Evolution and Evaluation of Ultra-Low Temperature Freezers: A Comprehensive Literature Review
Abstract
1. Introduction
2. History
2.1. History of Refrigeration
2.2. History of Refrigerants
3. Refrigerants
4. Importance and Application of ULT Freezers
4.1. Importance of ULT Freezers
4.2. Applications
5. Advanced ULT Freezer Technologies
5.1. Advantages and Challenges of ULT Freezers
5.2. Overview of ULT Freezers: Technologies, Configurations, and Energy Efficiency
5.3. Industrial Examples of Advanced ULT Freezer Technologies
6. Regulatory Compliance
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamenski, G.; Ayazseven, S.; Berndt, A.; Fink, W.; Kamenski, L.; Zehetmayer, S.; Pühringer, H. Clinical relevance of CYP2D6 polymorphisms in patients of an Austrian medical practice: A family practice-based observational study. Drugs Real World Outcomes 2020, 7, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Middelburg, R.A.; Borkent-Raven, B.A.; Janssen, M.P.; van de Watering, L.M.; Wiersum-Osselton, J.C.; Schipperus, M.R.; Beckers, E.A.; Briët, E.; van der Bom, J.G. Storage time of blood products and transfusion-related acute lung injury. Transfusion 2011, 52, 658–667. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, M.; Gehl, A.; Fricke, B.; Nawaz, K.; Gluesenkamp, K.; Shen, B.; Munk, J.; Hagerman, J.; Lapsa, M. COVID-19 vaccine distribution solution to the last mile challenge: Experimental and simulation studies of ultra-low temperature refrigeration system. Int. J. Refrig. 2022, 133, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Deng, B.; Wang, Y.; Liu, W.; Chen, G. Small, affordable, ultra-low-temperature vapor-compression and thermoelectric hybrid freezer for clinical applications. Cell Rep. Phys. Sci. 2023, 4, 101735. [Google Scholar] [CrossRef]
- Powell, S.; Molinolo, A.; Masmila, E.; Kaushal, S. Real-Time Temperature Mapping in Ultra-Low Freezers as a Standard Quality Assessment. Biopreserv. Biobank. 2019, 17, 139–142. [Google Scholar] [CrossRef]
- Ahamed, J.U.; Saidur, R.; Masjuki, H.H. A review on exergy analysis of vapor compression refrigeration system. Renew. Sustain. Energy Rev. 2011, 15, 1593–1600. [Google Scholar] [CrossRef]
- Barbosa, J.R.; Ribeiro, G.B.; de Oliveira, P.A. A State-of-the-Art Review of Compact Vapor Compression Refrigeration Systems and Their Applications. Heat Transf. Eng. 2011, 33, 356–374. [Google Scholar] [CrossRef]
- PHcbi. Choosing a Reliable ULT Freezer: What You Need to Know. Available online: https://www.phchd.com/us/biomedical/blog/choosing-a-reliable-ult-freezer-what-you-need-to-know?mtm_source=google&mtm_medium=cpc&mtm_campaign=21676150519&mtm_cid=21676150519&mtm_kwd=&mtm_content=&gad_source=1&gclid=Cj0KCQiAgdC6BhCgARIsAPWNWH3HWD6O4q4oY7PaoZsPUYSWoxC7salpyCivuOlz51WkYJ86su5nsRAaAgMxEALw_wcB (accessed on 20 November 2024).
- Zhao, H.; Hou, Y.; Chen, L. Experimental study on a small Brayton air refrigerator under −120 °C. Appl. Therm. Eng. 2009, 29, 1702–1706. [Google Scholar] [CrossRef]
- Gondrand, C.; Durand, F.; Delcayre, F.; Crispel, S.; Gistau Baguer, G.M. Overview of Air Liquide refrigeration systems between 1.8 K and 200 K. AIP Conf. Proc. 2014, 1573, 949–956. [Google Scholar] [CrossRef]
- Pan, M.; Zhao, H.; Liang, D.; Zhu, Y.; Liang, Y.; Bao, G. A review of the cascade refrigeration system. Energies 2020, 13, 2254. [Google Scholar] [CrossRef]
- Saravacos, G.; Kostaropoulos, A.E. Refrigeration and freezing equipment. In Handbook of Food Processing Equipment; Food Engineering Series; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- Arshad, M.O.; Azam, Q.; Ahmad, S.T.I.; Khan, F.; Wahid, M.A. Analysis of vapour compression refrigeration system with R-12, R-134a and R-22: An exergy approach. Mater. Today Proc. 2021, 46, 6748–6752. [Google Scholar] [CrossRef]
- Mota-Babiloni, A.; Mastani Joybari, M.; Navarro-Esbrí, J.; Mateu-Royo, C.; Barragán-Cervera, Á.; Amat-Albuixech, M.; Molés, F. Ultralow-temperature refrigeration systems: Configurations and refrigerants to reduce the environmental impact. Int. J. Refrig. 2020, 111, 147–158. [Google Scholar] [CrossRef]
- Wen, M.-Y.; Ho, C.-Y.; Hsieh, J.-M. Condensation heat transfer and pressure drop characteristics of R-290 (propane), R-600 (butane), and a mixture of R-290/R-600 in the serpentine small-tube bank. Appl. Therm. Eng. 2006, 26, 2045–2053. [Google Scholar] [CrossRef]
- Lim, J.Y.; Kim, N.A.; Lim, D.G.; Kim, K.H.; Choi, D.H.; Jeong, S.H. Process cycle development of freeze drying for therapeutic proteins with stability evaluation. J. Pharm. Investig. 2016, 46, 519–536. [Google Scholar] [CrossRef]
- Ye, W.; Yan, Y.; Zhou, Z.; Yang, P. Parametric analysis and performance prediction of an ultra-low temperature cascade refrigeration freezer based on an artificial neural network. Case Stud. Therm. Eng. 2024, 55, 104162. [Google Scholar] [CrossRef]
- Berchowitz, D.; Kwon, Y. Environmental profiles of stirling-cooled and cascade-cooled ultra-low temperature freezers. Sustainability 2012, 4, 2838–2851. [Google Scholar] [CrossRef]
- Saeed, M.Z.; Contiero, L.; Blust, S.; Allouche, Y.; Hafner, A.; Eikevik, T.M. Ultra-low-temperature refrigeration systems: A review and performance comparison of refrigerants and configurations. Energies 2023, 16, 7274. [Google Scholar] [CrossRef]
- Tan, H.; Xu, L.; Yang, L.; Bai, M.; Liu, Z. Operation performance of an ultralow temperature cascade refrigeration freezer with environmentally friendly refrigerants R290-R170. Environ. Sci. Pollut. Res. 2022, 30, 29790–29806. [Google Scholar] [CrossRef]
- ISPE. Good Practice Guide: Controlled Temperature Chamber Mapping and Monitoring; International Society for Pharmaceutical Engineering: Tampa, FL, USA, 2016. [Google Scholar]
- ISPE. Commissioning and Qualification, 2nd ed.; International Society for Pharmaceutical Engineering: Tampa, FL, USA, 2019; Volume 5. [Google Scholar]
- Moerman, F.; Fikiin, K. Hygienic design of air-blast freezing systems. In Handbook of Hygiene Control in the Food Industry, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 271–316. [Google Scholar] [CrossRef]
- Meir, K.; Cohen, Y.; Mee, B.; Gafney, E. Biobank networking for dissemination of data and resources: An overview. J. Bioreposit. Sci. Appl. Med. 2014, 2, 29. [Google Scholar] [CrossRef]
- Paradiso, A.V.; Daidone, M.G.; Canzonieri, V.; Zito, A. Biobanks and scientists: Supply and demand. J. Transl. Med. 2018, 16, 136. [Google Scholar] [CrossRef]
- Scudellari, M. Biobank managers bemoan underuse of collected samples. Nat. Med. 2013, 19, 253–263. [Google Scholar] [CrossRef]
- Dhiman, P.; Kumar, A. A situational based reliability indices estimation of ULT freezer using preventive maintenance under fuzzy environment. Int. J. Math. Eng. Manag. Sci. 2023, 8, 477–503. [Google Scholar] [CrossRef]
- Farley, M.; McTier, B.; Arnott, A.; Evans, A. Efficient ULT Freezer Storage. Soc. Responsib. Sustain. 2015, 18, 2024. Available online: https://www.ed.ac.uk/files/atoms/files/efficient_ult_freezer_storage.pdf (accessed on 18 November 2024).
- Hajagos, B. Life Cycle Assessment and Emission Reduction of Cascade and Stirling Ultra-Low Temperature Freezers. Master’s Thesis, Eindhoven University of Technology, Eindhoven, The Netherlands, 2021. Available online: https://pure.tue.nl/ws/portalfiles/portal/183343183/1492306_MasterThesisBenceHajagos.pdf (accessed on 18 November 2024).
