The Application of a High-Energy Fluidic Microfluidizer System Improves the Physicochemical and Antioxidant Properties of Whole Mulberry Juice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Whole Mulberry Juice by HEFM
2.3. Effect of HEFM on the Stability of WMBJ
2.3.1. Particle Size and Particle Size Distribution
2.3.2. Rheological Properties
2.3.3. Turbidity and Precipitate Weight Ratio
2.4. Effect of HEFM on the Physicochemical and Nutritional Indicators of WMBJ
2.4.1. pH, Soluble Solids, Total Acidity, and Color Attributes
2.4.2. Total Anthocyanin Content
2.4.3. Ascorbic Acid Content
2.4.4. Total Polyphenol Content
2.4.5. Polysaccharide Content
2.4.6. Soluble Pectin Content
2.4.7. Antioxidant Activities
2.4.8. Statistical Analysis
3. Results and Discussion
3.1. Effect of HEFM on Stability of WMBJ
3.1.1. Particle Size and PSD
3.1.2. Zeta Potential
3.1.3. Rheological Properties
3.1.4. Precipitate Weight Ratio and Turbidity
3.2. Effect of HEFM on Physicochemical and Nutritional Indicators of WMBJ
3.2.1. pH, Soluble Solids, Total Acidity, and Color Attributes
3.2.2. Total Anthocyanins
3.2.3. Ascorbic Acid Content
3.2.4. Total Polyphenols
3.2.5. Polysaccharides
3.2.6. Soluble Pectin
3.3. Antioxidant Activities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, F.; Chen, G.; Fu, X. Comparison of effect of gear juicer and colloid mill on microstructure, polyphenols profile, and bioactivities of mulberry (Morus indica L.). Food Bioprocess Technol. 2016, 9, 1233–1245. [Google Scholar] [CrossRef]
- Wen, P.; Hu, T.-G.; Linhardt, R.J.; Liao, S.-T.; Wu, H.; Zou, Y.-X. Mulberry: A review of bioactive compounds and advanced processing technology. Trends Food Sci. Technol. 2019, 83, 138–158. [Google Scholar] [CrossRef]
- Khalifa, I.; Zhu, W.; Li, K.-K.; Li, C.-M. Polyphenols of mulberry fruits as multifaceted compounds: Compositions, metabolism, health benefits, and stability—A structural review. J. Funct. Foods 2018, 40, 28–43. [Google Scholar] [CrossRef]
- Wang, K.; Kang, S.; Li, F.; Wang, X.; Xiao, Y.; Wang, J.; Xu, H. Relationship between fruit density and physicochemical properties and bioactive composition of mulberry at harvest. J. Food Compos. Anal. 2022, 106, 104322. [Google Scholar] [CrossRef]
