Influence of Environmental Conditions Associated with Low and High Altitudes on Economic and Quality Characteristics of Fruit Ripening of Camellia chekiangoleosa Hu
Abstract
1. Introduction
2. Materials and Method
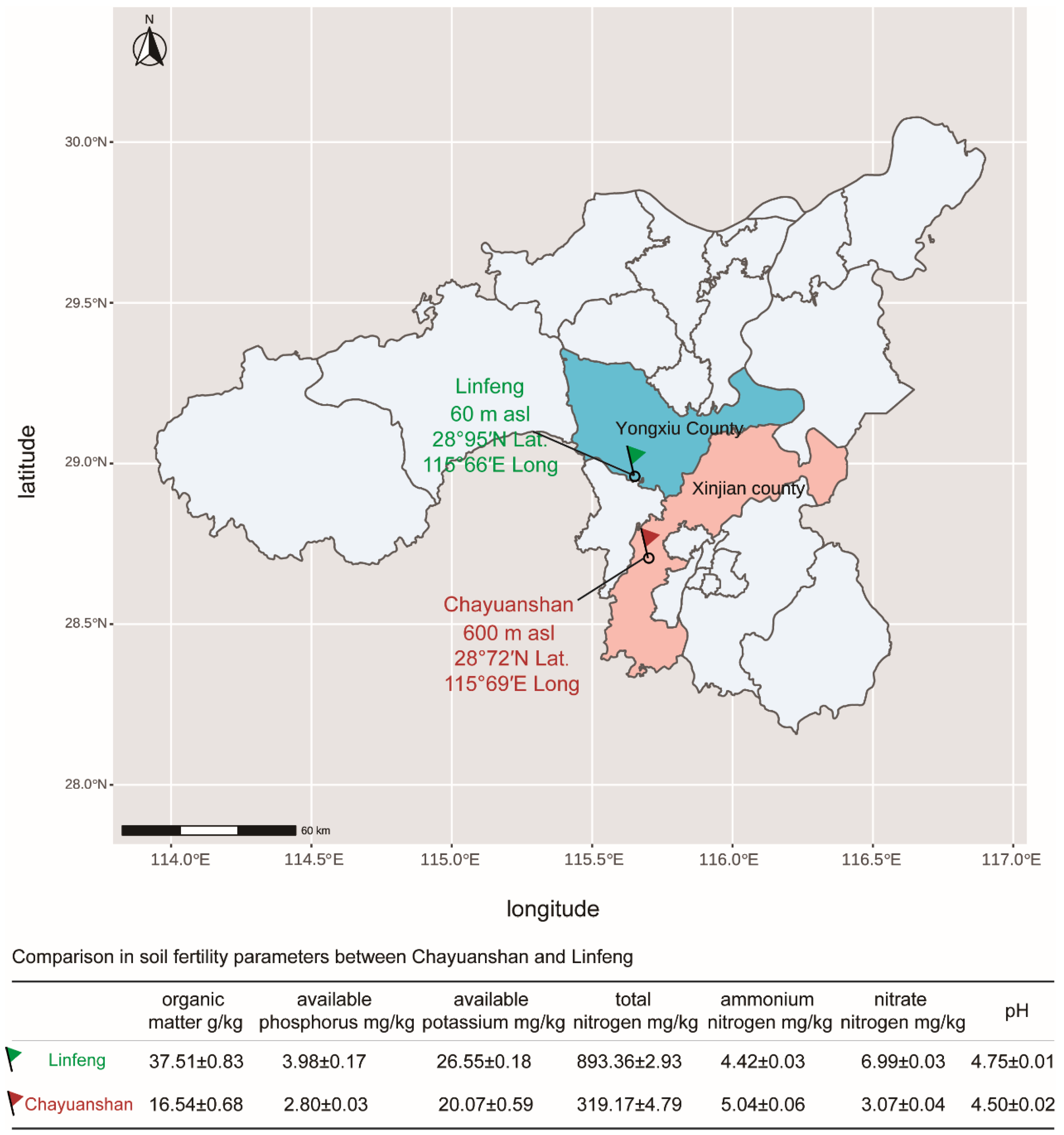
2.1. Sample Collection
2.2. Chemicals and Standards
2.3. Fruit Shell Macronutrient Analysis
2.3.1. Moisture Content
2.3.2. Oil Content
2.3.3. Soluble Sugar and Starch Content
2.3.4. Protein Content
2.4. Analysis of Total Polyphenol and Total Flavonoid Contents in Fruit Shell
2.5. Fruit Shell Tea Saponin Analysis
2.6. Fresh Seed Yield and Kernel Yield
2.7. Seed Oil Yield and Oil Extraction
2.8. Oil Component Determination
2.8.1. α-Tocopherol Determination
2.8.2. Analysis of β-Sitosterol, β-Amyrinol, and Squalene
2.8.3. Total Polyphenol and Total Flavonoid Contents
2.8.4. Fatty Acid Composition Analysis of Camellia Seed Oils
2.9. Statistical Analysis
3. Results and Discussion
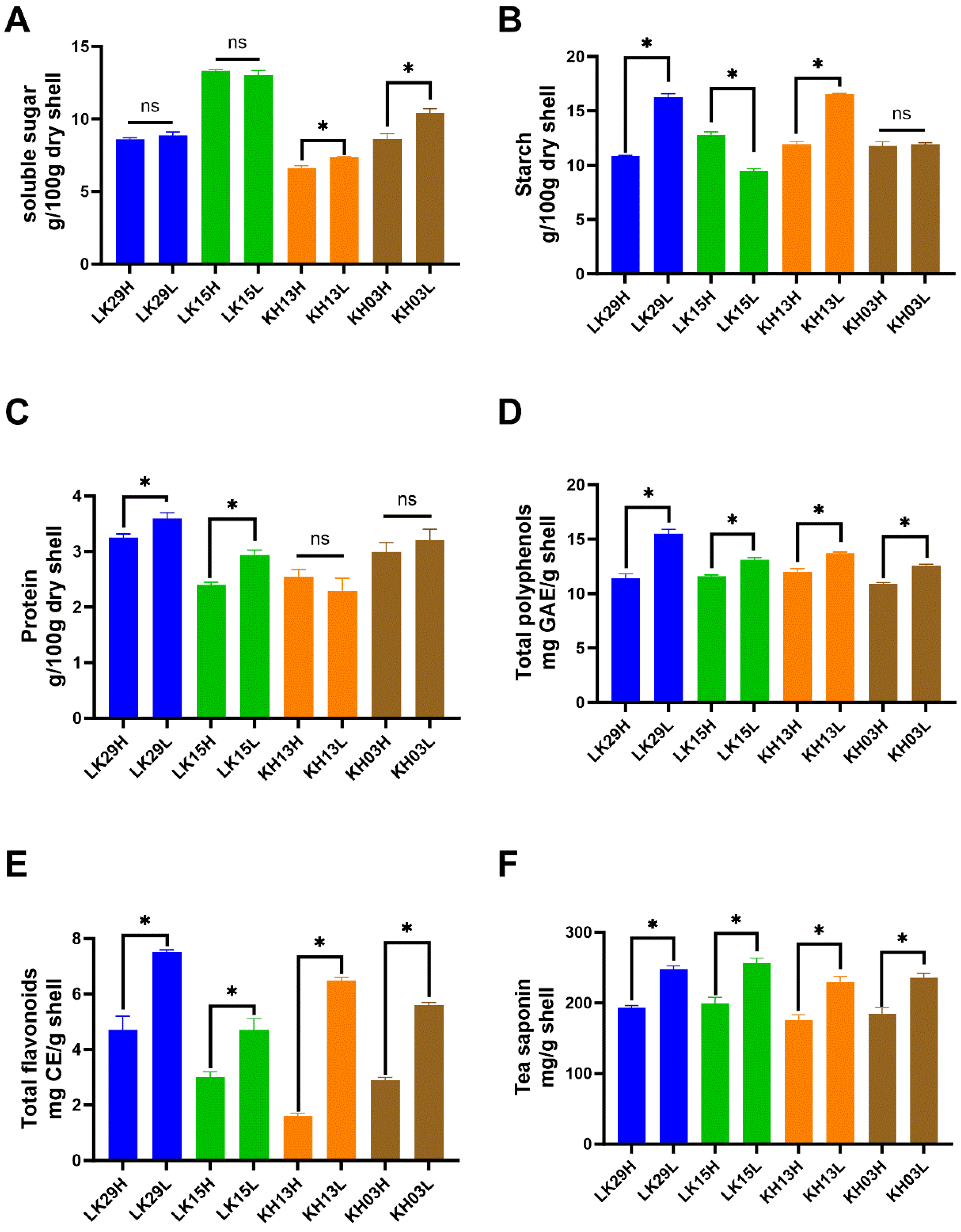
3.1. Effect of Altitude on Chemical Composition of C. chekiangoleosa Fruit Shell During the Ripening Process
3.2. Effect of Altitude on Shell Chemical Composition of Mature C. chekiangoleosa Fruit
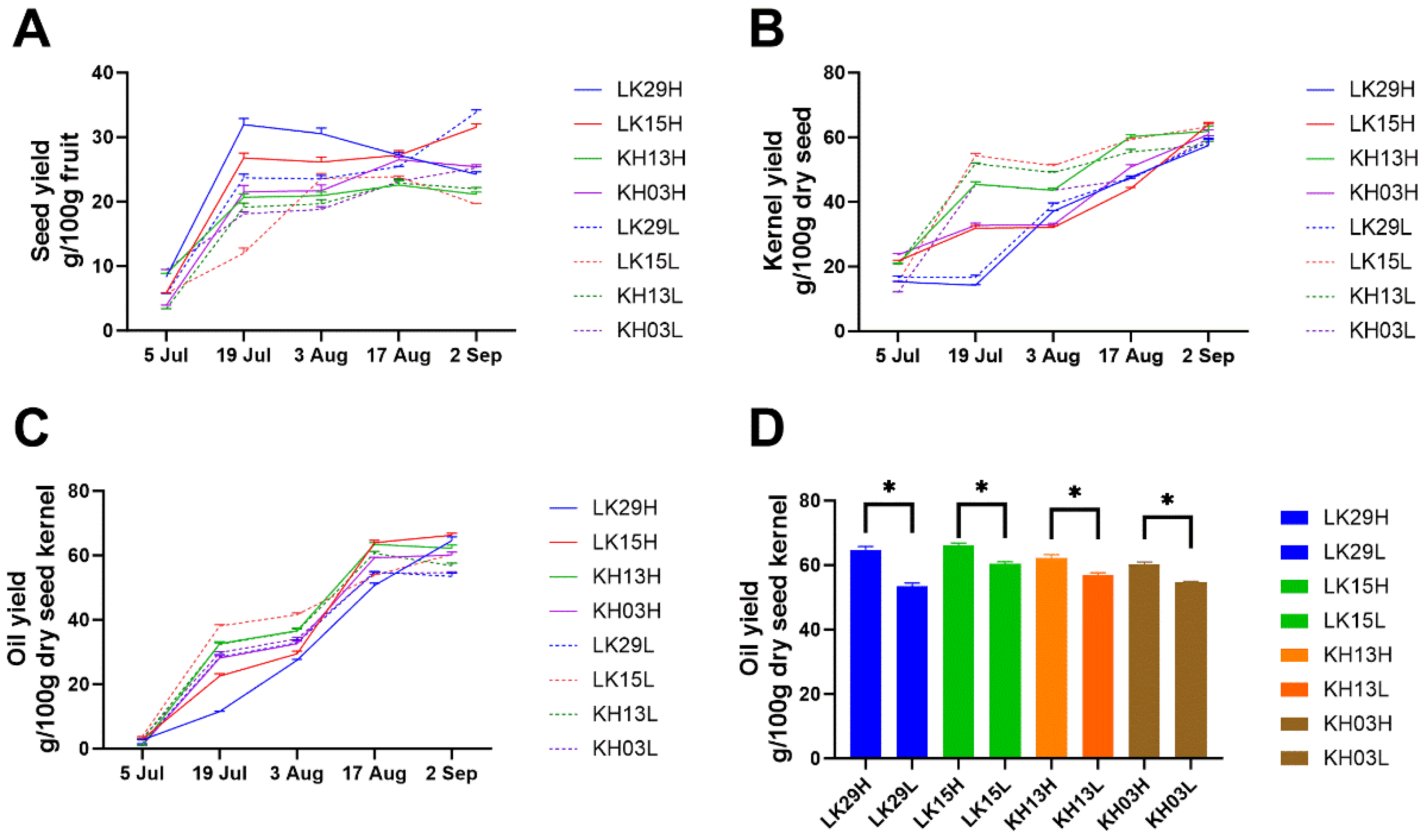
3.3. Effect of Altitude on the Seed Yield and Oil Yield of C. chekiangoleosa During the Ripening Process
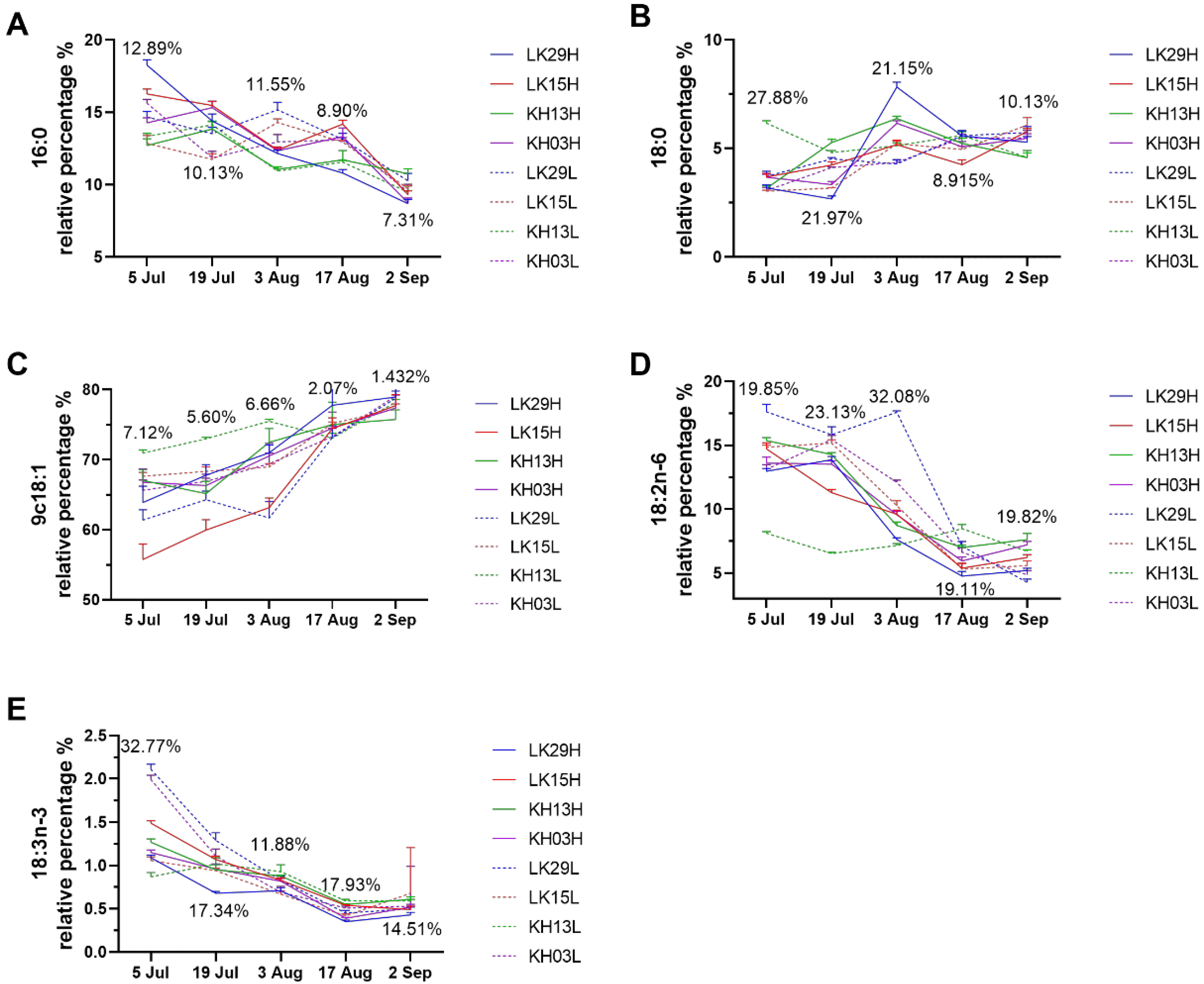
3.4. Effect of Altitude on Lipid Concomitant and Fatty Acid Composition of C. chekiangoleosa Seed Oils During the Ripening Process
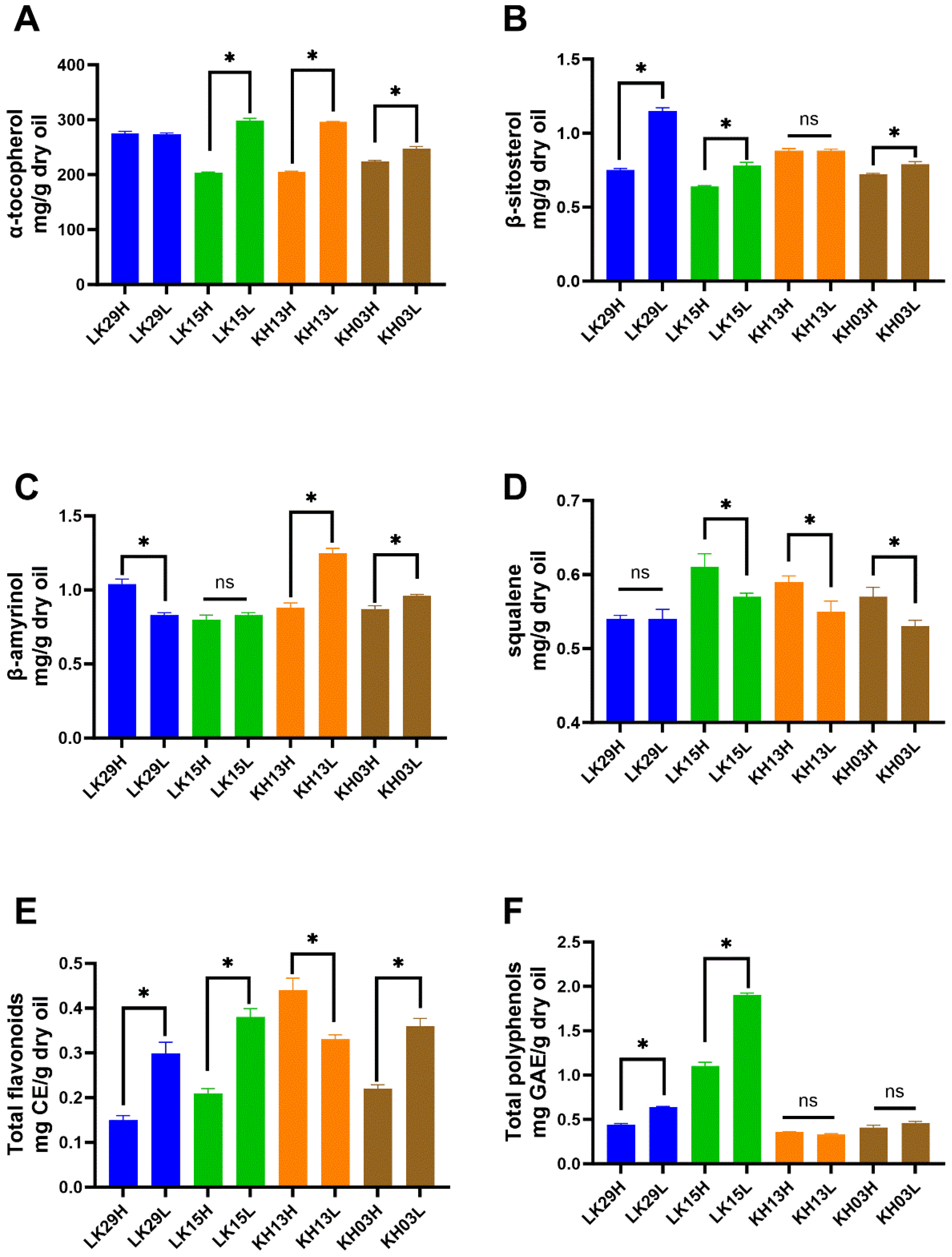
3.5. Effect of Altitude on Seed Oil’s Chemical Composition of Mature C. chekiangoleosa Seed Oil
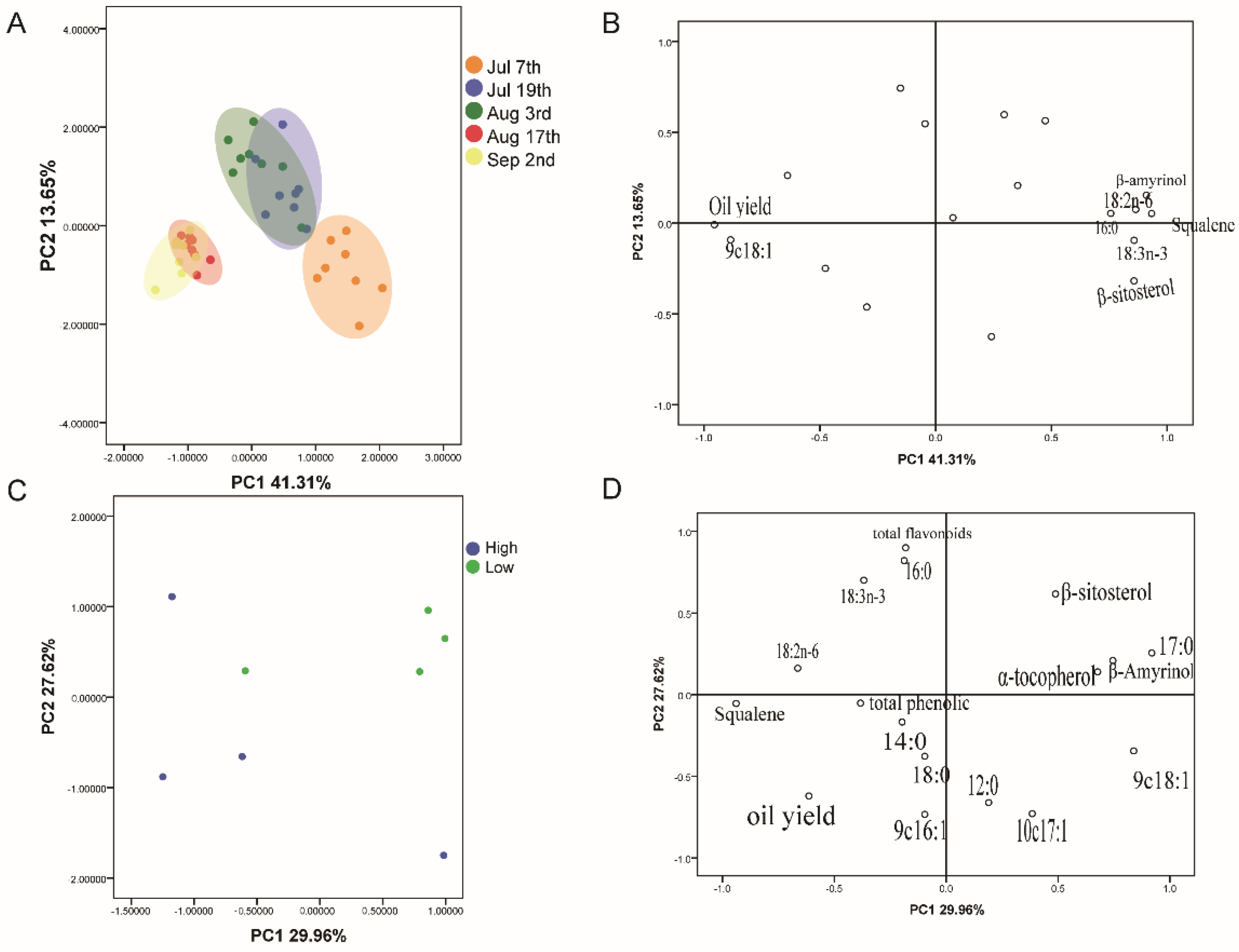
3.6. Cluster Analysis of C. chekiangoleosa Seed Oils
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, H.; Tan, H.; Zhou, J. Proximate composition of Camellia chekiangoleosa Hu fruit and fatty acid constituents of its seed oil. J. Zhejiang Univ. 2010, 36, 662–669. [Google Scholar]
- Zhou, W.; Wang, Z.; Dong, L.; Wen, Q.; Huang, W.; Li, T.; Ye, J.; Xu, L. Analysis on the character diversity of fruit and seed of Camellia chekiangoleosa. J. Nanjing Univ. Nat. Sci. Ed 2021, 45, 51–59. [Google Scholar]
- Cheng, X.; Yang, T.; Wang, Y.; Zhou, B.; Yan, L.; Teng, L.; Wang, F.; Chen, L.; He, Y.; Guo, K. New method for effective identification of adulterated Camellia oil basing on Camellia oleifera-specific DNA. Arab. J. Chem. 2018, 11, 815–826. [Google Scholar] [CrossRef]
- Cicero, A.F.; Derosa, G.; Pisciotta, L.; Barbagallo, C.; Group, S.-P.S. Testing the short-term efficacy of a lipid-lowering nutraceutical in the setting of clinical practice: A multicenter study. J. Med. Food 2015, 18, 1270–1273. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, L.; Hou, W.; Wu, J. β-Sitosterol alleviates inflammatory response via inhibiting the activation of ERK/p38 and NF-κB pathways in LPS-exposed BV2 cells. BioMed Res. Int. 2020, 2020, 7532306. [Google Scholar] [CrossRef]
- Wang, X.Q.; Zeng, Q.M.; del Mar Contreras, M.; Wang, L.J. Profiling and quantification of phenolic compounds in Camellia seed oils: Natural tea polyphenols in vegetable oil. Food Res. Int. 2017, 102, 184–194. [Google Scholar] [CrossRef]
- Huang, L.; Peng, H.; Xiao, Z.; Wu, H.; Fu, G.; Wan, Y.; Bi, H. Production of furfural and 5-hydroxymethyl furfural from Camellia oleifera fruit shell in [Bmim] HSO4/H2O/1, 4-dioxane biphasic medium. Ind. Crops Prod. 2022, 184, 115006. [Google Scholar] [CrossRef]
- Zhang, L.; He, Y.; Zhu, Y.; Liu, Y.; Wang, X. Camellia oleifera shell as an alternative feedstock for furfural production using a high surface acidity solid acid catalyst. Bioresour. Technol. 2018, 249, 536–541. [Google Scholar] [CrossRef]
