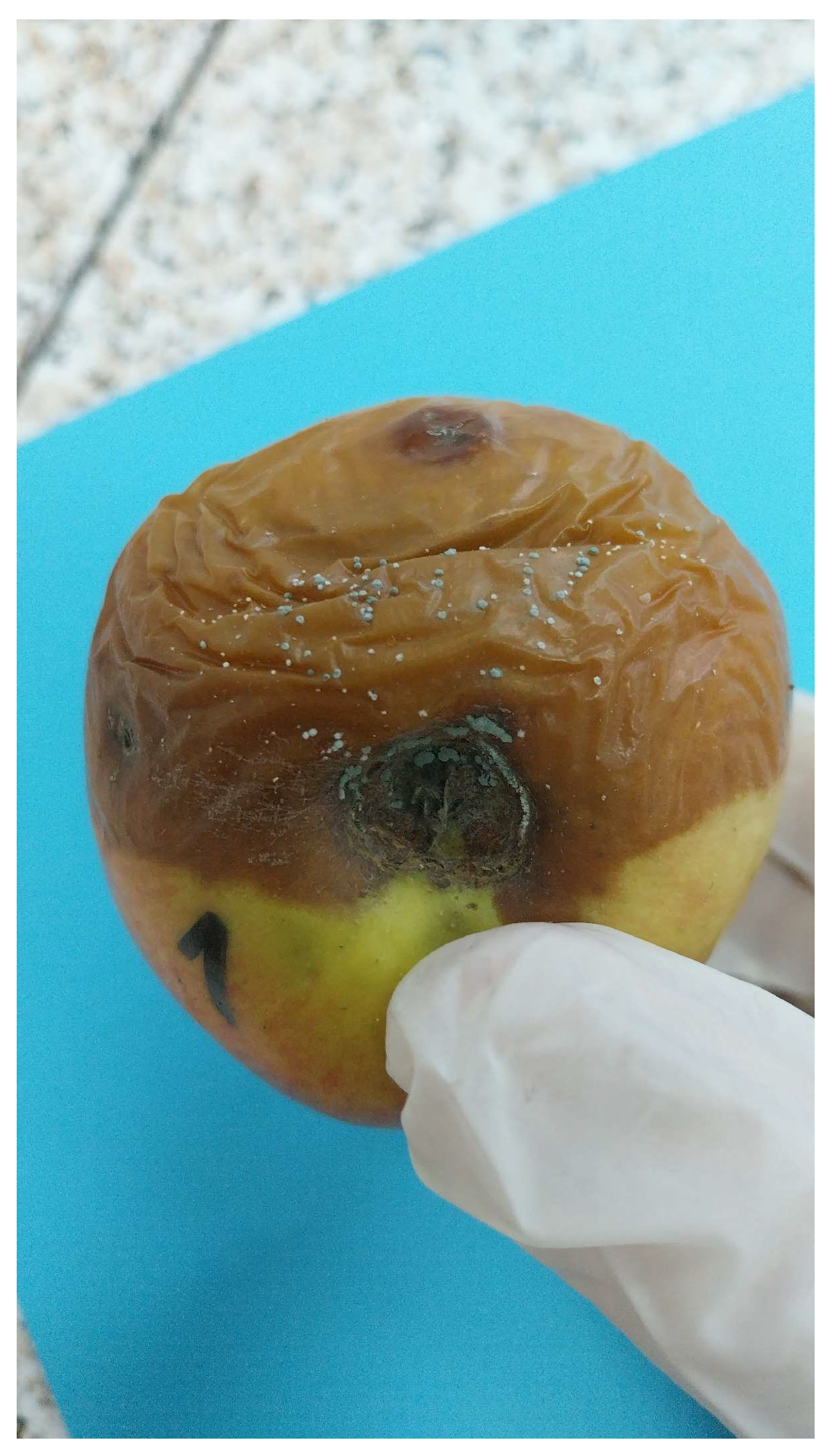
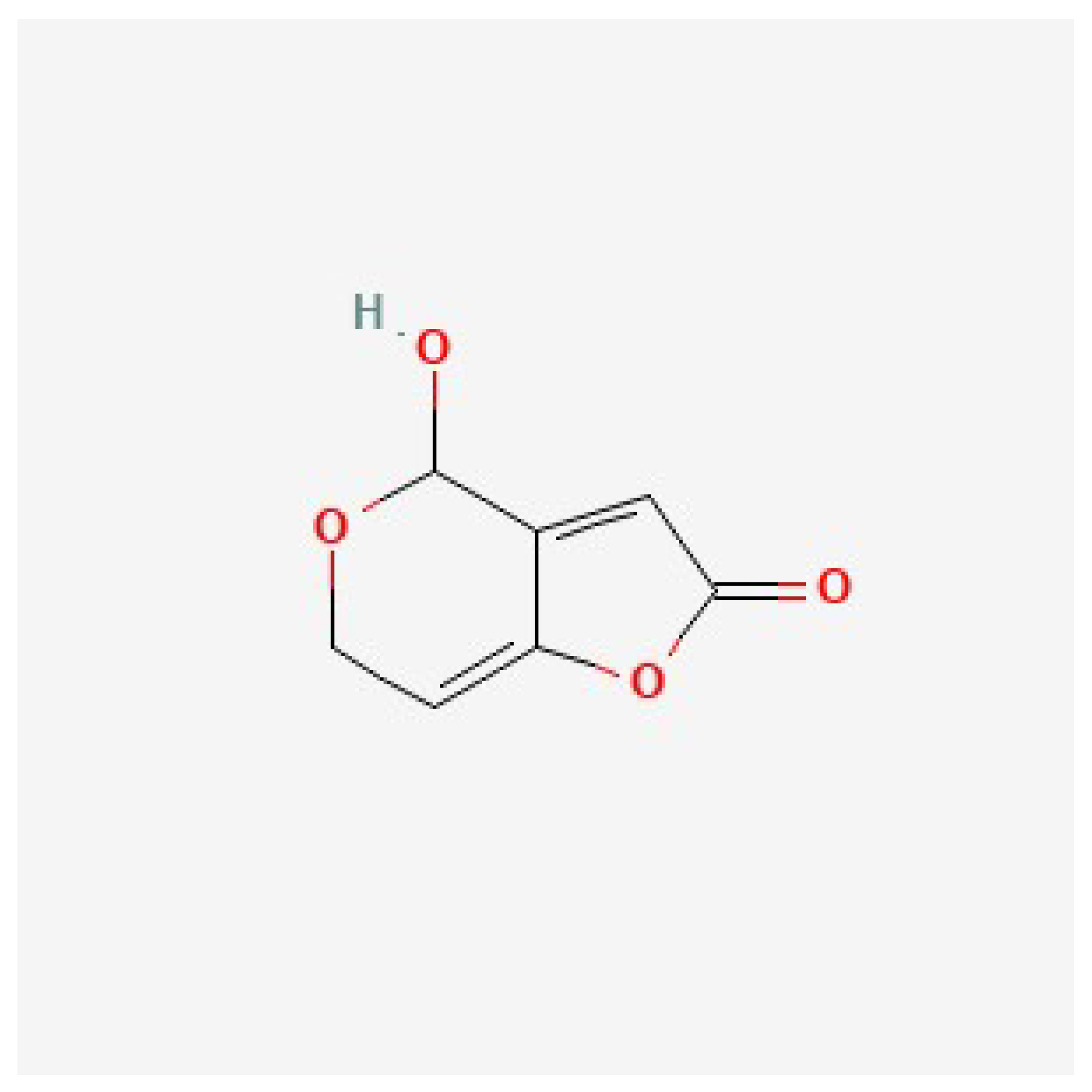
Factors Affecting Patulin Production by Penicillium expansum in Apples
Abstract
1. Introduction
2. External and Inherent Components That Drive Patulin Biosynthesis
2.1. Extrinsic Components That Influence Patulin Production
2.1.1. Storage Temperature
2.1.2. Atmospheric Regimes and Packaging Materials Used During Controlled Atmosphere (CA) Storage
2.1.3. Biocontrol Agents (BCAs)
2.1.4. Pathogen Load on the Fruits
2.2. Intrinsic Components That Influence PAT Production
2.2.1. Susceptibility of Different Cultivars
2.2.2. Physicochemical Properties of Apples
2.2.3. Chemical Composition of Apples
2.2.4. Genetics
2.2.5. Size of the Decay Area
2.2.6. Toxin-Producing Capacity of the P. expansum Strain
2.2.7. Ripening Degree
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PAT | Patulin |
| CA | Controlled atmosphere |
| PP | Polypropylene |
| PE | Polyethylene |
| HPLC | High-performance liquid chromatography |
| BCA | Biocontrol agent |
| aw | Water activity |
| ROS | Reactive oxygen species |
References
- Singh, D.; Sharma, R.R. Postharvest Diseases of Fruits and Vegetables and Their Management. In Postharvest Disinfection of Fruits and Vegetables; Siddiqui, M.W., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 1–52. [Google Scholar]
- Sharma, R.R.; Singh, D.; Singh, R. Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: A review. Biocontrol 2009, 50, 205–221. [Google Scholar] [CrossRef]
- Youssef, K.; Ippolito, A.; Roberto, S.R. Editorial: Post-harvest Diseases of Fruit and Vegetable: Methods and Mechanisms of Action. Front. Microbiol. 2022, 13, 900060. [Google Scholar] [CrossRef]
- Wenneker, M.; Thomma, B.P.H.J. Latent postharvest pathogens of pome fruit and their management: From single measures to a systems intervention approach. Eur. J. Plant Pathol. 2020, 156, 663–681. [Google Scholar] [CrossRef]
- Errampalli, D. Chapter 6—Penicillium expansum (Blue Mold). In Postharvest Decay; Bautista-Baños, S., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 189–231. [Google Scholar]
- Yu, L.; Qiao, N.; Zhao, J.; Zhang, H.; Tian, F.; Zhai, Q.; Chen, W. Postharvest control of Penicillium expansum in fruits: A review. Food Biosci. 2020, 36, 100633. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, N.; Wang, Y.; Jiang, D.; Feng, X. Characterization of Phenolic Compounds from Early and Late Ripening Sweet Cherries and Their Antioxidant and Antifungal Activities. J. Agric. Food Chem. 2017, 65, 5413–5420. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, A.K.; Singh, V.K.; Das, S.; Dubey, N.K. Nanoencapsulation of essential oils and their bioactive constituents: A novel strategy to control mycotoxin contamination in food system. Food Chem. Toxicol. 2021, 149, 112019. [Google Scholar] [CrossRef]
- Abdelfattah, A.; Freilich, S.; Bartuv, R.; Zhimo, V.Y.; Kumar, A.; Biasi, A.; Salim, S.; Feygenberg, O.; Burchard, E.; Dardick, C.; et al. Global analysis of the apple fruit microbiome: Are all apples the same? Environ. Microbiol. 2021, 23, 6038–6055. [Google Scholar] [CrossRef]
- Milošević, T.; Milošević, N.; Mladenović, J. The influence of organic, organo-mineral and mineral fertilizers on tree growth, yielding, fruit quality and leaf nutrient composition of apple cv. ‘Golden Delicious Reinders’. Sci. Hortic. 2022, 297, 110978. [Google Scholar] [CrossRef]
- Oyenihi, A.B.; Belay, Z.A.; Mditshwa, A.; Caleb, O.J. “An apple a day keeps the doctor away”: The potentials of apple bioactive constituents for chronic disease prevention. J. Food Sci. 2022, 87, 2291–2309. [Google Scholar] [CrossRef]