- Yi, J.; Kebede, B.; Kristiani, K.; Grauwet, T.; Van Loey, A.; Hendrickx, M. Minimizing quality changes of cloudy apple juice: The use of kiwifruit puree and high pressure homogenization. Food Chem. 2018, 249, 202–212. [Google Scholar] [CrossRef]
- Leite, T.S.; Augusto, P.E.; Cristianini, M. Frozen concentrated orange juice (FCOJ) processed by the high pressure homogenization (HPH) technology: Effect on the ready-to-drink juice. Food Bioprocess Technol. 2016, 9, 1070–1078. [Google Scholar] [CrossRef]
- Zhang, W.; Xie, F.; Lan, X.; Gong, S.; Wang, Z. Characteristics of pectin from black cherry tomato waste modified by dynamic high-pressure microfluidization. J. Food Eng. 2018, 216, 90–97. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, Y.; Meng, Y.; Liu, S.; Ding, Y.; Zhou, X.; Ding, Y. Synergistic effect of microfluidization and transglutaminase cross-linking on the structural and oil–water interface functional properties of whey protein concentrate for improving the thermal stability of nanoemulsions. Food Chem. 2023, 408, 135147. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, M.; Gao, Z.; Cheng, Y.; Yang, X.; Mu, S.; Qu, K. Effect of dynamic high-pressure microfluidization on the quality of not-from-concentrate cucumber juice. Foods 2024, 13, 2125. [Google Scholar] [CrossRef]
- Jin, X.; Huang, L.; Wang, H. Effects of dynamic high pressure microfluidization on the physical and chemical properties of carrot (Daucus carota L.). Bangladesh J. Bot. 2024, 53, 123–129. [Google Scholar] [CrossRef]
- Li, Y.-t.; Chen, M.-s.; Deng, L.-z.; Liang, Y.-z.; Liu, Y.-k.; Liu, W.; Chen, J.; Liu, C.-m. Whole soybean milk produced by a novel industry-scale micofluidizer system without soaking and filtering. J. Food Eng. 2021, 291, 110228. [Google Scholar] [CrossRef]
- He, X.; Dai, T.; Liang, R.; Liu, W.; Cheng, Y.; Liu, C.; Chen, J. A new partially-gelatinized granular starch prepared by industry-scale microfluidization treatment of pea starch. Innov. Food Sci. Emerg. Technol. 2023, 86, 103351. [Google Scholar] [CrossRef]
- Dai, T.; Shuai, X.; Chen, J.; Li, C.; Wang, J.; Liu, W.; Liu, C.; Wang, R. Whole peanut milk prepared by an industry-scale microfluidization system: Physical stability, microstructure, and flavor properties. LWT 2022, 171, 114140. [Google Scholar] [CrossRef]
