Characterization of Aroma-Active Compounds in Five Dry-Cured Hams Based on Electronic Nose and GC-MS-Olfactometry Combined with Odor Description, Intensity, and Hedonic Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Ham Samples
2.2. HS-SPME-GC-MS-O Analysis
2.3. E-Nose Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Volatile Compounds Analysis
3.2. Analysis of Aroma-Active Volatiles by GC-MS-O
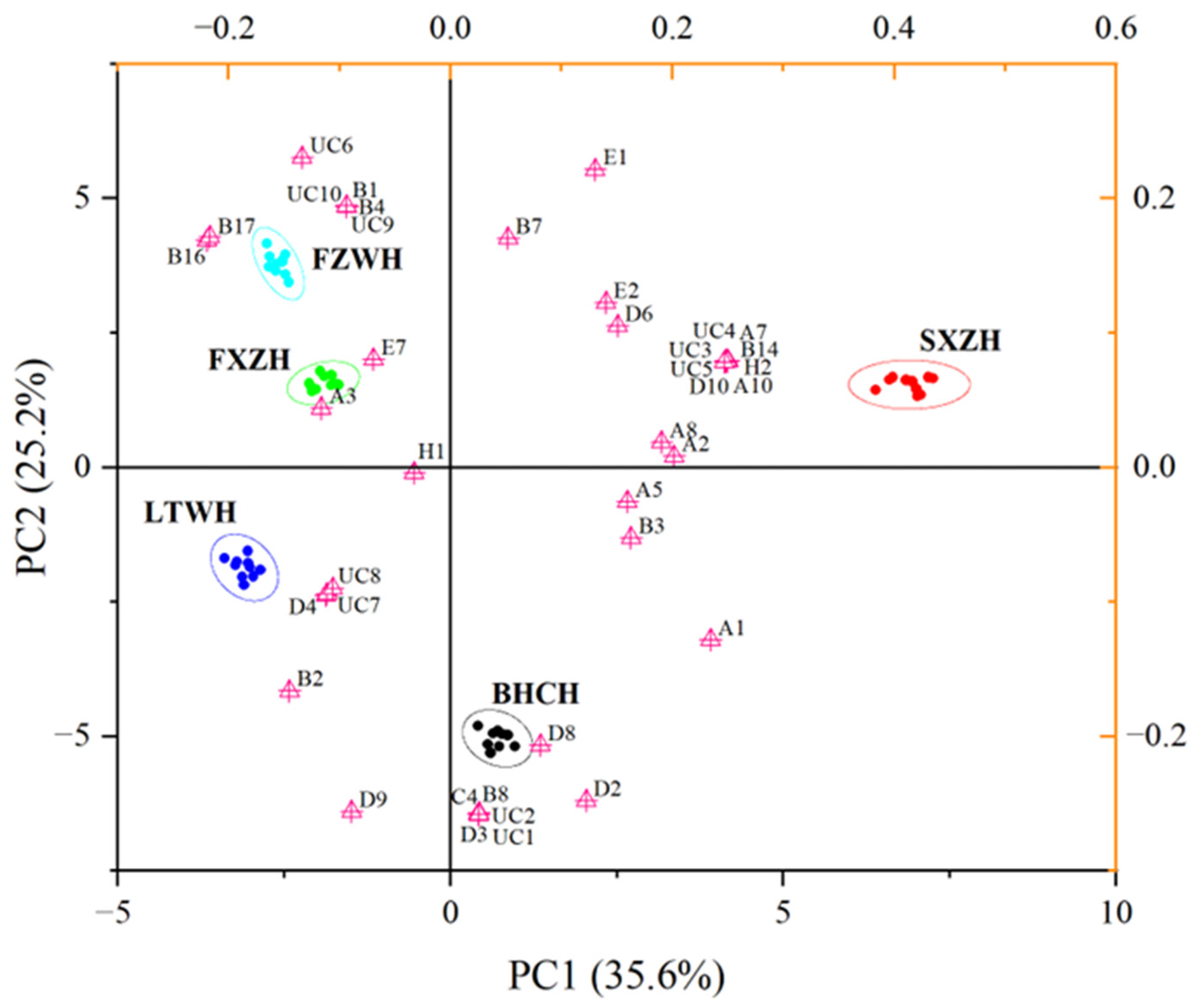
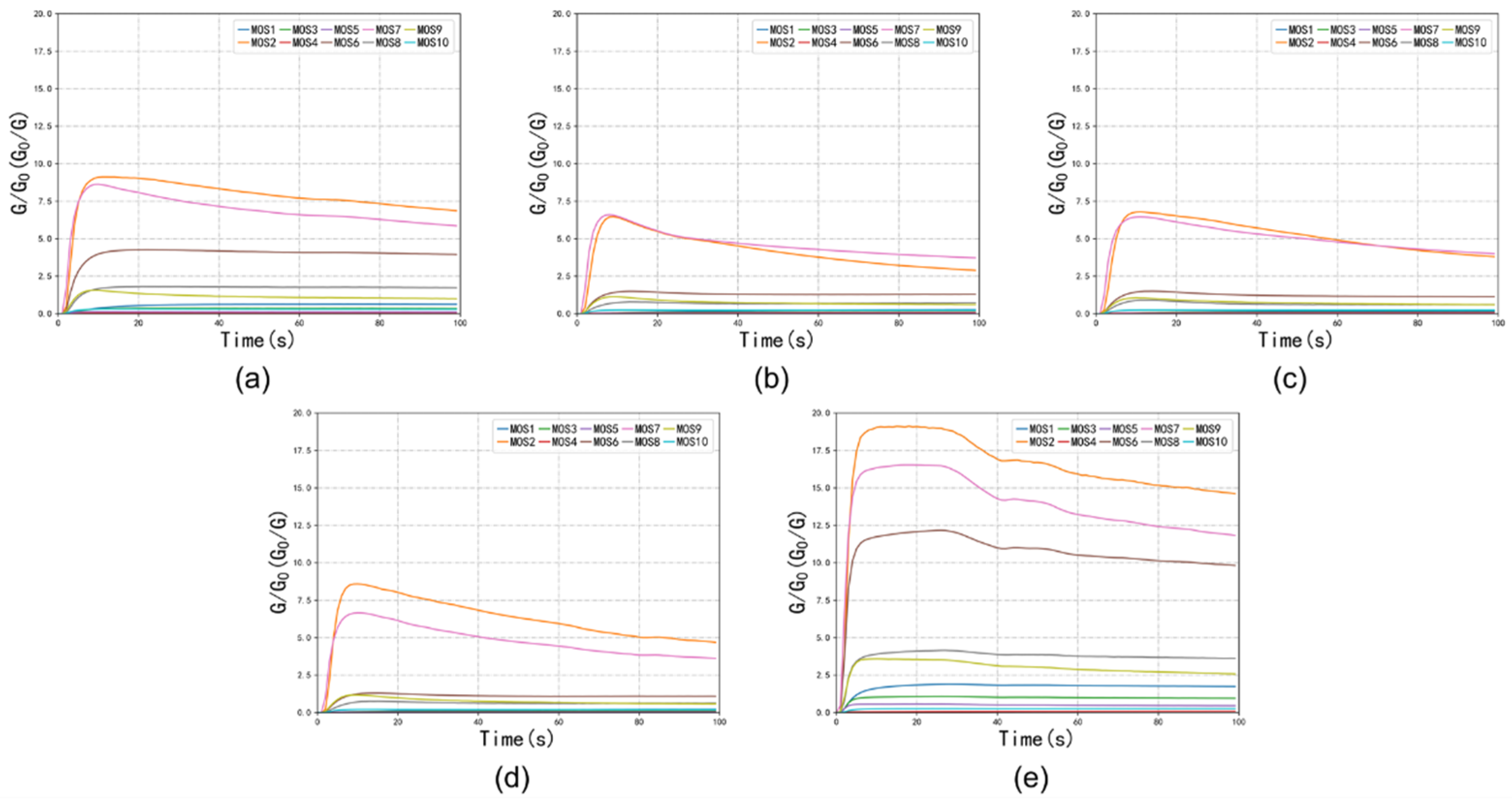
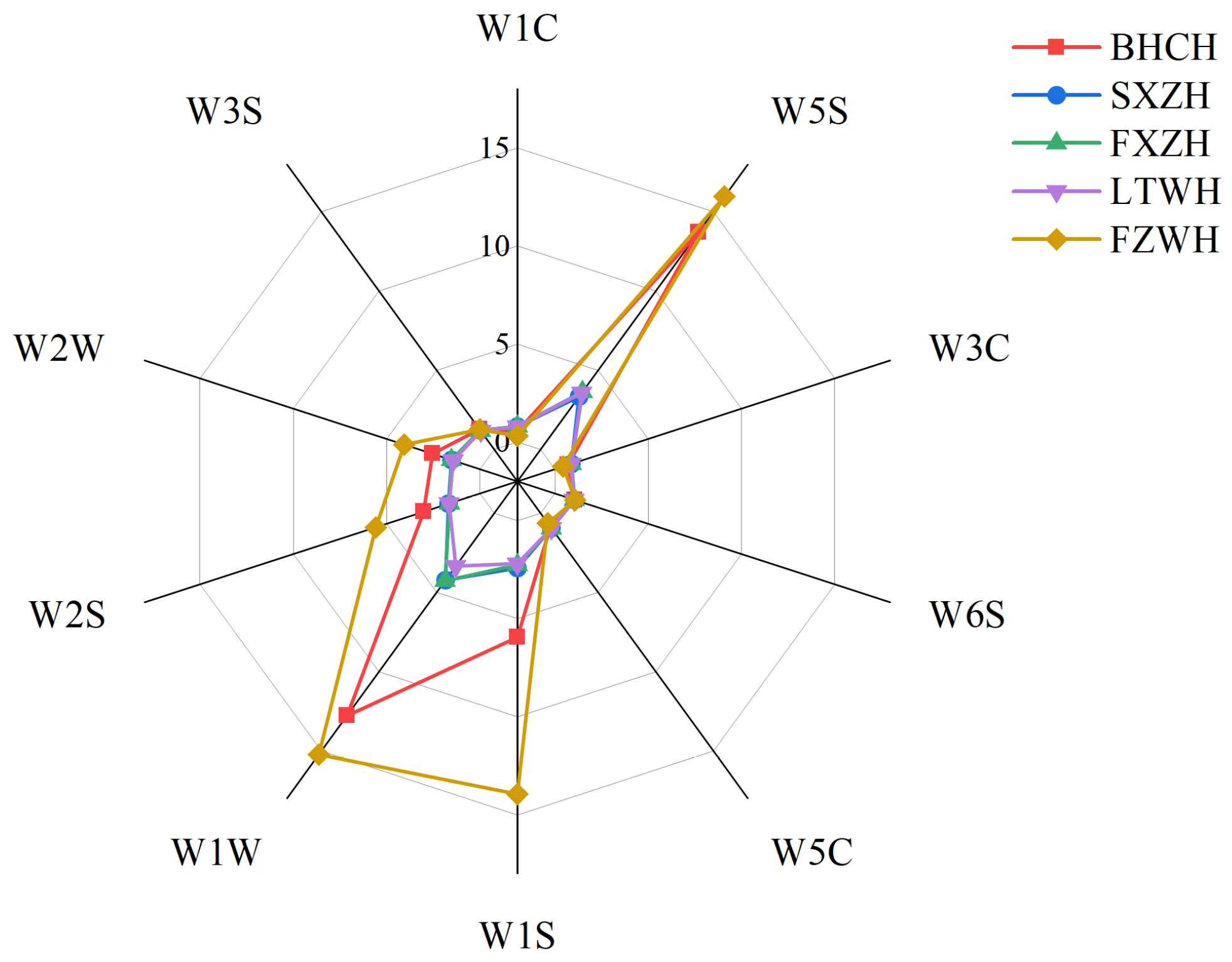
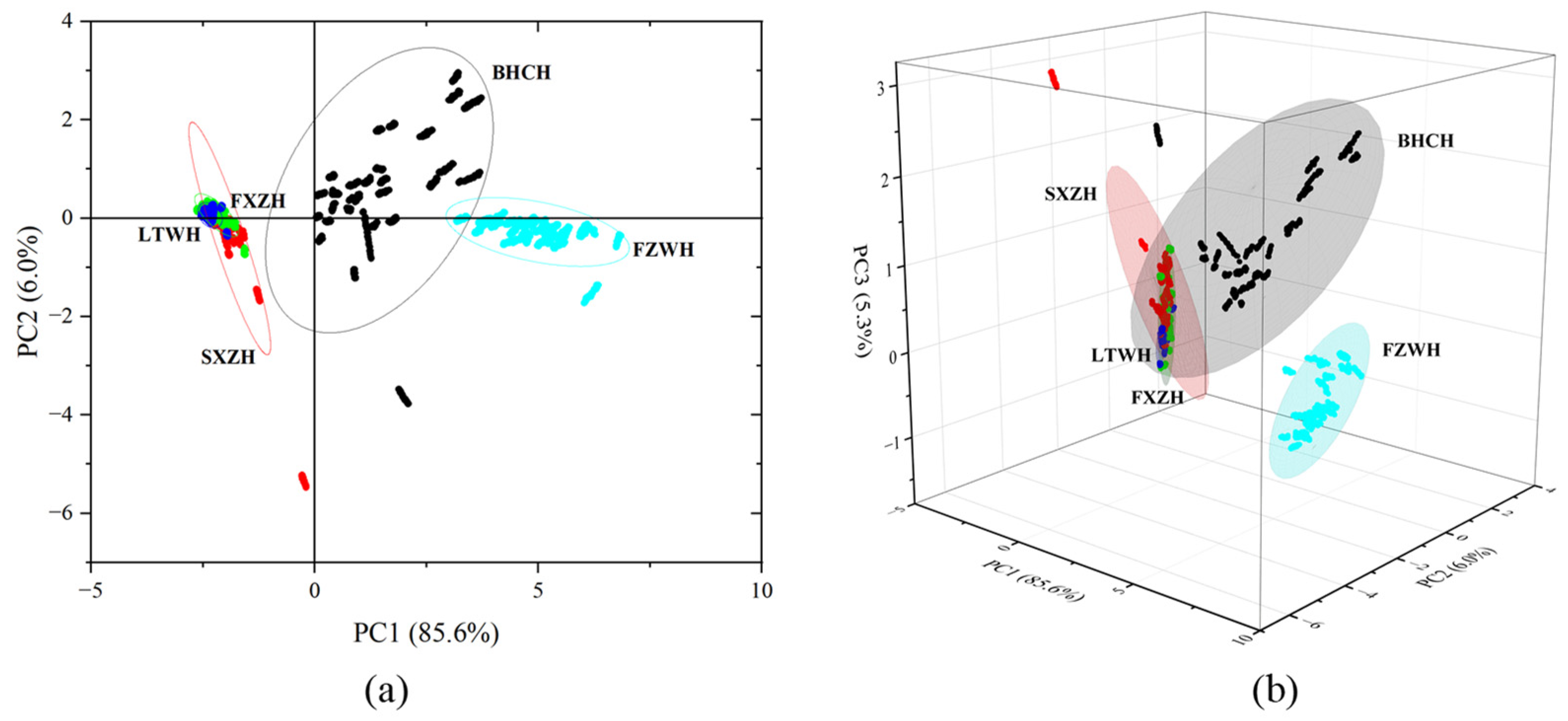
3.3. E-Nose Analysis
3.4. Correlation Between E-Nose and GC-MS-O
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GC-MS | Gas chromatography-mass spectrometry |
| E-Nose | Electronic nose |
| GC-MS-O | Gas chromatography-mass spectrometry-olfactometry |
| PCA | Principal component analysis |
| BHCH | Black Hoof Cured Ham |
| SXZH | Superior-grade Xuan-Zi Ham |
| FXZH | First-grade Xuan-Zi Ham |
| LTWH | Liang-Tou-Wu Ham |
| FZWH | Fei-Zhong-Wang Ham |
| HS-SPME | Headspace solid-phase microextraction |
References
- Liu, H.; Huang, J.; Hu, Q.; Chen, Y.P.; Lai, K.; Xu, J.; Ouyang, G.; Liu, Y. Dual-fiber solid-phase microextraction coupled with gas chromatography–mass spectrometry for the analysis of volatile compounds in traditional Chinese dry-cured ham. J. Chromatogr. B 2020, 1140, 121994–122000. [Google Scholar] [CrossRef]
- Jiang, S.; Xia, D.; Wang, X.; Zhu, Y.; Chen, G.; Liu, Y. Analysis of aroma-active compounds in four Chinese dry-cured hams based on GC-O combined with AEDA and frequency detection methods. LWT 2022, 153, 112497–112505. [Google Scholar] [CrossRef]
- Martín-Gómez, A.; Arroyo-Manzanares, N.; García-Nicolás, M.; López-Lorente, Á.I.; Cárdenas, S.; López-García, I.; Viñas, P.; Hernández-Córdoba, M.; Arce, L. Portable Raman spectrometer as a screening tool for characterization of Iberian dry-cured ham. Foods 2021, 10, 1177. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, W.; Yang, Y.; Ma, C.; Ahn, D.U.; Li, X.; Lei, J.; Du, M. Changes of hormone-sensitive lipase (HSL), adipose tissue triglyceride lipase (ATGL) and free fatty acids in subcutaneous adipose tissues throughout the ripening process of dry-cured ham. Food Chem. 2010, 121, 191–195. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; Martín-Gómez, A.; Jurado-Campos, N.; Garrido-Delgado, R.; Arce, C.; Arce, L. Target vs spectral fingerprint data analysis of Iberian ham samples for avoiding labelling fraud using headspace–gas chromatography–ion mobility spectrometry. Food Chem. 2018, 246, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Lammers, M.; Dietze, K.; Ternes, W. Headspace volatiles of dry-cured ham: A comparison of different manufacturing styles by spme and gc/ms analysis. J. Food Process. Pres. 2011, 35, 850–860. [Google Scholar] [CrossRef]
- Liu, X.S.; Liu, J.B.; Yang, Z.M.; Song, H.L.; Liu, Y.; Zou, T.T. Aroma-active compounds in jinhua ham produced with different fermentation periods. Molecules 2014, 19, 19097–19113. [Google Scholar] [CrossRef]
- Xia, D.; Zhang, D.N.; Gao, S.T.; Chen, L.; Li, N.; Zheng, F.P.; Liu, Y. Categorization of Chinese Dry-Cured Ham Based on Three Sticks’ Method by Multiple Sensory Technologies. J. Food Qual. 2017, 2017, 1701756–1701761. [Google Scholar] [CrossRef]
- Martínez-Onandi, N.; Rivas-Cañedo, A.; Nuñez, M.; Picon, A. Effect of chemical composition and high pressure processing on the volatile fraction of Serrano dry-cured ham. Meat Sci. 2016, 111, 130–138. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, J.; Chen, S.; Wu, D.; Liu, D.; Ye, X. Characterization of aroma-active volatiles in three Chinese bayberry (Myrica rubra) cultivars using GC–MS–olfactometry and an electronic nose combined with principal component analysis. Food Res. Int. 2015, 72, 8–15. [Google Scholar] [CrossRef]
