Abstract
The growing demand for more sustainable food systems has driven the development of solutions based on food microbiology, capable of integrating safety, functionality, and environmental responsibility. This paper presents a critical and up-to-date review of the most relevant advances at the interface between microbiology, sustainability, and food innovation. The analysis is structured around three main axes: (i) microbial fermentation, with a focus on traditional practices and precision technologies aimed at valorizing agro-industrial waste and producing functional foods; (ii) microbial biocontrol, including the use of bacteriocins, protective cultures, bacteriophages, and CRISPR-Cas (Clustered Regularly Interspaced Short Palindromic Repeats–CRISPR-associated)-based tools as alternatives to synthetic preservatives; and (iii) the development of functional foods containing probiotics, prebiotics, synbiotics, and postbiotics, with the potential to modulate the gut microbiota and promote metabolic, immune, and cognitive health. In addition to reviewing the microbiological and technological mechanisms involved, the paper discusses international regulatory milestones, scalability challenges, and market trends related to consumer acceptance and clean labeling. Finally, emerging trends and research gaps are addressed, including the use of omics technologies, artificial intelligence, and unexplored microbial resources. Food microbiology, by incorporating sustainable practices and advanced technologies, is positioned as a strategic pillar for building a healthy, circular, science-based food model.
1. Introduction
The pursuit of more sustainable food systems has become a critical global priority in the 21st century. Current estimates attribute up to 34% of global greenhouse gas emissions to the agro-food sector [1], which simultaneously places significant pressure on natural resources, driving environmental degradation, biodiversity loss, and depletion of soil and water reserves. Moreover, roughly one-third of all food produced worldwide is lost or wasted, intensifying both environmental and economic burdens [2]. These alarming statistics underscore the urgent necessity for a transformative shift toward food systems that harmonize productivity, technological innovation, and environmental stewardship.
Within this context, food microbiology emerges as a pivotal discipline to advance sustainability across the food production continuum. The application of microorganisms in fermentation, biocontrol, and functional food development has yielded promising sustainable solutions characterized by lower energy consumption, minimized waste generation, and enhanced the valorization of agro-industrial by-products [3]. Microorganisms have been indispensable throughout human history in food processing and preservation, from traditional fermentation techniques to cutting-edge approaches involving genetic engineering and synthetic biology.
Microbial fermentation constitutes a foundational pillar in this transition. Beyond the production of widely consumed fermented foods such as yogurt, sauerkraut, kefir, miso, and tempeh, fermentation processes are increasingly employed to convert agro-industrial residues into value-added products, thereby contributing to circular bioeconomy models with reduced environmental footprints [4,5]. These processes not only promote food diversification and safety but also support sustainable resource management.
Similarly, microbial biocontrol offers a natural and effective strategy to address food preservation challenges and reduce reliance on synthetic additives. Microorganisms that produce antimicrobial agents—including bacteriocins, organic acids, and peroxides—have demonstrated strong efficacy against pathogens and spoilage organisms while maintaining food quality and safety [6,7]. Recent advances, such as protective cultures, bacteriophage applications, and CRISPR-Cas technologies, have further enhanced the precision and sustainability of biocontrol measures, lowering environmental and toxicological risks.
The development of microbial functional foods represents another transformative avenue driven by advances in understanding the gut microbiota’s impact on human health, encompassing immunity, metabolism, cognitive function, and chronic disease risk [7,8]. Functional food formulations containing probiotics, prebiotics, synbiotics, and postbiotics not only improve nutritional profiles but also resonate with consumer demand for natural, health-promoting, and personalized dietary options.
In light of these developments, this review critically examines the latest sustainable innovations at the intersection of food microbiology and food systems. The discussion is organized around three thematic axes: (i) microbial fermentation for sustainable food production, (ii) microbial biocontrol as an eco-friendly alternative to chemical preservatives, and (iii) functional foods designed to enhance health outcomes. By synthesizing the current scientific evidence and identifying knowledge gaps, this work aims to guide future research and contribute to the design of resilient, circular food systems aligned with the United Nations Sustainable Development Goals (SDGs).
Thus, this review aims to provide a critical and integrative overview of recent sustainable innovations in food microbiology, focusing on microbial fermentation, microbial biocontrol, and functional foods. By synthesizing the current scientific knowledge and identifying technological and regulatory gaps, we intend to highlight how microbiological strategies can support circular food systems, health promotion, and environmental responsibility, thereby contributing to the scientific foundations of the Sustainable Development Goals (SDGs).
2. Microbial Fermentation for Sustainable Food Production
2.1. Traditional Fermented Foods: Cultural Heritage and Microbial Ecology
Traditional fermented foods constitute one of the oldest and most culturally rich forms of food biotechnology, rooted in empirical knowledge accumulated over thousands of years. These fermentations not only serve to preserve food but also enhance its digestibility, nutritional profile, and functional properties, contributing to human health while ensuring microbiological safety. Examples such as yogurt, kefir, tempeh, sauerkraut, miso, kimchi, and dadiah illustrate diverse regional practices shaped by local sociocultural contexts and ecological conditions.
The fermentation processes in these foods are driven by complex microbial consortia, predominantly composed of lactic acid bacteria (LAB), yeasts, and filamentous fungi. The composition of these microbial communities varies significantly according to the substrate, environmental factors, indigenous microbiota, and traditional production methods [4].
In yogurt production, controlled fermentation involves a symbiotic relationship between Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus, which cooperatively produce lactic acid to induce protein coagulation and develop volatile compounds responsible for its characteristic flavor and texture. Kefir grains form a natural symbiotic matrix of proteins and polysaccharides that harbor a diverse microbial consortium including LAB, yeasts such as Kluyveromyces marxianus, and acetic acid bacteria [9].
Tempeh, a traditional Indonesian fermented food, relies on the filamentous fungus Rhizopus oligosporus, which hydrolyzes soy proteins and lipids, releasing peptides with antioxidant, hypocholesterolemic, and antimicrobial activities [10]. Miso, a fermented soybean paste often combined with rice or barley, undergoes a multi-step fermentation starting with Aspergillus oryzae in the koji stage (i.e., the initial solid-state incubation step where Aspergillus oryzae grows on substrates such as soybeans or rice to generate a high-enzyme biomass inoculum), followed by fermentation by osmophilic yeasts (Zygosaccharomyces rouxii) and LAB, resulting in a product rich in free amino acids, vitamins, and aromatic compounds [11]. A summary of representative traditional fermented foods, their predominant microorganisms, fermentation methods, origin, and main metabolic products is provided in Table 1, which complements the microbiological and functional descriptions detailed in this section.

Table 1.
Overview of representative traditional fermented foods, their microbial ecology, fermentation type, origin, and key metabolic products.
Sauerkraut exemplifies spontaneous vegetable fermentation, where species such as Leuconostoc mesenteroides, Lactiplantibacillus plantarum, and Limosilactobacillus fermentum drive acidification, inhibit spoilage and pathogenic microbes, and stabilize the food matrix. This fermentation is characterized by natural microbial successions influenced by pH gradients and substrate availability [12].
Recent research by Thierry et al. (2023) [13] underscored the microbial diversity and ecological resilience in traditional fermentation systems. Their analysis of 75 homemade vegetable fermentation samples from France revealed over 20 dominant species well-adapted to acidic environments, including tolerance to low pH and the capacity to synthesize volatile metabolites. These dynamics are governed by fundamental ecological mechanisms such as selection, dispersal, genetic drift, and speciation, rendering fermented foods valuable model systems for applied microbial ecology studies [14].
Beyond their cultural and historical significance, traditional fermented foods deliver documented nutritional and functional benefits. These include enhanced mineral bioavailability, degradation of antinutritional factors, biosynthesis of B vitamins, production of bioactive compounds, and beneficial modulation of the gut microbiota [15,16]. Preserving and investigating these time-honored practices offers an effective strategy to promote sustainable food systems, leveraging microbial biodiversity and low-cost biotechnological innovation.
2.2. Controlled and Precision Fermentation
Controlled and precision fermentation represent important advances in modern food biotechnology, building upon the foundations of traditional fermentation by incorporating contemporary microbiological methods, genetic engineering, and synthetic biology. These innovations aim to optimize fermentation processes, ensuring greater consistency, safety, and reproducibility of the final products [17,18].
The use of starter cultures allows for targeted control over microbial growth by inoculating selected strains with desirable traits, such as rapid acidification, production of flavor compounds, vitamin biosynthesis, or the ability to inhibit spoilage and pathogenic microorganisms [17]. Furthermore, multi-species microbial consortia that engage in synergistic metabolic interactions have shown promising results in generating fermented foods with enhanced sensory profiles and improved ecological stability in both artisanal and industrial contexts [18,19].
Recent developments in genetic engineering and synthetic biology have made it possible to tailor microorganisms to produce specific metabolites efficiently. These include essential amino acids, vitamins, enzymes, organic acids, and functional proteins. A notable example is the genetic modification of yeasts for the production of recombinant casein, which enables the creation of animal-free cheeses with a substantially lower environmental footprint [17].
Advanced genome-editing technologies such as CRISPR-Cas are widely applied to insert, delete, or modulate genes relevant to fermentation processes, while synthetic genetic circuits provide dynamic regulation of microbial metabolism. These tools have significantly improved both the biochemical efficiency and scalability of fermentation at the industrial level [20].
Precision fermentation has also paved the way for innovative animal-free alternatives, including milk, eggs, and meat substitutes. Companies like Perfect Day, Formo, and The EVERY Company utilize genetically engineered microorganisms—such as yeasts and filamentous fungi—to produce proteins like beta-lactoglobulin and albumin. These proteins replicate the techno-functional and sensory qualities of their animal-derived equivalents, meeting the growing consumer demand for ethical, sustainable, and allergen- or intolerance-friendly food products [21].
Moreover, the integration of artificial intelligence (AI) with precision fermentation is enhancing the development of novel foods. For instance, the Chilean startup NotCo employs an AI platform named “Giuseppe” that matches plant-based ingredients with molecular signatures of animal-based products. By combining extensive molecular databases, machine learning algorithms, and fermentation biotechnology, NotCo creates plant-based foods that closely replicate the flavor, texture, and performance of traditional animal products. This synergy between biotechnology and data science is opening new avenues for innovation in sustainable food production [22].
