Development of Double-Film Composite Food Packaging with UV Protection and Microbial Protection for Cherry Preservation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
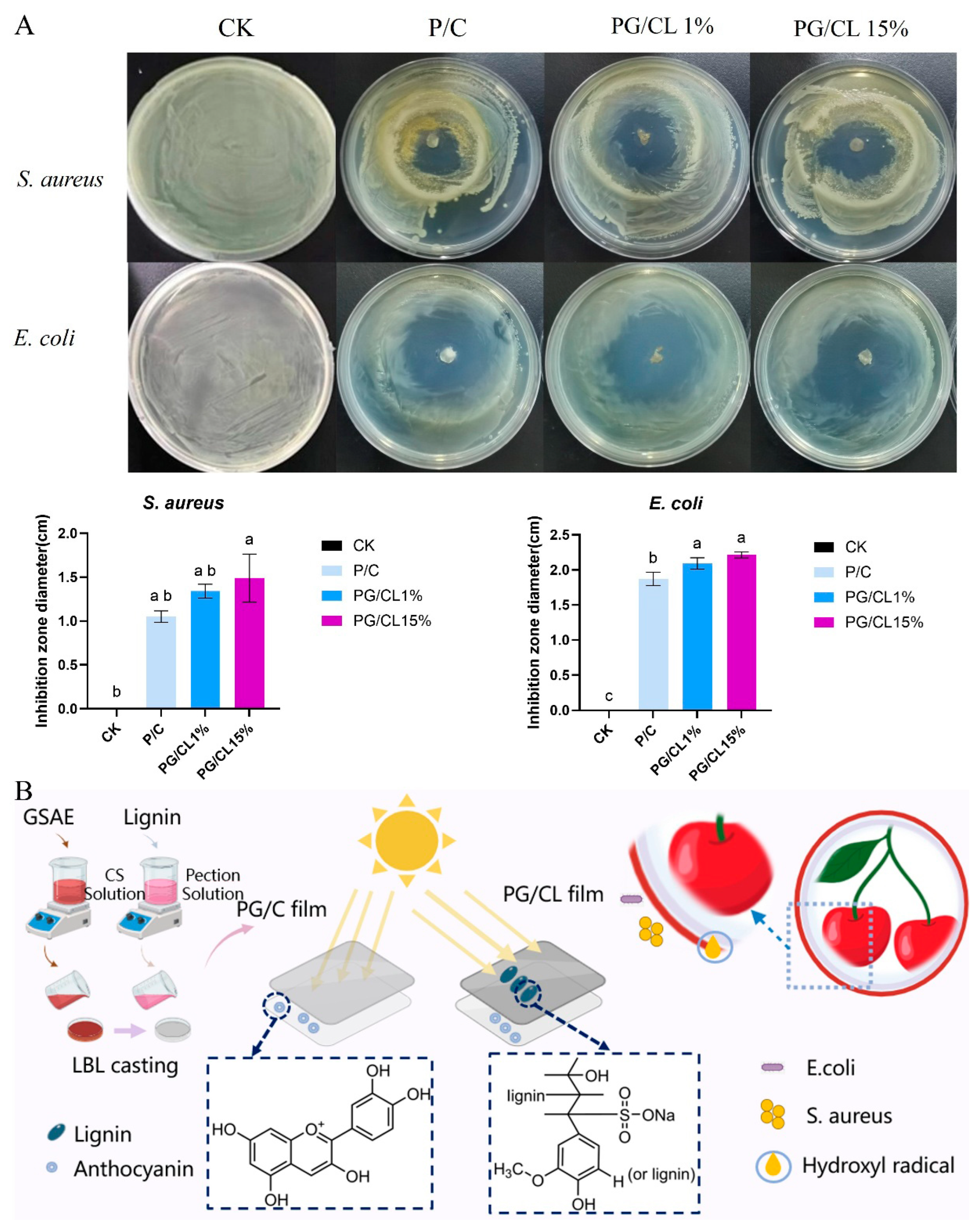
2.2. Extraction of GSAE and Lignin
2.3. Film Preparation
2.4. Analysis of Film Properties
2.4.1. Color
2.4.2. Optical Properties
2.4.3. Mechanical Properties
2.4.4. Water Contact Angle
2.4.5. Microstructure
2.4.6. Gas Barrier Properties
2.4.7. Antioxidant Activity
2.4.8. X-Ray Diffraction (XRD)
2.4.9. Fourier Transform Infrared (FT-IR) Spectroscopy
2.4.10. Thermogravimetric Analysis (TGA)
2.4.11. Antimicrobial Characteristics
2.4.12. Application of Double-Layer Composite Active Film in Cherry Preservation
2.5. Statistical Analysis
3. Results and Discussion
3.1. Optical Characteristics of Bilayer Films
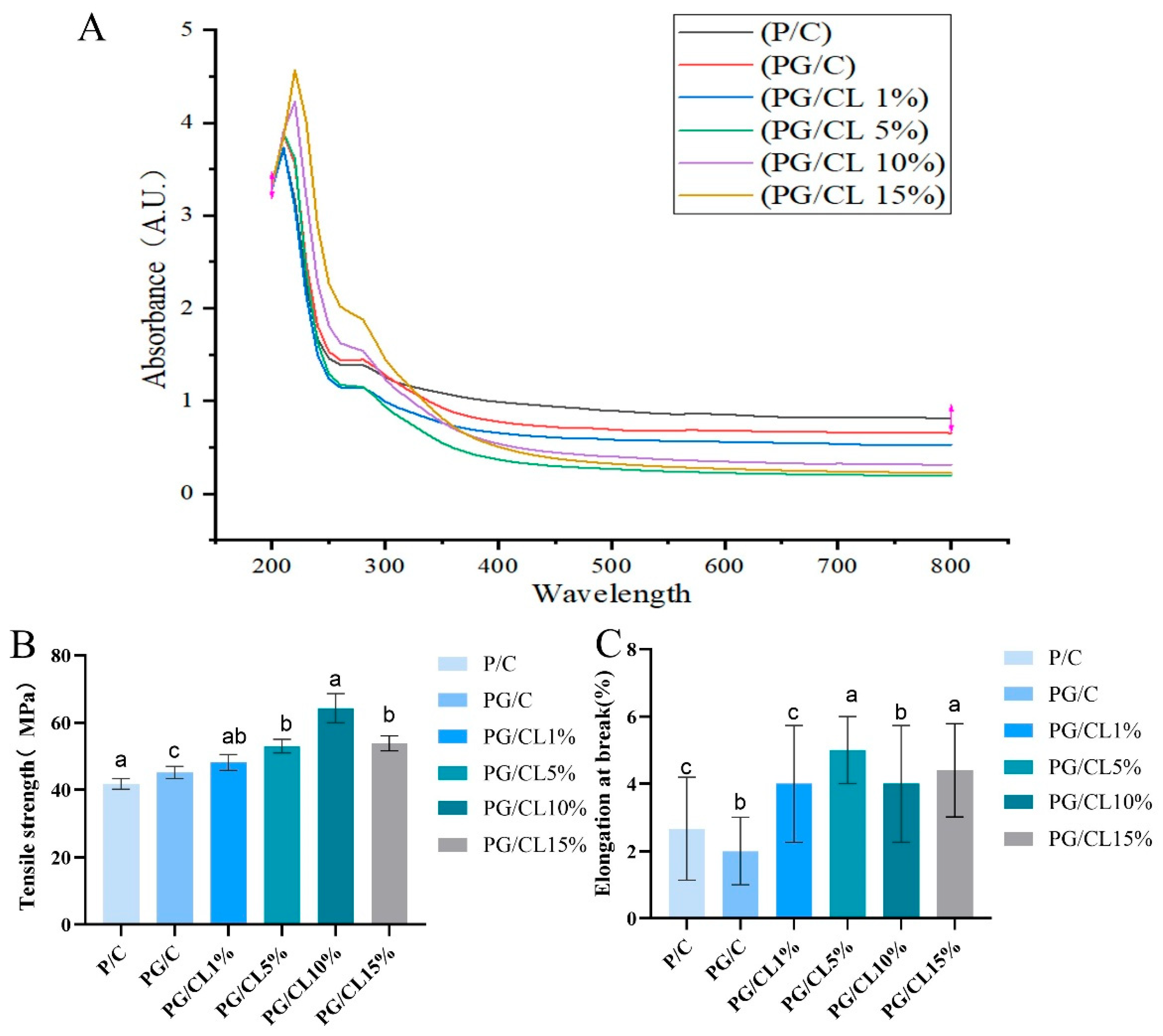
3.2. The Light-Shielding Properties of the Films
3.3. Mechanical Properties of Films
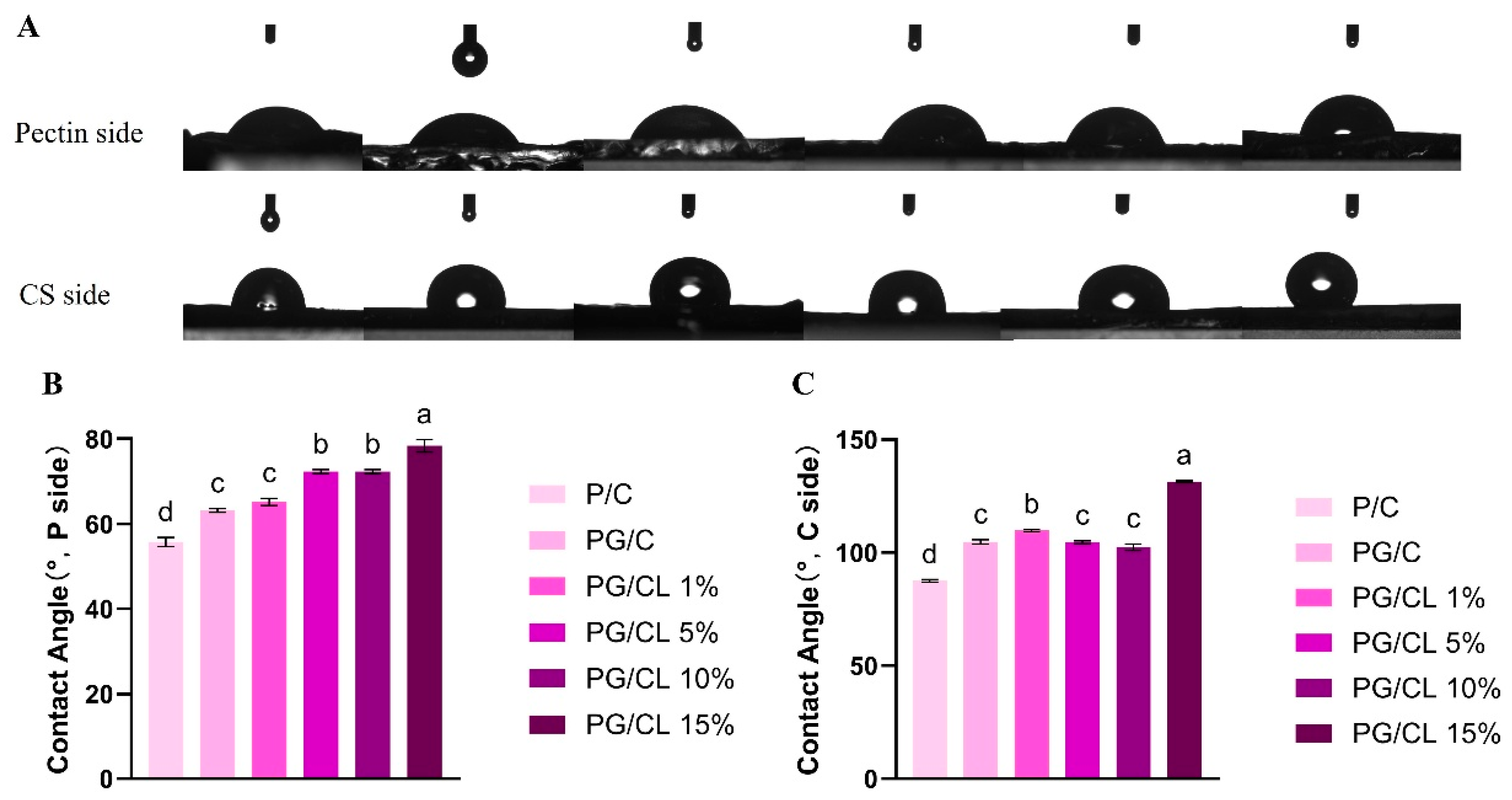
3.4. Measurement of Hydrophobicity of Films
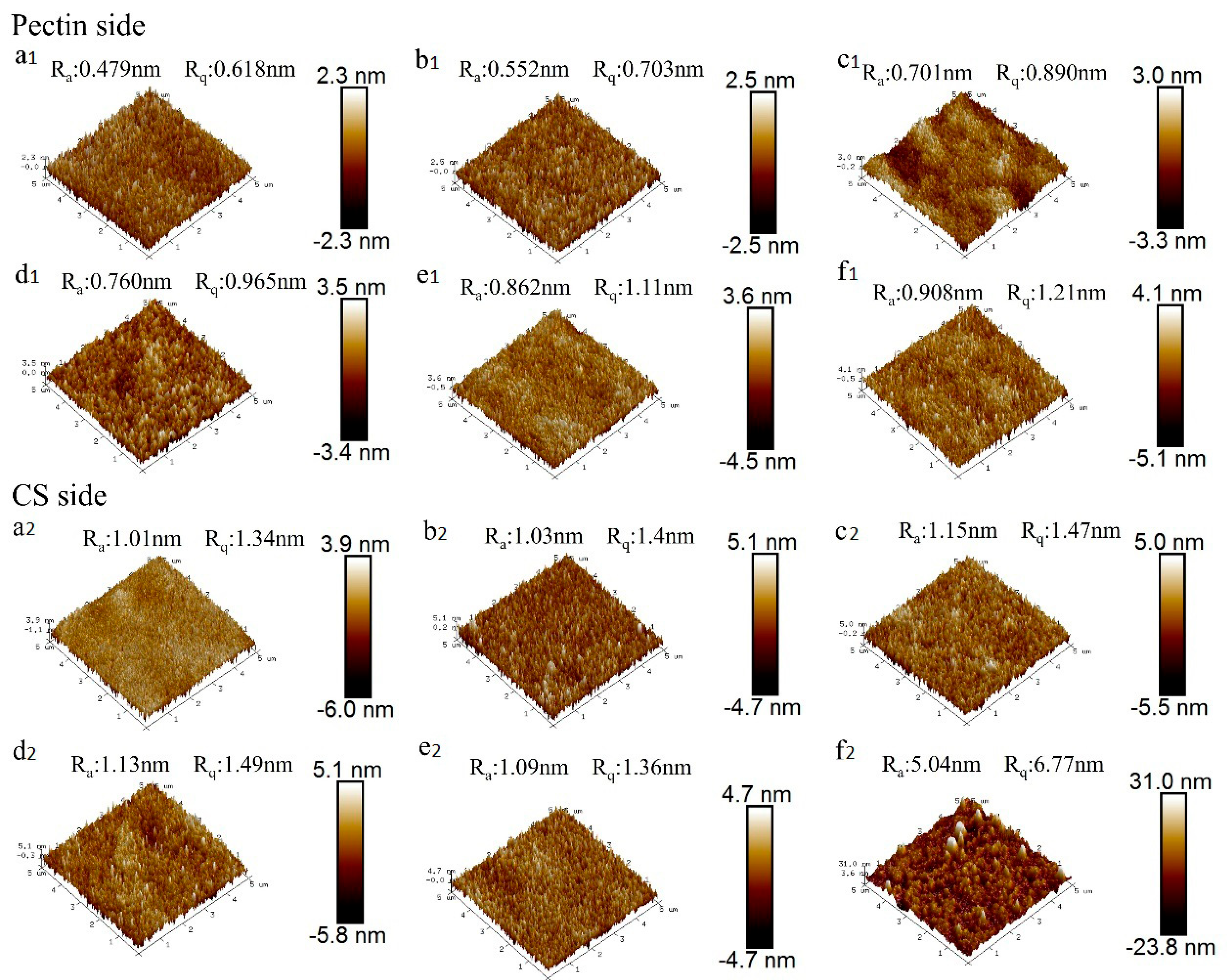
3.5. The Study of Film Morphology
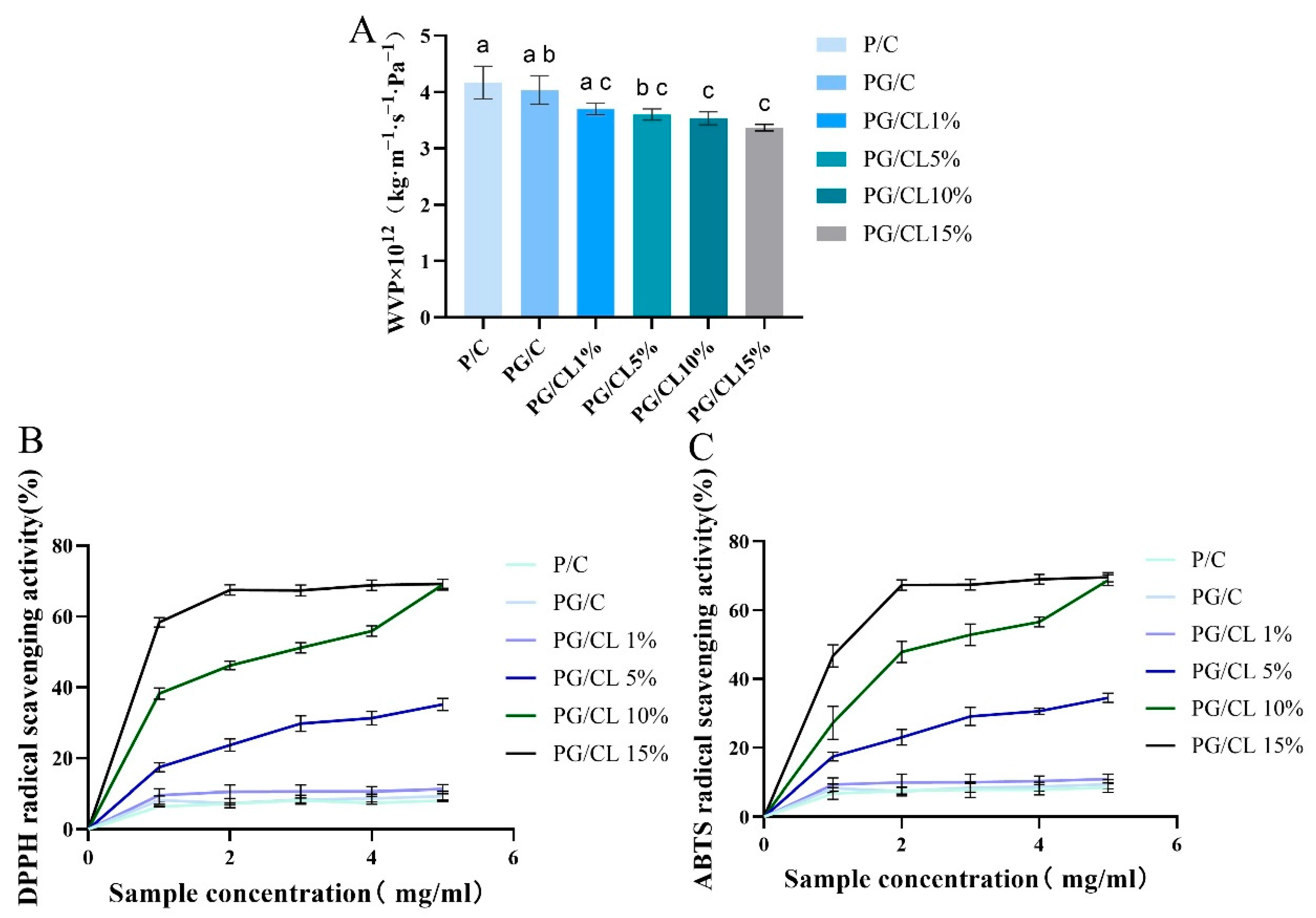
3.6. Properties Related to Gas Permeability
3.7. Activity of Antioxidants of Films
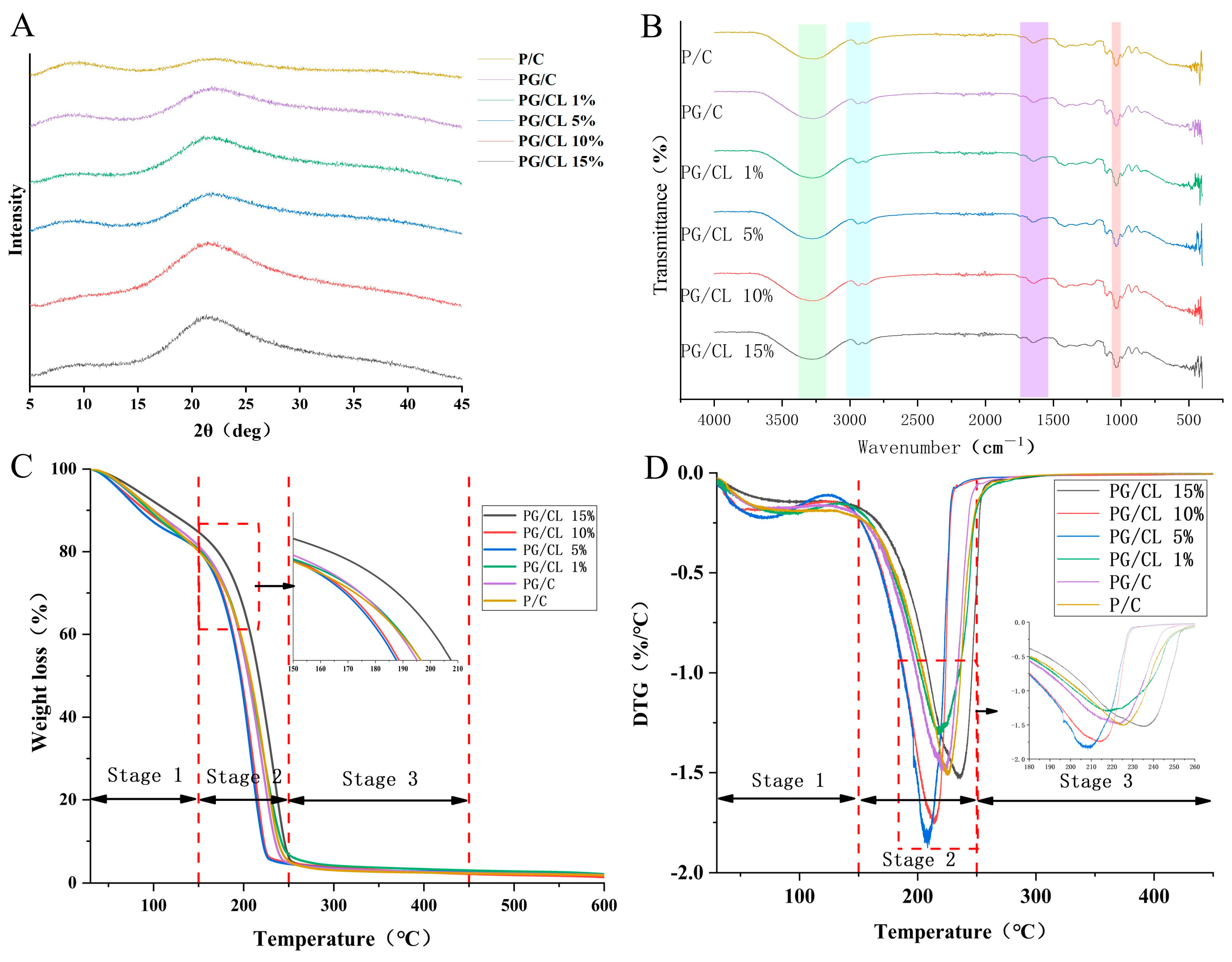
3.8. Crystalline Structure Analysis (XRD)
3.9. Chemical Structure Characterization (FT-IR)
3.10. Thermogravimetric Analysis (TGA)
3.11. Antimicrobial Properties of Films
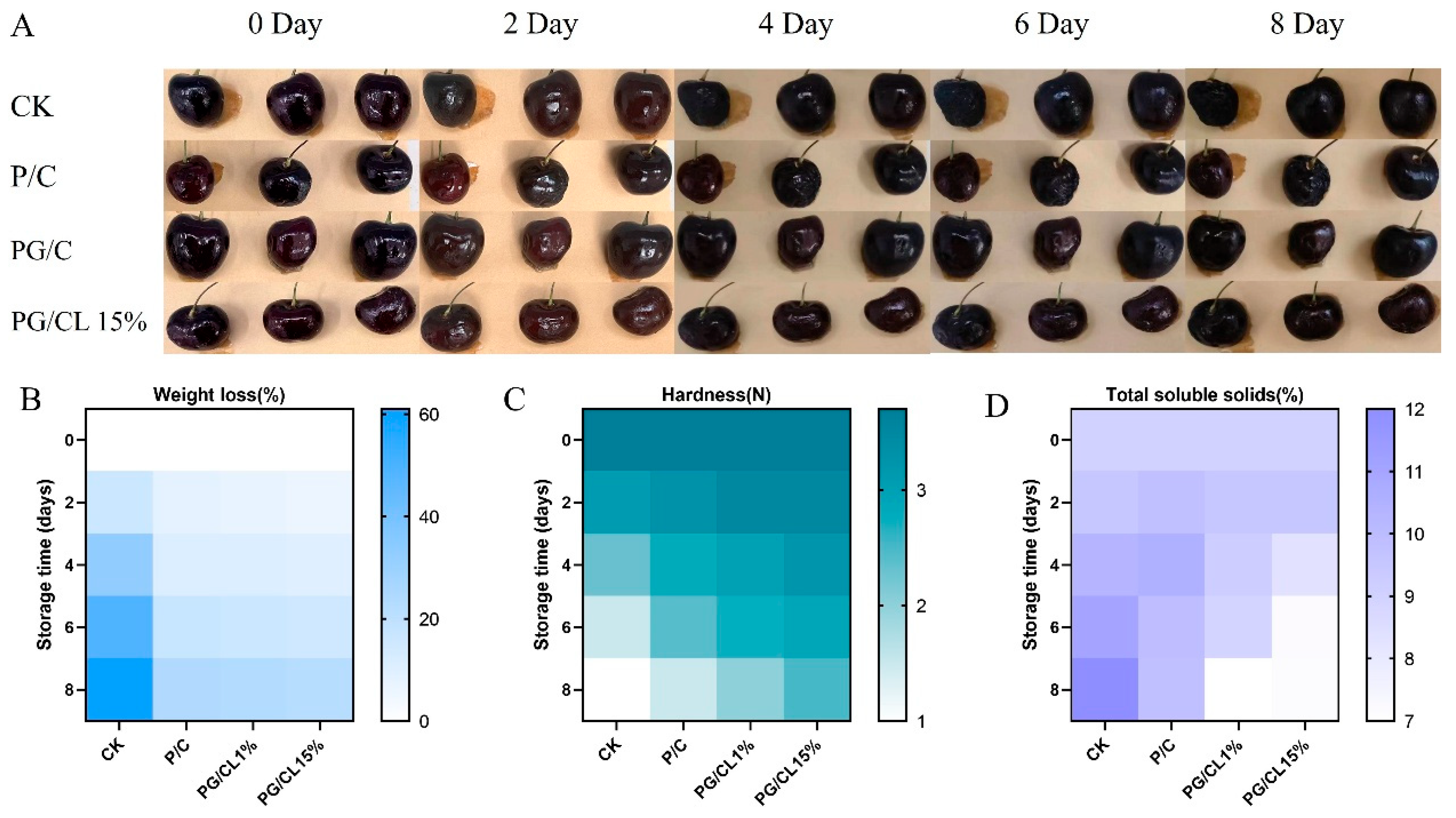
3.12. The Utilization of Multi-Active Packaging for the Preservation of Cherries
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goncalves, B.; Aires, A.; Oliveira, I.; Baltazar, M.; Cosme, F.; Afonso, S.; Pinto, T.; Anjos, M.R.; Ines, A.; Morais, M.C.; et al. From Orchard to Wellness: Unveiling the Health Effects of Sweet Cherry Nutrients. Nutrients 2024, 16, 3660. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, G.; Bacchetti, T.; Belleggia, A.; Neri, D. Cherry Antioxidants: From Farm to Table. Molecules 2010, 15, 6993–7005. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, H.; Zhang, J.; Chen, Q.; He, W.; Zhang, Y.; Luo, Y.; Tang, H.; Wang, Y.; Wang, X. Comparative metabolomics profiling highlights unique color variation and bitter taste formation of Chinese cherry fruits. Food Chem. 2024, 439, 138072. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Chang, X.; Xu, S.; Xu, H.; Ge, S.; Xie, Y.; Wang, R.; Xu, Y.; Luo, Z.; Shan, Y.; et al. Development of a chitosan/pectin-based multi-active food packaging with both UV and microbial defense functions for effectively preserving of strawberry. Int. J. Biol. Macromol. 2024, 254, 127968. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; An, J.; Su, H.; Li, B.; Liang, D.; Huang, C. Antimicrobial food packaging integrating polysaccharide-based substrates with green antimicrobial agents: A sustainable path. Food Res. Int. 2022, 155, 111096. [Google Scholar] [CrossRef]
- Yang, H.; Li, L.; Li, C.; Xu, Z.; Tao, Y.; Lu, J.; Xia, X.; Tan, M.; Du, J.; Wang, H. Multifunctional and antimicrobial carboxymethyl cellulose-based active hydrogel film for fruits packaging and preservation. Food Biosci. 2024, 59, 104005. [Google Scholar] [CrossRef]
- Bi, F.; Zhang, X.; Bai, R.; Liu, Y.; Liu, J.; Liu, J. Preparation and characterization of antioxidant and antimicrobial packaging films based on chitosan and proanthocyanidins. Int. J. Biol. Macromol. 2019, 134, 11–19. [Google Scholar] [CrossRef]
- Fu, X.; Chang, X.; Ding, Z.; Xu, H.; Kong, H.; Chen, F.; Wang, R.; Shan, Y.; Ding, S. Fabrication and Characterization of Eco-Friendly Polyelectrolyte Bilayer Films Based on Chitosan and Different Types of Edible Citrus Pectin. Foods 2022, 11, 3536. [Google Scholar] [CrossRef]
- Cordova, A.; Catalan, S.; Carrasco, V.; Farias, F.O.; Trentin, J.; Lopez, J.; Salazar, F.; Mussagy, C.U. Sustainable assessment of ultrasound-assisted extraction of anthocyanins with bio-based solvents for upgrading grape pomace Cabernet Sauvignon derived from a winemaking process. Ultrason. Sonochem. 2025, 112, 107201. [Google Scholar] [CrossRef]
- Jaradat, S.; Amr, A.; Hamadneh, I.; Alkhatib, H.; Alqaraleh, S.; Al-Omari, R.; Tarawneh, H. Improving Thermal and Light Stability of Black Grape Anthocyanins Using Cobalt Complexation. Prev. Nutr. Food Sci. 2024, 29, 495–503. [Google Scholar] [CrossRef]