- Wu, R.; Yang, C.; Xi, L.; Wang, T.; Zhang, J.; Kou, L.; Ding, W. Evaluation of the influence of flavor characteristics of cooked bacon with different sterilization methods by GC-IMS combined with HS-SPME-GC-MS and electronic nose. Foods 2022, 11, 3547. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhou, H.; Wang, Z.; Al-Dalali, S.; Nie, W.; Xu, F.; Li, C.; Li, P.; Cai, K.; Xu, B. Analysis of flavor formation during the production of Jinhua dry-cured ham using headspace-gas chromatography-ion mobility spectrometry (HS-GC-IMS). Meat Sci. 2022, 194, 108992–109001. [Google Scholar] [CrossRef]
- Wei, G.; Li, X.; Wang, D.; Huang, W.; Shi, Y.; Huang, A. Insights into free fatty acid profiles and oxidation on the development of characteristic volatile compounds in dry-cured ham from Dahe black and hybrid pigs. LWT 2023, 184, 115063–115074. [Google Scholar] [CrossRef]
- Martín-Gómez, A.; Segura-Borrego, M.P.; Ríos-Reina, R.; Cardador, M.J.; Callejón, R.M.; Morales, M.L.; Cardador, J.; Raquel, M.; Callejón, M. Discrimination of defective dry-cured Iberian ham determining volatile compounds by non-destructive sampling and gas chromatography. LWT 2022, 154, 112785–112792. [Google Scholar] [CrossRef]
- Qian, K.; Bao, Y.; Zhu, J.; Wang, J.; Wei, Z. Development of a portable electronic nose based on a hybrid filter-wrapper method for identifying the Chinese dry-cured ham of different grade. J. Food Eng. 2021, 290, 110250–110262. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, J.; Pei, Z.; Wei, P.; Xiang, D.; Cao, X.; Shen, X.; Li, C. Volatile flavour components and the mechanisms underlying their production in golden pompano (Trachinotus blochii) fillets subjected to different drying methods: A comparative study using an electronic nose, an electronic tongue and SDE-GC-MS. Food Res. Int. 2019, 123, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Brattoli, M.; Cisternino, E.; Dambruoso, P.R.; de Gennaro, G.; Giungato, P.; Mazzone, A.; Palmisani, J.; Tutino, M. Gas chromatography analysis with olfactometric detection (GC-O) as a useful methodology for chemical characterization of odorous compounds. Sensors 2013, 13, 16759–16800. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Wen, R.; Sun, F.; Wang, Y.; Kong, B.; Chen, Q. Collaborative analysis on differences in volatile compounds of Harbin red sausages smoked with different types of woodchips based on gas chromatography–mass spectrometry combined with electronic nose. LWT 2021, 143, 111144–111154. [Google Scholar] [CrossRef]
- Wilson, A.D.; Baietto, M. Applications and advances in electronic-nose technologies. Sensors 2009, 9, 5099–5148. [Google Scholar] [CrossRef]
- Lorido, L.; Pizarro, E.; Estévez, M.; Ventanas, S. Emotional responses to the consumption of dry-cured hams by Spanish consumers: A temporal approach. Meat Sci. 2019, 149, 126–133. [Google Scholar] [CrossRef]
- Tan, J.; Xu, J. Applications of electronic nose (e-nose) and electronic tongue (e-tongue) in food quality-related properties determination: A review. Artif. Intell. Agric. 2020, 4, 104–115. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, T.; Ni, W.; Zhang, Y.; Lv, W.; Zeng, M.; Yang, J.; Hu, N.; Zhan, R.; Li, G.; et al. Sensor array optimization for the electronic nose via different deep learning methods. Sens. Actuators B Chem. 2024, 410, 135579–135586. [Google Scholar] [CrossRef]
- Boeker, P. On ‘electronic nose’ methodology. Sens. Actuators B Chem. 2014, 204, 2–17. [Google Scholar] [CrossRef]
- Yu, D.; Gu, Y. A machine learning method for the fine-grained classification of green tea with geographical indication using a MOS-based electronic nose. Foods 2021, 10, 795. [Google Scholar] [CrossRef]
- García-González, D.L.; Aparicio, R.; Aparicio-Ruiz, R. Volatile and amino acid profiling of dry cured hams from different swine breeds and processing methods. Molecules 2013, 18, 3927–3947. [Google Scholar] [CrossRef]
- Cordente, A.G.; Espinase Nandorfy, D.; Solomon, M.; Schulkin, A.; Kolouchova, R.; Francis, I.L.; Schmidt, S.A. Aromatic Higher Alcohols in Wine: Implication on Aroma and Palate Attributes during Chardonnay Aging. Molecules 2021, 26, 4979. [Google Scholar] [CrossRef]
- Toldrá, F.; Flores, M. The role of muscle proteases and lipases in flavor development during the processing of dry-cured ham. Crit. Rev. Food Sci. 1998, 38, 331–352. [Google Scholar] [CrossRef]