- Juhņeviča-Radenkova, K.; Radenkovs, V. Assessment of Shelf-Life Ability of Apples cv. ‘Auksis’ after Long-term Storage Under Different Conditions. J. Hortic. Res. 2016, 24, 37–47. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Sun, M.; Wang, L.; Zou, Y.; Fu, L.; Han, C.; Li, A.; Li, L.; Zhu, C. Impact of vanillin on postharvest disease control of apple. Front. Microbiol. 2022, 13, 979737. [Google Scholar] [CrossRef] [PubMed]
- Kawhena, T.G.; Fawole, O.A.; Opara, U.L. Application of Dynamic Controlled Atmosphere Technologies to Reduce Incidence of Physiological Disorders and Maintain Quality of ‘Granny Smith’ Apples. Agriculture 2021, 11, 491. [Google Scholar] [CrossRef]
- Mditshwa, A.; Fawole, O.A.; Opara, U.L. Recent developments on dynamic controlled atmosphere storage of apples—A review. Food Packag. Shelf Life 2018, 16, 59–68. [Google Scholar] [CrossRef]
- Cameldi, I.; Neri, F.; Ventrucci, D.; Ceredi, G.; Muzzi, E.; Mari, M. Influence of harvest date on bull’s eye rot of ‘Cripps Pink’ apple and control chemical strategies. Plant Dis. 2016, 100, 2287–2293. [Google Scholar] [CrossRef]
- Vilanova, L.; Vall-llaura, N.; Torres, R.; Usall, J.; Teixidó, N.; Larrigaudière, C.; Giné-Bordonaba, J. Penicillium expansum (compatible) and Penicillium digitatum (non-host) pathogen infection differentially alter ethylene biosynthesis in apple fruit. Plant Physiol. Biochem. 2017, 120, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Leng, J.; Yu, L.; Dai, Y.; Leng, Y.; Wang, C.; Chen, Z.; Wisniewski, M.; Wu, X.; Liu, J.; Sui, Y. Recent advances in research on biocontrol of postharvest fungal decay in apples. Crit. Rev. Food Sci. Nutr. 2023, 63, 10607–10620. [Google Scholar] [CrossRef]
- Patriarca, A. Fungi and mycotoxin problems in the apple industry. Curr. Opin. Food Sci. 2019, 29, 42–47. [Google Scholar] [CrossRef]
- Soliman, S.; Li, X.Z.; Shao, S.; Behar, M.; Svircev, A.M.; Tsao, R.; Zhou, T. Potential mycotoxin contamination risks of apple products associated with fungal flora of apple core. Food Control 2015, 47, 585–591. [Google Scholar] [CrossRef]
- Sant’Ana, A.S.; Simas, R.C.; Almeida, C.A.A.; Cabral, E.C.; Rauber, R.H.; Mallmann, C.A.; Eberlin, M.N.; Rosenthal, A.; Massaguer, P.R. Influence of package, type of apple juice and temperature on the production of patulin by Byssochlamys nivea and Byssochlamys fulva. Int. J. Food Microbiol. 2010, 142, 156–163. [Google Scholar] [CrossRef]
- Errampalli, D. Effect of fludioxonil on germination and growth of Penicillium expansum and decay in apple cvs. Empire and Gala. Crop Prot. 2004, 23, 811–817. [Google Scholar] [CrossRef]
- Sant’Ana, A.; Rosenthal, A.; Massaguer, P. The fate of patulin in apple juice processing: A review. Food Res. Int. 2008, 41, 441–453. [Google Scholar] [CrossRef]
- Wang, K.; Ngea, G.L.N.; Godana, E.A.; Shi, Y.; Lanhuang, B.; Zhang, X.; Zhao, L.; Yang, Q.; Wang, S.; Zhang, H. Recent advances in Penicillium expansum infection mechanisms and current methods in controlling P. expansum in postharvest apples. Crit. Rev. Food Sci. Nutr. 2023, 63, 2598–2611. [Google Scholar] [CrossRef] [PubMed]
- Mahunu, G.K.; Zhang, H.; Yang, Q.; Li, C.; Zheng, X. Biological Control of Patulin by Antagonistic Yeast: A case study and possible model. Crit. Rev. Microbiol. 2016, 42, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Vico, I.; Duduk, N.; Vasic, M.; Lucev, M. Identification of Penicillium expansum causing postharvest blue mold decay of apple fruit. Pestic. Fitomed. 2014, 29, 257–266. [Google Scholar] [CrossRef]
