Screening for High-Yielding Pyruvate and Acetaldehyde Yeasts and Their Application in Improving the Stability of Anthocyanin in Mulberry Wine
Abstract
1. Introduction
2. Materials and Methods
2.1. Mulberry, Yeasts, and Reagents
2.2. Primary Screening of Yeast with High Yields of Pyruvate and Acetaldehyde
2.2.1. The Mehod of Detection of Acetaldehyde
2.2.2. The Mehod of Detection of Pyruvate
2.3. Secondary Screening for Yeast Strains with Strong Stress Resistance and Excellent Fermentation Performance
2.4. The Practical Fermentation and Aging Treatments with the Selected Strains
2.5. Analysis of Basic Physical and Chemical Indicators of Mulberry Juice and Wine
2.6. Determination of Total Sugar, Ethanol, and Malic Acid in Mulberry Juice and Mulberry Wine
2.7. Determination of Total Acidity and Volatile ACID in Mulberry Juice and Wine
2.8. Determination of Total Phenolics in Mulberry Juice and Wine
2.9. The Detection Methods of Color Index in Mulberry Juice and Wine
2.9.1. Determination of Color Parameters by CIELAB Method
2.9.2. Color Fitting by CIELAB Method
2.10. Analysis of Total Anthocyanin Content in Mulberry Wine
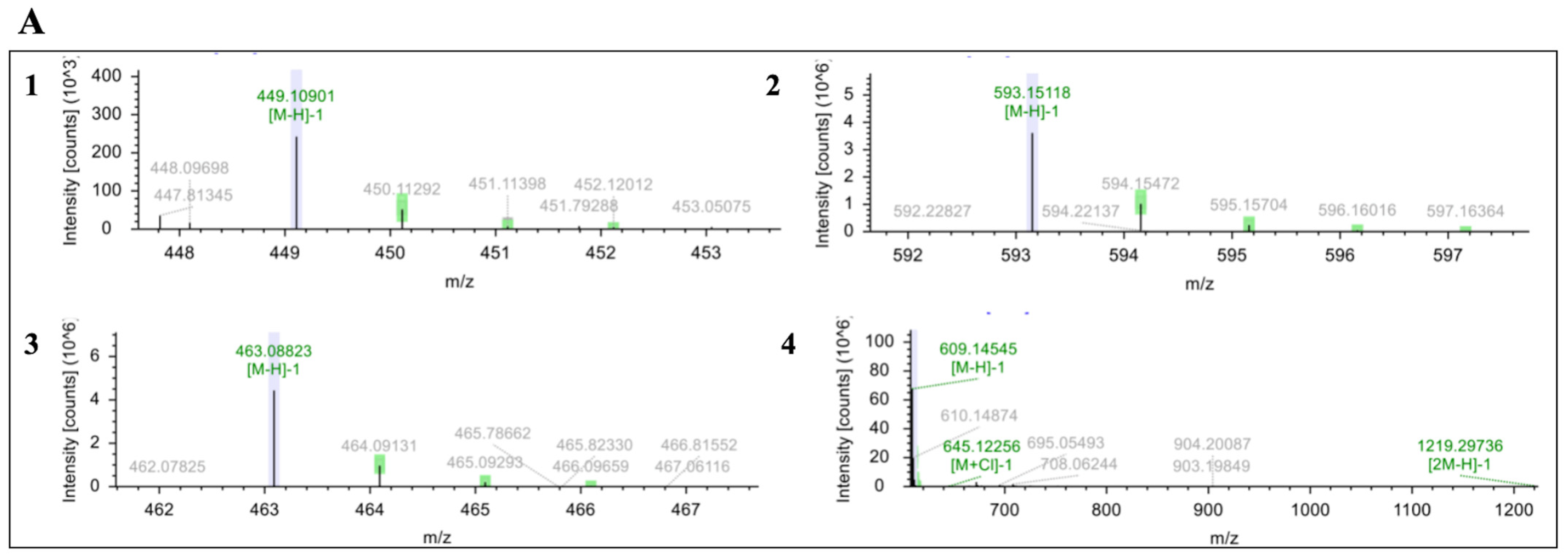
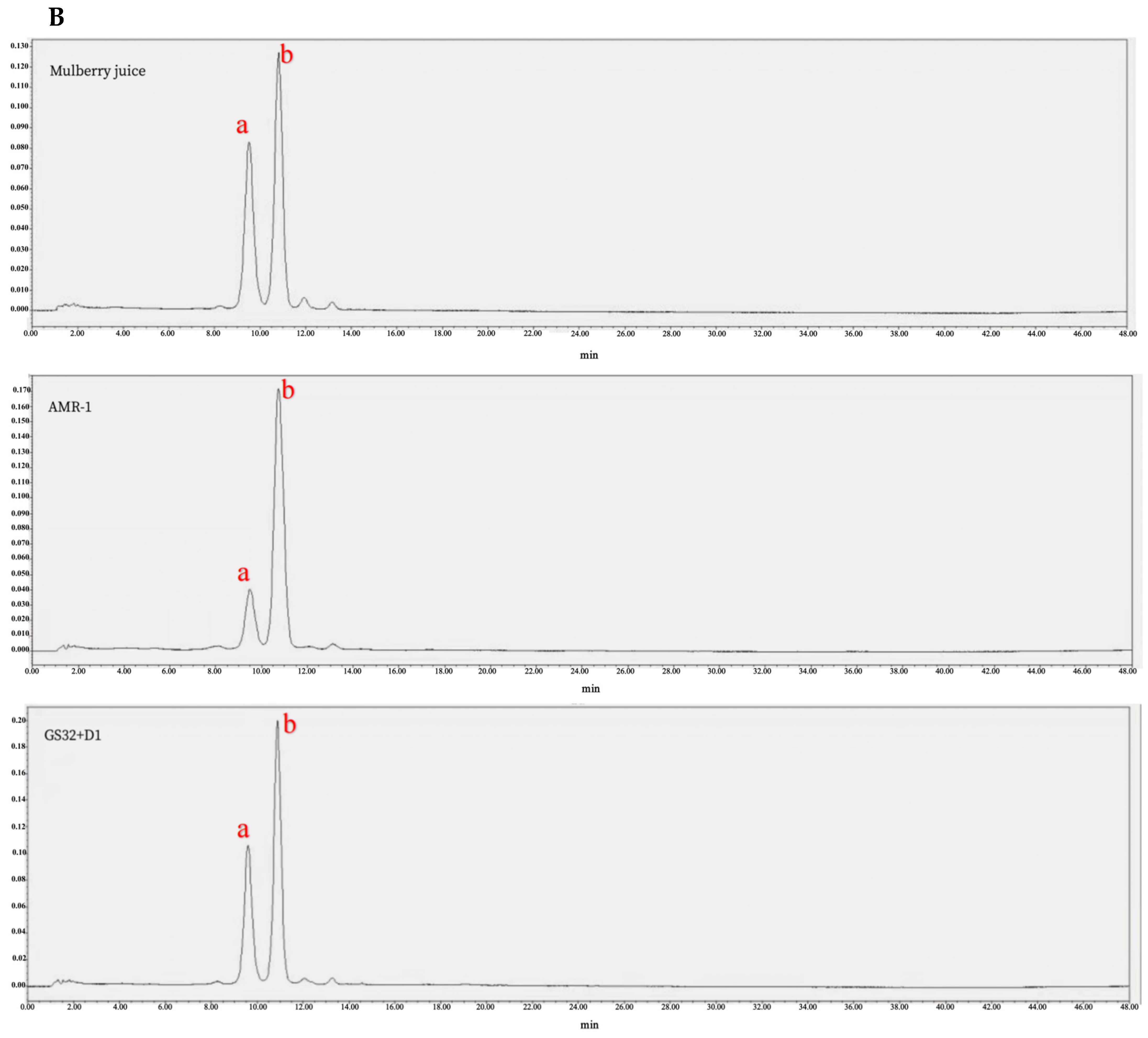
2.11. UPLC-MS/MS for Anthocyanin and Anthocyanin-Derived Compounds
2.12. HS-SPME-GC-MS for Volatile Compound Identification
2.13. Statistical Analysis
3. Results