- Jian, H.-l.; Liao, X.-x.; Zhu, L.-w.; Zhang, W.-m.; Jiang, J.-x. Synergism and foaming properties in binary mixtures of a biosurfactant derived from Camellia oleifera Abel and synthetic surfactants. J. Colloid Interface Sci. 2011, 359, 487–492. [Google Scholar] [CrossRef]
- Xie, Y.; Ge, S.; Jiang, S.; Liu, Z.; Chen, L.; Wang, L.; Chen, J.; Qin, L.; Peng, W. Study on biomolecules in extractives of Camellia oleifera fruit shell by GC–MS. Saudi J. Biol. Sci. 2018, 25, 234–236. [Google Scholar] [CrossRef]
- Zhao, Y.; Su, R.; Zhang, W.; Yao, G.-L.; Chen, J. Antibacterial activity of tea saponin from Camellia oleifera shell by novel extraction method. Ind. Crops Prod. 2020, 153, 112604. [Google Scholar] [CrossRef]
- Peng, J.; Wen, W.; Liang, G.; Huang, W.; Qiu, Z.; Wang, Q.; Xiao, G. Camellia oleifera shells polyphenols inhibit advanced glycation end-products (AGEs) formation and AGEs-induced inflammatory response in RAW264. 7 macrophages. Ind. Crops Prod. 2023, 197, 116589. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, S.; Shao, S.; Qian, L.; Xu, P. Studies on bioactivities of tea (Camellia sinensis L.) fruit peel extracts: Antioxidant activity and inhibitory potential against α-glucosidase and α-amylase in vitro. Ind. Crops Prod. 2012, 37, 520–526. [Google Scholar] [CrossRef]
- Huang, B.; Wang, Z.; Huang, J.; Li, X.; Zhu, H.; Wen, Q.; Xu, L.-A. Population Genetic Structure Analysis Reveals Significant Genetic Differentiation of the Endemic Species Camellia chekiangoleosa Hu. with a Narrow Geographic Range. Forests 2022, 13, 234. [Google Scholar] [CrossRef]
- Maowang, F. The Research on Genetic Variation Laws of Camellia chekiangoleosa Hu in Fujian Different Geographic Regions. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2013. [Google Scholar]
- Xuerui, R. Effects of Fertilization on Growth and Soil Nutrients in Camellia chekiangoleosa. Hubei For. Sci. Technol. 2022, 51, 14–18. [Google Scholar]
- Xue, H.; Li, Y. The successful introduction of Zhejiang safflower camellia and Tengchong safflower camellia on the northern edge of the subtropical zone. Shaanxi For. Sci. Technol. 1996, 4, 53–54. [Google Scholar]
- Zeng, M.; Ouyang, Y.; Liu, Y. Effect of altitude on the growth and yield of Camellia oleifera. J. Green Sci. Technol. 2023, 25, 137–144. [Google Scholar]
- Liu, B.T.; Ni, R.X.; Qin, Y.C.; Wang, Y.; Fang, R.; Wang, Y.B. Comparisons on Seeds of Changlin Series of Camellia oleifera at Different Elevation. J. Zhejiang For. Sci. Technol. 2017, 37, 49–53. [Google Scholar]
- Liu, X.Y.; Li, J.; Wen, Z.G.; Huang, X.M.; Rong, J.; Liu, X.R.; Deng, Z.Y. Comparison of Oil and Chemical Compositions of Wild Tea Seeds from Different Altitudes Above Sea Level in Lushan and Jinggangshan. J. Chin. Inst. Food Sci. Technol. 2019, 19, 248–256. [Google Scholar]
- Wei, T.; Dong, L.; Zhong, S.; Jing, H.; Deng, Z.; Wen, Q.; Li, J. Chemical composition of Camellia chekiangoleosa Hu. seeds during ripening and evaluations of seed oils quality. Ind. Crops Prod. 2022, 177, 114499. [Google Scholar] [CrossRef]
- Wang, H.; Pampati, N.; McCormick, W.M.; Bhattacharyya, L. Protein nitrogen determination by kjeldahl digestion and ion chromatography. J. Pharm. Sci. 2016, 105, 1851–1857. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Huang, B.; Wei, T.; Deng, Z.; Li, J.; Wen, Q. Comprehensive Evaluation of Quality Characteristics of Four Oil-Tea Camellia Species with Red Flowers and Large Fruit. Foods 2023, 12, 374. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.; Deng, Z.-Y.; Zhang, N.; Zou, Q.; Fan, Y.-W.; Liu, R.; Zhen, L.-F.; Li, J. Lipid profiles of Chinese soft-shell turtle eggs (Pelodiscus sinensis). J. Food Compost. Anal. 2020, 94, 103627. [Google Scholar] [CrossRef]