- Pham, A.J.; Schilling, M.W.; Mikel, W.B.; Williams, J.B.; Martin, J.M.; Coggins, P.C. Relationships between sensory descriptors, consumer acceptability and volatile flavor compounds of American dry-cured ham. Meat Sci. 2008, 80, 728–737. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Carballo, J.; Franco, D. Effect of the inclusion of chestnut in the finishing diet on volatile compounds of dry-cured ham from Celta pig breed. J. Integr. Agr. 2013, 12, 2002–2012. [Google Scholar] [CrossRef]
- Bosse, R.; Wirth, M.; Konstanz, A.; Becker, T.; Weiss, J.; Gibis, M. Determination of volatile marker compounds in raw ham using headspace-trap gas chromatography. Food Chem. 2017, 219, 249–259. [Google Scholar] [CrossRef]
- Pugliese, C.; Sirtori, F.; Calamai, L.; Franci, O. The evolution of volatile compounds profile of “Toscano” dry-cured ham during ripening as revealed by SPME-GC-MS approach. J. Mass Spectrom. 2010, 45, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.X.; Wang, Z.; Xu, S.Y. Characterization of odoractive compounds in Jinhua ham by GC-olfactometry. Food Ferment. Ind. (Chin.) 2004, 30, 117–123. [Google Scholar]
- Marušić Radovčić, N.; Vidaček, S.; Janči, T.; Medić, H. Characterization of volatile compounds, physico-chemical and sensory characteristics of smoked dry-cured ham. J. Food Sci. Technol. 2016, 53, 4093–4105. [Google Scholar] [CrossRef] [PubMed]
- Petrova, I.; Aasen, I.M.; Rustad, T.; Eikevik, T.M. Manufacture of dry-cured ham: A review. Part 1. Biochemical changes during the technological process. Eur. Food Res. Technol. 2015, 241, 587–599. [Google Scholar] [CrossRef]
- Petričević, S.; Radovčić, N.M.; Lukić, K.; Listeš, E.; Medić, H. Differentiation of dry-cured hams from different processing methods by means of volatile compounds, physico-chemical and sensory analysis. Meat Sci. 2018, 137, 217–227. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.P.; Blank, I.; Li, F.; Li, C.; Liu, Y. GC×GC-ToF-MS and GC-IMS based volatile profile characterization of the Chinese dry-cured hams from different regions. Food Res. Int. 2018, 142, 110222–110229. [Google Scholar] [CrossRef]
- Gaspardo, B.; Procida, G.; Toso, B.; Stefanon, B. Determination of volatile compounds in San Daniele ham using headspace GC–MS. Meat Sci. 2008, 80, 204–209. [Google Scholar] [CrossRef]
- Marušić, N.; Petrović, M.; Vidaček, S.; Petrak, T.; Medić, H. Characterization of traditional Istrian dry-cured ham by means of physical and chemical analyses and volatile compounds. Meat Sci. 2011, 88, 786–790. [Google Scholar] [CrossRef]
- Huan, Y.; Zhou, G.; Zhao, G.; Xu, X.; Peng, Z. Changes in flavor compounds of dry-cured Chinese Jinhua ham during processing. Meat Sci. 2005, 71, 291–299. [Google Scholar] [CrossRef]
- Narváez-Rivas, M.; Gallardo, E.; Léon-Camacho, M. Chemical changes in volatile aldehydes and ketones from subcutaneous fat during ripening of Iberian dry-cured ham. Prediction of the curing time. Food Res. Int. 2014, 55, 381–390. [Google Scholar] [CrossRef]
- Nunes, C.; Coimbra, M.A.; Saraiva, J.; Rocha, S.M. Study of the volatile components of a candied plum and estimation of their contribution to the aroma. Food Chem. 2008, 111, 897–905. [Google Scholar] [CrossRef]
- Wang, Y.; Li, F.; Chen, J.; Sun, Z.; Wang, F.; Wang, C.; Fu, L. High-throughput sequencing-based characterization of the predominant microbial community associated with characteristic flavor formation in Jinhua Ham. Food Microbiol. 2021, 94, 103643–103651. [Google Scholar] [CrossRef] [PubMed]
- Narváez-Rivas, M.; Gallardo, E.; León-Camacho, M. Analysis of volatile compounds from Iberian hams: A review. Grasas Y Aceites 2012, 63, 432–454. [Google Scholar] [CrossRef]
- Ramírez, R.; Cava, R. Volatile profiles of dry-cured meat products from three different Iberian X Duroc genotypes. J. Agric. Food Chem. 2007, 55, 1923–1931. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.; Ventanas, J.; Cava, R.; Andrés, A.; Garcıa, C. Volatile compounds of dry-cured Iberian ham as affected by the length of the curing process. Meat Sci. 1999, 52, 19–27. [Google Scholar] [CrossRef]
- Garćıa, C.; Berdagué, J.J.; Antequera, T.; López-Bote, C.; Córdoba, J.J.; Ventanas, J. Volatile components of dry cured Iberian ham. Food Chem. 2003, 41, 23–32. [Google Scholar] [CrossRef]
