Developing a Functional Triticale Noodle by Incorporating Silkworm (Antheraea pernyi and Bombyx mori) Pupae
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Triticale Flour and Insect Powders
2.3. Preparation of Triticale Noodles Fortified with Silkworm Pupae
2.4. Proximate Analysis
2.5. Texture Profile Analysis
2.6. Color Measurement
2.7. Analysis of Total Phenolic Content and Antioxidant Power
2.7.1. Extraction of Phenolic Compounds
2.7.2. Analysis of Total Phenolic Content
2.7.3. Analysis of DPPH Radical Scavenging Activity
2.7.4. Analysis of ABTS Radical Scavenging Activity
2.8. Starch Digestibility Analysis
2.9. Analysis of Protein Digestibility
2.10. E-Nose Analysis
2.11. E-Tongue Analysis
2.12. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition of Triticale and Silkworm Pupae
3.2. Proximate Composition of Triticale Noodles
3.3. Texture Profile
3.4. Color of Triticale Noodles
3.5. Antioxidant Properties of Triticale Noodles
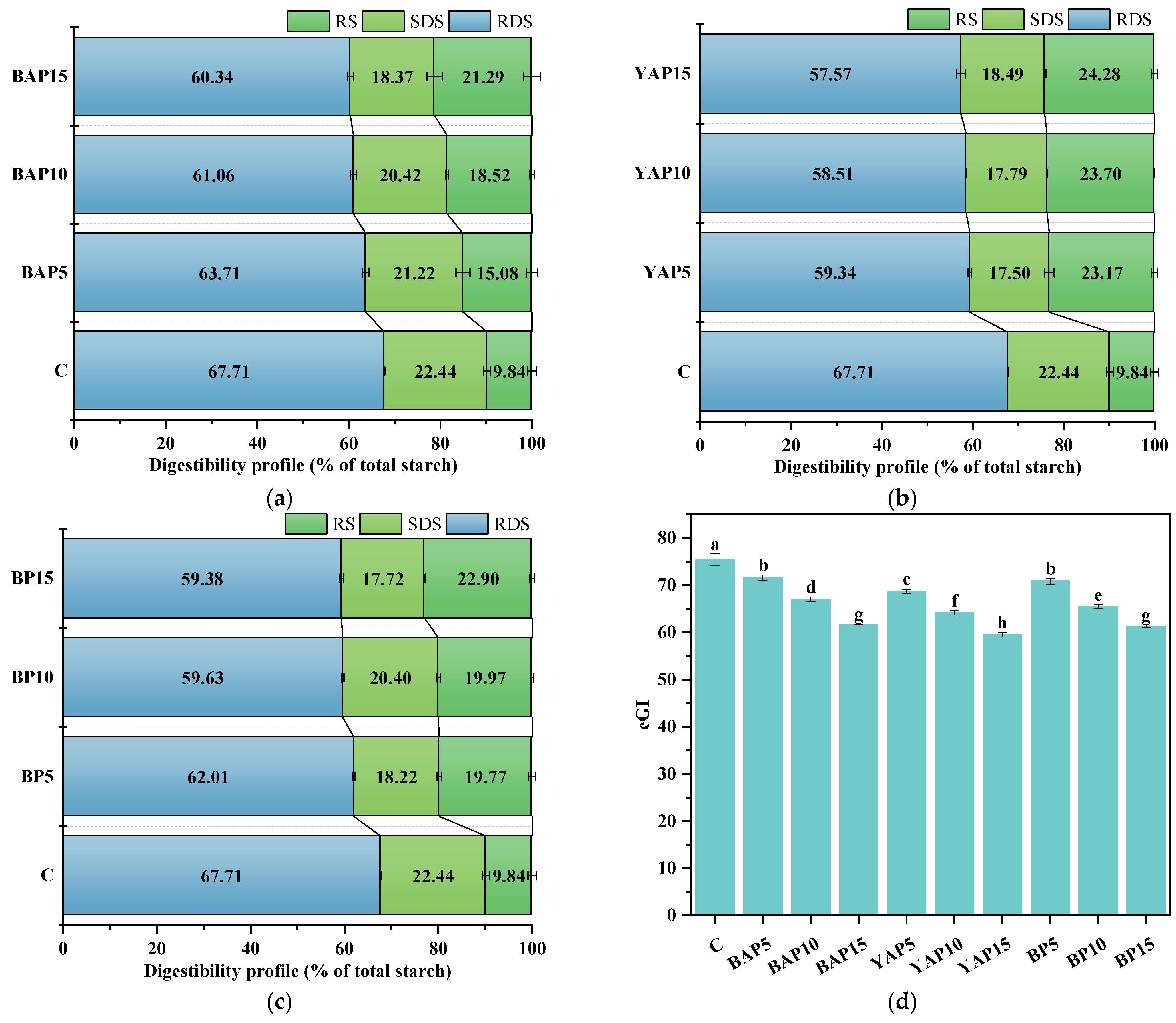
3.6. Starch Digestibility
3.7. In Vitro Protein Digestibility
3.8. E-Nose Analysis
3.9. E-Tongue Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, Q.; Ren, A.; Liu, R.; Xing, Y.; Yu, X.; Jiang, H. Flavor properties of Chinese noodles processed by dielectric drying. Front. Nutr. 2022, 9, 1007997. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Bhagya, S. Quality characterization of pasta enriched with mustard protein isolate. J. Food Sci. 2008, 73, S229–S237. [Google Scholar] [CrossRef]
- Roccatello, R.; Cerroni, S.; Dabbou, S. Sustainability of insect-based feed and consumer willingness to pay for novel food: A stated preference study. Future Foods 2024, 9, 100336. [Google Scholar] [CrossRef]
- Zielińska, E.; Pankiewicz, U. Nutritional, physiochemical, and antioxidative characteristics of shortcake biscuits enriched with Tenebrio molitor flour. Molecules 2020, 25, 5629. [Google Scholar] [CrossRef]
- Kouřimská, L.; Adámková, A. Nutritional and sensory quality of edible insects. NFS J. 2016, 4, 22–26. [Google Scholar] [CrossRef]
- Karnjanapratum, S.; Kaewthong, P.; Indriani, S.; Petsong, K.; Takeungwongreakul, S. Characteristics and nutritional value of silkworm (Bombyx mori) pupae-fortified chicken bread spread. Sci. Rep. 2022, 12, 1492. [Google Scholar] [CrossRef]
- Tomotake, H.; Katagiri, M.; Yamato, M. Silkworm pupae (Bombyx mori) are new sources of high quality protein and lipid. J. Nutr. Sci. Vitaminol. 2010, 56, 446–448. [Google Scholar] [CrossRef]
- Looy, H.; Dunkel, F.; Wood, J. How then shall we eat? Insect-eating attitudes and sustainable food ways. Agric. Hum. Values 2014, 31, 131–141. [Google Scholar] [CrossRef]