- Chen, X.; Xiao, N.; Xiang, H.; Li, S.; Zhu, Z.; Cong, X.; Chen, X.; Cheng, S. Fabrication and characterization of double-layer active intelligent film based on chitosan, polyvinyl alcohol, grape skin anthocyanin and selenium nanoparticle. Int. J. Biol. Macromol. 2024, 282, 137211. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, J.-Y.; Youn, H.J.; Choi, J.W. Utilization of lignin fractions in UV resistant lignin-PLA biocomposites via lignin-lactide grafting. Int. J. Biol. Macromol. 2019, 138, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Crouvisier-Urion, K.; Lagorce-Tachon, A.; Lauquin, C.; Winckler, P.; Tongdeesoontorn, W.; Domenek, S.; Debeaufort, F.; Karbowiak, T. Impact of the homogenization process on the structure and antioxidant properties of chitosan-lignin composite films. Food Chem. 2017, 236, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Wrzesniewska-Tosik, K.; Struszczyk, H.; Ratajska, M.; Tomaszewski, W. Biodegradation of lignin based resins and fibrous lignin composites. Fibres Text. East. Eur. 2002, 10, 22–26. [Google Scholar]
- Tan, J.; Han, Y.; Han, B.; Qi, X.; Cai, X.; Ge, S.; Xue, H. Extraction and purification of anthocyanins: A review. J. Agric. Food Res. 2022, 8, 100306. [Google Scholar] [CrossRef]
- Rizg, W.Y.; Alahmadi, A.A.; Baradwan, M.; Bairwan, R.D.; Marwan, M.; Mohamed, A.K.; El Saadany, S.; Abdullah, C.K.; Khalil, H.P.S.A. Lignin and biodegradable polymer blends with chemically treated biofiller for green thermoplastic composites. Express Polym. Lett. 2025, 19, 294–310. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; Chen, Y.; Zhang, L.; Kan, J.; Jin, C. Physical, mechanical and antioxidant properties of chitosan films grafted with different hydroxybenzoic acids. Food Hydrocoll. 2017, 71, 176–186. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Li, C.; Qin, Y.; Xiao, L.; Liu, J. Comparison of the structural, physical and functional properties of κ-carrageenan films incorporated with pomegranate flesh and peel extracts. Int. J. Biol. Macromol. 2020, 147, 1076–1088. [Google Scholar] [CrossRef]
