Multi-Mycotoxin Analyses by UPLC-MS/MS in Wheat: The Situation in Belgium in 2023 and 2024
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Collection
2.3. Meteorological Considerations
2.4. Sample Preparation
2.5. Standard Solutions and Calibration
2.6. UPLC-MS/MS Conditions
2.7. Data Treatment
3. Results
3.1. Mycotoxin Occurrence in Samples Collected in 2023 and 2024
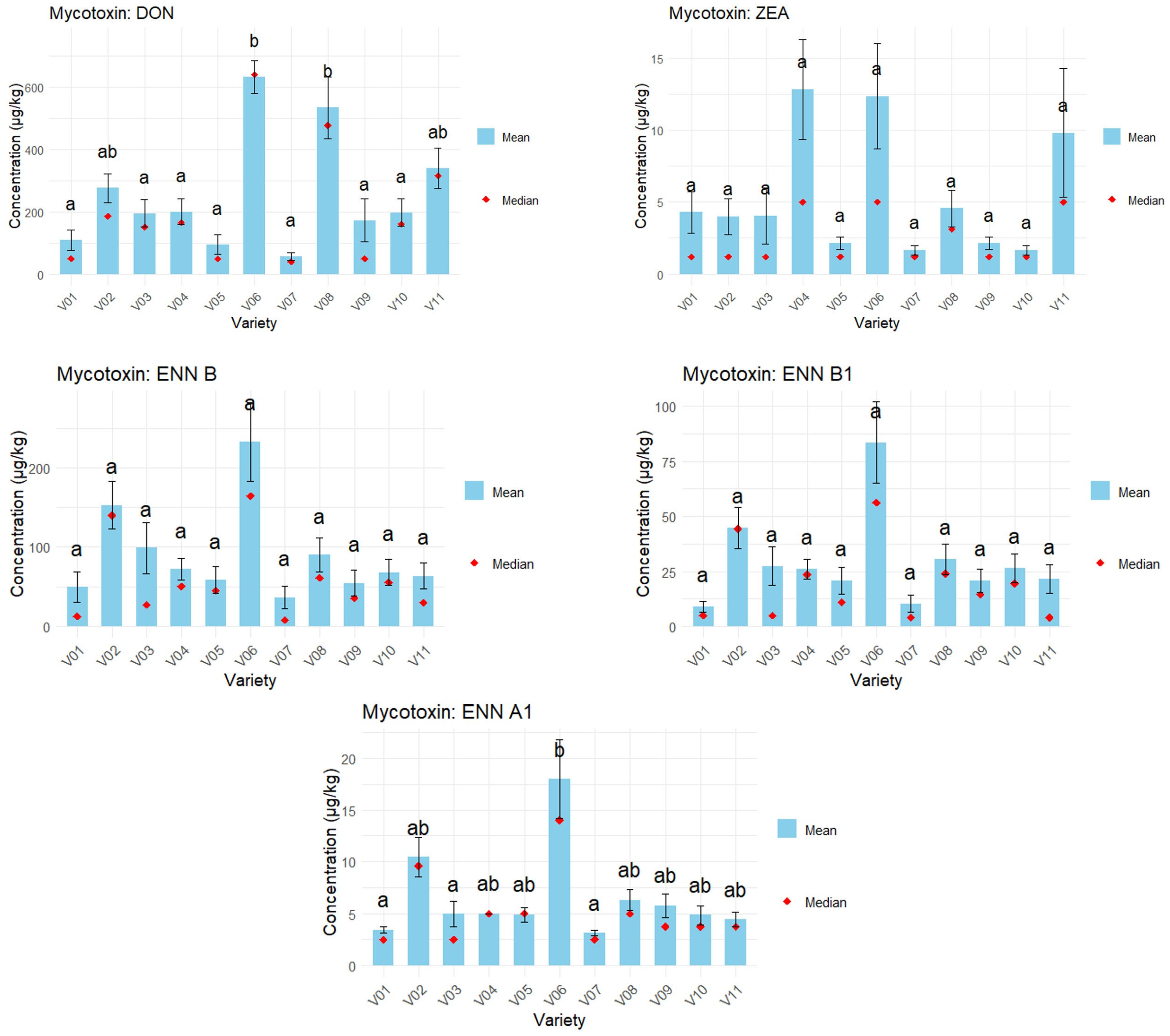
3.2. Differences in Concentration Between the Varieties in 2024 Wheat Samples
3.3. Differences in Mycotoxin Concentration Between the Cultivation Sites in 2024 Wheat Samples
4. Discussion
4.1. Effect of the Year of Harvest of Wheat on the Mycotoxin Contamination
4.2. Differences in Mycotoxin Concentration Between the Wheat Varieties
4.3. Differences in Mycotoxin Concentration Between the Wheat Cultivation Sites
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 15Ac-DON | 15-acetyldeoxynivalenol |
| 3Ac-DON | 3-acetyldeoxynivalenol |
| AF | aflatoxin |
| AFB1 | aflatoxin B1 |
| AME | alternariol-monomethyl-ether |
| AOH | alternariol |
| BEA | beauvericin |
| df | degree of freedom |
| DON | deoxynivalenol |
| EFSA | European Food Safety Authority |
| ENN | enniatin |
| FAO | Food and Agriculture Organization |
| FUM | fumonisin |
| HT2 | HT2 toxin |
| LC | liquid chromatography |
| LOD | limit of detection |
| LOQ | limit of quantification |
| ML | maximum level |
| MRM | Multiple Reaction Monitoring |
| ND | non-detected |
| OTA | ochratoxin A |
| OTB | ochratoxin B |
| S/N | signal-to-noise ratio |
| STE | sterygmatocystin |
| T2 | T2 toxin |
| UPLC-MS/MS | Ultra Performance Liquid Chromatography coupled with tandem mass spectrometry |
| ZEA | zearalenone |
References
- WHO (World Health Organization). Mycotoxins. Available online: https://www.who.int/news-room/fact-sheets/detail/mycotoxins (accessed on 19 May 2025).
- Tola, M.; Kebede, B. Occurrence, Importance and Control of Mycotoxins: A Review. Cogent Food Agric. 2016, 2, 1191103. [Google Scholar] [CrossRef]
- Smith, M.-C.; Madec, S.; Coton, E.; Hymery, N. Natural Co-Occurrence of Mycotoxins in Foods and Feeds and Their In Vitro Combined Toxicological Effects. Toxins 2016, 8, 94. [Google Scholar] [CrossRef]
- Pereira, V.L.; Fernandes, J.O.; Cunha, S.C. Mycotoxins in Cereals and Related Foodstuffs: A Review on Occurrence and Recent Methods of Analysis. Trends Food Sci. Technol. 2014, 36, 96–136. [Google Scholar] [CrossRef]
- Kolawole, O.; Siri-Anusornsak, W.; Petchkongkaew, A.; Elliott, C. A Systematic Review of Global Occurrence of Emerging Mycotoxins in Crops and Animal Feeds, and Their Toxicity in Livestock. Emerg. Contam. 2024, 10, 100305. [Google Scholar] [CrossRef]
