The Use of NIR Spectroscopy and Chemometrics to Identify the Thermal Treatment of Milk in Fiore Sardo PDO Cheese to Detect Fraud
Abstract
1. Introduction
2. Materials and Methods
2.1. Cheese-Making Procedure
2.2. Near-Infrared Spectra Acquisition
2.3. Chemometric Analysis and Genetic Algorithms
2.4. Sample Partitioning
3. Results
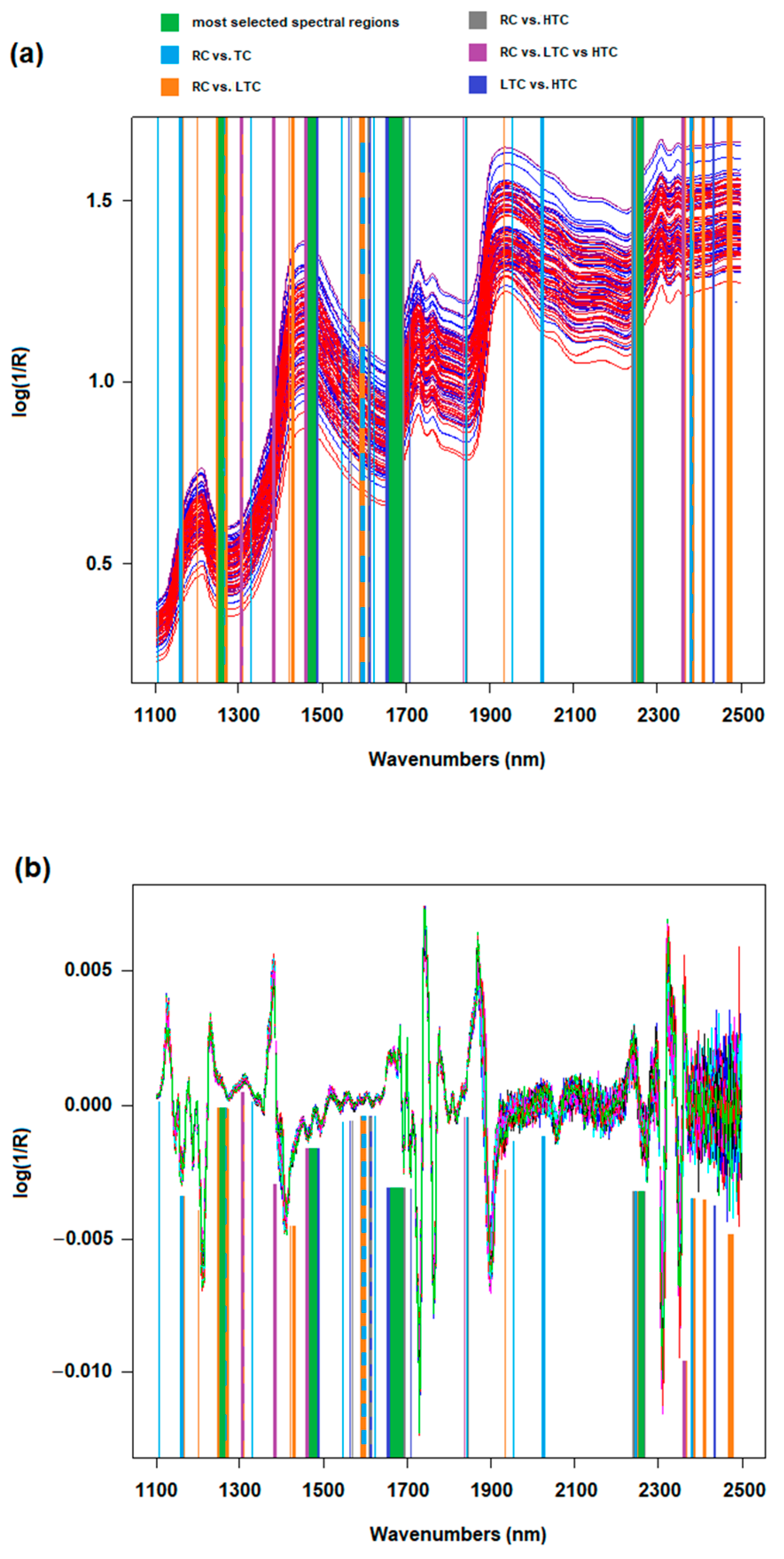
3.1. Analysis of NIR Spectra
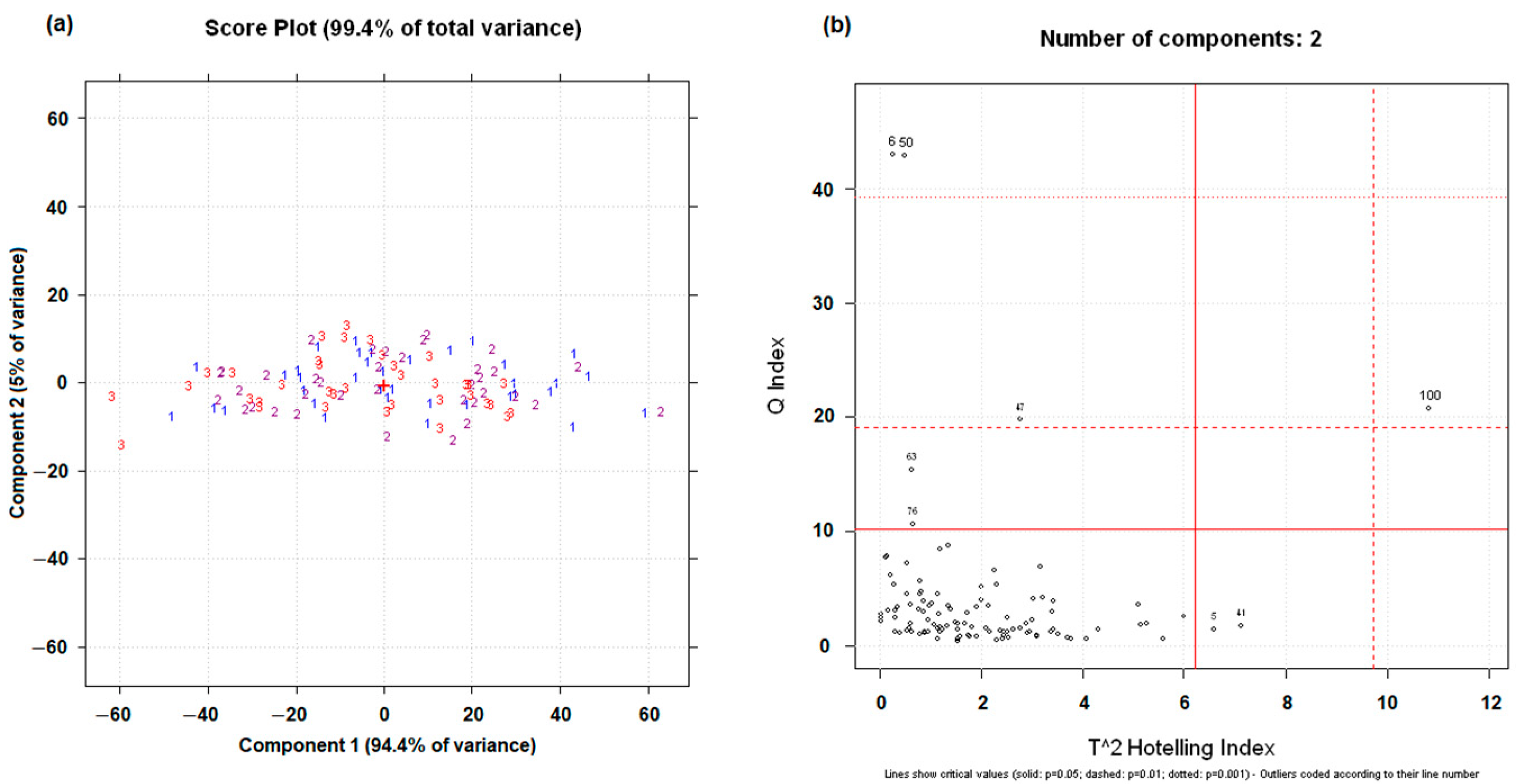
3.2. Principal Component Analysis
3.3. Building of LDA Models
3.3.1. Linear Discriminant Analysis to Discriminate RC, LTC, and HTC Samples
3.3.2. Linear Discriminant Analysis to Discriminate RC and LTC Samples
3.3.3. Linear Discriminant Analysis to Discriminate RC and HTC Samples
3.3.4. Linear Discriminant Analysis to Discriminate RC and TC Samples
3.3.5. Linear Discriminant Analysis to Discriminate LTC and HTC Samples
3.3.6. Misclassified Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PDO | Protected designation of origin |
| NIR | Near-infrared |
| ALP | Alkaline phosphatase |
| GGT | γ-glutamyltransferase |
| NMR | Nuclear magnetic resonance |
| MRI | Magnetic resonance imaging |
| GA | Genetic algorithm |
| FT-MIR | Fourier transform mid infrared |
| RM | Raw milk |
| LTM | Low-thermized milk |
| HTM | High-thermized milk |
| RC | Raw-milk cheeses |
| LTC | Low-thermized-milk cheeses |
| HTC | High-thermized-milk cheeses |
| PCA | Principal component analysis |
| LDA | Linear discriminant analysis |
| SNV | Standard normal variate |
| MSC | Multiplicative scatter correction |
| CV | Cross validation |
References
- Pinna, G.; Pirisi, A.; Piredda, G.; Addis, M.; Di Salvo, R. Effetto della termizzazione del latte sul formaggio DOP Fiore Sardo: 2. Andamento della Lipolisi nel Corso della Maturazione. Sci. Tec. Latt. Casearia 1999, 50, 366–377. [Google Scholar]
- Pirisi, A.; Pinna, G.; Papoff, C.M. Effetto della termizzazione del latte sul formaggio DOP Fiore Sardo: 1. Caratteristiche Fisico-Chimiche. Sci. Tec. Latt. Casearia 1999, 50, 353–366. [Google Scholar]
- Caboni, P.; Maxia, D.; Scano, P.; Addis, M.; Dedola, A.; Pes, M.; Murgia, A.; Casula, M.; Profumo, A.; Pirisi, A. A gas chromatography-mass spectrometry untargeted metabolomics approach to discriminate Fiore Sardo cheese produced from raw or thermized ovine milk. J. Dairy Sci. 2019, 102, 5005–5018. [Google Scholar] [CrossRef]
- Vélez, M.A.; Perotti, M.C.; Wolf, I.V.; Hynes, E.R.; Zalazar, C.A. Influence of milk pretreatment on production of free fatty acids and volatile compounds in hard cheeses: Heat treatment and mechanical agitation. J. Dairy Sci. 2010, 93, 4545–4554. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Xu, C.; Yao, W.; Yu, H.; Pan, X.; Chen, C. Changes of lactones in milk and their correlation with lipids under different heating treatments. Food Biosci. 2025, 66, 106190. [Google Scholar] [CrossRef]