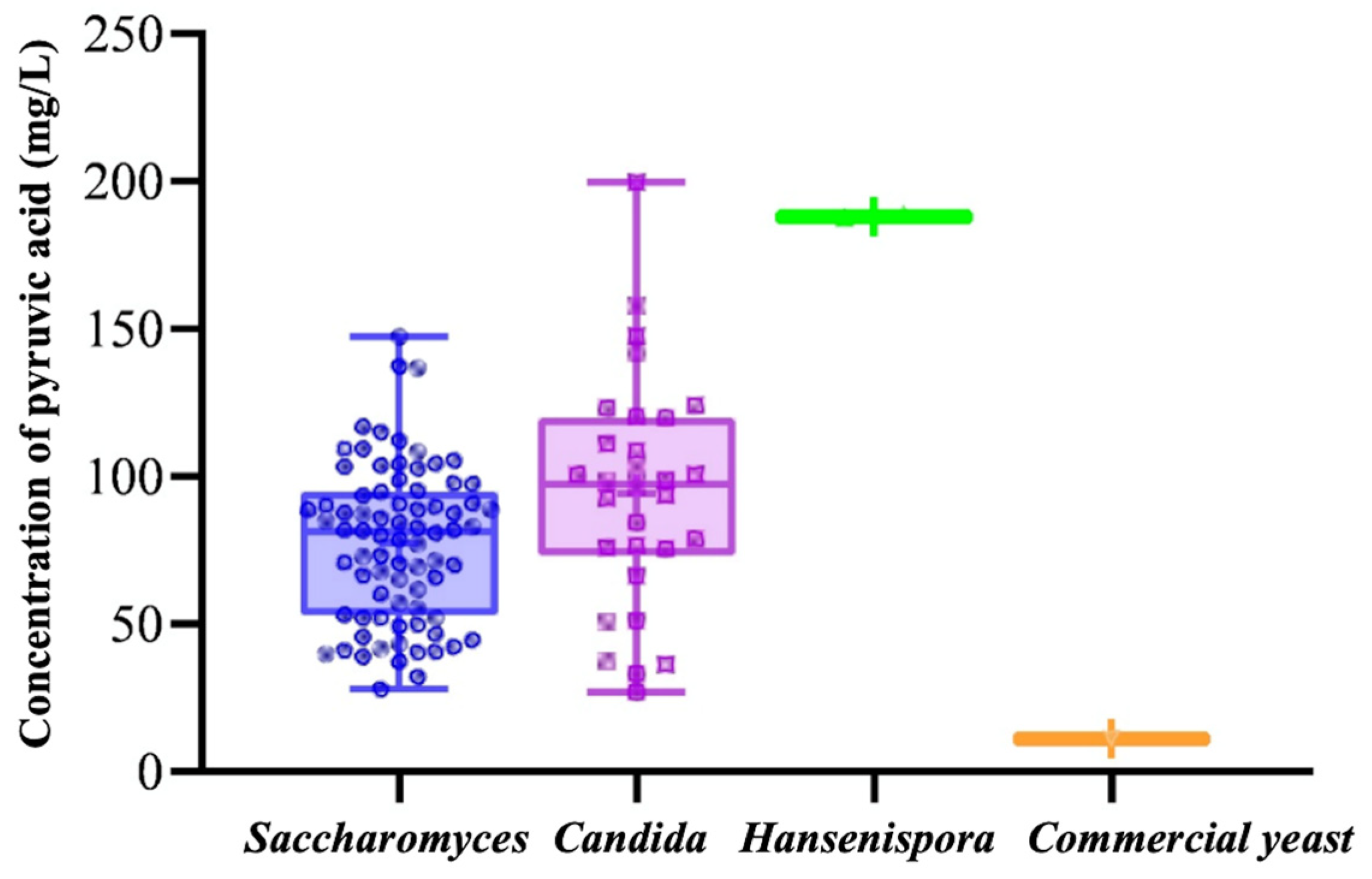
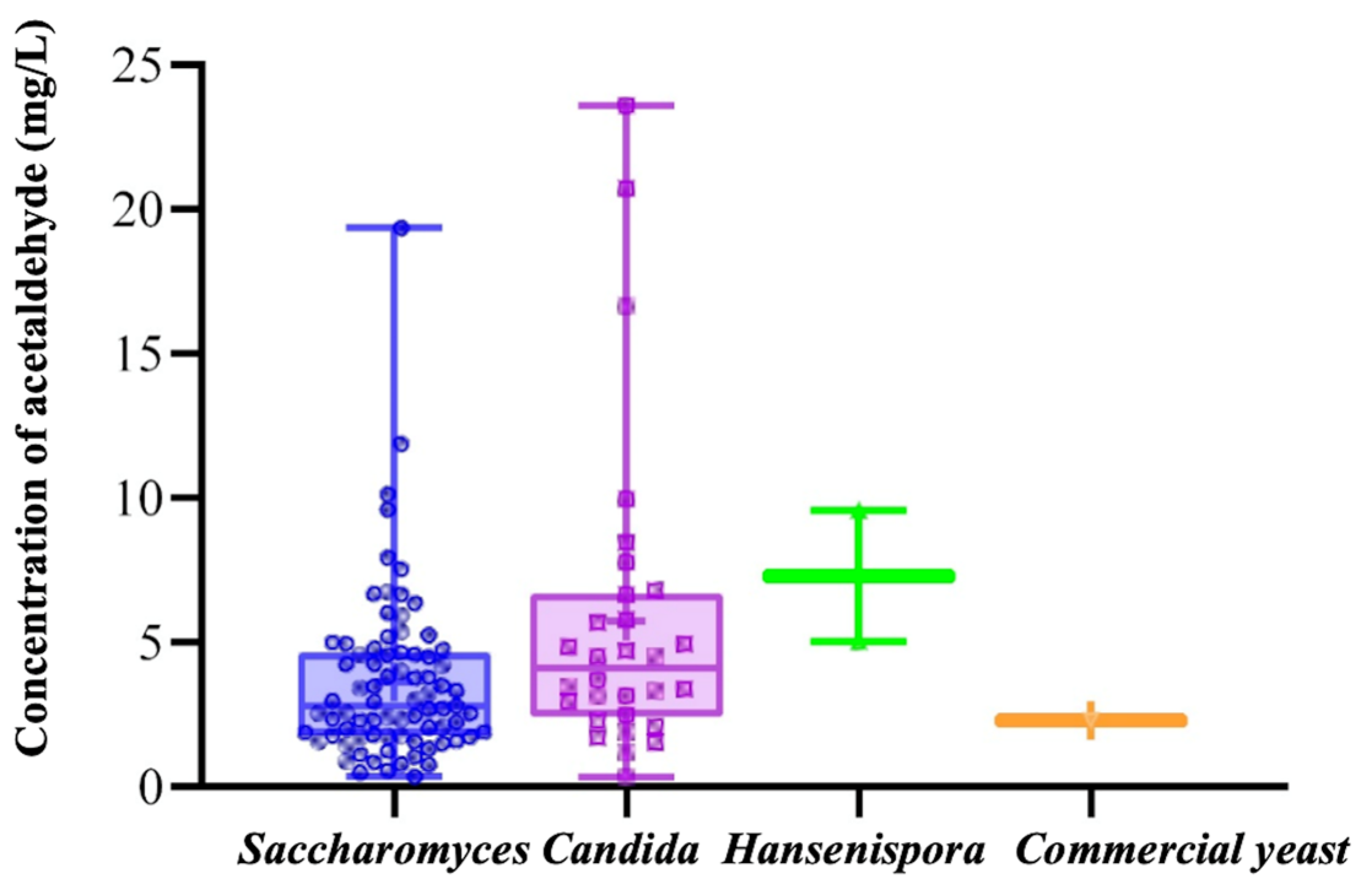
3.1. Four Strains Were Screened for High Yield of Pyruvate Acid, and Four Strains Were Screened for High Yield of Acetaldehyde
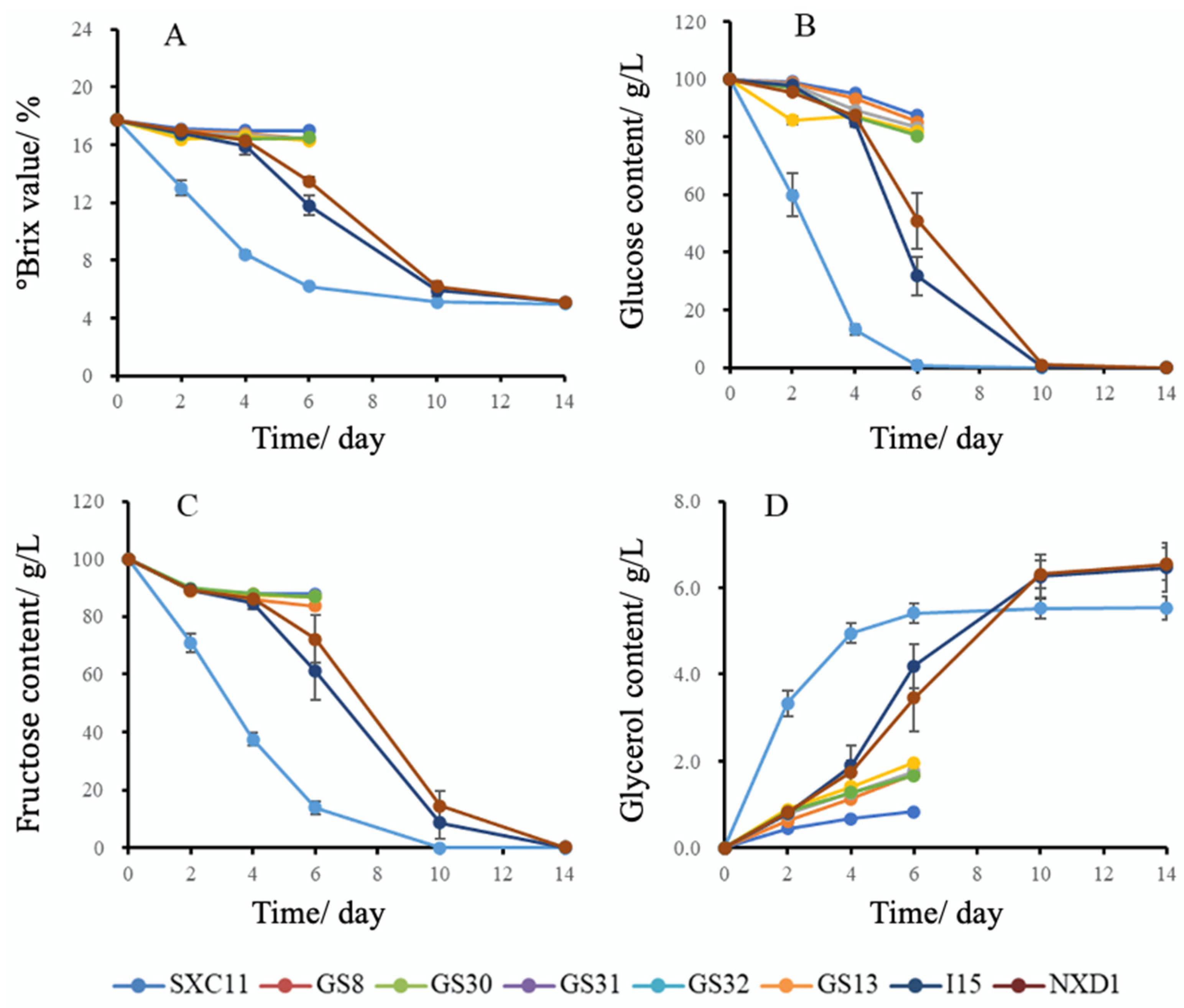
3.2. Three Strains Were Selected Based on Fermentation Characteristics and Stress Resistance
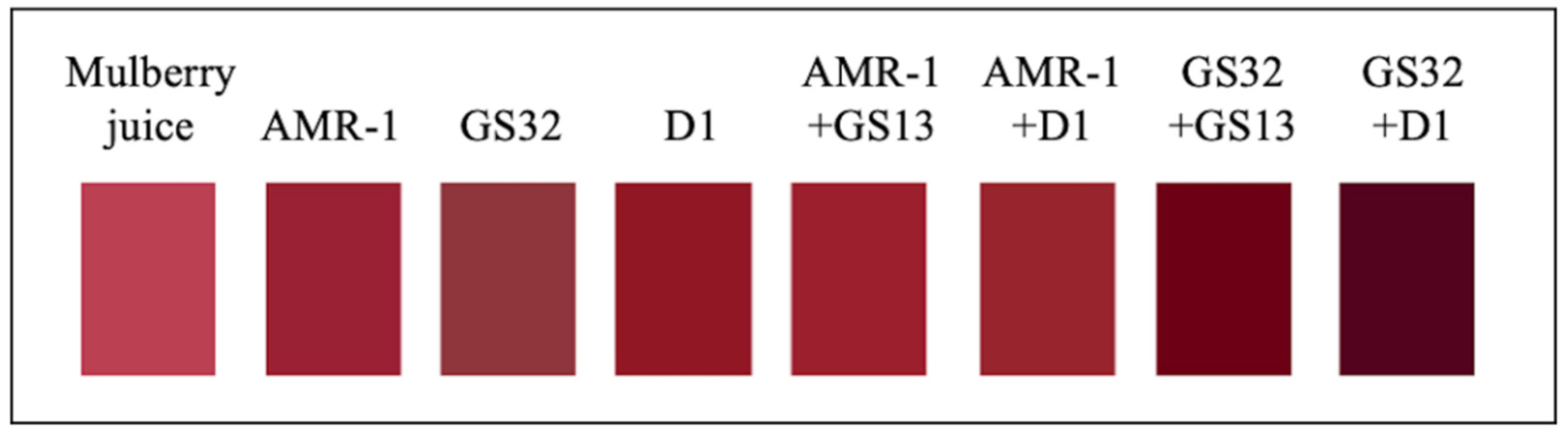
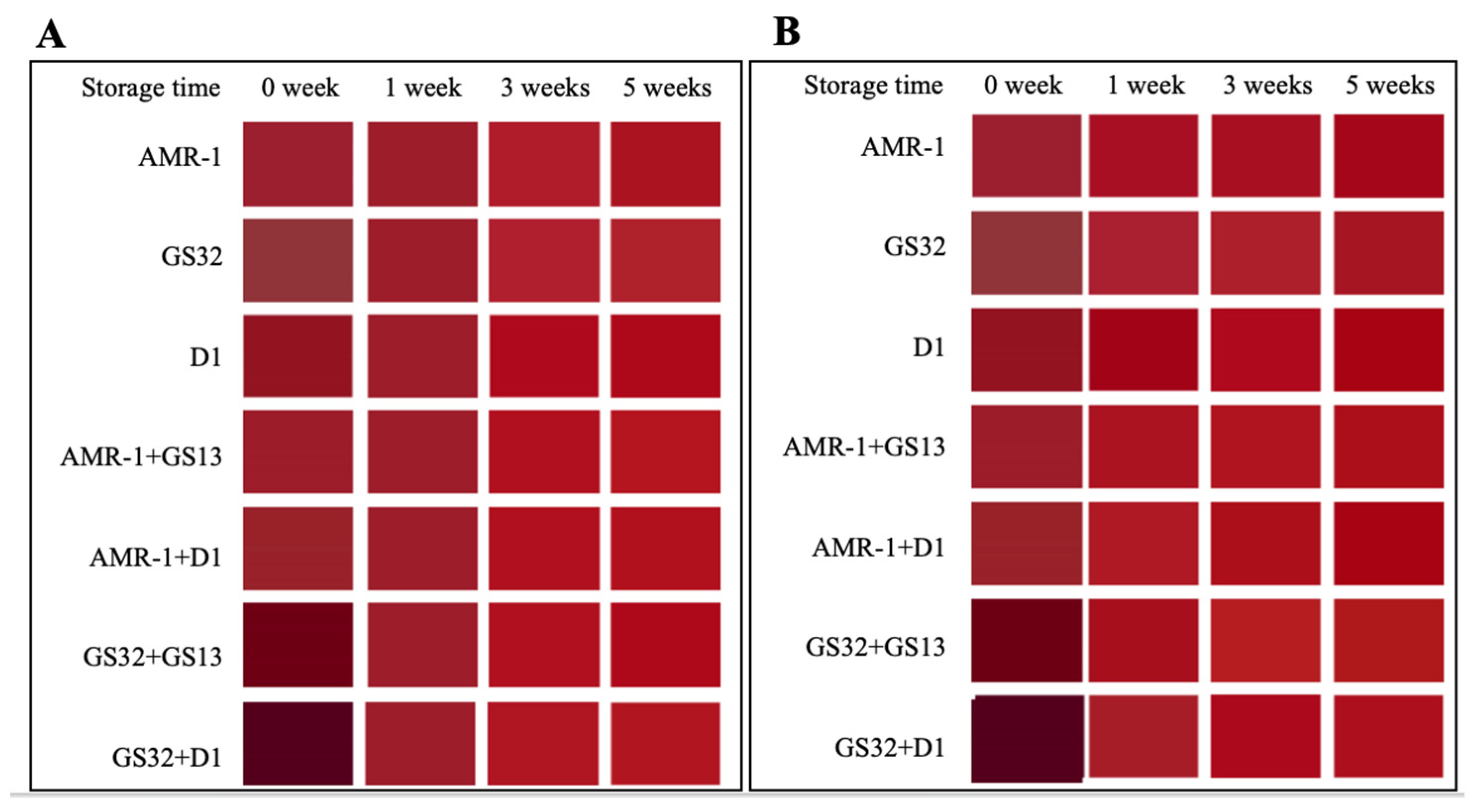
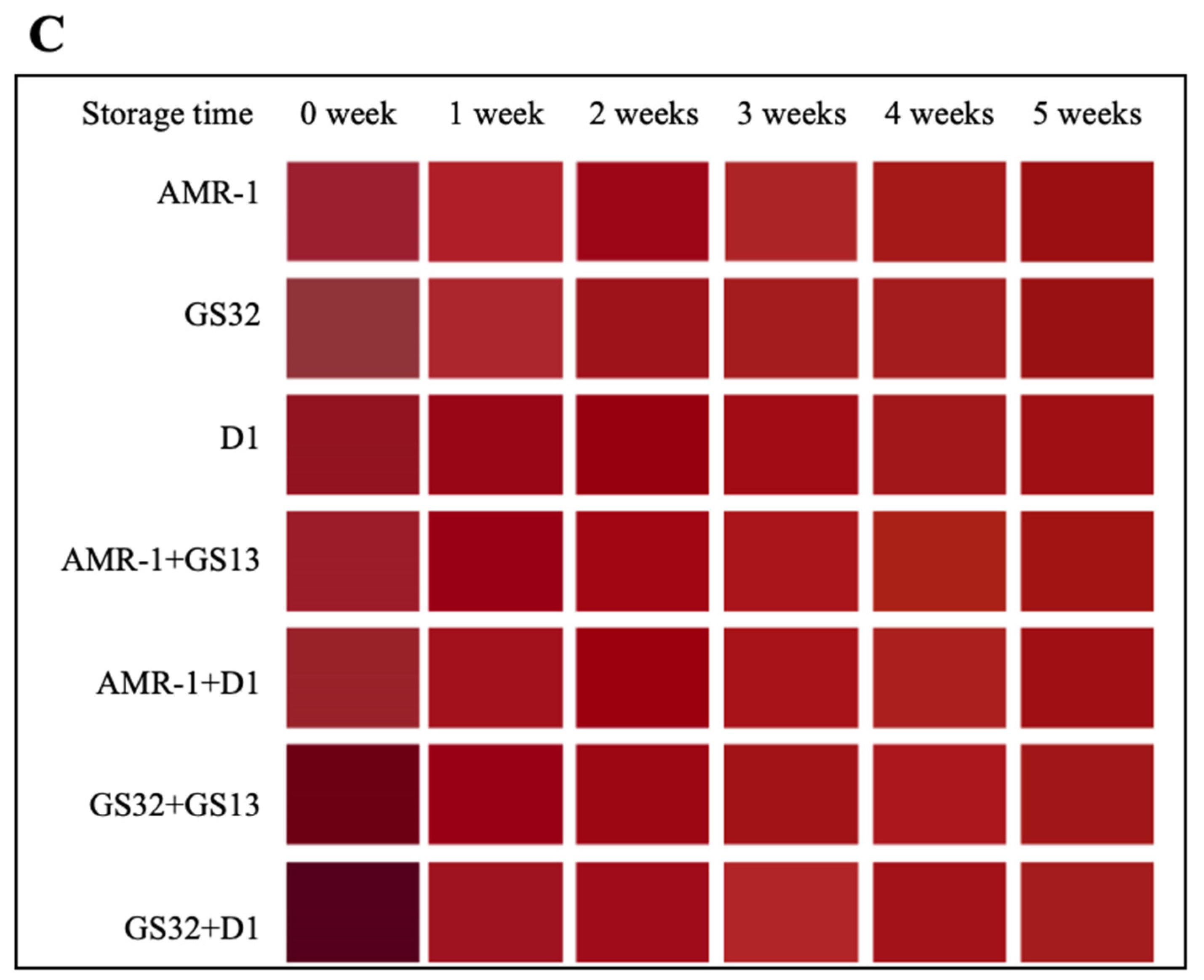
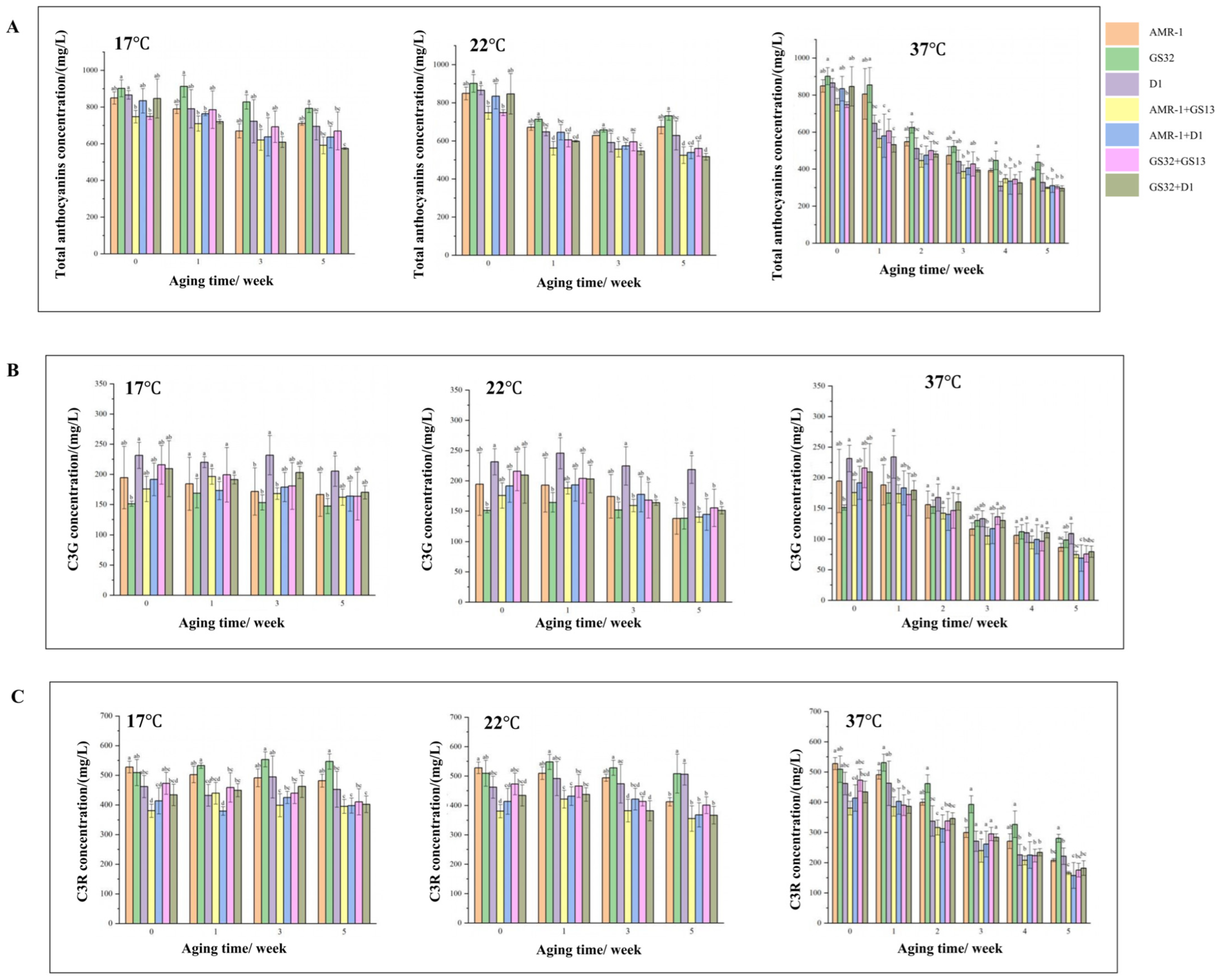
3.3. GS32 and D1 Significantly Improved the Total Anthocyanin Concentration and Color Stability of Mulberry Wine
3.4. GS32 Improve Total Anthocyanins (TA) Concentration After Fermentation, and Slow TA, C3G and C3R Degradation During Aging
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yuan, Q.; Zhao, L. The Mulberry (Morus alba L.) Fruit—A Review of Characteristic Components and Health Benefits. J. Agric. Food Chem. 2017, 65, 10383–10394. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, Z.F.; Luo, X.; Li, X. Effects of Mulberry Fruit (Morus alba L.) Consumption on Health Outcomes: A Mini-Review. Antioxidants 2018, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Wani, M.Y.; Ganie, N.A.; Wani, D.M.; Wani, A.W.; Dar, S.Q.; Khan, A.H.; Khan, N.A.; Manzar, M.S.; Dehghani, M.H. The phenolic components extracted from mulberry fruits as bioactive compounds against cancer: A review. Phytother. Res. 2023, 37, 1136–1152. [Google Scholar] [CrossRef]
