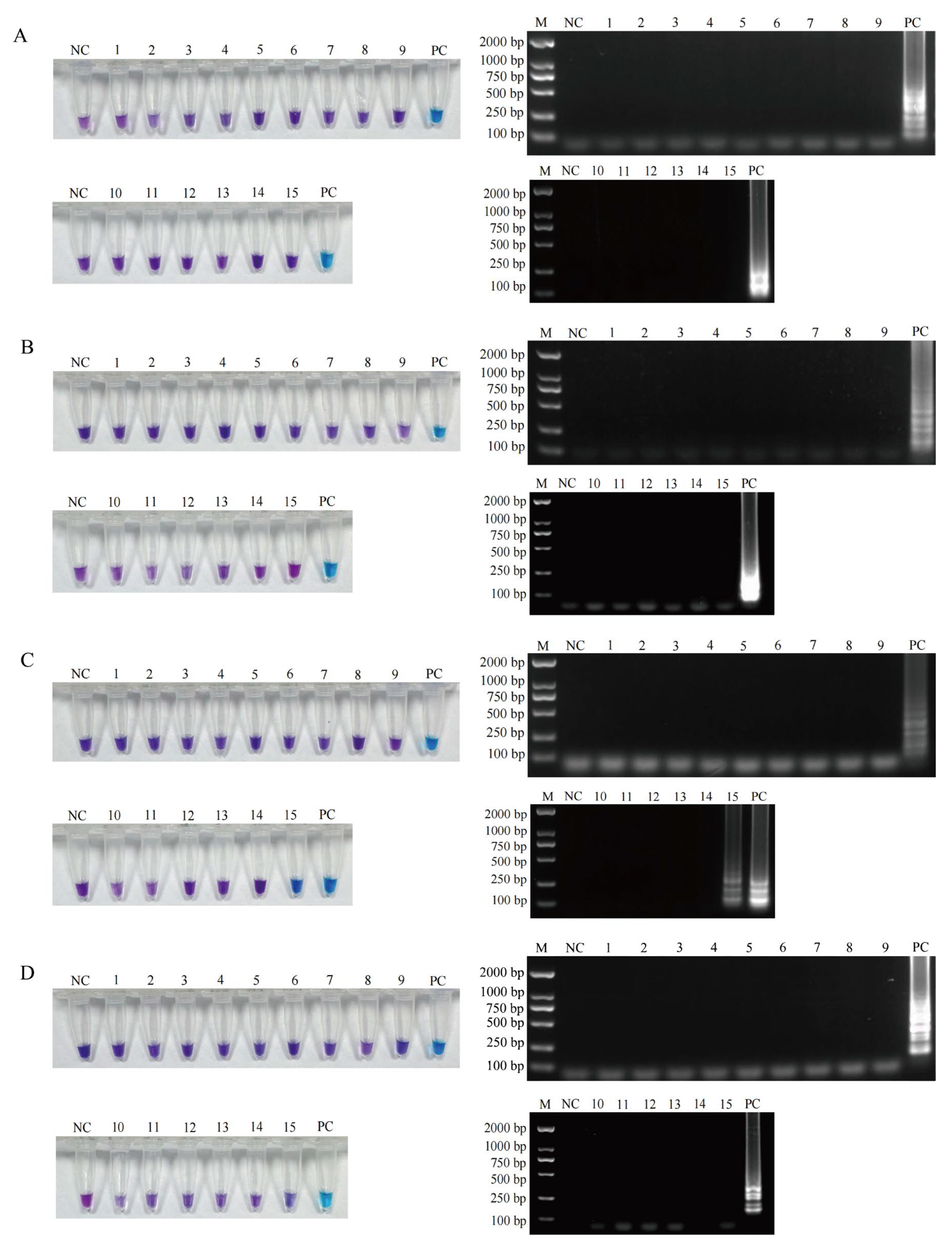
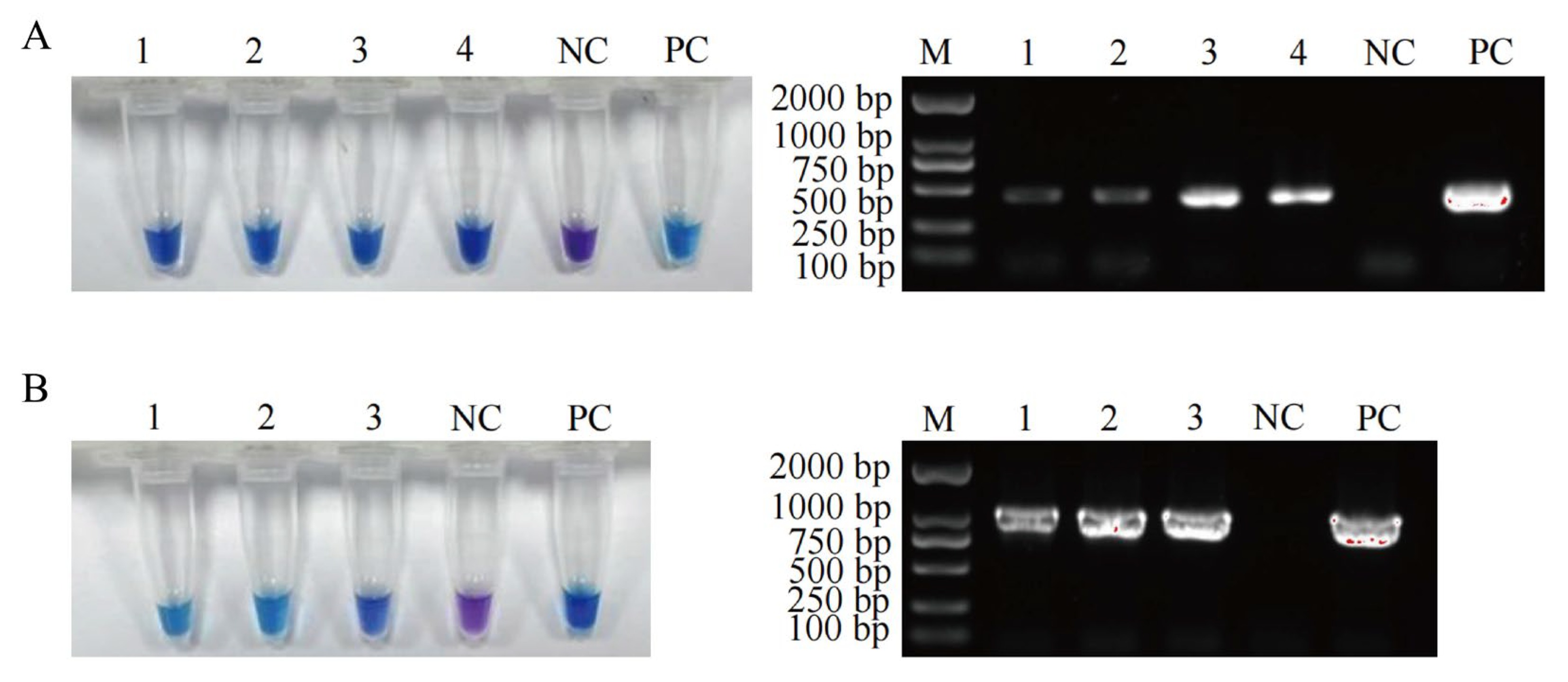
3.4. Detection Performance of the Established LAMP Methods in Meat and Meat Product Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stadnik, J. Nutritional value of meat and meat products and their role in human health. Nutrients 2024, 16, 1446. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Shankar, S.; Royon, F.; Salmieri, S.; Lacroix, M. Essential oils as natural antimicrobials applied in meat and meat products—A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 993–1009. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Kang, S. Foodborne pathogenic bacteria: Prevalence and control—Volume I. Foods 2024, 13, 1531. [Google Scholar] [CrossRef]
- Osek, J.; Lachtara, B.; Wieczorek, K. Listeria monocytogenes—How this pathogen survives in food-production environments? Front. Microbiol. 2022, 13, 866462. [Google Scholar] [CrossRef]
- Li, W.; Bai, L.; Fu, P.; Han, H.; Liu, J.; Guo, Y. The epidemiology of Listeria monocytogenes in China. Foodborne Pathog. Dis. 2018, 15, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.G.; Mills, K.B.; Crosby, H.A.; Horswill, A.R. The Staphylococcus aureus regulatory program in a human skin-like environment. mBio 2024, 15, e0045324. [Google Scholar] [CrossRef] [PubMed]
- Cieza, M.Y.R.; Bonsaglia, E.C.R.; Rall, V.L.M.; Santos, M.V.D.; Silva, N.C.C. Staphylococcal enterotoxins: Description and importance in food. Pathogens 2024, 13, 676. [Google Scholar] [CrossRef]
- Lu, J.; Wu, H.; Wu, S.; Wang, S.; Fan, H.; Ruan, H.; Qiao, J.; Caiyin, Q.; Wen, M. Salmonella: Infection mechanism and control strategies. Microbiol. Res. 2025, 292, 128013. [Google Scholar] [CrossRef]
- Teklemariam, A.D.; Al-Hindi, R.R.; Albiheyri, R.S.; Alharbi, M.G.; Alghamdi, M.A.; Filimban, A.A.R.; Al Mutiri, A.S.; Al-Alyani, A.M.; Alseghayer, M.S.; Almaneea, A.M.; et al. Human Salmonellosis: A continuous global threat in the farm-to-fork food safety continuum. Foods 2023, 12, 1756. [Google Scholar] [CrossRef]
- Jiang, L.; Yang, W.; Jiang, X.; Yao, T.; Wang, L.; Yang, B. Virulence-related O islands in enterohemorrhagic Escherichia coli O157:H7. Gut Microbes 2021, 13, 1992237. [Google Scholar] [CrossRef]