- Wang, F.-P.; Zhao, X.-J.; Wahid, F.; Zhao, X.-Q.; Qin, X.-T.; Bai, H.; Xie, Y.-Y.; Zhong, C.; Jia, S.-R. Sustainable, superhydrophobic membranes based on bacterial cellulose for gravity-driven oil/water separation. Carbohydr. Polym. 2021, 253, 117220. [Google Scholar] [CrossRef]
- Wu, H.; Lei, Y.; Lu, J.; Zhu, R.; Xiao, D.; Jiao, C.; Xia, R.; Zhang, Z.; Shen, G.; Liu, Y.; et al. Effect of citric acid induced crosslinking on the structure and properties of potato starch/chitosan composite films. Food Hydrocoll. 2019, 97, 105208. [Google Scholar] [CrossRef]
- Wang, X.; Yong, H.; Gao, L.; Li, L.; Jin, M.; Liu, J. Preparation and characterization of antioxidant and pH-sensitive films based on chitosan and black soybean seed coat extract. Food Hydrocoll. 2019, 89, 56–66. [Google Scholar] [CrossRef]
- Wong, L.-W.; Loke, X.-J.; Chang, C.-K.; Ko, W.-C.; Hou, C.-Y.; Hsieh, C.-W. Use of the plasma-treated and chitosan/gallic acid-coated polyethylene film for the preservation of tilapia (Orechromis niloticus) fillets. Food Chem. 2020, 329, 126989. [Google Scholar] [CrossRef]
- Yan, J.; Li, M.; Wang, H.; Lian, X.; Fan, Y.; Xie, Z.; Niu, B.; Li, W. Preparation and property studies of chitosan-PVA biodegradable antibacterial multilayer films doped with Cu2O and nano-chitosan composites. Food Control 2021, 126, 108049. [Google Scholar] [CrossRef]
- Jiang, L.; Jia, F.; Han, Y.; Meng, X.; Xiao, Y.; Bai, S. Development and characterization of zein edible films incorporated with catechin/β-cyclodextrin inclusion complex nanoparticles. Carbohydr. Polym. 2021, 261. [Google Scholar] [CrossRef]
- Ghadetaj, A.; Almasi, H.; Mehryar, L. Development and characterization of whey protein isolate active films containing nanoemulsions of Grammosciadium ptrocarpum Bioss. essential oil. Food Packag. Shelf Life 2018, 16, 31–40. [Google Scholar] [CrossRef]
- Cazon, P.; Antoniewska, A.; Rutkowska, J.; Vazquez, M. Evaluation of easy-removing antioxidant films of chitosan with Melaleuca alternifolia essential oil. Int. J. Biol. Macromol. 2021, 186, 365–376. [Google Scholar] [CrossRef]