- Naryal, A.; Dolkar, D.; Bhardwaj, A.K.; Kant, A.; Chaurasia, O.; Stobdan, T. Effect of altitude on the phenology and fruit quality attributes of apricot (Prunus armeniaca L.) fruits. Def. Life Sci. J. 2020, 5, 18–24. [Google Scholar] [CrossRef]
- Smith, A.J.; Rinne, R.W.; Seif, R.D. Phosphoenolpyruvate carboxylase and pyruvate kinase involvement in protein and oil biosynthesis during soybean seed development. Crop Sci. 1989, 29, 349–353. [Google Scholar] [CrossRef]
- Agoreyo, B.; Fregene, R. Variations in amylase and invertase activities in solanum species (eggplants) during ripening. J. Appl. Sci. Environ. Manag. 2014, 18, 283–290. [Google Scholar] [CrossRef][Green Version]
- Zhang, G.; Wng, Y.; Fu, C. Study on variation of several catalase activities during starwberry fruit ripeing. J. Anhui Agri. Sci. 2010, 38, 9180–9181. [Google Scholar]
- Xiang, X.F. Changes of Pericarp Development and Contents of Camellia oleifera. Master’s Thesis, Central South University of Forestry and Technology, Changsha, China, 2023. [Google Scholar]
- Alina, V.R.; Vlad, M.; Elena, M.A.; Carmen, M.C.; Adriana, P.; Viorel, M.; Maria, C.S.; Sevastita, M. The Changes of Polyphenols, Flavonoids, Anthocyanins and Chlorophyll Content in Plum Peels during Growth Phases: From Fructification to Ripening. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 46, 148. [Google Scholar]
- Xiang, X.; Chen, J.; Jiang, Y.; Gong, W. Changes of flavonoids during the development and maturation of Camellia oleifera peel. Non-Wood For. Res. 2023, 41, 83–90. [Google Scholar]
- Ning, R.; Cheng, X.; Zhang, F.; Chen, D.; Li, W.; Zhang, L.; Zhu, L.; Jiang, J. Comparative study on potentials of Camellia oleifera shell saponins as foam cleaning agents in the late growth stage. J. Clean. Prod. 2023, 420, 138326. [Google Scholar] [CrossRef]
- Andresen, M.; Cedergreen, N. Plant growth is stimulated by tea-seed extract: A new natural growth regulator? HortScience 2010, 45, 1848–1853. [Google Scholar] [CrossRef]
- Durán, A.G.; Calle, J.M.; Butrón, D.; Pérez, A.J.; Macías, F.A.; Simonet, A.M. Steroidal saponins with plant growth stimulation effects; Yucca schidigera as a commercial source. Plants 2022, 11, 3378. [Google Scholar] [CrossRef]
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in Plants: Structure, Biosynthesis, Abiotic Stress Regulation, and Practical Applications. Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef]
- Szakiel, A.; Pączkowski, C.; Henry, M. Influence of environmental abiotic factors on the content of saponins in plants. Phytochem. Rev. 2011, 10, 471–491. [Google Scholar] [CrossRef]
- Carrillo, L.C.; Londoño-Londoño, J.; Gil, A. Comparison of polyphenol, methylxanthines and antioxidant activity in Theobroma cacao beans from different cocoa-growing areas in Colombia. Food Res. Int. 2014, 60, 273–280. [Google Scholar] [CrossRef]
- Mateus, N.; Marques, S.; Gonçalves, A.C.; Machado, J.M.; De Freitas, V. Proanthocyanidin composition of red Vitis vinifera varieties from the Douro Valley during ripening: Influence of cultivation altitude. Am. J. Enol. Vitic. 2001, 52, 115–121. [Google Scholar] [CrossRef]
- Fujimura, S.; Shi, P.; Iwama, K.; Zhang, X.; Gopal, J.; Jitsuyama, Y. Effect of altitude on the response of net photosynthetic rate to carbon dioxide increase by spring wheat. Plant Prod. Sci. 2010, 13, 141–149. [Google Scholar] [CrossRef]