- Ahmadi-Afzadi, M.; Nybom, H.; Ekholm, A.; Tahir, I.; Rumpunen, K. Biochemical contents of apple peel and flesh affect level of partial resistance to blue mold. Postharvest Biol. Technol. 2015, 110, 173–182. [Google Scholar] [CrossRef]
- Tournas, V.H.; Memon, S. Internal contamination and spoilage of harvested apples by patulin-producing and other toxigenic fungi. Int. J. Food Microbiol. 2009, 133, 206–209. [Google Scholar] [CrossRef]
- Tannous, J.; Keller, N.P.; Atoui, A.; El Khoury, A.; Lteif, R.; Oswald, I.P.; Puel, O. Secondary metabolism in Penicillium expansum: Emphasis on recent advances in patulin research. Crit. Rev. Food Sci. Nutr. 2018, 58, 2082–2098. [Google Scholar] [CrossRef]
- Abramson, D.; Lombaert, G.; Clear, R.M.; Sholberg, P.; Trelka, R.; Rosin, E. Production of patulin and citrinin by Penicillium expansum from British Columbia (Canada) apples. Mycotoxin Res. 2009, 25, 85–88. [Google Scholar] [CrossRef]
- Zheng, X.; Yang, Q.; Zhang, X.; Apaliya, M.T.; Ianiri, G.; Zhang, H.; Castoria, R. Biocontrol Agents Increase the Specific Rate of Patulin Production by Penicillium expansum but Decrease the Disease and Total Patulin Contamination of Apples. Front. Microbiol. 2017, 8, 1240. [Google Scholar] [CrossRef]
- Saleh, I.; Goktepe, I. The characteristics, occurrence, and toxicological effects of patulin. Food Chem. Toxicol. 2019, 129, 301–311. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in Occurrence, Importance, and Mycotoxin Control Strategies: Prevention and Detoxification in Foods. Foods 2020, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.Z.; Malik, S.; Asi, M.R.; Selamat, J.; Malik, N. Natural occurrence of patulin in different fruits, juices and smoothies and evaluation of dietary intake in Punjab, Pakistan. Food Control 2018, 84, 370–374. [Google Scholar] [CrossRef]
- Zhong, L.; Carere, J.; Lu, Z.; Lu, F.; Zhou, T. Patulin in Apples and Apple-Based Food Products: The Burdens and the Mitigation Strategies. Toxins 2018, 10, 475. [Google Scholar] [CrossRef]
- Diao, E.; Ma, K.; Zhang, H.; Xie, P.; Qian, S.; Song, H.; Mao, R.; Zhang, L. Thermal Stability and Degradation Kinetics of Patulin in Highly Acidic Conditions: Impact of Cysteine. Toxins 2021, 13, 662. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Hong, S.-Y.; Kim, J.-Y.; Om, A.-S. Effects of temperature, pH, and relative humidity on the growth of Penicillium paneum OM1 isolated from pears and its patulin production. Fungal Biol. 2024, 128, 1885–1897. [Google Scholar] [CrossRef] [PubMed]
- Robert, H.; Payros, D.; Pinton, P.; Theodorou, V.; Mercier-Bonin, M.; Oswald, I. Impact of mycotoxins on the intestine: Are mucus and microbiota new targets? J. Toxicol. Environ. Health B Crit. Rev. 2017, 20, 249–275. [Google Scholar] [CrossRef]
- Pal, S.; Singh, N.; Ansari, K.M. Toxicological effects of patulin mycotoxin on the mammalian system: An overview. Toxicol. Res. 2017, 6, 764–771. [Google Scholar] [CrossRef]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20. [Google Scholar] [CrossRef]
- European Commission, E. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union. 2006, 364, 5–24. [Google Scholar]
- Raiola, A.; Tenore, G.C.; Manyes, L.; Meca, G.; Ritieni, A. Risk analysis of main mycotoxins occurring in food for children: An overview. Food Chem. Toxicol. 2015, 84, 169–180. [Google Scholar] [CrossRef]
- Chan-Hon-Tong, A.; Charles, M.-A.; Forhan, A.; Heude, B.; Sirot, V. Exposure to food contaminants during pregnancy. Sci. Total Environ. 2013, 458–460, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Judet-Correia, D.; Bollaert, S.; Duquenne, A.; Charpentier, C.; Bensoussan, M.; Dantigny, P. Validation of a predictive model for the growth of Botrytis cinerea and Penicillium expansum on grape berries. Int. J. Food Microbiol. 2010, 142, 106–113. [Google Scholar] [CrossRef]