- López-Pedrouso, M.; Pérez-Santaescolástica, C.; Franco, D.; Carballo, J.; Zapata, C.; Lorenzo, J.M. Molecular insight into taste and aroma of sliced dry-cured ham induced by protein degradation undergone high-pressure conditions. Food Res. Int. 2019, 122, 635–642. [Google Scholar] [CrossRef]
- Garćıa-Gonźalez, D.L.; Tena, N.; Aparicio-Ruiz, R.; Morales, M.T. Relationship between sensory attributes and volatile compounds qualifying dry-cured hams. Meat Sci. 2008, 80, 315–325. [Google Scholar] [CrossRef]
- Marušić, N.; Vidaček, S.; Janči, T.; Petrak, T.; Medić, H. Determination of volatile compounds and quality parameters of traditional Istrian dry-cured ham. Meat Sci. 2014, 96, 1409–1416. [Google Scholar] [CrossRef]
- Careri, M.; Mangia, A.; Barbieri, G.; Bouoni, L.; Virgili, R.; Parolari, G. Sensory property relationships to chemical data of Italian-type dry-cured ham. J. Food Sci. 1993, 58, 968–972. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef]
- Sirtori, F.; Dimauro, C.; Bozzi, R.; Aquilani, C.; Franci, O.; Calamai, L.; Pezzati, A.; Pugliese, C. Evolution of volatile compounds and physical, chemical and sensory characteristics of Toscano PDO ham from fresh to dry-cured product. Eur. Food Res. Technol. 2020, 246, 409–424. [Google Scholar] [CrossRef]
- Chen, X.; Luo, J.; Lou, A.; Wang, Y.; Yang, D.; Shen, Q.W. Duck breast muscle proteins, free fatty acids and volatile compounds as affected by curing methods. Food Chem. 2021, 338, 128138–128145. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.Z.; Zhao, J.L.; Tian, W.; Liu, Y.X.; Li, M.Y.; Zhao, G.M. Contribution of histidine and lysine to the generation of volatile compounds in Jinhua ham exposed to ripening conditions via Maillard reaction. J. Food Sci. 2017, 83, 46–52. [Google Scholar] [CrossRef]
- Gilles, G. Dry cured ham quality as related to lipid quality of raw material and lipid changes during processing: A review. Grasas Y Aceites 2009, 60, 297–307. [Google Scholar] [CrossRef]
- Wood, J.D.; Richardson, R.I.; Nute, G.R.; Fisher, A.V.; Campo, M.M.; Kasapidou, E.; Sheard, P.R.; Enser, M. Effects of fatty acids on meat quality: A review. Meat Sci. 2004, 66, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Creuly, C.; Larroche, C.; Gros, J.B. Bioconversion of fatty acids into methyl ketones by spores of Penicillium roquefortii in a waterorganic solvent, two-phase system. Enzyme Microb. Tech. 1992, 14, 669–678. [Google Scholar] [CrossRef]
- Nijssen, L.M. Volatile Compounds in Food: Qualitative and Quantitative Data; Wiley: Hoboken, NJ, USA, 1996. [Google Scholar]
- Hinrichsen, L.; Pedersen, S.B. Relationship among flavor, volatile compounds, chemical changes, and microflora in Italian-type dry-cured ham during processing. J. Agric. Food Chem. 1995, 43, 2932–2940. [Google Scholar] [CrossRef]
- Martínez-Onandi, N.; Rivas-Cãnedo, A.; Picon, A.; Nũnez, M. Influence of physicochemical parameters and high pressure processing on the volatile compounds of Serrano dry-cured ham after prolonged refrigerated storage. Meat Sci. 2016, 122, 101–108. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, M.; Xie, J.; Zhao, M.; Hou, L.; Liang, J.; Wang, S.; Cheng, J. Volatile flavor constituents in the pork broth of black-pig. Food Chem. 2017, 226, 51–60. [Google Scholar] [CrossRef]
- Pugliese, C.; Sirtori, F.; Skrlep, M.; Piasentier, E.; Calamai, L.; Franci, O.; Candek-Potokar, M. The effect of ripening time on the chemical, textural, volatile and sensorial traits of Bicep femoris and Semimembranosus muscles of the Slovenian dry-cured ham Kraski prsut. Meat Sci. 2015, 100, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Bolzoni, L.; Barbieri, G.; Virgili, R. Changes in volatile compounds of Parma ham during maturation. Meat Sci. 1996, 43, 301–310. [Google Scholar] [CrossRef]
- Sforza, S.; Galaverna, G.; Schivazappa, C.; Marchelli, R.; Dossena, A.; Virgili, R. Effect of extended aging of parma dry-cured ham on the content of oligopeptides and free amino acids. J Agric Food Chem. 2006, 54, 9422. [Google Scholar] [CrossRef]