Furthermore, the global market for precision fermentation ingredients was valued at approximately USD 2.8 billion in 2023 and is projected to reach over USD 36.3 billion by 2030, with a compound annual growth rate (CAGR) of around 44% [23]. This trend underscores the rapid industrial expansion and strong investment interest in this emerging field.
Despite the promise of these microbial technologies, their large-scale implementation in low- and middle-income countries (LMICs) faces significant challenges related to infrastructure, regulatory capacity, and investment. The high initial cost of bioreactors, need for specialized personnel, and limitations in cold-chain logistics can hinder scalability. Moreover, affordable access to microbial cultures and proprietary biotechnological tools remain restricted in many regions. Although comprehensive global data are still lacking, addressing these barriers requires public–private partnerships, technology transfer, and regionally adapted strategies to ensure equitable innovation in food systems [24].
However, the implementation of such innovative microbial technologies is not without potential drawbacks. Concerns have been raised regarding the unintended ecological consequences, such as the loss of native microbial diversity and the production of unexpected or harmful metabolites in complex food ecosystems. In particular, the use of genetically modified (GM) starter cultures, while offering benefits such as improved performance and safety, remains controversial. These concerns are often rooted in ethical debates, environmental risk perceptions, and consumer resistance, especially in regions where regulatory and labeling frameworks for GMOs are still evolving [25].
2.3. Valorization of Agro-Industrial Waste Through Fermentation
The growing generation of agro-industrial waste represents one of the main challenges for the sustainability of food systems. It is estimated that globally, around 1.3 billion tons of food are wasted annually, resulting in economic losses of approximately USD 940 billion and significant environmental impacts, such as greenhouse gas emissions, inefficient water use, and soil contamination [2]. In this context, microbial fermentation emerges as a promising biotechnological strategy for the valorization of these wastes, allowing their conversion into bioactive compounds, functional ingredients, and high-value biomaterials.
Among the most efficient approaches is solid-state fermentation (SSF), which uses low-moisture agro-industrial substrates as support for microbial growth. Studies show that SSF enables the production of industrial enzymes, such as cellulases, pectinases, and xylanases, from substrates like sugarcane bagasse, rice husks, and fruit residues, with potential applications in the food, textile, and pharmaceutical sectors [26]. Additionally, SSF has been employed in the production of bioactive compounds, including phenolic antioxidants and organic acids, with preservative and nutraceutical functions [27,28].
Quantitative studies report that SSF can convert 30–80% of lignocellulosic biomass into value-added compounds such as organic acids, enzymes, and phenolic extracts, depending on the substrate and microbial consortium used [24]. For example, the global availability of agro-industrial waste suitable for SSF-based valorization exceeds 5 billion tons annually, especially from sugarcane, wheat, rice, and fruit processing [25]. Yields from SSF processes include ~12 g/L of citric acid from sugarcane bagasse using Aspergillus niger and protease activities above 900 U/g of dry substrate from soybean meal fermentation. These values reinforce the industrial viability of SSF as an eco-efficient biotechnological platform.
The fermentation of agro-industrial waste has also been applied to the production of biopolymers and biofuels. Lignocellulosic wastes, such as corn straw, cassava peels, and sugarcane bagasse, can be used as substrates for the synthesis of polyhydroxybutyrate (PHB) by microorganisms like Bacillus megaterium or Cupriavidus necator, contributing to the replacement of conventional plastics with biodegradable [2]. Furthermore, carbohydrate-rich wastes, such as molasses, whey, and tropical fruit residues, have been successfully used in bioethanol production, promoting the diversification of the energy matrix and reducing dependence on fossil fuels [29].
In the food industry, the fermentation of waste has been explored for the development of functional and nutritionally enriched ingredients. The production of single-cell proteins (SCPs) from nutrient-rich waste, such as fruit peels, vegetable scraps, and by-products from the brewing industry, has been proposed as a sustainable alternative for obtaining proteins for human and animal nutrition [30].
In parallel, fermentation processes have been applied in the extraction or synthesis of prebiotic compounds, antioxidant polyphenols, exopolysaccharides, and bioactive peptides, which can be incorporated into food formulations with functional and health claims [31].
Thus, the valorization of agro-industrial waste through fermentation not only reduces the environmental impact of the agro-industry but also promotes circular bioeconomy, contributing to sustainable innovation in food, energy, and biomaterials.
3. Microbial Biocontrol for Food Safety and Preservation
3.1. Natural Antimicrobials Produced by Microorganisms
Increasing awareness of the potential health risks and environmental impacts associated with synthetic chemical preservatives has driven the demand for natural and sustainable alternatives in food preservation. In this context, natural antimicrobials produced by microorganisms, including bacteriocins, organic acids, hydrogen peroxide, and certain phenolic derivatives, have gained significant attention as effective tools for microbial control, particularly in minimally processed foods [6].
Bacteriocins represent the most extensively studied group of microbial-derived antimicrobial peptides. These ribosomally synthesized compounds, predominantly produced by lactic acid bacteria (LAB), are capable of inhibiting spoilage organisms and foodborne pathogens such as Listeria monocytogenes, Staphylococcus aureus, and Clostridium botulinum. A prominent example is nisin, synthesized by Lactococcus lactis, which has been approved for use in a variety of food products, including cheeses, processed meats, and canned goods, due to its broad antimicrobial spectrum and established safety profile [6].
In addition to bacteriocins, LAB contributes to food preservation through the production of organic acids—notably lactic, acetic, and propionic acids—which reduce the pH of the surrounding environment, thereby inhibiting the growth of undesirable microorganisms. These acids act synergistically with other LAB-derived metabolites such as hydrogen peroxide and diacetyl, exerting both bacteriostatic and bactericidal effects, especially against Gram-negative bacteria [32].
Furthermore, some microorganisms are capable of biotransforming plant-derived phenolic compounds into more potent antioxidants and antimicrobial derivatives. This metabolic capability extends the functional potential of microbial fermentation beyond preservation, offering added value in the development of functional foods. For instance, strains of Lactiplantibacillus plantarum and Pediococcus acidilactici have been shown to produce antimicrobial compounds during the fermentation of plant-based substrates such as fruit juices, peels, and pulps [7,32].
The application of natural microbial antimicrobials is particularly advantageous in minimally processed foods, including ready-to-eat vegetables, refrigerated juices, artisanal cheeses, cured meats, and fermented products. In these categories, where thermal treatments or synthetic additives are often limited due to sensory, nutritional, or regulatory concerns, microbial-derived compounds provide microbiological safety while supporting clean label formulations aligned with consumer preferences [33].
Nonetheless, several challenges remain for the broader industrial application of these natural antimicrobials. Limitations such as thermal instability, reduced efficacy across diverse food matrices, and narrow spectra of antimicrobial activity must be addressed. To overcome these barriers, strategies including microencapsulation, synergistic use with other preservation hurdles, and co-application with protective microbial cultures are being explored to enhance stability, efficacy, and scalability [34,35,36].
In conclusion, antimicrobials produced by microorganisms offer a promising avenue for the development of safe, natural, and eco-conscious food preservation systems. Their integration into modern food processing supports both the principles of barrier technology and the transition toward cleaner, more sustainable production models within the food industry [37,38].
3.2. Competitive Exclusion and Protective Cultures
Competitive exclusion is a fundamental ecological principle in food microbiology, asserting that two microorganisms occupying similar ecological niches cannot stably coexist in the same environment, as one will eventually outcompete the other [32]. This concept underpins the development of protective cultures, a consortia of selected microorganisms that rapidly colonize the food matrix and inhibit the proliferation of pathogens and spoilage organisms through non-antibiotic mechanisms.
The effectiveness of competitive exclusion in food preservation is attributed to several microbial strategies, including the production of antimicrobial metabolites (e.g., bacteriocins and organic acids), competition for nutrients, acidification of the environment, and the release of hydrogen peroxide, siderophores, and volatile inhibitory compounds. Additionally, physical occupation of ecological niches limits the ability of undesirable microorganisms to establish themselves [32,33,34,35,36,37,38,39]. These mechanisms are particularly relevant in minimally processed foods and under refrigerated storage, where psychotropic pathogens such as Listeria monocytogenes pose significant food safety risks.
Protective cultures are commonly composed of lactic acid bacteria (LAB), including Lactiplantibacillus plantarum, Lacticaseibacillus rhamnosus, Pediococcus acidilactici, and Leuconostoc mesenteroides. These strains are selected for their adaptability to diverse substrates and resilience under variable conditions of pH, temperature, and water activity [40]. The use of autochthonous strains, isolated from the same food matrix, is encouraged to enhance colonization efficiency and preserve the traditional sensory attributes of the final product [41,42,43,44].
Applications of protective cultures are particularly well established in meat products, fresh cheeses, and fermented sausages. For instance, the incorporation of Lactobacillus sakei or Carnobacterium maltaromaticum in white cheeses and refrigerated dairy products has been shown to effectively control Listeria monocytogenes without negatively affecting sensory quality [40]. In minimally processed vegetables, microbial consortia comprising Lactiplantibacillus and Pediococcus spp. have demonstrated efficacy against Salmonella and Escherichia coli through acidification and competitive metabolic interactions [44].
The use of protective cultures aligns with clean label initiatives, being recognized as safe and natural preservation strategies. In many jurisdictions, LAB used in foods are designated as GRAS (Generally Recognized As Safe) or hold QPS (Qualified Presumption of Safety) status by the European Food Safety Authority (EFSA), exempting them from specific regulatory approval provided they do not possess virulent traits or antibiotic resistance [45,46].
Beyond their antimicrobial function, protective cultures may offer additional technological and functional benefits. These include the production of exopolysaccharides with texturizing properties, reduction of biogenic amines such as histamine, and modulation of gut microbiota in fermented foods containing live cultures [15].
Commercial application of protective cultures in fermented dairy products, such as yogurts, has yielded improvements in microbiological stability, textural maintenance, and shelf life extension, all without compromising physicochemical integrity or sensory characteristics [47].
However, several challenges remain for their broader implementation, notably the standardization of culture composition, stability during storage, compatibility with other starter strains, and performance across varied food matrices. To address these issues, omics technologies (e.g., metagenomics and metabolomics) and ecological modeling approaches are increasingly employed to elucidate microbial interactions and optimize the rational selection and application of protective cultures in complex food systems [14].