- Breunig, S.; Fan, Z.; Keijzer, P.; Hettinga, K.; Bijl, E. Heating affects gelation properties and casein micelle structure in goat and cow milk differently. Food Struct. 2025, 44, 100438. [Google Scholar] [CrossRef]
- Pellegrino, L.; Resmini, P.; Luf, W. Assessment (indices) of heat treatment of milk. In Heat-Induced Changes in Milk, 2nd ed.; Fox, P.F., Ed.; International Dairy Federation: Brussels, Belgium, 1995; pp. 409–453. [Google Scholar]
- Piga, C.; Urgeghe, P.P.; Piredda, G.; Scintu, M.F.; Sanna, G. Analytical Methods for the Evaluation of α-L-Fucosidase Activity in Sheep Milk. Food. Anal. Methods 2010, 3, 17–21. [Google Scholar] [CrossRef]
- Piga, C.; Urgeghe, P.P.; Piredda, G.; Scintu, M.F.; Di Salvo, R.; Sanna, G. Thermal inactivation and variability of γ-glutamyltranferase and α-L-fucosidase enzymatic activity in sheep milk. LWT Food Sci. Technol. 2013, 54, 152–156. [Google Scholar] [CrossRef]
- Dedola, A.S.; Piras, L.; Addis, M.; Pirisi, A.; Piredda, G.; Mara, A.; Sanna, G. New Analytical Tools for Unmasking Frauds in Raw Milk-Based Dairy Products: Assessment, Validation and Application to Fiore Sardo PDO Cheese of a RP-HPLC Method for the Evaluation of the α-1-Fucosidase Activity. Separations 2020, 7, 40. [Google Scholar] [CrossRef]
- Aït-Kaddour, A.; Hassoun, A.; Bord, C.; Schmidt-Filgueras, R.; Biancolillo, A.; Di Donato, F.; Temiz, H.T.; Cozzolino, D. Application of Spectroscopic Techniques to Evaluate Heat Treatments in Milk and Dairy Products: An Overview of the Last Decade. Food Bioprocess Technol. 2021, 14, 781–803. [Google Scholar] [CrossRef]
- Curti, E.; Pardu, A.; Del Vigo, S.; Sanna, R.; Anedda, R. Non-invasive monitoring of curd syneresis upon renneting of raw and heat-treated cows’ and goats’ milk. Int. Dairy J. 2019, 90, 95–97. [Google Scholar] [CrossRef]
- Curti, E.; Pardu, A.; Melis, R.; Addis, M.; Pes, M.; Pirisi, A.; Anedda, R. Molecular mobility changes after high-temperature, short-time pasteurization: An extended time-domain nuclear magnetic resonance screening of ewe milk. J. Dairy Sci. 2020, 103, 9881–9892. [Google Scholar] [CrossRef] [PubMed]
- Mungkarndee, R.; Techakriengkrai, I.; Tumcharern, G.; Sukwattanasinitt, M. Fluorescence sensor array for identification of commercial milk samples according to their thermal treatments. Food Chem. 2016, 197, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Yazgan, N.N.; Genis, H.E.; Bulat, T.; Topcu, A.; Durna, S.; Yetisemiyen, A.; Boyaci, I.H. Discrimination of milk species using Raman spectroscopy coupled with partial least squares discriminant analysis in raw and pasteurized milk. J. Sci. Food Agr. 2020, 100, 4756–4765. [Google Scholar] [CrossRef] [PubMed]