- Riaz, A.; Lagnika, C.; Luo, H.; Dai, Z.; Nie, M.; Hashim, M.M.; Liu, C.; Song, J.; Li, D. Chitosan-based biodegradable active food packaging film containing Chinese chive (Allium tuberosum) root extract for food application. Int. J. Biol. Macromol. 2020, 150, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, Y.; Wang, M.; Li, R.; Dai, J.; Yan, J.; Qin, W.; Liu, Y. 3D printing of essential oil/β-cyclodextrin/popping candy modified atmosphere packaging for strawberry preservation. Carbohydr. Polym. 2022, 297, 120037. [Google Scholar] [CrossRef]
- Zhou, W.; He, Y.; Liu, F.; Liao, L.; Huang, X.; Li, R.; Zou, Y.; Zhou, L.; Zou, L.; Liu, Y.; et al. Carboxymethyl chitosan-pullulan edible films enriched with galangal essential oil: Characterization and application in mango preservation. Carbohydr. Polym. 2021, 256, 117579. [Google Scholar] [CrossRef]
- Zhou, X.; Cheng, R.; Wang, B.; Zeng, J.; Xu, J.; Li, J.; Kang, L.; Cheng, Z.; Gao, W.; Chen, K. Biodegradable sandwich-architectured films derived from pea starch and polylactic acid with enhanced shelf-life for fruit preservation. Carbohydr. Polym. 2021, 251, 117117. [Google Scholar] [CrossRef]
- Zhang, Z.; Argenziano, R.; Konate, A.; Shi, X.; Salazar, S.A.; Cerruti, P.; Panzella, L.; Terrasson, V.; Guenin, E. Preparation of chitosan/lignin nanoparticles-based nanocomposite films with high-performance and improved physicochemical properties for food packaging applications. Int. J. Biol. Macromol. 2025, 293, 139079. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ye, H.-C.; You, T.-T.; Xu, F. Sustainable and multifunctional cellulose-lignin films with excellent antibacterial and UV-shielding for active food packaging. Food Hydrocoll. 2023, 137, 108355. [Google Scholar] [CrossRef]
- Mariana, M.; Alfatah, T.; Khalil, A.H.P.S.; Yahya, E.B.; Olaiya, N.G.; Nuryawan, A.; Mistar, E.M.; Abdullah, C.K.; Abdulmadjid, S.N.; Ismail, H. A current advancement on the role of lignin as sustainable reinforcement material in biopolymeric blends. J. Mater. Res. Technol.-JmrT 2021, 15, 2287–2316. [Google Scholar] [CrossRef]
- Mirpoor, S.F.; Restaino, O.F.; Schiraldi, C.; Giosafatto, C.V.L.; Ruffo, F.; Porta, R. Lignin/Carbohydrate Complex Isolated from Posidonia oceanica Sea Balls (Egagropili): Characterization and Antioxidant Reinforcement of Protein-Based Films. Int. J. Mol. Sci. 2021, 22, 9147. [Google Scholar] [CrossRef]