- Zhu, D.; Kou, C.; Wei, L.; Xi, P.; Changxin, L.; Cao, X.; Liu, H. Effects of high pressure homogenization on the stability of cloudy apple juice. IOP Conf. Ser. Earth Environ. Sci. 2019, 358, 022059. [Google Scholar] [CrossRef]
- Ke, Y.; Dai, T.; Xiao, M.; Chen, M.; Liang, R.; Liu, W.; Liu, C.; Chen, J.; Deng, L. Industry-scale microfluidizer system produced whole mango juice: Effect on the physical properties, microstructure and pectin properties. Innov. Food Sci. Emerg. Technol. 2022, 75, 102887. [Google Scholar] [CrossRef]
- Weber, F.; Larsen, L.R. Influence of fruit juice processing on anthocyanin stability. Food Res. Int. 2017, 100, 354–365. [Google Scholar] [CrossRef]
- Ke, Y.; Chen, J.; Dai, T.; Liang, R.; Liu, W.; Liu, C.; Deng, L. Developing industry-scale microfluidization for cell disruption, biomolecules release and bioaccessibility improvement of Chlorella pyrenoidosa. Bioresour. Technol. 2023, 387, 129649. [Google Scholar] [CrossRef]
- Karacam, C.H.; Sahin, S.; Oztop, M.H. Effect of high pressure homogenization (microfluidization) on the quality of Ottoman Strawberry (F. ananassa) juice. LWT-Food Sci. Technol. 2015, 64, 932–937. [Google Scholar] [CrossRef]
- Zhang, A.; Shen, Y.; Cen, M.; Hong, X.; Shao, Q.; Chen, Y.; Zheng, B. Polysaccharide and crocin contents, and antioxidant activity of saffron from different origins. Ind. Crops Prod. 2019, 133, 111–117. [Google Scholar] [CrossRef]
- Wellala, C.K.D.; Bi, J.; Liu, X.; Liu, J.; Lyu, J.; Wu, X. Juice related water-soluble pectin characteristics and bioaccessibility of bioactive compounds in oil and emulsion incorporated mixed juice processed by high pressure homogenization. Food Hydrocoll. 2019, 93, 56–67. [Google Scholar] [CrossRef]
- Yu, Z.-Y.; Jiang, S.-W.; Cao, X.-M.; Jiang, S.-T.; Pan, L.-J. Effect of high pressure homogenization (HPH) on the physical properties of taro (Colocasia esculenta (L). Schott) pulp. J. Food Eng. 2016, 177, 1–8. [Google Scholar] [CrossRef]
- Dai, T.; McClements, D.J.; Niu, X.; Guo, X.; Sun, J.; He, X.; Liu, C.; Chen, J. Whole tomato juice produced by a novel industrial-scale microfluidizer: Effect on physical properties and in vitro lycopene bioaccessibility. Food Res. Int. 2022, 159, 111608. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liao, M.; Rao, L.; Zhao, L.; Wang, Y.; Liao, X. Effect of ultra-high pressure homogenization on microorganism and quality of composite pear juice. Food Sci. Nutr. 2022, 10, 3072–3084. [Google Scholar] [CrossRef]