- Anedda, R.; Melis, R.; Curti, E. Quality Control in Fiore Sardo PDO Cheese: Detection of Heat Treatment Application and Production Chain by MRI Relaxometry and Image Analysis. Dairy 2021, 2, 270–287. [Google Scholar] [CrossRef]
- Skeie, S.; Feten, G.; Almøy, T.; Østlie, H.; Isaksson, T. The use of near infrared spectroscopy to predict selected free amino acids during cheese ripening. Int. Dairy J. 2006, 16, 236–242. [Google Scholar] [CrossRef]
- Lucas, A.; Andueza, D.; Rock, E.; Martin, B. Prediction of Dry Matter, Fat, pH, Vitamins, Minerals, Carotenoids, Total Antioxidant Capacity, and Color in Fresh and Freeze-Dried Cheeses by Visible-Near-Infrared Reflectance Spectroscopy. J. Agric. Food Chem. 2008, 56, 6801–6808. [Google Scholar] [CrossRef]
- González-Martín, I.; Hernández-Hierro, J.M.; González-Pérez, C.; Revilla, I.; Vivar-Quintana, A.; Lobos Ortega, I. Potential of near infrared spectroscopy for the analysis of volatile components in cheeses. LWT Food Sci. Technol. 2014, 55, 666–673. [Google Scholar] [CrossRef]
- Manuelian, C.L.; Currò, S.; Penasa, M.; Cassandro, M.; De Marchi, M. Prediction of minerals, fatty acid composition and cholesterol content of commercial cheeses by near infrared transmittance spectroscopy. Int. Dairy J. 2017, 71, 107–113. [Google Scholar] [CrossRef]
- ISO 23291:2020|IDF 248:2020; Milk and Milk Products—Guidelines for the Application of in-Line and on-Line Infrared Spectrometry. ISO: Geneva, Switzerland, 2020.
- Parrini, S.; Crovetti, A.; Aquilani, C.; Nannucci, L.; Bozzi, R. Near-infrared spectroscopy to assess chemical composition of sheep and goat cheeses. Acta Fytotech. Zootech. 2020, 23, 97–104. [Google Scholar] [CrossRef]
- Bittante, G.; Patel, N.; Cecchinato, A.; Berzaghi, P. Invited review: A comprehensive review of visible and near-infrared spectroscopy for predicting the chemical composition of cheese. J. Dairy Sci. 2022, 105, 1817–1836. [Google Scholar] [CrossRef]
- Pillonel, L.; Luginbühl, W.; Picque, D.; Schaller, E.; Tabacchi, R.; Bosset, J.O. Analytical methods for the determination of the geographic origin of Emmental cheese: Mid- and near-infrared spectroscopy. Eur. Food Res. Technol. 2003, 216, 174–178. [Google Scholar] [CrossRef]
- Karoui, R.; Dufour, É.; Pillonel, L.; Schaller, E.; Picque, D.; Cattenoz, T.; Bosset, J.O. The potential of combined infrared and fluorescence spectroscopies as a method of determination of the geographic origin of Emmental cheeses. Int. Dairy J. 2005, 15, 287–298. [Google Scholar] [CrossRef]
- Cattaneo, T.M.P.; Tornelli, C.; Erini, S.; Panarelli, E.V. Relationship between sensory scores and near infrared absorptions in characterizing bitto, an Italian protected denomination of origin cheese. J. Near Infrared Spectrosc. 2008, 16, 173–178. [Google Scholar] [CrossRef]