- Cui, S.; Wei, Y.; Li, C.; Zhang, J.; Zhao, Y.; Peng, X.; Sun, F. Visual loop-mediated isothermal amplification (LAMP) assay for rapid on-site detection of Escherichia coli O157: H7 in milk products. Foods 2024, 13, 2143. [Google Scholar] [CrossRef]
- Omer, M.K.; Álvarez-Ordoñez, A.; Prieto, M.; Skjerve, E.; Asehun, T.; Alvseike, O.A. A systematic review of bacterial foodborne outbreaks related to red meat and meat products. Foodborne Pathog. Dis. 2018, 15, 598–611. [Google Scholar] [CrossRef] [PubMed]
- Aladhadh, M. A review of modern methods for the detection of foodborne pathogens. Microorganisms 2023, 11, 1111. [Google Scholar] [CrossRef] [PubMed]
- Park, D.G.; Ha, E.S.; Kang, B.; Choi, I.; Kwak, J.E.; Choi, J.; Park, J.; Lee, W.; Kim, S.H.; Kim, S.H.; et al. Development and evaluation of a next-generation sequencing panel for the multiple detection and identification of pathogens in fermented foods. J. Microbiol. Biotechnol. 2023, 33, 83–95. [Google Scholar] [CrossRef]
- Wang, D.; Chen, Q.; Huo, H.; Bai, S.; Cai, G.; Lai, W.; Lin, J. Efficient separation and quantitative detection of Listeria monocytogenes based on screen-printed interdigitated electrode, urease and magnetic nanoparticles. Food Control 2017, 73, 555–561. [Google Scholar] [CrossRef]
- Quintela, I.A.; Vasse, T.; Lin, C.S.; Wu, V.C.H. Advances, applications, and limitations of portable and rapid detection technologies for routinely encountered foodborne pathogens. Front. Microbiol. 2022, 13, 1054782. [Google Scholar] [CrossRef]
- Soroka, M.; Wasowicz, B.; Rymaszewska, A. Loop-mediated isothermal amplification (LAMP): The better sibling of PCR? Cells 2021, 10, 1931. [Google Scholar] [CrossRef]
- Yang, N.; Zhang, H.; Han, X.; Liu, Z.; Lu, Y. Advancements and applications of loop-mediated isothermal amplification technology: A comprehensive overview. Front. Microbiol. 2024, 15, 1406632. [Google Scholar] [CrossRef]
- Novi, V.T.; Meher, A.K.; Abbas, A. Visualization methods for loop mediated isothermal amplification (LAMP) assays. Analyst 2025, 150, 588–599. [Google Scholar] [CrossRef]
- Neshani, A.; Zare, H.; Sadeghian, H.; Safdari, H.; Riahi-Zanjani, B.; Aryan, E. A comparative study on visual detection of Mycobacterium tuberculosis by closed tube loop-mediated isothermal amplification: Shedding light on the use of Eriochrome Black T. Diagnostics 2023, 13, 155. [Google Scholar] [CrossRef]