- Prata, M.B.; Mussatto, S.I.; Rodrigues, L.R.; Teixeira, J.A. Fructooligosaccharide production by Penicillium expansum. Biotechnol. Lett. 2010, 32, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Salomão, B.C.M.; Aragão, G.M.F.; Churey, J.J.; Padilla-Zakour, O.I.; Worobo, R.W. Influence of Storage Temperature and Apple Variety on Patulin Production by Penicillium expansum. J. Food Prot. 2009, 72, 1030–1036. [Google Scholar] [CrossRef]
- Morales, H.; Marín, S.; Rovira, A.; Ramos, A.J.; Sanchis, V. Patulin accumulation in apples by Penicillium expansum during postharvest stages. Lett. Appl. Microbiol. 2007, 44, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Morales, H.; Marín, S.; Centelles, X.; Ramos, A.J.; Sanchis, V. Cold and ambient deck storage prior to processing as a critical control point for patulin accumulation. Int. J. Food Microbiol. 2007, 116, 260–265. [Google Scholar] [CrossRef]
- Baert, K.; Valero, A.; De Meulenaer, B.; Samapundo, S.; Ahmed, M.M.; Bo, L.; Debevere, J.; Devlieghere, F. Modeling the effect of temperature on the growth rate and lag phase of Penicillium expansum in apples. Int. J. Food Microbiol. 2007, 118, 139–150. [Google Scholar] [CrossRef]
- Reddy, K.R.N.; Spadaro, D.; Lore, A.; Gullino, M.L.; Garibaldi, A. Potential of patulin production by Penicillium expansum strains on various fruits. Mycotoxin Res. 2010, 26, 257–265. [Google Scholar] [CrossRef]
- Morales, H.; Sanchis, V.; Coromines, J.; Ramos, A.J.; Marín, S. Inoculum size and intraspecific interactions affects Penicillium expansum growth and patulin accumulation in apples. Food Microbiol. 2008, 25, 378–385. [Google Scholar] [CrossRef]
- Paster, N.; Huppert, D.; Barkai-Golan, R. Production of patulin by different strains of Penicillium expansum in pear and apple cultivars stored at different temperatures and modified atmospheres. Food Addit. Contam. 1995, 12, 51–58. [Google Scholar] [CrossRef]
- Baert, K.; Devlieghere, F.; Flyps, H.; Oosterlinck, M.; Ahmed, M.M.; Rajković, A.; Verlinden, B.; Nicolaï, B.; Debevere, J.; De Meulenaer, B. Influence of storage conditions of apples on growth and patulin production by Penicillium expansum. Int. J. Food Microbiol. 2007, 119, 170–181. [Google Scholar] [CrossRef]
- McCallum, J.L.; Tsao, R.; Zhou, T. Factors Affecting Patulin Production by Penicillium expansum†. J. Food Prot. 2002, 65, 1937–1942. [Google Scholar] [CrossRef]
- Welke, J.E.; Hoeltz, M.; Noll, I. Patulin accumulation in apples during storage by Penicillium expansum and Penicillium griseofulvum strains. Braz. J. Microbiol. 2011, 42, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.; Ramos, A.J.; Sanchis, V.; Marín, S. Intraspecific variability of growth and patulin production of 79 Penicillium expansum isolates at two temperatures. Int. J. Food Microbiol. 2011, 151, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Kingston, C.M. Maturity Indices for Apple and Pear. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1992; pp. 407–428. [Google Scholar]
- De Clercq, N.; Vlaemynck, G.; Pamel, E.; Colman, D.; Heyndrickx, M.; Van Hove, F.; Meulenaer, B.; Devlieghere, F.; Coillie, E. Patulin production by Penicillium expansum isolates from apples during different steps of long-term storage. World J. 2016, 9, 379–388. [Google Scholar] [CrossRef]
- dos Santos, I.D.; Pizzutti, I.R.; Dias, J.V.; Fontana, M.E.Z.; Brackmann, A.; Anese, R.O.; Thewes, F.R.; Marques, L.N.; Cardoso, C.D. Patulin accumulation in apples under dynamic controlled atmosphere storage. Food Chem. 2018, 255, 275–281. [Google Scholar] [CrossRef]