- Mu, Y.; Su, W.; Mu, Y.; Jiang, L. Combined application of high-throughput sequencing and metabolomics reveals metabolically active microorganisms during Panxian ham processing. Front. Microbiol. 2020, 10, 3012. [Google Scholar] [CrossRef] [PubMed]
- Gradinarska-Ivanova, D.; Mitreva, D.; Hadzhiyska, P.; Valkova-Yorgova, K.; Ivanova, I. Evolution of safety and quality aspects of dry-cured ham during aging under natural climatic conditions. J. Hyg. Eng. Des. 2020, 31, 53–60. [Google Scholar]
- Jin, S.K.; Moon, S.S. Quality and Shelf-Life Properties of Ready to Eat Dry-Cured Ham Slices under Different Packaging Systems during Storage. Food Sci. Anim. Resour. 2024, 44, 1358. [Google Scholar] [CrossRef]
- Flores, M. Understanding the implications of current health trends on the aroma of wet and dry cured meat products. Meat Sci. 2018, 144, 53–61. [Google Scholar] [CrossRef]
- Yoshii, F.; Yamada, Y.; Hoshi, T.; Hagiwara, H. The creation of a database of odorous compounds focused on molecular rigidity and analysis of the molecular features of the compounds in the database. Chemical Sens. 2002, 27, 399–405. [Google Scholar] [CrossRef]
- Pérez-Palacios, T.; Ruiz, J.; Martín, D.; Grau, R.; Antequera, T. Influence of pre-cure freezing on the profile of volatile compounds during the processing of Iberian hams. J. Sci. Food Agr. 2010, 90, 882–890. [Google Scholar] [CrossRef]
- Zhou, C.; Xia, Q.; Du, L.; He, J.; Sun, Y.; Dang, Y.; Geng, F.; Pan, D.; Cao, J.; Zhou, G. Recent developments in off-odor formation mechanism and the potential regulation by starter cultures in dry-cured ham. Crit. Rev. Food Sci. 2023, 63, 8781–8795. [Google Scholar] [CrossRef]


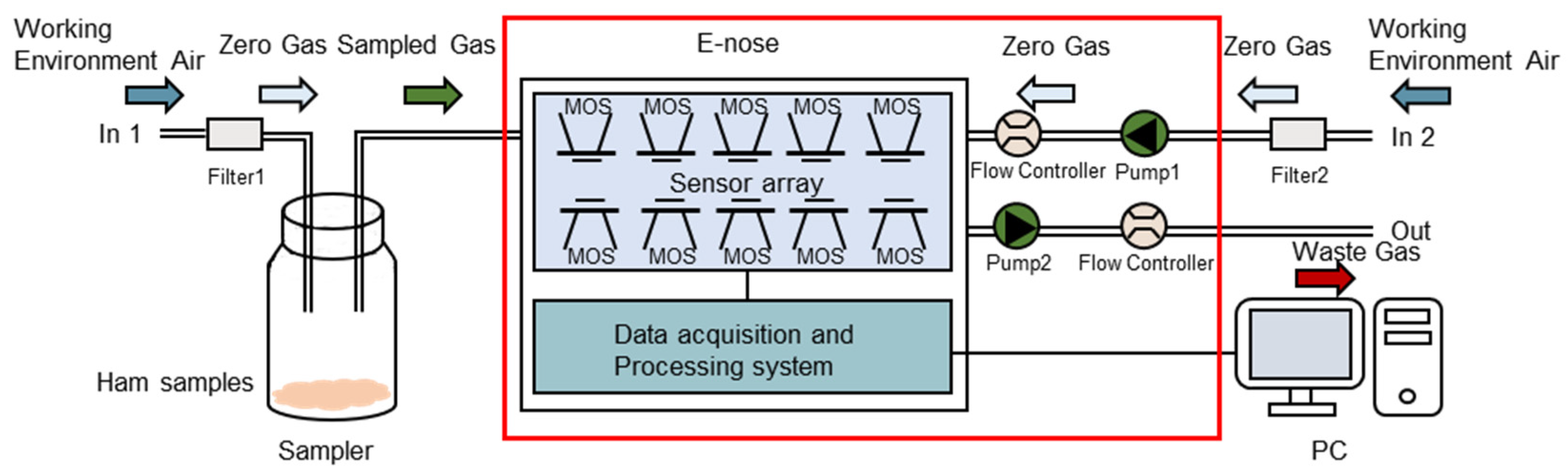

| No. | Name | Category | Producing Area | Pig Breed | Price (USD/100 g) |
|---|---|---|---|---|---|
| 1 | BHCH | Bama Ham | Spain | Spain white pig | 28.3 |
| 2 | SXZH | Xuanwei Ham | Yunnan, China | Highland Wujin pig | 22.0 |
| 3 | FXZH | Xuanwei Ham | Yunnan, China | Highland Wujin pig | 15.5 |
| 4 | LTWH | Jinhua Ham | Zhejiang, China | Two-Ends-Black pig | 6.1 |
| 5 | FZWH | Jinhua Ham | Zhejiang, China | Free-range local pig | 3.0 |
| NO. | Aroma Group | Main Characteristics |
|---|---|---|
| 1 | Floral | Aromatic, cologne, violet, subtle, rose, sweet |
| 2 | Woody/Resinous | Musty, strong, waxy, herbal, fatty, pungent, mushroom |
| 3 | Fruity, other than citrus | Sweet, strawberry, pineapple, banana, subtle, cucumber |
| 4 | Sickening | Putrid, sour milk, sweaty, excrement, strong, disgusting |
| 5 | Chemical | Anesthetic, cleaning fluid, medicine, paint, alcohol, pine oil |
| 6 | Minty | Cool, sweet, fennel, spice, medicine, aromatic |
| 7 | Sweet | Vanilla, caramel, chocolate, subtle, warm, malt |
| 8 | Popcorn | Burning, warm, strong, nutty, oil, peanut butter |
| 9 | Pungent | Onion, strong, household gas, burning, tartness, sulfide |
| 10 | Lemon | Citrus, sweet, cool, herbal, aromatic, subtle |
| NO. | Sensor | Main Performance |
|---|---|---|
| 1 | W1C | Sensitive to aromatic compounds |
| 2 | W5S | High sensitivity to nitrogen oxides, broad range sensitivity |
| 3 | W3C | Sensitive to ammonia and aromatic compounds |
| 4 | W6S | Sensitive mainly to hydrogen |
| 5 | W5C | Sensitive to alkanes and aromatic components and less sensitive to polar compounds |
| 6 | W1S | Sensitive to methane, broad range sensitivity |
| 7 | W1W | Sensitive primarily to sulfur compounds and many terpenes and organic sulfur compounds |
| 8 | W2S | Sensitive to ethanol and less sensitive to aromatic compounds |
| 9 | W2W | Sensitive to aromatic compounds and organic sulfur compounds |
| 10 | W3S | Highly sensitive to alkanes |
| No. | Compound | Retention Time (min) | CAS | Concentration (ng/g) | ||||
|---|---|---|---|---|---|---|---|---|
| BHCH a | SXZH b | FXZH c | LTWH d | FZWH e | ||||
| Alcohols | ||||||||
| A1 | 2-Heptanol | 9.96 | 543-49-7 | 1816.45 ± 5.39 | 3583.08 ± 1.67 | n.d. f | n.d. | n.d. |
| A2 | 1-Hexanol | 8.61 | 111-27-3 | 1318.91 ± 4.45 | 2084.91 ± 2.75 | n.d. | n.d. | 1776.81 ± 0.64 |
| A3 | 1-Octen-3-ol | 13.58 | 3391-86-4 | 970.42 ± 3.49 | n.d. | 299.66 ± 1.57 | n.d. | 4414.67 ± 13.68 |