- De Felice, B.; Spicer, L.J.; Caloni, F. Enniatin B1: Emerging Mycotoxin and Emerging Issues. Toxins 2023, 15, 383. [Google Scholar] [CrossRef] [PubMed]
- Ekwomadu, T.I.; Akinola, S.A.; Mwanza, M. Fusarium Mycotoxins, Their Metabolites (Free, Emerging, and Masked), Food Safety Concerns, and Health Impacts. Int. J. Environ. Res. Public Health 2021, 18, 11741. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food; Schrenk, D.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.-C.; Nebbia, C.S. Risk Assessment of Ochratoxin a in Food. EFSA J. 2020, 18, e06113. [Google Scholar] [CrossRef] [PubMed]
- IARC. Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins; International Agency for Research on Cancer, Ed.; This Publication Represents the Views and Expert Opinions of an IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Which Met in Lyon, 9–16 June 1992; IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans; IARC: Lyon, France, 1993; ISBN 978-92-832-1256-0. [Google Scholar]
- Zain, M.E. Impact of Mycotoxins on Humans and Animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Novak, B.; Nagl, V.; Berthiller, F. Emerging Mycotoxins: Beyond Traditionally Determined Food Contaminants. J. Agric. Food Chem. 2017, 65, 7052–7070. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food. Scientific Opinion on the Risks to Human and Animal Health Related to the Presence of Beauvericin and Enniatins in Food and Feed. EFSA J. 2014, 12, 3802. [Google Scholar] [CrossRef]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, Toxicology, and Exposure Assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef]
- Pleadin, J.; Frece, J.; Markov, K. Mycotoxins in Food and Feed. Adv. Food Nutr. Res. 2019, 89, 297–345. [Google Scholar] [CrossRef] [PubMed]
- Mousavi Khaneghah, A.; Fakhri, Y.; Gahruie, H.H.; Niakousari, M.; Sant’Ana, A.S. Mycotoxins in Cereal-Based Products During 24 Years (1983–2017): A Global Systematic Review. Trends Food Sci. Technol. 2019, 91, 95–105. [Google Scholar] [CrossRef]
- Ji, F.; He, D.; Olaniran, A.O.; Mokoena, M.P.; Xu, J.; Shi, J. Occurrence, Toxicity, Production and Detection of Fusarium Mycotoxin: A Review. Food Prod. Process. Nutr. 2019, 1, 6. [Google Scholar] [CrossRef]
- Bullerman, L.B.; Bianchini, A. Stability of Mycotoxins During Food Processing. Int. J. Food Microbiol. 2007, 119, 140–146. [Google Scholar] [CrossRef]
- Sun, L.-H.; Lei, M.; Zhang, N.-Y.; Zhao, L.; Krumm, C.S.; Qi, D.-S. Hepatotoxic Effects of Mycotoxin Combinations in Mice. Food Chem. Toxicol. 2014, 74, 289–293. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006; European Commission: Brussels, Belgium, 2023; Volume L119, pp. 103–157. [Google Scholar]