3.3. Phage Therapy and CRISPR-Based Biocontrol
The use of bacteriophages (phages) and CRISPR-Cas-based systems represents a cutting-edge approach in microbial biocontrol, offering highly specific and environmentally safe alternatives for pathogen management in food systems and processing environments. These strategies embody the principles of precision microbiology, enabling the targeted suppression of undesirable microorganisms without disturbing beneficial microbiota or compromising the sensory quality of foods [48,49].
Bacteriophages are viruses that infect and replicate exclusively within bacteria. Their application in food safety focuses on the selective elimination of pathogenic bacteria such as Listeria monocytogenes, Salmonella enterica, Escherichia coli O157:H7, and Campylobacter jejuni, with their efficacy demonstrated in various products, including ready-to-eat meats, minimally processed vegetables, dairy products, and seafood [42].
Several commercial phage-based products—such as ListShieldTM, EcoShieldTM, and PhageGuard—have been approved by regulatory bodies including the U.S. Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA) and are Generally Recognized As Safe (GRAS). These products can be applied via spraying, immersion, or integration into active packaging, without altering the organoleptic properties of foods [48].
Additionally, real-world applications of phage biocontrol are increasingly seen in the food industry. The U.S.-based company Intralytix has developed GRAS-approved phage products like ListShieldTM for Listeria monocytogenes and EcoShieldTM for E. coli, which are applied to ready-to-eat meats and vegetables. Likewise, Micreos BV (The Netherlands) manufactures PhageGuard, used in the dairy and meat industries to control pathogens like Listeria and Salmonella without affecting sensory properties
The mechanisms of action of phage therapy and CRISPR-Cas biocontrol are illustrated in Figure 1. This schematic representation is based on the models proposed by Sturino and Klaenhammer (2006) [49], who described engineered bacteriophage resistance systems in bioprocessing, and by Ghosh et al. (2019) [50], who highlighted phages and CRISPR-Cas systems as emerging alternatives to antibiotics.
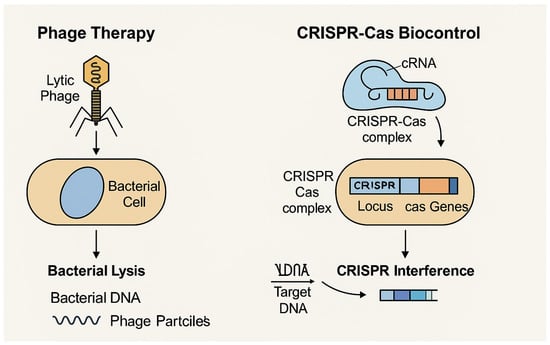
Figure 1.
Schematic illustration of biocontrol strategies based on bacteriophages and CRISPR-Cas systems. The mechanisms presented include direct bacterial lysis by lytic phages and gene-targeted disruption via CRISPR interference. Adapted from Sturino and Klaenhammer (2006) and Ghosh et al. (2019) [49,50].
Key advantages of phage therapy include:
- High specificity, which prevents disruption of non-target microbial communities.
- Self-limiting action, as phages only replicate in the presence of their specific bacterial hosts [51].
- Low risk of cross-resistance with conventional antibiotics.
- Compatibility with mild processing technologies, supporting clean label trends [52].
Nevertheless, some limitations persist. These include the rapid inactivation of phages in complex food matrices, the narrow host range of monovalent phages, and the need for strain-specific regulatory approvals [53]. To overcome these challenges, recent advancements include the development of phage cocktails and genetically engineered phages, which enhance stability, broaden the antibacterial spectrum, and improve the robustness of biocontrol interventions.
CRISPR-Cas systems, originally identified as adaptive immune mechanisms in prokaryotes, have been repurposed into high-precision tools for antimicrobial applications. These systems utilize guide RNAs to direct Cas nucleases to specific genomic sequences, allowing for the selective degradation of bacterial chromosomes, virulence genes, or antibiotic resistance plasmids [54].
Recent studies have demonstrated that CRISPR-Cas systems delivered via engineered bacteriophages can selectively eliminate target pathogens within complex microbial communities—such as those found in fermented foods or ready-to-eat meats—without disturbing the native microbiota [55]. This targeted approach is especially advantageous in products where microbial diversity is integral to sensory quality and functional properties.
Moreover, CRISPR-Cas technology has been employed to silence genes involved in biofilm formation, toxin production, and antimicrobial resistance, reinforcing its potential for use in food safety and industrial sanitation [56].
Despite its promise, the application of CRISPR-Cas in food systems faces regulatory, ethical, and societal challenges. These include concerns about the use of genetically modified organisms (GMOs), traceability, and consumer acceptance. However, the growing demand for precise, low-impact antimicrobial tools is driving translational research in this field, with tangible prospects for industrial implementation in the near future. Table 2 summarizes this information.

Table 2.
Applications of protective lactic acid bacteria (LAB) in food matrices, target pathogens, and inhibitory mechanisms. Data compiled by the authors based on sources [36,38,39,40,41,42,43,44,45,46,47,48,57].
4. Functional Foods and Health-Promoting Microorganisms
4.1. Probiotics, Prebiotics, Synbiotics, and Postbiotics
The growing understanding of the relationship between the gut microbiota and human health has intensified interest in dietary components capable of positively modulating this complex microbial ecosystem. In this context, probiotics, prebiotics, synbiotics, and, more recently, postbiotics have emerged as promising functional tools for health promotion, disease prevention, and innovation in the development of sustainable foods [54,55].
According to the joint definition by the FAO/WHO, probiotics are “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host” [56]. The most studied probiotic strains belong to the genera Lactobacillus, Bifidobacterium, Saccharomyces, Streptococcus, and Lactococcus, selected based on strict criteria such as resistance to gastric acidity and bile salts, adhesion to intestinal mucosa, immunomodulatory activity, antagonism against pathogens, and proven safety for human consumption [57].
Clinical studies have linked probiotic intake with a range of health benefits, including improvements in gastrointestinal function (e.g., reduction of diarrhea and constipation), modulation of immune responses, influence on the gut-brain axis, and support in the prevention and management of metabolic disorders such as obesity and type 2 diabetes [58]. In the food sector, probiotics have been incorporated into fermented dairy and plant-based products, infant formulas, functional beverages, and nutraceutical supplements, often requiring encapsulation technologies (e.g., microencapsulation or spray drying) to preserve cell viability during processing and storage until consumption.
Prebiotics are defined as “substrates that are selectively utilized by host microorganisms conferring a health benefit”. The most investigated prebiotics include fructooligosaccharides (FOS), galactooligosaccharides (GOS), inulin, and xylooligosaccharides (XOS), as well as plant-derived polyphenols from sources such as grapes, cocoa, and green tea [59]. These compounds preferentially stimulate the growth and metabolic activity of beneficial bacteria—particularly Bifidobacterium and Lactobacillus—enhancing the production of short-chain fatty acids (SCFAs) such as acetate, propionate, and butyrate, which play essential roles in intestinal homeostasis, immune modulation, and host metabolism.
Synbiotics combine probiotics and prebiotics to synergistically promote the survival and activity of beneficial microbes in the host. They are categorized into complementary synbiotics, where the components function independently, and synergistic synbiotics, in which the prebiotic selectively enhances the growth and metabolic activity of the co-administered probiotic strain [60]. Synbiotics have demonstrated efficacy in various clinical contexts, including inflammatory bowel disease, antibiotic-associated dysbiosis, and immunonutrition strategies for vulnerable populations such as the elderly and children.
Postbiotics represent a new generation of microbiome-derived ingredients, defined as “preparations of inactivated microorganisms and/or their metabolites that confer health benefits to the host” [61]. These compounds have attracted increasing attention due to their superior stability, safety profile, and compatibility with various food processing conditions when compared to live probiotics [62]. Unlike probiotics, postbiotics do not require viability to be effective, making them ideal for incorporation into heat-treated or shelf-stable food products. Key postbiotic components include short-chain fatty acids (SCFAs), organic acids, microbial cell fragments, peptides, and enzymes, which exhibit immunomodulatory, anti-inflammatory, and antioxidant activities.
Additionally, there is growing scientific consensus on the importance of clearly distinguishing probiotics, prebiotics, and postbiotics in functional food labeling and health claims. According to updated international guidelines, only microbial strains with proven health benefits—supported by human clinical trials and thorough taxonomic identification—should be designated as “probiotics” [63]. This distinction enhances consumer trust and promotes transparency while also supporting regulatory harmonization in the use of microbial ingredients in food systems.
Synbiotics combine probiotics and prebiotics in formulations aimed at improving the survival and metabolic activity of beneficial microorganisms in the host. They are categorized as complementary, where each component acts independently, or synergistic, where the prebiotic is specifically selected to support the probiotic strain [64]. Synbiotics have demonstrated clinical potential in inflammatory bowel disease, antibiotic-induced dysbiosis, and immunonutrition strategies for pediatric and elderly populations [65,66].
Postbiotics, recently defined by ISAPP as “preparations of inanimate microorganisms and/or their components that confer health benefits on the host,” represent a paradigm shift in the use of microbial-based interventions [67]. Unlike probiotics, postbiotics are not dependent on viability for efficacy, offering advantages in terms of safety, stability, and compatibility with industrial food processing [68]. They can be incorporated into heat-treated, shelf-stable, or non-refrigerated formulations while retaining their bioactivity [69].
Unlike probiotics, their functionality does not depend on microbial viability, offering enhanced stability, safety, and technological versatility, especially in food products that undergo thermal treatment or do not require refrigeration.
Key postbiotic components include antimicrobial peptides, organic acids, exopolysaccharides, vitamins, enzymes, and SCFAs, all of which have demonstrated anti-inflammatory, antioxidant, immunomodulatory, and gut barrier-protective effects [70]. Due to their stability and ease of incorporation, postbiotics present regulatory and industrial advantages over live probiotics, making them attractive candidates for the formulation of functional foods with a clean label and proven efficacy.
The future of functional nutrition is expected to move towards precision and personalized formulations, integrating omics technologies (e.g., metagenomics and metabolomics), bioinformatics, and targeted delivery systems. In this context, probiotics, prebiotics, synbiotics, and postbiotics will continue to play a central role in the design of sustainable and science-based food systems tailored to improve public health outcomes.
4.2. Technological Challenges and Stability of Functional Microbial Ingredients
The efficacy of probiotics and synbiotics largely depends on their viability and metabolic activity at the time of consumption. This poses significant formulation challenges, especially during food processing, storage, and gastrointestinal transit. Strategies such as microencapsulation, lyophilization, and the use of protective carriers (e.g., resistant starches or lipid-based matrices) are increasingly employed to enhance microbial survival and functional delivery. Moreover, ensuring strain-specific stability without compromising sensory quality remains a priority in the development of functional products [71,72,73,74].