- Martins, M.S.; Gonçalves, A.C.; Alves, G.; Silva, L.R. Blackberries and Mulberries: Berries with Significant Health-Promoting Properties. Int. J. Mol. Sci. 2023, 24, 12024. [Google Scholar] [CrossRef]
- Yousuf, B.; Gul, K.; Wani, A.A.; Singh, P. Health Benefits of Anthocyanins and Their Encapsulation for Potential Use in Food Systems: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2223–2230. [Google Scholar] [CrossRef]
- He, B.; Ge, J.; Yue, P.X.; Yue, X.Y.; Fu, R.Y.; Liang, J.; Gao, X.L. Loading of anthocyanins on chitosan nanoparticles influences anthocyanin degradation in gastrointestinal fluids and stability in a beverage. Food Chem. 2017, 221, 1671–1677. [Google Scholar] [CrossRef]
- Sendri, N.; Bhandari, P. Anthocyanins: A comprehensive review on biosynthesis, structural diversity, and industrial applications. Phytochem. Rev. 2024, 23, 1913–1974. [Google Scholar] [CrossRef]
- Kapsopoulou, K.; Kapaklis, A.; Spyropoulos, H. Growth and fermentation characteristics of a strain of the wine yeast Kluyveromyces thermotolerans isolated in Greece. World J. Microbiol. Biotechnol. 2005, 21, 1599–1602. [Google Scholar] [CrossRef]