| A4 | 1-Butanol, 3-methyl- | 4.64 | 123-51-3 | 579.03 ± 2.34 | 1033.38 ± 2.55 | n.d. | n.d. | 122.27 ± 3.60 |
| A5 | 1-Heptanol | 13.07 | 111-70-6 | 512.55 ± 4.16 | 865.27 ± 2.94 | 334.38 ± 1.54 | n.d. | 359.18 ± 3.18 |
| A6 | 1-Pentanol | 5.35 | 71-41-0 | 373.15 ± 2.65 | 529.05 ± 3.72 | n.d. | n.d. | 634.3 ± 2.69 |
| A7 | 1-Octanol | 18.12 | 111-87-5 | 353.85 ± 2.68 | 702.11 ± 3.32 | n.d. | n.d. | 305.68 ± 3.27 |
| A8 | Phenylethyl Alcohol | 20.12 | 60-12-8 | 337.77 ± 2.71 | 875.16 ± 2.92 | 60.91 ± 1.01 | 2.72 ± 0.36 | 137.56 ± 3.58 |
| A9 | Benzyl alcohol | 16.15 | 100-51-6 | 157.62 ± 2.98 | n.d. | n.d. | 98.47 ± 0.58 | 118.45 ± 3.61 |
| A10 | 2-Nonanol | 14.66 | 628-99-9 | n.d. | 3128.18 ± 1.44 | n.d. | n.d. | n.d. |
| A11 | 1-Tetradecanol | 43.55 | 112-72-1 | n.d. | 215.9 ± 2.44 | n.d. | n.d. | n.d. |
| A12 | Cyclooctyl alcohol | 17.95 | 696-71-9 | n.d. | 206.02 ± 2.46 | n.d. | 6.48 ± 0.36 | n.d. |
| A13 | 1-Octanol, 3,7-dimethyl- | 13.10 | 106-21-8 | n.d. | n.d. | 103.54 ± 0.98 | n.d. | n.d. |
| A14 | Ethanol | 2.24 | 64-17-5 | n.d. | n.d. | n.d. | n.d. | 359.18 ± 3.18 |
| A15 | 2-Octen-1-ol, (E)- | 17.94 | 18409-17-1 | n.d. | n.d. | n.d. | n.d. | 217.8 ± 3.43 |
| Aldehydes | ||||||||
| B1 | Hexanal | 6.16 | 66-25-1 | 7859.9 ± 19.90 | n.d. | 3310.9 ± 6.49 | 638.9 ± 0.67 | 13,283.48 ± 29.52 |
| B2 | Octanal | 14.64 | 124-13-0 | 1704.94 ± 3.87 | n.d. | n.d. | 292.9 ± 0.50 | 2078.68 ± 0.10 |
| B3 | Nonanal | 19.80 | 124-19-6 | 957.55 ± 3.51 | 1293.8 ± 1.52 | 356.3 ± 1.52 | 147.39 ± 0.56 | 1371.78 ± 1.37 |
| B4 | Benzaldehyde | 12.50 | 100-52-7 | 669.11 ± 2.21 | 776.27 ± 3.15 | 539.02 ± 1.37 | 157.42 ± 0.56 | 1077.55 ± 1.89 |
| B5 | 2-Heptenal, (E)- | 12.34 | 18829-55-5 | 411.76 ± 2.6 | n.d. | n.d. | n.d. | n.d. |
| B6 | 2-Octenal, (E)- | 17.38 | 2548-87-0 | 286.3 ± 2.79 | 238.98 ± 2.91 | 133.38 ± 1.71 | n.d. | n.d. |
| B7 | Phenylacetaldehyde | 16.59 | 122-78-1 | 41.54 ± 3.15 | 271.94 ± 4.31 | 60.79 ± 1.78 | 257.73 ± 0.51 | 133.73 ± 3.58 |
| B8 | 2-Nonenal, (E)- | 22.70 | 18829-56-6 | 196.51 ± 2.92 | n.d. | 10.13 ± 1.82 | n.d. | 80.81 ± 3.68 |
| B9 | 2-Decenal, (E)- | 30.65 | 3913-81-3 | 61.12 ± 3.12 | 365.88 ± 4.1 | 94.4 ± 0.99 | n.d. | 149.02 ± 3.55 |
| B10 | 2,4-Nonadienal, (E,E)- | 26.46 | 5910-87-2 | 24.66 ± 1.83 | n.d. | n.d. | n.d. | 80.24 ± 3.68 |
| B11 | 2-Dodecenal, (E)- | 37.67 | 20407-84-5 | 18.23 ± 1.83 | n.d. | n.d. | 1.67 ± 0.36 | n.d. |
| B12 | 2,4-Decadienal, (E,E)- | 35.10 | 25152-84-5 | 13.94 ± 1.86 | n.d. | n.d. | n.d. | 99.34 ± 3.64 |
| B13 | Decanal | 25.85 | 112-31-2 | 11.79 ± 1.84 | 161.52 ± 2.88 | n.d. | 2.93 ± 0.36 | 22.92 ± 3.78 |
| B14 | Tridecanal | 22.23 | 10486-19-8 | n.d. | 286.77 ± 4.28 | n.d. | n.d. | n.d. |
| B15 | Benzeneacetaldehyde, .alpha.-ethylidene- | 31.05 | 4411-89-6 | n.d. | 227.44 ± 4.42 | 149.83 ± 1.70 | n.d. | n.d. |
| B16 | Butanal, 3-methyl- | 3.36 | 590-86-3 | n.d. | n.d. | 1355.17 ± 1.67 | 505.52 ± 0.41 | 1180.72 ± 1.71 |
| B17 | Heptanal | 9.98 | 111-71-7 | n.d. | n.d. | 612.11 ± 1.30 | 90.94 ± 0.59 | 1409.99 ± 1.30 |
| B18 | cis-4-Decenal | 24.94 | 21662-9-9 | n.d. | n.d. | n.d. | n.d. | 103.17 ± 3.64 |
| Alkanes | ||||||||
| C1 | Heptane, 2,4-dimethyl- | 3.89 | 2213-23-2 | 2940.23 ± 14.08 | n.d. | n.d. | n.d. | n.d. |
| C2 | Cyclopentane, butyl- | 11.31 | 2040-95-1 | 128.67 ± 3.02 | 405.44 ± 4.01 | n.d. | n.d. | n.d. |
| C3 | Dodecane | 25.54 | 112-40-3 | 96.5 ± 3.07 | 355.99 ± 4.12 | 115.11 ± 1.73 | 5.85 ± 0.36 | 84.06 ± 3.67 |
| C4 | Pentadecane | 23.48 | 629-62-9 | 20.37 ± 1.86 | n.d. | 91.36 ± 1.75 | n.d. | n.d. |
| C5 | Tetradecane | 39.64 | 629-59-4 | 13.94 ± 1.86 | 174.7 ± 2.51 | 169.93 ± 1.68 | 10.03 ± 0.62 | 31.84 ± 2.16 |
| C6 | Heptadecane | 43.86 | 629-78-7 | 8.58 ± 1.85 | 192.83 ± 4.50 | 69.43 ± 1.77 | n.d. | n.d. |
| C7 | Hexane, 2,4-dimethyl- | 6.18 | 589-43-5 | n.d. | 4748.34 ± 18.47 | n.d. | n.d. | n.d. |
| C8 | Tridecane | 34.18 | 629-50-5 | n.d. | 257.1 ± 4.35 | 155.31 ± 1.69 | 4.6 ± 0.36 | n.d. |
| C9 | Heptane, 2,2,4,6,6-pentamethyl- | 13.99 | 13475-82-6 | n.d. | n.d. | 518.92 ± 1.38 | 723.78 ± 0.31 | n.d. |
| C10 | Nonane, 2,2,4,4,6,8,8-heptamethyl- | 15.84 | 4390-4-9 | n.d. | n.d. | 126.08 ± 1.72 | n.d. | n.d. |