- Morales, H.; Barros, G.; Marín, S.; Chulze, S.; Ramos, A.J.; Sanchis, V. Effects of apple and pear varieties and pH on patulin accumulation by Penicillium expansum. J. Sci. Food Agric. 2008, 88, 2738–2743. [Google Scholar] [CrossRef]
- Moodley, R.; Govinden, R.; Odhav, B. The Effect of Modified Atmospheres and Packaging on Patulin Production in Apples. J. Food Prot. 2002, 65, 867–871. [Google Scholar] [CrossRef]
- Morales, H.; Marín, S.; Ramos, A. Influence of post-harvest technologies applied during cold storage of apples in Penicillium expansum growth and patulin accumulation: A review. Food Control 2010, 21, 953–962. [Google Scholar] [CrossRef]
- Mari, M.; Neri, F.; Bertolini, P. Novel approaches to prevent and control postharvest diseases of fruits. Stewart Postharvest Rev. 2007, 3, 1–7. [Google Scholar] [CrossRef]
- Droby, S.; Wisniewski, M.; El-Ghaouth, A.; Wilson, C. Biological control of postharvest diseases of fruits and vegetables: Current achievements and future challenges. Acta Hortic. 2003, 628, 703–713. [Google Scholar] [CrossRef]
- Zhang, H.; Mahunu, G.K.; Castoria, R.; Apaliya, M.T.; Yang, Q. Augmentation of biocontrol agents with physical methods against postharvest diseases of fruits and vegetables. Trends Food Sci. Technol. 2017, 69, 36–45. [Google Scholar] [CrossRef]
- Calvo, A.M.; Wilson, R.A.; Bok, J.W.; Keller, N.P. Relationship between secondary metabolism and fungal development. Microbiol. Mol. Biol. Rev. 2002, 66, 447–459. [Google Scholar] [CrossRef]
- Coelho, A.R.; Celli, M.G.; Ono, E.Y.S.; Wosiacki, G.; Hoffmann, F.L.; Pagnocca, F.C.; Hirooka, E.Y. Penicillium expansum versus antagonist yeasts and patulin degradation in vitro. Braz. Arch. Biol. Technol. 2007, 50, 725–733. [Google Scholar] [CrossRef]
- Florianowicz, T. Antifungal activity of some microorganisms against Penicillium expansum. Eur. Food Res. Technol. 2001, 212, 282–286. [Google Scholar] [CrossRef]
- Castoria, R.; Morena, V.; Caputo, L.; Panfili, G.; De Curtis, F.; De Cicco, V. Effect of the Biocontrol Yeast Rhodotorula glutinis Strain LS11 on Patulin Accumulation in Stored Apples. Phytopathol. 2005, 95, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, D.; Lorè, A.; Garibaldi, A.; Gullino, M.L. A new strain of Metschnikowia fructicola for postharvest control of Penicillium expansum and patulin accumulation on four cultivars of apple. Postharvest Biol. Technol. 2013, 75, 1–8. [Google Scholar] [CrossRef]
- Marek, P.; Annamalai, T.; Venkitanarayanan, K. Detection of Penicillium expansum by polymerase chain reaction. Int. J. Food Microbiol. 2003, 89, 139–144. [Google Scholar] [CrossRef]
- Mahato, D.K.; Kamle, M.; Sharma, B.; Pandhi, S.; Devi, S.; Dhawan, K.; Selvakumar, R.; Mishra, D.; Kumar, A.; Arora, S.; et al. Patulin in food: A mycotoxin concern for human health and its management strategies. Toxicon 2021, 198, 12–23. [Google Scholar] [CrossRef]
- Morales, H.; Sanchis, V.; Usall, J.; Ramos, A.J.; Marín, S. Effect of biocontrol agents Candida sake and Pantoea agglomerans on Penicillium expansum growth and patulin accumulation in apples. Int. J. Food Microbiol. 2008, 122, 61–67. [Google Scholar] [CrossRef]
- Sarrocco, S.; Vannacci, G. Preharvest application of beneficial fungi as a strategy to prevent postharvest mycotoxin contamination: A review. Crop Prot. 2018, 110, 160–170. [Google Scholar] [CrossRef]
- Gholamnezhad, J.; Etebarian, H. Biological control of apple blue mold with Candida membranifaciens and Rhodotorula mucilaginosa. Afr. J. Food Sci. 2010, 4, 1–7. Available online: http://www.academicjournals.org/ajfs (accessed on 25 June 2025).