- Kapsopoulou, K.; Mourtzini, A.; Anthoulas, M.; Nerantzis, E. Biological acidification during grape must fermentation using mixed cultures of Kluyveromyces thermotolerans and Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2007, 23, 735–739. [Google Scholar] [CrossRef]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef]
- Benito, Á.; Calderón, F.; Palomero, F.; Benito, S. Combine Use of Selected Schizosaccharomyces pombe and Lachancea thermotolerans Yeast Strains as an Alternative to theTraditional Malolactic Fermentation in Red Wine Production. Molecules 2015, 20, 9510–9523. [Google Scholar] [CrossRef] [PubMed]
- Hranilovic, A.; Bely, M.; Masneuf-Pomarede, I.; Jiranek, V.; Albertin, W. The evolution of Lachancea thermotolerans is driven by geographical determination, anthropisation and flux between different ecosystems. PLoS ONE 2017, 12, e0184652. [Google Scholar] [CrossRef] [PubMed]
- You, Y.Y.; Li, N.; Han, X.; Guo, J.L.; Zhao, Y.; Liu, G.L.; Huang, W.D.; Zhan, J.C. Influence of different sterilization treatments on the color and anthocyanin contents of mulberry juice during refrigerated storage. Innov. Food Sci. Emerg. Technol. 2018, 48, 1–10. [Google Scholar] [CrossRef]
- You, Y.Y.; Li, N.; Han, X.; Guo, J.L.; Liu, G.J.; Huang, W.D.; Zhan, J.C. Influence of Tannin Extract and Yeast Extract on Color Preservation and Anthocyanin Content of Mulberry Wine. J. Food Sci. 2018, 83, 1084–1093. [Google Scholar] [CrossRef]