- Tuccillo, F.; Marino, M.; Torri, L. Italian consumers’ attitudes towards entomophagy: Influence of human factors and properties of insects and insect-based food. Food Res. Int. 2020, 137, 109619. [Google Scholar] [CrossRef]
- Akande, A.; Jolayemi, O.; Adelugba, V.; Akande, S. Silkworm pupae (Bombyx mori) and locusts as alternative protein sources for high-energy biscuits. J. Asia-Pac. Entomol. 2020, 23, 234–241. [Google Scholar] [CrossRef]
- Torres, K.; Sampaio, R.; Ferreira, T.; Argondoña, E. Development of cookie enriched with silkworm pupae (Bombyx mori). J. Food Meas. Charact. 2022, 16, 1540–1548. [Google Scholar] [CrossRef]
- Biró, B.; Fodor, R.; Szedljak, I.; Pásztor-Huszár, K.; Gere, A. Buckwheat-pasta enriched with silkworm powder: Technological analysis and sensory evaluation. LWT 2019, 116, 108542. [Google Scholar] [CrossRef]
- Piazza, L.; Ratti, S.; Girotto, F.; Cappellozza, S. Silkworm pupae derivatives as source of high value protein intended for pasta fortification. J. Food Sci. 2023, 88, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, A.; Cullere, M.; Zotte, A.; Pasini, G. Salt-soluble protein extracts from Hermetia illucens and Bombyx mori for high protein pasta production. LWT 2023, 190, 115507. [Google Scholar] [CrossRef]
- Hirunyophat, P.; Chalermchaiwat, P.; On-nom, N.; Prinyawiwatkul, W. Selected physicochemical properties and sensory acceptability as affected by addition of lecithin and calcium carbonate in extruded breakfast cereals made with silkworm pupae powder and rice flour. Int. J. Food Sci. Technol. 2022, 57, 631–642. [Google Scholar] [CrossRef]
- Suk, W.; Kim, J.; Kim, D.; Lim, H.; Choue, R. Effect of wheat flour noodles with Bombyx mori powder on glycemic response in healthy subjects. Prev. Nutr. Food Sci. 2016, 21, 165–170. [Google Scholar] [CrossRef]
- Mafu, A.; Ketnawa, S.; Phongthai, S.; Schönlechner, R.; Rawdkuen, S. Whole wheat bread enriched with cricket powder as an alternative protein. Foods 2022, 11, 2142. [Google Scholar] [CrossRef]
- Li, H.; Mao, Y.; Ma, D.; Li, H.; Liu, R.; Siriamornpun, S. Impact of cooking methods on phenolic acid composition, antioxidant activity, and starch digestibility of Chinese triticale porridges: A comparative study between atmospheric pressure and high pressure boiling. Foods 2024, 13, 230. [Google Scholar] [CrossRef]
- Zhu, F. Triticale: Nutritional composition and food uses. Food Chem. 2018, 241, 468–479. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; AOAC-Association of Analytical Chemists International: Gainthersburg, MD, USA, 2006. [Google Scholar]