- Leite, T.S.; Augusto, P.E.; Cristianini, M. Structural and rheological properties of frozen concentrated orange juice (FCOJ) by Multi-Pass High-Pressure Homogenisation (MP-HPH). Int. J. Food Prop. 2017, 20, 2107–2117. [Google Scholar] [CrossRef]
- Li, J.; Wu, M.; Wang, Y.; Li, K.; Du, J.; Bai, Y. Effect of pH-shifting treatment on structural and heat induced gel properties of peanut protein isolate. Food Chem. 2020, 325, 126921. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, S.; Wang, W.; Ge, Z.; Zhang, L.; Li, C.; Zhang, B.; Zong, W. Comparison of the effects of dynamic high-pressure microfluidization and conventional homogenization on the quality of peach juice. J. Sci. Food Agric. 2019, 99, 5994–6000. [Google Scholar] [CrossRef]
- Li, M.; Zhang, W.; Guo, C.; Hu, X.; Yi, J. Role of pectin characteristics in orange juice stabilization: Effect of high-pressure processing in combination with centrifugation pretreatments. Int. J. Biol. Macromol. 2022, 215, 615–624. [Google Scholar] [CrossRef]
- Zhou, L.; Guan, Y.; Bi, J.; Liu, X.; Yi, J.; Chen, Q.; Wu, X.; Zhou, M. Change of the rheological properties of mango juice by high pressure homogenization. LWT-Food Sci. Technol. 2017, 82, 121–130. [Google Scholar] [CrossRef]
- Yu, Z.-Y.; Jiang, S.-W.; Cai, J.; Cao, X.-M.; Zheng, Z.; Jiang, S.-T.; Wang, H.-L.; Pan, L.-J. Effect of high pressure homogenization (HPH) on the rheological properties of taro (Colocasia esculenta (L). Schott) pulp. Innov. Food Sci. Emerg. Technol. 2018, 50, 160–168. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, C.; Lozano-Sánchez, J.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Alternatives to conventional thermal treatments in fruit-juice processing. Part 2: Effect on composition, phytochemical content, and physicochemical, rheological, and organoleptic properties of fruit juices. Crit. Rev. Food Sci. Nutr. 2017, 57, 637–652. [Google Scholar] [CrossRef]
- Colle, I.; Van Buggenhout, S.; Van Loey, A.; Hendrickx, M. High pressure homogenization followed by thermal processing of tomato pulp: Influence on microstructure and lycopene in vitro bioaccessibility. Food Res. Int. 2010, 43, 2193–2200. [Google Scholar] [CrossRef]