- Liu, J.; Yu, J.; Yan, G. Experimental study on Joule-Thomson refrigeration system with R1150/R290/R601a for ultra-low temperature medical freezer. Appl. Therm. Eng. 2024, 255, 124015. [Google Scholar] [CrossRef]
- Xiao, R.; Zhang, Y.; Liu, X.; Yuan, Z. A life-cycle assessment of household refrigerators in China. J. Clean. Prod. 2015, 15, 301–310. [Google Scholar] [CrossRef]
- Wang, L.Y.; Cui, J.J.; Liu, J.Y.; Guo, A.X.; Zhao, Z.Y.; Liu, Y.Z.; Wu, J.C.; Li, M.; Hu, C.P.; Gao, Y.; et al. Gene-gene and gene-environment interaction data for platinum-based chemotherapy in non-small cell lung cancer. Sci. Data 2018, 5, 180284. [Google Scholar] [CrossRef]
- Authelin, J.-R.; Rodrigues, M.A.; Tchessalov, S.; Singh, S.K.; McCoy, T.; Wang, S.; Shalaev, E. Freezing of biologicals revisited: Scale, stability, excipients, and degradation stresses. J. Pharm. Sci. 2020, 109, 44–61. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, M.; Gehl, A.; Fricke, B.; Nawaz, K.; Gluesenkamp, K.; Shen, B.; Munk, J.; Hagerman, J.; Lapsa, M.; et al. Dataset of ultralow temperature refrigeration for COVID-19 vaccine distribution solution. Sci. Data 2022, 9, 67. [Google Scholar] [CrossRef]
- Owusu-Apenten, R.; Vieira, E.R. Low-Temperature Preservation. In Book Elementary Food Science, 5th ed.; Food Science Text Series; Springer: Berlin/Heidelberg, Germany, 2022; Chapter 13; pp. 289–316. [Google Scholar] [CrossRef]
- Gavroglu, K. Historiographical Issues in the History of Cold. In History of Artificial Cold, Scientific, Technological and Cultural Issues. Boston Studies in the Philosophy and History of Science; Gavroglu, K., Ed.; Springer: Dordrecht, The Netherlands, 2014; Volume 299. [Google Scholar] [CrossRef]
- Rees, J. Refrigeration Nation: A History of Ice, Appliances, and Enterprise in America; JHU Press: Baltimore, MD, USA, 2013; Available online: https://books.google.co.uk/books?id=JeoEAQAAQBAJ (accessed on 20 November 2024).
- Mallett, C.P. Frozen Food Technology; Springer: Berlin/Heidelberg, Germany, 1993; Available online: https://books.google.nl/books?hl=en&lr=&id=rzk9b3NpUzwC&oi=fnd&pg=PA20&dq=+William+Cullen+and+freezing&ots=1fbQor9ixv&sig=AhoQszLuugb-DMaNJxXQiGfpJ8g&redir_esc=y#v=onepage&q=William%20Cullen%20and%20freezing&f=false (accessed on 20 November 2024).
- Robinson, L.M. Safeguarded by your refrigerator: Mary Engle Pennington’s Struggle with the National Association of Ice Industries. In Rethinking Home Economics: Women and the History of a Profession; Stage, S., Vincenti, V.B., Eds.; Ithaca: Cornell University Press: New York, NY, USA, 1997; pp. 253–270. [Google Scholar]
- Prasad, U.S.; Mishra, R.S.; Das, R.K. Study of vapor compression refrigeration system with suspended nanoparticles in the low GWP refrigerant. Environ. Sci. Pollut. Res. 2024, 31, 1–26. [Google Scholar] [CrossRef]
- Thevenot, R.; Fidler, J.C. A History of Refrigeration Throughout the World; International Institute of Refrigeration: Paris, France, 1979. [Google Scholar]
- Pennington, M.E. The Hygienic and Economic Results of Refrigeration in the Conservation of Poultry and Eggs. Am. J. Public Health 1912, 2, 840–848. Available online: http://www.ncbi.nlm.nih.gov/pubmed/18008742 (accessed on 22 November 2024). [CrossRef]
- Thermo Scientific. Top Considerations for Selecting a ULT Freezer—Application. Available online: https://assets.thermofisher.com/TFS-Assets/LPD/Product-Information/Top-Considerations-ULT-Space-Constraints-TNTPCONSPACE-EN.pdf (accessed on 3 December 2024).
- Graham, M.; Samuel, G.; Farley, M. Roadmap for low-carbon ultra-low temperature storage in biobanking. J. Transl. Med. 2024, 22, 747. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.A. Frozen Food Science and Technology; Blackwell Publishing: Hoboken, NJ, USA, 2008. [Google Scholar] [CrossRef]
- James, C.; Purnell, G.; James, S.J. A Review of Novel and Innovative Food Freezing Technologies. Food Bioprocess Technol. 2015, 8, 1616–1634. [Google Scholar] [CrossRef]
- Koritsoglou, K.; Christou, V.; Ntritsos, G.; Tsoumanis, G.; Tsipouras, M.G.; Giannakeas, N.; Tzallas, A.T. Improving the Accuracy of Low-Cost Sensor Measurements for Freezer Automation. Sensors 2020, 20, 6389. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Sun, D.-W. Innovative applications of power ultrasound during food freezing processes—A review. Trends Food Sci. Technol. 2006, 17, 16–23. [Google Scholar] [CrossRef]
- Richmond Scientific. Reducing the Energy Used by ULT Freezers in the Lab. Richmond Scientific. Available online: https://www.richmondscientific.com/reduce-energy-used-by-ult-freezers-in-the-lab (accessed on 24 November 2024).
- Robertson, J.; Franzel, L.; Maire, D. Innovations in cold chain equipment for immunization supply chains. Vaccine 2017, 35, 2252–2259. [Google Scholar] [CrossRef]
- Legett, R. Field Demonstration of High-Efficiency Ultra-Low-Temperature Laboratory Freezers. Energy Effic. Renew. Energy 2014, 1–6. Available online: https://www.energy.gov/sites/default/files/2014/11/f19/ult_demo_report.pdf (accessed on 24 November 2024).
- Faugeroux, D. Ultra-Low Temperature Freezer Performance and Energy Use Tests; Office of Sustainability-University of California: Riverside, CA, USA, 2016; Available online: https://sels-network.org/wp-content/uploads/2022/09/Copy-of-ucr_ult_tests_report_-_2016_final_df1.pdf (accessed on 20 November 2024).