- Naseri, A.; Alirezalu, A.; Noruzi, P.; Alirezalu, K. The effect of different ammonium to nitrate ratios on antioxidant activity, morpho-physiological and phytochemical traits of Moldavian balm (Dracocephalum moldavica). Sci. Rep. 2022, 12, 16841. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Hajisolomou, E.; Xylia, P.; Tzortzakis, N. Ammonium to total nitrogen ratio affects the purslane (Portulaca oleracea L.) growth, nutritional, and antioxidant status. Heliyon 2023, 9, e21644. [Google Scholar] [CrossRef]
- Lü, X.; Ma, J.; Yan, H.; Yang, L.; Ren, X.; Guo, J.; Yong, Q.; Kong, W.; Deng, Y. Effect of degree of ripening on the quality of virgin olive oils produced in Longnan, China. J. Am. Oil Chem. Soc. 2021, 98, 1045–1056. [Google Scholar] [CrossRef]
- He, Y.; Wu, M.; Dong, L.; Wen, Q.; Li, T.; Li, X.; Zhu, H.; Xu, L.; Xu, L. Analysis of kernel oil content and variation of fatty acid composition of Camellia chekiangoleosa in the main producing areas. Non-Wood For. Res. 2020, 38, 37–45. [Google Scholar]
- Ding, X.N.; Shi, T.; Yang, L.F.; Yang, H.; Wang, K.X.; Guo, X.F.; Shi, G.A. Correlation between Seed Quality and Meteorological Factors of Oil Tree Peony Fengdan at Different Altitudes. J. Henan Agric. Sci. 2019, 48, 120–126. [Google Scholar]
- Xu, L. Response of photosynthetic physiology and oil production of spring rapeseed under different altitude conditions. Jiangsu Agric. Sci. 2017, 45, 92–94. [Google Scholar]
- Ben Brahim, S.; Kelebek, H.; Ammar, S.; Abichou, M.; Bouaziz, M. LC–MS phenolic profiling combined with multivariate analysis as an approach for the characterization of extra virgin olive oils of four rare Tunisian cultivars during ripening. Food Chem. 2017, 229, 9–19. [Google Scholar] [CrossRef]
- Bates, P.D.; Stymne, S.; Ohlrogge, J. Biochemical pathways in seed oil synthesis. Curr. Opin. Plant Biol. 2013, 16, 358–364. [Google Scholar] [CrossRef]
- Wang, Z.; Wng, B.; Dong, L.; Xu, L.; Tang, S.; Wen, Q. Cloning of CcFAD2 gene from Camellia chekiangoleosa and analysis on its expression during kernel development. J. Plant Resour. Environ. 2020, 29, 1–8. [Google Scholar]
- Zhang, Y.; Zhang, C.; Xu, C.; Deng, Y.; Wen, B.; Xie, P.; Huang, L. Effect of geographical location and soil fertility on main phenolic compounds and fatty acids compositions of virgin olive oil from Leccino cultivar in China. Food Res. Int. 2022, 157, 111207. [Google Scholar] [CrossRef]
- Nguyen, T.B.T.; Ketsa, S.; Doorn, W.G.V. Relationship between browning and the activities of polyphenoloxidase and phenylalanine ammonia lyase in banana peel during low temperature storage. Postharvest Biol. Technol. 2003, 30, 187–193. [Google Scholar] [CrossRef]
- He, X.Z.; Xu, H.N.; Long, J.; Chen, L.M.; Li, K.Z. Progresses on Phytosterols in Plant Stress. Life Sci. Res. 2013, 17, 267–273. [Google Scholar]
- Akbaba, E.; Erişir, F.E.; Aydın, S.; Sanyürek, N.K.; Çakır, A. Vitamin content and MDA levels of certain white grape varieties from different altitudes in Turkey. Emir. J. Food Agric. 2023, 35, 428–435. [Google Scholar] [CrossRef]
- Liu, J. The Research on Soil Fertility Quality Assessment of Camellia oleifera in Different Site Type and Quality of Tea Oil. Master’s Thesis, Central south university of forestry and technology, Changsha, China, 2018. [Google Scholar]
- Zhang, N.Y.; Huang, K.S.; Qin, Y.; Wang, N.X.; Zeng, W.J. Effects of major geographical and climatic factors on fatty acid composition of oil-tea camellia seed oil. China Oils Fats 2013, 38, 78–80. [Google Scholar]
- Erel, R.; Kerem, Z.; Ben-Gal, A.; Dag, A.; Schwartz, A.; Zipori, I.; Basheer, L.; Yermiyahu, U. Olive (Olea europaea L.) Tree Nitrogen Status Is a Key Factor for Olive Oil Quality. J. Agric. Food Chem. 2013, 61, 11261–11272. [Google Scholar] [CrossRef] [PubMed]

| LK29 | LK15 | KH13 | KH03 | |||||
|---|---|---|---|---|---|---|---|---|