- Bot, F.; Calligaris, S.; Cortella, G.; Nocera, F.; Peressini, D.; Anese, M. Effect of high pressure homogenization and high power ultrasound on some physical properties of tomato juices with different concentration levels. J. Food Eng. 2017, 213, 10–17. [Google Scholar] [CrossRef]
- Yu, W.; Cui, J.; Zhao, S.; Feng, L.; Wang, Y.; Liu, J.; Zheng, J. Effects of high-pressure homogenization on pectin structure and cloud stability of not-from-concentrate orange juice. Front. Nutr. 2021, 8, 647748. [Google Scholar] [CrossRef]
- Marszałek, K.; Trych, U.; Bojarczuk, A.; Szczepańska, J.; Chen, Z.; Liu, X.; Bi, J. Application of high-pressure homogenization for apple juice: An assessment of quality attributes and polyphenol bioaccessibility. Antioxidants 2023, 12, 451. [Google Scholar] [CrossRef]
- Szczepańska, J.; Skąpska, S.; Połaska, M.; Marszałek, K. High pressure homogenization with a cooling circulating system: The effect on physiochemical and rheological properties, enzymes, and carotenoid profile of carrot juice. Food Chem. 2022, 370, 131023. [Google Scholar] [CrossRef] [PubMed]
- Jing, S.; Li, Q.; Zheng, L.; Yue, L.; Fan, S.; Tao, G. Dynamic high pressure microfluidization-assisted extraction and bioactivities of Cyperus esculentus (C. esculentus L.) leaves flavonoids. Food Chem. 2016, 192, 319–327. [Google Scholar] [CrossRef]
- Guo, X.; McClements, D.J.; Chen, J.; He, X.; Liu, W.; Dai, T.; Liu, C. The nutritional and physicochemical properties of whole corn slurry prepared by a novel industry-scale microfluidizer system. LWT 2021, 144, 111096. [Google Scholar] [CrossRef]
- Kruszewski, B.; Zawada, K.; Karpiński, P. Impact of high-pressure homogenization parameters on physicochemical characteristics, bioactive compounds content, and antioxidant capacity of blackcurrant juice. Molecules 2021, 26, 1802. [Google Scholar] [CrossRef]
- Santhirasegaram, V.; Razali, Z.; George, D.S.; Somasundram, C. Effects of thermal and non-thermal processing on phenolic compounds, antioxidant activity and sensory attributes of chokanan mango (Mangifera indica L.) juice. Food Bioprocess Technol. 2015, 8, 2256–2267. [Google Scholar] [CrossRef]
- Corrales, M.; Butz, P.; Tauscher, B. Anthocyanin condensation reactions under high hydrostatic pressure. Food Chem. 2008, 110, 627–635. [Google Scholar] [CrossRef]