- Bian, H.; Gao, Y.; Wang, R.; Liu, Z.; Wu, W.; Dai, H. Contribution of lignin to the surface structure and physical performance of cellulose nanofibrils film. Cellulose 2018, 25, 1309–1318. [Google Scholar] [CrossRef]
- Li, X.; Hegyesi, N.; Zhang, Y.; Mao, Z.; Feng, X.; Wang, B.; Pukanszky, B.; Sui, X. Poly(lactic acid)/lignin blends prepared with the Pickering emulsion template method. Eur. Polym. J. 2019, 110, 378–384. [Google Scholar] [CrossRef]
- Ge, X.; Chang, M.; Jiang, W.; Zhang, B.; Xing, R.; Bulin, C. Investigation on two modification strategies for the reinforcement of biodegradable lignin/poly(lactic acid) blends. J. Appl. Polym. Sci. 2020, 137, 49354. [Google Scholar] [CrossRef]
- Ma, Z.; Xing, Z.; Zhao, Y.; Zhang, H.; Xin, Q.; Liu, J.; Qin, M.; Ding, C.; Li, J. Lotus leaf inspired sustainable and multifunctional Janus film for food packaging. Chem. Eng. J. 2023, 457, 141279. [Google Scholar] [CrossRef]
- Yu, T.; Wang, T.; Zhang, R.; Su, C.; Cao, S.; Zhao, Y.; Wang, H.; Li, J.; Niu, N.; Chen, L. Development of corn straw lignin-based cling films for enhanced preservation of strawberries. Food Packag. Shelf Life 2025, 49, 101529. [Google Scholar] [CrossRef]
- Dai, W.; Zhou, L.; Gu, S.; Wang, W.; Xu, Z.; Zhou, X.; Ding, Y. Preparation and characterization of chitosan films incorporating epigallocatechin gallate: Microstructure, physicochemical, and bioactive properties. Int. J. Biol. Macromol. 2022, 211, 729–740. [Google Scholar] [CrossRef]
- Rojo, E.; Peresin, M.S.; Sampson, W.W.; Hoeger, I.C.; Vartiainen, J.; Laine, J.; Rojas, O.J. Comprehensive elucidation of the effect of residual lignin on the physical, barrier, mechanical and surface properties of nanocellulose films. Green Chem. 2015, 17, 1853–1866. [Google Scholar] [CrossRef]
- Imani, M.; Ghasemian, A.; Dehghani-Firouzabadi, M.R.; Afra, E.; Borghei, M.; Johansson, L.S.; Gane, P.A.C.; Rojas, O.J. Coupling Nanofibril Lateral Size and Residual Lignin to Tailor the Properties of Lignocellulose Films. Adv. Mater. Interfaces 2019, 6, 1900770. [Google Scholar] [CrossRef]