- Yan, H.; Xu, B.; Gao, B.; Xu, Y.; Xia, X.; Ma, Y.; Qin, X.; Dong, Q.; Hirata, T.; Li, Z. Comparative analysis of in vivo and in vitro virulence among foodborne and clinical Listeria monocytogenes strains. Microorganisms 2025, 13, 191. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xia, D.; Ma, P.; Gao, X.; Kang, W.; Wei, J. Advances in the detection of virulence genes of Staphylococcus aureus originate from food. Food Sci. Hum. Wellness 2020, 9, 40–44. [Google Scholar] [CrossRef]
- Tian, S.; Wang, C.; Li, Y.; Bao, X.; Zhang, Y.; Tang, T. The impact of SlyA on cell metabolism of Salmonella typhimurium: A joint study of transcriptomics and metabolomics. J. Proteome Res. 2021, 20, 184–190. [Google Scholar] [CrossRef]
- Yang, X.; Qi, Y.; Feng, J.; Han, F.; Zhang, P.; Luo, L.; Zheng, Z.; Zhang, W.; Li, Z.; Tang, W. On-site monitoring of Escherichia coli O157:H7 in drinking water based on rapid detection of the rfbE gene at the single copy level. Sens. Actuators B Chem. 2024, 401, 135069. [Google Scholar] [CrossRef]
- Oslan, S.N.H.; Yusof, N.Y.; Lim, S.J.; Ahmad, N.H. Rapid and sensitive detection of Salmonella in agro-Food and environmental samples: A review of advances in rapid tests and biosensors. J. Microbiol. Methods 2024, 219, 106897. [Google Scholar] [CrossRef]
- Ngwa, G.A.; Schop, R.; Weir, S.; León-Velarde, C.G.; Odumeru, J.A. Detection and enumeration of E. coli O157:H7 in water samples by culture and molecular methods. J. Microbiol. Methods 2013, 92, 164–172. [Google Scholar] [CrossRef]
- Logeshwari, R.; Gopalakrishnan, C.; Kamalakannan, A.; Ramalingam, J.; Saraswathi, R. A colorimetric hydroxy naphthol blue based loop-mediated isothermal amplification detection assay targeting the β-tubulin locus of Sarocladium oryzae infecting rice seed. Front. Plant Sci. 2022, 13, 1077328. [Google Scholar] [CrossRef]
- Fiore, A.; Treglia, I.; Ciccaglioni, G.; Ortoffi, M.F.; Gattuso, A. Application of a loop-mediated isothermal amplification (LAMP) assay for the detection of Listeria monocytogenes in cooked ham. Foods 2023, 12, 193. [Google Scholar] [CrossRef]