- Adhikari, J.; Araghi, L.R.; Singh, R.; Adhikari, K.; Patil, B.S. Continuous-Flow High-Pressure Homogenization of Blueberry Juice Enhances Anthocyanin and Ascorbic Acid Stability during Cold Storage. J. Agric. Food Chem. 2024, 72, 11629–11639. [Google Scholar] [CrossRef]
- Benjamin, O.; Gamrasni, D. Microbial, nutritional, and organoleptic quality of pomegranate juice following high-pressure homogenization and low-temperature pasteurization. J. Food Sci. 2020, 85, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Bi, J.; Liu, X.; Liu, D.; Verkerk, R.; Dekker, M.; Lyu, J.; Wu, X. Modelling and optimization of high-pressure homogenization of not-from-concentrate juice: Achieving better juice quality using sustainable production. Food Chem. 2022, 370, 131058. [Google Scholar] [CrossRef] [PubMed]
- Szczepańska, J.; Skąpska, S.; Marszałek, K. Continuous high-pressure cooling-assisted homogenization process for stabilization of apple juice. Food Bioprocess Technol. 2021, 14, 1101–1117. [Google Scholar] [CrossRef]
- Sauceda-Gálvez, J.N.; Codina-Torrella, I.; Martinez-Garcia, M.; Hernández-Herrero, M.M.; Gervilla, R.; Roig-Sagués, A. Combined effects of ultra-high pressure homogenization and short-wave ultraviolet radiation on the properties of cloudy apple juice. LWT 2021, 136, 110286. [Google Scholar] [CrossRef]
- Wellala, C.K.D.; Bi, J.; Liu, X.; Liu, J.; Lyu, J.; Zhou, M.; Marszałek, K.; Trych, U. Effect of high pressure homogenization combined with juice ratio on water-soluble pectin characteristics, functional properties and bioactive compounds in mixed juices. Innov. Food Sci. Emerg. Technol. 2020, 60, 102279. [Google Scholar] [CrossRef]
- Mert, I.D. The applications of microfluidization in cereals and cereal-based products: An overview. Crit. Rev. Food Sci. Nutr. 2020, 60, 1007–1024. [Google Scholar] [CrossRef]
- Huang, Y.; Xie, W.; Tang, T.; Chen, H.; Zhou, X. Structural characteristics, antioxidant and hypoglycemic activities of polysaccharides from Mori fructus based on different extraction methods. Front. Nutr. 2023, 10, 1125831. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, S.; Zhou, T.; Waterhouse, G.I.; Du, Y.; Sun-Waterhouse, D.; Wu, P. Green approaches for dietary fibre-rich polysaccharide production from the cooking liquid of Adzuki beans: Enzymatic extraction combined with ultrasonic or high-pressure homogenisation. Food Hydrocoll. 2022, 130, 107679. [Google Scholar] [CrossRef]