- Silva Larissa, K.R.; Jesus Josane, C.; Onelli Rebeca, R.V.; Conceição Daniele, G.; Santos Leandro, S.; Ferrão Sibelli, P.B. Discriminating Coalho cheese by origin through near and middle infrared spectroscopy and analytical measures. Discrimination of Coalho cheese origin. Int. J. Dairy Technol. 2021, 74, 393–403. [Google Scholar] [CrossRef]
- González-Martín, M.I.; Hernández-Hierro, J.M.; Morón-Sancho, R.; Salvador-Esteban, J.; Vivar-Quintana, A.; Revilla, I. Determination of the percentage of milk (cow’s, ewe’s and goat’s) in cheeses with different ripening times using near infrared spectroscopy technology and a remote reflectance fibre-optic probe. Anal. Chim. Acta 2007, 604, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Ottavian, M.; Facco, P.; Barolo, M.; Berzaghi, P.; Segato, S.; Novelli, E.; Balzan, S. Near-infrared spectroscopy to assist authentication and labeling of Asiago d’allevo cheese. J. Food Eng. 2012, 113, 289–298. [Google Scholar] [CrossRef]
- Martín-Tornero, E.; Duràn-Meràs, I.; Alcaraz, M.R.; Munoz de la Pena, A.; Galeano-Díaz, T.; Goicoechea, H.C. Applying multivariate curve resolution modelling combined with discriminant tools on near-infrared spectra for distinguishing between cheese varieties and stages of ripening. Microchem. J. 2024, 204, 111039. [Google Scholar] [CrossRef]
- Andueza, D.; Agabriel, C.; Constant, I.; Lucas, A.; Martin, B. Using visible or near infrared spectroscopy (NIRS) on cheese to authenticate cow feeding regimes. Food Chem. 2013, 141, 209–214. [Google Scholar] [CrossRef]
- Spink, J.; Moyer, D.C. Defining the public health threat of food fraud. J. Food Sci. 2011, 76, R157–R163. [Google Scholar] [CrossRef]
- Everstine, K.; Spink, J.; Kennedy, S. Economically Motivated Adulteration (EMA) of Food: Common Characteristics of EMA Incidents. J. Food Prot. 2013, 76, 723–735. [Google Scholar] [CrossRef]
- Visconti, L.G.; Gonçalves Dias Diniz, P.H.; de Sousa Fernandes, D.D.; Rodriguez, M.S.; Di Anibal, C.V. Authentication of grated hard cheeses and quantification of adulteration by FT-NIR spectroscopy and multivariate analysis. Int. Dairy J. 2024, 158, 106035. [Google Scholar] [CrossRef]
- Atanassova, S.; Yorgov, D.; Veleva, P.; Stoyanchev, T.; Zlatev, Z. Cheese quality assessment by use of near-infrared spectroscopy—69th Scientific Conference with International Participation “Food Science, Engineering and Technology—2022”, Plovdiv, Bulgaria, 29–30 September 2022. BIO Web Conf. 2023, 58, 02007. [Google Scholar] [CrossRef]
- da Silva Medeiros, M.L.; Freitas Lima, A.; Gonçalves, M.C.; Teixeira Godoy, H.; Barbin, D.F. Portable near-infrared (NIR) spectrometer and chemometrics for rapid identification of butter cheese adulteration. Food Chem. 2023, 425, 136461. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, L.; Mlcek, J.; Sustova, K. Comparison of FT-NIR Spectroscopy and ELISA for Detection of Adulteration of Goat Cheeses with Cow’s Milk. J. AOAC Int. 2016, 99, 180–186. [Google Scholar] [CrossRef] [PubMed]