- Xu, D.; Zeng, H.; Wu, W.; Liu, H.; Wang, J. Isothermal amplification and CRISPR/Cas12a-system-based assay for rapid, sensitive and visual detection of Staphylococcus aureus. Foods 2023, 12, 4432. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, S.; Chen, C.; Jin, J.; Liu, H.; Bian, Z.; Qi, W.; Xie, Y.; Zhang, H. Development of a visual detection method for Salmonella based on loop-mediated isothermal amplification using pyrophosphatase. FEMS Microbiol. Lett. 2023, 370, fnad040. [Google Scholar] [CrossRef]
- Ren, X.; Yang, D.; Yang, Z.; Li, Y.; Yang, S.; Li, W.; Qiao, X.; Xue, C.; Chen, M.; Zhang, L.; et al. Prevalence and antimicrobial susceptibility of foodborne pathogens from raw livestock meat in China, 2021. Microorganisms 2024, 12, 2157. [Google Scholar] [CrossRef] [PubMed]
- Tosuncuk, Ö.; Bozatli, S.B.; Dikici, A. Investigation of efficient thermal inactivation parameters of Escherichia coli O157:H7 in meatballs by grilling. J. Food Sci. Technol. 2023, 60, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
| Gene | Primer Type | Primer Name | Sequence (5′ → 3′) |
|---|---|---|---|
| prfA | LAMP primer | LM-F3 | GTATTAGCGAGAACGGGAC |
| LM-B3 | TTGTTTTTGTAGGGTTTGGAA | ||
| LM-FIP | GCCAACCGATGTTTCTGTATCAATAATTTACAATACTACAAAGGGGCT | ||
| LM-BIP | GCAGGCTACCGCATACGTTAAAAGTGCGTAAGATTTTTGCTC | ||
| PCR primer | LM-F | ATGAACGCTCAAGCAGAAGAATTCAAA | |
| LM-R | TTAATTTAATTTTCCCCAAGTAGCAGGAC | ||
| nuc | LAMP primer | SA-F3 | AACAGTATATAGTGCAACTTCAA |
| SA-B3 | CTTTGTCAAACTCGACTTCAA | ||
| SA-FIP | ATGTCATTGGTTGACCTTTGTACATAAATTACATAAAGAACCTGCGA | ||
| SA-BIP | GTTGATACACCTGAAACAAAGCATCATTTTTTTCGTAAATGCACTTGC | ||
| PCR primer | SA-F | ATGACAGAATACTTATTAAGTGCTG | |
| SA-R | AAATATTTAATTTCTTTTTTTTCGCTTGTG | ||
| slyA | LAMP primer | SE-F3 | TTCTGATCTGGCACGGTTG |
| SE-B3 | GATCCAACGTGCGTACCAG | ||
| SE-FIP | TGACCCAATGTGTCTGCGTCAACATTTGGCGTGCTCTGATTG | ||
| SE-BIP | TCATCAATTGCCGCCTGACCACTGCTCAATGCCTATCGCTT | ||
| PCR primer | SE-F | AAGGAGATGAAATTGGAATCGCCA | |
| SE-R | TCAATCGTGAGAGTGCAATTCCATA | ||
| rfbE | LAMP primer | EC-F3 | AGCGTTAGGTATATCGGAAG |
| EC-B3 | GTTCCATATCACATGGATGTC | ||
| EC-FIP | TGGCTCCTGTGTATTTTATAGCATTGAGATGAAGTTATTGTTCCAACA | ||
| EC-BIP | TGAAACTTGGCAAATGTCTGTTAGTAATGGACACACATAATAGCTTTA | ||
| PCR primer | EC-F | ATGAAATATATACCAGTTTACCAACC | |
| EC-R | CTATTTATCACTATAAAATTCGTTAATAGA |
| Items | LAMP System for L. monocytogenes | LAMP System for S. aureus | LAMP System for S. enterica | LAMP System for E. coli O157:H7 |