| High Altitude | Low Altitude | High Altitude | Low Altitude | High Altitude | Low Altitude | High Altitude | Low Altitude | |
| Moisture (g/100 g) | ||||||||
| 5 July | 60.35 ± 1.39 a− | 66.37 ± 0.86 a+ | 62.74 ± 0.68 b+ | 61.86 ± 2.29 b+ | 64.18 ± 2.24 c+ | 62.73 ± 0.84 c+ | 62.57 ± 0.45 d+ | 64.59 ± 3.29 d+ |
| 19 July | 69.73 ± 2.33 a+ | 74.36 ± 1.57 a+ | 66.59 ± 1.49 b+ | 69.68 ± 1.38 b+ | 70.93 ± 1.46 c+ | 72.04 ± 2.24 c+ | 70.28 ± 1.22 d+ | 71.95 ± 1.72 d+ |
| 3 August | 75.42 ± 1.14 a+ | 75.9 ± 2.23 a+ | 75.22 ± 2.39 b+ | 75.27 ± 1.69 b+ | 76.17 ± 2.52 c+ | 76.92 ± 1.49 c+ | 77.42 ± 1.47 d+ | 76 ± 0.57 d+ |
| 17 August | 75.28 ± 2.78 a+ | 77.24 ± 2.17 a+ | 76.14 ± 1.39 b+ | 75.31 ± 0.55 b+ | 72.69 ± 3.11 c+ | 76.25 ± 2.65 c+ | 74.18 ± 0.95 d+ | 72.38 ± 0.47 d+ |
| 2 September | 78.57 ± 0.66 a+ | 74.56 ± 3.42 a+ | 75.86 ± 2.78 b+ | 75.12 ± 1.19 b+ | 77.86 ± 2.05 c+ | 78.6 ± 1.27 c+ | 78.17 ± 2.61 d+ | 78.46 ± 2.28 d+ |
| Oil (g/100 g) | ||||||||
| 5 July | 1.01 ± 0.03 a+ | 0.39 ± 0.01 a− | 0.45 ± 0.01 b− | 0.83 ± 0.02 b+ | 0.73 ± 0.02 c+ | 0.73 ± 0.01 c+ | 0.46 ± 0.01 d− | 0.54 ± 0.02 d+ |
| 19 July | 0.86 ± 0.02 a+ | 0.67 ± 0.04 a− | 0.74 ± 0.03 b+ | 0.63 ± 0.01 b− | 0.56 ± 0.01 c− | 0.92 ± 0.02 c+ | 0.79 ± 0.02 d+ | 0.83 ± 0.01 d+ |
| 3 August | 0.94 ± 0.02 a− | 1.44 ± 0.03 a+ | 1.01 ± 0 b− | 1.73 ± 0.01 b+ | 0.85 ± 0.03 c− | 1.53 ± 0.03 c+ | 1.24 ± 0.03 d− | 1.64 ± 0.03 d+ |
| 17 August | 1.51 ± 0.01 a− | 1.72 ± 0.06 a+ | 1.6 ± 0.01 b− | 2.03 ± 0.03 b+ | 1.45 ± 0.03 c− | 2.17 ± 0.02 c+ | 1.78 ± 0.02 d− | 2.33 ± 0.01 d+ |
| 2 September | 1.83 ± 0.02 a− | 2.03 ± 0.05 a+ | 2.07 ± 0.02 b− | 2.26 ± 0.04 b+ | 1.77 ± 0.03 c− | 2.4 ± 0.03 c+ | 2.02 ± 0.07 d− | 2.65 ± 0.02 d+ |
| Protein (g/100 g) | ||||||||
| 5 July | 1.42 ± 0.03 a+ | 0.83 ± 0.01 a− | 1.34 ± 0.01 b+ | 0.92 ± 0.02 b− | 1.28 ± 0.04 c+ | 0.77 ± 0.01 c− | 0.89 ± 0.01 d+ | 0.93 ± 0.02 d+ |
| 19 July | 0.68 ± 0.01 a+ | 0.73 ± 0.01 a+ | 1.49 ± 0.03 b+ | 0.86 ± 0.02 b− | 0.73 ± 0.02 c− | 1.03 ± 0.01 c+ | 1.02 ± 0.01 d+ | 1.12 ± 0.05 d+ |
| 3 August | 1.54 ± 0.3 a+ | 1.96 ± 0.13 a+ | 1.71 ± 0.11 b+ | 1.89 ± 0.05 b+ | 1.71 ± 0.07 c− | 2.36 ± 0.2 c+ | 1.84 ± 0.2 d+ | 2.21 ± 0.1 d+ |
| 17 August | 3.45 ± 0.09 a+ | 3.21 ± 0.1 a+ | 2.23 ± 0.06 b− | 3.17 ± 0.02 b+ | 2.09 ± 0.07 c+ | 2.35 ± 0.2 c+ | 2.64 ± 0.05 d− | 2.92 ± 0.05 d+ |
| 2 September | 3.25 ± 0.07 a+ | 3.59 ± 0.11 a+ | 2.4 ± 0.05 b− | 2.94 ± 0.09 b+ | 2.55 ± 0.13 c+ | 2.29 ± 0.23 c+ | 2.99 ± 0.17 d+ | 3.2 ± 0.2 d+ |
| Soluble sugar (g/100 g) | ||||||||
| 5 July | 9.04 ± 0.16 a+ | 9.48 ± 0.07 a+ | 8.47 ± 0.08 b− | 10.19 ± 0.14 b+ | 9.36 ± 0.15 c− | 10.33 ± 0.42 c+ | 9.18 ± 0.05 d+ | 9.36 ± 0.22 d+ |
| 19 July | 10.03 ± 0.33 a− | 11.94 ± 0.15 a+ | 10.25 ± 0.41 b+ | 10.27 ± 0.24 b+ | 10.38 ± 0.24 c+ | 9.86 ± 0.11 c+ | 12.27 ± 0.19 d+ | 12.76 ± 0.09 d+ |
| 3 August | 11.24 ± 0.19 a− | 13.16 ± 0.07 a+ | 13.57 ± 0.21 b− | 14.64 ± 0.2 b+ | 12.31 ± 0.21 c+ | 11.48 ± 0.24 c− | 12.76 ± 0.15 d− | 13.45 ± 0.16 d+ |
| 17 August | 10.15 ± 0.16 a− | 10.82 ± 0.13 a+ | 13.96 ± 0.15 b− | 15.14 ± 0.18 b+ | 10.08 ± 0.23 c+ | 9.57 ± 0.29 c+ | 11.56 ± 0.22 d− | 12.46 ± 0.15 d+ |
| 2 Sep | 8.6 ± 0.12 a+ | 8.87 ± 0.24 a+ | 13.33 ± 0.06 b+ | 13.03 ± 0.3 b+ | 6.62 ± 0.15 c− | 7.37 ± 0.05 c+ | 8.62 ± 0.37 d− | 10.4 ± 0.31 d+ |
| Starch (g/100 g) | ||||||||
| 5 July | 12.48 ± 0.11 a+ | 11.62 ± 0.14 a− | 13.29 ± 0.26 b+ | 13.25 ± 0.19 b+ | 12.86 ± 0.13 c− | 14.68 ± 0.17 c+ | 12.29 ± 0.25 d− | 13.16 ± 0.14 d+ |
| 19 July | 11.68 ± 0.21 a+ | 10.71 ± 0.2 a− | 10.26 ± 0.17 b− | 12.28 ± 0.16 b+ | 13.38 ± 0.24 c+ | 13.14 ± 0.15 c+ | 11.67 ± 0.18 d+ | 10.49 ± 0.21 d− |