- de Oliveira, C.F.; Giordani, D.; Lutckemier, R.; Gurak, P.D.; Cladera-Olivera, F.; Marczak, L.D.F. Extraction of pectin from passion fruit peel assisted by ultrasound. LWT-Food Sci. Technol. 2016, 71, 110–115. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Bi, J.; Yi, J.; Peng, J.; Ning, C.; Wellala, C.K.D.; Zhang, B. Effects of high pressure homogenization on pectin structural characteristics and carotenoid bioaccessibility of carrot juice. Carbohydr. Polym. 2019, 203, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Moelants, K.R.; Jolie, R.P.; Palmers, S.K.; Cardinaels, R.; Christiaens, S.; Van Buggenhout, S.; Van Loey, A.M.; Moldenaers, P.; Hendrickx, M.E. The effects of process-induced pectin changes on the viscosity of carrot and tomato sera. Food Bioprocess Technol. 2013, 6, 2870–2883. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Bi, J.; Cao, F.; Ding, Y.; Peng, J. Effects of high pressure homogenization on physical stability and carotenoid degradation kinetics of carrot beverage during storage. J. Food Eng. 2019, 263, 63–69. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant activity of l-adrenaline: A structure–activity insight. Chem.-Biol. Interact. 2009, 179, 71–80. [Google Scholar] [CrossRef]
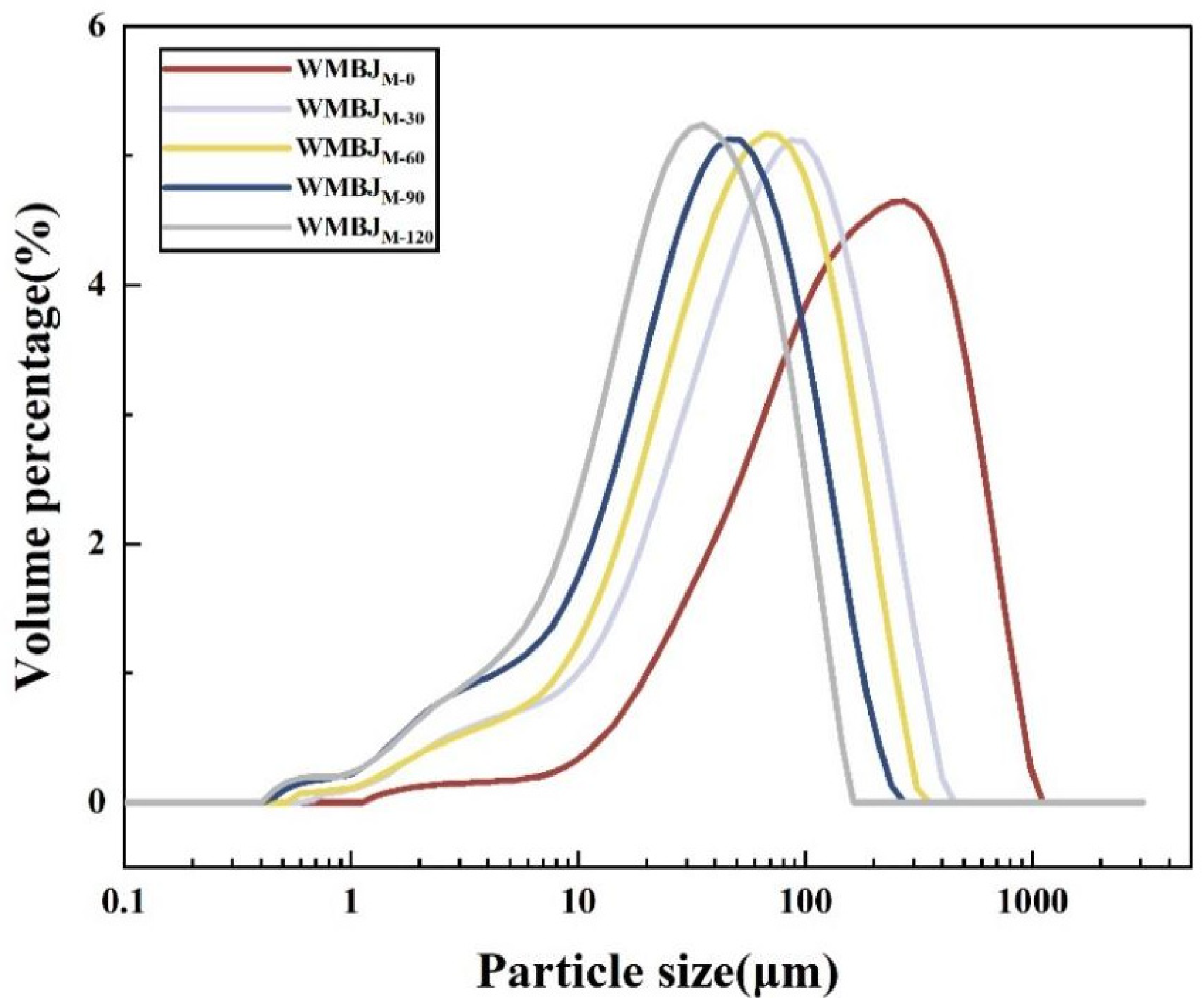
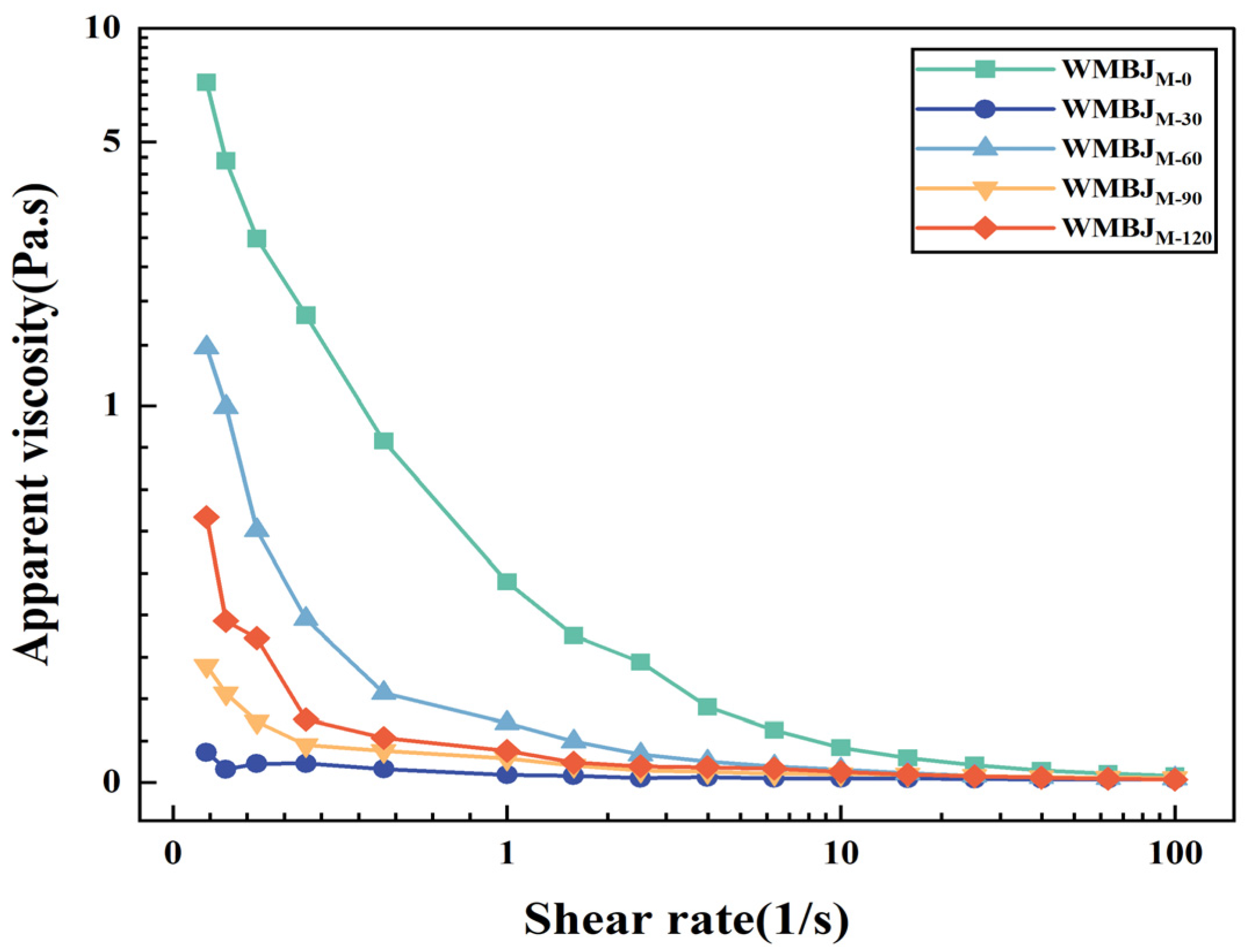


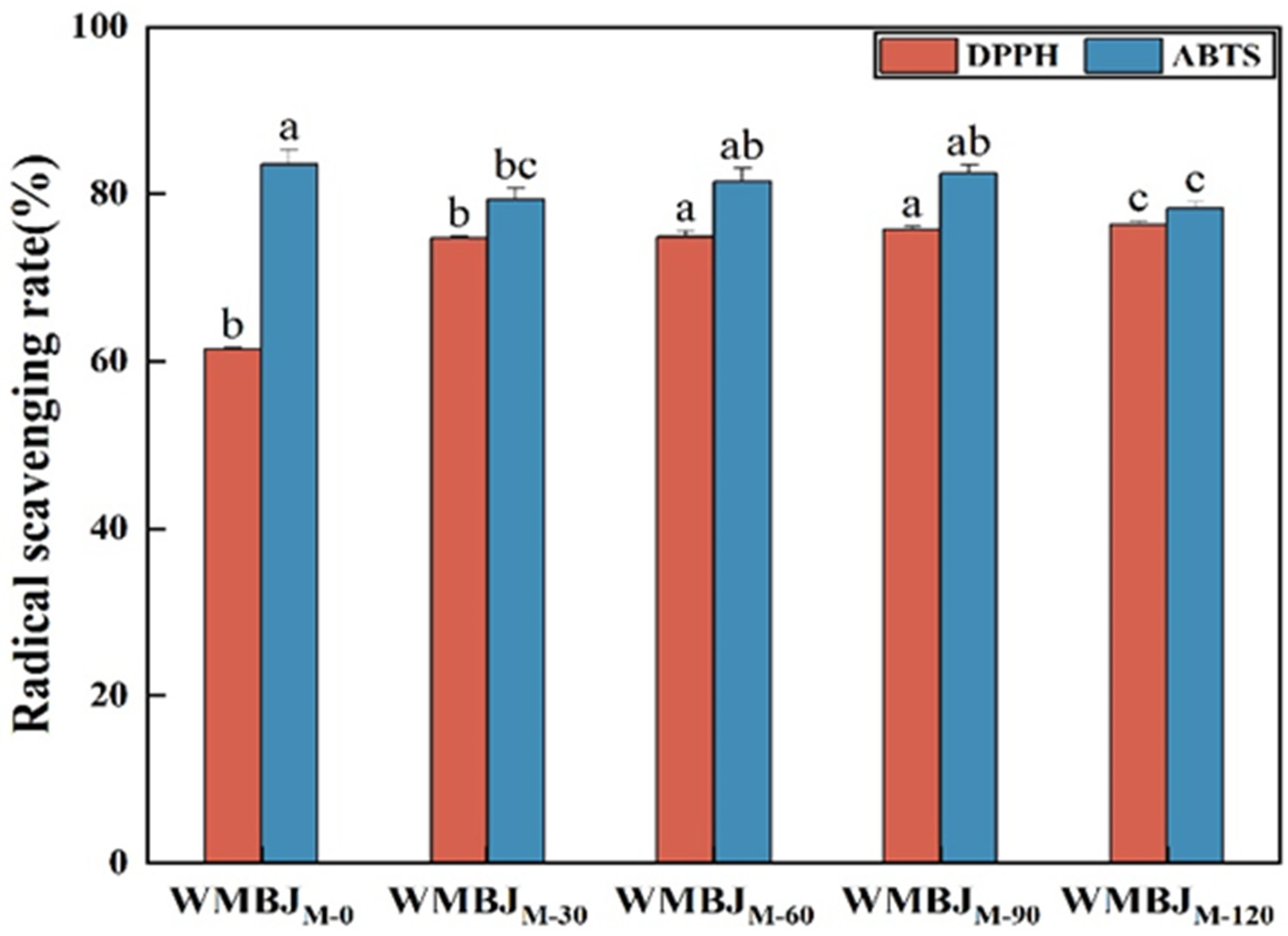
| Samples | D[3,2] (μm) | D[4,3] (μm) | D(10) (μm) | D(50) (μm) | D(90) (μm) |
|---|---|---|---|---|---|
| WMBJM-0 | 57.33 ± 3.21 a | 232.46 ± 4.09 a | 31.75 ± 3.21 a | 168.09 ± 1.17 a | 535.19 ± 12.42 a |
| WMBJM-30 | 22.24 ± 0.65 b | 91.31 ± 1.06 b | 10.95 ± 3.21 b | 68.83 ± 0.96 b | 205.79 ± 1.95 b |
| WMBJM-60 | 19.64 ± 0.63 b | 71.63 ± 2.45 c | 10.25 ± 3.21 b | 54.54 ± 1.76 c | 159.22 ± 5.54 c |
| WMBJM-90 | 12.68 ± 0.33 c | 50.48 ± 1.02 d | 5.62 ± 3.21 c | 37.98 ± 0.78 d | 113.38 ± 2.35 d |
| WMBJM-120 | 11.27 ± 0.05 c | 38.27 ± 0.16 e | 5.33 ± 3.21 c | 29.48 ± 0.08 e | 84.91 ± 0.37 e |
| Samples | Zeta Potential |
|---|---|
| WMBJM-0 | −7.27 ± 0.227 a |
| WMBJM-30 | −6.25 ± 0.089 cd |
| WMBJM-60 | −8.84 ± 0.070 bc |
| WMBJM-90 | −5.87 ± 0.596 d |
| WMBJM-120 | −7.34 ± 0.56 a |
| Samples | τ (Pa) | K | n | R2 |
|---|---|---|---|---|
| WMBJM-0 | 0.8436 ± 0.117 a | 0.1194 ± 0.088 a | 0.6043 ± 0.126 ab | 0.9966 |