- European Commission. Commission Regulation (EU) 2024/1022 of 8 April 2024 Amending Regulation (EU) 2023/915 as Regards Maximum Levels of Deoxynivalenol in Food; European Commission: Brussels, Belgium, 2024; Volume 9.4.2024, pp. 1–4. [Google Scholar]
- European Commission. Commission Regulation (EU) 2024/1038 of 9 April 2024 Amending Regulation (EU) 2023/915 as Regards Maximum Levels of T-2 and HT-2 Toxins in Food; European Commission: Brussels, Belgium, 2024; Volume 10.4.2024, pp. 1–5. [Google Scholar]
- European Commission. Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on Undesirable Substances in Animal Feed—Council Statement; European Commission: Brussels, Belgium, 2002; Volume L140, pp. 10–21. [Google Scholar]
- Mahato, D.K.; Devi, S.; Pandhi, S.; Sharma, B.; Maurya, K.K.; Mishra, S.; Dhawan, K.; Selvakumar, R.; Kamle, M.; Mishra, A.K.; et al. Occurrence, Impact on Agriculture, Human Health, and Management Strategies of Zearalenone in Food and Feed: A Review. Toxins 2021, 13, 92. [Google Scholar] [CrossRef]
- Khodaei, D.; Javanmardi, F.; Khaneghah, A.M. The Global Overview of the Occurrence of Mycotoxins in Cereals: A Three-Year Survey. Curr. Opin. Food Sci. 2021, 39, 36–42. [Google Scholar] [CrossRef]
- Marroquín-Cardona, A.G.; Johnson, N.M.; Phillips, T.D.; Hayes, A.W. Mycotoxins in a Changing Global Environment—A Review. Food Chem. Toxicol. 2014, 69, 220–230. [Google Scholar] [CrossRef]
- Moraes, W.B.; Madden, L.V.; Gillespie, J.; Paul, P.A. Environment, Grain Development, and Harvesting Strategy Effects on Zearalenone Contamination of Grain from Fusarium Head Blight-Affected Wheat Spikes. Phytopathology 2023, 113, 225–238. [Google Scholar] [CrossRef]
- Platzer, A.; Cherkaoui, Y.; Novak, B.; Schatzmayr, G. Investigating the Correlations Between Weather Factors and Mycotoxin Contamination in Corn: Evidence from Long-Term Data. Toxins 2025, 17, 77. [Google Scholar] [CrossRef]
- Erenstein, O.; Jaleta, M.; Mottaleb, K.A.; Sonder, K.; Donovan, J.; Braun, H.-J. Global Trends in Wheat Production, Consumption and Trade. In Wheat Improvement: Food Security in a Changing Climate; Reynolds, M.P., Braun, H.-J., Eds.; Springer International Publishing: Cham, Germany, 2022; pp. 47–66. ISBN 978-3-030-90673-3. [Google Scholar]
- Sarmast, E.; Fallah, A.A.; Jafari, T.; Mousavi Khaneghah, A. Occurrence and Fate of Mycotoxins in Cereals and Cereal-Based Products: A Narrative Review of Systematic Reviews and Meta-Analyses Studies. Curr. Opin. Food Sci. 2021, 39, 68–75. [Google Scholar] [CrossRef]
- FAO Cereal Supply and Demand Brief | Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/worldfoodsituation/csdb/en/ (accessed on 19 June 2024).