- Keri, C. Recycling cooling and freezing appliances. In Waste Electrical and Electronic Equipment (WEEE) Handbook, 2nd ed.; Goodship, V., Stevels, A., Huisman, J., Eds.; Woodhead Publishing: Cambridge, UK, 2019; Chapter 13; pp. 357–370. [Google Scholar] [CrossRef]
- Powell, R.L. CFC phase-out: Have we met the challenge? J. Fluor. Chem. 2002, 114, 237–250. [Google Scholar] [CrossRef]
- Tica, G.; Grubic, A.R. Mitigation of climate change from the aspect of controlling F-gases in the field of cooling technology. IOP Conf. Ser. Mater. Sci. Eng. 2019, 477, 012056. [Google Scholar] [CrossRef]
- Benhadid-Dib, S.; Benzaoui, A. Refrigerants and their environmental impact: Substitution of hydrochlorofluorocarbon (HCFC) and hydrofluorocarbon (HFC). Search for an adequate refrigerant. Energy Procedia 2012, 18, 807–816. [Google Scholar] [CrossRef]
- Muir, E.B. Commercial refrigeration and CFCs. Int. J. Refrig. 1990, 13, 106–111. [Google Scholar] [CrossRef]
- Calderazzi, L.; Colonna di Paliano, P. Thermal stability of R-134a, R-141b, R-13I1, R-7146, R-125 associated with stainless steel as a containing material. Int. J. Refrig. 1997, 20, 381–389. [Google Scholar] [CrossRef]
- Finberg, E.A.; Shiflett, M.B. Process designs for separating R-410A, R-404A, and R-407C using extractive distillation and ionic liquid entrainers. Ind. Eng. Chem. Res. 2021, 60, 16054–16067. [Google Scholar] [CrossRef]
- Zhang, W.; Li, W.; Cernicin, V.; Hrnjak, P. Theoretical and experimental investigation on the effects of internal heat exchangers on a reversible automobile R744 air-conditioning system under various operating conditions. Appl. Therm. Eng. 2024, 236, 121569. [Google Scholar] [CrossRef]
- Johnson, R.W. The effect of blowing agent choice on energy use and global warming impact of a refrigerator. Int. J. Refrig. 2004, 27, 794–799. [Google Scholar] [CrossRef]
- Lemmon, E.W. Pseudo-pure fluid equations of state for the refrigerant blends R-410A, R-404A, R-507A, and R-407C. Int. J. Thermophys. 2003, 24, 991–1006. [Google Scholar] [CrossRef]
- Liopis, R.; Sánchez, D.; Cabello, R.; Catalán-Gil, J.; Nebot-Andrés, L. Experimental analysis of R-450A and R-513A as replacements of R-134a and R-507A in a medium temperature commercial refrigeration system. Int. J. Refrig. 2017, 84, 52–66. [Google Scholar] [CrossRef]
- Liopis, R.; Calleja-Anta, D.; Sánchez, D.; Nebot-Andrés, L.; Catalán-Gil, J.; Cabello, R. R-454C, R-459B, R-457A and R-455A as low-GWP replacements of R-404A: Experimental evaluation and optimization. Int. J. Refrig. 2019, 106, 133–143. [Google Scholar] [CrossRef]
- Komarov, S.G.; Stankus, S.V. Experimental study of speed of sound in gaseous refrigerant R-507A. High Temp. 2011, 49, 150–153. [Google Scholar] [CrossRef]
- Reddy, V.S.; Panwar, N.L.; Kaushik, S.C. Exergetic analysis of a vapour compression refrigeration system with R134a, R143a, R152a, R404A, R407C, R410A, R502, and R507A. Clean Technol. Environ. Policy 2012, 14, 47–53. [Google Scholar] [CrossRef]
- Ema, M.; Naya, M.; Yoshida, K.; Nagaosa, R. Reproductive and developmental toxicity of hydrofluorocarbons used as refrigerants. Reprod. Toxicol. 2010, 29, 125–131. [Google Scholar] [CrossRef]
- Choi, T.Y.; Kim, Y.J.; Kim, M.S.; Ro, S.T. Evaporation heat transfer of R-32, R-134a, R-32/134a, and R-32/125/134a inside a horizontal smooth tube. Int. J. Heat Mass Transf. 2000, 43, 3651–3660. [Google Scholar] [CrossRef]
- Ong, K.S.; Haider-E-Alahi, M. Performance of a R-134a-filled thermosyphon. Appl. Therm. Eng. 2003, 23, 2373–2381. [Google Scholar] [CrossRef]
- Kim, C.-H.; Kim, N.-H. Condensation heat transfer and pressure drop of low GWP R-404A-alternative refrigerants (R-448A, R-449A, R-455A, R-454C) in a 7.0-mm outer-diameter horizontal microfin tube. Int. J. Refrig. 2021, 126, 181–194. [Google Scholar] [CrossRef]
- Kim, M.S.; Mulroy, W.J.; Didion, D.A. Performance evaluation of two azeotropic refrigerant mixtures of HFC-134a with R-290 (propane) and R-600a (isobutane). ASME J. Energy Resour. Technol. 1994, 116, 148–154. [Google Scholar] [CrossRef]
- Lee, B.-M.; Gook, H.-H.; Lee, S.-B.; Lee, Y.-W.; Park, D.-H.; Kim, N.-H. Condensation heat transfer and pressure drop of low GWP R-404A alternative refrigerants (R-448A, R-449A, R-455A, R-454C) in a 5.6 mm inner diameter horizontal smooth tube. Int. J. Refrig. 2021, 128, 71–82. [Google Scholar] [CrossRef]
- Cho, K.; Tae, S.-J. Evaporation heat transfer for R-22 and R-407C refrigerant–oil mixture in a microfin tube with a U-bend. Int. J. Refrig. 2000, 23, 219–231. [Google Scholar] [CrossRef]
- Cho, K.; Tae, S.-J. Condensation heat transfer for R-22 and R-407C refrigerant–oil mixtures in a microfin tube with a U-bend. Int. J. Heat Mass Transf. 2001, 44, 2043–2051. [Google Scholar] [CrossRef]
- Lee, J.H.; Bae, S.W.; Bang, K.H.; Kim, M.H. Experimental and numerical research on condenser performance for R-22 and R-407C refrigerants. Int. J. Refrig. 2002, 25, 372–382. [Google Scholar] [CrossRef]
- Guilherme, Í.,F.; Pico, D.F.M.; Santos, D.D.O.; Bandarra Filho, E.P. A review on the performance and environmental assessment of R-410A alternative refrigerants. J. Build. Eng. 2002, 47, 103847. [Google Scholar] [CrossRef]
- Ribeiro, R.P.P.L.; Sosa, J.E.; Araújo, J.M.M.; Pereiro, A.B.; Mota, J.P.B. Vacuum swing adsorption for R-32 recovery from R-410A refrigerant blend. Int. J. Refrig. 2023, 150, 253–264. [Google Scholar] [CrossRef]
- Calleja-Anta, D.; Martínez-Ángeles, M.; Nebot-Andres, L.; Sánchez, D.; Llopis, R. Optimizing R152a/R600 and R290/R600 mixtures for superior energy performance in vapor compression systems: Promising alternatives to Isobutane (R600a). Appl. Therm. Eng. 2024, 247, 123070. [Google Scholar] [CrossRef]
- Liu, Z.; Ji, S.; Tan, H.; Yang, D.; Cao, Z. An ultralow-temperature cascade refrigeration unit with natural refrigerant pair R290-R170: Performance evaluation under different ambient and freezing temperatures. Therm. Sci. Eng. Prog. 2023, 46, 102202. [Google Scholar] [CrossRef]
- Udroiu, C.-M.; Mota-Babiloni, A.; Navarro-Esbrí, J. Advanced two-stage cascade configurations for energy-efficient –80 °C refrigeration. Energy Convers. Manag. 2022, 267, 115907. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.-Y.; Ho, C.-Y. Evaporation heat transfer and pressure drop characteristics of R-290 (propane), R-600 (butane), and a mixture of R-290/R-600 in the three-lines serpentine small-tube bank. Appl. Therm. Eng. 2005, 25, 2921–2936. [Google Scholar] [CrossRef]
- Jones, A.; Wolf, A.; Kwark, S.M. Refrigeration system development with limited charge of flammable refrigerant, R-290. Therm. Sci. Eng. Prog. 2022, 34, 101392. [Google Scholar] [CrossRef]
- Pilla, T.S.; Sunkari, P.K.G.; Padmanabhuni, S.L.; Nair, S.S.; Dondapati, R.S. Experimental evaluation of mechanical performance of the compressor with mixed refrigerants R-290 and R-600a. Energy Procedia 2017, 109, 113–121. [Google Scholar] [CrossRef]
- Jeon, S.; Ko, J.; Lee, H.; Jeong, J.H. Advanced model for a non-adiabatic capillary tube considering both subcooled liquid and non-equilibrium two-phase states of R-600a. Int. J. Refrig. 2025, 169, 140–151. [Google Scholar] [CrossRef]
- Yang, C.-M.; Shen, B.; Muneeshwaran, M.; Nawaz, K.; Pickles, E.C.; Hartnett, C. Performance Evaluation of Various Configurations for Domestic Refrigerators with R-600a. International Refrigeration and Air Conditioning Conference. Paper 2656. Available online: https://docs.lib.purdue.edu/iracc/2656 (accessed on 5 January 2025).