- Wang, L.; Tang, H.; Li, Y.; Guo, Z.; Zou, L.; Li, Z.; Qiu, J. Milling of buckwheat hull to cell-scale: Influences on the behaviors of protein and starch in dough and noodles. Food Chem. 2023, 423, 136347. [Google Scholar] [CrossRef]
- Yu, K.; Huang, X.; He, W.; Ma, X.; Wu, D.; Ding, Z.; Li, P.; Du, C. Evaluation of the effects of thermal processing on antioxidant activity and digestibility of green tea noodles: Based on polyphenol stability and starch structure. J. Cereal Sci. 2023, 114, 103780. [Google Scholar] [CrossRef]
- Peñalver, R.; Nieto, G. Developing a functional gluten-free sourdough bread by incorporating quinoa, amaranth, rice and spirulina. LWT 2024, 201, 116162. [Google Scholar] [CrossRef]
- Farzana, T.; Abedin, M.; Abdullah, A.; Reaz, A. Exploring the impact of pumpkin and sweet potato enrichment on the nutritional profile and antioxidant capacity of noodles. J. Agric. Food Res. 2023, 14, 100849. [Google Scholar] [CrossRef]
- Tang, P.; Zhang, S.; Meng, L.; Wang, Z.; Yang, Y.; Shen, X.; Tang, X. Effects of different content of EGCG or caffeic acid addition on the structure, cooking, antioxidant characteristics and in vitro starch digestibility of extruded buckwheat noodles. Int. J. Biol. Macromol. 2023, 252, 126426. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, S33–S50. [Google Scholar]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food-an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Fan, X.; Zhong, M.; Feng, L.; Huo, Y.; Pan, L. Evaluation of flavor characteristics in tartary buckwheat (Fagopyrum tataricum) by E-nose, GC-IMS, and HS-SPME-GC-MS: Influence of different roasting temperatures. LWT 2024, 191, 115672. [Google Scholar] [CrossRef]
- Du, H.; Chen, Q.; Liu, Q.; Wang, Y.; Kong, B. Evaluation of flavor characteristics of bacon smoked with different woodchips by HS-SPME-GC-MS combined with an electronic tongue and electronic nose. Meat Sci. 2021, 182, 108626. [Google Scholar] [CrossRef]
- Piazza, I.; Carnevali, P.; Faccini, N.; Baronchelli, M.; Terzi, V.; Morcia, C.; Ghizzoni, R.; Patrone, V.; Morelli, L.; Cervini, M.; et al. Combining native and malted triticale flours in biscuits: Nutritional and technological implications. Foods 2023, 12, 3418. [Google Scholar] [CrossRef]
- Li, H.; Chumroenphat, T.; Boonarsa, P.; Yahuafai, J.; Wrigley, C.; Siriamornpun, S. Evaluation of roasting and grilling effects on chemical composition, volatile profiles, and toxicity of stink bugs (Tessaratoma papillosa): Implications for utilization as functional food ingredients. Foods 2023, 12, 3053. [Google Scholar] [CrossRef]
- Yeruva, T.; Jayaram, H.; Aurade, R.; Shunmugam, M.; Shinde, V.; Venkatesharao, S.; Azhiyakathu, M. Profiling of nutrients and bioactive compounds in the pupae of silkworm, Bombyx mori. Food Chem. Adv. 2023, 3, 100382. [Google Scholar] [CrossRef]