- Delcour, A.; Stilmant, D.; Burny, P.; Rabier, F.; Louppe, H.; Goffart, J.-P. État des lieux des flux céréaliers en Wallonie selon différentes filières d’utilisation. Biotechnol. Agron. Soc. Environ. 2014, 18, 181–192. [Google Scholar]
- Alshannaq, A.; Yu, J.-H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.I.; Miller, J.D. A Concise History of Mycotoxin Research. J. Agric. Food Chem. 2017, 65, 7021–7033. [Google Scholar] [CrossRef] [PubMed]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide Contamination of Food-Crops with Mycotoxins: Validity of the Widely Cited ‘FAO Estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Wang, X.; Liu, C.; Van Der Fels-Klerx, H.J. Regional Prediction of Multi-Mycotoxin Contamination of Wheat in Europe Using Machine Learning. Food Res. Int. 2022, 159, 111588. [Google Scholar] [CrossRef]
- Casu, A.; Camardo Leggieri, M.; Toscano, P.; Battilani, P. Changing Climate, Shifting Mycotoxins: A Comprehensive Review of Climate Change Impact on Mycotoxin Contamination. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13323. [Google Scholar] [CrossRef]
- Vargas Medina, D.A.; Bassolli Borsatto, J.V.; Maciel, E.V.S.; Lanças, F.M. Current Role of Modern Chromatography and Mass Spectrometry in the Analysis of Mycotoxins in Food. TrAC Trends Anal. Chem. 2021, 135, 116156. [Google Scholar] [CrossRef]
- Turner, N.W.; Bramhmbhatt, H.; Szabo-Vezse, M.; Poma, A.; Coker, R.; Piletsky, S.A. Analytical Methods for Determination of Mycotoxins: An Update (2009–2014). Anal. Chim. Acta 2015, 901, 12–33. [Google Scholar] [CrossRef]
- De Frutos Cachorro, J.; Gobin, A.; Buysse, J. Farm-Level Adaptation to Climate Change: The Case of the Loam Region in Belgium. Agric. Syst. 2018, 165, 164–176. [Google Scholar] [CrossRef]
- Zhang, J.-M.; Wu, Y.-L.; Lu, Y.-B. Simultaneous Determination of Carbamate Insecticides and Mycotoxins in Cereals by Reversed Phase Liquid Chromatography Tandem Mass Spectrometry Using a Quick, Easy, Cheap, Effective, Rugged and Safe Extraction Procedure. J. Chromatogr. B 2013, 915–916, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, W.; Zhang, Y.; Hu, X.; Wu, L.; Wang, B. QuEChERS Purification Combined with Ultrahigh-Performance Liquid Chromatography Tandem Mass Spectrometry for Simultaneous Quantification of 25 Mycotoxins in Cereals. Toxins 2016, 8, 375. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, L.; Stramenga, A.; Visciano, P.; Schirone, M.; De Colli, L.; Colagrande, M.N.; Campana, G.; Scortichini, G. Simultaneous Determination of Aflatoxins, T-2 and HT-2 Toxins, and Fumonisins in Cereal-Derived Products by QuEChERS Extraction Coupled with LC-MS/MS. Anal. Bioanal. Chem. 2017, 409, 5143–5155. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzo, M.; Gambacorta, L.; Bibi, R.; Ciriaci, M.; Paoloni, A.; Pecorelli, I. Multimycotoxin Analysis by LC-MS/MS in Cereal Food and Feed: Comparison of Different Approaches for Extraction, Purification, and Calibration. J. AOAC Int. 2018, 101, 647–657. [Google Scholar] [CrossRef]
- González-Jartín, J.M.; Rodríguez-Cañás, I.; Alfonso, A.; Sainz, M.J.; Vieytes, M.R.; Gomes, A.; Ramos, I.; Botana, L.M. Multianalyte Method for the Determination of Regulated, Emerging and Modified Mycotoxins in Milk: QuEChERS Extraction Followed by UHPLC–MS/MS Analysis. Food Chem. 2021, 356, 129647. [Google Scholar] [CrossRef]
- Perrone, G.; Ferrara, M.; Medina, A.; Pascale, M.; Magan, N. Toxigenic Fungi and Mycotoxins in a Climate Change Scenario: Ecology, Genomics, Distribution, Prediction and Prevention of the Risk. Microorganisms 2020, 8, 1496. [Google Scholar] [CrossRef]
- Cargill. Cargill World Mycotoxin Report. 2024. Available online: https://mycotoxins.com/assets/content/knowledgecenter/survey/2024%20Cargill%20World%20Mycotoxin%20Report.pdf (accessed on 18 May 2025).
- DSM-Firmenich. World Mycotoxin Survey: The Global Threat–January–December 2024. 2024. Available online: https://www.dsm-firmenich.com/content/dam/dsm/anh/en/documents/REP_MTXsurvey_Q4_0525_63898.pdf (accessed on 18 May 2025).
- Medina, Á.; Rodríguez, A.; Magan, N. Climate Change and Mycotoxigenic Fungi: Impacts on Mycotoxin Production. Curr. Opin. Food Sci. 2015, 5, 99–104. [Google Scholar] [CrossRef]
- Liu, C.; Van der Fels-Klerx, H.J. Quantitative Modeling of Climate Change Impacts on Mycotoxins in Cereals: A Review. Toxins 2021, 13, 276. [Google Scholar] [CrossRef]