| C11 | Hexadecane | 46.79 | 544-76-3 | n.d. | n.d. | 91.36 ± 1.75 | 7.11 ± 0.36 | n.d. |
| C12 | Dodecane, 2,6,10-trimethyl- | 38.50 | 3891-98-3 | n.d. | n.d. | 68.22 ± 1.00 | 2.09 ± 0.36 | 49.67 ± 3.73 |
| C13 | Heneicosane | 46.78 | 629-94-7 | n.d. | n.d. | n.d. | 8.15 ± 0.62 | 106.99 ± 3.63 |
| C14 | Octadecane | 43.84 | 593-45-3 | n.d. | n.d. | n.d. | 2.72 ± 0.36 | 72.6 ± 3.69 |
| Ketones | ||||||||
| D1 | 2-Heptanone | 9.37 | 110-43-0 | 1291.03 ± 3.03 | 979 ± 2.68 | n.d. | n.d. | 202.51 ± 3.46 |
| D2 | 2-Nonanone | 19.09 | 821-55-6 | 975.78 ± 3.48 | 1166.89 ± 2.24 | n.d. | 21.12 ± 0.35 | n.d. |
| D3 | 3-Octanone | 13.75 | 106-68-3 | 839.6 ± 1.95 | n.d. | n.d. | n.d. | n.d. |
| D4 | 2(3H)-Furanone, 5-ethyldihydro- | 16.94 | 695-6-7 | 79.87 ± 3.10 | n.d. | 21.09 ± 1.81 | 135.47 ± 0.57 | n.d. |
| D5 | 2-Hexanone | 5.83 | 591-78-6 | 270.21 ± 2.81 | 266.99 ± 4.33 | n.d. | n.d. | n.d. |
| D6 | Dihydro-5-pentyl-2(3H)-furanone | 37.37 | 104-61-0 | 70.77 ± 3.11 | 326.33 ± 4.19 | 96.84 ± 1.74 | 13.59 ± 0.36 | 133.73 ± 3.58 |
| D7 | 2-Decanone | 24.81 | 693-54-9 | 50.4 ± 1.79 | 405.44 ± 4.01 | n.d. | n.d. | n.d. |
| D8 | 5-Butyldihydro-2(3H)-furanone | 29.71 | 104-50-7 | 47.18 ± 1.80 | 375.77 ± 4.07 | 102.32 ± 1.74 | 12.96 ± 0.36 | 30.56 ± 3.77 |
| D9 | 2(3H)-Furanone, dihydro-5-propyl- | 22.04 | 105-21-5 | 34.31 ± 1.81 | n.d. | 11.56 ± 1.82 | 15.02 ± 0.62 | n.d. |
| D10 | 2-Tetradecanone | 18.63 | 2345-27-9 | n.d. | 598.27 ± 3.56 | n.d. | n.d. | n.d. |
| D11 | Acetophenone | 17.71 | 98-86-2 | n.d. | 187.88 ± 4.51 | 70.65 ± 1.00 | n.d. | n.d. |
| D12 | Amyl cyclopentenone | 21.49 | 4819-67-4 | n.d. | 184.59 ± 2.49 | n.d. | n.d. | n.d. |
| D13 | 3,5-Octadien-2-one | 17.97 | 38284-27-4 | n.d. | n.d. | 184.55 ± 1.67 | n.d. | n.d. |
| D14 | 3,5-Octadien-2-one, (E,E)- | 19.18 | 30086-02-3 | n.d. | n.d. | 158.97 ± 1.69 | n.d. | 412.68 ± 3.08 |
| D15 | 3-Octen-2-one | 16.37 | 1669-44-9 | n.d. | n.d. | n.d. | n.d. | 431.78 ± 3.05 |
| Esters | ||||||||
| E1 | Octanoic acid, ethyl ester | 25.17 | 106-32-1 | n.d. | 499.38 ± 3.79 | 116.94 ± 1.73 | 3.34 ± 0.36 | 82.79 ± 2.08 |
| E2 | Butanoic acid, 3-methyl-, ethyl ester | 8.00 | 108-64-5 | n.d. | 370.83 ± 4.09 | 363.61 ± 1.52 | n.d. | n.d. |
| E3 | Heptanoic acid, ethyl ester | 19.47 | 106-30-9 | n.d. | 234.04 ± 2.90 | 71.26 ± 1.77 | n.d. | 76.42 ± 3.68 |
| E4 | Nonanoic acid, ethyl ester | 33.77 | 123-29-5 | n.d. | 217.55 ± 4.44 | n.d. | n.d. | n.d. |
| E5 | Acetic acid, hexyl ester | 15.12 | 142-92-7 | n.d. | 174.7 ± 2.51 | n.d. | n.d. | n.d. |
| E6 | Decanoic acid, ethyl ester | 39.36 | 110-38-3 | n.d. | 159.87 ± 2.54 | 126.08 ± 1.72 | n.d. | 58.59 ± 2.11 |
| E7 | Hexanoic acid, ethyl ester | 14.43 | 123-66-0 | n.d. | n.d. | 1207.78 ± 0.79 | n.d. | n.d. |
| Acids | ||||||||
| F1 | Hexanoic acid | 15.35 | 142-62-1 | n.d. | n.d. | n.d. | 311.09 ± 0.49 | 982.02 ± 2.06 |
| F2 | Acetic acid | 2.67 | 64-19-7 | n.d. | n.d. | n.d. | n.d. | 371.92 ± 1.66 |
| Terpenes | ||||||||
| G1 | Naphthalene, 1,2,4a,5,8,8a-hexahydro-4,7-dimethyl-1-(1-methylethyl)-,[1S-(1.alpha.,4a.beta.,8a.alpha.)]- | 44.45 | 523-47-7 | n.d. | n.d. | 69.43 ± 0.06 | 2.72 ± 0.36 | n.d. |
| G2 | D-Limonene | 15.94 | 5989-27-5 | n.d. | n.d. | n.d. | 137.35 ± 0.57 | 393.57 ± 3.12 |
| G3 | Naphthalene, 1,2,3,5,6,8a-hexahydro-4,7-dimethyl-1-(1-methylethyl)-, (1S-cis)- | 44.43 | 483-76-1 | n.d. | n.d. | n.d. | n.d. | 76.42 ± 3.68 |
| Others | ||||||||
| H1 | 2,6-Dimethylpyrazine | 10.44 | 108-50-9 | 160.84 ± 2.97 | 583.44 ± 3.59 | 484.21 ± 1.41 | 87.81 ± 0.59 | 197.42 ± 1.91 |
| H2 | Furan, 2-pentyl- | 14.04 | 3777-69-3 | n.d. | 8534.15 ± 24.7 | n.d. | n.d. | n.d. |
| H3 | Pyrazine, tetramethyl- | 18.84 | 1124-11-4 | n.d. | n.d. | 87.7 ± 1.75 | n.d. | n.d. |
| H4 | Dimethyl trisulfide | 12.83 | 3658-80-8 | n.d. | n.d. | 75.52 ± 1.00 | n.d. | n.d. |
| No. | Quality | BHCH a | SXZH b | FXZH c | LTWH d | FZWH e | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DF f | OI g | HA h | DF | OI | HA | DF | OI | HA | DF | OI | HA | DF | OI | HA | ||
| A1 | Woody/Resinous | 10 | S i | 4.60 ± 0.52 | 10 | S | 5.20 ± 0.42 | |||||||||
| A2 | Pungent | 10 | S | 1.80 ± 0.42 | 10 | S | 2.50 ± 0.53 | 10 | S | 1.50 ± 0.53 | ||||||