- Menniti, A.M.; Neri, F.; Gregori, R.; Maccaferri, M. Some factors influencing patulin production by Penicillium expansum in pome fruits. J. Sci. Food Agric. 2010, 90, 2183–2187. [Google Scholar] [CrossRef]
- Konstantinou, S.; Karaoglanidis, G.; Bardas, G.A.; Minas, I.S.; Doukas, E.; Markoglou, A. Postharvest Fruit Rots of Apple in Greece: Pathogen Incidence and Relationships Between Fruit Quality Parameters, Cultivar Susceptibility, and Patulin Production. Plant Dis. 2011, 95, 666–672. [Google Scholar] [CrossRef]
- Marín, S.; Morales, H.; Hasan, H.A.; Ramos, A.J.; Sanchis, V. Patulin distribution in Fuji and Golden apples contaminated with Penicillium expansum. Food Addit. Contam. 2006, 23, 1316–1322. [Google Scholar] [CrossRef]
- Cunha, S.C.; Faria, M.A.; Pereira, V.L.; Oliveira, T.M.; Lima, A.C.; Pinto, E. Patulin assessment and fungi identification in organic and conventional fruits and derived products. Food Control 2014, 44, 185–190. [Google Scholar] [CrossRef]
- Marín, S.; Morales, H.; Ramos, A.J.; Sanchis, V. Evaluation of growth quantification methods for modelling the growth of Penicillium expansum in an apple-based medium. J. Sci. Food Agric. 2006, 86, 1468–1474. [Google Scholar] [CrossRef]
- Kumar, D.; Tannous, J.; Sionov, E.; Keller, N.; Prusky, D. Apple Intrinsic Factors Modulating the Global Regulator, LaeA, the Patulin Gene Cluster and Patulin Accumulation During Fruit Colonization by Penicillium expansum. Front. Plant Sci. 2018, 9, 1094. [Google Scholar] [CrossRef]
- Bok, J.W.; Keller, N.P. LaeA, a Regulator of Secondary Metabolism in Aspergillus spp. Eukaryot. Cell. 2004, 3, 527–535. [Google Scholar] [CrossRef]
- Tannous, J.; Atoui, A.; El Khoury, A.; Francis, Z.; Oswald, I.P.; Puel, O.; Lteif, R. A study on the physicochemical parameters for Penicillium expansum growth and patulin production: Effect of temperature, pH, and water activity. Food Sci. Nutr. 2015, 4, 611–622. [Google Scholar] [CrossRef]
- Lindroth, S.; Niskanen, A.; Pensala, O. Patulin production during storage of blackcurrant, blueberry and strawberry jams inoculated with Penicillium expansum mould. J. Food Sci. 1978, 43, 1427–1429. [Google Scholar] [CrossRef]
- Patterson, M.; Damoglou, A.P. The effect of water activity and pH on the production of mycotoxins by fungi growing on a bread analogue. Lett. Appl. Microbiol. 1986, 3, 123–125. [Google Scholar] [CrossRef]
- Zong, Y.; Li, B.; Tian, S. Effects of carbon, nitrogen and ambient pH on patulin production and related gene expression in Penicillium expansum. Int. J. Food Microbiol. 2015, 206, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Barad, S.; Espeso, E.A.; Sherman, A.; Prusky, D. Ammonia activates pacC and patulin accumulation in an acidic environment during apple colonization by Penicillium expansum. Mol. Plant Pathol. 2016, 17, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, B.; Xu, X.; Zhang, Z.; Tian, S. The pH-responsive PacC transcription factor plays pivotal roles in virulence and patulin biosynthesis in Penicillium expansum: Functional characterization of PePacC. Environ. Microbiol. 2018, 20, 4063–4078. [Google Scholar] [CrossRef]
- Zhuo, R.; Chen, Y.; Xing, M.; Zhang, Z.; Tian, S.; Li, B. Ena Proteins Respond to PacC-Mediated pH Signaling Pathway and Play a Crucial Role in Patulin Biosynthesis. J. Fungi 2023, 9, 806. [Google Scholar] [CrossRef]
- Prusky, D.; McEvoy, J.L.; Saftner, R.; Conway, W.S.; Jones, R. Relationship Between Host Acidification and Virulence of Penicillium spp. on Apple and Citrus Fruit. Phytopathology 2004, 94, 44–51. [Google Scholar] [CrossRef]
- Jimdjio, C.K.; Xue, H.; Bi, Y.; Nan, M.; Li, L.; Zhang, R.; Liu, Q.; Pu, L. Effect of Ambient pH on Growth, Pathogenicity, and Patulin Production of Penicillium expansum. Toxins 2021, 13, 550. [Google Scholar] [CrossRef]
- Wei, J.; Ma, F.; Shi, S.; Qi, X.; Zhu, X.; Yuan, J. Changes and postharvest regulation of activity and gene expression of enzymes related to cell wall degradation in ripening apple fruit. Postharvest Biol. Technol. 2010, 56, 147–154. [Google Scholar] [CrossRef]
- Vilanova, L.; Viñas, I.; Torres, R.; Usall, J.; Buron-Moles, G.; Teixidó, N. Increasing maturity reduces wound response and lignification processes against Penicillium expansum (pathogen) and Penicillium digitatum (non-host pathogen) infection in apples. Postharvest Biol. Technol. 2014, 88, 54–60. [Google Scholar] [CrossRef]