- Yang, M.-H.; Yeh, R.-H. Investigation of the potential of R717 blends as working fluids in the organic Rankine cycle (ORC) for ocean thermal energy conversion (OTEC). Energy 2022, 245, 123317. [Google Scholar] [CrossRef]
- Söylemez, E. Energy and Conventional Exergy Analysis of an Integrated Transcritical CO2 (R-744) Refrigeration System. Energies 2024, 17, 479. [Google Scholar] [CrossRef]
- Kanbur, B.B.; Kriezi, E.E.; Markussen, W.B.; Kærn, M.R.; Busch, A.; Kristófersson, J. Framework for prediction of two-phase R-744 ejector performance based on integration of thermodynamic models with multiphase mixture CFD simulations. Appl. Therm. Eng. 2025, 258, 124888. [Google Scholar] [CrossRef]
- Macrì, C.; De León, Á.; Flohr, F. Comparison of performance and efficiency of different refrigerants at high load conditions and their impact on CO2eq emissions. In Proceedings of the CO2 Reduction for Transportation Systems Conference, Daikin Chemical Europe GmbH, Turin, Italy, 12–13 June 2024; pp. 1–7. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, J.-H.; Heo, I.-Y.; Yoon, J.-I.; Son, C.-H.; Nam, J.-W.; Kim, H.J.; Cha, S.-Y.; Seol, S.-H. Optimal charge amount for semiconductor chiller applying eco-friendly refrigerant R-744. Case Stud. Therm. Eng. 2024, 59, 104461. [Google Scholar] [CrossRef]
- Ye, G.; Ye, M.; Yang, J.; Wu, X.; Yan, Y.; Guo, Z.; Han, X. Investigation on absorption and separation performance of R-32, R-125, R-134a, and R-1234yf refrigerants using EMIM-based ionic liquids. ACS Sustain. Chem. Eng. 2024, 12, 1822–1835. [Google Scholar] [CrossRef]
- Wang, N.; Carlozo, M.N.; Marin-Rimoldi, E.; Befort, B.J.; Dowling, A.W.; Maginn, E.J. Machine learning-enabled development of accurate force fields for refrigerants. J. Chem. Theory Comput. 2023, 19. [Google Scholar] [CrossRef] [PubMed]
- Midzic Kurtagic, S.; Kadric, D.; Alispahic, M.; Blazevic, R.; Hadziahmetovic, H. Inventory of refrigerants in use for commercial purposes in BiH. In Proceedings of the 33rd DAAAM International Symposium, Vienna, Austria, 27–28 October 2022; Katalinic, B., Ed.; DAAAM International: Vienna, Austria, 2022; pp. 143–150. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linteris, G.T. Air humidity influence on combustion of R-1234yf (CF3CFCH2), R-1234ze(E) (trans-CF3CHCHF), and R-134a (CH2FCF3) refrigerants. Combust. Flame 2024, 262, 113352. [Google Scholar] [CrossRef]
- Ha, S.-J.; Lee, J.-H. Heat transfer characteristics of R-1234yf, an eco-friendly alternative refrigerant, in condenser for semiconductor chiller. Appl. Therm. Eng. 2024, 239, 122106. [Google Scholar] [CrossRef]
- Maqbool, S.; Maddali, R. Effect of system configuration on the performance of a hybrid air conditioning system based on R-1234yf. Appl. Therm. Eng. 2024, 236, 121624. [Google Scholar] [CrossRef]
- Kumar, A.; Lakshmi, B.J.; Yang, S.-Y.; Wang, C.-C. Effect of viscosity grade (POE) on the smooth-tube pool boiling performance with R-1234ze(E) refrigerant. Appl. Therm. Eng. 2024, 241, 122328. [Google Scholar] [CrossRef]
- Robaczewski, C.; Leehey, M.H.; DeDeker, Z. Material compatibility of seal materials with low GWP refrigerants and lubricant. In Proceedings of the International Refrigeration and Air Conditioning Conference, West Lafayette, IN, USA, 15 July 2024; Purdue University: West Lafayette, IN, USA, 2024. Paper 2826. Available online: https://docs.lib.purdue.edu/icec/2826 (accessed on 10 January 2025).
- Miyawaki, K.; Shikazono, N. Experimental evaluation of NIR spectroscopic characteristics of liquid R32, R1234yf, and R454C refrigerants. Int. Commun. Heat Mass Transf. 2024, 156, 107633. [Google Scholar] [CrossRef]
- Six, P.; Valtz, A.; Zhou, Y.; Yang, Z.; Coquelet, C. Experimental measurements and correlation of vapor–liquid equilibrium data for the difluoromethane (R32) + 1,3,3,3-tetrafluoropropene (R1234ze(E)) binary system from 254 to 348 K. Fluid Phase Equilibria 2024, 581, 114072. [Google Scholar] [CrossRef]
- Fabris, F.; Fabrizio, M.; Marinetti, S.; Rossetti, A.; Minetto, S. Evaluation of the carbon footprint of HFC and natural refrigerant transport refrigeration units from a life-cycle perspective. Int. J. Refrig. 2024, 159, 17–27. [Google Scholar] [CrossRef]
- Leehey, M.H.; Kujak, S.; Collins, C. Chemical Stability of HFO and HCO Refrigerants. In Proceedings of the International Refrigeration and Air Conditioning Conference, West Lafayette, IN, USA, 15 July 2024; Purdue University: West Lafayette, IN, USA, 2024. Paper 2555. Available online: https://docs.lib.purdue.edu/iracc/2555 (accessed on 4 January 2025).
- Cavallini, A. The state-of-the-art on refrigerants. J. Phys. Conf. Ser. 2020, 1599, 012001. [Google Scholar] [CrossRef]
- Mickoleit, E.; Breitkopf, C.; Jäger, A. Influence of equations of state and mixture models on the design of a refrigeration process. Int. J. Refrig. 2021, 121, 193–205. [Google Scholar] [CrossRef]
- Sharma, V.; Fricke, B.; Cheekatamarla, P.; Abdelaziz, O.; Baxter, V. Refrigerants for a sustainable future. Encyclopedia 2025, 5, 5. [Google Scholar] [CrossRef]
- Narute, S.; Joshi, K.; Rane, V.; Kokate, P. A brief review on development of refrigerants and their applications. Int. Res. J. Eng. Technol. 2021, 8, 4885. Available online: http://www.irjet.net (accessed on 13 January 2025).
- Long, N.V.D.; Lee, D.Y.; Han, T.H.; Sunyong, P.; Bong, H.B.; Lee, M. Purification of R-12 for refrigerant reclamation using existing industrial-scale batch distillation: Design, optimization, simulation, and experimental studies. Korean J. Chem. Eng. 2020, 37, 1823–1828. [Google Scholar] [CrossRef]
- Naskar, R.; Mandal, R. A competitive study of all natural refrigerants implementation on 1.5-ton domestic air conditioners using Cool Pack software. Ind. Eng. J. 2023, 52, 150–157. [Google Scholar]
- McLinden, M.O.; Huber, M.L. (R)Evolution of refrigerants. J. Chem. Eng. Data 2020, 65, 4176–4193. [Google Scholar] [CrossRef]
- Nederhand, R.J.; Droog, S.; Kluft, C.; Simoons, M.L.; de Maat, M.P.; Investigators of the EUROPA Trial. Logistics and quality control for DNA sampling in large multicenter studies. J. Thromb. Hemost. 2003, 1, 987–991. [Google Scholar] [CrossRef]
- Nasarabadi, S.; Hogan, M.; Nelson, J. Biobanking in precision medicine. Curr. Pharmacol. Rep. 2018, 4, 91–101. [Google Scholar] [CrossRef]
- She, R.C.; Petti, C.A. Procedures for the storage of microorganisms. In Manual of Clinical Microbiology, 11th ed.; Jorgensen, J.H., Carroll, K.C., Funke, G., Pfaller, M.A., Landry, M.L., Richter, S.S., Warnock, D.W., Eds.; American Society for Microbiology Press: Washington, DC, USA, 2015. [Google Scholar] [CrossRef]
- Karim, A.S.; Jewett, M.C. Cell-free synthetic biology for pathway prototyping. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2018; Volume 608, pp. 31–57. [Google Scholar] [CrossRef]
- Landor, L.A.; Stevenson, T.; Mayers, K.M.; Fleming, M.S.; Le Moine Bauer, S.; Babel, H.R.; Thiele, S. DNA, RNA, and prokaryote community sample stability at different ultra-low temperature storage conditions. Environ. Sustain. 2024, 7, 77–83. [Google Scholar] [CrossRef]
- Bao, J.; Zhao, L. Experimental research on the influence of system parameters on the composition shift for zeotropic mixture (isobutane/pentane) in a system occurring phase change. Energy Convers. Manag. 2016, 113, 1–15. [Google Scholar] [CrossRef]
- Ajmani, P.S. Storage of blood. In Immunohematology and Blood Banking; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–15. [Google Scholar] [CrossRef]
- Sato, K.; Tamaki, K.; Okajima, H.; Katsumata, Y. Long-term storage of blood samples as whole blood at extremely low temperatures for methemoglobin determination. Forensic Sci. Int. 1988, 37, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Hu, Z.; Muallem, H.; Gulley, M.L. Quality assurance of RNA expression profiling in clinical laboratories. J. Mol. Diagn. 2012, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shaw, G.F. The Low Down on Ultralow Temperature Freezers. Endocrine News, May 2021; pp. 40+. Available online: https://link.gale.com/apps/doc/A688004802/AONE?u=anon~103b3ad0&sid=googleScholar&xid=d263f033 (accessed on 9 February 2025).