- Wang, S.; Meng, Y.; Wang, D. Nutritional profile changes in an insect-fungus complex of Antheraea pernyi pupa infected by Samsoniella hepiali. Foods 2023, 12, 2796. [Google Scholar] [CrossRef] [PubMed]
- Brogan, E.; Park, Y.; Shen, C.; Matak, K.; Jaczynski, J. Characterization of lipids in insect powders. LWT 2023, 184, 115040. [Google Scholar] [CrossRef]
- Srivastava, D.; Tripathi, D.; Singh, V.; Poluri, K.; Kumar R, V. Insights into the characterization and therapeutic potential of Tasar silkworm pupal oil. Biocatal. Agric. Biotechnol. 2024, 55, 102985. [Google Scholar] [CrossRef]
- Chieco, C.; Morrone, L.; Bertazza, G.; Cappellozza, S.; Saviane, A.; Gai, F.; Virgilio, N.; Rossi, F. The effect of strain and rearing medium on the chemical composition, fatty acid profile and carotenoid content in silkworm (Bombyx mori) pupae. Animals 2019, 9, 103. [Google Scholar] [CrossRef]
- Hirunyophat, P.; Chalermchaiwat, P.; On-nom, N.; Prinyawiwatkul, W. Selected nutritional quality and physicochemical properties of silkworm pupae (frozen or powdered) from two species. Int. J. Food Sci. Technol. 2021, 56, 3578–3587. [Google Scholar] [CrossRef]
- Mihaly Cozmuta, A.; Uivarasan, A.; Peter, A.; Nicula, C.; Kovacs, D.; Mihaly Cozmuta, L. Yellow mealworm (Tenebrio molitor) powder promotes a high bioaccessible protein fraction and low glycaemic index in biscuits. Nutrients 2023, 15, 997. [Google Scholar] [CrossRef]
- Tzompa-Sosa, D.A.; Yi, L.; van Valenberg, H.J.F.; van Boekel, M.A.J.S.; Lakemond, C.M.M. Insect lipid profile: Aqueous versus organic solvent-based extraction methods. Food Res. Int. 2014, 62, 1087–1094. [Google Scholar] [CrossRef]
- Ho, I.; Peterson, A.; Madden, J.; Huang, E.; Amin, S.; Lammert, A. Will it cricket? Product development and evaluation of cricket (Acheta domesticus) powder replacement in sausage, pasta, and brownies. Foods 2022, 11, 3128. [Google Scholar] [CrossRef]
- Duda, A.; Adamczak, J.; Chełmińska, P.; Juszkiewicz, J.; Kowalczewski, P. Quality and nutritional/textural properties of durum wheat pasta enriched with cricket powder. Foods 2019, 8, 46. [Google Scholar] [CrossRef]
- Pasini, G.; Cullere, M.; Vegro, M.; Simonato, B.; Zotte, A. Potentiality of protein fractions from the house cricket (Acheta domesticus) and yellow mealworm (Tenebrio molitor) for pasta formulation. LWT 2022, 164, 113638. [Google Scholar] [CrossRef]
- Fu, B. Asian noodles: History, classification, raw materials, and processing. Food Res. Int. 2008, 41, 888–902. [Google Scholar] [CrossRef]