- Medina, A. Chapter 22—Emerging Mycotoxin Risks Due to Climate Change. What to Expect in the Coming Decade? In Present Knowledge in Food Safety; Knowles, M.E., Anelich, L.E., Boobis, A.R., Popping, B., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 309–314. ISBN 978-0-12-819470-6. [Google Scholar]
- Michel, S.; Steiner, B.; Buerstmayr, H. Efficiency of Indirect Selection for Fusarium Head Blight Resistance and Mycotoxin Accumulation in Winter Wheat (Triticum aestivum L.). Plant Breed. 2024, 143, 34–43. [Google Scholar] [CrossRef]
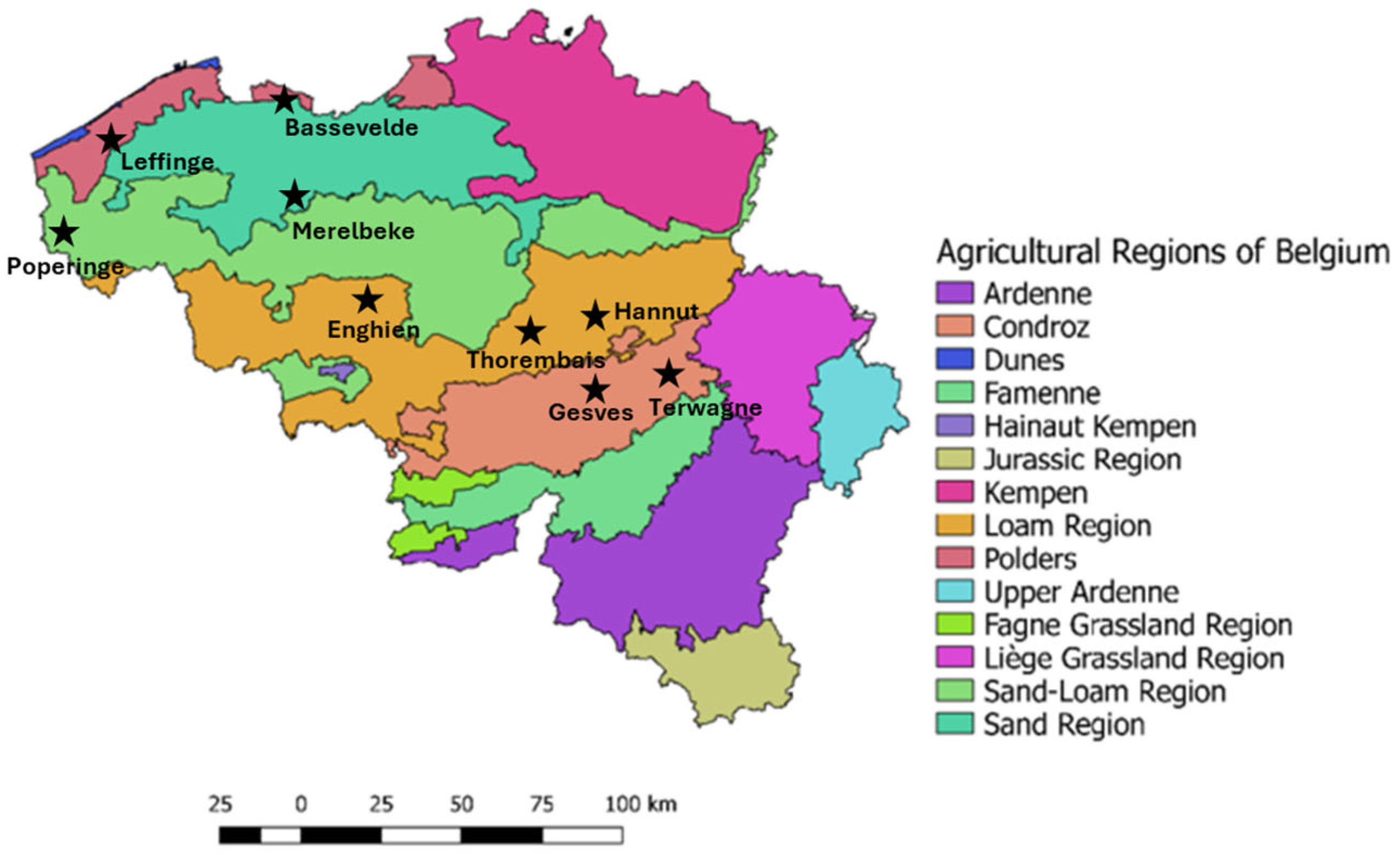



| (a) | ||||
| Harvest 2023 | ||||
| Sowing Date | Harvest Date | Previous Crop | Ploughing | |
| Gesves | 5 November 2022 | 10 August 2023 | Potatoes | No |
| Terwagne | 31 October 2022 | 14 August 2023 | Sugar beet | No |
| Hannut | 25 October 2022 | 13 August 2023 | Flax | No |
| Thorembais | 26 October 2022 | 11 August 2023 | Flax | Yes |
| Enghien | 16 October 2022 | 20 July 2023 | Flax | No |
| Merelbeke | 18 October 2022 | 18 July 2023 | Potatoes | Yes |
| Poperinge | 19 October 2022 | 18 July 2023 | Corn silage | Yes |
| Leffinge | 19 October 2022 | 26 July 2023 | Potatoes | Yes |
| Bassevelde | 19 October 2022 | 19 July 2023 | Grain corn | Yes |
| (b) | ||||
| Harvest 2024 | ||||
| Sowing Date | Harvest Date | Previous Crop | Ploughing | |
| Gesves | 18 December 2023 | 12 August 2024 | Potatoes | Yes |
| Terwagne | 17 October 2023 | 06 August 2024 | Rapeseed | No |
| Hannut | 18 December 2023 | 06 August 2024 | Sugar beet | No |
| Thorembais | 22 November 2023 | 30 July 2024 | Flax | Yes |
| Enghien | 1 December 2023 | 30 July 2024 | Canned peas | Yes |
| Merelbeke | 18 October 2023 | 29 July 2024 | Potatoes | Yes |
| Poperinge | 18 December 2023 | 30 July 2024 | Sugar beet | Yes |
| Leffinge | 18 October 2023 | 30 July 2024 | Potatoes | Yes |
| 1 [µg/kg] | 2 [µg/kg] | 3 [µg/kg] | |
|---|---|---|---|
| Standard 01 | 0.5 | 10 | 100 |
| Standard 02 | 1.0 | 20 | 200 |
| Standard 03 | 2.5 | 50 | 500 |
| Standard 04 | 5.0 | 100 | 1000 |
| Standard 05 | 7.5 | 150 | 1500 |
| Standard 06 | 10 | 200 | 2000 |
| Standard 07 | 15 | 300 | 3000 |
| Mycotoxin | Molecular Mass | MODE | m/z | Cone (V) | Daughter 1 | Energy1 (eV) | Daughter 2 | Energy2 (eV) | RT (min) |