- Li, R.; Johnson, R.; Yu, G.; McKenna, D.H.; Hubel, A. Preservation of cell-based immunotherapies for clinical trials. Cytotherapy 2019, 21, 943–957. [Google Scholar] [CrossRef]
- Shabihkhani, M.; Lucey, G.M.; Wei, B.; Mareninov, S.; Lou, J.J.; Vinters, H.V.; Singer, E.J.; Cloughesy, T.F.; Yong, W.H. The procurement, storage, and quality assurance of frozen blood and tissue biospecimens in pathology, biorepository, and biobank settings. Clin. Biochem. 2014, 47, 258–266. [Google Scholar] [CrossRef]
- Kaushal, S.; Molinolo, A. Potential use of College of American Pathologists accredited biorepositories to bridge unmet need for medical refrigeration using ultralow temperature storage for COVID-19 vaccine or drug storage. Biopreserv. Biobank. 2021, 19, 154–155. [Google Scholar] [CrossRef]
- Cabra, J.; Castro, D.; Colorado, J.; Mendez, D.; Trujillo, L. An IoT approach for wireless sensor networks applied to e-health environmental monitoring. In Proceedings of the 2017 IEEE International Conference on Internet of Things (iThings) and IEEE Green Computing and Communications (GreenCom) and IEEE Cyber, Physical and Social Computing (CPSCom) and IEEE Smart Data (SmartData), Exeter, UK, 21–23 June 2017; IEEE: New York, NY, USA, 2017; pp. 578–583. [Google Scholar] [CrossRef]
- Samuel, G.; Sims, J.M. Drivers and constraints to environmental sustainability in UK-based biobanking: Balancing resource efficiency and future value. BMC Med. Ethics 2023, 24, 36. [Google Scholar] [CrossRef]
- Isaacs, E.; Schmelz, M. Come in out of the cold: Alternatives to freezing for microbial biorepositories. Clin. Microbiol. Newsl. 2017, 39, 27–34. [Google Scholar] [CrossRef]
- Hutchison, J.R.; Brooks, S.M.; Kennedy, Z.C.; Pope, T.R.; Deatherage Kaiser, B.L.; Victry, K.D.; Warner, C.L.; Oxford, K.L.; Omberg, K.M.; Warner, M.G. Polysaccharide-based liquid storage and transport media for non-refrigerated preservation of bacterial pathogens. PLoS ONE 2019, 14, e0221831. [Google Scholar] [CrossRef]
- Grasedieck, S.; Schöler, N.; Bommer, M.; Niess, J.H.; Tumani, H.; Rouhi, A.; Bloehdorn, J.; Liebisch, P.; Mertens, D.; Döhner, H.; et al. Impact of serum storage conditions on microRNA stability. Leukemia 2012, 26, 2414–2416. [Google Scholar] [CrossRef]
- Groelz, D.; Sobin, L.; Branton, P.; Compton, C.; Wyrich, R.; Rainen, L. Non-formalin fixative versus formalin-fixed tissue: A comparison of histology and RNA quality. Exp. Mol. Pathol. 2013, 94, 188–194. [Google Scholar] [CrossRef]
- Lou, J.J.; Mirsadraei, L.; Sanchez, D.E.; Wilson, R.W.; Shabihkhani, M.; Lucey, G.M.; Wei, B.; Singer, E.J.; Mareninov, S.; Yong, W.H. A review of room temperature storage of biospecimen tissue and nucleic acids for anatomic pathology laboratories and biorepositories. Clin. Biochem. 2014, 47, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Shehu, D.; Kim, M.-O.; Rosendo, J.; Krogan, N.; Morgan, D.O.; Guglielmo, B.J. Institutional conversion to energy-efficient ultra-low freezers decreases carbon footprint and reduces energy costs. Biopreserv. Biobank. 2024, 23. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.; Graves, S.; Murray, T. The Biophile sample process management system—Automated sample access at ultra low temperatures. JALA J. Assoc. Lab. Autom. 2001, 6, 28–31. [Google Scholar] [CrossRef]
- Kasi, P.; Cheralathan, M. Review of cascade refrigeration systems for vaccine storage. J. Phys. Conf. Ser. 2021, 2054, 012041. [Google Scholar] [CrossRef]
- Bakaltcheva, I.; Reid, T. Effects of blood product storage protectants on blood coagulation. Transfus. Med. Rev. 2003, 17, 263–271. [Google Scholar] [CrossRef]
- Greening, D.W.; Glenister, K.M.; Sparrow, R.L.; Simpson, R.J. International blood collection and storage: Clinical use of blood products. J. Proteom. 2010, 73, 386–395. [Google Scholar] [CrossRef]
- Hess, J.R. Conventional blood banking and blood component storage regulation: Opportunities for improvement. Blood Transfus. 2010, 8 (Suppl. S3), S9–S15. [Google Scholar] [CrossRef]
- Radin, J. Life on Ice: A History of New Uses for Cold Blood; The University of Chicago Press: Chicago, IL, USA, 2017. [Google Scholar]
- Sperling, S.; Vinholt, P.J.; Sprogøe, U.; Yazer, M.H.; Frederiksen, H.; Nielsen, C. The effects of storage on platelet function in different blood products. Hematology 2019, 24, 89–96. [Google Scholar] [CrossRef]
- Du, C.; Xu, J.; Song, H.; Tao, L.; Lewandowski, A.; Ghose, S.; Borys, M.C.; Li, Z.J. Mechanisms of color formation in drug substance and mitigation strategies for the manufacture and storage of therapeutic proteins produced using mammalian cell culture. Process Biochem. 2019, 86, 127–135. [Google Scholar] [CrossRef]
- Kao, G.S.; Kim, H.T.; Daley, H.; Ritz, J.; Burger, S.R.; Kelley, L.; Vierra-Green, C.; Flesch, S.; Spellman, S.; Miller, J.; et al. Validation of short-term handling and storage conditions for marrow and peripheral blood stem cell products. Transfusion 2011, 51, 1129–1138. [Google Scholar] [CrossRef]
- Sugrue, M.W.; Hutcheson, C.E.; Fisk, D.D.; Roberts, C.G.; Mageed, A.; Wingard, J.R.; Moreb, J.S. The effect of overnight storage of leukapheresis stem cell products on cell viability, recovery, and cost. J. Hematother. 2009, 7, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Jewell, S.D.; Srinivasan, M.; McCart, L.M.; Williams, N.; Grizzle, W.H.; LiVolsi, V.; MacLennan, G.; Sedmak, D.D. Analysis of the molecular quality of human tissues: An experience from the Cooperative Human Tissue Network. Am. J. Clin. Pathol. 2002, 118, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Mager, S.R.; Oomen, M.H.A.; Morente, M.M.; Ratcliffe, C.; Knox, K.; Kerr, D.J.; Pezzella, F.; Riegman, P.H.J. Standard operating procedure for the collection of fresh frozen tissue samples. Eur. J. Cancer 2007, 43, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Troyer, D. Biorepository Standards and Protocols for Collecting, Processing, and Storing Human Tissues. In Tissue Proteomics. Methods in Molecular Biology™; Liu, B.C.S., Ehrlich, J.R., Eds.; Humana Press: Totowa, NJ, USA, 2008; Volume 441. [Google Scholar] [CrossRef]
- International Society for Biological and Environmental Repositories (ISBER). Collection, storage, retrieval, and distribution of biological materials for research. Cell Preserv. Technol. 2008, 6, 3–58. [Google Scholar] [CrossRef]
- Wolf, L.E.; Bouley, T.A.; McCulloch, C.E. Genetic Research with Stored Biological Materials: Ethics and Practice. IRB 2010, 32, 7–18. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC3052851/ (accessed on 1 April 2025).
- Zimkus, B.M.; Ford, L.S. Best practices for genetic resources associated with natural history collections: Recommendations for practical implementation. Collect. Forum 2014, 28, 77–112. [Google Scholar] [CrossRef]
- Elliott, M.A.; Halbert, G.W. Maintaining the cold chain shipping environment for Phase I clinical trial distribution. Int. J. Pharm. 2005, 299, 49–54. [Google Scholar] [CrossRef]
- Harada, L.M.; Rodrigues, E.F.; Ferreira Wde, P.; Maniçoba da Silva, A.; Kawamoto Júnior, L.T. Storage management of clinical research supplies of a phase IIB/III, national, multi-centre, double-blind and randomized study. Braz. J. Oper. Prod. Manag. 2016, 13, 430–441. [Google Scholar][Green Version]
- Li, Q.C.; Qiu, F.; Cohen, K.; Tougas, T.; Li, J.; McCaffrey, J.; Purdue, T.; Song, J.J.; Swanek, F.; Abelaira, S. Best practices for drug substance stress and stability studies during early-stage development Part I—Conducting drug substance solid stress to support phase Ia clinical trials. J. Pharm. Innov. 2012, 7, 214–224. [Google Scholar] [CrossRef]
- Lloyd, J.; Lydon, P.; Ouhichi, R.; Zaffran, M. Reducing the loss of vaccines from accidental freezing in the cold chain: The experience of continuous temperature monitoring in Tunisia. Vaccine 2015, 33, 902–907. [Google Scholar] [CrossRef]
- Liu, Z.; Bai, M.; Tan, H.; Ling, Y.; Cao, Z. Experimental test on the performance of a −80 °C cascade refrigeration unit using refrigerants R290-R170 for COVID-19 vaccines storage. J. Build. Eng. 2023, 63, 105537. [Google Scholar] [CrossRef]
- Groover, K.; Kulina, L.; Franke, J. Effect of Increasing Thermal Mass on Chamber Temperature Stability Within Ultra-Low Freezers in Bio-Repository Operations. Available online: https://biospecimens.cancer.gov/meeting/brnsymposium/2009/docs/posters/poster%2015%20groover.pdf (accessed on 3 February 2025).