|---|---|---|---|---|
| 10× ThermPol Buffer | 2.5 μL | 2.5 μL | 2.5 μL | 2.5 μL |
| MgSO4 (100 mM) | 1.5 μL | 1.5 μL | 1.5 μL | 1.5 μL |
| dNTP Mix (10 mM) | 3.0 μL | 2.5 μL | 2.5 μL | 3.0 μL |
| F3/B3 primers (10 µM) | 0.5 μL | 0.5 μL | 0.5 μL | 0.5 μL |
| FIP/BIP primers (10 µM) | 4.0 μL | 4.0 μL | 4.0 μL | 4.0 μL |
| Bst DNA polymerase | 0.4 μL | 0.8 μL | 0.6 μL | 0.8 μL |
| HNB (3 mM) | 1.0 μL | 0.75 μL | 0.75 μL | 0.75 μL |
| Genomic DNA template | 100 ng | 100 ng | 100 ng | 100 ng |
| ddH2O | Fill up to 25 μL | Fill up to 25 μL | Fill up to 25 μL | Fill up to 25 μL |
| Reaction temperature | 64 °C | 62 °C | 62 °C | 61 °C |
| Reaction time | 50 min | 40 min | 40 min | 50 min |
| Strains | LAMP Detection Limit (CFU/mL) | PCR Detection Limit (CFU/mL) | Fold Change |
|---|---|---|---|
| L. monocytogenes | 1.8 × 101 | 1.8 × 103 | 100 |
| S. aureus | 5.1 × 101 | 5.1 × 103 | 100 |
| S. enterica | 1.2 × 101 | 1.2 × 104 | 1000 |
| E. coli O157:H7 | 3.3 × 103 | 3.3 × 104 | 10 |
| Strains | Total Samples | LAMP Detection | Accuracy Rate | |
|---|---|---|---|---|
| Positive Samples | Negative Samples | |||
| L. monocytogenes | 52 | 0 | 52 | 100% |
| S. aureus | 52 | 0 | 52 | 100% |
| S. enterica | 52 | 4 | 48 | 100% |
| E. coli O157:H7 | 52 | 3 | 49 | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhu, M.; Wang, S.; Li, W.; Ren, B.; Qu, L.; Zhang, X. Loop-Mediated Isothermal Amplification for Detecting Four Major Foodborne Pathogens in Meat and Meat Products. Foods 2025, 14, 2321. https://doi.org/10.3390/foods14132321
Li X, Zhu M, Wang S, Li W, Ren B, Qu L, Zhang X. Loop-Mediated Isothermal Amplification for Detecting Four Major Foodborne Pathogens in Meat and Meat Products. Foods. 2025; 14(13):2321. https://doi.org/10.3390/foods14132321
Chicago/Turabian StyleLi, Xin, Mingxue Zhu, Siyuan Wang, Weijia Li, Baohong Ren, Lingbo Qu, and Xiaoling Zhang. 2025. "Loop-Mediated Isothermal Amplification for Detecting Four Major Foodborne Pathogens in Meat and Meat Products" Foods 14, no. 13: 2321. https://doi.org/10.3390/foods14132321
APA StyleLi, X., Zhu, M., Wang, S., Li, W., Ren, B., Qu, L., & Zhang, X. (2025). Loop-Mediated Isothermal Amplification for Detecting Four Major Foodborne Pathogens in Meat and Meat Products. Foods, 14(13), 2321. https://doi.org/10.3390/foods14132321