| 3 August | 9.08 ± 0.11 a− | 10.71 ± 0.11 a+ | 9.74 ± 0.12 b+ | 8.05 ± 0.07 b− | 10.39 ± 0.13 c− | 11.65 ± 0.25 c+ | 10.71 ± 0.15 d+ | 10.68 ± 0.18 d+ |
| 17 August | 8.37 ± 0.19 a− | 11.76 ± 0.36 a+ | 9.59 ± 0.24 b+ | 8.28 ± 0.14 b− | 9.13 ± 0.01 c− | 12.93 ± 0.25 c+ | 8.77 ± 0.23 d− | 10.31 ± 0.25 d+ |
| 2 September | 10.86 ± 0.07 a− | 16.25 ± 0.33 a+ | 12.75 ± 0.32 b+ | 9.51 ± 0.17 b− | 11.93 ± 0.27 c− | 16.55 ± 0.06 c+ | 11.75 ± 0.41 d+ | 11.93 ± 0.13 d+ |
| Total polyphenols (mg GAE/g) | ||||||||
| 5 July | 18.8 ± 0.2 a− | 20.3 ± 0.2 a+ | 23.2 ± 0.3 b− | 27.5 ± 0.3 b+ | 19.8 ± 0.3 c+ | 19.5 ± 0.2 c+ | 21.5 ± 0.1 d− | 23.6 ± 0.1 d+ |
| 19 July | 22.6 ± 0.2 a+ | 15.8 ± 0.3 a− | 18.8 ± 0.1 b− | 32.5 ± 0.2 b+ | 31.5 ± 0.2 c+ | 29.5 ± 0.3 c− | 24.7 ± 0.4 d− | 34.7 ± 0.5 d+ |
| 3 August | 10.7 ± 0.2 a− | 15.9 ± 0.3 a+ | 9.8 ± 0.1 b− | 11.9 ± 0.1 b+ | 10.4 ± 0.1 c− | 12.5 ± 0.1 c+ | 10 ± 0.1 d− | 11.7 ± 0.1 d+ |
| 17 August | 14.1 ± 0.2 a− | 22.8 ± 0 a+ | 14.6 ± 0 b+ | 14.9 ± 0.1 b+ | 14.4 ± 0.2 c− | 16.9 ± 0.1 c+ | 13.6 ± 0.1 d− | 14.4 ± 0.1 d+ |
| 2 September | 11.4 ± 0.4 a− | 15.5 ± 0 a+ | 11.6 ± 0.1 b− | 13.1 ± 0.2 b+ | 12 ± 0.3 c− | 13.7 ± 0.1 c+ | 10.9 ± 0.1 d− | 12.6 ± 0.1 d+ |
| Total flavonoids (mg CE/g) | ||||||||
| 5 July | 8.3 ± 0.2 a+ | 6.5 ± 0.1 a− | 7.6 ± 0.1 b+ | 6.9 ± 0.2 b+ | 6.3 ± 0.1 c− | 9.2 ± 0.3 c+ | 7.2 ± 0.2 d− | 8.5 ± 0.4 d+ |
| 19 July | 11.7 ± 0.2 a− | 13.4 ± 0.3 a+ | 12.6 ± 0.2 b+ | 12.7 ± 0.2 b+ | 10.8 ± 0.3 c− | 14.5 ± 0.33 c+ | 11.6 ± 0.3 d+ | 11.5 ± 0.2 d+ |
| 3 August | 15.6 ± 0.3 a− | 18.5 ± 0.1 a+ | 14.5 ± 0.1 b− | 16.5 ± 0.2 b+ | 15 ± 0.2 c− | 18 ± 0.2 c+ | 17.4 ± 0.2 d+ | 16.6 ± 0.2 d− |
| 17 August | 18.1 ± 0.5 a− | 24.2 ± 0.5 a+ | 17 ± 0.2 b− | 18 ± 0.4 b+ | 13.8 ± 0.3 c− | 20.4 ± 0.1 c+ | 16 ± 0.1 d− | 18.5 ± 0.1 d+ |
| 2 September | 4.7 ± 0.5 a− | 7.5 ± 0.1 a+ | 3 ± 0.2 b− | 4.7 ± 0.4 b+ | 1.6 ± 0.1 c− | 6.5 ± 0.1 c+ | 2.9 ± 0.1 d− | 5.6 ± 0.1 d+ |
| Tea saponin (mg/g) | ||||||||
| 5 July | 143.9 ± 0.8 a+ | 134.9 ± 2.6 a− | 164.7 ± 1.6 b+ | 167.3 ± 1.1 b+ | 167.2 ± 0.9 c+ | 153.4 ± 1 c− | 157.9 ± 1.8 d+ | 134.9 ± 2.4 d− |
| 19 July | 113.5 ± 1.9 a+ | 102.7 ± 1.5 a− | 105.7 ± 2.4 b− | 117.3 ± 2.2 b+ | 132.6 ± 2.2 c+ | 121.7 ± 1.5 c− | 98.6 ± 1.4 d+ | 103.9 ± 2.7 d+ |
| 3 August | 162.4 ± 1.9 a+ | 164.3 ± 3.2 a+ | 149.8 ± 2.9 b− | 172.4 ± 4.4 b+ | 147.6 ± 2.8 c− | 174.7 ± 1.8 c+ | 152.4 ± 5.3 d− | 165.3 ± 3.3 d+ |
| 17 August | 143.6 ± 2.8 a− | 171.6 ± 4.4 a+ | 151.1 ± 4.3 b− | 169.5 ± 2.6 b+ | 134.7 ± 3.2 c− | 183.6 ± 2.4 c+ | 146.3 ± 5.3 d− | 173.6 ± 5.5 d+ |
| 2 September | 193.4 ± 3 a− | 248 ± 4.7 a+ | 199.2 ± 8.8 b+ | 256.4 ± 7.4 b+ | 175.8 ± 7.6 c− | 229.4 ± 8.2 c+ | 184.8 ± 8.5 d− | 235.3 ± 6.5 d+ |
| LK29 | LK15 | KH13 | KH03 | |||||
|---|---|---|---|---|---|---|---|---|
| High Altitude | Low Altitude | High Altitude | Low Altitude | High Altitude | Low Altitude | High Altitude | Low Altitude | |
| Total flavonoids (mg CE/g) | ||||||||
| 5 Julyy | 0.69 ± 0.017 a− | 0.96 ± 0.038 a+ | 0.89 ± 0.027 b+ | 0.53 ± 0.01 b− | 1.07 ± 0.014 c− | 1.31 ± 0.047 c+ | 1.48 ± 0.028 d− | 0.61 ± 0.025 d+ |
| 19 Julyy | 1.72 ± 0.04 a− | 1.98 ± 0.024 a+ | 1.33 ± 0.043 b+ | 1.19 ± 0.041 b− | 1.47 ± 0.013 c− | 2.5 ± 0.041 c+ | 0.96 ± 0.026 d− | 1.41 ± 0.014 d+ |
| 3 August | 0.65 ± 0.034 a− | 1.71 ± 0.022 a+ | 1.92 ± 0.022 b+ | 2 ± 0.032 b+ | 1.3 ± 0.028 c− | 1.7 ± 0.044 c+ | 1.24 ± 0.034 d+ | 0.93 ± 0.021 d− |
| 17 August | 0.3 ± 0.027 a− | 0.48 ± 0.02 a+ | 0.63 ± 0.043 b− | 1.51 ± 0.029 b+ | 0.4 ± 0.035 c− | 0.59 ± 0.01 c+ | 0.41 ± 0.018 d− | 0.52 ± 0.01 d+ |
| 2 September | 0.15 ± 0.01 a− | 0.3 ± 0.024 a+ | 0.21 ± 0.01 b− | 0.38 ± 0.019 b+ | 0.44 ± 0.027 c+ | 0.33 ± 0.01 c− | 0.22 ± 0.009 d+ | 0.36 ± 0.017 d− |
| Total polyphenols (mg GAE/g) | ||||||||