- Rogers, J.; Carolin, T.; Vaught, J.; Compton, C. Biobankonomics: A taxonomy for evaluating the economic benefits of standardized centralized human biobanking for translational research. JNCI Monogr. 2011, 2011, 32–38. [Google Scholar] [CrossRef]
- Labmode. Nordic Modular ULT Freezer System. Labmode. Available online: https://labmode.co.uk/product/nordic-modular-ult-freezer-system/ (accessed on 5 February 2025).
- Lucas, P.; Pries, J.; Wei, S.; Wuttig, M. The glass transition of water, insight from phase change materials. J. Non-Cryst. Solids 2022, 1, 100084. [Google Scholar] [CrossRef]
- Kypraiou, C. Comparative Analysis of Temperature Uniformity and Efficiency in Low Temperature Refrigeration Systems of Hermetic Compressor, Free Piston Engine and Multicompressors. Master’s Thesis, Hellenic Open University, Patras, Greece, 2025. [Google Scholar]
- ISO 14067:2018; Greenhouse Gases-Carbon Footprint of Products—Requirements and Guidelines for Quantification. International Organization for Standardisation: Geneva, Switzerland, 2018.
- Muenz, R. How to Operate and Maintain an Ultralow Temperature Freezer. Lab Manager. Available online: https://www.labmanager.com/big-picture/lab-ultralow-cold-storage/how-to-operate-and-maintain-an-ultralow-temperature-freezer-24970 (accessed on 9 February 2025).
- Pharmaceutical Inspection Co-Operation Scheme. Guide to Good Manufacturing Practice for Medicinal Products Part I (PE 009-17). PIC/S Secretariat. Available online: https://www.picscheme.org (accessed on 6 April 2025).
- Tsimpoukis, D.; Syngounas, E.; Bellos, E.; Koukou, M.; Tzivanidis, C.; Anagnostatos, S.; Vrachopoulos, M.G. Data-driven energy efficiency comparison between operating R744 and R448A supermarket refrigeration systems based on hybrid experimental-simulation analysis. Therm. Sci. Eng. Prog. 2024, 53, 102776. [Google Scholar] [CrossRef]
- McColloster, P.J.; Martin-de-Nicolas, A. Vaccine refrigeration: Thinking outside of the box. Hum. Vaccines Immunother. 2014, 10, 1126–1128. [Google Scholar] [CrossRef]
- Li, Y.; Pan, X.; Liao, X.; Xing, Z. A data-driven energy management strategy based on performance prediction for cascade refrigeration systems. Int. J. Refrig. 2022, 136, 114–123. [Google Scholar] [CrossRef]
- Gumapas, L.A.M.; Simons, G. Factors affecting the performance, energy consumption, and carbon footprint for ultra low temperature freezers: Case study at the National Institutes of Health. World Rev. Sci. Technol. Sustain. Dev. 2013, 10, 129. [Google Scholar] [CrossRef]
- Stirling Ultracold. SU780XLE Operating Manual (Version 061824P). Available online: https://www.stirlingultracold.com/wp-content/uploads/2024/06/SU780XLE_Operating_Manual_Stirling_Ultracold_061824P.pdf (accessed on 10 April 2025).
- Klinge Corporation. PTI NMF-372 Prod 063 Rev C. Available online: https://klingecorp.com/wp-content/uploads/2019/09/PTI-Form-NMF-372-Prod-063-Rev-C.pdf (accessed on 10 April 2025).
- Koncept Media. Model K66 HPL [PDF]. Available online: https://www.konceptmedia.hr/admin/dokumenti/docfiles/model-K66-HPL.pdf (accessed on 10 April 2025).
- WHO. Temperature Mapping of Storage Areas; Technical Report Series; No. 961, 2011 Annex 9; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- WHO. Temperature Mapping of Storage Areas; Technical Report Series; No. 961, 2011, Supplement 8; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Javeri-Shahreza, I.; Abdolmaleki, L.; Sadrameli, S.M.; Fakhroleslam, M. Dynamic modeling and experimental validation of household refrigerators/freezers equipped with phase change materials towards improved energy efficiency. Therm. Sci. Eng. Prog. 2023, 46, 102227. [Google Scholar] [CrossRef]
- European Commission. Eudralex—Volume 4: Good Manufacturing Practice (GMP) Guidelines. Available online: https://health.ec.europa.eu/medicinal-products/eudralex/eudralex-volume-4_en (accessed on 10 April 2025).
- European Commission. Guidelines of 19 March 2015 on Principles of Good Distribution Practice of Active Substances for Medicinal Products for Human Use (Text with EEA Relevance) (2015/C 95/01). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52015XC0321(01) (accessed on 10 April 2025).
- European Parliament & Council. Directive 2000/54/EC of 18 September 2000 on the Protection of Workers from Risks Related to Exposure to Biological Agents at Work (Seventh Individual Directive Within the Meaning of Article 16(1) of Directive 89/391/EEC). OJ L 262, 17.10.2000. 2000, pp. 21–45. Available online: http://data.europa.eu/eli/dir/2000/54/oj (accessed on 10 April 2025).
- European Parliament & Council. Directive 2009/41/EC of 6 May 2009 on the Contained Use of Genetically Modified Micro-Organisms (Recast) (Text with EEA Relevance). OJ L 125, 21.5.2009. 2009, pp. 75–97. Available online: http://data.europa.eu/eli/dir/2009/41/oj (accessed on 9 April 2025).
- European Medicines Agency. Guideline on Process Validation for Finished Products—Information and Data to be Provided in Regulatory Submissions (EMA/CHMP/CVMP/QWP/BWP/70278/2012-Rev1,Corr.1). 2016. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-process-validation-finished-products-information-data-be-provided-regulatory-submissions_en.pdf (accessed on 10 April 2025).
- US Food and Drug Administration. Title 21—Food and Drugs, Chapter I—Food and Drug Administration, Department of Health and Human Services, Subchapter A—General, Part 11 Electronic Records; Electronic Signatures. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=11 (accessed on 10 April 2025).
- US Food and Drug Administration. Title 21—Food and Drugs, Chapter I—Food and Drug Administration, Department of Health and Human Services, Subchapter C—Drugs: General, Part 210 Current Good Manufacturing Practice in Manufacturing, Processing, Packing, or Holding of Drugs; General. Retrieved from URL The American Society of Mechanical Engineers (2024). Bioprocessing Equipment. Available online: https://files.asme.org/Catalog/Codes/PrintBook/35606.pdf (accessed on 15 April 2025).
- Evans, A. ULT Freezer Best Practice: Impacts. Green Light Labs. 2022. Available online: https://www.scientificlabs.co.uk/file/1991/Defrost%20the%20Freezer%20Regularly%20to%20Prevent%20a%20Build-up%20of%20Frost (accessed on 9 April 2025).