- da Paixão Teixeira, J.L.; dos Santos Caramês, E.T.; Parra Baptista, D.; Gigante, M.L.; Azevedo Lima Pallone, J. Rapid adulteration detection of yogurt and cheese made from goat milk by vibrational spectroscopy and chemometric tools. J. Food Compos. Anal. 2021, 96, 103712. [Google Scholar] [CrossRef]
- Alinovi, M.; Mucchetti, G.; Tidona, F. Application of NIR spectroscopy and image analysis for the characterisation of grated Parmigiano-Reggiano cheese. Int. Dairy J. 2019, 92, 50–58. [Google Scholar] [CrossRef]
- Calvini, R.; Michelini, S.; Pizzamiglio, V.; Foca, G.; Ulrici, A. Exploring the potential of NIR hyperspectral imaging for automated quantification of rind amount in grated Parmigiano Reggiano cheese. Food Control 2020, 112, 107111. [Google Scholar] [CrossRef]
- Hebling e Tavares, J.P.; da SilvaMedeiros, M.L.; Barbin, D.F. Near-infrared techniques for fraud detection in dairy products: A review. J. Food Sci. 2022, 87, 1943–1960. [Google Scholar] [CrossRef]
- Abedini, A.; Salimi, M.; Mazaheri, Y.; Sadighara, P.; Sani, M.A.; Assadpour, E.; Jafari, S.M. Assessment of cheese frauds, and relevant detection methods: A systematic review. Food Chem. X 2023, 19, 100825. [Google Scholar] [CrossRef]
- Leardi, R. Genetic Algorithms in Feature Selection. In Genetic Algorithms in Molecular Modeling; A volume in Principles of QSAR and Drug Design; Devillers, J., Ed.; Academic Press: Lyon, France, 1996; pp. 67–86. [Google Scholar] [CrossRef]
- Leardi, R. Application of genetic algorithm-PLS for feature selection in spectral data sets. J. Chemom. 2000, 14, 5–6, 643–655. [Google Scholar] [CrossRef]
- Leardi, R. Genetic algorithms. In Comprehensive Chemometrics; Brown, S.D., Tauler, R., Walczak, B., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2009; pp. 631–653. [Google Scholar] [CrossRef]
- Ferreiro, B.; Leardi, R.; Farinini, E.; Andrade, J.M. Supervised classification combined with genetic algorithm variable selection for a fast identification of polymeric microdebris using infrared reflectance. Mar. Pollut. Bull. 2023, 195, 115540. [Google Scholar] [CrossRef] [PubMed]
- Caredda, M.; Addis, M.; Ibba, I.; Leardi, R.; Scintu, M.F.; Piredda, G.; Sanna, G. Prediction of fatty acid content in sheep milk by Mid-Infrared spectrometry with a selection of wavelengths by Genetic Algorithms. LWT Food Sci. Technol. 2016, 65, 503–510. [Google Scholar] [CrossRef]
- Caredda, M.; Addis, M.; Ibba, I.; Leardi, R.; Scintu, M.F.; Piredda, G.; Sanna, G. Building of prediction models by using Mid-Infrared spectroscopy and fatty acid profile to discriminate the geographical origin of sheep milk. LWT Food Sci. Technol. 2017, 75, 131–136. [Google Scholar] [CrossRef]