| 5 July | 4.86 ± 0.049 a+ | 1.95 ± 0.049 a− | 4.2 ± 0.02 b+ | 0.95 ± 0.024 b− | 4.43 ± 0.034 c+ | 2.58 ± 0.013 c− | 0.53 ± 0.017 d− | 2.27 ± 0.014 d+ |
| 19 July | 2.12 ± 0.031 a+ | 1.4 ± 0.03 a− | 2.32 ± 0.03 b+ | 1.63 ± 0.049 b− | 2.23 ± 0.014 c− | 2.63 ± 0.025 c+ | 2.88 ± 0.019 d+ | 2.36 ± 0.034 d− |
| 3 August | 7.42 ± 0.013 a+ | 2.15 ± 0.023 a− | 8.77 ± 0.047 b+ | 4.72 ± 0.015 b− | 8.27 ± 0.044 c+ | 4.63 ± 0.03 c− | 9.81 ± 0.034 d+ | 5.92 ± 0.027 d− |
| 17 August | 1.01 ± 0.012 a+ | 1.07 ± 0.023 a+ | 1.2 ± 0.041 b− | 1.41 ± 0.045 b+ | 0.72 ± 0.021 c− | 1.19 ± 0.024 c+ | 0.8 ± 0.022 d− | 1.29 ± 0.038 d+ |
| 2 September | 0.44 ± 0.014 a− | 0.64 ± 0.007 a+ | 1.1 ± 0.045 b− | 1.9 ± 0.026 b+ | 0.36 ± 0.002 c+ | 0.33 ± 0.011 c+ | 0.41 ± 0.024 d+ | 0.46 ± 0.018 d+ |
| α-tocopherol mg/g | ||||||||
| 5 July | 103.59 ± 0.68 a− | 108.73 ± 0.85 a+ | 82.17 ± 0.57 b− | 93.67 ± 0.95 b+ | 113.57 ± 0.24 c+ | 88.26 ± 1.09 c− | 93.16 ± 1.27 d− | 102.38 ± 2.26 d+ |
| 19 July | 593.46 ± 4.24 a+ | 531.46 ± 3.26 a− | 644.39 ± 0.58 b+ | 568.63 ± 0.62 b− | 617.26 ± 0.57 c+ | 559.47 ± 0.89 c− | 601.73 ± 4.71 d+ | 584.19 ± 0.57 d− |
| 3 August | 539.24 ± 0.67 a+ | 433.18 ± 3.33 a− | 573.16 ± 1.79 b+ | 466.72 ± 2.12 b− | 427.75 ± 2.27 c− | 503.29 ± 3.51 c+ | 469.36 ± 2.61 d+ | 473.28 ± 2.62 d+ |
| 17 August | 436.49 ± 4.27 a+ | 316.13 ± 2.64 a− | 542.61 ± 0.58 b+ | 267.39 ± 0.62 b− | 309.68 ± 0.57 c+ | 290.39 ± 0.94 c− | 409.48 ± 2.47 d+ | 275.29 ± 1.27 d− |
| 2 September | 275.13 ± 3.74 a+ | 273.96 ± 1.74 a+ | 203.99 ± 0.93 b− | 298.91 ± 4.07 b+ | 205.61 ± 0.86 c− | 296.52 ± 0.41 c+ | 224.28 ± 1.74 d− | 247.39 ± 3.82 d+ |
| β-sitosterol mg/g | ||||||||
| 5 July | 3.23 ± 0.023 a− | 4.92 ± 0.019 a+ | 5.7 ± 0.032 b+ | 5.29 ± 0.032 b− | 4.85 ± 0.018 c− | 5.44 ± 0.024 c+ | 3.85 ± 0.02 d− | 4.33 ± 0.019 d+ |
| 19 July | 2.19 ± 0.025 a+ | 2.18 ± 0.019 a+ | 1.61 ± 0.029 b− | 2.33 ± 0.023 b+ | 2.41 ± 0.027 c+ | 1.63 ± 0.02 c− | 1.96 ± 0.02 d− | 2.18 ± 0.029 d+ |
| 3 August | 0.98 ± 0.017 a− | 1.63 ± 0.026 a+ | 1.84 ± 0.028 b+ | 1.22 ± 0.018 b− | 1.83 ± 0.026 c+ | 1.27 ± 0.016 c− | 1.1 ± 0.025 d− | 1.4 ± 0.033 d+ |
| 17 August | 1.21 ± 0.024 a+ | 0.84 ± 0.012 a− | 1.49 ± 0.018 b+ | 1.22 ± 0.025 b− | 0.96 ± 0.014 c+ | 0.7 ± 0.011 c− | 1.17 ± 0.032 d+ | 0.97 ± 0.01 d− |
| 2 September | 0.75 ± 0.012 a− | 1.15 ± 0.022 a+ | 0.64 ± 0.005 b− | 0.78 ± 0.023 b+ | 0.88 ± 0.015 c+ | 0.88 ± 0.013 c+ | 0.72 ± 0.008 d− | 0.79 ± 0.018 d+ |
| β-amyrinol mg/g | ||||||||
| 5 July | 3.77 ± 0.018 a+ | 2.52 ± 0.013 a− | 3.18 ± 0.009 b− | 3.47 ± 0.027 b+ | 3.56 ± 0.014 c− | 3.98 ± 0.027 c+ | 2.71 ± 0.026 d− | 3.15 ± 0.02 d+ |
| 19 July | 2.9 ± 0.016 a+ | 2.76 ± 0.02 a− | 2.56 ± 0.015 b+ | 2.35 ± 0.014 b− | 2.67 ± 0.027 c+ | 2.16 ± 0.026 c− | 2.28 ± 0.028 d− | 2.61 ± 0.015 d+ |
| 3 August | 2.61 ± 0.03 a+ | 2.56 ± 0.03 a+ | 2.7 ± 0.013 b+ | 1.82 ± 0.031 b− | 2.48 ± 0.027 c+ | 1.67 ± 0.011 c− | 2.03 ± 0.009 d− | 2.43 ± 0.007 d+ |
| 17 August | 1 ± 0.009 a+ | 0.99 ± 0.012 a+ | 1.25 ± 0.014 b+ | 1.21 ± 0.011 b− | 1.08 ± 0.014 c+ | 0.74 ± 0.006 c− | 1.17 ± 0.017 d+ | 0.86 ± 0.023 d− |
| 2 September | 1.04 ± 0.033 a+ | 0.96 ± 0.027 a− | 0.8 ± 0.03 b+ | 0.83 ± 0.015 b+ | 0.88 ± 0.033 c− | 1.25 ± 0.03 c+ | 0.87 ± 0.024 d− | 0.96 ± 0.009 d+ |
| Squalene mg/g | ||||||||
| 5 July | 2.56 ± 0.024 a+ | 3.49 ± 0.028 a− | 2.03 ± 0.011 b− | 2.81 ± 0.016 b+ | 2.57 ± 0.026 c− | 2.82 ± 0.019 c+ | 2.11 ± 0.035 d− | 2.31 ± 0.027 d+ |
| 19 July | 1.54 ± 0.019 a+ | 1.59 ± 0.019 a+ | 1.39 ± 0.028 b+ | 1.24 ± 0.032 b− | 1.45 ± 0.011 c+ | 1.33 ± 0.039 c− | 1.86 ± 0.022 d+ | 1.92 ± 0.031 d+ |
| 3 August | 1.19 ± 0.017 a− | 1.9 ± 0.026 a+ | 1.92 ± 0.025 b+ | 1.86 ± 0.022 b+ | 1.13 ± 0.019 c− | 1.69 ± 0.033 c+ | 1.44 ± 0.022 d+ | 1.9 ± 0.031 d− |