4.3. Applications in Personalized Nutrition
Advancements in microbiome research have revealed significant inter-individual variability in microbial composition and metabolic responses to dietary interventions. This has opened the door for personalized nutrition strategies, where probiotics, prebiotics, and synbiotics are tailored to individual gut profiles or health conditions. Tools such as metagenomic sequencing and machine learning are being integrated into clinical practice to guide targeted formulations that optimize host–microbe interactions for specific outcomes [75,76,77,78].
4.4. Role in Mental Health and the Gut-Brain Axis
Emerging evidence highlights the influence of gut microbiota on neurological function through the gut-brain axis, implicating microbial metabolites such as SCFAs, neurotransmitter precursors, and immune mediators. Certain probiotic strains—sometimes referred to as “psychobiotics”—have shown promise in modulating mood, reducing anxiety, and improving cognitive performance. These findings suggest potential for probiotic and postbiotic interventions in mental health management, although further clinical validation is needed [79,80,81].
4.5. Environmental and Sustainability Perspectives
Incorporating microbiome-based ingredients into food systems also contributes to sustainability goals. Fermentation processes using probiotic or postbiotic-producing microbes can valorize agricultural by-products, reduce food waste, and lower the environmental footprint of production. Additionally, the development of self-stable, non-refrigerated postbiotic formulations aligns with the reduction of energy demands across the food supply chain [82,83,84,85]. Table 3 summarizes this content.

Table 3.
Overview of the functional food components, their mechanisms of action, and health-related applications. Data compiled by the authors based on Verma et al. (2022) [84], Abedin et al. (2024) [85], and additional literature sources [66,67,68,69,70,71,72,73,74,75,76].
5. Regulatory and Technological Advances in Microbial Ingredients for Food Innovation
The advancement of microbial innovations applied to food—including functional fermented foods, protective cultures, biocontrol, and precision fermentation—requires not only technological development but also alignment with specific regulatory frameworks, overcoming technical barriers and understanding the expectations of modern consumers. The consolidation of these products in the market depends on the interaction between science, regulation, and social acceptance [86,87,88].
In the European Union, the authorization of microorganisms or their derivatives for use in food follows the criteria established by the EFSA, through the QPS (Qualified Presumption of Safety) status. This system evaluates toxicological safety, usage history, and the absence of virulence and antimicrobial resistance genes [38]. Products such as probiotics, microbial enzymes, and bacteriocins can also be classified as novel foods, requiring specific evaluation before commercialization [89,90,91].
In the United States, the FDA system classifies substances and microorganisms as GRAS (Generally Recognized As Safe). Companies can submit safety assessment dossiers or use strains with a proven history of use. Postbiotic ingredients and bioprotective cultures have been classified based on scientific data and traditional usage precedents [92,93].
In Brazil, ANVISA regulates probiotics and functional ingredients through Normative Instruction No. 60/2020, requiring scientific evidence of efficacy and safety, as well as the standardization of strains and dosages. Labeling and health claims must follow the guidelines of RDC No. 429/2020, promoting transparency and traceability [18,94].
Despite regulatory advances, there are still gaps in regulations for ingredients such as postbiotics, proteins produced by precision fermentation, phages, and CRISPR systems, which incorporation depends on case-by-case evaluations and updated legal frameworks [21,95].
The transition of fermentation processes from the laboratory to industrial scale presents challenges such as the selection and maintenance of stable strains, reproducible control of fermentation conditions, ensuring cell viability (in the case of probiotics), and optimizing the yield of target metabolites [67].
Technologies such as automated bioreactors, kinetic modeling, genetic editing of industrial strains, and spray drying or fluidized bed encapsulation have been incorporated to improve the efficiency, cost-effectiveness, and functional stability of products with live or inactivated microorganisms [18].
Furthermore, the application of omics tools (metagenomics, proteomics, and metabolomics) associated with artificial intelligence has enabled the customization of ingredients based on the target microbiome profile, opening up opportunities for functional foods with individualized applications and greater clinical efficacy [15,95,96].
The global market for microbial functional foods has grown exponentially, driven by consumers seeking natural products with health claims, clean labeling, and lower environmental impact. It is estimated that the probiotics and synbiotics sector will generate over USD 80 billion by 2026, with a focus on yogurts, plant-based beverages, snacks, and encapsulated supplements [97].
Behavioral studies indicate that consumers value attributes such as naturalness of ingredients, local or artisanal origin, sustainability in production, and transparency in functional claims [58].
However, the acceptance of emerging technologies, such as precision fermentation, synthetic postbiotics, and foodborne phages, still faces resistance from more conservative consumers, requiring communication strategies based on scientific education, trust in certifications, and regulatory transparency [98].
6. Evidence-Based Insights into Microbial Innovations
Food microbiology stands at the forefront of some of the most promising strategies for building sustainable, resilient, and functional food systems. Throughout this review, we have examined evidence highlighting the transformative potential of microorganisms in food production, preservation, and functionalization, bringing together technological innovation, public health goals, and environmental responsibility [83,84,99].
Microbial fermentation, whether in its traditional forms or through precision approaches, has shown remarkable versatility and low environmental impact in converting plant-based substrates and agro-industrial by-products into safe, nutritious, and value-added foods. The use of starter cultures, synergistic microbial consortia, and metabolic engineering enables process control, standardization, and functional customization across different production scales and cultural contexts [90,100,101].
Microbial biocontrol, through mechanisms such as competitive exclusion, bacteriocins, bacteriophages, and CRISPR-Cas-based technologies, offers a promising alternative to chemical preservatives. These strategies provide natural, targeted solutions for enhancing food safety and shelf life, especially in minimally processed products, and are generally well accepted by consumers seeking clean label alternatives [97,98,99,100,101,102].
In the field of functional foods, the integration of probiotics, prebiotics, synbiotics, and postbiotics has been associated with improvements in gut microbiota composition and a range of health indicators, including metabolic, immune, and cognitive functions. Developing such products with sustainability in mind—using local raw materials, intelligent encapsulation methods, and waste valorization—reinforces their relevance as essential components of a healthier, more ethical, and adaptable diet for the 21st century [83,90,100,101,102,103,104,105,106,107].
Despite their potential, the widespread adoption of these microbial innovations still faces key challenges, including methodological harmonization, clinical validation of health benefits, regulatory alignment, and consumer education. The integrated application of omics technologies, artificial intelligence, and environmental bioprospecting could accelerate discoveries; support personalized interventions; and broaden the ethical, safe, and scalable application of microbial solutions [108,109,110].
In conclusion, food microbiology—when grounded in sustainability and powered by emerging technologies—has the capacity to drive the transition toward a new food paradigm that is healthier, regenerative, and evidence-based. Achieving this vision will require interdisciplinary collaboration among researchers, regulatory bodies, industry, and society, as well as sustained investment in applied science, open innovation, and public policies that promote the bioeconomy and global food security (Figure 2).
Figure 2.
Sustainable innovations in food microbiology.
7. Future Trends and Research Gaps
Food microbiology, increasingly aligned with sustainability and precision health, is entering a transformative phase driven by the convergence of advanced biotechnological platforms; unconventional microbial resources; and the pressing need for scientific, technological, and regulatory standardization. This multifaceted evolution is pivotal for translating laboratory discoveries into robust, safe, and scalable innovations with real-world applications across the food sector [111,112,113,114].
The rapid development and integration of omics technologies—including metagenomics, transcriptomics, proteomics, and metabolomics—have profoundly reshaped our understanding of microbial ecosystems in food matrices. These tools allow for high-resolution analyses of the composition, functionality, and ecological interactions of microbial consortia. Advanced techniques such as next-generation sequencing (NGS) and high-resolution mass spectrometry are now routinely employed to characterize microbial communities in fermented foods, detect emerging spoilage or pathogenic agents, and identify novel bioactive compounds or resistance mechanisms [115,116,117].
Simultaneously, the integration of artificial intelligence (AI) and machine learning (ML) into microbiome research has opened new frontiers in predictive modeling, enabling the simulation of microbial interactions, metabolic pathway optimization, and customization of functional foods in accordance with individual microbiome profiles [118,119,120]. Computational platforms such as QIIME, PICRUSt, and GNPS are being increasingly linked with expanding genomic and metabolomic databases, enhancing the accuracy of metabolic function prediction and enabling the design of microbial consortia with targeted health applications [74,121,122,123,124].
These multidisciplinary strategies are driving the development of a new generation of precision-functional foods—tailored formulations incorporating microbial strains or metabolites aimed at specific physiological goals such as glycemic regulation, modulation of inflammation, immune enhancement, or neurological well-being [67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126].
In parallel, extreme environments, including hydrothermal vents, hypersaline lakes, polar ice caps, marine sediments, and arid ecosystems, are being increasingly explored as reservoirs of novel microbial biodiversity. Microorganisms adapted to extreme conditions (e.g., halotolerant, thermophilic, or psychrophilic strains) possess unique enzymatic systems and bioactive metabolites, many of which exhibit antimicrobial, antioxidant, or biopreservative activities with potential applications in food preservation and functionalization [127,128,129,130,131].
Tropical regions, especially biodiversity hotspots such as the Brazilian Atlantic Forest, Amazon Basin, and Cerrado biome, represent strategic frontiers for the bioprospecting of autochthonous probiotics, new bacteriocins, and biocontrol agents adapted to local climatic conditions and traditional food substrates [132,133,134]. However, the ethical and sustainable exploration of these microbial resources must strictly adhere to international frameworks, particularly the Nagoya Protocol and national legislation governing access to genetic heritage and benefit-sharing [135,136,137,138,139,140,141,142].
Despite notable advances, there are still considerable research and regulatory gaps that hinder the consolidation of microbiome-derived innovations in food systems. One major limitation is the lack of methodological standardization in the isolation, characterization, and application of microbial strains and consortia. This heterogeneity impedes cross-study comparisons, reduces reproducibility, and poses significant barriers to clinical validation and regulatory approval [15,62].
There is an urgent need for internationally harmonized guidelines encompassing key parameters such as accurate taxonomic identification and strain-level quantification, determination of cell viability and bioactivity in vivo, design and execution of robust randomized clinical trials (RCTs), evaluation of long-term stability during processing and storage, and comprehensive toxicological and genomic safety assessments [137,138].