|---|---|---|---|---|---|---|---|---|---|
| AME | 272.3 | [M+H]+ | 273.0 | 20 | 128.0 | 60 | 228.0 | 40 | 10.9 |
| AOH | 258.2 | [M+H]+ | 259.0 | 10 | 185.0 | 28 | 213.0 | 28 | 9.2 |
| DON | 296.3 | [M+H]+ | 297.1 | 15 | 231.1 | 18 | 249.1 | 18 | 3.9 |
| 3Ac-DON | 338.4 | [M+H]+ | 339.1 | 25 | 137.0 | 15 | 203.1 | 12 | 7.0 |
| 15Ac-DON | 338.4 | [M+NH4]+ | 356.2 | 5 | 137.0 | 16 | 261.0 | 14 | 7.0 |
| ZEA | 318.4 | [M+H]+ | 319.2 | 20 | 185.1 | 25 | 187.1 | 19 | 10.6 |
| T2 | 466.5 | [M+NH4]+ | 484.2 | 7 | 215.0 | 18 | 305.1 | 12 | 10.4 |
| HT2 | 424.5 | [M+NH4]+ | 442.2 | 5 | 215.0 | 12 | 263.1 | 10 | 9.8 |
| OTA | 403.8 | [M+H]+ | 404.1 | 25 | 239.1 | 25 | 357.9 | 15 | 10.5 |
| OTB | 369.4 | [M+H]+ | 370.0 | 10 | 187.0 | 30 | 204.8 | 20 | 9.7 |
| Enniatin A | 681.9 | [M+H]+ | 682.6 | 30 | 99.6 | 40 | 209.8 | 25 | 11.8 |
| Enniatin A1 | 667.9 | [M+H]+ | 669.1 | 30 | 99.6 | 40 | 210.0 | 25 | 11.7 |
| Enniatin B | 639.8 | [M+H]+ | 640.7 | 15 | 85.9 | 40 | 196.0 | 20 | 11.5 |
| Enniatin B1 | 653.8 | [M+H]+ | 655.2 | 20 | 85.9 | 40 | 196.1 | 25 | 11.6 |
| Fumonisin B1 | 721.8 | [M+H]+ | 722.3 | 15 | 334.3 | 35 | 352.3 | 35 | 10.0 |
| Sterigmatocystin | 324.28 | [M+H]+ | 325.0 | 15 | 253.1 | 38 | 281.1 | 35 | 10.8 |
| Mycotoxins | Recovery [%] | LOQ [µg/kg] | LOD [µg/kg] |
|---|---|---|---|
| 15Ac-DON | 49 | 10 | 3.8 |
| 3Ac-DON | 59 | 10 | 3.3 |
| DON | 88 | 100 | 60 |
| AOH | 90 | 10 | 8 |
| AME | 90 | 10 | 8 |
| T2 + HT2 | 84 | 10 | 0.3 |
| ZEA | 96 | 10 | 2.4 |
| OTA | 104 | 1 | 0.5 |
| OTB | 99 | 1 | 0.5 |
| ENN A | 91 | 10 | 8 |
| ENN A1 | 93 | 10 | 5 |
| ENN B | 93 | 10 | 1 |
| ENN B1 | 92 | 10 | 6 |
| FUM B1 | 58 | 10 | 3 |
| STE | 91 | 10 | 1.5 |
| Mycotoxin | ND (n) | <LOQ (n) | >LOQ (n) | Mean ± SD (µg/kg) | Min–Max of Samples > LOQ (µg/kg) | Median of Samples > LOQ (µg/kg) |
|---|---|---|---|---|---|---|
| DON | 39 | 1 | 5 | 63 ± 104 | 171–567 | 317 |
| ZEA | 40 | 0 | 5 | 2.7 ± 4.4 | 12–18 | 15 |
| ENN B | 38 | 5 | 2 | 4.5 ± 19.7 | 28–131 | 79 |
| ENN B1 | 44 | 1 | 0 | 3.0 ± 0.3 | All < LOQ | All < LOQ |
| Mycotoxin | ND (n) | <LOQ (n) | >LOQ (n) | Mean ± SD (µg/kg) | Min–Max of Samples > LOQ (µg/kg) | Median of Samples > LOQ (µg/kg) |