- You, Y.Y.; Li, N.; Han, X.; Guo, J.L.; Zhao, Y.; Huang, W.D.; Zhan, J.C. The effects of six phenolic acids and tannic acid on colour stability and the anthocyanin content of mulberry juice during refrigerated storage. Int. J. Food Sci. Technol. 2019, 54, 2141–2150. [Google Scholar] [CrossRef]
- Gao, Y.X.; Wang, X.; Ai, J.Y.; Huang, W.D.; Zhan, J.C.; You, Y.Y. Formation of vinylphenolic pyranoanthocyanins by selected indigenous yeasts displaying high hydroxycinnamate decarboxylase activity during mulberry wine fermentation and aging. Food Microbiol. 2023, 113, 104272. [Google Scholar] [CrossRef]
- Romano, P.; Suzzi, G.; Turbanti, L.; Polsinelli, M. Acetaldehyde production in Saccharomyces cerevisiae wine yeasts. FEMS Microbiol. Lett. 1994, 118, 213–218. [Google Scholar] [CrossRef]
- Wolff, F.; El Khattabi, C.; Bourdon, F.; Willems, D. Comparison of high-performance liquid chromatography with fluorescence detection versus enzymatic assay to measure blood pyruvate in clinical practice. Clin. Biochem. 2009, 42, 1099–1103. [Google Scholar] [CrossRef]
- Benito, S.; Palomero, F.; Morata, A.; Calderón, F.; Palmero, D.; Suárez-Lepe, J.A. Physiological features of Schizosaccharomyces pombe of interest in making of white wines. Eur. Food Res. Technol. 2013, 236, 29–36. [Google Scholar] [CrossRef]
- GB/T 15038-2006; Analytical Methods of Wine and Fruit Wine. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. Standards Press of China: Beijing, China, 2006.