- Barakat, H.; Shama, A.; Denev, P.; Khalifa, I. Incorporation of quinoa seeds accessions in instant noodles improves their textural and quality characteristics. J Food Sci Technol. 2022, 59, 1912–1921. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Seehua, N.; Prakitchaiwattana, C.; Liu, R.; Zheng, J.; Siriamornpun, S. Fortification of cricket and silkworm pupae powders to improve nutrition quality and digestibility of rice noodles. Food Chem.-X. 2025, 26, 102279. [Google Scholar] [CrossRef]
- Zielińska, E.; Pankiewicz, U.; Sujka, M. Nutritional, physiochemical, and biological value of muffins enriched with edible insects flour. Antioxidants 2021, 10, 1122. [Google Scholar] [CrossRef]
- Kowalski, S.; Gumul, D.; Oracz, J.; Rosicka-Kaczmarek, J.; Mikulec, A.; Mickowska, B.; Skotnicka, M.; Zborowski, M. Chemical composition, antioxidant properties and sensory aspects of sponge cakes supplemented with edible insect flours. Antioxidants 2023, 12, 1912. [Google Scholar] [CrossRef]
- Zielińska, E.; Baraniak, B.; Karas, M. Antioxidant and anti-inflammatory activities of hydrolysates and peptide fractions obtained by enzymatic hydrolysis of selected heat-treated edible insects. Nutrients 2017, 9, 970. [Google Scholar] [CrossRef]
- Rocchetti, G.; Zengin, G.; Giuberti, G.; Cervini, M.; Lucini, L. Impact of in vitro gastrointestinal digestion on the phenolic bioaccessibility and bioactive properties of insect-containing beef burgers. Antioxidants 2024, 13, 365. [Google Scholar] [CrossRef]
- Gumul, D.; Oracz, J.; Kowalski, S.; Mikulec, A.; Skotnicka, M.; Karwowska, K.; Areczuk, A. Bioactive compounds and antioxidant composition of nut bars with addition of various edible insect flours. Molecules 2023, 28, 3556. [Google Scholar] [CrossRef]
- Di Mattia, C.; Battista, N.; Sacchetti, G.; Serafini, M. Antioxidant activities in vitro of water and liposoluble extracts obtained by different species of edible insects and invertebrates. Front. Nutr. 2019, 6, 106. [Google Scholar] [CrossRef]
- Fu, X.; Chai, C.; Li, Y.; Li, P.; Luo, S.; Li, Q.; Li, M.; Liu, Y. Metabolomics reveals abundant flavonoids in edible insect Antheraea pernyi. J. Asia-Pac. Entomol. 2021, 24, 711–715. [Google Scholar] [CrossRef]
- David-Birman, T.; Moshe, H.; Lesmes, U. Impact of thermal processing on physicochemical properties of silk moth pupae (Bombyx mori) flour and in-vitro gastrointestinal proteolysis in adults and seniors. Food Res. Int. 2019, 123, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.; Kendall, C.; Augustin, L.; Franceschi, S.; Hamidi, M.; Marchie, A.; Jenkins, A.; Axelsen, M. Glycemic index: Overview of implications in health and disease. Am. J. Clin. Nutr. 2002, 76, 266S–273S. [Google Scholar] [CrossRef] [PubMed]