| Group | Type | Attribute | Strengths | Weaknesses | Opportunities | Threats | References |
|---|---|---|---|---|---|---|---|
| Hydrofluorocarbons (HFCs) | R-134a | Tetrafluoroethane | Good energy efficiency, widely available | High GWP, climate impact | Transitional use in systems already reliant on HFCs | Global HFC phase-down, regulatory pressure | [58,66,67,68,69] |
| R-404A | HFC-125, HFC-143a, and HFC-134a blend | Strong low-temperature performance | Very high GWP | Use in existing industrial setups | Obsolescence due to environmental regulations | [59,64,66,70,71,72] | |
| R-407C | HFC-32, HFC-125, and HFC-134a blend | Replacement for R-22, lower GWP than R-22 | Still relatively high GWP | Retrofit applications | Market shift to lower-GWP alternatives | [59,66,73,74,75] | |
| R-410A | HFC-32 and HFC-125 blend | High capacity, quiet operation | High GWP, facing phase-out | Short-term high-performance use | Regulatory bans and alternatives like HFOs | [59,76,77] | |
| R-507A | HFC-125 and HFC-143a mixture | Stable performance at low temperatures | High GWP | Specialized industrial systems | Environmental and legislative pressure | [62,63,65,66] | |
| Hydrocarbons | R-290 | Propane | Very low GWP, high efficiency | Flammable | Domestic/commercial systems with proper safety | Safety regulations in populated areas | [67,71,78,79,80,81,82,83] |
| R-600a | Isobutane | Low GWP, good performance | Flammable | Home refrigeration, small systems | Public perception, installation restrictions | [67,77,80,81,83,84,85] | |
| R-170 | Ethane | Extremely low GWP, high efficiency | Flammable, limited use | Niche cryogenic applications | Narrow market scope due to safety limits | [67,79,80,81] | |
| Ammonia | R-717 | Ammonia | High efficiency, low cost, No GWP | Toxicity concerns | Large-scale refrigeration, food storage | Risk of leaks, regulatory burden | [80,86] |
| Carbon Dioxide | R-744 | Carbon dioxide | Low GWP, non-toxic, non-flammable | High pressure requirements | Supermarkets, heat pumps, mobile A/C | Cost of high-pressure systems | [80,87,88,89,90] |
| Hydrofluoroolefins (HFOs) | R-1234yf | 2,3,3,3-Tetrafluoropropene | Ultra-low GWP | Limited infrastructure, new tech | Automotive A/C, OEM adoption | Environmental uncertainty (e.g., TFA formation) | [80,91,92,93,94,95,96,97,98,99,100] |
| R-1234ze | Dia-1,3,3,3-tetrafluoropropene | Ultra-low GWP | Infrastructure not yet mature | Stationary air conditioning | Uncertain long-term performance data | [80,94,95,96,97,98,99] | |
| Natural Cooling Taps | R-600 | Butane | Low GWP, effective cooling | Flammable | Household refrigerators | Safety standards and market limitations | [42,67,77,80,81,83,84,85] |
| Special Cooling Taps | R-401A | HFC-125, HFC-143a, and HCFC-22 blend | R-22 replacement | High GWP, transitional | Temporary retrofit use | Not sustainable long-term | [42,101,102] |
| R-421A | Azeotropic mixture to replace R-22 | Lower GWP than R-22 | Limited market availability | Retrofit for older R-22 systems | Competing low-GWP solutions becoming standard | [42,75] | |
| Inert Gas Refrigerant Taps | R-40 | Ethylene | Low GWP, non-toxic | Very limited applications | Experimental or niche systems | Minimal commercial demand | [103,104,105] |
| Other Cooling Taps | R-12 | Dichlorodifluoromethane—has been withdrawn due to ozone depletion | Good low-temp performance | Ozone depletion, high GWP | Only in legacy systems | Banned, legally restricted | [106,107] |
| R-22 | Chlorodifluoromethane—withdrawn due to HFC regulations | Good efficiency, historic widespread use | High GWP, phased out | Retrofitting with replacements | Global withdrawal under Montreal Protocol | [73,74,75] |
| Category | Product Type | Storage Temperature | Examples | References |
|---|---|---|---|---|
| Pharmaceuticals | -Small molecular drugs | −80 °C to −20 °C | Acetaminophen, ibuprofen, aspirin | [1,32,111] |
| -Organic (e.g., peptides) | −80 °C to −20 °C | Insulin, glucagon, vasopressin | [1,32,111] | |
| Large Molecular/Biological | -Monoclonal antibodies | −80 °C to −20 °C | Adalimumab (Humira), trastuzumab (Herceptin) | [113,125,126] |
| -Recombinant proteins | −80 °C to −20 °C | Recombinant human growth hormone (rhGH), erythropoietin | [112,113,114,125] | |
| -Therapeutic proteins | −80 °C | Interferon-beta, tissue plasminogen activator (tPA) | [112,113,114,125] | |
| -Enzymes | −80 °C to −20 °C | DNA polymerase, reverse transcriptase, trypsin | [12,35,41,48] | |
| -Viruses | −80 °C or −196 °C | Adenovirus vectors, influenza virus stocks, lentivirus | [10,64] | |
| -Antibodies against RNA lines | −80 °C to −20 °C | Anti-miRNA antibodies, anti-snoRNA antibodies | [44,122,131] | |
| Biological Samples | -DNA/RNA | −196 °C to −80 °C | Genomic DNA from blood, total RNA from tissues | [144,145,146] |
| -Proteins | −80 °C to −20 °C | Purified histones, protein lysates from cells | [64] | |
| Vaccines | -mRNA vaccines | −80 °C to −60 °C | Pfizer-BioNTech (BNT162b2), Moderna COVID-19 vaccine | [34,119,132] |
| Blood Products | -Blood | −80 °C or cooling (<4 °C) | Whole blood units, red blood cell concentrates | [2,133,134,135,136,137] |
| -Platelets | 4 °C for short-term storage; −80 °C for long-term storage | Apheresis platelets, pooled platelet concentrates | [2,133,134,135,136,137] | |
| Cell Culture | -Cell lines | −196 °C to −80 °C | HEK293, CHO cells, HeLa cells | [126,138,139,140] |
| -Fetal/stem cells | −196 °C to −80 °C | Human embryonic stem cells, mesenchymal stem cells | [126,138,139,140] | |
| Tissue Samples | -Fresh frozen samples | −196 °C to −80 °C | Frozen liver biopsy, tumor tissue slices | [142,143] |
| -Paraffin (formalin) samples | −80 °C to −20 °C (for long-term storage) | FFPE tumor sections, preserved kidney samples | [142,143] | |
| Genetic Material | -DNA plasmid | −80 °C | Plasmids used for gene expression, cloning vectors | [144,145,146] |
| -Oligodynamic | −80 °C to −20 °C | siRNA oligos, antisense oligonucleotides | [144,145,146] | |
| Research Samples | -Environment samples | −80 °C | Air particulate filters, contaminated soil extracts | [147,148,149] |
| -Clinical samples | −80 °C | Nasopharyngeal swabs, serum from patients | [147,148,149] | |
| -Experimental observations | −196 °C to −80 °C | Cryopreserved test specimens, bioassay controls | [147,148,149] | |
| Food (Perishables) | -Frozen fruits and vegetables | −40 °C to −20 °C (for long-term storage; in ultra-low freezers) | Frozen strawberries, green beans, corn | [12,35,41,48] |
| -Meat and seafood (frozen) | −30 °C to −18 °C | Frozen beef cuts, salmon fillets | [12,35,41,48] | |
| -Dairy products (frozen) | −30 °C to −20 °C | Frozen cheese, ice cream, butter blocks | [12,35,41,48] | |
| -Ready-to-eat frozen meals | −30 °C to −18 °C | Frozen lasagna, chicken stir-fry packs | [12,35,41,48] |
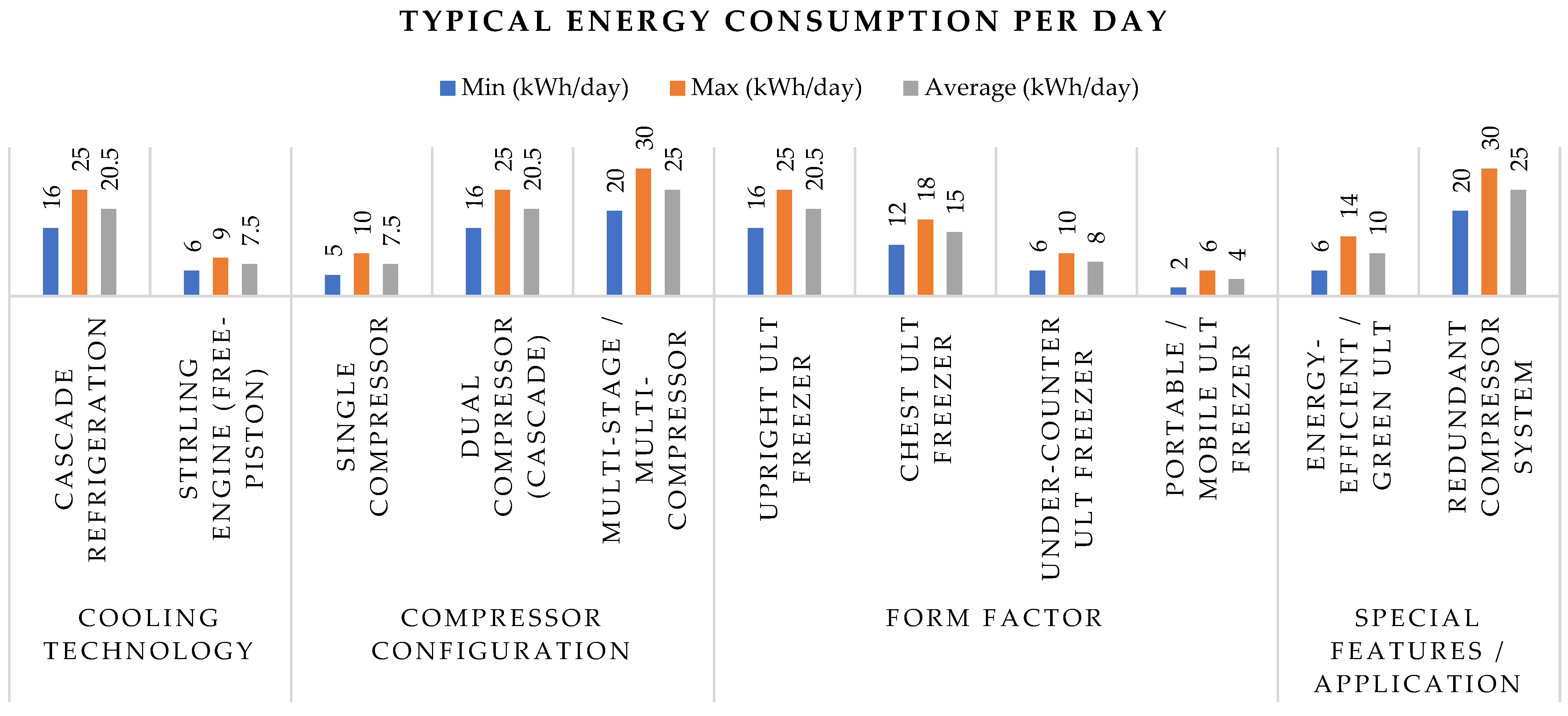
| Category | Type | Specifications | References |
|---|---|---|---|