- Jayaprakasha, G.K.; Singh, R.P.; Sakariah, K.K. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem. 2001, 73, 285–290. [Google Scholar] [CrossRef]
- Pérez-Caballero, V.; Ayala, F.; Echávarri, J.F.; Negueruela, A.I. Proposal for a new standard OIV method for determination of chromatic characteristics of wine. Am. J. Enol. Vitic. 2003, 54, 59–62. [Google Scholar] [CrossRef]
- Han, X.Y.; Qing, X.; Yang, S.Y.; Li, R.; Zhan, J.C.; You, Y.Y.; Huang, W.D. Study on the diversity of non-Saccharomyces yeasts in Chinese wine regions and their potential in improving wine aroma by β-glucosidase activity analyses. Food Chem. 2021, 360, 129886. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, V.; Mateus, N. Chemical transformations of anthocyanins yielding a variety of colours (Review). Environ. Chem. Lett. 2006, 4, 175–183. [Google Scholar] [CrossRef]
- Morata, A.; Calderón, F.; González, M.C.; Gómez-Cordovés, M.C.; Suárez, J.A. Formation of the highly stable pyranoanthocyanins (vitisins A and B) in red wines by the addition of pyruvic acid and acetaldehyde. Food Chem. 2007, 100, 1144–1152. [Google Scholar] [CrossRef]
- Marquez, A.; Serratosa, M.P.; Merida, J. Pyranoanthocyanin Derived Pigments in Wine: Structure and Formation during Winemaking. J. Chem. 2013, 2013, 713028. [Google Scholar] [CrossRef]
- Liu, S.; Laaksonen, O.; Kortesniemi, M.; Kalpio, M.; Yang, B. Chemical composition of bilberry wine fermented with non-Saccharomyces yeasts (Torulaspora delbrueckii and Schizosaccharomyces pombe) and Saccharomyces cerevisiae in pure, sequential and mixed fermentations. Food Chem. 2018, 266, 262–274. [Google Scholar] [CrossRef]
- Wang, Z.; Svyantek, A.; Miller, Z.; Watrelot, A.A. Assessment of Sequential Yeast Inoculation for Blackcurrant Wine Fermentation. Fermentation 2024, 10, 184. [Google Scholar] [CrossRef]
- Cheng, S.Q.; Wu, T.Y.; Gao, J.; Han, X.Y.; Huang, W.D.; You, Y.Y.; Zhan, J.C. Color myth anthocyanins reactions and enological approaches achieving their stabilization in the aging process of red wine. Food Innov. Adv. 2023, 2, 255–271. [Google Scholar] [CrossRef]
- Li, E.; Mira De Orduña, R. Evaluation of the acetaldehyde production and degradation potential of 26 enological Saccharomyces and non-Saccharomyces yeast strains in a resting cell model system. J. Ind. Microbiol. Biotechnol. 2011, 38, 1391–1398. [Google Scholar] [CrossRef]
- Morata, A.; Gómez-Cordovés, M.C.; Colomo, B.; Suárez, J.A. Pyruvic Acid and Acetaldehyde Production by Different Strains of Saccharomyces cerevisiae: Relationship with Vitisin A and B Formation in Red Wines. J. Agric. Food Chem. 2003, 51, 7402–7409. [Google Scholar] [CrossRef]
- Fulcrand, H.; Benabdeljalil, C.; Rigaud, J.; Cheynier, V.; Moutounet, M. A new class of wine pigments generated by reaction between pyruvic acid and grape anthocyanins. Phytochemistry 1998, 47, 1401–1407. [Google Scholar] [CrossRef]
- Oliveira, J.; Fernandes, V.; Miranda, C.; Santos-Buelga, C.; Silva, A.; De Freitas, V.; Mateus, N. Color properties of four cyanidin-pyruvic acid adducts. J. Agric. Food Chem. 2006, 54, 6894–6903. [Google Scholar] [CrossRef]
| Identification Number | Strain Generus | Pyruvate Acid Concentration (mg/L) |
|---|---|---|
| I15 | Candida | 199.63 ± 5.35 |
| D1 | Candida | 157.78 ± 5.45 |
| LN10 | Candida | 147.68 ± 8.61 |
| LN22 | Candida | 141.73 ± 7.68 |
| D18 | Candida | 124.12 ± 7.00 |
| GS18 | Candida | 123.25 ± 0.89 |
| GS10 | Candida | 120.22 ± 6.36 |
| GS31 | Candida | 119.92 ± 6.67 |
| YT4 | Candida | 111.06 ± 3.43 |
| BG3 | Candida | 108.58 ± 0.31 |
| GS16 | Candida | 102.30 ± 7.74 |
| D16 | Candida | 100.70 ± 3.48 |
| GS20 | Candida | 100.70 ± 7.45 |
| GS13 | Candida | 98.83 ± 6.71 |
| GS27 | Candida | 98.39 ± 6.66 |
| LN9 | Candida | 96.25 ± 4.41 |
| LN12 | Candida | 93.67 ± 2.87 |
| YT25 | Candida | 92.54 ± 0.41 |
| GS15 | Candida | 84.58 ± 3.43 |
| GS14 | Candida | 78.79 ± 2.35 |
| F19 | Candida | 76.66 ± 5.36 |
| LN13 | Candida | 75.94 ± 5.26 |
| YT2 | Candida | 75.44 ± 3.00 |
| GS11 | Candida | 66.45 ± 6.30 |
| GS30 | Candida | 51.22 ± 5.03 |
| YT10 | Candida | 50.76 ± 5.21 |
| GS12 | Candida | 37.40 ± 1.28 |
| GS22 | Candida | 36.45 ± 3.73 |
| YT3 | Candida | 33.25 ± 6.02 |
| D40 | Candida | 26.93 ± 5.44 |
| SXC11 | Hanseniaspora | 188.69 ± 7.92 |
| GS8 | Hanseniaspora | 187.51 ± 1.46 |
| GS39 | Saccharomyces | 147.42 ± 5.41 |
| D30 | Saccharomyces | 137.32 ± 7.16 |
| CL16 | Saccharomyces | 136.75 ± 6.87 |
| GS40 | Saccharomyces | 117.01 ± 5.08 |