- Bas, A.; El, S.N. Nutritional evaluation of biscuits enriched with cricket flour (Acheta domesticus). Int. J. Gastron. Food Sci. 2022, 29, 100583. [Google Scholar] [CrossRef]
- Ronoh, A.; Serrem, C.; Tumwebaze, S.; Were, G. Effect of fortifying sorghum and wheat with Longhorn grasshopper (Ruspolia differens) powder on nutritional composition and consumer acceptability of biscuits. Food Sci. Nutr. 2024, 12, 3492–3507. [Google Scholar] [CrossRef]
- Cheng, J.; Zheng, L.; Zhao, J.; Yu, M.; Cao, R.; Wang, D.; Li, J.; Zhou, L. Study on the effect of microwaved brewer’s spent grains on the quality and flavor characteristics of bread. Foods 2024, 13, 461. [Google Scholar] [CrossRef]
- Zhao, X.; Lai, B.; Jiang, Y.; Sun, X.; Luo, Z.; Ge, Q.; Chen, J.; Yu, H. Characterization of flavor profiles of water-boiled pork meatballs at different ultrasonic powers using solid-phase microextraction gas chromatography-mass spectrometry combined with electronic nose. Meat Sci. 2025, 222, 109756. [Google Scholar] [CrossRef]
- Karolkowski, A.; Belloir, C.; Briand, L.; Salles, C. Non-volatile compounds involved in bitterness and astringency of pulses: A review. Molecules 2023, 28, 3298. [Google Scholar] [CrossRef]









| Sample Code | Triticale Flour (g/100 g) | BAP Powder (g/100 g) | YAP Powder (g/100 g) | BP Powder (g/100 g) |
|---|---|---|---|---|
| C | 100 | 0 | 0 | 0 |
| BAP5 | 95 | 5 | 0 | 0 |
| BAP10 | 90 | 10 | 0 | 0 |
| BAP15 | 85 | 15 | 0 | 0 |
| YAP5 | 95 | 0 | 5 | 0 |
| YAP10 | 90 | 0 | 10 | 0 |
| YAP15 | 85 | 0 | 15 | 0 |
| BP5 | 95 | 0 | 0 | 5 |
| BP10 | 90 | 0 | 0 | 10 |
| BP15 | 85 | 0 | 0 | 15 |
| Sensor Name | Aroma Type |
|---|---|
| W1C | Aromatic components |
| W5S | Nitrogen oxides |
| W3C | Aromatic and ammonia components |
| W6S | Hydrides |
| W5C | Aromatic components (short-chain alkanes) |
| W1S | Methyl compounds |
| W1W | Inorganic sulfides, terpenes |
| W2S | Alcohols, aldehydes, and ketones |
| W2W | Aromatic components (organic sulfides) |
| W3S | Long chain alkanes |
| Sample | Protein (%) | Fat (%) | Ash (%) | Carbohydrate (%) |
|---|---|---|---|---|
| TF | 19.32 ± 0.57 d | 1.51 ± 0.06 d | 2.13 ± 0.09 b | 77.05 ± 0.67 a |
| BAP | 55.88 ± 0.55 b | 25.56 ± 0.08 c | 2.18 ± 0.07 b | 16.38 ± 0.42 b |
| YAP | 57.31 ± 0.65 a | 28.18 ± 0.63 b | 1.98 ± 0.08 b | 12.54 ± 0.26 c |
| BP | 49.22 ± 0.93 c | 32.80 ± 0.70 a | 6.09 ± 0.68 a | 11.89 ± 0.90 c |
| Sample | Protein (%) | Fat (%) | Ash (%) | Carbohydrate (%) |
|---|---|---|---|---|
| C | 19.69 ± 0.59 h | 1.60 ± 0.08 h | 1.33 ± 0.02 g | 77.38 ± 0.66 a |
| BAP5 | 21.89 ± 0.02 fg | 1.93 ± 0.07 g | 1.37 ± 0.02 fg | 74.82 ± 0.07 b |
| BAP10 | 23.60 ± 0.09 d | 3.15 ± 0.04 e | 1.43 ± 0.02 ef | 71.81 ± 0.10 e |