Furthermore, while the therapeutic potential of postbiotics, personalized synbiotics, and next-generation fermented foods has been increasingly documented, the lack of long-term studies and high-quality clinical trials—especially in vulnerable populations such as elderly individuals, children, and immunocompromised patients—continues to limit their clinical translation and acceptance by regulatory authorities [31,81,142,143].
Bridging these gaps requires the development of integrated translational research platforms that connect basic science, industrial development, and regulatory science. These platforms should promote multi-stakeholder collaboration, ensure equitable benefit-sharing, and support the democratization of sustainable microbial solutions in food systems. Ultimately, aligning microbiome innovation with public health priorities, consumer values, and planetary boundaries will be key to unlocking its full potential in shaping the future of food [81,108,144,145].
8. Conclusions
The integration of traditional fermented foods with innovative microbial biocontrol strategies provides a robust and sustainable foundation for advancing food quality, safety, and functionality. Fermentation processes, driven by diverse microbial consortia, not only preserve food but also enhance its nutritional and functional properties, contributing to both human health and cultural continuity. At the same time, emerging precision biocontrol tools—such as protective cultures, bacteriophages, and CRISPR-Cas systems—represent a clear evolution from conventional preservation techniques toward a more targeted and adaptive microbiology.
These innovations align with clean label trends, evolving regulatory frameworks, and the urgent need for resilient food systems in the face of global challenges. Nevertheless, their widespread implementation requires further investigation into strain specificity, process integration, consumer acceptance, and policy alignment. By bridging traditional ecological knowledge with advanced molecular technologies, the food sector is poised to deliver safer, more effective, and personalized solutions. Interdisciplinary research will not only optimize these tools across diverse food matrices but will also shape the future of sustainable and functional food innovation.
Funding
This work is supported by National Funds by the FCT—Portuguese Foundation for Science and Technology, under the projects UID/04033/2023: Centre for the Research and Technology of Agro-Environmental and Biological Sciences and LA/P/0126/2020 (https://doi.org/10.54499/LA/P/0126/2020).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Crippa, M.; Solazzo, E.; Guizzardi, D.; Monforti-Ferrario, F.; Tubiello, F.N.; Leip, A. Os sistemas alimentares são responsáveis por um terço das emissões antropogênicas globais de GEE. Nat. Food 2021, 2, 198–209. [Google Scholar] [CrossRef] [PubMed]
- FAO—Food and Agriculture Organization of the United Nations. The State of Food and Agriculture 2021: Making Agrifood Systems More Resilient to Shocks and Stresses; FAO: Rome, Italy, 2021; Available online: https://www.fao.org/documents/card/en/c/cb4476en (accessed on 20 May 2025).
- Leeuwendaal, N.K.; Stanton, C.; O’Toole, P.W.; Beresford, T.P. Alimentos Fermentados, Saúde e o Microbioma Intestinal. Nutrients 2022, 14, 1527. [Google Scholar] [CrossRef]
- Tamang, J.P.; Cotter, P.D.; Endo, A.; Han, N.S.; Kort, R.; Liu, S.Q.; Mayo, B.; Westerik, N.; Hutkins, R. Fermented foods in a global age: East meets West. Compr. Rev. Food Sci. Food Saf. 2020, 19, 184–217. [Google Scholar] [CrossRef]
- Swain, M.R.; Anandharaj, M.; Ray, R.C.; Rani, R.P. Fermented fruits and vegetables of Asia: A potential source of probiotics. Biotechnol. Res. Int. 2014, 2014, 1–19. [Google Scholar] [CrossRef]
- Silva, C.C.G.; Silva, S.P.M.; Ribeiro, S.C. Application of bacteriocins and protective cultures in dairy food preservation. Front. Microbiol. 2018, 9, 594. [Google Scholar] [CrossRef] [PubMed]
- Markowiak, P.; Śliżewska, K. Efeitos de probióticos, prebióticos e simbióticos na saúde humana. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Suez, J.; Suez, J.; Zmora, N.; Segal, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef]
- Leite, A.M.d.O.; Miguel, M.A.L.; Peixoto, R.S.; Rosado, A.S.; Silva, J.T.; Paschoalin, V.M.F. Microbiological, technological and therapeutic properties of kefir: A natural probiotic beverage. Braz. J. Microbiol. 2013, 44, 341–349. [Google Scholar] [CrossRef]
- Nout, M.J.R.; Kiers, J.L. Tempe fermentation, innovation and functionality: Update into the third millennium. J. Appl. Microbiol. 2005, 98, 789–805. [Google Scholar] [CrossRef]
- Tomohiro, W.; Koji, Y.; Minoru, O. Production of miso, soy sauce, and other traditional Japanese seasonings. In Handbook of Food Products Manufacturing: Health, Meat, Milk, Poultry, Seafood, and Vegetables; Hui, Y.H., Ed.; Wiley: Hoboken, NJ, USA, 2007; Volume 2, pp. 545–566. [Google Scholar]
- Spiros, P.; Eleftherios, H.D.; Effie, T. Fermentation of vegetables. In Microbiology and Technology of Fermented Foods, 2nd ed.; Hutkins, R.W., Ed.; Wiley-Blackwell: Ames, IA, USA, 2020; ca11; pp. 311–340. [Google Scholar]
- Thierry, A.; Madec, M.-N.; Chuat, V.; Bage, A.-S.; Picard, O.; Grondin, C.; Rué, O.; Mariadassou, M.; Marché, L.; Valence, F. Microbial communities of a variety of 75 homemade fermented vegetables. Front. Microbiol. 2023, 14, 1323424. [Google Scholar] [CrossRef]
- Louw, N.L.; Lele, K.; Ye, R.; Edwards, C.B.; Wolfe, B.E. Microbiome Assembly in Fermented Foods. Annu. Rev. Microbiol. 2023, 77, 381–402. [Google Scholar] [CrossRef] [PubMed]
- Marco, M.L.; Sanders, M.E.; Gänzle, M.; Arrieta, M.C.; Cotter, P.D.; De Vuyst, L.; Hill, C.; Holzapfel, W.; Lebeer, S.; Merenstein, D.; et al. Declaração de consenso da Associação Científica Internacional para Probióticos e Prebióticos (ISAPP) sobre alimentos fermentados. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 196–208. [Google Scholar] [CrossRef]
- Rezac, S.; Kok, C.R.; Heermann, M.; Hutkins, R. Fermented foods as a dietary source of live organisms. Front. Microbiol. 2018, 9, 1785. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.B.; Meyer, A.S.; Arnau, J. The next food revolution is here: Recombinant microbial production of milk and egg proteins by precision fermentation. Annu. Rev. Food Sci. Technol. 2024, 15, 173–187. [Google Scholar] [CrossRef]
- Augustin, M.A.; Hartley, C.J.; Maloney, G.; Tyndall, S. Innovation in precision fermentation for food ingredients. Crit. Rev. Food Sci. Nutr. 2023, 64, 6218–6238. [Google Scholar] [CrossRef] [PubMed]
- Leroy, F.; De Vuyst, L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol. 2004, 15, 67–78. [Google Scholar] [CrossRef]
- Rogers, J.K.; Taylor, N.D. Synthetic biology approaches for engineering metabolism and production of value-added compounds. Curr. Opin. Biotechnol. 2021, 67, 1–8. [Google Scholar] [CrossRef]
- Eastham, J.L.; Leman, A.R. Precision fermentation for food proteins: Ingredient innovations, bioprocess considerations, and outlook. Curr. Opin. Food Sci. 2024, 58, 101194. [Google Scholar] [CrossRef]
- Kuhl, E. IA para alimentos: Acelerando e democratizando a descoberta e a inovação. NPJ Sci. Food 2025, 9, 82. [Google Scholar] [CrossRef]
- MarketsandMarkets. Precision Fermentation Ingredients Market by Ingredient (Egg, Dairy Proteins, Collagen, Heme), Microbe (Yeast, Algae, Bacteria, Fungi), Application (Dairy Alternatives, Meat Alternatives, Egg Alternatives), and Region—Global Forecast to 2030. MarketsandMarketsTM. 2025. Available online: https://www.marketsandmarkets.com/Market-Reports/precision-fermentation-market-147520320.html (accessed on 25 May 2025).