| SH29 | Saccharomyces | 115.17 ± 3.33 |
| AC29 | Saccharomyces | 112.20 ± 3.44 |
| D22 | Saccharomyces | 109.45 ± 6.14 |
| B47 | Saccharomyces | 109.44 ± 6.81 |
| BH8 | Saccharomyces | 108.39 ± 5.89 |
| XJA1 | Saccharomyces | 105.59 ± 4.46 |
| B43 | Saccharomyces | 104.49 ± 1.04 |
| I43 | Saccharomyces | 104.46 ± 6.66 |
| XJA8 | Saccharomyces | 103.86 ± 0.75 |
| B38 | Saccharomyces | 103.41 ± 4.76 |
| SC37 | Saccharomyces | 102.87 ± 4.29 |
| E68 | Saccharomyces | 99.10 ± 3.07 |
| H68 | Saccharomyces | 97.86 ± 9.50 |
| GS9 | Saccharomyces | 97.54 ± 2.90 |
| E67 | Saccharomyces | 95.22 ± 6.16 |
| SXC9 | Saccharomyces | 94.91 ± 9.45 |
| E63 | Saccharomyces | 93.57 ± 0.97 |
| LH21 | Saccharomyces | 90.90 ± 2.89 |
| GS32 | Saccharomyces | 90.70 ± 7.03 |
| SXC8 | Saccharomyces | 90.31 ± 4.09 |
| D35 | Saccharomyces | 89.95 ± 5.89 |
| YT28 | Saccharomyces | 88.91 ± 6.12 |
| CL1 | Saccharomyces | 88.81 ± 4.82 |
| H66 | Saccharomyces | 88.78 ± 1.49 |
| NC3 | Saccharomyces | 87.90 ± 4.18 |
| H63 | Saccharomyces | 87.53 ± 5.58 |
| CL34 | Saccharomyces | 87.43 ± 6.04 |
| D39 | Saccharomyces | 85.84 ± 3.94 |
| D28 | Saccharomyces | 84.90 ± 5.48 |
| D20 | Saccharomyces | 84.45 ± 1.90 |
| AH39 | Saccharomyces | 83.11 ± 5.34 |
| GS5 | Saccharomyces | 82.76 ± 4.01 |
| E19 | Saccharomyces | 81.90 ± 6.33 |
| E74 | Saccharomyces | 81.88 ± 8.90 |
| E58 | Saccharomyces | 81.79 ± 8.45 |
| GS29 | Saccharomyces | 80.98 ± 2.06 |
| B49 | Saccharomyces | 80.09 ± 7.21 |
| D27 | Saccharomyces | 78.92 ± 7.12 |
| LN21 | Saccharomyces | 77.09 ± 6.46 |
| CL19 | Saccharomyces | 73.16 ± 9.66 |
| E65 | Saccharomyces | 73.12 ± 0.96 |
| E12 | Saccharomyces | 71.60 ± 5.02 |
| GS33 | Saccharomyces | 71.00 ± 2.23 |
| CL14 | Saccharomyces | 70.58 ± 5.09 |
| SXC13 | Saccharomyces | 70.02 ± 6.31 |
| E77 | Saccharomyces | 69.48 ± 2.35 |
| XJA2 | Saccharomyces | 67.82 ± 7.55 |
| B50 | Saccharomyces | 66.62 ± 8.70 |
| BH33 | Saccharomyces | 65.93 ± 2.48 |
| NW9 | Saccharomyces | 65.23 ± 6.24 |
| B46 | Saccharomyces | 61.84 ± 8.24 |
| CL7 | Saccharomyces | 60.24 ± 2.22 |
| SXC6 | Saccharomyces | 57.27 ± 5.97 |
| L59 | Saccharomyces | 55.25 ± 2.87 |
| LH39 | Saccharomyces | 53.33 ± 3.88 |
| LB1 | Saccharomyces | 52.25 ± 5.81 |
| D41 | Saccharomyces | 52.20 ± 1.21 |
| I61 | Saccharomyces | 52.11 ± 4.71 |
| B42 | Saccharomyces | 49.85 ± 1.19 |
| CL8 | Saccharomyces | 49.31 ± 5.60 |
| I42 | Saccharomyces | 46.83 ± 3.21 |
| CL10 | Saccharomyces | 45.74 ± 2.34 |
| NC1 | Saccharomyces | 44.77 ± 2.89 |
| I52 | Saccharomyces | 43.37 ± 4.00 |
| I34 | Saccharomyces | 42.49 ± 6.91 |
| H70 | Saccharomyces | 41.81 ± 4.88 |
| NW12 | Saccharomyces | 41.05 ± 2.60 |
| CL27 | Saccharomyces | 40.79 ± 2.85 |
| I68 | Saccharomyces | 40.52 ± 4.28 |
| H58 | Saccharomyces | 39.99 ± 3.22 |
| YT13 | Saccharomyces | 39.15 ± 5.85 |
| CL26 | Saccharomyces | 37.42 ± 5.23 |
| E11 | Saccharomyces | 32.12 ± 2.80 |
| I56 | Saccharomyces | 28.01 ± 8.45 |
| AMR-1 * | Saccharomyces | 11.11 ± 0.76 |
| Identification Number | Strain Generus | Acetaldehyde Concentration (mg/L) |
|---|---|---|
| GS13 | Candida | 23.60 ± 2.43 |
| GS30 | Candida | 20.71 ± 2.82 |
| GS31 | Candida | 16.65 ± 0.54 |
| LN22 | Candida | 9.96 ± 1.99 |
| D1 | Candida | 8.46 ± 0.89 |
| GS14 | Candida | 7.79 ± 1.27 |
| D18 | Candida | 6.81 ± 0.04 |
| LN12 | Candida | 6.66 ± 0.45 |
| GS16 | Candida | 5.78 ± 0.79 |
| I15 | Candida | 5.69 ± 2.85 |
| GS10 | Candida | 4.94 ± 0.62 |
| YT4 | Candida | 4.85 ± 0.98 |
| GS20 | Candida | 4.71 ± 1.00 |
| LN9 | Candida | 4.52 ± 0.56 |
| YT25 | Candida | 4.52 ± 1.05 |
| F19 | Candida | 3.69 ± 0.61 |
| GS15 | Candida | 3.46 ± 0.70 |
| GS12 | Candida | 3.39 ± 0.41 |
| LN13 | Candida | 3.33 ± 0.40 |
| BG3 | Candida | 3.16 ± 0.77 |
| YT3 | Candida | 3.14 ± 0.65 |
| YT2 | Candida | 2.94 ± 0.46 |
| D16 | Candida | 2.48 ± 0.59 |
| YT10 | Candida | 2.28 ± 0.76 |
| GS18 | Candida | 2.09 ± 0.45 |
| GS11 | Candida | 1.88 ± 0.34 |
| LN10 | Candida | 1.72 ± 0.42 |
| GS27 | Candida | 1.53 ± 0.13 |
| GS22 | Candida | 1.20 ± 0.11 |
| D40 | Candida | 0.34 ± 0.11 |
| SXC11 | Hanseniaspora | 9.58 ± 0.13 |
| GS8 | Hanseniaspora | 5.04 ± 0.67 |
| GS32 | Saccharomyces | 19.37 ± 4.29 |
| D35 | Saccharomyces | 11.88 ± 2.15 |
| LN21 | Saccharomyces | 6.04 ± 0.90 |
| H68 | Saccharomyces | 7.56 ± 0.86 |
| CL1 | Saccharomyces | 6.76 ± 2.43 |
| LH39 | Saccharomyces | 6.67 ± 1.00 |
| CL10 | Saccharomyces | 6.37 ± 0.40 |
| SXC8 | Saccharomyces | 5.95 ± 0.54 |
| D27 | Saccharomyces | 5.26 ± 1.10 |