|---|---|---|---|---|---|---|
| DON | 11 | 25 | 52 | 255 ± 268 | 131–1478 | 370 |
| ZEA | 53 | 25 | 10 | 5.4 ± 9.5 | 16–55 | 28 |
| ENN A1 | 35 | 36 | 17 | 6.5 ± 7.0 | 10–49 | 14 |
| ENN B | 3 | 17 | 68 | 89 ± 109 | 12–488 | 66 |
| ENN B1 | 21 | 18 | 49 | 29 ± 37 | 7–185 | 38 |
| Mean Concentration ± SD (µg/kg) | Kruskal–Wallis Χ2 | df | p-Value | Significance | |
|---|---|---|---|---|---|
| DON | 255 ± 268 | 42.239 | 10 | 6.80 × 10−6 | *** |
| ZEA | 5.4 ± 9.5 | 23.644 | 10 | 0.008604 | ** |
| ENN A1 | 6.5 ± 7.0 | 24.693 | 10 | 0.005958 | ** |
| ENN B | 89 ± 109 | 16.969 | 10 | 0.07506 | n.s. |
| ENN B1 | 29 ± 37 | 19.086 | 10 | 0.03918 | * |
| Mean Concentration ± SD (µg/kg) | Kruskal–Wallis Χ2 | df | p-Value | Significance | |
|---|---|---|---|---|---|
| DON | 255 ± 268 | 15.108 | 7 | 0.03464 | * |
| ZEA | 5.4 ± 9.5 | 29.384 | 7 | 0.0001231 | *** |
| ENN A1 | 6.5 ± 7.0 | 25.715 | 7 | 0.0005662 | *** |
| ENN B | 89 ± 109 | 40.970 | 7 | 8.21 × 10−7 | *** |
| ENN B1 | 29 ± 37 | 34.123 | 7 | 1.63 × 10−5 | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jonard, C.; Chandelier, A.; Eylenbosch, D.; Pannecoucque, J.; Godin, B.; Douny, C.; Scippo, M.-L.; Gofflot, S. Multi-Mycotoxin Analyses by UPLC-MS/MS in Wheat: The Situation in Belgium in 2023 and 2024. Foods 2025, 14, 2300. https://doi.org/10.3390/foods14132300
Jonard C, Chandelier A, Eylenbosch D, Pannecoucque J, Godin B, Douny C, Scippo M-L, Gofflot S. Multi-Mycotoxin Analyses by UPLC-MS/MS in Wheat: The Situation in Belgium in 2023 and 2024. Foods. 2025; 14(13):2300. https://doi.org/10.3390/foods14132300
Chicago/Turabian StyleJonard, Camille, Anne Chandelier, Damien Eylenbosch, Joke Pannecoucque, Bruno Godin, Caroline Douny, Marie-Louise Scippo, and Sébastien Gofflot. 2025. "Multi-Mycotoxin Analyses by UPLC-MS/MS in Wheat: The Situation in Belgium in 2023 and 2024" Foods 14, no. 13: 2300. https://doi.org/10.3390/foods14132300
APA StyleJonard, C., Chandelier, A., Eylenbosch, D., Pannecoucque, J., Godin, B., Douny, C., Scippo, M.-L., & Gofflot, S. (2025). Multi-Mycotoxin Analyses by UPLC-MS/MS in Wheat: The Situation in Belgium in 2023 and 2024. Foods, 14(13), 2300. https://doi.org/10.3390/foods14132300