- Soccol, C.R.; Pandey, A.; Larroche, C. (Eds.) Current Developments in Biotechnology and Bioengineering: Solid-State Fermentation for Foods and Beverages; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Thomas, L.; Larroche, C.; Pandey, A. Current developments in solid-state fermentation. Biochem. Eng. J. 2013, 81, 146–161. [Google Scholar] [CrossRef]
- Rana, P.; Inbaraj, B.S.; Gurumayum, S.; Sridhar, K. Produção sustentável de enzimas lignocelulolíticas na fermentação em estado sólido de resíduos agroindustriais: Aplicação na clarificação do suco de abóbora (Cucurbita Maxima). Agron 2021, 11, 2379. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Khanashyam, A.C.; Mundanat, A.S.; Shah, K.; Babu, K.S.; Thorakkattu, P.; Al-Asmari, F.; Pandiselvam, R. Valorização de Resíduos de Frutas para Compostos Bioativos e Suas Aplicações na Indústria Alimentícia. Foods 2023, 12, 556. [Google Scholar] [CrossRef]
- Senila, L.; Gál, E.; Kovacs, E.; Cadar, O.; Dan, M.; Senila, M.; Roman, C. Produção de poli(3-hidroxibutirato) a partir de resíduos lignocelulósicos usando Bacillus megaterium ATCC 14581. Polymers 2023, 15, 4488. [Google Scholar] [CrossRef] [PubMed]
- de Abreu, Í.B.S.; Silva, R.K.; Siqueira, J.G.W.; Silva, P.K.N.d.; Sonego, J.L.S.; de Souza, R.B.; Antonino, A.C.D.; Menezes, R.S.C.; Dutra, E.D. Resíduos de Alimentos Brasileiros como Substrato para Produção de Bioetanol. Alimentos 2024, 13, 4032. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Wang, L.; Liu, J.; Wang, Z.; Tang, Y.; Li, M.; Zhou, X.; Hu, X.; Jia, Y.; et al. Valorization of food waste into single-cell protein: An innovative approach for sustainable protein production. NPJ Sci. Food 2024, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Neylon, E.; Nyhan, L.; Zannini, E.; Monin, T.; Münch, S.; Sahin, A.W.; Arendt, E.K. Ingredientes Alimentares para o Futuro: Análise Aprofundada dos Efeitos da Fermentação com Bactérias Ácido-Láticas em Raízes de Cevada Usadas. Fermentation 2023, 9, 78. [Google Scholar] [CrossRef]
- Sorathiya, K.B.; Melo, A.; Hogg, M.C.; Pintado, M. Ácidos orgânicos na preservação de alimentos: Explorando sinergias, insights moleculares e aplicações sustentáveis. Sustentabilidade 2025, 17, 3434. [Google Scholar] [CrossRef]
- Yarmolinsky, L.; Nakonechny, F.; Haddis, T.; Khalfin, B.; Dahan, A.; Ben-Shabat, S. Compostos Antimicrobianos Naturais como Conservantes Promissores: Um Olhar para um Problema Antigo sob Novas Perspectivas. Molecules 2024, 29, 5830. [Google Scholar] [CrossRef]
- Yarmolinsky, L.; Nakonechny, F.; Haddis, T.; Khalfin, B.; Dahan, A.; Ben-Shabat, S. Application of essential oils and polyphenols as natural antimicrobial agents in postharvest treatments: Advances and challenges. Food Sci. Nutr. 2020, 8, 2555–2568. [Google Scholar] [CrossRef]
- Soni, A.; Brightwell, G. Nature-Inspired Antimicrobial Surfaces and Their Potential Applications in Food Industries. Foods 2022, 11, 844. [Google Scholar] [CrossRef]
- Qian, M.; Ismail, B.B.; He, Q.; Zhang, X.; Yang, Z.; Ding, T.; Ye, X.; Liu, D.; Guo, M. Inhibitory mechanisms of promising antimicrobials from plant byproducts: A review. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2523–2590. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, D.; Palop, A. Applications of Natural Antimicrobials in Food Packaging and Preservation. Foods 2021, 10, 568. [Google Scholar] [CrossRef]
- Pinto, L.; Tapia-Rodríguez, M.R.; Baruzzi, F.; Ayala-Zavala, J.F. Plant Antimicrobials for Food Quality and Safety: Recent Views and Future Challenges. Foods 2023, 12, 231. [Google Scholar] [CrossRef] [PubMed]
- Woraprayote, W.; Malila, Y.; Sorapukdee, S.; Swetwiwathana, A.; Benjakul, S.; Visessanguan, W. Bacteriocins from lactic acid bacteria and their applications in meat and meat products. Meat Sci. 2016, 120, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-H.; Kim, G.-B. Inhibition of Listeria monocytogenes in fresh cheese using a bacteriocin-producing Lactococcus lactis CAU2013 strain. Korean J. Food Sci. Anim. Resour. 2022, 42, 1009–1018. Available online: https://www.kosfaj.org/archive/view_article?pid=kosfa-42-6-1009 (accessed on 20 May 2025). [CrossRef]
- Zapaśnik, A.; Sokołowska, B.; Bryła, M. Role of Lactic Acid Bacteria in Food Preservation and Safety. Foods 2022, 11, 1283. [Google Scholar] [CrossRef]
- Hakim, B.; Xuan, N.; Oslan, S. A Comprehensive Review of Bioactive Compounds from Lactic Acid Bacteria: Potential Functions as Functional Food in Dietetics and the Food Industry. Foods 2023, 12, 2850. [Google Scholar] [CrossRef]
- Gaggia, F.; Di Gioia, D.; Baffoni, L.; Biavati, B. The role of protective and probiotic cultures in food and feed and their impact in food safety. Trends Food Sci. Technol. 2011, 22 (Suppl. S1), S58–S66. [Google Scholar] [CrossRef]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Use of autochthonous starters for safety improvement in minimally processed fruits and vegetables. Trends Food Sci. Technol. 2013, 30, 38–49. [Google Scholar] [CrossRef]
- EFSA—European Food Safety Authority. Update of the list of QPS-recommended biological agents for safety risk assessments. EFSA J. 2020, 18, e06266. [Google Scholar] [CrossRef]
- Mélo, E.D.d.; e Silva, P.I.S.; do Oriente, S.F.; Almeida, R.D.; Pessoa, J.M.; França, K.B.; de Gusmão, T.A.S.; de Gusmão, R.P.; Lisboa Oliveira, H.M.; Nascimento, A.P.S. Efeito de culturas lácticas bioprotetoras comerciais nas propriedades físico-químicas, microbiológicas e texturais do iogurte. Fermentação 2024, 10, 585. [Google Scholar] [CrossRef]
- Imran, A.; Shehzadi, U.; Islam, F.; Afzaal, M.; Ali, R.; Ali, Y.A.; Chauhan, A.; Biswas, S.; Khurshid, S.; Usman, I.; et al. Bacteriophages and food safety: An updated overview. Food Sci. Nutr. 2023, 11, 2023–2035. [Google Scholar] [CrossRef] [PubMed]
- Farinati, S.; Devillars, A.; Gabelli, G.; Vannozzi, A.; Scariolo, F.; Palumbo, F.; Barcaccia, G. Quão útil pode ser um sistema baseado em CRISPR/Cas para rastreabilidade de alimentos? Foods 2024, 13, 3397. [Google Scholar] [CrossRef]
- Sturino, J.M.; Klaenhammer, T.R. Engineered bacteriophage-defence systems in bioprocessing. Nat. Rev. Microbiol. 2006, 4, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, C.; Sarkar, P.; Issa, R.; Haldar, J. Alternatives to Conventional Antibiotics in the Era of Antimicrobial Resistance. Trends Microbiol. 2019, 27, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Moye, Z.D.; Woolston, J.; Sulakvelidze, A. Bacteriophage applications for food production and processing. Viruses 2018, 10, 205. [Google Scholar] [CrossRef]
- Díaz-Muñoz, S.L.; Koskella, B. Bacteria–phage interactions in natural environments. Adv. Appl. Microbiol. 2014, 89, 135–183. [Google Scholar] [CrossRef]
- Mirzaei, M.; Maurice, C. Ménage à trois no intestino humano: Interações entre hospedeiro, bactérias e fagos. Nat. Rev. Microbiol. 2017, 15, 397–408. [Google Scholar] [CrossRef]
- Barrangou, R.; Van Pijkeren, J.-P. Exploiting CRISPR-Cas immune systems for genome editing in bacteria. Curr. Opin. Biotechnol. 2016, 37, 61–68. [Google Scholar] [CrossRef]
- Yosef, I.; Goren, M.G.; Qimron, U. Proteins and DNA elements essential for phage-assisted CRISPR-Cas antibacterial activity. Sci. Rep. 2012, 7, 42581. [Google Scholar] [CrossRef]
- Citorik, R.J.; Mimee, M.; Lu, T.K. Sequence-specific antimicrobials using efficiently delivered RNA-guided nucleases. Nat. Biotechnol. 2014, 32, 1141–1145. [Google Scholar] [CrossRef] [PubMed]
- Asioli, D.; Aschemann-Witzel, J.; Caputo, V.; Vecchio, R.; Annunziata, A.; Næs, T.; Varela, P. Making sense of the “clean label” trends: A review of consumer food choice behavior and discussion of industry implications. Food Res. Int. 2017, 99, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Polônio, M.L.T.; Peres, F. Consumo de aditivos alimentares e efeitos à saúde: Desafios para a saúde pública brasileira. Cad. De Saúde Pública 2009, 25, 1653–1666. [Google Scholar] [CrossRef] [PubMed]
- Lisboa, H.M.; Pasquali, M.B.; dos Anjos, A.I.; Sarinho, A.M.; de Melo, E.D.; Andrade, R.; Batista, L.; Lima, J.; Diniz, Y.; Barros, A. Técnicas Inovadoras e Sustentáveis de Conservação de Alimentos: Melhorando a Qualidade Alimentar, Segurança e Sustentabilidade Ambiental. Sustentabilidade 2024, 16, 8223. [Google Scholar] [CrossRef]
- EFSA—European Food Safety Authority. The use of biological agents in food production: Risk-based approaches and regulatory perspectives. EFSA J. 2023, 21, e07890. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Declaração de consenso da Associação Científica Internacional para Probióticos e Prebióticos sobre o escopo e o uso apropriado do termo probiótico. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Binda, S.; Hill, C.; Johansen, E.; Obis, D.; Pot, B.; Sanders, M.E.; Tremblay, A.; Ouwehand, A.C. Criteria to qualify microorganisms as “probiotic” in food and dietary supplements. Front. Microbiol. 2020, 11, 1662. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. Declaração de consenso da Associação Científica Internacional de Probióticos e Prebióticos (ISAPP) sobre a definição e o escopo dos pós-bióticos. Nat. Rev. Gastroenterol. Hepatol 2021, 18, 649–667. [Google Scholar] [CrossRef]
- FAO/WHO—Food and Agriculture Organization of the United Nations; World Health Organization. Guidelines for the Evaluation of Probiotics in Food; FAO: Rome, Italy; WHO: London, UK, 2002. Available online: https://www.fao.org/home/en (accessed on 20 May 2025).