- Guo, T.; Gu, L.; Zhang, Y.; Chen, H.; Jiang, B.; Zhao, H.; Jin, Y.; Xiao, H. Bioinspired self-assembled films of carboxymethyl cellulose-dopamine/montmorillonite. J. Mater. Chem. A 2019, 7, 14033–14041. [Google Scholar] [CrossRef]
- Sun, M.; Liu, N.; Ni, S.; Xu, Q.; Gao, H.; Dai, H.; Fu, Y.; Qin, M. Enhanced strength, antioxidant and barrier properties of chitosan-based film by bentonite and lignosulfonate. Food Packag. Shelf Life 2024, 42, 101246. [Google Scholar] [CrossRef]
- Pires, J.R.A.; Souza, V.G.L.; Gomes, L.A.; Coelhoso, I.M.; Godinho, M.H.; Fernando, A.L. Micro and nanocellulose extracted from energy crops as reinforcement agents in chitosan films. Ind. Crops Prod. 2022, 186, 115247. [Google Scholar] [CrossRef]
- Costa, S.M.; Ferreira, D.P.; Teixeira, P.; Ballesteros, L.F.; Teixeira, J.A.; Fangueiro, R. Active natural-based films for food packaging applications: The combined effect of chitosan and nanocellulose. Int. J. Biol. Macromol. 2021, 177, 241–251. [Google Scholar] [CrossRef]
- Lu, X.; Gu, X.; Shi, Y. A review on lignin antioxidants: Their sources, isolations, antioxidant activities and various applications. Int. J. Biol. Macromol. 2022, 210, 716–741. [Google Scholar] [CrossRef]
- Mesgari, M.; Aalami, A.H.; Sahebkar, A. Antimicrobial activities of chitosan/titanium dioxide composites as a biological nanolayer for food preservation: A review. Int. J. Biol. Macromol. 2021, 176, 530–539. [Google Scholar] [CrossRef]
- Alzagameem, A.; Klein, S.E.; Bergs, M.; Do, X.T.; Korte, I.; Dohlen, S.; Huewe, C.; Kreyenschmidt, J.; Kamm, B.; Larkins, M.; et al. Antimicrobial Activity of Lignin and Lignin-Derived Cellulose and Chitosan Composites against Selected Pathogenic and Spoilage Microorganisms. Polymers 2019, 11, 670. [Google Scholar] [CrossRef]
- Huewe, C.; Schmeichel, J.; Brodkorb, F.; Dohlen, S.; Kalbfleisch, K.; Kreyenschmidt, M.; Lorenz, R.; Kreyenschmidt, J. Potential of antimicrobial treatment of linear low-density polyethylene with poly((tert-butyl-amino)-methyl-styrene) to reduce biofilm formation in the food industry. Biofouling 2018, 34, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Dohlen, S.; Braun, C.; Brodkorb, F.; Fischer, B.; Ilg, Y.; Kalbfleisch, K.; Lorenz, R.; Kreyenschmidt, M.; Kreyenschmidt, J. Effect of different packaging materials containing poly-2-(tert-butylamino) methylstyrene on the growth of spoilage and pathogenic bacteria on fresh meat. Int. J. Food Microbiol. 