- Chávez, R.A.S.; Peniche, R.Á.M.; Medrano, S.A.; Muñoz, L.S.; Ortíz, M.d.S.C.; Espasa, N.T.; Sanchis, R.T. Effect of maturity stage, ripening time, harvest year and fruit characteristics on the susceptibility to Penicillium expansum link of apple genotypes from Queretaro, Mexico. Sci. Hortic. 2014, 180, 86–93. [Google Scholar] [CrossRef]
- Tahir, I.; Nybom, H.; Ahmadi-Afzadi, M.; Røen, K.; Sehic, J.; Røen, D. Susceptibility to blue mold caused by Penicillium expansum in apple cultivars adapted to a cool climate. Eur. J. Hortic. Sci. 2015, 80, 117–127. [Google Scholar] [CrossRef]
- Shao, X.; Tu, K.; Tu, S.; Su, J.; Zhao, Y. Effects of Heat Treatment on Wound Healing in Gala and Red Fuji Apple Fruits. J. Agric. Food Chem. 2010, 58, 4303–4309. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.; Hewett, E.; Hertog, M.; Harker, F. Temperature induces differential softening responses in apple cultivars. Postharvest Biol. Technol. 2001, 23, 185–196. [Google Scholar] [CrossRef]
- Sun, J.; Janisiewicz, W.J.; Nichols, B.; Jurick Ii, W.M.; Chen, P. Composition of phenolic compounds in wild apple with multiple resistance mechanisms against postharvest blue mold decay. Postharvest Biol. Technol. 2017, 127, 68–75. [Google Scholar] [CrossRef]
- Lončarić, A.; Šarkanj, B.; Gotal, A.-M.; Kovač, M.; Nevistić, A.; Fruk, G.; Skendrović Babojelić, M.; Babić, J.; Miličević, B.; Kovač, T. Penicillium expansum Impact and Patulin Accumulation on Conventional and Traditional Apple Cultivars. Toxins 2021, 13, 703. [Google Scholar] [CrossRef]
- Li, B.; Chen, Y.; Zhang, Z.; Qin, G.; Chen, T.; Tian, S. Molecular basis and regulation of pathogenicity and patulin biosynthesis in Penicillium expansum. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3416–3438. [Google Scholar] [CrossRef]
- Delgado, J.; Ballester, A.R.; Núñez, F.; González-Candelas, L. Evaluation of the activity of the antifungal PgAFP protein and its producer mould against Penicillium spp. postharvest pathogens of citrus and pome fruits. Food Microbiol. 2019, 84, 103266. [Google Scholar] [CrossRef]
- Sanzani, S.; Schena, L.; Nigro, F.; De Girolamo, A.; Ippolito, A. Effect of quercetin and umbelliferone on the transcript level of Penicillium expansum genes involved in patulin biosynthesis. Eur. J. Plant Pathol. 2009, 125, 223–233. [Google Scholar] [CrossRef]
- Kumar, D.; Barad, S.; Chen, Y.; Luo, X.; Tannous, J.; Dubey, A.; Glam Matana, N.; Tian, S.; Li, B.; Keller, N.; et al. LaeA regulation of secondary metabolism modulates virulence in Penicillium expansum and is mediated by sucrose. Mol. Plant Pathol. 2017, 18, 1150–1163. [Google Scholar] [CrossRef]
- Dombrink-Kurtzman, M.A.; Engberg, A.E. Byssochlamys nivea with patulin-producing capability has an isoepoxydon dehydrogenase gene (idh) with sequence homology to Penicillium expansum and P. griseofulvum. Mycol. Res. 2006, 110, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Dombrink-Kurtzman, M.A. The sequence of the isoepoxydon dehydrogenase gene of the patulin biosynthetic pathway in Penicillium species. Antonie Van Leeuwenhoek 2007, 91, 179–189. [Google Scholar] [CrossRef]
- Snini, S.P.; Tadrist, S.; Laffitte, J.; Jamin, E.L.; Oswald, I.P.; Puel, O. The gene PatG involved in the biosynthesis pathway of patulin, a food-borne mycotoxin, encodes a 6-methylsalicylic acid decarboxylase. Int. J. Food Microbiol. 2014, 171, 77–83. [Google Scholar] [CrossRef]
- Norelli, J.L.; Wisniewski, M.; Fazio, G.; Burchard, E.; Gutierrez, B.; Levin, E.; Droby, S. Genotyping-by-sequencing markers facilitate the identification of quantitative trait loci controlling resistance to Penicillium expansum in Malus sieversii. PLoS ONE 2017, 12, e0172949. [Google Scholar] [CrossRef] [PubMed]
- Snini, S.; Tannous, J.; Heuillard, P.; Bailly, S.; Lippi, Y.; Zehraoui, E.; Barreau, C.; Oswald, I.; Puel, O. The patulin is a cultivar dependent aggressiveness factor favoring the colonization of apples by Penicillium expansum. Mol. Plant Pathol. 2015, 17, 920–930. [Google Scholar] [CrossRef] [PubMed]
- Janisiewicz, W.J.; Saftner, R.A.; Conway, W.S.; Forsline, P.L. Preliminary Evaluation of Apple Germplasm from Kazakhstan for Resistance to Postharvest Blue Mold in Fruit Caused by Penicillium expansum. HortScience 2008, 43, 420–426. [Google Scholar] [CrossRef]