- Molle, G.; Cabiddu, A.; Decandia, M.; Sitzia, M.; Ibba, I.; Giovanetti, V.; Scanu, G.; Addis, M.; Caredda, M. Can FT-Mid-Infrared Spectroscopy of Milk Samples Discriminate Different Dietary Regimens of Sheep Grazing With Restricted Access Time? Front. Vet. Sci. 2021, 8, 623823. [Google Scholar] [CrossRef] [PubMed]
- Caredda, M.; Mara, A.; Ciulu, M.; Floris, I.; Pilo, M.I.; Spano, N.; Sanna, G. Use of genetic algorithms in the wavelength selection of FT-MIR spectra to classify unifloral honeys from Sardinia. Food Control 2023, 146, 109559. [Google Scholar] [CrossRef]
- Caredda, M.; Ciulu, M.; Tilocca, F.; Langasco, I.; Núñez, O.; Sentellas, S.; Saurina, J.; Pilo, M.I.; Spano, N.; Sanna, G.; et al. Portable NIR Spectroscopy to Simultaneously Trace Honey Botanical and Geographical Origins and Detect Syrup Adulteration. Foods 2024, 13, 3062. [Google Scholar] [CrossRef]
- CAT (Chemometric Agile Tool). Available online: https://gruppochemiometria.it/index.php/software (accessed on 8 May 2025).
- Rodriguez-Otero, J.L.; Hermida, M.; Centeno, J. Analysis of Dairy Products by Near-Infrared Spectroscopy: A Review. J. Agric. Food Chem. 1997, 45, 2815–2819. [Google Scholar] [CrossRef]
- Stuart, B. Organic Molecules. In Infrared Spectroscopy: Fundamentals and Applications; Wiley, J. & Sons: Wiley Hoboken, NJ, USA, 2004; pp. 71–93. [Google Scholar] [CrossRef]
- Niemöller, A.; Behmer, D. Use of near infrared spectroscopy in the food industry. In Nondestructive Testing of Food Quality; Irudayaraj, J., Reh, C., Eds.; Blackwell Publishing and the Institute of Food Technologists: Ames, IA, USA, 2008; pp. 67–118. [Google Scholar]
- Schwanninger, M.; Rodrigues, J.C.; Fackler, K. A review of band assignments in near infrared spectra of wood and wood components. J. Near Infrared Spectrosc. 2011, 19, 287–308. [Google Scholar] [CrossRef]
- Bobozka, L. Near infrared spectroscopy. J. Near Infrared Spectrosc. 1998, 6, 3–17. [Google Scholar] [CrossRef]
- Clawin-Rädecker, I.; De Block, J.; Egger, L.; Willis, C.; Da Silva Felicio, M.T.; Messens, W. The use of alkaline phosphatase and possible alternative testing to verify pasteurisation of raw milk, colostrum, dairy and colostrum-based products. EFSA J. 2021, 19, 6576. [Google Scholar] [CrossRef]
- Leardi, R. Genetic algorithm-PLS as a tool for wavelength selection in spectral data sets. In Nature-Inspired Methods in Chemometrics: Genetic Algorithms and Artificial Neural Networks, Data Handling in Science and Technology Series; Leardi, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 23, pp. 169–196. [Google Scholar] [CrossRef]

| Categories to Be Discriminated | Spectral Pretreatment | Accuracy (%) | Accuracy (%) | Accuracy (%) |
|---|---|---|---|---|