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Documento de consenso de especialistas: Declaração de consenso da Associação Científica Internacional para Probióticos e Prebióticos (ISAPP) sobre a definição e o escopo dos prebióticos. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. Declaração de consenso da Associação Científica Internacional para Probióticos e Prebióticos (ISAPP) sobre a definição e o escopo dos simbióticos. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Asefa, Z.; Belay, A.; Welelaw, E.; Haile, M. Postbiotics and their biotherapeutic potential for chronic disease and their feature perspective: A review. Front. Microbiomes 2025, 2, 1489339. [Google Scholar] [CrossRef]
- Morazzoni, C.; Sirel, M.; Allesina, S.; Garcia, M.; Kragh, K.; Pane, M.; Beilharz, K. Proof of concept: Real-time viability and metabolic profiling of probiotics with isothermal microcalorimetry. Front. Microbiol. 2024, 15, 1391688. [Google Scholar] [CrossRef]
- Li, H.; Zhou, D.; Gan, R.; Huang, S.; Zhao, C.; Shang, A.; Xu, X.; Li, H. Effects and Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics on Metabolic Diseases Targeting Gut Microbiota: A Narrative Review. Nutrients 2021, 13, 3211. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Muthuramalingam, K.; Kim, Y.; Park, S.; Kim, S.; Lee, J.; Hyun, C.; Unno, T.; Cho, M. Synbiotic supplementation with prebiotic Schizophyllum commune derived β-(1,3/1,6)-glucan and probiotic concoction benefits gut microbiota and its associated metabolic activities. Appl. Biol. Chem. 2021, 64, 1–10. [Google Scholar] [CrossRef]
- Wastyk, H.C.; Franks, A.F.; Haas, K.N.; Cohen, S.E.; Livingston, R.; Varma, Y.; Gardner, C.D.; Sonnenburg, E.D.; Sonnenburg, J.L. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153.e14. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Dhaneshwar, S. Role of prebiotics, probiotics, and synbiotics in management of inflammatory bowel disease: Current perspectives. World J. Gastroenterol. 2023, 29, 2078–2100. [Google Scholar] [CrossRef]
- Sergeev, I.; Aljutaily, T.; Walton, G.; Huarte, E. Effects of Synbiotic Supplement on Human Gut Microbiota, Body Composition and Weight Loss in Obesity. Nutrients 2020, 12, 222. [Google Scholar] [CrossRef]
- Olas, B. Probiotics, Prebiotics and Synbiotics—A Promising Strategy in Prevention and Treatment of Cardiovascular Diseases? Int. J. Mol. Sci. 2020, 21, 9737. [Google Scholar] [CrossRef]
- Dahiya, D.; Nigam, P. Probiotics, Prebiotics, Synbiotics, and Fermented Foods as Potential Biotics in Nutrition Improving Health via Microbiome-Gut-Brain Axis. Fermentation 2022, 8, 303. [Google Scholar] [CrossRef]
- Morales-Torres, R.; Carrasco-Gubernatis, C.; Grasso-Cladera, A.; Cosmelli, D.; Parada, F.; Palacios-García, I. Psychobiotic Effects on Anxiety Are Modulated by Lifestyle Behaviors: A Randomized Placebo-Controlled Trial on Healthy Adults. Nutrients 2023, 15, 1706. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.; Simões, I.; Cordeiro, C.; Martins, P. Hidden role of gut microbiome in mental health. Eur. Psychiatry 2022, 65, S695. [Google Scholar] [CrossRef]
- Dziedzic, A.; Maciak, K.; Bliźniewska-Kowalska, K.; Gałecka, M.; Kobierecka, W.; Saluk, J. The Power of Psychobiotics in Depression: A Modern Approach through the Microbiota–Gut–Brain Axis: A Literature Review. Nutrients 2024, 16, 1054. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Liu, T.; Al-Ansi, W.; Qian, H.; Fan, M.; Li, Y.; Wang, L. Functional microbiome assembly in food environments: Addressing sustainable development challenges. Comprehens. Rev. Food Sci. Food Saf. 2025, 24, e70074. [Google Scholar] [CrossRef]
- Jahn, L.; Rekdal, V.; Sommer, M. Microbial foods for improving human and planetary health. Cell 2023, 186, 469–478. [Google Scholar] [CrossRef]
- Frontiers Production Office. Erratum: Microbiome-based solutions to address new and existing threats to food security, nutrition, health, and agrifood systems’ sustainability. Front. Sustain. Food Syst. 2023, 7, 1143808. [Google Scholar] [CrossRef]
- Verma, D.K.; Thakur, M.; Singh, S.; Tripathy, S.; Gupta, A.K.; Baranwal, D.; Patel, A.R.; Shah, N.; Utama, G.L.; Niamah, A.K.; et al. Bacteriocins as antimicrobial and preservative agents in food: Biosynthesis, separation and application. Food Biosci. 2022, 46, 101594. [Google Scholar] [CrossRef]
- Abedin, M.M.; Chourasia, R.; Phukon, L.C.; Sarkar, P.; Ray, R.C.; Singh, S.P.; Rai, A.K. Lactic acid bacteria in the functional food industry: Biotechnological properties and potential applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 10730–10748. [Google Scholar] [CrossRef]
- Mahmud, N.; Taiwo, K.; Usack, J. Decarbonizing the Food System with Microbes and Carbon-Neutral Feedstocks. Annu. Rev. Food Sci. Technol. 2024, 16, 81–104. [Google Scholar] [CrossRef]
- Jin, R.; Song, J.; Liu, C.; Lin, R.; Liang, D.; Aweya, J.; Weng, W.; Zhu, L.; Shang, J.; Yang, S. Synthetic microbial communities: Novel strategies to enhance the quality of traditional fermented foods. Compreh. Rev. Food Sci. Food Saf. 2024, 23, e13388. [Google Scholar] [CrossRef] [PubMed]
- Praveen, M.; Brogi, S. Microbial Fermentation in Food and Beverage Industries: Innovations, Challenges, and Opportunities. Foods 2025, 14, 114. [Google Scholar] [CrossRef] [PubMed]
- EFSA. EFSA statement on the requirements for whole genome sequence analysis of microorganisms intentionally used in the food chain. EFSA J. 2024, 22, e8912. [Google Scholar] [CrossRef]
- Lähteenmäki-Uutela, A.; Rahikainen, M.; Lonkila, A.; Yang, B. Alternative proteins and EU food law. Food Control. 2021, 130, 108336. [Google Scholar] [CrossRef]
- Cruz, J.; Vasconcelos, V. Legal Aspects of Microalgae in the European Food Sector. Foods 2023, 13, 124. [Google Scholar] [CrossRef]
- FDA—U.S. Food and Drug Administration. GRAS Notices Inventory and Guidance for Industry; FDA: Silver Spring, MA, USA, 2023. Available online: https://www.fda.gov (accessed on 22 May 2025).
- Salinas, N.; Gomes, L. Open Exceptions: Why Does the Brazilian Health Regulatory Agency (ANVISA) Exempt RIA and Ex Post Reviews? J. Benefit-Cost Anal. 2024, 15, 87–104. [Google Scholar] [CrossRef]
- Danielski, R.; Da Silva, C.; Camargo, A. The gap between scientific evidence and food regulation: Analyzing the updated Brazilian normative on the use of bioactives in food supplements. J. Food Bioact. 2020, 12, 12247. [Google Scholar] [CrossRef]
- Ferrocino, I.; Rantsiou, K.; McClure, R.; Kostić, T.; De Souza, R.; Lange, L.; FitzGerald, J.; Kriaa, A.; Cotter, P.; Maguin, E.; et al. The need for an integrated multi-OMICs approach in microbiome science in the food system. Compreh. Rev. Food Sci. Food Saf. 2023, 22, 1082–1103. [Google Scholar] [CrossRef]
- Shankar, A.; Sharma, K. Fungal secondary metabolites in food and pharmaceuticals in the era of multi-omics. Appl. Microbiol. Biotechnol. 2022, 106, 3465–3488. [Google Scholar] [CrossRef]
- Grand View Research. Probiotics Market Size Report, 2023–2030; Grand View Research: San Francisco, CA, USA, 2023; Available online: https://www.grandviewresearch.com (accessed on 22 May 2025).
- Fanhani, F.C. Emerging Technologies in the Food Industry. Master’s Thesis, Federal University of Paraná, Curitiba, Brazil, 2023. [Google Scholar]
- Graham, A.; Ledesma-Amaro, R. The microbial food revolution. Nat. Commun. 2023, 14, 2231. [Google Scholar] [CrossRef]
- Pereira, A.; Yaverino-Gutiérrez, M.; Monteiro, M.; Souza, B.; Bachheti, R.; Chandel, A. Precision fermentation in the realm of microbial protein production: State-of-the-art and future insights. Food Res. Int. 2024, 200, 115527. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Qiao, M. Towards microbial consortia in fermented foods for metabolic engineering and synthetic biology. Food Res. Int. 2025, 201, 115677. [Google Scholar] [CrossRef]
- Kim, Y.; Huang, L.; Nitin, N. Bio-based antimicrobial compositions and sensing technologies to improve food safety. Curr. Opin. Biotechnol. 2023, 79, 102871. [Google Scholar] [CrossRef] [PubMed]
- Comitini, F.; Canonico, L.; Agarbati, A.; Ciani, M. Biocontrol and probiotic function of non-Saccharomyces yeasts: New insights in agri-food industry. Microorganisms 2023, 11, 1450. [Google Scholar] [CrossRef]
- Bukvički, D.; D’Alessandro, M.; Rossi, S.; Siroli, L.; Gottardi, D.; Braschi, G.; Patrignani, F.; Lanciotti, R. Essential oils and their combination with lactic acid bacteria and bacteriocins to improve the safety and shelf life of foods: A review. Foods 2023, 12, 3288. [Google Scholar] [CrossRef]
- Al-Habsi, N.; Al-Khalili, M.; Haque, S.; Elias, M.; Olqi, N.; Uraimi, T. Health benefits of prebiotics, probiotics, synbiotics, and postbiotics. Nutrients 2024, 16, 3955. [Google Scholar] [CrossRef]
- Zhou, P.; Chen, C.; Patil, S.; Dong, S. Unveiling the therapeutic symphony of probiotics, prebiotics, and postbiotics in gut-immune harmony. Front. Nutr. 2024, 11, 1355542. [Google Scholar] [CrossRef]
- Fekete, M.; Lehoczki, A.; Major, D.; Fazekas-Pongor, V.; Csípő, T.; Tarantini, S.; Csizmadia, Z.; Varga, J. Exploring the influence of gut–brain axis modulation on cognitive health: A comprehensive review of prebiotics, probiotics, and symbiotics. Nutrients 2024, 16, 789. [Google Scholar] [CrossRef] [PubMed]