| 1. Based on Cooling Technology | Cascade Refrigeration System (Traditional) | Most common ULT freezer technology. Uses two hermetically sealed compressors and refrigerants in a cascade cycle. Can reach temperatures of –80 °C to –86 °C. Pros: Proven, reliable. Cons: Higher energy use, more moving parts. Energy Consumption: 16–25 kWh/day. Examples: Thermo Fisher TSX, PHCbi MDF series, Haier DW-86 series. | [11,17,18,79] |
| Stirling Engine (Free-Piston) | Uses a free-piston Stirling engine with no oil or traditional compressor. Environmentally friendly (helium gas as the working fluid). Ideal for energy efficiency and low maintenance. Energy Consumption: 6–9 kWh/day. Examples: Stirling Ultracold SU780XLE. | [164] | |
| 2. Based on Compressor Configuration | Single Compressor | Not typical for –86 °C but used in –40 °C to –60 °C units. Simpler and cheaper design, often used for transport or backup. Energy Consumption: 5–10 kWh/day. | [83,98] |
| Dual Compressor (Cascade) | Two-stage cascade system, standard for most –80 °C to –86 °C ULT freezers. Reliable performance with moderate energy consumption. Energy Consumption: 16–25 kWh/day. | [83,98,132] | |
| Multi-Stage or Multi-Compressor | More than two compressors, often used for large-capacity units or those requiring redundancy. Provides increased cooling power and failsafe operation. Energy Consumption: 20–30+ kWh/day. | [165] | |
| 3. Based on Form Factor | Upright ULT Freezers | Most common in labs. Easy access with adjustable shelves or racks. Capacity typically ranges from 300–800+ liters. Energy Consumption: 16–25 kWh/day. Examples: Thermo TSX Series, PHCbi MDF-DU702VH. | [4,8,83,98] |
| Chest ULT Freezers | Top-opening design offers better insulation and temperature stability. Generally more energy-efficient than upright models. Capacity: 300–900 L. Energy Consumption: 12–18 kWh/day. Examples: So-Low U85-25, Stirling SU105UE. | [164] | |
| Under-Counter ULT Freezers | Compact and space-saving, often used in clinical or point-of-care labs. Capacity: 100–200 L. Energy Consumption: 6–10 kWh/day. Examples: PHCbi MDF-U33, Thermo Fisher TSX Series Compact. | [8] | |
| Portable/Mobile ULT Freezers | Designed for safe transport of biological samples at ultra-low temperatures. May be battery-powered, compressor-driven, or use dry ice. Energy Consumption: 2–6 kWh/day. Examples: Stirling Ultracold ULT25NEU. | [164] | |
| 4. Based on Application or Special Features | Energy-Efficient or Green ULT Freezers | Use hydrocarbon refrigerants (e.g., R-170, R-290) with low global warming potential (GWP). ENERGY STAR® certified, optimized for low power consumption. Energy Consumption: 6–14 kWh/day. Examples: PHCbi VIP ECO Series, Thermo TSX Series. | [51,56,98] |
| ULT Freezers with Redundant Compressor Systems | High-reliability systems featuring backup compressors or modular components for critical storage applications such as biobanking or clinical trials. Energy Consumption: 20–30+ kWh/day. Examples: Custom or pharma-grade configurations. | [165] |
| Features | Hermetic Compressor | Free-Piston Engine | Multi-Compressors |
|---|---|---|---|
| References | [156,164,165,166] | [156,164,165,166] | [156,164,165,166] |
| Capacity | 706 L | 780 L | 59,720 L |
| Temperature Range | −85 °C to −40 °C | −86 °C to −20 °C | −60 °C to 0 °C |
| Construction Material | AISI 304 stainless steel | Blank insulation panels | Special design with two systems |
| Cooling Technology | Hermetic compressors | Free-piston Stirling | Dual cooling system |
| Energy Consumption | Energy saving strategies | ~6.67 kWh/d, up to 40% less energy | Requires maintenance for stable operation |
| Connectivity | USB, SIM, Wi-Fi, Ethernet | Remote monitoring | Limited options |
| Temperature Stability | Superior thermal performance | ±1 °C | Constant temperature control |
| Operating Safety | Safety thermostats | Lock and PIN for access | Diagnostics and warnings |
| Maintenance Procedures | Regular programs necessary | Maintenance with GUI and automated monitoring | Regular maintenance and checks with automated diagnostics |
| Useful Life | 10–12 years | 12 years | 10–12 years |
| Refrigerant Safety | HCFC or CFC-free R-170 and R-1270 | Uses R-170 (Ethane), eco-friendly | Uses HFCs, requires caution due to flammability |
| Operating Noise | Noise during operation | <48 dB(A) | Noise during operation |
| Environmental Policy | Eco-friendly refrigerants | Uses natural refrigerants | Low ozone depletion potential |
| Resistance to Temperature Fluctuations | High | High | High via dual compressors |
| Reliability Price | High reliability | Variable operation with minimal maintenance | Excellent due to redundancy |
| Measurement | 1990 × 1060 × 1000 mm | 1994 × 870 × 915 mm | 10,945 × 2154 × 2896 mm |
| Source | Title of the Regulation | Article | References |
|---|---|---|---|
| EU | Eudralex; Volume 4 GMP Guidelines | Volume 4 | [170] |
| Good Distribution Practice of Active Substances for Medicinal Products for Human Use (2015/C 95/01) | 2015/C 95/01 | [171] | |
| Directive 2000/54/EC Protection of Workers from Risks Related to Exposure to Biologic Agents at Work | 2000/54/EC Annex V and VI | [172] | |
| Directive 2009/41/EC on the Contained Use of Genetically modified Micro-organisms | 2009/41/EC | [173] | |
| European Medicines Agency Scientific Guidance Documents on Biological Drug Substances | N/A | [174] | |
| US FDA | Title 21 Code of Federal Regulations, Electronic Records, and Electronic Signatures | Part 11 | [175] |
| Title 21 Current Good Manufacturing Practice In Manufacturing, Processing, Packing, Or Holding Of Drugs; General | Part 210 | [176] | |
| Title 21 Code of Federal Regulations, Current Good Manufacturing Practice for Finished Pharmaceuticals | Part 211 | [176] | |
| ASME | Bioprocessing Equipment | ASME BPE-2014 | [159] |
| PIC/S | Guide to Good Manufacturing Practice for Medicinal Products, Part I | PE-009-9 | [159] |
| Guide to Good Manufacturing Practice for Medicinal Products, Part II | PE-009-11 | [159] | |
| ISPE | Baseline Guide: Biopharmaceuticals | Volume 6, 2nd | [21,22] |
| Baseline Guide: Commissioning and Qualification | Volume 5, 2nd | [21,22] | |
| Good Practice Guide—Cold Chain Management | 2011 | [21,22] | |
| Good Practice Guide—Controlled Temperature Chamber Mapping and Monitoring | 2016 | [21,22] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kypraiou, C.; Varzakas, T. Evolution and Evaluation of Ultra-Low Temperature Freezers: A Comprehensive Literature Review. Foods 2025, 14, 2298. https://doi.org/10.3390/foods14132298
Kypraiou C, Varzakas T. Evolution and Evaluation of Ultra-Low Temperature Freezers: A Comprehensive Literature Review. Foods. 2025; 14(13):2298. https://doi.org/10.3390/foods14132298
Chicago/Turabian StyleKypraiou, Christos, and Theodoros Varzakas. 2025. "Evolution and Evaluation of Ultra-Low Temperature Freezers: A Comprehensive Literature Review" Foods 14, no. 13: 2298. https://doi.org/10.3390/foods14132298
APA StyleKypraiou, C., & Varzakas, T. (2025). Evolution and Evaluation of Ultra-Low Temperature Freezers: A Comprehensive Literature Review. Foods, 14(13), 2298. https://doi.org/10.3390/foods14132298