| A3 | Woody/Resinous | 9 | M j | 4.67 ± 0.50 | 9 | M | 4.22 ± 0.67 | 7 | M | 5.86 ± 0.38 | ||||||
| A5 | Woody/Resinous, pungent, chemical | 7 | M | 5.43 ± 0.53 | 9 | M | 4.67 ± 0.50 | 10 | W k | 3.50 ± 0.53 | 8 | W | 3.25 ± 0.71 | |||
| A7 | Popcorn | 5 | W | 6.60 ± 0.55 | ||||||||||||
| A8 | Chemical | 7 | M | 3.71 ± 0.49 | 4 | M | 4.75 ± 0.50 | 4 | M | 3.50 ± 0.58 | ||||||
| A10 | Woody/Resinous, fruit, other than citrus | 10 | S | 5.70 ± 0.48 | ||||||||||||
| B1 | Lemon | 7 | W | 5.71 ± 0.76 | ||||||||||||
| B2 | Woody/Resinous, minty, chemical | 9 | M | 5.67 ± 0.71 | 8 | W | 6.63 ± 0.52 | 10 | M | 4.70 ± 0.48 | ||||||
| B3 | Sweet, popcorn | 10 | S | 7.30 ± 0.48 | 10 | S | 7.60 ± 0.52 | 10 | S | 7.30 ± 0.48 | 10 | S | 6.30 ± 0.48 | 10 | S | 6.20 ± 0.42 |
| B4 | Sweet, popcorn | 9 | W | 4.56 ± 0.53 | ||||||||||||
| B7 | Woody/Resinous | 10 | M | 3.30 ± 0.48 | 9 | M | 2.67 ± 0.50 | 7 | W | 3.43 ± 0.53 | ||||||
| B8 | Woody/Resinous | 9 | M | 4.89 ± 0.60 | ||||||||||||
| B14 | Woody/Resinous | 8 | M | 5.38 ± 0.52 | ||||||||||||
| B16 | Woody/Resinous, chemical | 9 | M | 4.22 ± 0.44 | 10 | W | 3.70 ± 0.48 | 7 | W | 4.57 ± 0.53 | ||||||
| B17 | Woody/Resinous, popcorn | 10 | M | 5.30 ± 0.48 | 10 | M | 4.40 ± 0.70 | 10 | S | 5.60 ± 0.52 | ||||||
| C4 | Woody/Resinous | 10 | M | 4.70 ± 0.48 | ||||||||||||
| D2 | Woody/Resinous | 9 | M | 4.44 ± 0.53 | 10 | S | 3.60 ± 0.52 | 10 | M | 3.70 ± 0.48 | ||||||
| D3 | Minty | 10 | W | 6.50 ± 0.53 | ||||||||||||
| D4 | Woody/Resinous, sweet | 10 | W | 7.60 ± 0.52 | ||||||||||||
| D6 | Sweet | 10 | W | 7.40 ± 0.52 | 10 | M | 8.90 ± 0.57 | 9 | W | 7.44 ± 0.53 | 9 | W | 7.44 ± 0.53 | |||
| D8 | Sweet, popcorn, lemon | 10 | W | 8.20 ± 0.42 | 10 | M | 8.00 ± 0.47 | 9 | W | 6.22 ± 0.44 | 10 | W | 8.50 ± 0.53 | 10 | W | 6.60 ± 0.52 |
| D9 | Sweet, popcorn | 10 | M | 5.70 ± 0.48 | 9 | W | 8.33 ± 0.50 | |||||||||
| D10 | Woody/Resinous | 10 | M | 5.10 ± 0.57 | ||||||||||||
| H1 | Popcorn | 9 | M | 5.56 ± 0.53 | 10 | S | 5.10 ± 0.32 | 10 | S | 5.60 ± 0.52 | 10 | M | 5.00 ± 0.67 | 9 | M | 5.44 ± 0.53 |
| H2 | Lemon, minty | 10 | M | 7.60 ± 0.52 | ||||||||||||
| E1 | Woody/Resinous | 9 | M | 5.56 ± 0.53 | 7 | W | 5.43 ± 0.53 | |||||||||
| E2 | Sickening, pungent | 10 | M | 1.60 ± 0.52 | 10 | M | 1.60 ± 0.52 | |||||||||
| E7 | Fruity, other than citrus | 6 | W | 4.50 ± 0.55 | ||||||||||||
| UC1 l | Minty | 7 | W | 6.57 ± 0.53 | ||||||||||||
| UC2 | Popcorn | 8 | M | 5.63 ± 0.52 | ||||||||||||
| UC3 | Woody/Resinous, chemical | 10 | M | 4.60 ± 0.52 | ||||||||||||
| UC4 | Floral, woody/resinous | 8 | M | 5.75 ± 0.46 | ||||||||||||
| UC5 | Woody/Resinous, chemical | 10 | S | 3.40 ± 0.52 | ||||||||||||
| UC6 | Woody/Resinous | 9 | M | 4.33 ± 0.50 | 9 | M | 5.11 ± 0.33 | |||||||||
| UC7 | Minty, pungent | 10 | M | 3.30 ± 0.48 | ||||||||||||
| UC8 | Pungent | 10 | S | 1.50 ± 0.53 | ||||||||||||
| UC9 | Woody/Resinous | 10 | S | 3.80 ± 0.42 | ||||||||||||
| UC10 | Sweet | 10 | S | 8.90 ± 0.57 | ||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, D.; Gu, Y. Characterization of Aroma-Active Compounds in Five Dry-Cured Hams Based on Electronic Nose and GC-MS-Olfactometry Combined with Odor Description, Intensity, and Hedonic Assessment. Foods 2025, 14, 2305. https://doi.org/10.3390/foods14132305
Yu D, Gu Y. Characterization of Aroma-Active Compounds in Five Dry-Cured Hams Based on Electronic Nose and GC-MS-Olfactometry Combined with Odor Description, Intensity, and Hedonic Assessment. Foods. 2025; 14(13):2305. https://doi.org/10.3390/foods14132305
Chicago/Turabian StyleYu, Dongbing, and Yu Gu. 2025. "Characterization of Aroma-Active Compounds in Five Dry-Cured Hams Based on Electronic Nose and GC-MS-Olfactometry Combined with Odor Description, Intensity, and Hedonic Assessment" Foods 14, no. 13: 2305. https://doi.org/10.3390/foods14132305
APA StyleYu, D., & Gu, Y. (2025). Characterization of Aroma-Active Compounds in Five Dry-Cured Hams Based on Electronic Nose and GC-MS-Olfactometry Combined with Odor Description, Intensity, and Hedonic Assessment. Foods, 14(13), 2305. https://doi.org/10.3390/foods14132305