- De Clercq, N.; Vlaemynck, G.; Van Pamel, E.; Van Weyenberg, S.; Herman, L.; Devlieghere, F.; De Meulenaer, B.; Van Coillie, E. Isoepoxydon dehydrogenase (idh) gene expression in relation to patulin production by Penicillium expansum under different temperature and atmosphere. Int. J. Food Microbiol. 2016, 220, 50–57. [Google Scholar] [CrossRef]
- Damoglou, A.P.; Campbell, D.S.; Button, J.E. Some factors governing the production of patulin in apples. Food Microbiol. 1985, 2, 3–10. [Google Scholar] [CrossRef]
- Coton, M.; Bregier, T.; Poirier, E.; Debaets, S.; Arnich, N.; Coton, E.; Dantigny, P. Production and migration of patulin in Penicillium expansum molded apples during cold and ambient storage. Int. J. Food Microbiol. 2019, 313, 108377. [Google Scholar] [CrossRef]
- Neri, F.; Donati, I.; Veronesi, F.; Mazzoni, D.; Mari, M. Evaluation of Penicillium expansum isolates for aggressiveness, growth and patulin accumulation in usual and less common fruit hosts. Int. J. Food Microbiol. 2010, 143, 109–117. [Google Scholar] [CrossRef]
- Sanzani, S.; Susca, A.; Mastrorosa, S.; Solfrizzo, M. Patulin risk associated with blue mould of pome fruit marketed in southern Italy. Qual. Assur. Saf. Crops Foods 2017, 9, 23–29. [Google Scholar] [CrossRef]
- Baert, K.; Devlieghere, F.; Amiri, A.; De Meulenaer, B. Evaluation of strategies for reducing patulin contamination of apple juice using a farm to fork risk assessment model. Int. J. Food Microbiol. 2012, 154, 119–129. [Google Scholar] [CrossRef]
- Rizzolo, A.; Grassi, M.; Zerbini, P.E. Influence of harvest date on ripening and volatile compounds in the scab-resistant apple cultivar ‘Golden Orange’. J. Hortic. Sci. Biotechnol. 2006, 81, 681–690. [Google Scholar] [CrossRef]
- Lafer, G. Storability and fruit quality of ‘Golden Delicious’ as affected by harvest date, AVG and 1-MCP treatments. J. Fruit. Ornament. 2006, 14, 203–212. [Google Scholar]
- Torres, R.; Valentines, M.C.; Usall, J.; Viñas, I.; Larrigaudiere, C. Possible involvement of hydrogen peroxide in the development of resistance mechanisms in ‘Golden Delicious’ apple fruit. Postharvest Biol. Technol. 2003, 27, 235–242. [Google Scholar] [CrossRef]
- Vilanova, L.; Teixidó, N.; Torres, R.; Usall, J.; Viñas, I. The infection capacity of P. expansum and P. digitatum on apples and histochemical analysis of host response. Int. J. Food Microbiol. 2012, 157, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi-Afzadi, M.; Tahir, I.; Nybom, H. Impact of harvesting time and fruit firmness on the tolerance to fungal storage diseases in an apple germplasm collection. Postharvest Biol. Technol. 2013, 82, 51–58. [Google Scholar] [CrossRef]
- Goulao, L.F.; Santos, J.; de Sousa, I.; Oliveira, C.M. Patterns of enzymatic activity of cell wall-modifying enzymes during growth and ripening of apples. Postharvest Biol. Technol. 2007, 43, 307–318. [Google Scholar] [CrossRef]
- Shi, Y.; Jiang, L.; Zhang, L.; Kang, R.; Yu, Z. Dynamic changes in proteins during apple (Malus × domestica) fruit ripening and storage. Hortic. Res. 2014, 1, 6. [Google Scholar] [CrossRef]
- Zheng, Q.; Song, J.; Campbell-Palmer, L.; Thompson, K.; Li, L.; Walker, B.; Cui, Y.; Li, X. A proteomic investigation of apple fruit during ripening and in response to ethylene treatment. J. Proteom. 2013, 93, 276–294. [Google Scholar] [CrossRef]
- Bobelyn, E.; Serban, A.-S.; Nicu, M.; Lammertyn, J.; Nicolai, B.M.; Saeys, W. Postharvest quality of apple predicted by NIR-spectroscopy: Study of the effect of biological variability on spectra and model performance. Postharvest Biol. Technol. 2010, 55, 133–143. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gal, T.E.; Alexa, E.C.; Șumălan, R.M.; Dascălu, I.; Iordănescu, O.A. Factors Affecting Patulin Production by Penicillium expansum in Apples. Foods 2025, 14, 2310. https://doi.org/10.3390/foods14132310
Gal TE, Alexa EC, Șumălan RM, Dascălu I, Iordănescu OA. Factors Affecting Patulin Production by Penicillium expansum in Apples. Foods. 2025; 14(13):2310. https://doi.org/10.3390/foods14132310
Chicago/Turabian StyleGal, Tamara Edina, Ersilia Călina Alexa, Renata Maria Șumălan, Ionuț Dascălu, and Olimpia Alina Iordănescu. 2025. "Factors Affecting Patulin Production by Penicillium expansum in Apples" Foods 14, no. 13: 2310. https://doi.org/10.3390/foods14132310
APA StyleGal, T. E., Alexa, E. C., Șumălan, R. M., Dascălu, I., & Iordănescu, O. A. (2025). Factors Affecting Patulin Production by Penicillium expansum in Apples. Foods, 14(13), 2310. https://doi.org/10.3390/foods14132310