| WMBJM-30 | 0.0358 ± 0.019 cd | 0.0788 ± 0.089 a | 0.8197 ± 0.101 a | 0.9962 |
| WMBJM-60 | 0.1708 ± 0.063 bc | 0.0229 ± 0.006 a | 0.7509 ± 0.737 ab | 0.9911 |
| WMBJM-90 | 0.0221 ± 0.002 d | 0.0246 ± 0.009 a | 0.7686 ± 0.085 ab | 0.9984 |
| WMBJM-120 | 0.2505 ± 0.027 a | 0.0805 ± 0.017 a | 0.5697 ± 0.074 b | 0.9654 |
| Samples | WMBJM-0 | WMBJM-30 | WMBJM-60 | WMBJM-90 | WMBJM-120 |
|---|---|---|---|---|---|
| pH | 3.89 ± 0.01 a | 3.86 ± 0.01 a | 3.84 ± 0.01 a | 3.83 ± 0.01 a | 3.84 ± 0.01 a |
| TSS (Brix°) | 5.10 ± 0.00 c | 5.10 ± 0.00 c | 5.13 ± 0.05 b | 5.20 ± 0.00 b | 6.03 ± 0.05 a |
| TA (%) | 0.46 ± 0.02 c | 0.47 ± 0.01 c | 0.52 ± 0.01 b | 0.54 ± 0.01 ab | 0.56 ± 0.01 a |
| L* | 24.51 ± 0.20 d | 25.21 ± 0.02 d | 25.61 ± 0.01 b | 25.82 ± 0.06 bc | 25.92 ± 0.02 a |
| a* | 0.31 ± 0.07 a | 0.32 ± 0.02 a | 0.33 ± 0.07 a | 0.34 ± 0.04 a | 0.37 ± 0.15 b |
| b* | −3.56 ± 0.26 d | −3.19 ± 0.06 c | −2.71 ± 0.01 b | −2.51 ± 0.13 b | −1.83 ± 0.08 a |
| ΔE | - | 1.22 ± 0.04 d | 1.82 ± 0.02 c | 2.11 ± 0.13 b | 2.78 ± 0.02 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Li, X.; Tang, Y.; Meng, X.; Wei, Z.; Li, B.; Dai, T.; Shuai, X.; Wang, Z.; Zhang, X. The Application of a High-Energy Fluidic Microfluidizer System Improves the Physicochemical and Antioxidant Properties of Whole Mulberry Juice. Foods 2025, 14, 2311. https://doi.org/10.3390/foods14132311
He X, Li X, Tang Y, Meng X, Wei Z, Li B, Dai T, Shuai X, Wang Z, Zhang X. The Application of a High-Energy Fluidic Microfluidizer System Improves the Physicochemical and Antioxidant Properties of Whole Mulberry Juice. Foods. 2025; 14(13):2311. https://doi.org/10.3390/foods14132311
Chicago/Turabian StyleHe, Xuemei, Xinyi Li, Yayuan Tang, Xiaolin Meng, Zhen Wei, Baoshen Li, Taotao Dai, Xixiang Shuai, Zhenxing Wang, and Xuechun Zhang. 2025. "The Application of a High-Energy Fluidic Microfluidizer System Improves the Physicochemical and Antioxidant Properties of Whole Mulberry Juice" Foods 14, no. 13: 2311. https://doi.org/10.3390/foods14132311
APA StyleHe, X., Li, X., Tang, Y., Meng, X., Wei, Z., Li, B., Dai, T., Shuai, X., Wang, Z., & Zhang, X. (2025). The Application of a High-Energy Fluidic Microfluidizer System Improves the Physicochemical and Antioxidant Properties of Whole Mulberry Juice. Foods, 14(13), 2311. https://doi.org/10.3390/foods14132311