| LDA Model Using the Wavelengths Selected on the Entire Spectra (By Averaging over 4 Adjacent Variables) | LDA Model Using the Wavelengths Selected on Two Portions of the Spectra (By Averaging over 2 Adjacent Variables) | LDA Model Using the Wavelengths Selected on Four Portions of the Spectra (Without Averaging Variables) | ||
| RC vs. LTC vs. HTC | None | 47 | 57 | 63 |
| None + 1st derivative | 53 | 58 | 59 | |
| None + 2nd derivative | 43 | 51 | 67 | |
| SNV | 57 | 56 | 60 | |
| SNV + 1st derivative | 46 | 63 | 74 | |
| SNV + 2nd derivative | 53 | 51 | 76 | |
| MSC | 41 | 60 | 64 | |
| MSC + 1st derivative | 48 | 54 | 65 | |
| MSC + 2nd derivative | 45 | 67 | 65 | |
| RC vs. LTC | None | 74 | 76 | 73 |
| None + 1st derivative | 54 | 70 | 76 | |
| None + 2nd derivative | 53 | 67 | 93 | |
| SNV | 70 | 73 | 77 | |
| SNV + 1st derivative | 71 | 59 | 81 | |
| SNV + 2nd derivative | 79 | 79 | 81 | |
| MSC | 56 | 64 | 77 | |
| MSC + 1st derivative | 71 | 79 | 84 | |
| MSC + 2nd derivative | 80 | 80 | 81 | |
| RC vs. HTC | None | 67 | 71 | 81 |
| None + 1st derivative | 70 | 77 | 90 | |
| None + 2nd derivative | 77 | 81 | 83 | |
| SNV | 71 | 67 | 80 | |
| SNV + 1st derivative | 81 | 89 | 89 | |
| SNV + 2nd derivative | 90 | 86 | 87 | |
| MSC | 70 | 70 | 80 | |
| MSC + 1st derivative | 64 | 91 | 84 | |
| MSC + 2nd derivative | 70 | 83 | 91 | |
| RC vs. TC | None | 66 | 73 | 74 |
| None + 1st derivative | 64 | 72 | 80 | |
| None + 2nd derivative | 54 | 65 | 89 | |
| SNV | 61 | 77 | 79 | |
| SNV + 1st derivative | 73 | 71 | 79 | |
| SNV + 2nd derivative | 71 | 68 | 84 | |
| MSC | 59 | 75 | 73 | |
| MSC + 1st derivative | 74 | 78 | 80 | |
| MSC + 2nd derivative | 67 | 76 | 81 | |
| LTC vs. HTC | None | 66 | 71 | 69 |
| None + 1st derivative | 81 | 71 | 83 | |
| None + 2nd derivative | 86 | 73 | 84 | |
| SNV | 61 | 73 | 80 | |
| SNV + 1st derivative | 73 | 74 | 79 | |
| SNV + 2nd derivative | 64 | 86 | 86 | |
| MSC | 66 | 73 | 76 | |
| MSC + 1st derivative | 67 | 77 | 84 | |
| MSC + 2nd derivative | 63 | 90 | 84 |
| RC | LTC | HTC | Sensitivity | Specificity | F1 Score | Accuracy (%) | Selected Spectral Regions (2nd Derivative SNV Spectra) | ||
| RC | 27 | 5 | 3 | 0.771 | 0.914 | 0.794 | 1256–1258 nm, 1306–1308 nm, 1378–1380 nm, 1466–1470 nm, 1474 nm, 1676 nm, 1680–1688 nm, 1832 nm, 2356–2360 nm | ||
| LTC | 4 | 25 | 6 | 0.714 | 0.857 | 0.714 | 76 | ||
| HTC | 2 | 5 | 28 | 0.800 | 0.871 | 0.778 | |||
| RC | LTC | Sensitivity | Specificity | F1 Score | Accuracy (%) | Selected Spectral Regions (2nd Derivative Spectra) | |||
| RC | 34 | 1 | 0.971 | 0.886 | 0.931 | 93 | 1136 nm, 1198 nm, 1254–1262 nm, 1306 nm, 1322–1324 nm, 1432 nm, 1436–1438 nm, 1470–1472 nm, 1594–1598 nm, 1930 nm, 2260–2264 nm, 2362 nm, 2376–2380 nm, 2408–2414 nm, 2460–2468 nm | ||
| LTC | 4 | 31 | 0.886 | 0.971 | 0.925 | ||||