- Sampara, P.; Lawson, C.; Scarborough, M.; Ziels, R. Advancing environmental biotechnology with microbial community modeling rooted in functional ‘omics. Curr. Opin. Biotechnol. 2024, 88, 103165. [Google Scholar] [CrossRef]
- Jacob, S.; Rajeswari, G.; Rai, A.; Tripathy, S.; Gopal, S.; Das, E.; Kumar, V.; Kumar, S.; Aminabhavi, T.; Garlapati, V. Paradigm of integrative OMICS of microbial technology towards biorefinery prospects. Biocatal. Agric. Biotechnol. 2024, 58, 103226. [Google Scholar] [CrossRef]
- McElhinney, J.; Catacutan, M.; Mawart, A.; Hasan, A.; Dias, J. Interfacing machine learning and microbial omics: A promising means to address environmental challenges. Front. Microbiol. 2022, 13, 851450. [Google Scholar] [CrossRef] [PubMed]
- Avourez, U.; Matassa, S.; Vlaeminck, S.; Verstraete, W. Ruminations on sustainable and safe food: Championing for open symbiotic cultures ensuring resource efficiency, eco-sustainability and affordability. Microb. Biotechnol. 2024, 17, e14436. [Google Scholar] [CrossRef] [PubMed]
- Macori, G.; Fanning, S. The next-generation tools for risk assessment and precision food safety in the One Health continuum. Eur. J. Public Health 2023, 33, e1032. [Google Scholar] [CrossRef]
- Mitra, D.; Kumar, R.; Kamboj, N. Microbial innovations in agriculture: Interdisciplinary approaches to leveraging microbes for food sustainability and security. Indian J. Microbiol. Res. 2024, 11, 129–139. [Google Scholar] [CrossRef]
- Carlino, N.; Blanco-MígUEZ, A.; Punčochář, M.; Mengoni, C.; Segata, N.; Pasolli, E. Unexplored microbial diversity from 2500 food metagenomes and links with the human microbiome. Cell 2024, 187, 5775–5795.e15. [Google Scholar] [CrossRef]
- Shi, H.; An, F.; Lin, H.; Li, M.; Wu, J.; Wu, R. Advances in fermented foods revealed by multi-omics: A new direction toward precisely clarifying the roles of microorganisms. Front. Microbiol. 2022, 13, 1044820. [Google Scholar] [CrossRef]
- Bertola, M.; Ferrarini, A.; Visioli, G. Improvement of soil microbial diversity through sustainable agricultural practices and its evaluation by -Omics approaches: A perspective for the environment, food quality and human safety. Microorganisms 2021, 9, 1400. [Google Scholar] [CrossRef]
- Borges, F.; Briandet, R.; Callon, C.; Champomier-Vergès, M.; Christieans, S.; Chuzeville, S.; Denis, C.; Desmasures, N.; Desmonts, M.; Feurer, C.; et al. Contribution of omics to biopreservation: Toward food microbiome engineering. Front. Microbiol. 2022, 13, 951182. [Google Scholar] [CrossRef]
- Wang, X.; Wang, T.; Liu, Y. Artificial Intelligence for Microbiology and Microbiome Research. arXiv 2024, arXiv:2411.01098. [Google Scholar] [CrossRef]
- Papoutsoglou, G.; Tarazona, S.; Lopes, M.; Klammsteiner, T.; Ibrahimi, E.; Eckenberger, J.; Novielli, P.; Tonda, A.; Simeon, A.; Shigdel, R.; et al. Machine learning approaches in microbiome research: Challenges and best practices. Front. Microbiol. 2023, 14, 1261889. [Google Scholar] [CrossRef]
- Cheng, X.; Joe, B. Artificial Intelligence in Medicine: Microbiome-Based Machine Learning for Phenotypic Classification. Methods Mol. Biol. 2023, 2649, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Dakal, T.C.; Xu, C.; Kumar, A. Advanced computational tools, artificial intelligence and machine-learning approaches in gut microbiota and biomarker identification. Front. Med. Technol. 2025, 6, 1434799. [Google Scholar] [CrossRef] [PubMed]
- Silva-Andrade, C.; Rodriguez-Fernandez, M.; Garrido, D.; Martin, A. Using metabolic networks to predict cross-feeding and competition interactions between microorganisms. Microbiol. Spectr. 2024, 12, e0228723. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Han, W.; Han, M.; Liu, X.; Dai, J.; Dong, Y.; Sun, T.; Xu, J. Intratumoral and fecal microbiota reveals microbial markers associated with gastric carcinogenesis. Front. Cell. Infect. Microbiol. 2024, 14, 1397466. [Google Scholar] [CrossRef]
- Zhang, X.; Borjigin, Q.; Gao, J.; Yu, X.; Hu, S.; Zhang, B.; Han, S. Community succession and functional prediction of microbial consortium with straw degradation during subculture at low temperature. Sci. Res. 2022, 12, 20163. [Google Scholar] [CrossRef]
- Pannerchelvan, S.; Rios-Solis, L.; Wasoh, H.; Sobri, M.; Wong, F.; Mohamed, M.; Mohamad, R.; Halim, M. Functional yogurt: A comprehensive review of its nutritional composition and health benefits. Food Funct. 2024, 15, 10927–10955. [Google Scholar] [CrossRef]
- Marquez-Paradas, E.; Torrecillas-López, M.; Barrera-Chamorro, L.; Del Rio-Vazquez, J.; La Rosa, T.; La Paz, M. Microbiota-derived extracellular vesicles: Current knowledge, gaps, and challenges in precision nutrition. Front. Immunol. 2025, 16, 1514726. [Google Scholar] [CrossRef]
- Medeiros, W.; Hidalgo, K.; Leão, T.; De Carvalho, L.; Ziemert, N.; Oliveira, V. Unlocking the biosynthetic potential and taxonomy of the Antarctic microbiome along temporal and spatial gradients. Microbiol. Spectr. 2024, 12, e0024424. [Google Scholar] [CrossRef]
- De Freitas, P.; Jacinavicius, F.; Passos, L.; De Souza, A.; Dextro, R.; Pinto, E. Exploring the biodiversity of Antarctic cyanobacteria: A review of secondary metabolites and their applications. Algal Res. 2024, 82, 103617. [Google Scholar] [CrossRef]
- Rawat, M.; Chauhan, M.; Pandey, A. Extremophiles and their expanding biotechnological applications. Arch. Microbiol. 2024, 206, 247. [Google Scholar] [CrossRef]
- Rothschild, L.J.; Mancinelli, R.L. Life in extreme environments. Nature 2021, 409, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Jordan, H.; Older, C.; Griffin, M.; Allen, P.; Wise, D.; Goodman, P.; Reifers, J.; Yamamoto, F. Lactococcus lactis MA5 is a potential autochthonous probiotic for nutrient digestibility enhancement and bacterial pathogen inhibition in hybrid catfish (Ictalurus punctatus × I. furcatus). J. Fish Dis. 2024, 47, e13997. [Google Scholar] [CrossRef]
- Persson, K.; Onyema, V.; Nwafor, I.; Peri, K.; Otti, C.; Nnaemeka, P.; Onyishi, C.; Okoye, S.; Moneke, A.; Amadi, O.; et al. Lactose-assimilating yeasts with high fatty acid accumulation uncovered by untargeted bioprospecting. Appl. Environ. Microbiol. 2024, 91, e0161524. [Google Scholar] [CrossRef]
- Goswami, D.; Mondal, S.; Hor, P.; Santra, S.; Jana, H.; Gauri, S.; Halder, S.; Mondal, K. Bioprospecting of probiotic bacteria from traditional food of high-altitude Himalayan region. Food Biosci. 2023, 57, 103257. [Google Scholar] [CrossRef]
- Brasil. Lei nº 13.123, de 20 de maio de 2015. In Regulamenta o Acesso ao Patrimônio Genético e aos Conhecimentos Tradicionais Associados; Diário Oficial da União: Brasília, DF, Brasil, 2015. [Google Scholar]
- Silvestri, L.; Mason, P. Improved access to biological control genetic resources: Navigating through the Convention on Biological Diversity and the Nagoya Protocol. BioControl 2023, 68, 299–310. [Google Scholar] [CrossRef]
- Mason, P.; Hill, M.; Smith, D.; Silvestri, L.; Weyl, P.; Brodeur, J.; Vitorino, M. Best practices in the use and exchange of microorganism biological control genetic resources. BioControl 2023, 68, 311–327. [Google Scholar] [CrossRef]
- Hirai, T.; Yasuda, S.; Umezawa, A.; Sato, Y. Country-specific regulation and international standardization of cell-based therapeutic products derived from pluripotent stem cells. Stem Cell Rep. 2023, 18, 1573–1591. [Google Scholar] [CrossRef]
- Heijer, J.; Heuberger, J.; Hijma, H.; Kruithof, A.; Van Smeden, J.; Groeneveld, G.; Burggraaf, J.; Cohen, A. Good Clinical Trials by removing defensive interpretation of Good Clinical Practice guidelines. Br. J. Clin. Pharmacol. 2021, 87, 4552–4559. [Google Scholar] [CrossRef]
- Sedgwick, H.; Gibson, G.; Adams, J.; Wijeyesekera, A. Seaweed-derived bioactives: Gut microbiota targeted interventions for immune function. J. Funct. Foods 2025, 125, 106696. [Google Scholar] [CrossRef]
- Mishra, N.; Garg, A.; Ashique, S.; Bhatt, S. Potential of postbiotics for the treatment of metabolic disorders. Drug Discov. Today 2024, 29, 103921. [Google Scholar] [CrossRef]
- Seidler, Y.; Rimbach, G.; Lüersen, K.; Vinderola, G.; Ipharraguerre, I. The postbiotic potential of Aspergillus oryzae—A narrative review. Front. Microbiol. 2024, 15, 1452725. [Google Scholar] [CrossRef] [PubMed]
- Clagnan, E.; Costanzo, M.; Visca, A.; Di Gregorio, L.; Tabacchioni, S.; Colantoni, E.; Sevi, F.; Sbarra, F.; Bindo, A.; Nolfi, L.; et al. Culturomics- and metagenomics-based insights into the soil microbiome preservation and application for sustainable agriculture. Front. Microbiol. 2024, 15, 1473666. [Google Scholar] [CrossRef] [PubMed]
- Glockow, T.; Kaster, A.; Rabe, K.; Niemeyer, C. Sustainable agriculture: Leveraging microorganisms for a circular economy. Appl. Microbiol. Biotechnol. 2024, 108, 452. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zeng, Z.; Contreras, A.; Cui, C. Editorial: Innovative microbial technologies for future and sustainable food science. Front. Microbiol. 2023, 14, 1215775. [Google Scholar] [CrossRef]
- Niamah, A.K.; Al-Sahlany, S.T.G.; Abdul-Sada, H.K.; Prabhakar, P.; Tripathy, S.; Dadrwal, B.K.; Singh, S.; Verma, D.K.; Gupta, A.K.; Shukla, R.M.; et al. Phytophagous Probiotic Foods: Exploring the Intersection of Characteristics, Quality Implications, Health Benefits, and Market Dynamics. Trends Food Sci. Technol. 2024, 154, 104795. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).