2017, 257, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Chen, F.; Lai, S.; Yang, H. Influence of chitosan-based coatings on the physicochemical properties and pectin nanostructure of Chinese cherry. Postharvest Biol. Technol. 2017, 133, 64–71. [Google Scholar] [CrossRef]
| Film | Color | Opacity (mm−1) | Physical Appearance | ||||
|---|---|---|---|---|---|---|---|
| L | a | b | ΔE | WI | |||
| P/C | 34.15 | −0.15 | 0.70 | 59.3 | 34.1 | 0.22 |  |
| PG/C | 32.59 | 0.04 | 1.32 | 60.9 | 32.6 | 0.35 |  |
| PG/CL1% | 32.88 | 0 | 1.46 | 60.6 | 32.9 | 0.39 |  |
| PG/CL5% | 32.59 | 0.05 | 1.68 | 60.9 | 32.6 | 0.53 |  |
| PG/CL10% | 33.83 | −0.03 | 1.05 | 59.7 | 33.8 | 0.63 |  |
| PG/CL15% | 33.37 | −0.04 | 1.71 | 60.1 | 33.3 | 0.77 |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Liao, Y.; Zhu, G.; Wang, L.; Chen, Z.; Li, X.; Wang, C.; Yu, J.; Song, P. Development of Double-Film Composite Food Packaging with UV Protection and Microbial Protection for Cherry Preservation. Foods 2025, 14, 2283. https://doi.org/10.3390/foods14132283
Wang H, Liao Y, Zhu G, Wang L, Chen Z, Li X, Wang C, Yu J, Song P. Development of Double-Film Composite Food Packaging with UV Protection and Microbial Protection for Cherry Preservation. Foods. 2025; 14(13):2283. https://doi.org/10.3390/foods14132283
Chicago/Turabian StyleWang, Han, Yanjing Liao, Guida Zhu, Longwen Wang, Zihan Chen, Xue Li, Chao Wang, Jing Yu, and Ping Song. 2025. "Development of Double-Film Composite Food Packaging with UV Protection and Microbial Protection for Cherry Preservation" Foods 14, no. 13: 2283. https://doi.org/10.3390/foods14132283
APA StyleWang, H., Liao, Y., Zhu, G., Wang, L., Chen, Z., Li, X., Wang, C., Yu, J., & Song, P. (2025). Development of Double-Film Composite Food Packaging with UV Protection and Microbial Protection for Cherry Preservation. Foods, 14(13), 2283. https://doi.org/10.3390/foods14132283