| 17 August | 0.53 ± 0.017 a− | 0.67 ± 0.013 a+ | 0.47 ± 0.008 b− | 0.55 ± 0.012 b+ | 0.45 ± 0.006 c− | 0.49 ± 0.023 c+ | 0.51 ± 0.015 d− | 0.55 ± 0.011 d+ |
| 2 September | 0.54 ± 0.005 a+ | 0.54 ± 0.013 a+ | 0.61 ± 0.018 b+ | 0.57 ± 0.005 b− | 0.59 ± 0.008 c+ | 0.55 ± 0.014 c− | 0.57 ± 0.013 d+ | 0.53 ± 0.008 d− |
| LK29 | LK15 | KH13 | KH03 | |||||
|---|---|---|---|---|---|---|---|---|
| High Altitude | Low Altitude | High Altitude | Low Altitude | High Altitude | Low Altitude | High Altitude | Low Altitude | |
| 12:0 | 0.96 ± 0.16 a+ | 0.05 ± 0.008 a− | 0.04 ± 0.003 b− | 0.24 ± 0.01 b+ | 0.28 ± 0.02 c+ | 0.02 ± 0.003 c− | 0.26 ± 0.02 d+ | 0.1 ± 0.004 d− |
| 14:0 | 0.11 ± 0.02 a+ | 0.08 ± 0.009 a− | 0.12 ± 0.01 b− | 0.15 ± 0.01 b+ | 0.09 ± 0.002 | 0.11 ± 0.003 | 0.09 ± 0.01 | 0.11 ± 0.01 |
| 16:0 | 8.7 ± 0.29 a− | 10.27 ± 0.51 a+ | 9.3 ± 0.28 | 9.72 ± 0.22 | 10.76 ± 0.34 c+ | 9.49 ± 0.43 c− | 8.76 ± 0.34 | 9.52 ± 0.51 |
| 9 t16:1 | 0.04 ± 0.002 | 0.04 ± 0.002 | 0.05 ± 0.001 b+ | 0.03 ± 0.001 b− | 0.06 ± 0.003 c+ | 0.05 ± 0.001 c− | 0.06 ± 0.003 d+ | 0.03 ± 0.001 d− |
| 9 c16:1 | 0.12 ± 0.01 | 0.11 ± 0.01 | 0.11 ± 0.005 b+ | 0.08 ± 0.001 b− | 0.09 ± 0.001 c+ | 0.07 ± 0.001 c− | 0.12 ± 0.01 d+ | 0.08 ± 0.001 d− |
| 17:0 | 0.11 ± 0.01 | 0.11 ± 0.008 | 0.08 ± 0.004 | 0.09 ± 0.003 | 0.09 ± 0.006 c− | 0.12 ± 0.01 c+ | 0.08 ± 0.01 d− | 0.11 ± 0.01 d+ |
| 10 c17:1 | 0.11 ± 0.002 a+ | 0.06 ± 0.001 a− | 0.07 ± 0.003 b− | 0.08 ± 0.004 b+ | 0.06 ± 0.004 c− | 0.07 ± 0.002 c+ | 0.07 ± 0.003 d− | 0.08 ± 0.001 d+ |
| 18:0 | 5.29 ± 0.26 | 5.74 ± 0.27 | 5.79 ± 0.13 | 6.08 ± 0.35 | 4.58 ± 0.21 | 4.59 ± 0.31 | 5.47 ± 0.21 | 5.49 ± 0.35 |
| 9 c18:1 | 78.93 ± 2.59 | 78.77 ± 1.02 | 77.72 ± 1.55 | 77.27 ± 0.7 | 75.74 ± 1.38 | 78.19 ± 0.41 | 77.36 ± 1.85 | 79.09 ± 1.01 |
| 18:2 n-6 | 5.2 ± 0.17 a+ | 4.27 ± 0.26 a− | 6.22 ± 0.22 | 5.58 ± 0.38 | 7.63 ± 0.46 c+ | 6.71 ± 0.11 c− | 7.21 ± 0.27 d+ | 4.86 ± 0.33 d− |
| 18:3 n-3 | 0.43 ± 0.03 | 0.50 ± 0.02 | 0.49 ± 0.04 | 0.68 ± 0.53 | 0.61 ± 0.03 | 0.59 ± 0.02 | 0.52 ± 0.03 | 0.53 ± 0.46 |
| Parameters | AUC |
|---|---|
| α-tocopherol | 0.875 * |
| Squalene | 0.813 * |
| β-sitosterol | 0.844 * |
| β-amyrinol | 0.688 |
| Oil yield | 0.938 * |
| Total flavonoids | 0.750 |
| Total polyphenols | 0.625 |
| 16:0 | 0.750 |
| 18:0 | 0.688 |
| 9 c18:1 | 0.688 |
| 18:2 n-6 | 0.823 * |
| 18:3 n-3 | 0.750 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, T.; Zhong, S.; Huang, B.; Zha, K.; Li, J.; Wen, Q. Influence of Environmental Conditions Associated with Low and High Altitudes on Economic and Quality Characteristics of Fruit Ripening of Camellia chekiangoleosa Hu. Foods 2025, 14, 2266. https://doi.org/10.3390/foods14132266
Wei T, Zhong S, Huang B, Zha K, Li J, Wen Q. Influence of Environmental Conditions Associated with Low and High Altitudes on Economic and Quality Characteristics of Fruit Ripening of Camellia chekiangoleosa Hu. Foods. 2025; 14(13):2266. https://doi.org/10.3390/foods14132266
Chicago/Turabian StyleWei, Teng, Shengyue Zhong, Bin Huang, Kang Zha, Jing Li, and Qiang Wen. 2025. "Influence of Environmental Conditions Associated with Low and High Altitudes on Economic and Quality Characteristics of Fruit Ripening of Camellia chekiangoleosa Hu" Foods 14, no. 13: 2266. https://doi.org/10.3390/foods14132266
APA StyleWei, T., Zhong, S., Huang, B., Zha, K., Li, J., & Wen, Q. (2025). Influence of Environmental Conditions Associated with Low and High Altitudes on Economic and Quality Characteristics of Fruit Ripening of Camellia chekiangoleosa Hu. Foods, 14(13), 2266. https://doi.org/10.3390/foods14132266