| GS39 | Saccharomyces | 4.97 ± 0.40 |
| E74 | Saccharomyces | 4.76 ± 1.29 |
| E68 | Saccharomyces | 4.66 ± 1.94 |
| B38 | Saccharomyces | 4.58 ± 1.53 |
| SXC13 | Saccharomyces | 4.50 ± 0.62 |
| D41 | Saccharomyces | 4.25 ± 0.36 |
| CL19 | Saccharomyces | 4.02 ± 0.61 |
| SXC6 | Saccharomyces | 3.81 ± 0.64 |
| AH39 | Saccharomyces | 10.13 ± 0.58 |
| NW9 | Saccharomyces | 9.61 ± 0.39 |
| B43 | Saccharomyces | 6.70 ± 1.25 |
| L59 | Saccharomyces | 6.04 ± 0.90 |
| D30 | Saccharomyces | 5.38 ± 0.56 |
| I43 | Saccharomyces | 5.19 ± 0.72 |
| BH8 | Saccharomyces | 5.02 ± 1.92 |
| E12 | Saccharomyces | 4.81 ± 0.05 |
| SXC9 | Saccharomyces | 4.59 ± 0.02 |
| BH33 | Saccharomyces | 4.57 ± 1.96 |
| NC3 | Saccharomyces | 4.26 ± 0.17 |
| H63 | Saccharomyces | 4.22 ± 1.05 |
| I52 | Saccharomyces | 3.82 ± 0.31 |
| B46 | Saccharomyces | 3.78 ± 0.96 |
| E63 | Saccharomyces | 3.52 ± 0.98 |
| D22 | Saccharomyces | 3.49 ± 0.55 |
| B47 | Saccharomyces | 3.43 ± 1.39 |
| I42 | Saccharomyces | 3.35 ± 0.53 |
| SC37 | Saccharomyces | 3.24 ± 0.15 |
| CL27 | Saccharomyces | 2.98 ± 0.47 |
| CL14 | Saccharomyces | 2.71 ± 0.56 |
| I34 | Saccharomyces | 2.58 ± 0.78 |
| XJA8 | Saccharomyces | 2.55 ± 0.71 |
| CL34 | Saccharomyces | 2.46 ± 0.45 |
| H58 | Saccharomyces | 2.36 ± 0.57 |
| AC29 | Saccharomyces | 2.33 ± 0.81 |
| H70 | Saccharomyces | 2.08 ± 0.66 |
| E77 | Saccharomyces | 1.90 ± 0.33 |
| YT28 | Saccharomyces | 1.78 ± 0.27 |
| I61 | Saccharomyces | 1.74 ± 0.19 |
| D28 | Saccharomyces | 1.58 ± 0.52 |
| GS29 | Saccharomyces | 1.44 ± 0.10 |
| GS33 | Saccharomyces | 1.23 ± 0.07 |
| B50 | Saccharomyces | 1.14 ± 0.13 |
| E67 | Saccharomyces | 0.89 ± 0.24 |
| SH29 | Saccharomyces | 0.81 ± 0.27 |
| E58 | Saccharomyces | 0.57 ± 0.18 |
| CL16 | Saccharomyces | 0.37 ± 0.21 |
| CL7 | Saccharomyces | 3.04 ± 0.33 |
| CL8 | Saccharomyces | 2.96 ± 0.51 |
| I68 | Saccharomyces | 2.81 ± 0.36 |
| XJA2 | Saccharomyces | 2.70 ± 0.03 |
| E65 | Saccharomyces | 2.56 ± 0.45 |
| NC1 | Saccharomyces | 2.42 ± 0.59 |
| I56 | Saccharomyces | 2.34 ± 0.55 |
| CL26 | Saccharomyces | 2.31 ± 0.40 |
| D39 | Saccharomyces | 2.27 ± 0.20 |
| D20 | Saccharomyces | 2.08 ± 0.20 |
| B49 | Saccharomyces | 2.04 ± 0.28 |
| GS9 | Saccharomyces | 1.89 ± 0.22 |
| GS5 | Saccharomyces | 1.80 ± 0.25 |
| GS40 | Saccharomyces | 1.76 ± 0.14 |
| E19 | Saccharomyces | 1.72 ± 0.05 |
| LH21 | Saccharomyces | 1.60 ± 0.36 |
| LB1 | Saccharomyces | 1.57 ± 0.16 |
| NW12 | Saccharomyces | 1.51 ± 0.36 |
| XJA1 | Saccharomyces | 1.32 ± 0.23 |
| YT13 | Saccharomyces | 1.05 ± 0.16 |
| B42 | Saccharomyces | 0.86 ± 0.19 |
| H66 | Saccharomyces | 0.79 ± 0.20 |
| E11 | Saccharomyces | 0.52 ± 0.04 |
| AMR-1 * | Saccharomyces | 2.32 ± 0.25 |
| Mulberry Juice | Mulberry Wine | |||||||
|---|---|---|---|---|---|---|---|---|
| AMR-1 (CK) | GS32 | D1 | AMR-1 + GS13 | AMR-1 + D1 | GS32 + GS13 | GS32 + D1 | ||
| Total anthocyanins (mg/L) | 753.67 ± 47.00 b | 849.36 ± 41.21 ab | 901.70 ± 56.52 a | 866.02 ± 28.79 a | 747.93 ± 41.56 b | 834.24 ± 81.05 ab | 748.11 ± 19.45 b | 846.27 ± 77.40 ab |
| C3G (mg/L) | 240.85 ± 0.36 a | 194.73 ± 51.89 ab | 151.39 ± 4.47 b | 231.47 ± 21.71 a | 175.86 ± 20.99 ab | 191.81 ± 27.08 ab | 215.78 ± 31.91 ab | 209.43 ± 46.33 ab |
| C3R (mg/L) | 354.61 ± 18.72 d | 528.01 ± 19.81 a | 509.29 ± 43.95 a | 462.07 ± 37.21 ab | 380.87 ± 22.60 cd | 413.72 ± 43.90 bcd | 473.09 ± 36.89 ab | 434.09 ± 36.04 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Chai, Y.; Huang, W.; Zhan, J.; You, Y. Screening for High-Yielding Pyruvate and Acetaldehyde Yeasts and Their Application in Improving the Stability of Anthocyanin in Mulberry Wine. Foods 2025, 14, 2278. https://doi.org/10.3390/foods14132278
Zhou H, Chai Y, Huang W, Zhan J, You Y. Screening for High-Yielding Pyruvate and Acetaldehyde Yeasts and Their Application in Improving the Stability of Anthocyanin in Mulberry Wine. Foods. 2025; 14(13):2278. https://doi.org/10.3390/foods14132278
Chicago/Turabian StyleZhou, Hui, Yajie Chai, Weidong Huang, Jicheng Zhan, and Yilin You. 2025. "Screening for High-Yielding Pyruvate and Acetaldehyde Yeasts and Their Application in Improving the Stability of Anthocyanin in Mulberry Wine" Foods 14, no. 13: 2278. https://doi.org/10.3390/foods14132278
APA StyleZhou, H., Chai, Y., Huang, W., Zhan, J., & You, Y. (2025). Screening for High-Yielding Pyruvate and Acetaldehyde Yeasts and Their Application in Improving the Stability of Anthocyanin in Mulberry Wine. Foods, 14(13), 2278. https://doi.org/10.3390/foods14132278