| RC | HTC | Sensitivity | Specificity | F1 Score | Accuracy (%) | Selected Spectral Regions (2nd Derivative MSC Spectra) | |||
| RC | 32 | 3 | 0.914 | 0.914 | 0.914 | 91 | 1574–1576 nm, 1606–1612 nm, 1678–1690 nm, 1840–1844 nm, 2152–2156 nm, 2262–2266 nm | ||
| HTC | 3 | 32 | 0.914 | 0.914 | 0.914 | ||||
| RC | TC | Sensitivity | Specificity | F1 Score | Accuracy (%) | Selected Spectral Regions (2nd Derivative Spectra) | |||
| RC | 31 | 4 | 0.886 | 0.886 | 0.838 | 89 | 1106 nm, 1132–1134 nm, 1256–1260 nm, 1472 nm, 1540 nm, 1596–1598 nm, 1618–1622 nm, 1838–1840 nm, 1950 nm, 2020–2024 nm, 2158 nm, 2262–2264 nm, 2376–2378 nm | ||
| TC | 8 | 62 | 0.886 | 0.886 | 0.912 | ||||
| LTC | HTC | Sensitivity | Specificity | F1 Score | Accuracy (%) | Selected Spectral Regions (2nd Derivative MSC Spectra) | |||
| LTC | 31 | 4 | 0.886 | 0.914 | 0.899 | 90 | 1472–1476 nm, 1568–1570 nm, 1612–1614 nm, 1672–1686 nm, 1704–1706 nm, 2436–2438 nm | ||
| HTC | 3 | 32 | 0.914 | 0.886 | 0.901 | ||||
| Category | Models | ||||
|---|---|---|---|---|---|
| RC vs. LTC vs. HTC | RC vs. TC | RC vs. LTC | RC vs. HTC | LTC vs. HTC | |
| RC | 3 | ||||
| 4 | 4 | ||||
| 7 | |||||
| 11 | 11 | 11 | |||
| 15 | |||||
| 16 | 16 | ||||
| 20 | 20 | ||||
| 21 | 21 | 21 | |||
| 25 | 25 | 25 | 25 | ||
| 27 | 27 | ||||
| 31 | 31 | 31 | |||
| LTC | 38 | ||||
| 41 | |||||
| 42 | |||||
| 45 | 45 | ||||
| 48 | 48 | 48 | |||
| 53 | |||||
| 55 | 55 | 55 | |||
| 57 | 57 | ||||
| 60 | |||||
| 61 | |||||
| 62 | 62 | ||||
| 64 | |||||
| 65 | |||||
| 67 | |||||
| 70 | |||||
| HTC | 71 | 71 | 71 | ||
| 80 | |||||
| 81 | 81 | ||||
| 82 | |||||
| 84 | |||||
| 85 | |||||
| 86 | 86 | ||||
| 94 | |||||
| 98 | |||||
| 100 | 100 | ||||
| 101 | |||||
| 105 | |||||
| 71 | 71 | 71 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caredda, M.; Dedola, A.S.; Pes, M.; Addis, M. The Use of NIR Spectroscopy and Chemometrics to Identify the Thermal Treatment of Milk in Fiore Sardo PDO Cheese to Detect Fraud. Foods 2025, 14, 2288. https://doi.org/10.3390/foods14132288
Caredda M, Dedola AS, Pes M, Addis M. The Use of NIR Spectroscopy and Chemometrics to Identify the Thermal Treatment of Milk in Fiore Sardo PDO Cheese to Detect Fraud. Foods. 2025; 14(13):2288. https://doi.org/10.3390/foods14132288
Chicago/Turabian StyleCaredda, Marco, Alessio Silvio Dedola, Massimo Pes, and Margherita Addis. 2025. "The Use of NIR Spectroscopy and Chemometrics to Identify the Thermal Treatment of Milk in Fiore Sardo PDO Cheese to Detect Fraud" Foods 14, no. 13: 2288. https://doi.org/10.3390/foods14132288
APA StyleCaredda, M., Dedola, A. S., Pes, M., & Addis, M. (2025). The Use of NIR Spectroscopy and Chemometrics to Identify the Thermal Treatment of Milk in Fiore Sardo PDO Cheese to Detect Fraud. Foods, 14(13), 2288. https://doi.org/10.3390/foods14132288