| BAP15 | 25.49 ± 0.11 b | 4.38 ± 0.03 c | 1.50 ± 0.02 e | 68.63 ± 0.13 g |
| YAP5 | 22.06 ± 0.10 f | 2.27 ± 0.17 f | 1.37 ± 0.03 fg | 74.30 ± 0.25 c |
| YAP10 | 23.96 ± 0.13 d | 3.59 ± 0.03 d | 1.45 ± 0.06 e | 71.01 ± 0.13 f |
| YAP15 | 25.94 ± 0.18 a | 4.92 ± 0.08 b | 1.57 ± 0.08 d | 67.56 ± 0.18 h |
| BP5 | 21.58 ± 0.04 g | 3.05 ± 0.14 e | 1.73 ± 0.03 c | 73.64 ± 0.17 d |
| BP10 | 22.89 ± 0.17 e | 4.46 ± 0.05 c | 1.92 ± 0.03 b | 70.73 ± 0.25 f |
| BP15 | 24.57 ± 0.19 c | 6.12 ± 0.14 a | 2.16 ± 0.03 a | 67.16 ± 0.27 h |
| Sample | Hardness (g) | Springiness | Cohesiveness | Chewiness | Resilience |
|---|---|---|---|---|---|
| C | 689.37 ± 39.78 f | 0.68 ± 0.19 b | 0.92 ± 0.07 ab | 442.65 ± 167.44 e | 0.57 ± 0.05 cd |
| BAP5 | 947.84 ± 17.81 e | 0.85 ± 0.03 a | 0.95 ± 0.01 a | 767.73 ± 39.62 cd | 0.57 ± 0.02 d |
| BAP10 | 1120.01 ± 86.34 cde | 0.94 ± 0.04 a | 0.95 ± 0.01 a | 994.94 ± 97.21 bcd | 0.62 ± 0.06 abcd |
| BAP15 | 1724.09 ± 272.08 a | 0.92 ± 0.02 a | 0.94 ± 0.02 ab | 1490.52 ± 178.40 a | 0.60 ± 0.03 bcd |
| YAP5 | 992.57 ± 163.54 de | 0.89 ± 0.03 a | 0.96 ± 0.02 a | 842.16 ± 138.39 cd | 0.68 ± 0.01 a |
| YAP10 | 1292.53 ± 80.82 bc | 0.91 ± 0.03 a | 0.96 ± 0.02 a | 1136.84 ± 78.10 b | 0.63 ± 0.02 abc |
| YAP15 | 1435.64 ± 82.88 b | 0.93 ± 0.03 a | 0.95 ± 0.03 a | 1158.21 ± 308.27 b | 0.57 ± 0.01 d |
| BP5 | 911.45 ± 101.08 e | 0.85 ± 0.03 a | 0.95 ± 0.01 ab | 735.04 ± 91.57 d | 0.66 ± 0.02 ab |
| BP10 | 1199.89 ± 123.55 cd | 0.91 ± 0.02 a | 0.94 ± 0.03 ab | 1033.07 ± 120.97 bc | 0.60 ± 0.03 bcd |
| BP15 | 1490.73 ± 106.67 b | 0.91 ± 0.04 a | 0.89 ± 0.02 b | 1212.22 ± 162.24 b | 0.62 ± 0.03 abcd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liu, R.; Phuseerit, O.; Li, H.; Siriamornpun, S. Developing a Functional Triticale Noodle by Incorporating Silkworm (Antheraea pernyi and Bombyx mori) Pupae. Foods 2025, 14, 2282. https://doi.org/10.3390/foods14132282
Liu Y, Liu R, Phuseerit O, Li H, Siriamornpun S. Developing a Functional Triticale Noodle by Incorporating Silkworm (Antheraea pernyi and Bombyx mori) Pupae. Foods. 2025; 14(13):2282. https://doi.org/10.3390/foods14132282
Chicago/Turabian StyleLiu, Yu, Ruixin Liu, Onanong Phuseerit, Hua Li, and Sirithon Siriamornpun. 2025. "Developing a Functional Triticale Noodle by Incorporating Silkworm (Antheraea pernyi and Bombyx mori) Pupae" Foods 14, no. 13: 2282. https://doi.org/10.3390/foods14132282
APA StyleLiu, Y., Liu, R., Phuseerit, O., Li, H., & Siriamornpun, S. (2025). Developing a Functional Triticale Noodle by Incorporating Silkworm (Antheraea pernyi and Bombyx mori) Pupae. Foods, 14(13), 2282. https://doi.org/10.3390/foods14132282






